Abstract
Purpose
Gastro-entero-pancreatic neuroendocrine tumors (GEPNETs) are increasing in incidence, and accurate staging is important for selecting the appropriate treatment. 68Ga-DOTATATE imaging is a promising approach for detecting GEPNETs and could help in selecting optimal therapeutic strategies. The aim of this study was to prospectively determine the clinical utility of 68Ga-DOTATATE positron emission tomography (PET)/computed tomography (CT) in detecting unknown primary and metastatic GEPNETs.
Patients and Methods
One hundred thirty-one patients were enrolled in a prospective study of patients undergoing 68Ga-DOTATATE PET/CT, 111In-pentetreotide single-photon emission computed tomography (SPECT)/CT and multiphasic CT scan, and/or magnetic resonance imaging in a blinded fashion with comprehensive biochemical testing. The primary outcome measure was the detection of lesions by each imaging study.
Results
68Ga-DOTATATE PET/CT imaging detected 95.1% of lesions (95% CI, 92.4% to 96.8%) with an average maximum standardized uptake value of 65.4 ± 47 (range, 6.9 to 244), anatomic imaging detected 45.3% of lesions (95% CI, 37.9% to 52.9%), and 111In-pentetreotide SPECT/CT detected 30.9% of lesions (95% CI, 25.0% to 37.5%), with a significant difference between imaging modalities (P < .001). In four of 14 patients (28.6%), 68Ga-DOTATATE PET/CT found a previously unknown primary tumor, and detected primary GEPNET, lymph node, and distant metastases correctly in 72 of 113 lesions (63.7%) when compared with histopathology, with 22.1% and 38.9% detected by using 111In-pentetreotide SPECT/CT and anatomic imaging, respectively. On the basis of findings with 68Ga-DOTATATE PET/CT, 43 of 131 patients (32.8%) had a change in management recommendation. In patients with carcinoid symptoms but negative biochemical testing, 68Ga-DOTATATE PET/CT detected lesions in 65.2% of patients, 40% of which were detected neither by anatomic imaging nor by 111In-pentetreotide SPECT/CT.
Conclusion
68Ga-DOTATATE PET/CT imaging provides important information for accurate staging of GEPNETs and selection of appropriate treatment interventions even in the absence of biochemical evidence of disease in symptomatic patients.
INTRODUCTION
Neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms that arise from the cells of the endocrine system. The incidence of gastro-entero-pancreatic NETs (GEPNETs) is about 7.8 per 100,000 persons/y with a prevalence of 35 per 100,000 persons.1,2 Most GEPNETs are sporadic, but can be part of a familial cancer syndrome, such as multiple endocrine neoplasia type 1, neurofibromatosis type 1, von Hippel-Lindau, and tuberous sclerosis complex.3-5
The clinical presentation of GEPNETs depends on the anatomic sites involved, tumor biology, and functional status. Surgical resection is the only curative treatment option for patients with early-stage disease; however, the extent, timing, and effect of surgical intervention for advanced, metastatic GEPNETs remain controversial. Most management guidelines emphasize that resection should be the first-line treatment option for patients with advanced GEPNETs if up to 90% of the disease burden is resectable6; however, only 5% to 20% of patients with advanced GEPNETs meet this criterion.7,8 The identification of metastatic disease and tumor grade are the most important prognostic factors in patients with advanced GEPNETs.9,10 Therefore, precise staging and evaluation of disease burden with a reliable imaging method is crucial for determining the best treatment strategy, especially as there are new treatment alternatives for locally advanced and metastatic GEPNETs.
A unique feature of NETs is the expression of somatostatin receptors (SSTR),11,12 which can be targeted with radiolabeled peptides for imaging and treatment.13 Imaging modalities currently approved by the US Food and Drug Administration (FDA) for evaluation of patients with GEPNETs include 111In-pentetreotide single-photon emission computed tomography (SPECT)/computed tomography (CT) scintigraphy with a diagnostic sensitivity of 65% to 100%, depending on tumor site,14,15 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET), 123I-metaiodobenzylguanidine scintigraphy, CT, and endoscopic ultrasound and magnetic resonance imaging (MRI). A relatively new PET/CT technique using somatostatin analogs labeled with the positron emitting isotope, 68Ga (68Ga-DOTA peptides), has been shown to offer advantages over conventional imaging modalities as well as additional important quantitative and qualitative diagnostic information.16,17 This makes it an important tool in the clinical decision-making process for patients with GEPNETs.18 68Ga-DOTA imaging is still investigational in the United States and there have been no large prospective studies with comprehensive biochemical screening to evaluate its clinical utility when specifically focused on GEPNETs.
