Abstract
Purpose
CALGB 49907 showed the superiority of standard therapy, which included either cyclophosphamide/doxorubicin (AC) or cyclophosphamide/methotrexate/fluorouracil over single-agent capecitabine in the treatment of patients age ≥ 65 with early-stage breast cancer. The treatment allowed dosing adjustments of methotrexate and capecitabine for pretreatment renal function. The purpose of the current analysis was to assess the relationship between pretreatment renal function and five end points: toxicity, dose modification, therapy completion, relapse-free survival, and overall survival.
Methods
Pretreatment renal function was defined as creatinine clearance (CrCl) using the Cockcroft-Gault equation. Multivariable logistic and proportional hazards regression were used to model separately for each regimen the relationship between CrCl and the first three binary end points and the last two time-to-event end points, respectively, after adjusting for variables of prognostic importance.
Results
Six hundred nineteen assessable patients were analyzed. The incidence of stage III (moderate) or stage IV (severe) renal dysfunction was 72%, 64%, and 75% for treatment with cyclophosphamide/methotrexate/fluorouracil, AC, and capecitabine, respectively. There was no relationship for any regimen between pretreatment renal function and the five end points. For AC, as CrCl increased, the odds of nonhematologic toxicity decreased (P = .008), whereas for capecitabine, as CrCl increased, the odds of experiencing toxicity of any type also increased (P = .035). Patients with renal insufficiency who received dose modifications were not at increased risk for complications compared with those who did not have renal insufficiency and received a full dose.
Conclusion
Excluding from clinical trials patients with renal insufficiency but good performance status on the basis of concern of excessive hematologic toxicity or poor outcomes may not be justified with appropriate dosing modifications. Results should be considered in the design of clinical trials for older patients.
INTRODUCTION
Renal dysfunction is a common comorbidity in older cancer patients.1-5 It has significant consequences, including exclusion from clinical trials, increased toxicity from therapy, and potentially poorer clinical outcomes. Much of the information regarding the association of renal insufficiency and treatment tolerance in older adult patients with cancer has been derived from retrospective subset analyses of clinical trials in which older patients were a small fraction of participants. Cancer and Leukemia Group B study (CALGB/CTSU) 49907, which was designed exclusively for older patients, thus provides an opportunity to evaluate the effect of baseline renal dysfunction on various clinical outcomes. CALGB 49907 was a prospective phase III trial for the adjuvant treatment of women age ≥ 65 with breast cancer. Patients were randomly assigned to either standard combination therapy or single-agent treatment. Standard therapy consisted of either CMF (cyclophosphamide/methotrexate/fluorouracil) or AC (cyclophosphamide/doxorubicin), chosen at physician discretion. Single-agent treatment was the oral fluoropyrimidine, capecitabine.6 The purpose of the current study (A171201, Alliance for Clinical Trials in Oncology) was to determine the relationship between pretreatment renal function and clinical outcomes as well as toxicity in patients treated in CALGB 499076.
METHODS
Eligibility
Women age ≥ 65 who had operable, histologically confirmed adenocarcinoma of the breast, with a performance status of 0 to 2 (according to National Cancer Institute criteria) and a tumor diameter that was greater than 1 cm were eligible for the treatment on protocol CALGB 49907; status with respect to estrogen receptor, progesterone receptor, and human epidermal growth factor receptor type 2 was not specified as an eligibility criterion. Adequate hematologic, renal (CrCl ≥ 30 mL/min using Cockcroft-Gault [C-G] equation), and hepatic function as well as clear surgical margins for the invasive component of the tumor were required. Patients with hormone receptor–positive tumors were offered tamoxifen or an aromatase inhibitor after chemotherapy. Patients had to have an expected survival of greater than 5 years and no medical condition that would make treatment with this protocol unreasonably hazardous. Exclusion criteria included any other active cancer or a previous cancer with a risk of relapse greater than 30%.
Treatment
Patients were randomly assigned with equal probability to standard chemotherapy (CMF or AC) or capecitabine. The choice of standard chemotherapy regimen was made at the discretion of the patient or her physician. The CMF regimen consisted of cyclophosphamide 100 mg/m2 administered orally from days 1 through 14 and methotrexate 40 mg/m2 and fluorouracil 600 mg/m2 administered intravenously on days 1 and 8. The cycle was repeated every 28 days for six cycles. The AC regimen consisted of doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 administered intravenously on day 1. The cycle was repeated every 21 days for four cycles. Capecitabine 2,000 mg/m2 was administered daily for 14 days followed by a 7-day rest period for six cycles.
As a measure of renal function, pretreatment CrCl was calculated using the modified C-G equation.7 Eligibility required a calculated CrCl of greater than 30 mL/min. Initial dose calculations in the protocol took the following into account: methotrexate doses were adjusted if the initial CrCl was 30 to 80 mL/min (30-50 mL/min, 20 mg/m2; 51-80 mL/min, 30 mg/m2; > 80 mL/min, 40 mg/m2); capecitabine doses were adjusted if CrCl was 30 to 50 mL/min (1,500 mg/m2); and the actual weight of the patient was adjusted to ideal body weight if the actual weight was greater than 30% of ideal body weight.
