Abstract
Purpose
Over the past decade, intensity-modulated radiation therapy (IMRT) has replaced conventional radiation techniques in the management of head-and-neck cancers (HNCs). We conducted this population-based study to evaluate the influence of radiation oncologist experience on outcomes in patients with HNC treated with IMRT compared with patients with HNC treated with conventional radiation therapy.
Methods
We identified radiation providers from Medicare claims of 6,212 Medicare beneficiaries with HNC treated between 2000 and 2009. We analyzed the impact of provider volume on all-cause mortality, HNC mortality, and toxicity end points after treatment with either conventional radiation therapy or IMRT. All analyses were performed by using either multivariable Cox proportional hazards or Fine-Gray regression models controlling for potential confounding variables.
Results
Among patients treated with conventional radiation, we found no significant relationship between provider volume and patient survival or any toxicity end point. Among patients receiving IMRT, those treated by higher-volume radiation oncologists had improved survival compared with those treated by low-volume providers. The risk of all-cause mortality decreased by 21% for every additional five patients treated per provider per year (hazard ratio [HR], 0.79; 95% CI, 0.67 to 0.94). Patients treated with IMRT by higher-volume providers had decreased HNC-specific mortality (subdistribution HR, 0.68; 95% CI, 0.50 to 0.91) and decreased risk of aspiration pneumonia (subdistribution HR, 0.72; 95% CI, 0.52 to 0.99).
Conclusion
Patients receiving IMRT for HNC had improved outcomes when treated by higher-volume providers. These findings will better inform patients and providers when making decisions about treatment, and emphasize the critical importance of high-quality radiation therapy for optimal treatment of HNC.
INTRODUCTION
Radiation therapy is a mainstay of treatment in patients with head-and-neck cancer (HNC); however, the proximity of the tumor to sensitive normal tissues poses unique challenges in the delivery of radiation. Radiation fields that are too small can lead to cancer progression, whereas radiation fields that are too big can lead to long-term toxicity that can dramatically impact patient quality of life. The perceived skill required to treat this cancer is illustrated by the National Comprehensive Cancer Network clinical guidelines, which specify that “…all patients need access to specialists with expertise in the management of patients with [HNC] for optimal treatment.”1(p9)
Prior research regarding radiation therapy in patients with HNC demonstrates a potential link between treatment center experience and survival. A report from the Trans-Tasman Radiation Oncology Group found that noncompliant radiation plans were seen more often from cancer centers that had a low volume of patients enrolled in clinical trials, and were associated with decreased survival.2 More recently, the Radiation Therapy Oncology Group (RTOG) found worse overall survival among patients treated at low-volume accruing centers.3 These two studies, however, included only patients treated with conventional, three-dimensional (3D), conformal radiation therapy, whereas, over the past decade, intensity-modulated radiation therapy (IMRT) has become the standard of care for HNC.1,4,5
Compared with classic radiation techniques, IMRT markedly increases the complexity of target delineation and treatment planning. In fact, results of the first RTOG study to evaluate IMRT in HNC suggested a learning curve for the use of this technology6; therefore, the impact of physician experience on patient outcomes in the IMRT era could increase substantially compared with physician experience and the use of older radiation techniques. The purpose of this population-based study was to evaluate the influence of radiation oncology physician experience on survival and toxicity in patients with HNC treated with IMRT compared with patients treated with conventional conformal radiation therapy.
METHODS
Data Source
We identified patients with HNC from the SEER-Medicare linked database. Managed by the National Cancer Institute, SEER pools data from individual cancer registries across the United States, covering approximately 28% of the US population. Medicare is a federally-funded health insurance program for individuals older than 65 years. The SEER-Medicare linkage includes Medicare claims for Medicare beneficiaries within SEER. Medicare claims for services such as radiation include physician identifiers, which makes this dataset ideal for tracking and studying radiation providers. In addition, the longitudinal aspect of Medicare data gives researchers the ability to track long-term health outcomes across a patient's disease course. The Institutional Review Board of the University of California San Diego deemed this study exempt from review.
Study Population
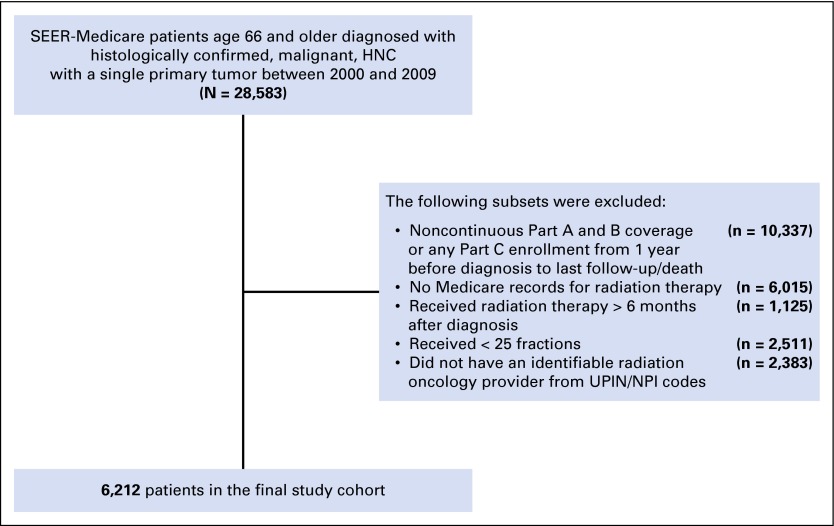
An initial query of the SEER database identified 28,583 patients at least 66 years old with histologically confirmed HNC treated with radiation for a single primary tumor diagnosed between 2000 and 2009. Patients were required to have complete Medicare claims data, which included continuous Part A and B coverage from one year before diagnosis until death or the end of the study period (December 2010), to allow for the ascertainment of comorbidities before diagnosis and health outcomes after radiation. Patients with incomplete Part A or B during our study period were excluded. Patients with Part C coverage were also excluded from the study as managed care organizations do not routinely submit claims information. Additional patient exclusion criteria are detailed under Radiation Therapy, and the complete patient selection schema is illustrated in Figure 1. The final study cohort included 6,212 patients.
Fig 1.
Patient selection process. HNC, head-and-neck cancer; NPI, National Physician Identifier; UPIN, Unique Physician Identification Number.
Study Covariates
SEER data were used to identify patient characteristics, such as age at diagnosis, gender, race, marital status, year of diagnosis, primary tumor size and grade, and median household income, determined from 2000 US Census tract data. As specific TNM staging data were not captured for all years studied, the SEER historic staging system was used. SEER historic staging classifies patients into three primary disease categories representing local, regional, or distant disease. SEER historic staging of HNC for distant disease includes locally advanced patients (T4a and T4b) as well as patients with distant metastatic disease (M1)7; however, the fraction of M1 patients is likely small, and excluding these patients does not influence the analysis (Appendix, online only).
Inpatient and outpatient Medicare claims from the year before diagnosis were used to assess pre-existing comorbidity by using the Deyo adaptation of the Charlson comorbidity index.8 The administration of chemotherapy was ascertained by using previously described methods.9 Specific chemotherapeutic drugs, such as cisplatin and cetuximab, were identified by using Healthcare Common Procedure Coding System J codes (Appendix). Care at a teaching hospital was defined as any indirect medical education payment noted during a hospitalization after the diagnosis of cancer. Positron emission tomography imaging prior to radiation was identified to account for potential stage migration. Surgeries prior to radiation were determined from Common Procedural Terminology and International Classification of Diseases, Ninth Revision, codes. Patient characteristics, including treatment-related variables, are presented in Table 1.
