Abstract
Purpose
The International Neuroblastoma Response Criteria (INRC) require serial measurements of primary tumors in three dimensions, whereas the Response Evaluation Criteria in Solid Tumors (RECIST) require measurement in one dimension. This study was conducted to identify the preferred method of primary tumor response assessment for use in revised INRC.
Patients and Methods
Patients younger than 20 years with high-risk neuroblastoma were eligible if they were diagnosed between 2000 and 2012 and if three primary tumor measurements (antero-posterior, width, cranio-caudal) were recorded at least twice before resection. Responses were defined as ≥ 30% reduction in longest dimension as per RECIST, ≥ 50% reduction in volume as per INRC, or ≥ 65% reduction in volume.
Results
Three-year event-free survival for all patients (N = 229) was 44% and overall survival was 58%. The sensitivity of both volume response measures (ability to detect responses in patients who survived) exceeded the sensitivity of the single dimension measure, but the specificity of all response measures (ability to identify lack of response in patients who later died) was low. In multivariable analyses, none of the response measures studied was predictive of outcome, and none was predictive of the extent of resection.
Conclusion
None of the methods of primary tumor response assessment was predictive of outcome. Measurement of three dimensions followed by calculation of resultant volume is more complex than measurement of a single dimension. Primary tumor response in children with high-risk neuroblastoma should therefore be evaluated in accordance with RECIST criteria, using the single longest dimension.
INTRODUCTION
Neuroblastoma is the most common extracranial solid tumor of childhood and is a heterogeneous malignancy. The International Neuroblastoma Staging System (INSS) and the International Neuroblastoma Response Criteria (INRC) were developed to compare results of trials for children with neuroblastoma conducted around the world.1,2 However, difficulties associated with INSS became apparent over time,3 and, in 2009, the International Neuroblastoma Risk Group Staging System (INRGSS) was adopted. Whereas INSS was a surgical-pathologic staging system, INRGSS relies upon radiologic characteristics to determine stage. Because INRGSS is imaging-based, and because imaging modalities have changed substantially over time, modernization of the INRC is required. This is particularly true with respect to imaging of primary tumors, as both anatomic imaging (computed tomography [CT] and magnetic resonance imaging [MRI]) and functional imaging (diffusion-weighted MRI, nuclear medicine single-photon emission CT, and positron emission tomography) have evolved. Determining the method used to assess changes in primary tumor size is a vital step in revising the INRC. INRC requires serial measurement of lesions in three dimensions to compute volume. In contrast, Response Evaluation Criteria in Solid Tumors (RECIST), requires measurement of index lesions in one dimension.4,5 This study was conducted to determine the best approach for measurement of primary tumors in the updated INRC.
PATIENTS AND METHODS
This study was conducted at seven centers: Texas Children’s Hospital, Great Ormond Street Hospital for Children, Children’s Hospital of Philadelphia, Universitatsklinikum Koln, Hopital Necker-Enfants Malades, Instituto Giannina Gaslini, and Dr von Hauner Children’s Hospital. Medical records and imaging studies were reviewed after ethics board approval. Subjects were eligible if the following criteria were met: younger than 20 years of age at diagnosis; initial imaging studies performed between January 1, 2000, and June 30, 2012; and availability of serial anatomic imaging studies and clinical outcome data. Study radiologists at participating sites measured primary tumors in three dimensions for each subject; central review was not performed. To permit assessment of the relationship between response by imaging and event-free survival (EFS) and overall survival (OS), only patients with high-risk neuroblastoma were included. Because INSS criteria were in use during most of the period in which patients were diagnosed, INSS stage designation was used. For this study, high-risk neuroblastoma was defined as INSS stage 4 neuroblastoma diagnosed at an age greater than 18 months, INSS stage ≥ 2 disease with MYCN amplification, and INSS stage 3 disease and unfavorable histology per the International Neuroblastoma Pathology Classification (INPC) system6,7 diagnosed at an age greater than 18 months. Patients were treated per institutional standards or were enrolled in clinical trials.
Patients comprising the analytic cohort included those for whom three primary tumor measurements (antero-posterior, width, and cranio-caudal) were recorded at least twice before tumor resection. Tumor size reduction was the difference in maximum tumor diameter, measured serially in the same orthogonal plane, or the difference in tumor volume observed upon comparison of imaging performed at diagnosis and imaging performed at the time point closest to primary tumor resection. If a primary tumor formed a single conglomerate mass with enlarged regional lymph nodes, the entire mass was measured. If clear separation between the primary tumor and regional nodes became apparent after treatment or regional nodes disappeared, only the primary tumor itself was measured. A complete response was defined as absence of residual tumor. A partial response (PR) on the basis of a comparison of maximum tumor diameters was defined, per RECIST, as a ≥ 30% reduction in longest tumor dimension (Diam30). For volume assessment, the formula, volume = (π/6) × antero-posterior (depth) × width × cranio-caudal, was used. A PR on the basis of a comparison of volumes was defined, per INRC, as ≥ 50% reduction in primary tumor volume following treatment (Vol50). Because ≥ 30% reduction in the diameter of a sphere corresponds to a ≥ 65% reduction in volume (Vol65), this definition of PR was also evaluated (Table 1).