The first 56 patients assigned to capecitabine received a dose of 2,000 mg/m2 per day in two divided doses for 14 consecutive days, every 3 weeks, for a total of six cycles, and the dose was increased to 2,500 mg/m2 if they had no toxic effects after the first course. Because the toxicity of this regimen was unacceptable, the protocol was amended to eliminate the dose escalation. Dose modifications for all regimens were based on standard National Cancer Institute toxicity criteria. All patients provided written institutional review board–approved informed consent that met state, federal, and institutional guidelines.
Statistical Analysis
In the current analysis, the primary independent variable of interest was baseline renal function, which was defined as pretreatment CrCl, calculated using the modified C-G formula. CrCl was also categorized into levels of renal dysfunction defined by the National Kidney Foundation.8 The level of renal dysfunction with their corresponding CrCl ranges were as follows: 1, normal (≥ 90 mL/min); 2, mild (60-89 mL/min); 3, moderate (30-59 mL/min); and 4, severe (15-29 mL/min). Because of the small number of 1 and 4 patients, renal dysfunction was split into early (normal and mild, 1 and 2) and late (moderate and severe, 3 and 4). Renal function was analyzed both on a continuous scale as CrCl and as a dichotomous variable as stage of kidney dysfunction (early 1 and 2 versus late 3 and 4). Because results were similar for both the continuous and categorical forms but the continuous was more predictive, the results shown are from modeling the continuous variable. There were five end points, the occurrence or not of any treatment-related grade ≥ 3 toxicity9; the occurrence or not of any dose modification; the completion or not of all protocol therapy; relapse-free survival (RFS); and overall survival (OS). The toxicity end point was further categorized by to hematologic, nonhematologic, and overall (any type) toxicity. RFS was defined as the interval from study entry until first disease recurrence regardless of site or death from any cause, whichever occurred first. Recurrence-free survivors were censored at the date last known to be alive and free from recurrence. OS was defined as the interval from study entry until death from any cause. Surviving patients were censored at the last date known to be alive. Multiple logistic regression was used to model the relationship between pretreatment renal function and the first three dichotomous end points. Adjusted odds ratios (aORs) and their 95% CIs were taken from corresponding logistic models. Multiple proportional hazards regression was used to model the relationship between pretreatment renal function and OS and RFS; adjusted hazard ratios (aHRs) and their 95% CIs were taken from corresponding models. The statistical significance of aORs and aHRs were obtained from Wald χ2 tests from respective multivariable models. Multivariable models included as covariables the demographics of patient age and race and the clinical variables used in the primary treatment analysis6 of number of positive nodes, tumor size, and hormone receptor status. Although CrCl was analyzed on a linear scale, tables show aORs and aHRs for 10-unit increments for descriptive purposes. Comparison of two or more proportions was assessed by χ2 test. Because CALGB 49907 reported differences in both efficacy and toxicity by treatment regimens of AC, CMF, and capecitabine, each end point was assessed separately by treatment regimen.
All tests were performed under a two-sided α level of .05 and are presented as statistically significant if their P values were less than .05. However, as they are unadjusted for multiplicities, the true α level is indeterminate but larger than .05. Study data were collected by CALGB/Alliance data coordinators and stored in the CALGB/Alliance database. Analyses of data available in the database as of February 2015 were performed by CALGB/Alliance statisticians using SAS (SAS/STAT User’s Guide, Version 9.2; SAS Institute, Cary, NC).
RESULTS
Between September 2001 and December 2006, CALGB 49907 accrued 633 patients, 622 of whom received protocol therapy. Among treated patients, 619 had pretreatment CrCl data and they comprise the analyzable sample for this report. In this sample, 204 patients died and 415 were censored (388 patients were alive and in active follow-up, 21 withdrew consent to be followed, and six were lost to follow-up). The median follow-up time for censored patients was 8.7 years, with a maximum of 12.4 years.
Table 1 shows demographics and baseline laboratory results. Patient age, Zubrod (ECOG) performance score, and tumoral hormone receptor were well balanced among the three regimens. More than one half of the patients were between age 70 and 79. Nearly all had a performance score of 0 or 1. Approximately one third of the sample had hormone receptor (estrogen receptor/progesterone receptor)–negative tumors. The incidence of node-negative disease was slightly lower for patients receiving CMF (23%) compared with those receiving AC (32%) or capecitabine (31%). Overall, patients presented with a baseline CrCl range of between 22 and 112 mL/min. The distribution of pretreatment CrCl was similar among the three regimens with 25th percentiles, medians, and 75th percentiles of 41, 51, and 62 for CMF; 43, 53, and 67 for AC; and 42, 50, and 60 for capecitabine, respectively. In each regimen, most patients had moderate baseline renal dysfunction (CrCl, 30-59 mL/min); few patients had normal renal function (CrCl, > 90 mL/min) or severe renal function (CrCl, 15-29 mL/min). Seventy-three percent of patients receiving CMF and 75% of patients receiving capecitabine had high stage (moderate/severe) renal dysfunction compared with only 64% of patients receiving AC (P = .030). Errors in creatinine clearance calculations led to nine patients being treated with CrCl less than 30 mL/min.
Table 1.