Table 1.
Patient Characteristics for the Overall Cohort and Stratified by Provider Volume and Conventional Radiation Versus IMRT
| Characteristic | No. of Patients | Radiation Type | |||||
|---|---|---|---|---|---|---|---|
| Conventional | IMRT | ||||||
| Low-Volume Provider, No. (%) | High-Volume Provider, No. (%) | P | Low-Volume Provider, No. (%) | High-Volume Provider, No. (%) | P | ||
| Total | 6,212 | 1,986 (50.0) | 1,984 (50.0) | — | 1,119 (49.9) | 1,123 (50.1) | — |
| Age at diagnosis, years | |||||||
| 66-74 | 3,460 | 1,090 (54.9) | 1,045 (52.6) | .28 | 647 (57.8) | 678 (60.4) | .41 |
| 75-79 | 1,340 | 419 (21.1) | 455 (22.9) | 236 (21.1) | 230 (20.5) | ||
| ≥ 80 | 1,412 | 475 (24.0) | 486 (24.5) | 236 (21.1) | 215 (19.1) | ||
| Male | 4,373 | 1,391 (70.1) | 1,436 (72.3) | .13 | 775 (69.3) | 771 (68.7) | .76 |
| Race | |||||||
| White | 5,373 | 1,685 (84.9) | 1,738 (87.5) | < .001 | 949 (84.8) | 1,001 (89.1) | < .001 |
| Black | 487 | 153 (7.7) | 180 (9.1) | 76 (6.8) | 78 (7.0) | ||
| Other | 352 | 146 (7.4) | 68 (3.4) | 94 (8.4) | 44 (3.9) | ||
| Marital status | |||||||
| Married | 3,481 | 1,105 (55.7) | 1,110 (55.9) | .63 | 628 (56.1) | 638 (56.8) | .14 |
| Divorced | 540 | 152 (7.7) | 149 (7.5) | 111 (9.9) | 128 (11.4) | ||
| Single | 576 | 205 (10.3) | 183 (9.2) | 108 (9.7) | 80 (7.1) | ||
| Other | 1,615 | 553 (26.3) | 544 (27.4) | 272 (24.3) | 277 (24.7) | ||
| Charlson comorbidity score | |||||||
| 0 | 3,289 | 1,032 (52.0) | 1,062 (53.5) | .67 | 603 (53.9) | 592 (52.7) | .68 |
| 1 | 1,711 | 570 (28.7) | 538 (27.1) | 303 (27.1) | 300 (26.7) | ||
| 2 | 675 | 216(10.9) | 224 (11.3) | 117 (10.4) | 118 (10.5) | ||
| ≥ 3 | 537 | 166 (8.4) | 162 (8.1) | 96 (8.6) | 113 (10.1) | ||
| Pre-existing comorbidities or procedures | |||||||
| Gastrostomy tube placement | 671 | 191 (9.6) | 198 (10.0) | .72 | 147 (13.1) | 135 (12.0) | .43 |
| Dysphagia | 617 | 83 (4.2) | 67 (3.4) | .18 | 230 (20.6) | 237 (21.1) | .74 |
| Aspiration pneumonia | 136 | 37 (1.9) | 47 (2.4) | .27 | 25 (2.2) | 27 (2.4) | .79 |
| Metropolitan area | 5,127 | 1,646 (83.0) | 1,580 (53.2) | .01 | 950 (84.9) | 951 (84.7) | .89 |
| Teaching hospital | 3,346 | 1,067 (53.8) | 1,056 (53.2) | .70 | 662 (55.6) | 601 (52.5) | .33 |
| Region | |||||||
| East | 1,493 | 444 (22.4) | 511 (25.7) | < .001 | 232 (20.7) | 306 (27.2) | < .001 |
| Midwest | 548 | 221 (11.1) | 167 (8.4) | 84 (7.5) | 76 (6.8) | ||
| South | 1,871 | 416 (21.0) | 822 (41.4) | 234 (20.9) | 399 (35.5) | ||
| West | 2,300 | 903 (45.5) | 486 (24.5) | 569 (50.9) | 342 (30.5) | ||
| Year of diagnosis | |||||||
| 2000-2003 | 2,211 | 1,007 (50.8) | 1,027 (51.7) | .71 | 85 (7.6) | 92 (8.2) | .84 |
| 2004-2006 | 1,958 | 598 (30.1) | 599 (30.2) | 384 (34.3) | 377 (33.6) | ||
| 2007-2009 | 2,043 | 379 (19.1) | 260 (18.1) | 650 (58.1) | 654 (58.2) | ||
| Tumor site | |||||||
| Hypopharynx | 402 | 97 (4.9) | 118 (5.9) | .45 | 82 (7.3) | 105 (9.4) | .51 |
| Larynx | 2,561 | 1,051 (51.2) | 1,028 (51.8) | 251 (22.4) | 267 (23.8) | ||
| Nasopharynx | 136 | 26 (1.3) | 26 (1.3) | 43 (3.8) | 41 (3.6) | ||
| Oral cavity | 1,202 | 336 (16.9) | 345 (17.4) | 264 (23.6) | 257 (22.9) | ||
| Oropharynx | 1,325 | 332 (16.7) | 293 (14.7) | 362 (32.4) | 338 (30.1) | ||
| Other | 586 | 178 (9.0) | 176 (8.9) | 117 (10.5) | 115 (10.2) | ||
| Historic stage | |||||||
| Localized | 2,400 | 937 (47.2) | 923 (46.5) | .24 | 256 (22.8) | 284 (25.3) | .45 |
| Regional | 2,941 | 805 (40.6) | 810 (40.8) | 669 (59.8) | 657 (58.5) | ||
| Distant | 630 | 149 (7.5) | 177 (8.9) | 160 (14.3) | 144 (12.8) | ||
| Unknown | 241 | 93 (4.7) | 76 (3.8) | 34 (3.1) | 38 (3.4) | ||
| Size of primary tumor, cm | |||||||
| 0-5 | 4,245 | 1,388 (70.0) | 1,412 (71.1) | .38 | 726 (64.9) | 719 (64.0) | .89 |
| > 5 | 180 | 36 (1.8) | 26 (1.3) | 57 (5.1) | 61 (5.4) | ||
| Unknown | 1,787 | 560 (28.2) | 548 (27.6) | 336 (30.0) | 343 (30.6) | ||
| Grade | |||||||
| Well or moderately differentiated | 3,404 | 1,178 (59.4) | 1,121 (56.5) | .01 | 578 (51.7) | 527 (46.9) | .08 |
| Poorly or undifferentiated | 1,612 | 453 (22.8) | 436 (22.0) | 346 (17.4) | 337 (33.6) | ||
| Unknown | 1,196 | 353 (17.8) | 429 (21.6) | 195 (17.4) | 219 (19.5) | ||
| PET | 3,432 | 814 (41.0) | 807 (40.6) | .80 | 916 (81.9) | 895 (79.7) | .19 |
| Surgery prior to radiation | 2,192 | 669 (35.2) | 686 (34.5) | .65 | 405 (36.2) | 402 (35.8) | .84 |
| Types of chemotherapy | |||||||
| Cisplatin | 1,669 | 433 (21.8) | 458 (23.1) | .35 | 391 (34.9) | 387 (34.5) | .81 |
| Cetuximab | 729 | 86 (4.3) | 87 (4.4) | .94 | 319 (28.5) | 237 (21.1) | < .001 |
NOTE. The distribution of patient characteristics across provider volumes for each radiation type was evaluated using χ2 tests.