Table 1.
Measurements of Primary Tumor Response
| No. of Dimensions Measured | Definition of Partial Response | Abbreviation Used in Study |
|---|---|---|
| Single dimension | ≥ 30% reduction in longest diameter | Diam30 |
| Three dimensions | ≥ 50% reduction in volume* | Vol50 |
| Three dimensions | ≥ 65% reduction in volume* | Vol65 |
Volume = (π/6) × antero-posterior (depth) × width × cranio-caudal.
Survival plots, life tables, and log-rank tests were used to compare OS and EFS. Risk of relapse was evaluated using known prognostic factors. These included age (< 18 months v ≥ 18 months), INSS stage (non–stage 4 v stage 4), MYCN status (nonamplified v amplified), and INPC histology (favorable v unfavorable). The Kaplan-Meier method was used to generate survival curves with SEs per Peto et al.8,9 For EFS, an event was defined as relapse, progression, or death from any cause. For OS, only death was considered. Time to event or death was calculated from time of diagnosis. In the absence of an event or death, survival time was censored at time of last contact.
The sensitivity and specificity of response measures in predicting death were calculated. A χ2 test was performed for each response measure to determine if a statistically significant relationship existed between tumor reduction and extent of tumor resection, and between maximum diameter or volume reduction and prognostic factors. Patients were classified as having a complete surgical resection if ≥ 90% of the tumor mass was removed, otherwise patients were classified as having an incomplete resection. To determine the independent prognostic strength of response measures for survival in the presence of prognostic factors, multivariable Cox proportional hazards (PH) regression models with the Efron method of handling tied event times were fit. Because INPC histology is confounded with age, models were fit to include these variables separately. Any apparent violations of the PH assumption were tested, and if found significant, were handled by treating the covariate as time dependent, which was accomplished by including a survival-time interaction term in the model.10 Backward selection was used to determine the most parsimonious model.
Analyses were performed using SAS (SAS/STAT User’s Guide, Version 9.2; SAS Institute, Cary, NC). Life tables and survival curves were created using R (R Project for Statistical Computing; https://www.r-project.org/). P values less than .05 were considered statistically significant.
RESULTS
Patient Characteristics and Outcome
Data for 252 children with high-risk neuroblastoma were collected. Twenty-three patients were excluded because of the absence of complete tumor measurements. The final analytic cohort consisted of 229 patients. Median time between baseline and presurgical imaging was 110 days (range, 25 to 693 days).
Of the 229 patients, 189 (83%) had INSS stage 4 disease. Eighty-two percent (187 of 229 patients) were age ≥ 18 months at diagnosis. Fifty-two percent of patients had MYCN amplified tumors, and 87% had tumors with unfavorable histology (Table 2). Patients without an event (n = 101) had a median follow-up time of 2.9 years (range, 88 days to 12.6 years). Patients with an event (n = 128) had a median time to event of 1.1 years (range, 81 days to 12.1 years). Patients who remained alive (n = 134) had a median follow-up time of 2.8 years (range, 88 days to 12.6 years). Patients who died (n = 95) had a median time to death of 1.2 years (range, 96 days to 10.9 years).
Table 2.
Patient Characteristics and Outcome
| Patient Cohort | No. (%) | 3-Year EFS ± SE (%) | P (EFS Log-Rank) | 3-Year OS ± SE (%) | P (OS Log-Rank) |
|---|---|---|---|---|---|
| Overall | 229 | 44.4 ± 4.3 | N/A | 58.2 ± 4.4 | N/A |
| Age at diagnosis, months | |||||
| < 18 | 42 (18) | 53.1 ± 9.7 | .284 | 60.7 ± 9.8 | .943 |
| ≥ 18 | 187 (82) | 42.4 ± 4.7 | 57.6 ± 4.9 | ||
| INSS stage | |||||
| Non–stage 4 | 40 (17) | 67.0 ± 9.9 | .002 | 76.4 ± 9.0 | .023 |
| Stage 4 | 189 (83) | 39.4 ± 4.6 | 54.0 ± 4.9 | ||
| MYCN status | |||||
| Nonamplified | 105 (48) | 52.4 ± 7.0 | .006 | 70.5 ± 6.5 | < .001 |
| Amplified | 112 (52) | 37.0 ± 5.4 | 45.7 ± 5.8 | ||
| Histology | |||||
| Favorable | 18 (13) | 81.3 ± 12.4 | .035 | 80.8 ± 12.5 | .133 |
| Unfavorable | 107 (87) | 48.9 ± 6.3 | 60.1 ± 6.5 |
Abbreviations: EFS, event-free survival; INSS, International Neuroblastoma Staging System; N/A, not applicable; OS, overall survival.