Patient Demographics and Baseline Disease Features
| Variable | Regimen | ||
|---|---|---|---|
| CMF | AC | Capecitabine | |
| Patients | 135 (100) | 182 (100) | 302 (100) |
| Age, years | |||
| 65-69 | 36 (27) | 73 (40) | 108 (36) |
| 70-80 | 92 (68) | 102 (56) | 180 (60) |
| ≥ 80 | 7 (5) | 7 (4) | 14 (5) |
| Performance score | |||
| 0 or 1 | 131 (97) | 177 (97) | 290 (96) |
| 2 | 4 (3) | 5 (3) | 12 (4) |
| Race | |||
| White | 117 (87) | 153 (84) | 258 (85) |
| Black | 16 (12) | 25 (14) | 28 (9) |
| Other | 2 (1) | 4 (3) | 16 (6) |
| Receptor | |||
| Both ER/PgR-negative | 43 (32) | 60 (33) | 95 (31) |
| Either ER/PgR-positive | 91 (67) | 122 (67) | 206 (68) |
| Missing | 1 (1) | 0 (0) | 1 (< 1) |
| Tumor size, cm | |||
| ≤ 2 | 68 (50) | 85 (47) | 120 (40) |
| > 2 and ≤ 5 | 57 (42) | 88 (48) | 165 (55) |
| > 5 | 9 (7) | 9 (5) | 17 (6) |
| Missing | 1 (1) | 0 (0) | 0 (0) |
| No. of positive nodes | |||
| 0 | 31 (23) | 58 (32) | 95 (31) |
| 1-3 | 73 (54) | 103 (57) | 154 (51) |
| ≥ 4 | 31 (23) | 20 (11) | 49 (16) |
| Missing | 0 (0) | 1 (1) | 4 (1) |
| Calculated CrCl, mL/min | |||
| ≤ 40 | 27 (20) | 31 (17) | 56 (19) |
| 41-49 | 35 (26) | 45 (25) | 88 (29) |
| 50-59 | 36 (27) | 40 (22) | 82 (27) |
| ≥ 60 | 37 (27) | 66 (36) | 76 (25) |
| Renal function by level | |||
| 1 (normal) | 3 (2) | 5 (3) | 5 (2) |
| 2 (mild) | 34 (25) | 61 (34) | 71 (24) |
| 3 (moderate) | 95 (70) | 112 (62) | 224 (74) |
| 4 (severe) | 3 (2) | 4 (2) | 2 (1) |
| 5 (renal failure) | 0 (0) | 0 (0) | 0 (0) |
| Renal function dichotomized by level | |||
| Level 1 and 2 | 62 (46) | 76 (42) | 144 (48) |
| Level 3 and 4 | 73 (54) | 106 (58) | 158 (52) |
NOTE. Data are given as No. (%) unless otherwise noted.
Abbreviations: AC, cyclophosphamide/doxorubicin; CMF, cyclophosphamide/methotrexate/fluorouracil; CrCl, creatinine clearance; ER, estrogen receptor; PgR, progesterone receptor.
Dose Modification
Dose modification refers to modification at any time during treatment. Overall, the incidence of any dose modification on the AC regimen was considerably lower than that on either the CMF or capecitabine regimens. However, within each regimen, the incidence of dose modification was similar for patients with early versus late stages of renal dysfunction (Table 2). Baseline renal function was not related to dose modification for any regimen.
Table 2.
Adjusted Effect of Baseline Creatinine Clearance on Study End Points by Regimen
| End Point by Regimen | % Who Met End Point* | OR/HR (95% CI)† | P‡ |
|---|---|---|---|
| Was dose modified | |||
| CMF | 71/70 | 1.14 (0.85 to 1.52) | NS |
| AC | 18/12 | 1.13 (0.84 to 1.49) | NS |
| Capecitabine | 68/61 | 1.10 (0.91 to 1.34) | NS |
| Completed all treatment | |||
| CMF | 65/65 | 0.90 (0.69 to 1.18) | NS |
| AC | 94/94 | 0.96 (0.62 to 1.48) | NS |
| Capecitabine | 84/80 | 1.15 (0.90 to 1.47) | NS |
| Experienced grade ≥ 3 AE | |||
| Hematologic | |||
| CMF | 49/52 | 0.92 (0.72 to 1.18) | NS |
| AC | 56/53 | 0.97 (0.78 to 1.20) | NS |
| Capecitabine | 3/2 | 1.37 (0.80 to 2.35) | NS |
| Nonhematologic | |||
| CMF | 41/40 | 1.07 (0.83 to 1.38) | NS |
| AC | 14/31 | 0.69 (0.52 to 0.91) | .008 |
| Capecitabine | 39/31 | 1.21 (1.00 to 1.47) | .052 |
| Either hematologic or nonhematologic | |||
| CMF | 70/68 | 1.05 (0.80 to 1.38) | NS |
| AC | 59/59 | 0.93 (0.75 to 1.15) | NS |
| Capecitabine | 40/32 | 1.23 (1.01 to 1.45) | .035 |
| RFS§ | |||
| CMF | 27/39 | 1.06 (0.86 to 1.33) | NS |
| AC | 34/36 | 1.08 (0.90 to 1.28) | NS |
| Capecitabine | 34/45 | 0.93 (0.80 to 1.08) | NS |
| OS§ | |||
| CMF | 22/33 | 1.10 (0.87 to 1.41) | NS |
| AC | 27/33 | 1.05 (0.87 to 1.27) | NS |
| Capecitabine | 29/38 | 0.92 (0.78 to 1.09) | NS |
Abbreviations: AC, cyclophosphamide/doxorubicin; AE, adverse event; CMF, cyclophosphamide/methotrexate/fluorouracil; HR, hazard ratio; NS, not significant; OR, odds ratio; OS, overall survival; RFS, relapse-free survival.
