Abstract
Purpose
CD33 is variably expressed on acute myeloid leukemia (AML) blasts and is targeted by gemtuzumab ozogamicin (GO). GO has shown benefit in both adult and pediatric AML trials, yet limited data exist about whether GO response correlates with CD33 expression level.
Patients and Methods
CD33 expression levels were prospectively quantified by multidimensional flow cytometry in 825 patients enrolled in Children’s Oncology Group AAML0531 and correlated with response to GO.
Results
Patients with low CD33 expression (lowest quartile of expression [Q1]) had no benefit with the addition of GO to conventional chemotherapy (relapse risk [RR]: GO 36% v No-GO 34%, P = .731; event-free survival [EFS]: GO 53% v No-GO 58%, P = .456). However, patients with higher CD33 expression (Q2 to Q4) had significantly reduced RR (GO 32% v No-GO 49%, P < .001) and improved EFS (GO 53% v No-GO 41%, P = .005). This differential effect was observed in all risk groups. Specifically, low-risk (LR), intermediate-risk (IR), and high-risk (HR) patients with low CD33 expression had similar outcomes regardless of GO exposure, whereas the addition of GO to conventional chemotherapy resulted in a significant decrease in RR and disease-free survival (DFS) for patients with higher CD33 expression (LR RR, GO 13% v No-GO 35%, P = .001; LR DFS, GO 79% v No-GO 59%, P = .007; IR RR, GO 44% v No-GO 57%, P = .044; IR DFS, GO 51% v No-GO 40%, P = .078; HR RR, GO 40% v No-GO 73%, P = .016; HR DFS, GO 47% v No-GO 28%, P = .135).
Conclusion
We demonstrate that GO lacks clinical benefit in patients with low CD33 expression but significantly reduces RR and improves EFS in patients with high CD33 expression, which suggests a role for CD33-targeted therapeutics in subsets of pediatric AML.
INTRODUCTION
The majority of patients with acute myeloid leukemia (AML) expresses the myeloid antigen CD33 on leukemic blasts.1 CD33, a 67-kDa transmembrane glycoprotein, is a member of the sialic acid–binding immunoglobulin-like lectins (Siglecs) and is targeted by gemtuzumab ozogamicin (GO), a toxin-conjugated humanized immunoglobulin G4 anti-CD33 monoclonal antibody that has efficacy within subsets of adult patients with de novo AML, particularly those with favorable or normal cytogenetics.1-10 GO has also been studied in a number of pediatric oncology trials,11-16 which include three conducted within the Children’s Oncology Group (COG).11,13,16 The first, AAML00P2, determined the maximum tolerated dose of GO when used in combination with conventional chemotherapy for patients whose condition had relapsed.11 The subsequent study, COG AAML03P1, was a pilot in which patients with de novo AML received GO in combination with Medical Research Council–based conventional chemotherapy.13 The successor study, COG AAML0531, used the same chemotherapy regimen as that for AAML03P1, but patients were randomly assigned to receive standard chemotherapy alone or in combination with GO. This study found that GO recipients had significantly improved event-free survival (EFS) as well as relapse risk (RR) and disease-free survival (DFS) compared with patients treated only with conventional chemotherapy.16
We previously demonstrated within the context of AAML03P1, in which all patients received GO, that high CD33 expression was correlated with negative prognostic features and significantly lower overall survival (OS) and DFS from complete remission (CR). In a multivariable model, high CD33 expression remained a negative predictor of outcome.17 The aim of the current study was to determine, within the context of the GO randomization trial COG AAML0531, whether GO had efficacy in patients with the lowest CD33 expression and, conversely, whether it significantly improved outcomes in patients with higher CD33 expression compared with the control arm. Toward that end, we prospectively quantified CD33 expression on the surface of leukemic blasts and correlated these findings with disease characteristics and clinical outcome by treatment arm.
PATIENTS AND METHODS
Patients and Treatment
Pediatric patients with de novo AML enrolled in COG AAML0531 were eligible for this study. Details of the treatment regimen have been described previously.16 In brief, patients were randomly assigned to one of two study arms: a backbone of standard chemotherapy alone (No-GO arm) or in combination with 3 mg/m2 GO administered on day 6 of induction I and day 7 of intensification II (GO arm). Patients designated as high risk (HR) received the best allogeneic hematopoietic stem cell transplantation (HSCT) after intensification I chemotherapy. Selection of an alternative donor was by the discretion of the treating transplantation center. Intermediate-risk (IR) patients, defined as those without low-risk (LR) or HR features, underwent HSCT if a matched family donor was available. IR patients without a matched family donor and LR patients did not undergo HSCT; their therapy was limited to five chemotherapy courses. There was no threshold of CD33 expression for enrollment in this clinical protocol. All samples from patients enrolled in AAML0531 were eligible for our correlative study if consent for biology studies was obtained. The institutional review boards of all participating institutions approved the clinical protocol, and the COG Myeloid Disease Biology Committee approved this research.
Risk Stratification
For the purposes of this correlative biology study, cytogenetic and molecular abnormalities were used to stratify the study population into risk groups. The LR group included patients with core-binding factor AML [t(8;21) or inv(16)/t(16;16)] and/or nucleophosmin 1 (NPM1) or CEBPA mutations without FLT3/ITD mutations. The HR group included patients with high allelic ratio (> 0.4) FLT3/ITD+ disease and/or monosomy 5, del(5q), or monosomy 7. The remaining patients with known cytogenetics were designated as IR.
Assessment of CD33 Expression
By using flow cytometry, CD33 mean fluorescent intensity (MFI) of myeloid progenitor cells, as defined by CD45 low and side scatter, was determined with a previously described protocol.17-19
Statistical Analyses
Clinical outcome data for patients enrolled in COG AAML051 were analyzed as of September 30, 2014. The median follow-up for eligible patients who were alive at last contact and included in our analysis was 1,856 days (range, 4 to 2,829 days). Patients were considered in CR if they had less than 5% blasts and an absence of extramedullary disease after one course of induction chemotherapy. Minimal residual disease (MRD) was defined by using flow cytometry and considered positive if 0.1% or more disease was detected at the end of induction I.20 OS was defined as the time from study entry or from the end of course I for patients in CR to death. EFS was defined as the time from study entry until death, induction failure, or relapse of any type. DFS was defined as the time from the end of course I for patients in CR until relapse or death of any cause. RR was defined as the time from the end of course I for patients in CR to relapse, where deaths without a relapse were considered competing events.21 The significance of predictor variables was tested with the log-rank statistic for OS, EFS, and DFS and with the Gray statistic for RR. Patients lost to follow-up were censored at their date of last known contact. The significance of observed difference in proportions was tested by the χ2 test between patient groups. Alternatively, the exact test was used if data were sparse. The Kruskal-Wallis test was used to determine the significance between differences in medians of the groups.