We conducted a prospective study to assess the clinical utility of 68Ga-DOTATATE imaging for detecting unknown primary tumors and metastatic disease in patients with suspected or known GEPNETs with comprehensive biochemical testing, and compared it to current FDA-approved imaging modalities, 111In-pentetreotide SPECT/CT, CT, and/or MRI.
PATIENTS AND METHODS
Patients
Patients suspected or known to have GEPNETs on imaging (CT, MRI, 18F-FDG PET) and/or biochemical evidence of GEPNETs, and/or a familial predisposition to NET (multiple endocrine neoplasia type 1 or von Hippel-Lindau) were included. The study was performed under an Investigational New Drug approval from the FDA, and Eligibility criteria are outlined in Table 1. The study was approved by the National Cancer Institute Institutional Review Board and the National Institutes of Health Radiation Safety Committee (NCT01967537). Written informed consent was obtained from all study participants.
Table 1.
Study Eligibility Criteria
| Factors Determining Eligibility |
|---|
| Inclusion criteria |
| Patient with any one of the following: |
| Suspicion of NET on imaging (CT/MRI/18F-FDG PET); and/or |
| Biochemical evidence of NET (serum/urinary) on the basis of elevated levels of chromogranin A, pancreatic polypeptide, neuron-specific enolase, vasoactive intestinal polypeptide, serotonin (urinary 5-HIAA), gastrin, somatostatin, catecholamines, metanephrines, calcitonin, fasting insulin, C-peptide (proinsulin), glucagon; and/or |
| Familial predisposition to NET in patient with clinical or genetic proof of mutation in MEN1 or VHL (symptomatic and/or asymptomatic case; with biochemical or anatomic imaging evidence of disease). |
| Age ≥ 18 years. |
| For women: Negative urine pregnancy test or postmenopausal for at least 2 years or if patient has had a hysterectomy. |
| Patient must be willing to return to NIH for follow-up. |
| Ability of patient or legally authorized representative (if the patient is deemed by the treating physician to be cognitively impaired or questionably impaired in such a way that the ability of the patient to give informed consent is questionable) to understand and the willingness to sign a written informed consent document indicating that he or she is aware of the investigational nature of this study. |
| Exclusion criteria |
| Patient unwilling to undergo serial noninvasive imaging. |
| Pregnant or lactating woman: Pregnant women are excluded from this study because the effects of 68Ga-DOTATATE in pregnancy are not known. Because there is an unknown but potential risk for adverse events in nursing infants secondary to administration of 68Ga-DOTATATE in the mother, breastfeeding should be discontinued for at least 1 day if the mother receives 68Ga-DOTATATE. |
| Patient has recognized concurrent active infection. |
| Patient has had the use of any investigational product or device, excluding F-DOPA scans, within 30 days before dosing. |
Abbreviations: CT, computed tomography; 18F-FDG, 8F-fluorodeoxyglucose; 5-HIAA, 5-hydroxyindoleacetic acid; MEN1, multiple endocrine neoplasia type 1; MRI, magnetic resonance imaging; NET, neuroendocrine tumor; NIH, National Institutes of Health; PET, positron emission tomography; VHL, von Hippel-Lindau.
Biochemical and Imaging Evaluation in Patients With GEPNET
All patients underwent testing for serum chromogranin A, pancreatic polypeptide, neuron-specific enolase and vasoactive intestinal polypeptide, urinary 5-hydroxyindoleacetic acid (5-HIAA), and fasting serum gastrin, somatostatin, insulin, C-peptide (proinsulin), and glucagon.
For 68Ga-DOTATATE PET/CT imaging, 68Ga-DOTATATE 185 MBq (5 mCi) was administered through a peripheral vein. After approximately 60 minutes, the patient was positioned supine in a PET/CT scanner, and images were obtained from the area of the upper thighs to midskull. A low-dose, noncontrast enhanced CT was used for attenuation correction and anatomic localization. Maximum standardized uptake values (SUVmax) were measured on the basis of patient total body weight. Patients treated with long-acting octreotide were scanned before the next scheduled monthly dose.19
An 111In-pentetreotide SPECT/CT scan was performed after intravenous administration of 111In-pentetreotide 222 MBq (6 mCi) within 4 weeks of 68Ga-DOTATATE PET/CT. Planar whole body 111In-pentetreotide with SPECT/CT scans of the chest (at 24 hours) and abdomen and pelvis (at 4 hours and repeated at 24 hours) were used for analyses. A low-dose, noncontrast enhanced CT was used for attenuation correction and anatomic localization. Anatomic imaging (chest, abdominal, and pelvic CT scan) was performed using LightSpeed Ultra and LightSpeed QX/i scanners (General Electric Healthcare Technologies, Waukesha, WI) as well as an Mx8000 IDT scanner (Philips Medical Systems, Andover, MA) with the following settings: noncontrast, arterial and portal venous phase, 2-mm slices, with rapid infusion of nonionic, water-soluble contrast agent (130 mL injected at 2 mL/s), and oral contrast. In patients with severe contrast allergy (n = 6) or renal insufficiency (n = 4), an abdominal and pelvic MRI was performed, with slices at a thickness of 6 mm, to include T2 series with and without fat saturation and short tau inversion recovery and T1 pre- and postcontrast series, after intravenous injection of gadolinium-diethylentriamine pentaacetic acid (Siemens Verio 1.5 Tesla; Siemens Medical Solution, Malvern, PA; and Philips Achieva 1.5 and 3 Tesla; Philips Medical Systems). One hundred seven patients had all three imaging studies within 14 days, 15 patients within 1 month, and 9 patients had the CT scan within 3 months of the two functional imaging modalities.