Stage of renal function at baseline (early stage/late stage).
OR for the following end points: was dose modified, completed all treatment, and experienced grade ≥ 3 toxicity; taken from the corresponding logistic regression. The OR represents a 10-unit increase in baseline creatinine clearance. HR for the following end points: RFS and OS; taken from the corresponding proportional hazards regression. The HR represents a 10-unit increase in baseline creatinine clearance. ORs and HRs are adjusted for race, age, number of positive nodes, tumor size, and hormone receptor status.
From Wald χ2 test of main effect of creatinine clearance from multivariable models.
Met end point for RFS or OS means the patient experienced an RFS or OS event, respectively.
Completion of All Protocol Treatment
Table 2 also shows the relationship of renal function with treatment protocol completion: whether patients completed all treatment per protocol or terminated treatment early for any reason. Overall, the CMF regimen had the lowest proportion of patients who completed treatment, whereas the AC regimen had the highest proportion. Renal function was not related to treatment completion.
Toxicity
Of the 619 assessable patients, 614 had adverse event data. Table 2 summarizes the incidence of any hematologic toxicity, any nonhematologic toxicity, and any toxicity regardless of the type that occurred during protocol therapy. Pretreatment renal function did not influence the occurrence of hematologic toxicity regardless of regimen. Within treatment regimens, the incidence of hematologic toxicity was similar for early and late renal dysfunction. Baseline renal function was highly related to the occurrence of nonhematologic toxicity for