RESULTS
CD33 Expression Levels and Correlation With Disease Characteristics
We prospectively evaluated CD33 expression levels in samples from 825 pediatric patients with de novo AML enrolled in COG AAML0531. CD33 MFI of the blast population varied more than two-log-fold, with a median MFI of 146.00 (range, 2.68 to 1,351.00). The study population was divided into four quartiles on the basis of CD33 expression, and expression levels were correlated with clinical characteristics and outcomes. Median MFI for quartile (Q) 1 to 4 was as follows: Q1 (n = 208), 34.61 (range, 2.68 to 67.00); Q2 (n = 205), 100.7 (range, 67.13 to 146.94); Q3 (n = 206), 207.01 (range, 147.00 to 296.38); and Q4 (n = 206), 435.9 (range, 296.98 to 1,351.00). Correlation of CD33 expression with somatic mutations revealed that FLT3/ITD, NPM1, and CEBPA mutations were detected in 16%, 8%, and 6% of evaluable samples, respectively. Similar to our previous analysis,17 there was a statistically significant increase in the proportion of FLT3/ITD and NPM1 mutations with increasing CD33 expression and a trend toward decreased numbers of patients with CEBPA mutations with high levels of CD33 expression (Fig 1A; Table 1). This translated into higher median CD33 expression for patients with FLT3/ITD+ disease (median MFI, 215.31; range, 4.27 to 1,225.87) compared with patients with the wild-type FLT3 (median MFI, 135; range, 2.68 to 1,351; P < .001). Similar findings were observed for patients with NPM1+ disease (median MFI, 275.6; range, 6.84 to 1,160) compared with those with the wild-type NPM1 (median MFI, 139; range, 2.68 to 1,351; P < .001).
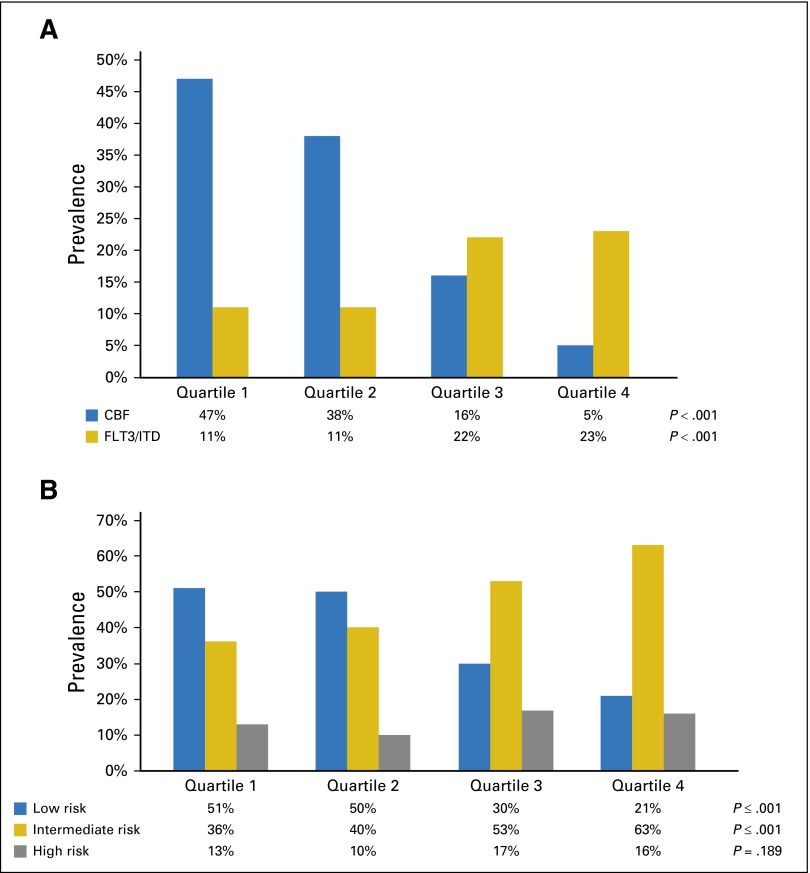
Fig 1.
Distribution of CD33 expression in patients. (A) Correlation of CD33 expression data with specific cytogenetic/molecular disease characteristics by quartile. (B) CD33 expression and disease risk group classification by quartile. CBF, core-binding factor; FLT3/ITD, FLT3 internal tandem duplication.
Table 1.