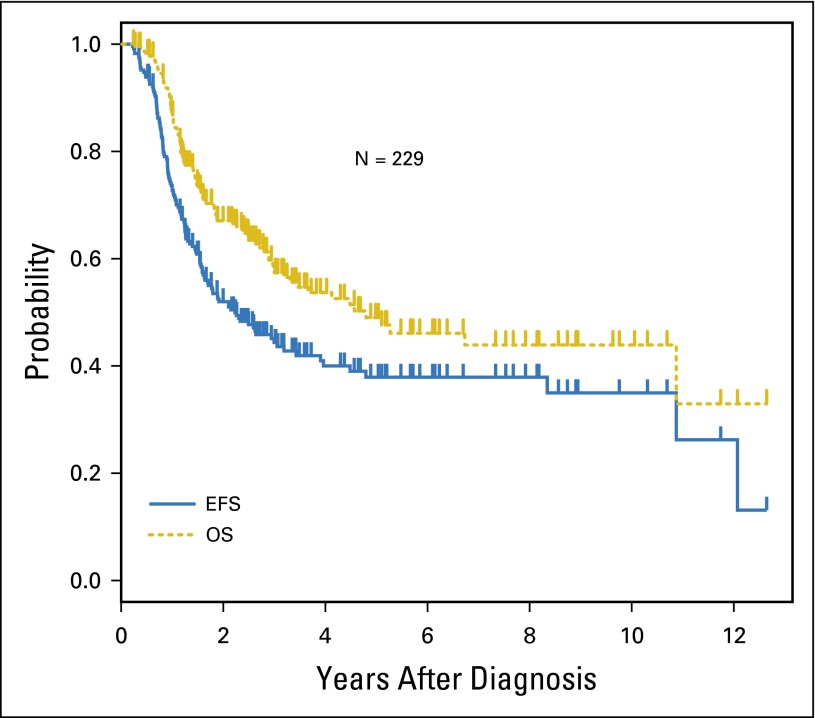
The 3-year EFS and OS for all 229 patients was 44.4% ± 4.3% and 58.2% ± 4.4%, respectively (Fig 1). Three-year EFS and OS for patients with non–stage 4 disease were significantly higher than the EFS and OS for patients with stage 4 disease (67.0% ± 9.9% v 39.4 ± 4.6%; P = .002; and 76.4% ± 9.0% v 54.0% ± 4.9%; P = .023, respectively). Patients whose tumors were MYCN nonamplified fared better than those whose tumors were MYCN amplified (3-year EFS 52.4% ± 7.0% v 37.0 ± 5.4%; P = .006; 3-year OS 70.5% ± 6.5% v 45.7% ± 5.8%; P < .001, respectively).
Fig 1.
Event-free survival (EFS) and overall survival (OS) for all patients (n = 229).
Response Measures and Outcome
Sensitivity and specificity of response measures with respect to life status (alive v dead) are shown in Table 3. The sensitivity of each measure reflects the ability of that measure to detect a PR or greater in patients who go on to survive. The specificity of each measure reflects the ability of that measure to identify less than a PR among patients who ultimately die. The sensitivity of Vol50 was 79% and the sensitivity of Vol65 was 72%; both were greater than the sensitivity associated with Diam30. However, the specificity of all three measures was low: Diam30, 23%; Vol50, 17%; and Vol65, 28%. None of the methods of response assessment was significantly associated with extent of resection.
Table 3.
Sensitivity and Specificity of Response Measures
| Response Measure | Overall Cohort (N = 229) | MYCN Amplified (n = 112) | Stage 4 (n = 189) | |||
|---|---|---|---|---|---|---|
| Sensitivity, % (95% CI) | Specificity, % (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | |
| > 30% maximum diameter reduction | 61.9 (53.7 to 70.2) | 23.2 (14.7 to 31.6) | 89.1 (80.9 to 97.3) | 15.8 (6.3 to 25.3) | 65.4 (56.2 to 74.5) | 21.2 (12.5 to 29.9) |
| > 50% volume reduction | 79.1 (72.2 to 86.0) | 16.8 (9.3 to 24.4) | 96.4 (87.5 to 99.6) | 12.3 (3.8 to 21.0) | 82.7 (74.0 to 89.4) | 15.3 (7.6 to 23.0) |