the AC regimen and very mildly for the capecitabine regimen, but not related for the CMF regimen. For patients treated with AC, every 10-unit increase in CrCl was associated with a 31% decrease in the odds of experiencing at least one nonhematologic toxicity (aOR, 0.69; 95% CI, 0.52 to 0.91; P = .008). For capecitabine-treated patients, each 10-unit increase in CrCl was associated with a 21% increase in the odds of experiencing nonhematologic toxicity (aOR, 1.21; 95% CI, 1.00 to 1.47; P = .052). This unusual finding occurred because 26% of patients with higher CrCl (early renal function level 1 and 2) experienced grade ≥ 3 rash (hand-foot reaction) compared with 12% of patients with lower CrCl (late renal function level 3 and 4). The incidence of other toxicities was similar between early and late stage renal function. The one toxicity of rash affected the overall nonhematologic toxicity finding for capecitabine.
The probability of experiencing any toxicity increased at a steady rate over the six-cycle capecitabine treatment beginning at the end of cycle one. By the end of treatment, the probability of first experiencing at least one toxicity was approximately 47%. In contrast, the probability of first experiencing rash was highest at the end of cycles two (approximately 5%) and three (approximately 11%), and thereafter generally plateaued.
OS and RFS
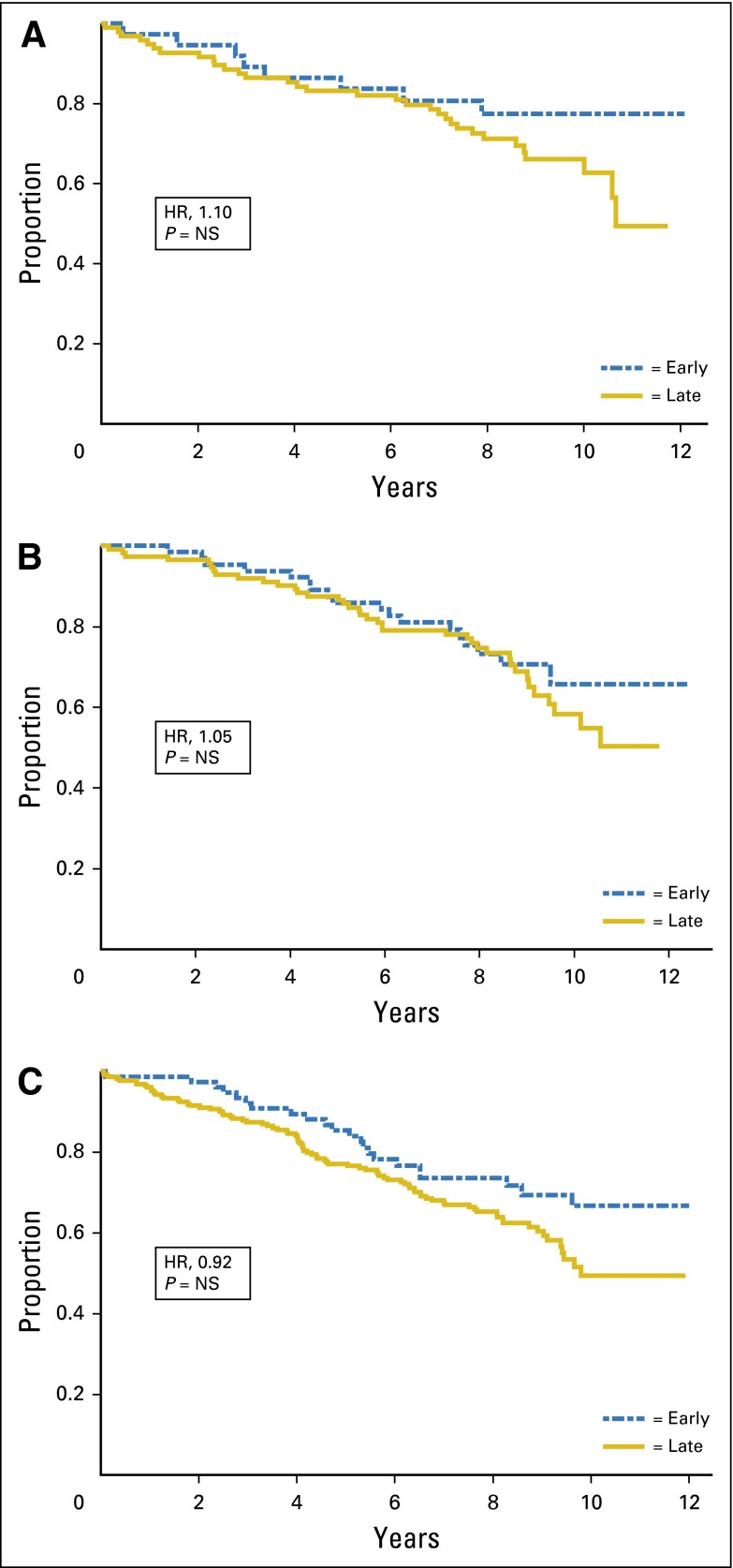
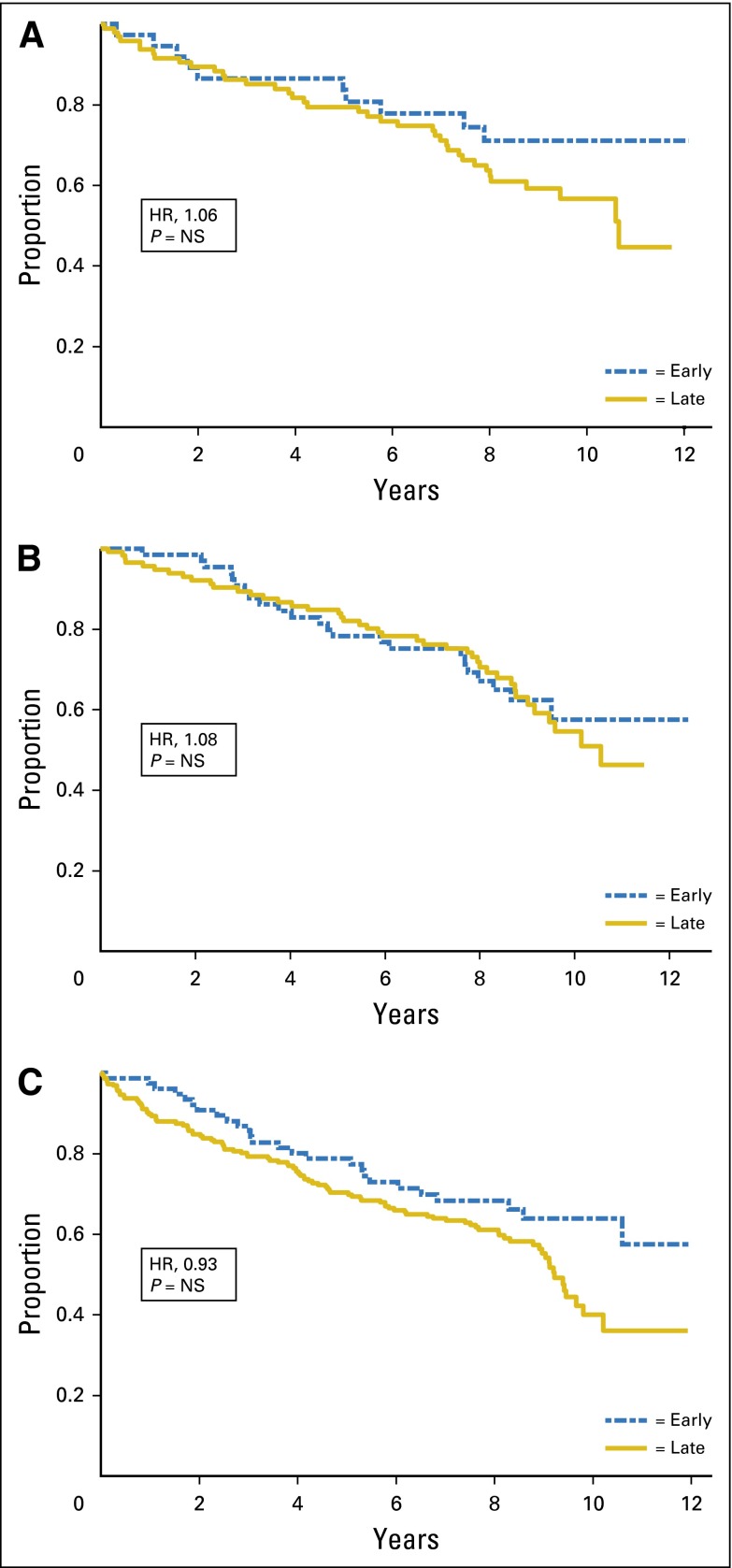
After controlling for standard prognostic factors, renal function was not predictive of either OS or RFS regardless of regimen. Figures 1 and 2 show the relationship of renal function and OS and RFS, respectively, by stage of renal function, unadjusted for prognostic variables.