Disease Characteristics and Induction Response by Quartile of CD33 Expression
| CD33 Q1 (n = 208) | CD33 Q2 (n = 205) | CD33 Q3 (n = 206) | CD33 Q4 (n = 206) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No. | % | No. | % | No. | % | No. | % | P |
| Sex | |||||||||
| Male | 110 | 53 | 105 | 51 | 96 | 47 | 108 | 52 | .563 |
| Female | 98 | 47 | 100 | 49 | 110 | 53 | 98 | 48 | |
| Treatment arm | |||||||||
| No-GO | 115 | 55 | 99 | 48 | 96 | 47 | 101 | 49 | .310 |
| GO | 93 | 45 | 106 | 52 | 110 | 53 | 105 | 51 | |
| Cytogenetics | |||||||||
| Normal | 28 | 14 | 34 | 17 | 60 | 30 | 60 | 30 | < .001 |
| t(8;21) | 64 | 32 | 40 | 20 | 13 | 6 | 0 | 0 | < .001 |
| inv(16) | 31 | 15 | 34 | 17 | 20 | 10 | 11 | 5 | .001 |
| t(9;11)/11q23 | 16 | 8 | 32 | 16 | 50 | 25 | 66 | 33 | < .001 |
| t(6;9)(p23;q34) | 2 | 1 | 0 | 0 | 5 | 2 | 5 | 2 | .112 |
| Monosomy 7 | 6 | 3 | 5 | 3 | 2 | 1 | 3 | 1 | .462 |
| del(7q) | 4 | 2 | 3 | 2 | 3 | 1 | 2 | 1 | .880 |
| -5/5q- | 1 | 0 | 6 | 3 | 4 | 2 | 0 | 0 | .035 |
| +8 | 12 | 6 | 7 | 4 | 16 | 8 | 17 | 8 | .183 |
| Other | 38 | 19 | 36 | 18 | 28 | 14 | 38 | 19 | .505 |
| Unknown | 6 | 8 | 5 | 4 | |||||
| FLT3/ITD status | |||||||||
| ITD+ | 23 | 11 | 22 | 11 | 45 | 22 | 46 | 23 | < .001 |
| ITD− | 183 | 89 | 179 | 89 | 157 | 78 | 156 | 77 | |
| Unknown | 2 | 4 | 4 | 4 | |||||
| CEBPA status | |||||||||
| CEBPA mutant | 10 | 5 | 17 | 8 | 16 | 8 | 6 | 3 | .065 |
| CEBPA WT | 197 | 95 | 185 | 92 | 186 | 92 | 197 | 97 | |
| Unknown | 1 | 3 | 4 | 3 | |||||
| NPM1 status | |||||||||
| NPM1 mutant | 5 | 2 | 11 | 5 | 17 | 8 | 31 | 15 | < .001 |
| NPM1 WT | 202 | 98 | 190 | 95 | 185 | 92 | 172 | 85 | |
| Unknown | 1 | 4 | 4 | 3 | |||||
| Risk group (cyto/molecular) | |||||||||
| Intermediate | 73 | 36 | 80 | 40 | 108 | 53 | 129 | 63 | < .001 |
| Low | 105 | 51 | 99 | 50 | 60 | 30 | 43 | 21 | < .001 |
| High | 27 | 13 | 20 | 10 | 34 | 17 | 33 | 16 | .189 |
| Unknown | 3 | 8 | 4 | 1 | |||||
| Induction I response | |||||||||
| CR | 153 | 74 | 151 | 75 | 143 | 70 | 145 | 73 | .701 |
| Not in CR | 54 | 26 | 50 | 25 | 61 | 30 | 54 | 27 | |
| Not evaluable | 1 | 4 | 2 | 7 | |||||
| Induction II response | |||||||||
| CR | 183 | 90 | 169 | 87 | 171 | 86 | 169 | 86 | .684 |
| Not in CR | 21 | 10 | 26 | 13 | 27 | 14 | 27 | 14 | |
| Not evaluable | 4 | 10 | 8 | 10 | |||||
| No-GO patients only | |||||||||
| Induction I response | |||||||||
| CR | 84 | 73 | 73 | 74 | 59 | 62 | 65 | 67 | .208 |
| Not in CR | 31 | 27 | 25 | 26 | 36 | 38 | 32 | 33 | |
| Not evaluable | 0 | 1 | 1 | 4 | |||||
| Induction II response | |||||||||
| CR | 101 | 90 | 82 | 84 | 73 | 81 | 84 | 88 | .228 |
| Not in CR | 11 | 10 | 16 | 16 | 17 | 19 | 11 | 12 | |
| Not evaluable | 3 | 1 | 6 | 6 | |||||
| GO patients only | |||||||||
| Induction I response | |||||||||
| CR | 69 | 75 | 78 | 76 | 84 | 77 | 80 | 78 | .945 |
| Not in CR | 23 | 25 | 25 | 24 | 25 | 23 | 22 | 22 | |
| Not evaluable | 1 | 3 | 1 | 3 | |||||
| Induction II response | |||||||||
| CR | 82 | 89 | 87 | 90 | 98 | 91 | 85 | 84 | .065 |
| Not in CR | 10 | 11 | 10 | 10 | 10 | 9 | 16 | 16 | |
| Not evaluable | 1 | 9 | 2 | 4 | |||||
Abbreviations: CR, complete remission; cyto, cytogenetic; GO, gemtuzumab ozogamicin; ITD, internal tandem duplication; Q, quartile; WT, wild type.
Cytogenetic data were available for 799 of 825 patient samples (97%). Prevalence of core-binding factor AML was inversely associated with CD33 expression, with a prevalence of 47% in patients with the lowest (Q1) and 5% in patients with the highest (Q4) CD33 expression (P < .001; Fig 1A). The prevalence of KMT2A gene alterations (11q23) also increased with increase in CD33 expression (P < .001; Table 1). There was no association between CD33 expression and HR cytogenetics; however, analysis was limited by the small number of patients (27 of 825 [3%]) with such alterations.
For risk group classification, complete cytogenetic and molecular data were available for 811 of 825 patients (98%); 307 (38%) were classified as LR, 390 (48%) as IR, and 114 (14%) as HR. LR disease was associated with low CD33 expression (P < .001; Fig 1B; Table 1), whereas the prevalence of IR disease increased significantly with increasing quartile (P ≤ .001, Fig 1B; Table 1). There was no clear trend in prevalence by quartile for HR disease (P = .189; Fig 1B; Table 1), although there was a significantly higher median CD33 MFI with HR (median MFI, 191.05; range, 4.27 to 1,225.87) versus LR disease (median MFI, 98; range, 5 to 876; P < .001).
Association of CD33 Expression and GO Response
Induction I and induction II remission rates were determined for all patients independent of risk group classification and were similar across all quartiles (Table 1). CR rates across quartiles also lacked statistical significance when analysis was conducted by treatment arm (Table 1). Given the mechanism of action of GO, we were particularly interested in determining whether GO had differential efficacy in patients who expressed low (Q1) versus higher (Q2 to Q4) levels of CD33. For patients with low CD33 expression, CR rates were not significantly different for those receiving and not receiving GO (75% v 73%; P = .750). However, for patients with higher CD33 expression, end–induction I CR rates were significantly higher for those who received GO than those who did not (77% v 68%; P = .012). Similarly, patients with low CD33 expression did not have significantly different rates of end–induction I MRD when compared by treatment arm (GO 27% v No-GO 33%; P = .426). However, patients with higher (Q2 to Q4) CD33 expression treated with GO had lower rates of MRD than those treated with conventional chemotherapy only (GO 28% v No-GO 36%; P = .052).