| > 65% volume reduction | 72.4 (64.8 to 80.0) | 28.4 (19.4 to 37.5) | 92.7 (82.4 to 98.0) | 19.3 (9.1 to 29.5) | 76.9 (67.6 to 84.6) | 28.2 (18.7 to 37.8) |
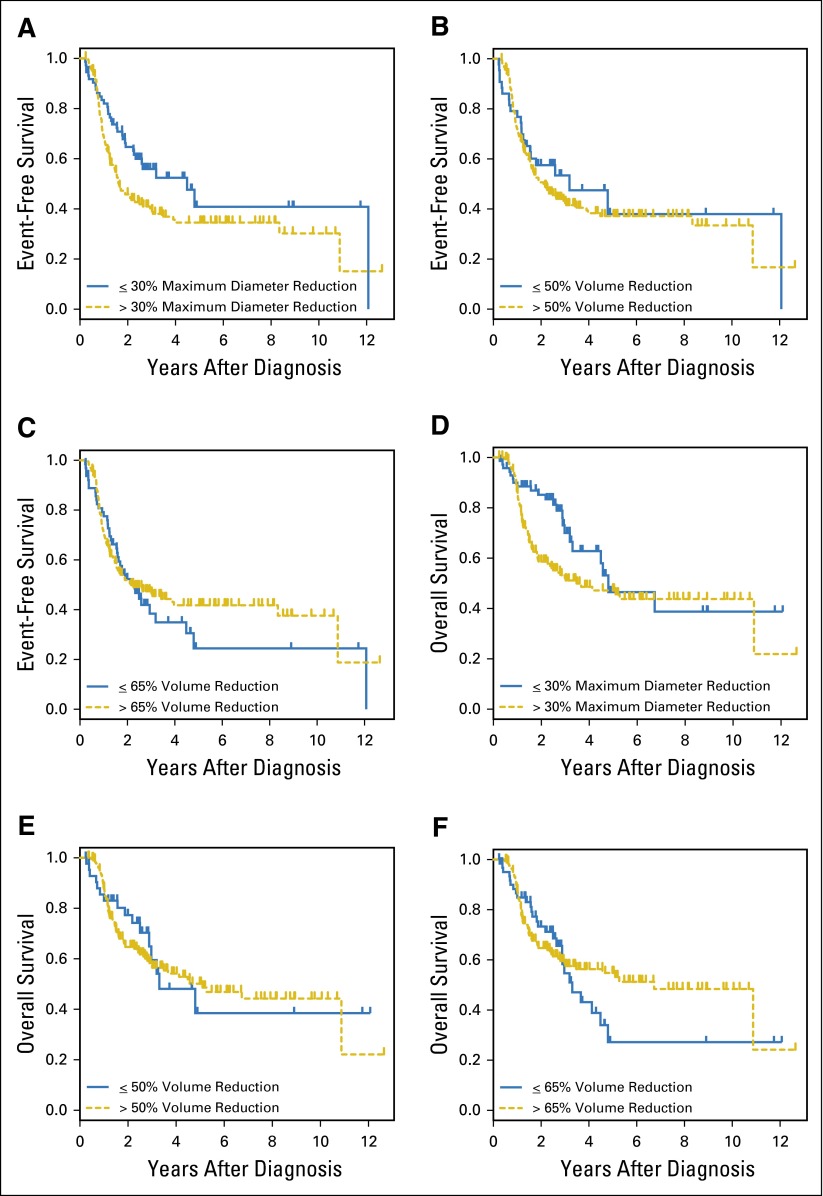
EFS and OS on the basis of response to initial therapy are shown in Figures 2A to 2F. Because survival curves cross, PH assumptions were tested; however, no statistically significant results were found, indicating that PH may be assumed and that log-rank P values are valid. In both EFS and OS multivariable Cox models, only INSS stage and MYCN status were predictive of outcome; neither a change in the single longest diameter nor changes in tumor volume were retained in the respective models.
Fig 2.
Event-free survival and overall survival by method of response assessment. (A to C) Event-free survival and (D to F) overall survival on the basis of response as defined by a (A, D) greater than 30% reduction in longest diameter, (B, E) greater than 50% reduction in volume, (C, F) greater than 65% reduction in volume.
Primary Site Response in MYCN Amplified Neuroblastoma
Three-year EFS and OS for patients with MYCN amplified tumors (n = 112) were 37.0% ± 5.4% and 45.7% ± 5.8%, respectively (Table 4). The majority of patients with MYCN amplified tumors responded to initial treatments; 87% were classified as responders by Diam30, 92% were responders using Vol50, and 87% were responders using Vol65 as the response benchmark. Within this subgroup, the sensitivity of all measures in detecting response among survivors was high (Table 3). However, the specificity of all measures was low, which indicated that a lack of a PR did not identify patients who would go on to die of disease, regardless of method of assessment used. In this subgroup, as in the cohort as a whole, none of the response measures was significantly associated with extent of tumor resection.
Table 4.