Abbreviations: IMRT, intensity-modulated radiation therapy; PET, positron emission tomography.
Radiation Therapy
Radiation therapy was identified from Medicare claims by using relevant Healthcare Common Procedure Coding System codes for each step in the delivery of a course of radiation.9 The individual components of a radiation course included the radiation simulation, radiation treatment planning, daily radiation treatments, and weekly management activities. A course of radiation therapy was defined as a cluster of claims. Because patients who recur or develop distant metastatic disease can receive additional radiation, we assumed that a break of 30 days or more between sequential radiation codes indicated an additional course of radiation. As defined elsewhere,10 the administration of IMRT was defined as the presence of any IMRT planning or treatment code during the course of radiation. Patients without an IMRT code received two-dimensional or 3D conformal radiation therapy and were grouped in the conventional radiation (non-IMRT) cohort. To reduce the likelihood of including patients treated with palliative intent for metastatic disease, we included only patients treated within six months of diagnosis who received at least 25 individual days (fractions) of radiation.11
Survival and Toxicities
The primary objective of this study was to determine the impact of radiation provider volume, and, therefore, experience, on all-cause mortality. Secondary objectives included exploring the impact of provider volume on HNC mortality and the incidence of radiation-associated toxicities, including dysphagia, aspiration pneumonia, and gastrostomy tube (G-tube) placement.12 International Classification of Diseases, Ninth Revision, codes and Common Procedural Terminology codes were used to identify toxicity events in the inpatient, outpatient, and Carrier Claims files (Appendix Table A1, online only). Many of these clinical toxicity end points, by nature, are not exclusively caused by radiation and could instead be caused by the cancer itself or its associated risk factors. Our multivariable analyses of toxicity end points, described further under Statistical Analysis, controlled for these pre-existing factors, and we defined these as the presence of dysphagia, aspiration pneumonia, and G-tube placement during the year prior to diagnosis through the start of radiation.
Provider Volume
Provider volume was defined as the number of patients with HNC treated by a physician in a year. This approach was based on published methodology using Medicare data to determine hospital and surgeon volume.13-16 Prior research demonstrates a high degree of concordance between volumes estimated by using this approach and actual physician volumes.14,17,18 We identified the specific provider with the Unique Physician Identification Number or National Physician Identifier on the weekly management code, 77247. This code is provider specific as opposed to other technical codes that link to facilities or organizations. Provider volume was expressed as patients treated per year, which was defined as the total number of patients with HNC treated by the provider divided by the time interval between the treatment dates of the first and the last patient. Patient volume was based on all patients (IMRT and conventional) to capture the total HNC experience of a provider; however, we performed a sensitivity analysis and redefined provider volume separately for the IMRT and non-IMRT cohorts as a measure of internal validity. This analysis yielded similar results, and only the primary analysis is presented.
Statistical Analysis
Provider volume was analyzed as a continuous variable to be consistent with other research15-17 and to avoid choosing arbitrary cut points for reclassification of provider volume into lower and higher categories. However, we categorized provider volume into two groups divided by the median value to demonstrate trends and differences in tables and figures. Differences in patient characteristics between high and low provider volume were assessed with χ2 tests. The association between provider volume and all-cause mortality was assessed by using adjusted Cox proportional hazards regression models. To examine the impact of provider volume on HNC-specific mortality and radiation-associated toxicities, competing-risk models were used to assess the adjusted subdistribution hazard ratio (HR) associated with provider volume while accounting for the competing risk of noncancer death or any death, respectively.19 For all multivariable analyses, the HR and subdistribution HRs were presented per every five patients treated. We analyzed the IMRT and conventional (non-IMRT) cohorts separately to understand the role of experience for each radiation type.
Covariates controlled for in the multivariable models were defined a priori and included age at diagnosis, marital status, gender, race, treatment at a teaching hospital, median income quartile, Charlson comorbidity index, year of diagnosis, tumor size, stage, and grade, node status, prior head-and-neck surgery, chemotherapy, length of radiation treatment (expressed continuously as the number of fractions delivered), and the use of positron emission tomography for staging prior to radiation. Unless noted, all covariates were categorical with subgroups presented in Table 1. To account for differences in regional practices, we stratified all multivariable models by hospital service area, which is a standard metric in health outcomes research that represents a collection of ZIP codes surrounding a local health care market.20 In addition to the primary Cox proportional hazards regression models, we conducted secondary propensity score analyses, which did not impact our findings (details and results are in the Appendix). All statistical tests performed were two sided with P < .05 considered significant. Analyses were conducted using SAS (SAS/STAT User’s Guide, Version 9.4; SAS Institute, Cary, NC).
RESULTS
A total of 6,212 patients in this study were treated by 788 radiation oncologists, with provider volume ranging from 1 to 70 patients throughout the study period. Patient characteristics stratified by median provider volume and conventional radiation versus IMRT are presented in Table 1. Patients treated with conventional radiation therapy by high-volume providers were more likely to be white, live in the South, and less likely to have well- or moderately differentiated tumors compared with those treated by low-volume providers. A similar pattern was observed for patients receiving IMRT who were treated by high-volume providers with geographic location and race; these patients were also less likely to receive cetuximab chemotherapy.
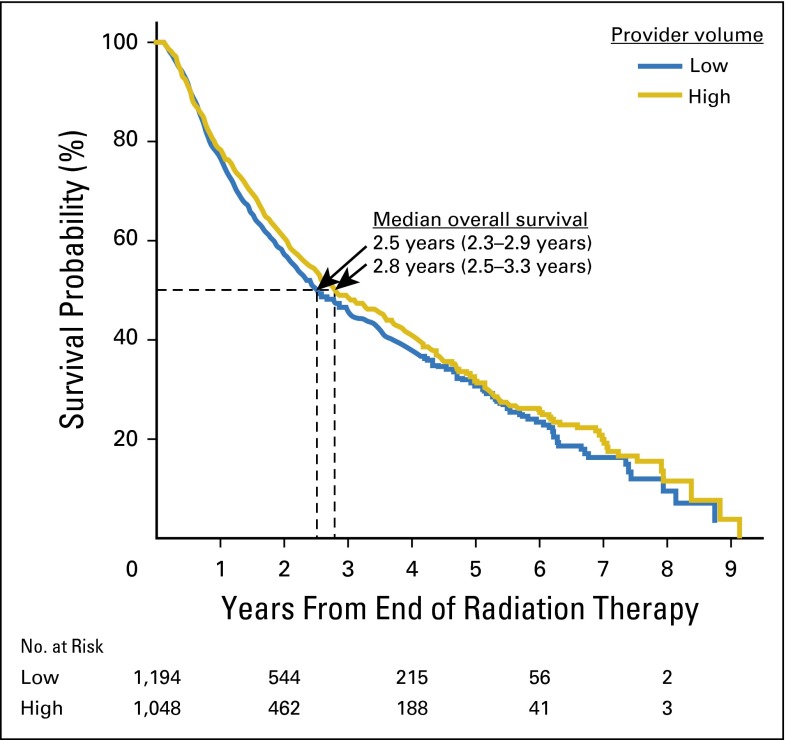
When examining with multivariable analysis the relationship between all-cause mortality and provider volume, patients receiving IMRT treated by high-volume providers had decreased all-cause mortality. For every additional five patients treated per provider per year, the risk of death decreased by 21% (HR, 0.79; 95% CI, 0.67 to 0.94). For patients treated with conventional radiation therapy, there was no significant impact on all-cause mortality from provider experience (HR, 0.95; 95% CI, 0.87 to 1.04). Results of the full multivariable models are presented in Appendix Figure A1 and Appendix Table A2 (online only). Kaplan-Meier curves for patients treated with IMRT (Fig 2) illustrate a small but significant difference in overall survival for low- versus high-volume providers. The observed results also held with HNC-specific mortality, for which providers with higher volumes had improved outcomes among patients receiving IMRT (subdistribution HR, 0.68; 95% CI, 0.50 to 0.91). Select subset analyses are presented in Appendix Figure A2.