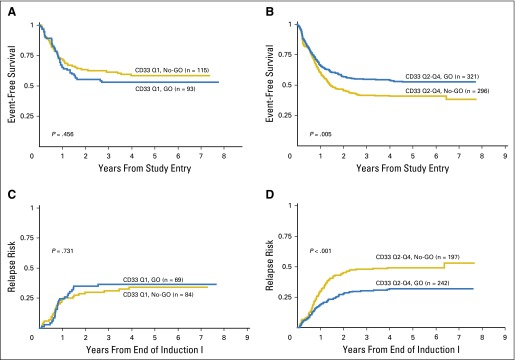
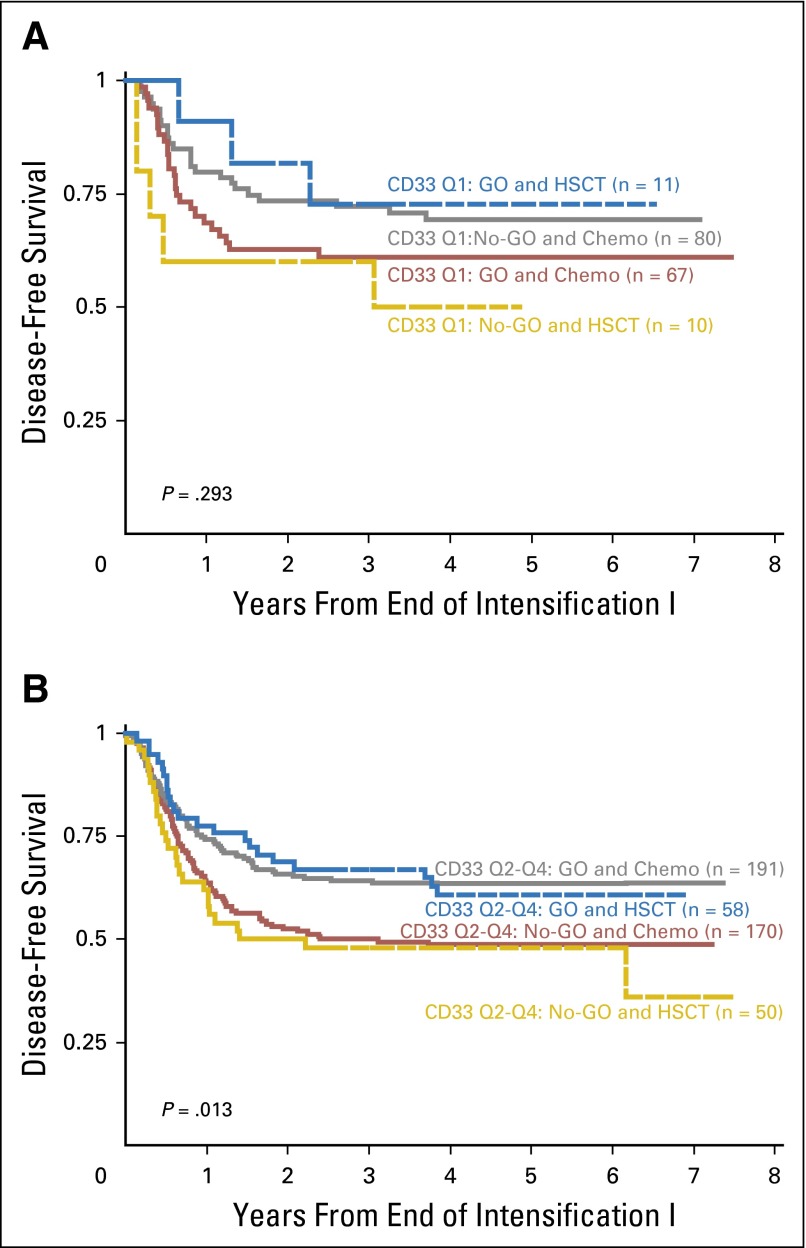
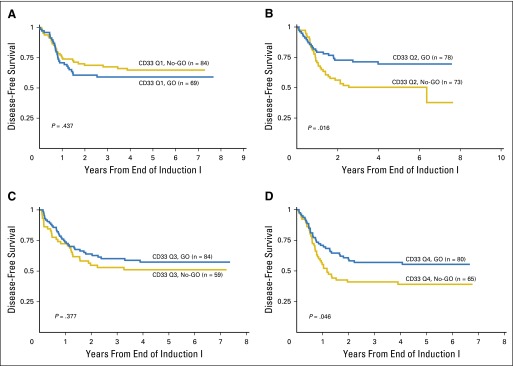
Patients with the lowest CD33 expression had similar survival outcomes regardless of GO exposure (Table 2; Figs 2A and 2C). In contrast, patients with higher CD33 expression (Q2 to Q4) who received GO had a significant improvement in clinical outcome compared with those who did not receive GO, with an RR of 32 ± 6% versus 49% ± 7% (P < .001) and a corresponding EFS of 53 ± 6% versus 41 ± 6% (P = .005) observed for those treated with and without GO therapy (Table 2; Figs 2B and 2D). The relative impact of consolidation with HSCT versus chemotherapy was also determined. Analysis of DFS from time of consolidation therapy supported our observation that GO provided clinical benefit for patients with high CD33 expression. However, consolidation with HSCT versus chemotherapy resulted in similar DFS from CR (Appendix Fig A1, online only).
Table 2.