Fig 1.
Overall survival by renal function stage. (A) Cyclophosphamide/methotrexate/fluorouracil; (B) cyclophosphamide/doxorubicin; and (C) capecitabine. HR, hazard ratio; NS, not significant.
Fig 2.
Relapse-free survival by renal function stage. (A) Cyclophosphamide/methotrexate/fluorouracil; (B) cyclophosphamide/doxorubicin; and (C) capecitabine. HR, hazard ratio; NS, not significant.
DISCUSSION
Baseline CrCl did not predict whether a patient would receive dose modification, complete treatment per protocol, or experience hematologic toxicity for any regimen. It did, however, predict nonhematologic toxicity as follows. For AC, increased CrCl was associated with decreased risk (P = .008); for capecitabine, the relationship was reversed, with increased CrCl predicting increased risk (P = .052). The finding among patients receiving capecitabine was attributed to the incidence of rash (hand-foot reaction) among early stage 1 and 2 patients being double that of late stage 3 and 4 patients (26% versus 12%, respectively). After adjusting for relevant covariates, baseline renal function was not predictive of OS and RFS for any regimen.
Patients receiving AC with worse renal function had increased risk of grade 3 nonhematologic toxicity. It is difficult to determine whether this increased toxicity resulted from renal insufficiency or other factors. Dose reduction for AC was not required for renal insufficiency in this trial as it is usually not recommended for the range of renal function seen in patients participating in clinical trials. Doxorubicin and its main active metabolite, doxorubicinol, are not predominantly eliminated in the urine. Studies on renal insufficiency show that drug exposure, as measured by area under the curve, is greater in patients with renal insufficiency than in patients with normal renal function. The half-lives of these two compounds are the same in both groups; therefore, doxorubicin may not need dose adjustment in patients with renal insufficiency.10-12 For cyclophosphamide, nonrenal mechanisms of elimination predominate and dose reduction with renal insufficiency is not required. Dose reductions may be important for patients with more severe renal impairment (ie, CrCl < 30 mL/min). Whereas patients with CrCl less than 30 mL/min were not eligible for the trial, calculations of renal function are imperfect, and there can be significant intrapatient variability over time.13,14
There seems to be a more complex relationship with capecitabine and renal function. The increase in grade ≥ 3 rash in patients with renal dysfunction levels 1 and 2 at baseline can be a reflection of an increased dose in patients who had required a dose modification at baseline. These conclusions are speculative and deserve further study.
The increased comorbidity associated with aging often prevents participation in clinical trials because patients with end organ dysfunction are excluded. Renal dysfunction is a common comorbidity resulting from the predictable physiologic decline with aging and is often compounded by other issues, such as hypertension, vascular disease, and medications; it has been associated with excess mortality.15 Two studies have observed a high prevalence of renal insufficiency of 33% glomerular filtration rate (GFR; < 80 mL/min) and 27% (GFR < 90 mL/min) among patients with cancer.1,16 A total of 50% to 60% of patients with cancer in the IRMA-1 study had a GFR less than 90 mL/min, whereas serum creatinine levels were normal in most patients.4,17 The importance of these findings is that many anticancer drugs are excreted primarily in the urine as unchanged drug or active metabolites. Therefore, reduction in renal function can potentially lead to alterations in pharmacokinetics, elevated blood levels of the drugs, and increased toxicity. This has led to the development of safety guidelines in patients with renal insufficiency.2,3
There have been few studies to evaluate the effects of renal insufficiency on toxicity and treatment outcomes. A Gynecologic Oncology Group database analysis examined carboplatin dosing. There was no excess of toxicity among patients with CrCl less than 60 mL/min, and such a restriction would have unnecessarily excluded 15% of patients from the trial.18 In a study of bladder cancer, the association between CrCl measured by 12 to 24 urine collections and calculated CrCl was examined. The ability to complete at least three cycles was statistically significantly related with a measured CrCl greater than 60 mL/min (P = .02), but not with calculated CrCl greater than 60 mL/min. The authors concluded that the current formulas estimating CrCl tend to underestimate measured CrCl, especially in patients older than 65 years, and that these patients may be inappropriately excluded from cisplatin-based therapy.19
There are a number of limitations of this data analysis—this was a retrospective analysis, which has inherent limitations. However, this evaluation was performed on a prospective trial that exclusively enrolled older patients and not on a subset analysis of a larger trial in which older patients were a minority. The primary variable of interest, renal function, was calculated using the modified C-G equation at protocol enrollment. There have been various renal function equations proposed as the preferred method of calculation.20,21 Each of these methods has their limitations as does measured CrCl.22 Because the C-G equation is the most common in practice, it was taken to be the most appropriate. The development of the toxicities could have been a result of other comorbidities or an interaction of other comorbidities and renal insufficiency. Therapy-related toxicity is complex, and factors such as performance status, functional status, end organ function, bone marrow reserve, and genetics can also play a significant role.