Fig 2.
Overall survival for patients with head-and-neck cancer receiving intensity-modulated radiation therapy stratified by high- versus low-volume providers.
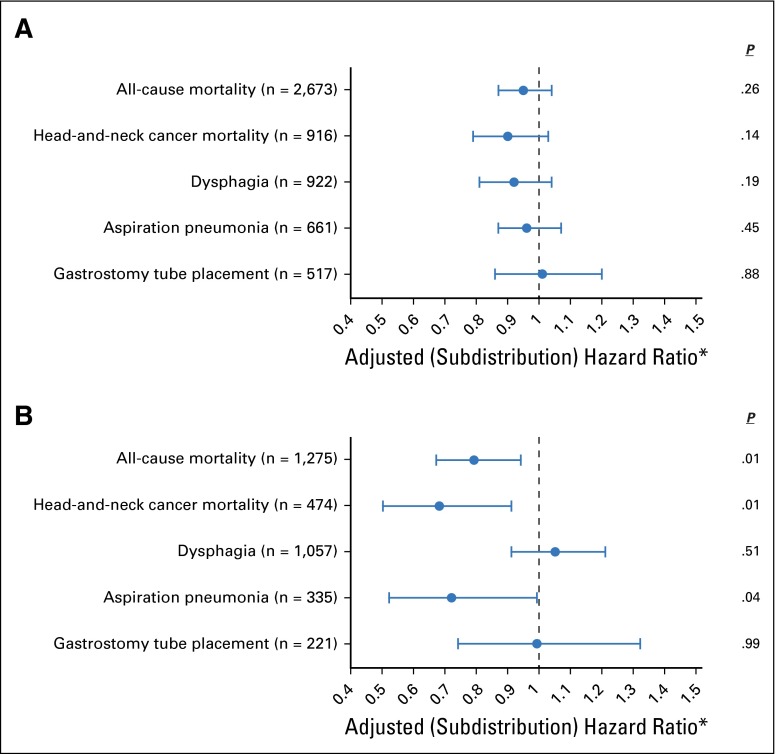
Analysis of toxicity outcomes found that high-volume providers had decreased rates of aspiration pneumonia among patients treated with IMRT (subdistribution HR, 0.72; 95% CI, 0.52 to 0.99). No significant relationships were observed with other toxicity end points in either the conventional or IMRT cohorts (Fig 3).
Fig 3.
Impact of radiation provider volume on survival and toxicity after radiation stratified by (A) conventional radiation (n = 3,970) or (B) intensity-modulated radiation therapy (n = 2,242). The points on the plot represent adjusted hazard ratios and subdistribution hazard ratios for the impact of provider experience. *In the case of all-cause mortality, the adjusted hazard ratio is presented, whereas for all other outcomes the adjusted subdistribution hazard ratio is presented. Horizontal bars represent 95% CIs and P-values are reported on the right. Numbers in parentheses represent number of events.
DISCUSSION
The key finding of this retrospective population-based analysis relates to the impact of radiation provider experience on survival in patients receiving IMRT for HNC. Among patients treated with IMRT, for every five additional patients treated per provider per year, the risk of all-cause mortality decreased by 21%. The findings in of this study have the potential to impact both the patient and provider. From a patient perspective, choosing the physician who will treat a cancer is one of the principal decisions any patient with cancer will make. This study provides patients with valuable data to better inform this decision process. In addition, this study offers guidance to oncologists and surgeons who refer patients with HNC to radiation oncology.
Our study complements previous work by Wuthrick et al3 that reported on a secondary analysis of the HNC radiation therapy trial RTOG 0129. Wuthrick et al found that patients treated at historically low-volume accrual centers had a significantly greater risk of death (HR, 1.91; 95% CI, 1.37 to 2.65). Additional research by Peters et al,2 who performed a secondary analysis of the phase III chemoradiation therapy trial TROG 0202, further supports the relationship between treatment center volume and patient survival. However, the studies by Wuthrick et al3 and Peters et al2 observed a benefit among patients treated with 3D conformal radiation (IMRT was not included in either study), whereas we found no difference among patients treated with conventional radiation. The underlying explanation for our differing results with 3D conformal radiation is not clear, but could be a result of the fact that clinical trial participants may be different from the general Medicare population. Clinical trial participants are a highly selected group of patients who, in general, are younger and have better performance status and fewer comorbidities than do Medicare patients older than 65 years. In addition, this study evaluated differences at the level of the provider, whereas the studies by Wuthrick et al3 and Peters et al2 evaluated outcome at the level of the treating institution.
The finding of improved survival among patients treated by high-volume providers raises questions about how best to provide care for this population and reduce inequality among patients. Regionalization of care presents one potential solution and has shown improved outcomes in other health care areas, such as trauma21; however, regionalization in radiation oncology would have to address the logistical issues of treatment. From a patient perspective, traveling or relocating for the typical 6- to 7-week course of head-and-neck radiation could create a significant personal or financial burden for the patient or their family. Other potential solutions might involve improved training or novel educational resources for practicing physicians. The constant evolution of technology in radiation oncology—from two-dimensional to 3D conformal radiation, to IMRT, to stereotactic radiation—underscores the need for continuing education. Groups like the American Society for Radiation Oncology already promote continuing education with skills-based educational sessions at national meetings.22 Advances in technology could also offer potential solutions such as semiautomated target delineation,23 knowledge base planning,24,25 or telemedicine, any of which could enable the transfer of expert knowledge to local radiation therapy centers without requiring physician or patient travel.
With any research evaluating the impact of provider experience on patient outcomes, identifying the specific elements of treatment that directly improve outcomes becomes a key area of focus. In the current study, one must consider the impact of the radiation oncologist in every part of patient care, including ordering and interpreting staging studies, defining radiation target volumes, overseeing radiation treatment planning and treatment, managing toxicity, and arranging appropriate cancer follow-up and survivorship care. Variability in any one factor in this complex chain could influence outcomes after treatment; however, our observation that experience impacts outcome only in the IMRT cohort strongly suggests a level of provider proficiency with IMRT is required for optimal outcomes. This assertion is backed by the RTOG 0022 study, which evaluated IMRT in oropharyngeal cancer and noted higher failure rates among patients with major protocol violations in IMRT radiation plans.6 In addition, whereas tumor delineation and radiation plan quality have been implicated as sources of poor patient outcomes, existing research also suggests that IMRT may be more sensitive to setup errors than conventional radiation therapy.26
Our study has limitations that must be acknowledged. Our method of estimating provider volume underestimates the true provider experience for a given radiation oncologist as our study population was limited to Medicare patients over the age of 65. This method introduces the possibility of misclassification of provider volume, which could have an unpredictable impact on our results. However, similar methods used to estimate surgeon and hospital volume have been externally validated in the surgical literature.14,17,18 This research also focused on patients who were at least 66 years, and therefore, our findings might not be generalizable to a younger population. The retrospective nonrandomized nature of this study creates the potential for selection bias, in which healthier patients or those with more favorable disease could seek out more experienced providers. In addition, because of a lack of data, we cannot comment on potentially confounding risk factors, such as smoking, body habitus, human papillomavirus status, or the specifics of radiation therapy, including dose and target, all of which affect outcomes. Furthermore, although we controlled for median income, residual confounding could bias the results as patients with higher socioeconomic status could seek care from high-volume providers and better manage the post-treatment care, resulting in improved survival. Finally, the findings in this study do not necessarily indicate that the individual radiation oncology provider impacts patient outcomes. Higher-volume radiation oncologists may simply reside in more adept multispecialty hospitals or health care networks better suited to caring for the complex needs of patients with HNC. Treatment of HNC requires a multidisciplinary team that relies heavily on the infrastructure of the treatment center to provide the necessary support throughout the course of disease.