Clinical Outcome Analysis by Treatment Arm (No-GO v GO) and Quartile as Well as Aggregate Analysis for Q2 to Q4 for All Patients
| All Patients (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD33 Q1 (n = 208) | P | CD33 Q2 (n = 205) | P | CD33 Q3 (n = 206) | P | CD33 Q4 (n = 206) | P | CD33 Q2-Q4 (n = 617) | P | ||||||
| No-GO (n = 115) | GO (n = 93) | No-GO (n = 99) | GO (n = 106) | No-GO (n = 96) | GO (n = 110) | No-GO (n = 101) | GO (n = 105) | No-GO (n = 296) | GO (n = 321) | ||||||
| 5-year OS from study entry, mean ± SE | 75 ± 8 | 72 ± 9 | .495 | 59 ± 10 | 71 ± 9 | .174 | 62 ± 10 | 64 ± 9 | .758 | 54 ± 10 | 60 ± 10 | .341 | 58 ± 6 | 65 ± 6 | .132 |
| 5-year EFS from study entry, mean ± SE | 58 ± 9 | 53 ± 10 | .456 | 43 ± 10 | 61 ± 10 | .010 | 43 ± 10 | 53 ± 10 | .151 | 36 ± 10 | 44 ± 10 | .359 | 41 ± 6 | 53 ± 6 | .005 |
| No-GO (n = 84) | GO (n = 69) | No-GO (n = 73) | GO (n = 78) | No-GO (n = 59) | GO (n = 84) | No-GO (n = 65) | GO (n = 80) | No-GO (n = 197) | GO (n = 242) | ||||||
| 5-year DFS from end of induction I, mean ± SE | 65 ± 11 | 59 ± 12 | .437 | 50 ± 12 | 70 ± 11 | .016 | 51 ± 13 | 57 ± 11 | .377 | 39 ± 12 | 55 ± 11 | .046 | 47 ± 7 | 61 ± 6 | .003 |
| 5-year RR from end of induction I, mean ± SE | 34 ± 11 | 36 ± 12 | .731 | 47 ± 12 | 21 ± 10 | < .001 | 45 ± 13 | 39 ± 11 | .361 | 55 ± 13 | 34 ± 11 | .016 | 49 ± 7 | 32 ± 6 | < .001 |
NOTE. SE is two standard errors of the mean (± 2SE %).
Abbreviations: DFS, disease-free survival; EFS, event-free survival; GO, gemtuzumab ozogamicin; OS, overall survival; Q, quartile; RR, relapse risk.
Fig 2.
Association of CD33 levels and GO response. (A) Event-free survival from study entry for patients with low CD33 expression levels (Q1) when analyzed by treatment arm. (B) Event-free survival from study entry for patients with higher CD33 expression levels (Q2 to Q4) when analyzed by treatment arm. (C) Relapse risk from complete remission for patients with low CD33 expression levels (Q1) when analyzed by treatment arm. (D) Relapse risk from complete remission for patients with higher CD33 expression levels (Q2 to Q4) when analyzed by treatment arm. GO, gemtuzumab ozogamicin; Q, quartile.
Association of CD33 Expression and GO Response By Disease Risk Group
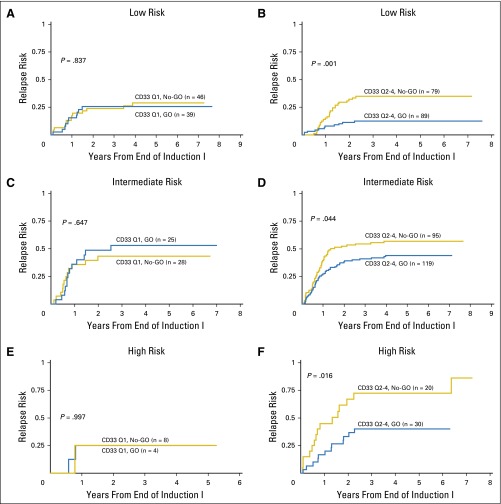
We also assessed the clinical impact of GO within the context of CD33 expression and risk group. LR patients with the lowest CD33 expression (Q1) had comparable outcomes regardless of GO (Table 3; Fig 3A), whereas LR patients with higher CD33 expression (Q2 to Q4) had improved EFS from study entry as well as DFS from CR when treated with GO (EFS from study entry: GO 77 ± 8% versus No-GO 60 ± 10%, P = .020; DFS from CR: GO 79 ± 9% versus No-GO 59 ± 11%, P = 0.007; Table 3). This translated into a lower RR for patients with higher CD33 expression treated with GO therapy (RR from CR: GO 13 ± 7% v No-GO 35 ± 11%, P = .001; Table 3; Fig 3B). Outcomes for patients with IR disease also demonstrated a differential GO effect. Specifically, IR patients with the lowest CD33 expression had similar outcomes regardless of GO therapy (Table 3; Fig 3C), whereas IR patients with higher CD33 expression seemed to have a significant improvement in EFS from study entry, a trend toward improved DFS from CR, and a reduction in RR when treated with GO versus conventional chemotherapy alone (EFS from study entry: GO 44 ± 8% v No-GO 33 ± 8%, P = .021; DFS from CR: GO 51 ± 9% v No-GO 40 ± 10%, P = .078; RR from CR: GO 44 ± 9% v No-GO 57 ± 10%, P = .044; Table 3; Fig 3D). HR patients with low CD33 expression had no benefit from GO (Table 3; Fig 3E). However, HR patients with high CD33 expression treated with GO had an RR from CR of 40 ± 18% compared with 73 ± 22% for patients treated without GO (P = .016) with a corresponding DFS from CR of 47% ± 18% v 28 ± 21% (P = 0.135; Table 3; Fig 3F).
Table 3.