The results of this analysis suggest that with appropriate dose modification, patients with varying degrees of renal insufficiency should be able to tolerate standard therapies when combined with appropriate supportive care measure. Older patients eligible for clinical trials are a small subset of the overall older patient population and would be expected to have less toxicity and better outcomes. However, these results can provide guidance and some reassurance to clinicians treating patients with renal insufficiency. Because of the increased incidence of renal dysfunction in older patients, these findings are particularly important. The current analysis emphasizes that prior to the administration of chemotherapy, an estimate of renal function should be obtained. When a formula such as C-G is used, the results should be evaluated with the known shortcomings of this calculation23,24; however, it can still add valuable information and can assist in appropriate dose modification. The general exclusion of patients with renal insufficiency from studies may not be justified, particularly if the drug of interest does not have renal excretion as its primary mechanism of elimination. This has unnecessarily decreased the already low participation of older patients in studies. Clinical trial design, data reporting, and treatment guidelines need to emphasize these factors.25-27
Footnotes
Supported by the National Cancer Institute of the National Institutes of Health under Grants No. U10CA180821, U10CA180882, and 1UG1CA189823 to the Alliance for Clinical Trials in Oncology, as well as the following: 1U10CA180791, 1U10CA180790, 1U10CA180802, 1U10CA180867, 1U10CA180857, and 1U10CA180838.
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Stuart M. Lichtman, Constance T. Cirrincione, Arti Hurria, Harvey Jay Cohen, Hyman B. Muss
Provision of study materials or patients: Hyman B. Muss
Collection and assembly of data: Stuart M. Lichtman, Constance T. Cirrincione, Arti Hurria, Harvey Jay Cohen, Hyman B. Muss
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Effect of Pretreatment Renal Function on Treatment and Clinical Outcomes in the Adjuvant Treatment of Older Women With Breast Cancer: Alliance A171201, an Ancillary Study of CALGB/CTSU 49907
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or www.jco.ascopubs.org/site/ifc.
Stuart M. Lichtman
Consulting or Advisory Role: Icore Health Care, Bayer AG,
Speakers' Bureau: Plexus Communications
Travel, Accommodations, Expenses: Bayer AG
Constance T. Cirrincione
No relationship to disclose
Arti Hurria
Consulting or Advisory Role: GTx, Seattle Genetics, Boehringer Ingelheim, On Q Health, OptumHealth Care Solutions
Research Funding: GlaxoSmithKline (Inst), Celgene (Inst)
Aminah Jatoi
Research Funding: AVEO, Boston Biologics
Maria Theodoulou
No relationship to disclose
Antonio C. Wolff
Consulting or Advisory Role: Mersana
Research Funding: Myriad Genetics (Inst), Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: cMethDNA assay
Julie Gralow
Consulting or Advisory Role: Novartis, Genentech, Eli Lilly, Bayer AG, Pfizer, Merck
Research Funding: Genentech (Inst), Novartis (Inst), Amgen (Inst)
Daniel E. Morganstern
No relationship to disclose
Gustav Magrinat
No relationship to disclose
Harvey Jay Cohen
No relationship to disclose
Hyman B. Muss
Consulting or Advisory Role: Pfizer
REFERENCES
- 1.Launay-Vacher V, Izzedine H, Rey JB, et al. Incidence of renal insufficiency in cancer patients and evaluation of information available on the use of anticancer drugs in renally impaired patients. Med Sci Monit. 2004;10:CR209–CR212. [PubMed] [Google Scholar]
- 2.Lichtman SM, Wildiers H, Launay-Vacher V, et al. International Society of Geriatric Oncology (SIOG) recommendations for the adjustment of dosing in elderly cancer patients with renal insufficiency. Eur J Cancer. 2007;43:14–34. doi: 10.1016/j.ejca.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Launay-Vacher V, Chatelut E, Lichtman SM, et al. International Society of Geriatric Oncology Renal insufficiency in elderly cancer patients: International Society of Geriatric Oncology clinical practice recommendations. Ann Oncol. 2007;18:1314–1321. doi: 10.1093/annonc/mdm011. [DOI] [PubMed] [Google Scholar]
- 4.Launay-Vacher V, Oudard S, Janus N, et al. Renal Insufficiency and Cancer Medications (IRMA) Study Group Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: The Renal Insufficiency and Anticancer Medications (IRMA) study. Cancer. 2007;110:1376–1384. doi: 10.1002/cncr.22904. [DOI] [PubMed] [Google Scholar]
- 5.Launay-Vacher V, Spano JP, Janus N, et al. Renal Insufficiency and Anticancer Medications (IRMA) Study Group Renal insufficiency and anticancer drugs in elderly cancer patients: A subgroup analysis of the IRMA study. Crit Rev Oncol Hematol. 2009;70:124–133. doi: 10.1016/j.critrevonc.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Muss HB, Berry DA, Cirrincione CT, et al. CALGB Investigators Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–2065. doi: 10.1056/NEJMoa0810266. [Erratum: N Engl J Med 361:1714, 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 8.National Kidney Foundation KDOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. http://www2.kidney.org/professionals/KDOQI/guidelines_ckd/toc.htm. [DOI] [PubMed]
- 9.National Cancer Institute Cancer therapy evaluation program: Common toxicity criteria manual, version 2.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcmanual_v4_10-4-99.pdf.