This study, to our knowledge, represents the first evaluation of how the patient volume of a radiation oncologist can affect patient outcomes after radiation therapy. We found that patients treated with IMRT potentially benefit from treatment by a provider with experience in managing patients with HNC. This information has the ability to better inform patients and providers alike, and suggests the importance of high-quality radiation therapy to optimally manage patients with HNC. Despite our findings, however, unmeasured confounders and selection bias could influence our results; future research should attempt to validate these findings.
Appendix
Subgroup Analysis
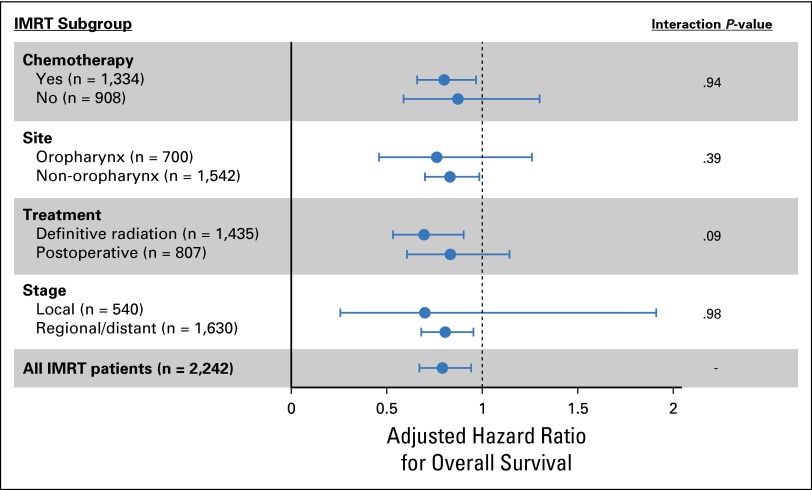
Among the intensity-modulated radiation therapy (IMRT) cohort, we conducted further subgroup analysis to determine whether the impact of provider volume on overall survival was consistent across various subgroups. We looked for statistical interaction between subgroups in an attempt to identify significant differences between subgroups. Figure A2 demonstrates the results of multivariable Cox proportional hazards regression analyses on each subset. Overall, the impact of radiation provider volume did not vary substantially between subgroups (nonsignificant interaction). The error bars for select subgroups crossed 1.0, but this was partly because of smaller sample sizes that reduced the power of these subgroup analyses.
Impact of SEER Historic Staging
American Joint Committee on Cancer (AJCC) staging was not available for patients across our entire study cohort; therefore, SEER historic staging was used to classify cancer stage into local, regional, or distant. By SEER historic staging, the designation of distant disease included patients with stage T4a and T4b disease per AJCC, as well as those with distant metastatic disease (M1 per AJCC). We further evaluated the cohort of patients with distant disease per SEER historic staging.
Our study cohort included patients diagnosed between 2000 and 2009; however, SEER started recording AJCC staging for head-and neck-cancer in 2004. Therefore, we have both SEER historic staging and TNM staging (AJCC 6th edition) for the subset of patients from 2004 to 2009. Among the subset of patients diagnosed from 2004 to 2009 with distant SEER historic stage disease (n = 483), only 17.8% had M1 disease per AJCC staging. Of our entire cohort, only 10.1% of patients had distant disease by SEER historic staging, which suggests that the number of M1 patients in the study cohort is likely small.
We conducted a sensitivity analysis on the cohort of patients with AJCC stage M0 disease (n = 2,014) who received IMRT and were diagnosed between 2004 and 2009. Among this cohort we found that increased provider volume among patients receiving IMRT was associated with improved overall survival (hazard ratio [HR], 0.79; 95% CI, 0.63 to 0.98).
Sensitivity Analysis Excluding Low-Volume Providers
A fraction of our patients (24.7%) received treatment from radiation oncology providers with little experience treating head-and-neck cancer (defined as treating five or fewer patients). We conducted a sensitivity analysis with overall survival on the IMRT cohort for which we excluded these low-volume providers, yielding results similar to the primary analysis. The multivariable Cox proportional hazards regression model found that patients receiving IMRT who were treated by higher-volume radiation providers had a lower risk of death (HR, 0.76; 95% CI, 0.60 to 0.95).
Propensity Score Analysis
To further address the issue of selection bias, we conducted a propensity score analysis for overall survival. First, a multivariable logistic regression with all relevant covariates (Table 1) was used to predict treatment. Individual patient propensity scores were defined as the estimated probability of the treatment from the logistic regression. We analyzed propensity scores with three different techniques: study subjects were divided into quintiles by their propensity scores, each stratum was analyzed separately, and a pooled HR was determined; the propensity scores were added directly as a covariate into the Cox proportional hazards regression model; and a propensity score weight was defined as the inverse of the propensity score normalized to the size of the treatment group, and an HR accounting for these weights was determined. Results of these propensity score analyses (Table A3) demonstrate results similar to our primary analysis, indicating that IMRT delivered by more experienced radiation providers was associated with improved survival.
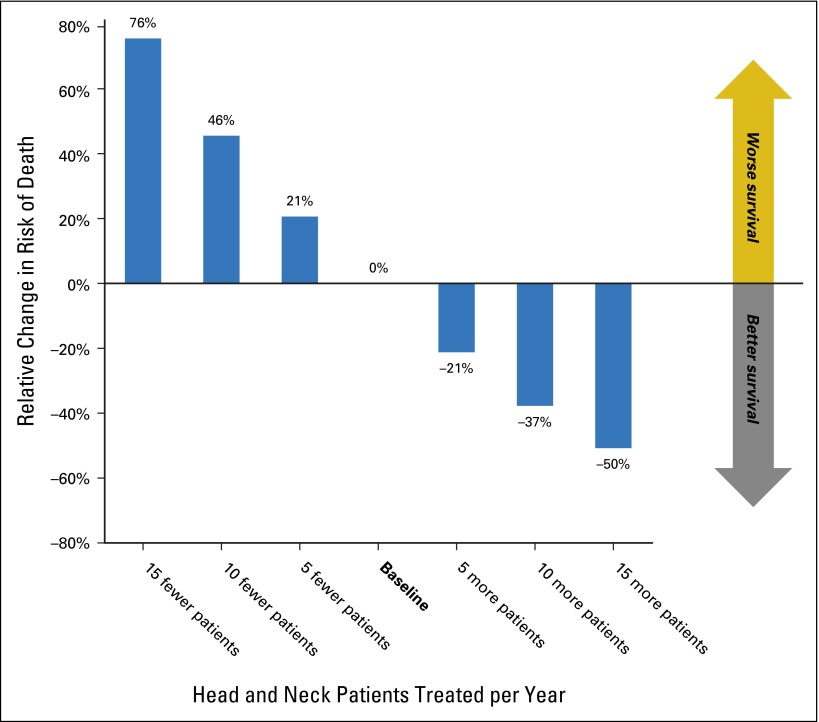
Fig A1.