Clinical Outcome Analysis by Treatment Arm (No-GO v GO) and Risk Group for Patients With Low (Q1) Versus Higher (Q2 to Q4) CD33 Expression
| All Patients (%) | ||||||
|---|---|---|---|---|---|---|
| CD33 Q1 (n = 105) | P | CD33 Q2-Q4 (n = 202) | P | |||
| Low-risk patients | No-GO (n = 57) | GO (n = 48) | No-GO (n = 96) | GO (n = 106) | ||
| 5-year OS from study entry, mean ± SE | 83 ± 11 | 85 ± 10 | .959 | 78 ± 9 | 83 ± 8 | .425 |
| 5-year EFS from study entry, mean ± SE | 69 ± 12 | 67 ± 14 | .750 | 60 ± 10 | 77 ± 8 | .020 |
| No-GO (n = 46) | GO (n = 39) | No-GO (n = 79) | GO (n = 89) | |||
| 5-year DFS from end of induction I, mean ± SE | 71 ± 14 | 69 ± 15 | .725 | 59 ± 11 | 79 ± 9 | .007 |
| 5-year RR from end of induction I, mean ± SE | 29 ± 14 | 26 ± 14 | .837 | 35 ± 11 | 13 ± 7 | .001 |
| CD33 Q1 (n = 73) | CD33 Q2-Q4 (n = 317) | |||||
| Intermediate-risk patients | No-GO (n = 40) | GO (n = 33) | No-GO (n = 157) | GO (n = 160) | ||
| 5-year OS from study entry, mean ± SE | 78 ± 13 | 59 ± 18 | .116 | 50 ± 8 | 59 ± 8 | .100 |
| 5-year EFS from study entry, mean ± SE | 52 ± 16 | 41 ± 17 | .290 | 33 ± 8 | 44 ± 8 | .021 |
| No-GO (n = 28) | GO (n = 25) | No-GO (n = 95) | GO (n = 119) | |||
| 5-year DFS from end of induction I, mean ± SE | 57 ± 19 | 43 ± 20 | .428 | 40 ± 10 | 51 ± 9 | .078 |
| 5-year RR from end of induction I, mean ± SE | 43 ± 19 | 53 ± 21 | .647 | 57 ± 10 | 44 ± 9 | .044 |
| CD33 Q1 (n = 27) | CD33 Q2-Q4 (n = 87) | |||||
| High-risk patients | No-GO (n = 16) | GO (n = 11) | No-GO (n = 39) | GO (n = 48) | ||
| 5-year OS from study entry, mean ± SE | 41 ± 26 | 61 ± 31 | .251 | 44 ± 16 | 48 ± 15 | .805 |
| 5-year EFS from study entry, mean ± SE | 38 ± 24 | 36 ± 29 | .977 | 24 ± 14 | 31 ± 14 | .608 |
| No-GO (n = 8) | GO (n = 4) | No-GO (n = 20) | GO (n = 30) | |||
| 5-year DFS from end of induction I, mean ± SE | 63 ± 34 | 75 ± 43 | .653 | 28 ± 21 | 47 ± 18 | .135 |
| 5-year RR from end of induction I, mean ± SE | 25 ± 33 | 25 ± 50 | .997 | 73 ± 22 | 40 ± 18 | .016 |
NOTE. SE is two standard errors of the mean (± 2SE %).
Abbreviations: DFS, disease-free survival; EFS, event-free survival; GO, gemtuzumab ozogamicin; OS, overall survival; Q, quartile; RR, relapse risk.
Fig 3.
Association of CD33 levels and GO response by treatment arm and risk group. Relapse risk from complete remission by treatment arm. (A) Low-risk patients with low CD33 expression (Q1). (B) Low-risk patients with high CD33 expression (Q2 to Q4). (C) Intermediate-risk patients with low CD33 expression (Q1). (D) Intermediate-risk patients with high CD33 expression (Q2 to Q4). (E) High-risk patients with low CD33 expression (Q1). (F) High-risk patients with high CD33 expression (Q2 to Q4). GO, gemtuzumab ozogamicin; Q, quartile.
DISCUSSION
In this prospective clinical trial in which pediatric patients with de novo AML were randomly assigned to receive GO in combination with conventional chemotherapy, we demonstrate that GO has limited benefit in patients with low CD33 expression but significantly decreases relapse and improves EFS and DFS in patients with high CD33 expression. This differential effect in which GO benefits only patients with higher CD33 expression was observed in all risk groups.
Previous in vitro analysis found a direct quantitative relationship between CD33 expression and GO-induced cytoxicity in an AML cell line forced to express different levels of CD33 by way of lentivirus-mediated gene transfer.22 Furthermore, Jager et al23 demonstrated in an in vitro model that high CD33 production predicted high intracellular GO exposure, a surrogate marker for GO response. Culture of CD34+/CD38−/CD123+ leukemia stem/progenitor cells with GO was also affected by CD33 expression; those samples with high CD33 expression grew less in the presence of GO.24 Despite these findings, initial correlative analyses of single-agent phase II AML trials of GO in adults suggested that GO response is independent of CD33 expression levels.25,26 However, reanalysis of these data with the use of a larger sample size and different methodology for CD33 analysis refuted the results and demonstrated a direct relation between CD33 expression and GO response.27
To date, five large adult de novo AML trials have included GO randomization. Four of the five suggested that GO treatment during induction, particularly for LR and IR patients, significantly improves outcome.1-5,7 The fifth trial, Southwest Oncology Group S0106, did not show a clinical benefit of GO. However, this study used lower anthracycline doses for patients on the GO arm compared with the control arm, which may have affected the ability to detect GO benefit.28 Despite this study design limitation, patients with favorable cytogenetics demonstrated a trend toward improved survival with GO treatment. A meta-analysis of these five randomized trials suggested that GO significantly reduced RR and improved relapse-free survival (RFS) and OS despite slightly higher rates of early mortality.7,9 LR patients, and to some degree IR patients, experienced the most pronounced GO effect.9 A second meta-analysis of 11 randomized clinical trials of GO suggested that GO improved RFS without clear benefit on OS as a result of increased induction deaths. Analysis by risk classification suggested that GO improved OS in patients with favorable cytogenetics but not IR or HR cytogenetics.8 The current data further support the benefit of GO for LR patients but as demonstrated in Table 3, suggest that the benefit of GO may be restricted to LR patients with higher CD33 expression.
Initial single-agent GO trials mandated a threshold level of CD33 expression for patients to be eligible for study enrollment. Subsequent adult combination chemotherapy trials did not exclude CD33-negative disease, which raises the question of whether GO is efficacious in all patients or only in those with a threshold level of CD33 expression. To date, only the Medical Research Council has published data on this issue. Analysis of the study cohort by treatment arm and CD33 positivity versus negativity found that CR, OS, and cumulative incidence of death in CR were comparable for patients with CD33-negative versus -positive disease and was independent of GO exposure. However, RFS was better and RR lower for patients with CD33-positive disease receiving GO treatment.2
Pediatric studies of GO plus combination chemotherapy also suggested that GO may provide benefit. In the COG pilot study AAML03P1, 350 patients with de novo AML received GO and had 3-year OS and EFS rates of 66% and 53%, respectively, which compared favorably to previously published cooperative pediatric AML trials.13 High CD33 expression was correlated with negative prognostic features, and patients with the highest CD33 expression had significantly lower OS and DFS from CR.17 COG AAML0531, the GO randomization trial, found that GO significantly improved 3-year EFS (GO 53% v No-GO 47%, P = .04) but not OS (69% v 65%, P = .39) possibly due to increased postremission toxic mortality for those patients who received GO (7% v 4%, P = .09). Notably, RR was significantly reduced among GO recipients (33% v 41%, P = .006), which translated into improved DFS for those receiving GO (61% v 55%, P = .07).16 Two additional pediatric studies explored the value of GO as postremission therapy in AML. Hasle et al14 failed to show a clear benefit for using GO during consolidation therapy, although time to relapse was prolonged, but not significantly, with GO treatment. A second study demonstrated that GO consolidation was effective at reducing MRD levels pre-HSCT without increasing transplant-related toxicity.12,15 Taken together, CD33-targeted agents may show promise in pediatric AML within the context of induction treatment and possibly during consolidation cycles.