Response Measures
| Response Measure | No. (%) | 3-Year EFS ± SE, % | P (EFS) | 3-Year OS ± SE, % | P (OS) |
|---|---|---|---|---|---|
| Overall cohort | |||||
| Maximum diameter reduction | |||||
| ≤ 30% | 73 (32) | 55.9 ± 8.5 | .023* | 70.0 ± 8.0 | .016* |
| > 30% | 156 (68) | 39.0 ± 4.8 | 52.2 ± 5.1 | ||
| Volume reduction | |||||
| ≤ 50% | 44 (19) | 53.4 ± 12.2 | .648* | 59.5 ± 11.4 | .664* |
| > 50% | 185 (81) | 42.4 ± 4.5 | 57.4 ± 4.7 | ||
| Volume reduction | |||||
| ≤ 65% | 64 (28) | 38.4 ± 9.1 | .791* | 54.6 ± 9.5 | .899* |
| > 65% | 165 (72) | 46.4 ± 4.9 | 58.5 ± 4.9 | ||
| MYCN amplified | |||||
| Overall | 112 | 37.0 ± 5.4 | N/A | 45.7 ± 5.8 | N/A |
| Maximum diameter reduction | |||||
| ≤ 30% | 15 (13) | 26.7 ± 13.2 | .254 | 40.2 ± 15.5 | .335 |
| > 30% | 97 (87) | 38.9 ± 5.6 | 47.0 ± 6.3 | ||
| Volume reduction | |||||
| ≤ 50% | 9 (8) | 11.1 ± 10.5 | .001 | 12.5 ± 11.7 | .001 |
| > 50% | 103 (92) | 39.4 ± 5.7 | 48.7 ± 6.1 | ||
| Volume reduction | |||||
| ≤ 65% | 15 (13) | 6.7 ± 6.4 | < .001† | 10.3 ± 9.7 | .001 |
| > 65% | 97 (87) | 42.2 ± 6.0 | 50.8 ± 6.2 | ||
| Stage 4 | |||||
| Overall | 189 | 39.4 ± 4.6 | N/A | 54.0 ± 4.9 | N/A |
| Maximum diameter reduction | |||||
| ≤ 30% | 54 (29) | 50.2 ± 9.1 | .029 | 68.6 ± 8.8 | PH assumption violated |
| > 30% | 135 (71) | 35.0 ± 5.2 | 48.3 ± 5.6 | ||
| Volume reduction | |||||
| ≤ 50% | 31 (19) | 44.0 ± 13.4 | .905* | 54.4 ± 13.0 | .638* |
| > 50% | 158 (81) | 38.6 ± 4.8 | 53.7 ± 5.2 | ||
| Volume reduction | |||||
| ≤ 65% | 48 (25) | 27.7 ± 8.9 | .539* | 47.4 ± 10.4 | .762* |
| > 65% | 141 (75) | 43.5 ± 5.3 | 55.7 ± 5.5 |
Abbreviations: EFS, event-free survival; N/A, not applicable; OS, overall survival; PH, proportional hazards.
Apparent violation of the PH assumption tested but not statistically significant; log-rank test P values remain valid.
P < .001 using time-dependent covariate adjusted Cox model.
In contrast with the finding for the overall cohort, however, a statistically significant association between volume-based response assessment and survival was observed in the MYCN amplified subgroup (Table 4). Diam30 was not associated with a statistically significant difference in EFS or OS in this subgroup. Differences in outcome related to volume response measures were further explored by fitting Cox PH regression models that included age, histology, and INSS stage. Vol50 or Vol65 remained predictive of both EFS and OS. Patients with MYCN amplified neuroblastoma who did not have a ≥ 50% reduction in tumor volume in response to initial systemic therapy were at more than three times greater risk for an event and for death than were patients who had such a reduction in volume. The hazard ratios associated with a 65% volume reduction were 2.79 and 2.73 for EFS and OS, respectively.
Primary Site Response in Stage 4 Neuroblastoma
The 3-year EFS and OS for patients with stage 4 disease (n = 189) were 39.4% ± 4.6% and 54.0% ± 4.9%, respectively (Table 3). The majority of stage 4 patients responded to initial therapy; 71% were responders using Diam30, 81% were responders using Vol50, and 75% were responders using Vol65. In this group, the sensitivities of all measures were lower (Diam30, 65.4%; Vol50, 82.7%; and Vol65, 76.9%) than the sensitivities observed in the MYCN amplified subgroup. The specificity of Vol50 was the lowest of the measures evaluated (15.3%). The specificity of Vol65 and Diam30 were 28.2% and 21.2%, respectively (Table 2). Again, none of the response measures was significantly associated with extent of resection.
EFS and OS in this subgroup were also evaluated in light of method of response assessment. Cox models for EFS and OS did not reveal a statistically significant violation of the PH assumption for volume response measures; the log-rank test remains valid for these analyses. However, PH assumptions were violated for the longest diameter response measure, and Cox models were therefore used for additional analyses. The final backward-selected Cox model showed that only MYCN status was predictive of EFS. Among patients with INSS stage 4 disease, those with MYCN amplified tumors had an increased risk of event of 1.473. Diam30 was dropped from the EFS model as a result of a lack of statistical significance. For OS, stage 4 patients whose tumors decreased by greater than 30% in longest diameter unexpectedly seemed to have a higher risk of death, with all other prognostic factors dropping out of the final backward-selected model. Within the stage 4 subgroup, MYCN status and response as assessed by diameter reduction are highly correlated (P < .001), which could explain, in part, this finding. Of 127 patients with stage 4 disease who had a PR by Diam30, 82 (65%) had MYCN amplified tumors. Of 93 patients with stage 4 disease with MYCN amplified tumors, 82 (88%) had a greater than 30% reduction in maximum tumor diameter.
DISCUSSION
This study was undertaken to build international consensus regarding measurement of primary tumor response using current imaging technology. When the INRC were published in 1988, recommended modalities for primary site imaging included ultrasound, CT, and MRI.1 By 1993, ultrasound was no longer recommended for volume assessment.2 Since the last INRC revision, there have been dramatic changes in imaging techniques. Multidetector CT has made submillimeter section thickness scanning routine, and isovolumetric resolution allows rapid measurement of regions of interest in all three orthogonal planes. Increased availability of MRI with sequences in all planes has also greatly improved accuracy of depiction. To our knowledge, the present cohort represents the largest group of patients with high-risk neuroblastoma in whom current approaches to primary tumor response have been evaluated. In the cohort as a whole, no clear advantage for use of three-dimensional rather than one-dimensional measurement was observed, and neither change in volume nor change in longest diameter was predictive of outcome.