Impact of radiation provider volume on overall survival of patients treated with intensity-modulated radiation therapy (n = 2,242). Relative change in overall survival associated with providers who treat more or fewer patients with head-and-neck cancer. These numbers represent an extrapolation of the hazard ratio for provider volume in the multivariable Cox proportional hazards regression model for overall survival. For example, for every 10 additional patients treated by a radiation oncologist, the relative risk of death decreases by 37%.
Fig A2.
Subgroup analysis evaluating the impact of radiation provider experience on the cohort of patients with head-and-neck cancer treated with intensity-modulated radiation therapy ( = 2,242). Plot represents the results of multivariable Cox proportional hazards regression models for overall survival among select subgroups. Each dot on the plot represents the adjusted hazard ratio for provider experience. Hazard ratios less than 1 imply that higher provider volume was associated with a decreased risk of death.
Table A1.
Medicare Codes Used in the Study
| Variable | Codes |
|---|---|
| Radiotherapy | HCPCS codes: 61796-61800, 63620-63621, 77371-77373, 77401-77416, 77418, 77421-77423, 77470, 77520, 77522, 77523, 77525, 0197T, G0173-G0174, G0243, G0251, and G0339-G0340 |
| Surgery | ICD-9 procedure codes: 21.5-21.6, 22.31, 22.42, 22.60-22.66, 24.31, 25.1-25.4, 26.2, 26.29-26.32, 27.3, 27.32, 27.4, 27.42-27.43, 27.49, 27.72, 28.92, 29.33, 29.39, 30.0, 30.09, 30.1, 30.21-30.22, 30.29, 30.3-30.5, 31.5, 40.40-40.42, 76.2, 76.31, 76.39-76.42, or 76.44-76.45 |
| CPT codes: 21044-21045, 21555-21557, 30117-30118, 30130, 30140, 30150, 31200-31201, 31205, 31225, 31230, 31299, 31365, 31367-31368, 31370, 31375, 31380, 31382, 31390, 31395, 31420, 38700, 38720, 38724, 40810, 40812, 40814, 40816, 40819, 41110, 41112-41114, 41116, 41120, 41130, 41135, 41140, 41145, 41150, 41153, 41155, 41825-41827, 42104, 42106-42107, 42120, 42140, 42410, 42415, 42420, 42425-42456, 42440, 42450, 42842, 42844-42845, or 42890 | |
| Chemotherapy | ICD-9 procedure code: 99.25 |
| ICD-9 diagnosis codes: V58.1, V58.11, V58.12, V66.2, or V67.2 | |
| HCPCS codes: 96400 to 96599, J8999 to J9999, J8520, J8521, or Q0083 to Q0085 | |
| Revenue Center codes: 0331, 0332, or 0335. | |
| NDC: 00004110013, 00004110020, 00004110051, 00004110113, 00004110116, 00004110150, 00004110151 | |
| BETOS codes: 01D | |
| Cisplatin | HCPCS codes: J9060, J9062 |
| Cetuximab | HCPCS codes: J9055 |
| IMRT | HCPCS codes: 77301, 77338, 77418, G0174, G0178 |
| PET | HCPCS codes: 78810-78816, G0223-G0225 |
| Dysphagia | ICD-9 diagnosis code: 787.20, 787.21, 787.22, 787.23, 787.24 |
| Gastrostomy tube placement | ICD-9 procedure code: 43.11 |
| CPT codes: 43246, 43750, 44500, 44372, 44373, 74355, 74350 | |
| Aspiration pneumonia | ICD-9 diagnosis code: 507.0 |
Abbreviations: BETOS, Berenson-Eggers type of service; CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System; ICD-9, International Classification of Diseases, Ninth Revision; IMRT, intensity-modulated radiation therapy; NDC, National Drugs Codes; PET, positron emission tomography.
Table A2.
Full Results of Multivariable Models
| Covariable | Overall Survival,* HR (95% CI) | Cancer-Specific Survival,† SDHR (95% CI) | Aspiration Pneumonia,† SDHR (95% CI) | |||
|---|---|---|---|---|---|---|
| Conventional Radiation | IMRT | Conventional Radiation | IMRT | Conventional Radiation | IMRT | |
| Age at diagnosis, years | ||||||
| 66-74 | 1 | 1 | 1 | 1 | 1 | 1 |
| 75-79 | 1.31 (1.18 to 1.46) | 1.34 (1.15 to 1.56) | 1.14 (0.96 to 1.35) | 1.07 (0.84 to 1.37) | 1.46 (1.21 to 1.78) | 1.33 (1.00 to 1.78) |
| ≥ 80 | 1.91 (1.73 to 2.11) | 1.83 (1.56 to 2.15) | 1.47 (1.24 to 1.73) | 1.33 (1.02 to 1.73) | 1.70 (1.40 to 2.08) | 1.58 (1.18 to 2.11) |
| Female | 1.06 (0.96 to 1.17) | 1.01 (0.88 to 1.16) | 1.11 (0.96 to 1.30) | 1.35 (1.09 to 1.66) | 0.87 (0.72 to 1.06) | 0.78 (0.59 to 1.03) |
| Race | ||||||
| White | 1 | 1 | 1 | 1 | 1 | 1 |
| Black | 1.24 (1.05 to 1.47) | 0.99 (0.77 to 1.27) | 1.24 (0.96 to 1.61) | 0.76 (0.49 to 1.19) | 0.86 (0.63 to 1.19) | 1.08 (0.70 to 1.69) |
| Other | 0.92 (0.75 to 1.11) | 1.00 (0.77 to 1.30) | 1.11 (0.82 to 1.50) | 1.34 (0.89 to 2.00) | 1.18 (0.85,1.64) | 0.78 (0.49 to 1.26) |
| Marital status | ||||||
| Married | 1 | 1 | 1 | 1 | 1 | 1 |
| Divorced | 1.21 (1.04 to 1.42) | 1.16 (0.95 to 1.41) | 1.19 (0.92 to 1.53) | 0.98 (0.72 to 1.34) | 1.16 (0.87 to 1.55) | 0.82 (0.55 to 1.22) |
| Single | 1.27 (1.09 to 1.47) | 1.10 (0.87 to 1.38) | 1.21 (0.95 to 1.52) | 0.93 (0.64 to 1.34) | 0.96 (0.72 to 1.26) | 0.82 (0.55 to 1.23) |
| Other | 1.25 (1.13 to 1.39) | 1.06 (0.91 to 1.23) | 1.39 (1.18 to 1.63) | 0.98 (0.77 to 1.26) | 0.94 (0.77 to 1.15) | 1.04 (0.77 to 1.39) |
| Median income | ||||||
| 1st quartile | 1 | 1 | 1 | 1 | 1 | 1 |