We acknowledge that the current data did not demonstrate a discrete biologically defined threshold of CD33 expression in which CD33-targeted therapeutics would be clearly justified. However, analysis of outcome by individual quartile (Table 2; Appendix Fig A2, online only) suggests that patients with the lowest CD33 expression do not benefit from GO. In contrast, Q2 patients had significantly improved EFS, DFS, and RR when treated with GO. Patients in Q3 and Q4 had either a statistically significant improvement or a trend toward better outcome with GO therapy (Table 2; Appendix Fig A2), which suggests that GO can abrogate inferior clinical outcomes observed in patients with higher (Q2 to Q4) CD33 expression. Analysis with alternative cut points (Q1 to Q2 v Q3 to Q4; Appendix Table A1, online only) demonstrates a clear benefit of GO for patients in Q3 to Q4 as well a relative contribution of GO toward reduction of RR within Q1 to Q2, which likely reflects the positive impact GO has on Q2 treatment response (Table 2).
The current study is significant as the first to our knowledge to demonstrate, within the context of a large randomized pediatric AML trial, that GO therapy is unlikely to have clinical benefit in patients with low CD33 expression. Conversely, in patients with high CD33 expression, GO seems to significantly improve disease-free response in all risk groups. Given limited commercial access to GO, additional studies of novel CD33-targeted agents, like SGN-CD33A, are needed in pediatric AML. However, the efficacy of SGN-CD33A needs to be critically evaluated in patients with intrinsically low CD33 expression in light of preclinical data that suggest it may have limited efficacy when CD33 expression is low or absent.29 If second-generation CD33-targeted therapeutics further demonstrate a selective benefit in patients with high CD33 expression, future clinical trials may warrant conventional therapy de-escalation for this subset of patients.
Acknowledgment
We thank Vani J. Shanker for scientific editing and the COG AML Reference Laboratory, particularly Sommer Castro, for the provision of diagnostic specimens. We also thank the patients and families who provided consent for the use of biologic specimens in this trial.
Appendix
Fig A1.
Association of CD33 levels and GO response by treatment arm (GO v No-GO) and consolidation therapy (HSCT v chemotherapy) for patients with (A) low (Q1) CD33 expression and (B) higher (Q2to Q4) CD33 expression. Chemo, chemotherapy; GO, gemtuzumab ozogamicin; HSCT, hematopoietic stem cell transplantation; Q, quartile.
Fig A2.
Disease-free survival by quartile and treatment arm (GO v No-GO) for (A) Q1, (B) Q2, (C) Q3, and (D) Q4. Chemo, chemotherapy; GO, gemtuzumab ozogamicin; HSCT, hematopoietic stem cell transplantation; Q, quartile.
Table A1.
Clinical Outcome Analysis by Treatment Arm (No-GO v GO) for Lower (Q1 to Q2) Versus Higher (Q2 to Q4) CD33 Expression
| All Patients (%) | ||||||
|---|---|---|---|---|---|---|
| CD33 Q1-Q2 (n = 413) | P | CD33 Q3-Q4 (n = 412) | P | |||
| No-GO (n = 214) | GO (n = 199) | No-GO (n = 197) | GO (n = 215) | |||
| 5-year OS from study entry, mean ± SD | 68 ± 7 | 71 ± 7 | .706 | 58 ± 7 | 62 ± 7 | .350 |
| 5-year EFS from study entry, mean ± SD | 51 ± 7 | 57 ± 7 | .230 | 40 ± 7 | 49 ± 7 | .086 |
| No-GO (n = 157) | GO (n = 147) | No-GO (n = 124) | GO (n = 164) | |||
| 5-year DFS from end of induction I, mean ± SD | 58 ± 8 | 65 ± 8 | .281 | 45 ± 9 | 56 ± 8 | .035 |
| 5-year RR from end of induction I, mean ± SD | 40 ± 8 | 28 ± 8 | .036 | 50 ± 9 | 37 ± 8 | .016 |
Abbreviations: DFS, disease-free survival; EFS, event-free survival; GO, gemtuzumab ozogamicin; OS, overall survival; Q, quartile; RR, relapse risk.
Footnotes
Supported by a St Baldrick’s Career Development Award (J.A.P.), Children’s Oncology Group Chairs Grants No. U10CA180886-01 and U10CA98543, Statistics and Data Center Grant No. CA98413-08, and National Cancer Institute Grant No. R01CA114563 (S.M.).