Use of one versus three dimensions for assessment of response in pediatric tumors has been studied previously in patients with rhabdomyosarcoma. Two studies showed that use of volume in assessment of response to initial therapy did not more accurately predict outcome than did use of single dimension measurements.11,12 In children with neuroblastoma, Yoo et al13 evaluated the relationship between primary tumor response and outcome, but did not compare methods of response assessment. Our study, to our knowledge, is the first to compare reduction in volume versus reduction in longest diameter as response measures in children with neuroblastoma.
Early response in sites of metastatic disease is predictive of outcome in patients with high-risk neuroblastoma14-16; however, the fact that primary tumor response after initial chemotherapy did not predict outcome in our study is not surprising given the nature of modern-era therapy. Multiagent chemotherapy remains a cornerstone of treatment, but primary tumor control includes use of other therapeutic modalities. Although there is debate regarding the extent to which aggressive surgery alters outcome in patients with metastatic disease,17-19 surgery remains a key component of primary tumor treatment. Radiation of the primary tumor bed in all patients20,21 or those with residual disease at end induction is also standard.22 Because of local tumor control measures and addition of effective postconsolidation therapy (isotretinoin and immunotherapy), chemoresponsiveness is not the sole determinant of outcome. Indeed, 5-year local relapse-free survival in the Children’s Oncology Group A3973 trial was 87.3%, whereas overall EFS at 5 years was 43.5%.19 Thus, although control of primary tumors is important, control of other sites of disease is also essential.
Yoo et al13 have reported that more favorable primary tumor response is associated with improved relapse-free survival in patients with high-risk neuroblastoma. The discrepancy between those findings and the results of the current study may in part be a result of differences in treatment. Children described by Yoo et al received postconsolidation therapy (isotretinoin and interleukin-2), but GD2-directed antibody therapy was not included. Dose-intensified chemotherapy was the central component of treatment of the Yoo et al cohort, and the majority of patients underwent two cycles of high-dose chemotherapy with stem cell rescue. Therefore, an early measure of chemoresponsiveness might be expected to have greater predictive value in the context of treatment that is chemotherapy focused.
Differences in response assessment methodology may also explain differences between the current results and those reported by Yoo et al.13 In the latter study, a good response to initial therapy was defined as a ≥ 60% reduction in volume. In contrast, our study used standard response definitions including the RECIST definition of PR (Diam30), a corresponding reduction in volume (Vol65), and the INRC definition of PR (Vol50). Finally, Yoo et al computed tumor volume by outlining regions of interest in each slice of stacked CT or MRI images. Areas of interest were summed and multiplied by slice thickness to determine volume. In our study, the formula for volume of a spheroid was used to estimate tumor volume, as is more typically done in everyday radiology practice. Although this strategy is more accurate than the use of formulae for cubes or spheres, volumes calculated are approximations of the irregularly shaped lesions commonly encountered in children with neuroblastoma. Volumetric approaches, rather than formula-based approximations, have been used in other pediatric studies23-26 but volumetrics have not been broadly incorporated into clinical practice. Because our study was designed to facilitate a common approach to response assessment internationally, we focused on measurement methods that can be used around the globe today, acknowledging that volumetric techniques may be used more widely in the future.
There are several limitations of this work. Response was evaluated in patients for whom paired imaging studies were available. Although missing data may have led to exclusion of some potential subjects, the characteristics of patients comprising this cohort are consistent with those of published high-risk cohorts with respect to age, stage, MYCN status, and histology.15,27,28 Key components of high-risk neuroblastoma therapy were included in regimens delivered at participating centers; however, treatment protocols were not identical across sites. The timing of induction cycles varied by institution (Appendix Table A1, online only), and although most centers delivered high-dose chemotherapy with autologous stem cell rescue as part of standard high-risk therapy, not all patients underwent autologous stem cell rescue. Similarly, not all patients received GD2-directed immunotherapy. Additional prospective studies with central radiology review focused on uniformly treated patients should be pursued, as should studies with extended follow-up times. Only patients with high-risk disease were included in this study; evaluation of response measures in patients with non–high-risk neuroblastoma should be considered, particularly as response by imaging may be less dramatic in more differentiated tumors. Finally, this study focused on assessment of primary tumor response to frontline therapy. An effort to address response in the relapse setting is in progress.
In summary, extent of reduction in primary tumor size did not accurately predict outcome in children with high-risk neuroblastoma, whether assessed by change in tumor volume or by reduction in single longest diameter. However, primary tumor response must be considered in overall response evaluation, as primary site progression must be captured. In practical terms, a single measurement is easier to perform than is measurement of three dimensions followed by calculation of volume. In light of our findings, it is recommended that primary tumor response be measured in the upcoming, revised INRC in accordance with RECIST criteria, using the single longest tumor dimension. This approach will be studied prospectively in forthcoming cooperative group trials.
Appendix
Table A1.