| 2nd quartile | 1.14 (1.00 to 1.30) | 1.21 (1.01 to 1.45) | 1.03 (0.82 to 1.29) | 1.56 (1.16 to 2.10) | 0.97 (0.76 to 1.24) | 1.15 (0.82 to 1.60) |
| 3rd quartile | 1.06 (0.94 to 1.20) | 1.13 (0.96 to 1.33) | 1.07 (0.88 to 1.31) | 1.29 (0.97 to 1.71) | 0.91 (0.73 to 1.14) | 0.92 (0.68 to 1.25) |
| 4th quartile | 1.06 (0.92 to 1.23) | 1.35 (1.09 to 1.66) | 1.11 (0.87 to 1.41) | 1.59 (1.14 to 2.20) | 0.94 (0.71 to 1.25) | 0.87 (0.60 to 1.27) |
| Charlson comorbidity score | ||||||
| 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 1.34 (1.22 to 1.47) | 1.25 (1.08 to 1.43) | 1.13 (0.96 to 1.32) | 1.13 (0.91 to 1.41) | 1.16 (0.97 to 1.39) | 1.01 (0.85 to 1.43) |
| 2 | 1.47 (1.29 to 1.68) | 1.43 (1.17 to 1.75) | 1.04 (0.83 to 1.30) | 1.12 (0.80 to 1.58) | 1.26 (0.98 to 1.61) | 1.12 (0.79,1.59) |
| ≥ 3 | 1.94 (1.68 to 2.24) | 1.81 (1.49 to 2.20) | 1.04 (0.80 to 1.35) | 1.20 (0.86 to 1.67) | 1.31 (0.98 to 1.74) | 1.22 (0.84 to 1.76) |
| Teaching hospital | 1.07 (0.97 to 1.17) | 1.09 (0.95 to 1.24) | 1.03 (0.88 to 1.19) | 1.17 (0.94 to 1.44) | 1.82 (1.51 to 2.18) | 2.15 (1.67 to 2.75) |
| Year of diagnosis | ||||||
| 2000-2003 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2004-2006 | 0.99 (0.87 to 1.12) | 0.87 (0.68 to 1.11) | 0.84 (0.69 to 1.02) | 0.82 (0.58 to 1.16) | 0.90 (0.71 to 1.16) | 0.67 (0.45 to 1.00) |
| 2007-2009 | 0.98 (0.84 to 1.15) | 1.14 (0.89 to 1.47) | 0.62 (0.48 to 0.81) | 0.72 (0.50 to 1.04) | 0.65 (0.46 to 0.90) | 0.60 (0.40 to 0.89) |
| Tumor site | ||||||
| Hypopharynx | 0.86 (0.70 to 1.04) | 0.88 (0.70 to 1.12) | 0.88 (0.66 to 1.18) | 0.75 (0.50 to 1.11) | 1.25 (0.87 to 1.80) | 1.84 (1.23 to 2.75) |
| Larynx | 0.61 (0.53 to 0.70) | 0.63 (0.52 to 0.75) | 0.63 (0.51 to 0.77) | 0.82 (0.60 to 1.10) | 1.24 (0.96 to 1.59) | 1.15 (0.80 to 1.67) |
| Nasopharynx | 0.91 (0.64 to 1.28) | 0.64 (0.47 to 0.88) | 1.15 (0.74 to 1.77) | 0.62 (0.37 to 1.04) | 1.22 (0.65 to 2.27) | 1.54 (0.92 to 2.58) |
| Oral cavity | 1 | 1 | 1 | 1 | 1 | 1 |
| Oropharynx | 0.65 (0.56 to 0.75) | 0.57 (0.48 to 0.68) | 0.74 (0.59 to 0.92) | 0.67 (0.50 to 0.90) | 0.92 (0.69 to 1.22) | 1.03 (0.73 to 1.47) |
| Other | 0.71 (0.60 to 0.85) | 0.66 (0.52 to 0.84) | 0.55 (0.42 to 0.74) | 0.68 (0.46 to 1.00) | 0.79 (0.55 to 1.14) | 0.72 (0.44 to 1.17) |
| Historic stage | ||||||
| Localized | 1 | 1 | 1 | 1 | 1 | 1 |
| Regional | 1.63 (1.46 to 1.81) | 1.43 (1.22 to 1.67) | 1.85 (1.53 to 2.22) | 1.70 (1.29 to 2.25) | 1.32 (1.09 to 1.63) | 1.06 (0.78 to 1.45) |
| Distant | 2.37 (2.01 to 2.80) | 2.24 (1.82 to 2.75) | 2.68 (2.06 to 3.47) | 2.58 (1.84 to 3.61) | 1.29 (0.93 to 1.78) | 1.12 (0.77 to 1.67) |
| Unknown | 1.10 (0.88 to 1.38) | 1.32 (0.91 to 1.91) | 1.63 (1.15 to 2.32) | 1.27 (0.67 to 2.40) | 1.11 (0.74 to 1.66) | 0.65 (0.30 to 1.41) |
| Size of primary tumor, cm | ||||||
| 0-5 | 1 | 1 | 1 | 1 | 1 | 1 |
| > 5 | 1.64 (1.14 to 2.36) | 1.36 (1.08 to 1.72) | 1.64 (1.03 to 2.60) | 1.36 (0.92 to 2.01) | 0.59 (0.24 to 1.43) | 1.32 (0.86 to 2.01) |
| Unknown | 0.87 (0.75 to 0.99) | 1.16 (1.01 to 1.34) | 0.85 (0.67 to 1.08) | 1.03 (0.82 to 1.30) | 0.99 (0.76 to 1.29) | 1.29 (0.99 to 1.68) |
| Grade | ||||||
| Well or moderately differentiated | 1 | 1 | 1 | 1 | 1 | 1 |
| Poorly or undifferentiated | 1.10 (0.99 to 1.23) | 0.86 (0.75 to 0.98) | 1.20 (1.02 to 1.42) | 0.95 (0.76 to 1.19) | 1.12 (0.92 to 1.36) | 0.81 (0.63 to 1.05) |
| Unknown | 1.06 (0.95 to 1.18) | 0.97 (0.83 to 1.14) | 1.05 (0.87 to 1.27) | 0.97 (0.74 to 1.28) | 1.00 (0.80 to 1.24) | 0.86 (0.64 to 1.16) |
| PET | 0.81 (0.74 to 0.89) | 0.86 (0.73 to 1.02) | 0.98 (0.84 to 1.14) | 0.84 (0.65 to 1.09) | 1.11 (0.93 to 1.32) | 0.99 (0.73 to 1.35) |
| Surgery prior to radiation | 0.75 (0.68 to 0.82) | 0.88 (0.76 to 1.02) | 0.85 (0.73 to 1.00) | 0.90 (0.71 to 1.14) | 0.96 (0.80 to 1.14) | 0.62 (0.48 to 0.82) |
| Types of chemotherapy | ||||||
| None | 1 | 1 | 1 | 1 | 1 | 1 |
| Cisplatin | 1.02 (0.85 to 1.23) | 0.99 (0.81 to 1.20) | 1.25 (0.95 to 1.64) | 0.91 (0.66 to 1.26) | 0.84 (0.58 to 1.22) | 1.17 (0.81 to 1.69) |
| Cetuximab | 1.23 (0.95 to 1.59) | 1.34 (1.11 to 1.63) | 1.40 (0.95 to 2.06) | 1.53 (1.13 to 2.07) | 1.35 (0.77 to 2.35) | 1.90 (1.33 to 2.71) |
| Other | 1.16 (1.03 to 1.31) | 0.98 (0.83 to 1.16) | 1.25 (1.04 to 1.50) | 1.12 (0.86 to 1.45) | 0.126 (1.02 to 1.57) | 1.26 (0.94 to 1.69) |
| Aspiration pneumonia prior to radiation | N/A | N/A | N/A | N/A | 2.00 (1.31 to 3.08) | 2.18 (1.30 to 3.64) |
| Number of radiation fractions (continuous) | 1.01 (0.99 to 1.02) | 1.00 (0.98 to 1.02) | 1.01 (0.99 to 1.02) | 1.01 (0.99 to 1.04) | 1.02 (1.00 to 1.04) | 0.98 (0.95 to 1.01) |
| Provider volume (continuous) | 0.95 (0.87 to 1.04) | 0.79 (0.67 to 0.94) | 0.90 (0.79 to 1.03) | 0.68 (0.50 to 0.91) | 0.96 (0.87 to 1.07) | 0.72 (0.52 to 0.99) |
Abbreviations: HR, hazard ratio; IMRT, intensity-modulated radiation therapy; N/A, not applicable; PET, positron emission tomography; SDHR, subdistribution hazard ratio.