Presented in part at the 55th American Society of Hematology Annual Meeting and Exposition, New Orleans, LA, December 7-10, 2013.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Jessica A. Pollard, Michael Loken, Irwin D. Bernstein, Alan S. Gamis, Todd A. Alonzo, Soheil Meshinchi
Collection and assembly of data: Jessica A. Pollard, Michael Loken, Robert B. Gerbing, Susana C. Raimondi, Betsy A. Hirsch, Richard Aplenc, Alan S. Gamis, Todd A. Alonzo, Soheil Meshinchi
Data analysis and interpretation: Jessica A. Pollard, Robert B. Gerbing, Richard Aplenc, Irwin D. Bernstein, Alan S. Gamis, Todd A. Alonzo, Soheil Meshinchi
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
CD33 Expression and Its Association With Gemtuzumab Ozogamicin Response: Results From the Randomized Phase III Children’s Oncology Group Trial AAML0531
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Jessica A. Pollard
No relationship to disclose
Michael Loken
Employment: Hematologics
Leadership: Hematologics
Stock or Other Ownership: Hematologics
Robert B. Gerbing
No relationship to disclose
Susana C. Raimondi
No relationship to disclose
Betsy A. Hirsch
No relationship to disclose
Richard Aplenc
Honoraria: Sigma-Tau
Travel, Accommodations, Expenses: Sigma-Tau
Irwin D. Bernstein
Patents, Royalties, Other Intellectual Property: Royalties from CD33 antibody used for leukemia diagnosis from Becton Dickinson
Alan S. Gamis
Consulting or Advisory Role: Pfizer, Novartis
Todd A. Alonzo
No relationship to disclose
Soheil Meshinchi
No relationship to disclose
REFERENCES
- 1.Walter RB, Appelbaum FR, Estey EH, et al. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood. 2012;119:6198–6208. doi: 10.1182/blood-2011-11-325050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: Results of the MRC AML15 trial. J Clin Oncol. 2011;29:369–377. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- 3.Delaunay J, Recher C, Pigneux A, et al. Addition of gemtuzumab ozogamycin to chemotherapy improves event-free survival but not overall survival of AML patients with intermediate cytogenetics not eligible for allogeneic transplantation. Results of the GOELAMS AML 2006 IR study. Blood. 2011;118:79. [Google Scholar]
- 4.Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol. 2012;30:3924–3931. doi: 10.1200/JCO.2012.42.2964. [DOI] [PubMed] [Google Scholar]
- 5.Castaigne S, Pautas C, Terré C, et al. Acute Leukemia French Association Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet. 2012;379:1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 6.Burnett AK, Hills RK, Hunter AE, et al. UK National Cancer Research Institute AML Working Group The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: Results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia. 2013;27:75–81. doi: 10.1038/leu.2012.229. [DOI] [PubMed] [Google Scholar]
- 7.Laszlo GS, Estey EH, Walter RB. The past and future of CD33 as therapeutic target in acute myeloid leukemia. Blood Rev. 2014;28:143–153. doi: 10.1016/j.blre.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Loke J, Khan JN, Wilson JS, et al. Mylotarg has potent anti-leukaemic effect: A systematic review and meta-analysis of anti-CD33 antibody treatment in acute myeloid leukaemia. Ann Hematol. 2015;94:361–373. doi: 10.1007/s00277-014-2218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15:986–996. doi: 10.1016/S1470-2045(14)70281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thol F, Schlenk RF. Gemtuzumab ozogamicin in acute myeloid leukemia revisited. Expert Opin Biol Ther. 2014;14:1185–1195. doi: 10.1517/14712598.2014.922534. [DOI] [PubMed] [Google Scholar]
- 11.Aplenc R, Alonzo TA, Gerbing RB, et al. Children’s Oncology Group Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukemia: A report from the Children’s Oncology Group. J Clin Oncol. 2008;26:2390–2395. doi: 10.1200/JCO.2007.13.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: Results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–552. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: A report from the Children’s Oncology Group. Cancer. 2012;118:761–769. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- 14.Hasle H, Abrahamsson J, Forestier E, et al. Nordic Society of Paediatric Haematology and Oncology (NOPHO) Gemtuzumab ozogamicin as postconsolidation therapy does not prevent relapse in children with AML: Results from NOPHO-AML 2004. Blood. 2012;120:978–984. doi: 10.1182/blood-2012-03-416701. [DOI] [PubMed] [Google Scholar]
- 15.O’Hear C, Inaba H, Pounds S, et al. Gemtuzumab ozogamicin can reduce minimal residual disease in patients with childhood acute myeloid leukemia. Cancer. 2013;119:4036–4043. doi: 10.1002/cncr.28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: Results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32:3021–3032. doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollard JA, Alonzo TA, Loken M, et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood. 2012;119:3705–3711. doi: 10.1182/blood-2011-12-398370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walter RB, Alonzo TA, Gerbing RB, et al. High expression of the very late antigen-4 integrin independently predicts reduced risk of relapse and improved outcome in pediatric acute myeloid leukemia: A report from the Children’s Oncology Group. J Clin Oncol. 2010;28:2831–2838. doi: 10.1200/JCO.2009.27.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells DA, Loken MR. Flow cytometric mean fluorescence intensity: The biophysics behind the number. Leuk Res. 2008;32:845–846. doi: 10.1016/j.leukres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Loken MR, Alonzo TA, Pardo L, et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: A report from Children’s Oncology Group. Blood. 2012;120:1581–1588. doi: 10.1182/blood-2012-02-408336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Hoboken, NJ,: John Wiley & Sons; 2002. [Google Scholar]
- 22.Walter RB, Raden BW, Kamikura DM, et al. Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood. 2005;105:1295–1302. doi: 10.1182/blood-2004-07-2784. [DOI] [PubMed] [Google Scholar]
- 23.Jager E, van der Velden VH, te Marvelde JG, et al. Targeted drug delivery by gemtuzumab ozogamicin: Mechanism-based mathematical model for treatment strategy improvement and therapy individualization. PLoS One. 2011;6:e24265. doi: 10.1371/journal.pone.0024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jawad M, Seedhouse C, Mony U, et al. Analysis of factors that affect in vitro chemosensitivity of leukaemic stem and progenitor cells to gemtuzumab ozogamicin (Mylotarg) in acute myeloid leukaemia. Leukemia. 2010;24:74–80. doi: 10.1038/leu.2009.199. [DOI] [PubMed] [Google Scholar]
- 25.Sievers EL, Appelbaum FR, Spielberger RT, et al. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: A phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood. 1999;93:3678–3684. [PubMed] [Google Scholar]
- 26.Sievers EL, Larson RA, Stadtmauer EA, et al. Mylotarg Study Group Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 27.Walter RB, Gooley TA, van der Velden VH, et al. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood. 2007;109:4168–4170. doi: 10.1182/blood-2006-09-047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kung Sutherland MS, Walter RB, Jeffrey SC, et al. SGN-CD33A: A novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood. 2013;122:1455–1463. doi: 10.1182/blood-2013-03-491506. [DOI] [PubMed] [Google Scholar]