- 10.Launay-Vacher V, Gligorov J, Le Tourneau C, et al. Renal Insufficiency and Anticancer Medications (IRMA) Study Group Prevalence of renal insufficiency in breast cancer patients and related pharmacological issues. Breast Cancer Res Treat. 2010;124:745–753. doi: 10.1007/s10549-008-0131-1. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida H, Goto M, Honda A, et al. Pharmacokinetics of doxorubicin and its active metabolite in patients with normal renal function and in patients on hemodialysis. Cancer Chemother Pharmacol. 1994;33:450–454. doi: 10.1007/BF00686499. [DOI] [PubMed] [Google Scholar]
- 12.Janus N, Thariat J, Boulanger H, et al. Proposal for dosage adjustment and timing of chemotherapy in hemodialyzed patients. Ann Oncol. 2010;21:1395–1403. doi: 10.1093/annonc/mdp598. [DOI] [PubMed] [Google Scholar]
- 13.Haubitz M, Bohnenstengel F, Brunkhorst R, et al. Cyclophosphamide pharmacokinetics and dose requirements in patients with renal insufficiency. Kidney Int. 2002;61:1495–1501. doi: 10.1046/j.1523-1755.2002.00279.x. [DOI] [PubMed] [Google Scholar]
- 14.Juma FD, Rogers HJ, Trounce JR. Effect of renal insufficiency on the pharmacokinetics of cyclophosphamide and some of its metabolites. Eur J Clin Pharmacol. 1981;19:443–451. doi: 10.1007/BF00548589. [DOI] [PubMed] [Google Scholar]
- 15.Breton G, Froissart M, Janus N, et al. Inappropriate drug use and mortality in community-dwelling elderly with impaired kidney function: The Three-City population-based study. Nephrol Dial Transplant. 2011;26:2852–2859. doi: 10.1093/ndt/gfq827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dogan E, Izmirli M, Ceylan K, et al. Incidence of renal insufficiency in cancer patients. Adv Ther. 2005;22:357–362. doi: 10.1007/BF02850082. [DOI] [PubMed] [Google Scholar]
- 17.Janus N, Launay-Vacher V, Byloos E, et al. Cancer and renal insufficiency results of the BIRMA study. Br J Cancer. 2010;103:1815–1821. doi: 10.1038/sj.bjc.6605979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Cearbhaill RE, Miller A, Muggia F, et al. Carboplatin dosing in the treatment of epithelial ovarian cancer (EOC): A Gynecologic Oncology Group (GOG) study; J Clin Oncol; 2012. (abstr 5041) [Google Scholar]
- 19.Raj GV, Iasonos A, Herr H, et al. Formulas calculating creatinine clearance are inadequate for determining eligibility for cisplatin-based chemotherapy in bladder cancer. J Clin Oncol. 2006;24:3095–3100. doi: 10.1200/JCO.2005.04.3091. [DOI] [PubMed] [Google Scholar]
- 20.Berman N, Hostetter TH. Comparing the Cockcroft-Gault and MDRD equations for calculation of GFR and drug doses in the elderly. Nat Clin Pract Nephrol. 2007;3:644–645. doi: 10.1038/ncpneph0627. [DOI] [PubMed] [Google Scholar]
- 21.Marx GM, Blake GM, Galani E, et al. Evaluation of the Cockroft-Gault, Jelliffe and Wright formulae in estimating renal function in elderly cancer patients. Ann Oncol. 2004;15:291–295. doi: 10.1093/annonc/mdh079. [DOI] [PubMed] [Google Scholar]
- 22.Friedman JR, Norman DC, Yoshikawa TT. Correlation of estimated renal function parameters versus 24-hour creatinine clearance in ambulatory elderly. J Am Geriatr Soc. 1989;37:145–149. doi: 10.1111/j.1532-5415.1989.tb05873.x. [DOI] [PubMed] [Google Scholar]
- 23.Nicoll SR, Sainsbury R, Bailey RR, et al. Assessment of creatinine clearance in healthy subjects over 65 years of age. Nephron. 1991;59:621–625. doi: 10.1159/000186654. [DOI] [PubMed] [Google Scholar]
- 24.Smythe M, Hoffman J, Kizy K, et al. Estimating creatinine clearance in elderly patients with low serum creatinine concentrations. Am J Hosp Pharm. 1994;51:198–204. [PubMed] [Google Scholar]
- 25.Lichtman SM. Call for changes in clinical trial reporting of older patients with cancer. J Clin Oncol. 2012;30:893–894. doi: 10.1200/JCO.2011.41.0696. [DOI] [PubMed] [Google Scholar]
- 26.Lichtman SM. Clinical trial design in older adults with cancer—The need for new paradigms. J Geriatr Oncol. 2012;3:368–375. [Google Scholar]
- 27.Wildiers H, Mauer M, Pallis A, et al. End points and trial design in geriatric oncology research: A joint European Organisation for Research and Treatment of Cancer–Alliance for Clinical Trials in Oncology–International Society of Geriatric Oncology position article. J Clin Oncol. 2013;31:3711–3718. doi: 10.1200/JCO.2013.49.6125. [DOI] [PubMed] [Google Scholar]