Analysis with overall survival shows the results of a multivariable Cox proportional hazards regression model for both patients treated without IMRT and those treated with IMRT.
Analysis with cancer-specific survival and aspiration pneumonia shows the results of a multivariable Fine-Gray regression model for both patients treated without IMRT and those treated with IMRT.
Table A3.
Propensity Score Analysis Evaluating the Impact of Radiation Provider Experience Among the Cohort of Patients With HNC Treated With IMRT (n = 2,242)
| Propensity Technique | Overall Survival, HR (95% CI) |
|---|---|
| Pooled HR | 0.94 (0.894 to 0.995) |
| Covariate in model | 0.93 (0.866 to 0.995) |
| Inverse weighting | 0.93 (0.868 to 0.995) |
NOTE. Results of three different propensity score techniques. HRs reflect the impact of provider experience on overall survival. HRs less than 1 imply that more experienced radiation providers were associated with improved survival.
Abbreviations: HNC, head-and-neck cancer; HR, hazard ratio; IMRT, intensity-modulated radiation therapy.
Footnotes
See accompanying editorial on page 653
Supported by the National Institutes of Health Grants No. KL2 TR001444 (to J.D.M.) and TL1 TR001443 (to I.B.).
Presented in part at the American Society of Radiation Oncology (ASTRO) Annual Meeting, San Antonio, TX, October 18-21, 2015.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Isabel J. Boero, James D. Murphy
Collection and assembly of data: Isabel J. Boero, Anthony J. Paravati, Beibei Xu, James D. Murphy
Data analysis and interpretation: Isabel J. Boero, Ezra E.W. Cohen, Loren K. Mell, Quynh-Thu Le, James D. Murphy
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Importance of Radiation Oncologist Experience Among Patients With Head-and-Neck Cancer Treated With Intensity-Modulated Radiation Therapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Isabel J. Boero
No relationship to disclose
Anthony J. Paravati
Consulting or Advisory Role: Varian Medical Systems
Beibei Xu
No relationship to disclose
Ezra E.W. Cohen
Consulting or Advisory Role: Merck, Bristol-Myers Squibb, AstraZeneca, Eisai, Pfizer
Speakers' Bureau: Bayer AG, Eisai
Loren K. Mell
No relationship to disclose
Quynh-Thu Le
Stock or Other Ownership: Aldea
Research Funding: Amgen (Inst)
James D. Murphy
No relationship to disclose
REFERENCES
- 1.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in oncology: Head and neck cancers. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 2.Peters LJ, O’Sullivan B, Giralt J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: Results from TROG 02.02. J Clin Oncol. 2010;28:2996–3001. doi: 10.1200/JCO.2009.27.4498. [DOI] [PubMed] [Google Scholar]
- 3.Wuthrick EJ, Zhang Q, Machtay M, et al. Institutional clinical trial accrual volume and survival of patients with head and neck cancer. J Clin Oncol. 2015;33:156–164. doi: 10.1200/JCO.2014.56.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 5.Nutting CM, Morden JP, Harrington KJ, et al. PARSPORT Trial Management Group Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisbruch A, Harris J, Garden AS, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22) Int J Radiat Oncol Biol Phys. 2010;76:1333–1338. doi: 10.1016/j.ijrobp.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.VanderWalde NA, Meyer AM, Liu H, et al. Patterns of care in older patients with squamous cell carcinoma of the head and neck: A surveillance, epidemiology, and end results-medicare analysis. J Geriatr Oncol. 2013;4:262–270. doi: 10.1016/j.jgo.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 9.Virnig BA, Warren JL, Cooper GS, et al. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40:IV-49–IV-54. doi: 10.1097/00005650-200208001-00007. (suppl 8) [DOI] [PubMed] [Google Scholar]
- 10.Sher DJ, Neville BA, Chen AB, et al. Predictors of IMRT and conformal radiotherapy use in head and neck squamous cell carcinoma: A SEER-Medicare analysis. Int J Radiat Oncol Biol Phys. 2011;81:e197–e206. doi: 10.1016/j.ijrobp.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Fesinmeyer MD, Mehta V, Tock L, et al. Completion of radiotherapy for local and regional head and neck cancer in Medicare. Arch Otolaryngol Head Neck Surg. 2009;135:860–867. doi: 10.1001/archoto.2009.108. [DOI] [PubMed] [Google Scholar]
- 12.Xu B, Boero IJ, Hwang L, et al. Aspiration pneumonia after concurrent chemoradiotherapy for head and neck cancer. Cancer. 2015;121:1303–1311. doi: 10.1002/cncr.29207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birkmeyer JD, Finlayson SR, Tosteson AN, et al. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. 1999;125:250–256. [PubMed] [Google Scholar]
- 14.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–1144. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 15.Schrag D, Earle C, Xu F, et al. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. J Natl Cancer Inst. 2006;98:163–171. doi: 10.1093/jnci/djj018. [DOI] [PubMed] [Google Scholar]
- 16.Schrag D, Panageas KS, Riedel E, et al. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg. 2002;236:583–592. doi: 10.1097/00000658-200211000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 18.Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–188. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 19.Fine J, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 20.Wennberg J, Cooper M. The Dartmouth Atlas of Health Care in the United States. Chicago, IL: American Hospital Publishing; 1996. [PubMed] [Google Scholar]
- 21.MacKenzie EJ, Rivara FP, Jurkovich GJ, et al. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med. 2006;354:366–378. doi: 10.1056/NEJMsa052049. [DOI] [PubMed] [Google Scholar]
- 22.American Society for Radiation Oncology eContouring webinar series. https://www.astro.org/Educational-Resources/Webinars/eContouring-Webinar-Series.aspx.
- 23.Sjöberg C, Lundmark M, Granberg C, et al. Clinical evaluation of multi-atlas based segmentation of lymph node regions in head and neck and prostate cancer patients. Radiat Oncol. 2013;8:229. doi: 10.1186/1748-717X-8-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tol JP, Delaney AR, Dahele M, et al. Evaluation of a knowledge-based planning solution for head and neck cancer. Int J Radiat Oncol Biol Phys. 2015;91:612–620. doi: 10.1016/j.ijrobp.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Moore KL, Brame RS, Low DA, et al. Experience-based quality control of clinical intensity-modulated radiotherapy planning. Int J Radiat Oncol Biol Phys. 2011;81:545–551. doi: 10.1016/j.ijrobp.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Ploquin N, Lau H, Dunscombe P. Intensity modulated and three-dimensional conformal radiation therapy plans for oropharyngeal cancer: A comparison of their sensitivity to set-up errors and uncertainties. Curr Oncol. 2006;13:61–66. doi: 10.3390/curroncol13020005. [DOI] [PMC free article] [PubMed] [Google Scholar]