Common Induction Regimens
| Regimen Name | Reference |
|---|---|
| Rapid COJEC | Pearson et al27 |
| GPOH NB97 | Simon T, et al: Pediatr Blood Cancer 56:578-583, 2011 |
| GPOH NB2004 | Simon T, et al: Pediatr Blood Cancer 56:578-83, 2011 |
| Modified NB87 | Coze C, et al: J Clin Oncol 15:3433-3440, 1997 |
| COG A3973 | Kreissman SG, et al: Lancet Oncol 14:999-1008, 2013 |
| COG ANBL00P1 | Seif AE, et al: Bone Marrow Transplant 48:947-952, 2013 |
| CHOP/DFCI Tandem Trial | George RE, et al: J Clin Oncol 24:2891-2896, 2006 |
| COG ANBL02P1/ANBL0532 | Park JR, et al: J Clin Oncol 29:4351-4357, 2011 |
| Texas PEPI Trial | NCT00578864 |
Footnotes
Listen to the podcast by Dr Irwin at www.jco.org/podcasts
Supported by the National Cancer Institute Clinical Trials Planning Meeting; Alex’s Lemonade Stand Foundation; The Ben Towne Foundation; Deutsche Krebshilfe; NIH Grants No. U10 CA98413, U10 CA98543, and U10 CA180899; Little Heroes Pediatric Cancer Research Foundation; and CureSearch for Children’s Cancer.
Presented at the Advances in Neuroblastoma Research Congress, Cologne, Germany, May 16, 2014.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Rochelle Bagatell, Kieran McHugh, Arlene Naranjo, Chaim Kirby, Penelope Brock, Thorsten Simon, Sabine Sarnacki, Julie R. Park, Jed Nuchtern
Provision of study materials or patients: Rochelle Bagatell, Kieran McHugh, Penelope Brock, Karen A. Lyons, Lisa J. States, Thorsten Simon, Barbara Krug, Sabine Sarnacki, Dominique Valteau-Couanet, Dietrich von Schweinitz, Birgit Kammer, Claudio Granata, Luca Pio, Jed Nuchtern
Collection and assembly of data: Rochelle Bagatell, Kieran McHugh, Collin Van Ryn, Chaim Kirby, Penelope Brock, Karen A. Lyons, Lisa J. States, Yesenia Rojas, Alexandra Miller, Sam L. Volchenboum, Thorsten Simon, Barbara Krug, Sabine Sarnacki, Dominique Valteau-Couanet, Claudio Granata, Luca Pio, Jed Nuchtern
Data analysis and interpretation: Rochelle Bagatell, Kieran McHugh, Arlene Naranjo, Collin Van Ryn, Penelope Brock, Thorsten Simon, Barbara Krug, Sabine Sarnacki, Dietrich von Schweinitz, Birgit Kammer, Julie R. Park, Jed Nuchtern
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHOR'S DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Assessment of Primary Site Response in Children With High-Risk Neuroblastoma: An International Multicenter Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Rochelle Bagatell
No relationship to disclose
Kieran McHugh
No relationship to disclose
Arlene Naranjo
No relationship to disclose
Collin Van Ryn
No relationship to disclose
Chaim Kirby
No relationship to disclose
Penelope Brock
No relationship to disclose
Karen A. Lyons
No relationship to disclose
Lisa J. States
No relationship to disclose
Yesenia Rojas
No relationship to disclose
Alexandra Miller
No relationship to disclose
Sam L. Volchenboum
No relationship to disclose
Thorsten Simon
No relationship to disclose
Barbara Krug
No relationship to disclose
Sabine Sarnacki
No relationship to disclose
Dominique Valteau-Couanet
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Dietrich von Schweinitz
No relationship to disclose
Birgit Kammer
No relationship to disclose
Claudio Granata
No relationship to disclose
Luca Pio
No relationship to disclose
Julie R. Park
No relationship to disclose
Jed Nuchtern
Stock or Other Ownership: Dexcom, Johnson & Johnson, Lexicon Pharmaceuticals, McKesson, CVS Health Corp, Insulet, Tandem Diabetes Care
REFERENCES
- 1.Brodeur GM, Seeger RC, Barrett A, et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol. 1988;6:1874–1881. doi: 10.1200/JCO.1988.6.12.1874. [DOI] [PubMed] [Google Scholar]
- 2.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 3.Monclair T, Brodeur GM, Ambros PF, et al. INRG Task Force The International Neuroblastoma Risk Group (INRG) staging system: An INRG Task Force report. J Clin Oncol. 2009;27:298–303. doi: 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Shimada H, Ambros IM, Dehner LP, et al. Terminology and morphologic criteria of neuroblastic tumors: Recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999;86:349–363. [PubMed] [Google Scholar]
- 7.Shimada H, Ambros IM, Dehner LP, et al. The International Neuroblastoma Pathology Classification (the Shimada system) Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- 8.Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 9.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allison PD, Inc SI, editors. Survival Analysis Using the SAS System: A Practical Guide. Cary, NC,: SAS Institute; 1995. [Google Scholar]
- 11.Ferrari A, Miceli R, Meazza C, et al. Comparison of the prognostic value of assessing tumor diameter versus tumor volume at diagnosis or in response to initial chemotherapy in rhabdomyosarcoma. J Clin Oncol. 2010;28:1322–1328. doi: 10.1200/JCO.2009.25.0803. [DOI] [PubMed] [Google Scholar]
- 12.Schoot RA, McHugh K, van Rijn RR, et al. Response assessment in pediatric rhabdomyosarcoma: Can response evaluation criteria in solid tumors replace three-dimensional volume assessments? Radiology. 2013;269:870–878. doi: 10.1148/radiol.13122607. [DOI] [PubMed] [Google Scholar]
- 13.Yoo SY, Kim JS, Sung KW, et al. The degree of tumor volume reduction during the early phase of induction chemotherapy is an independent prognostic factor in patients with high-risk neuroblastoma. Cancer. 2013;119:656–664. doi: 10.1002/cncr.27775. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Simon T, Hero B, et al. The prognostic impact of functional imaging with (123)I-mIBG in patients with stage 4 neuroblastoma >1 year of age on a high-risk treatment protocol: Results of the German Neuroblastoma Trial NB97. Eur J Cancer. 2008;44:1552–1558. doi: 10.1016/j.ejca.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Yanik GA, Parisi MT, Shulkin BL, et al. Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: A report from the Children’s Oncology Group. J Nucl Med. 2013;54:541–548. doi: 10.2967/jnumed.112.112334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decarolis B, Schneider C, Hero B, et al. Iodine-123 metaiodobenzylguanidine scintigraphy scoring allows prediction of outcome in patients with stage 4 neuroblastoma: Results of the Cologne Interscore Comparison Study. J Clin Oncol. 2013;31:944–951. doi: 10.1200/JCO.2012.45.8794. [DOI] [PubMed] [Google Scholar]
- 17.La Quaglia MP, Kushner BH, Su W, et al. The impact of gross total resection on local control and survival in high-risk neuroblastoma. J Pediatr Surg. 2004;39:412–417, discussion 412-417. doi: 10.1016/j.jpedsurg.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Simon T, Häberle B, Hero B, et al. Role of surgery in the treatment of patients with stage 4 neuroblastoma age 18 months or older at diagnosis. J Clin Oncol. 2013;31:752–758. doi: 10.1200/JCO.2012.45.9339. [DOI] [PubMed] [Google Scholar]
- 19.Von Allmen D, Davidoff AM, London WB, et al. Influence of Extent of Resection on Survival in High Risk Neuroblastoma Patients: A Report from the COG A3973 Study. Cologne, Germany,: Advances in Neuroblastoma Research Association; 2014. [Google Scholar]
- 20.Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): A randomised phase 3 trial. Lancet Oncol. 2013;14:999–1008. doi: 10.1016/S1470-2045(13)70309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaze MN, Boterberg T, Dieckmann K, et al. Results of a quality assurance review of external beam radiation therapy in the International Society of Paediatric Oncology (Europe) Neuroblastoma Group’s High-risk Neuroblastoma Trial: A SIOPEN study. Int J Radiat Oncol Biol Phys. 2013;85:170–174. doi: 10.1016/j.ijrobp.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Simon T, Hero B, Bongartz R, et al. Intensified external-beam radiation therapy improves the outcome of stage 4 neuroblastoma in children > 1 year with residual local disease. Strahlenther Onkol. 2006;182:389–394. doi: 10.1007/s00066-006-1498-8. [DOI] [PubMed] [Google Scholar]
- 23.Solomon J, Warren K, Dombi E, et al. Automated detection and volume measurement of plexiform neurofibromas in neurofibromatosis 1 using magnetic resonance imaging. Comput Med Imaging Graph. 2004;28:257–265. doi: 10.1016/j.compmedimag.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Harris GJ, Plotkin SR, Maccollin M, et al. Three-dimensional volumetrics for tracking vestibular schwannoma growth in neurofibromatosis type II. Neurosurgery. 2008;62:1314–1319, discussion 1319-1320. doi: 10.1227/01.neu.0000333303.79931.83. [DOI] [PubMed] [Google Scholar]
- 25.Warren KE, Patronas N, Aikin AA, et al. Comparison of one-, two-, and three-dimensional measurements of childhood brain tumors. J Natl Cancer Inst. 2001;93:1401–1405. doi: 10.1093/jnci/93.18.1401. [DOI] [PubMed] [Google Scholar]
- 26.Shah GD, Kesari S, Xu R, et al. Comparison of linear and volumetric criteria in assessing tumor response in adult high-grade gliomas. Neuro-oncol. 2006;8:38–46. doi: 10.1215/S1522851705000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson AD, Pinkerton CR, Lewis IJ, et al. European Neuroblastoma Study Group. Children’s Cancer and Leukaemia Group (CCLG formerly United Kingdom Children’s Cancer Study Group) High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: A randomised trial. Lancet Oncol. 2008;9:247–256. doi: 10.1016/S1470-2045(08)70069-X. [DOI] [PubMed] [Google Scholar]
- 28.Berthold F, Boos J, Burdach S, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: A randomised controlled trial. Lancet Oncol. 2005;6:649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]