Data Analyses
Images were reviewed independently by radiology and nuclear medicine physicians in a blinded fashion. All imaging studies were analyzed and correlated with clinical information in an unblinded fashion by a multidisciplinary team to determine the optimal treatment. Treatment strategies took into account the functional status of GEPNET, risk of malignancy (primary tumor size and grade), and/or the presence of metastatic disease.
Patient-to-patient and lesion-to-lesion analyses were performed. Body areas were analyzed separately and grouped as pancreas, liver, bowel, lung and mediastinum (parenchyma and lymph nodes), abdomen and retroperitoneal lymph nodes (excluding liver, pancreas, adrenal glands, and bowel), and bone. The neck and brain area were excluded from data analysis, as no initial anatomic brain scan was performed for comparison. Because of the known physiologic uptake of 68Ga-DOTATATE in the spleen and adrenal glands, these organs were also excluded from analysis.
The total number of lesions on all three imaging modalities was taken to be the imaging denominator and this number represents the sum of lesions of all anatomic and functional imaging. Two analyses were performed, one in the entire study cohort and one in patients who underwent surgery with histopathologic diagnosis.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA). Data of continuous variables are presented as mean ± standard deviation (SD) or median (range). Spearman rank correlation coefficient (r) was used to measure and test the association of two continuous variables. Differences in proportions between subgroups of patients were compared by using the two-sample binomial test. Generalized estimating equations with a logit link function and working independence correlation structure were used to estimate and compare the proportions of positive lesions between 68Ga-DOTATATE and 111In-pentetreotide as well as between 68Ga-DOTATATE and CT/MRI. The standard errors of the proportions and the Wald test were based on robust variance estimates under a working independence model assumption. Generalized estimating equations analysis was done using R version 3.1.0 and the R package geepack (The R Project for Statistical Computing, Vienna, Austria). A two-tailed P value < .05 was considered statistically significant.
RESULTS
Study Cohort Biochemical Profile and Imaging Results
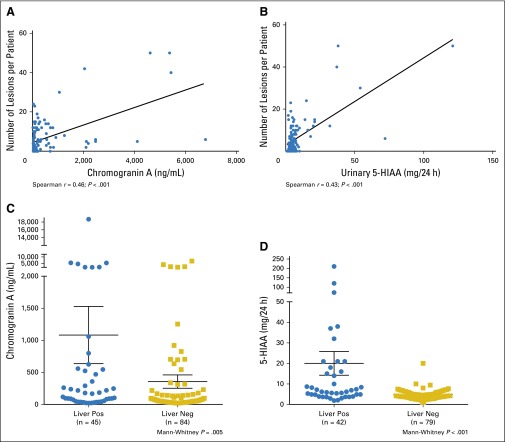
The clinical and biochemical characteristics of the study cohort are summarized in Table 2. A total of 131 patients with biochemical or radiologic suspicion and/or known diagnosis of GEPNET were enrolled. Serum chromogranin A and urinary 5-HIAA levels correlated with the number of lesions detected with 68Ga-DOTATATE PET/CT imaging (r = 0.46; P < .001; n = 128; and r = 0.43; P < .001; n = 119, respectively; Figs 1A and 1B). There was a weak correlation between serum neuron-specific enolase level and the number of lesions (r = 0.22; P = .016; n = 123). There was also a correlation between chromogranin A and 5-HIAA and the number of lesions on CT/MRI and 111In-pentetreotide SPECT/CT scanning (chromogranin A: r = 0.54; P < .001; and r = 0.49; P <.001; 5-HIAA: r = 0.32; P < .001; and r = 0.32; P < .001, respectively). Levels of chromogranin A and 5-HIAA were significantly higher in patients with liver metastases (Mann-Whitney P = .005 and P < .001, respectively; Figs 1C and 1D).
Table 2.
Clinical and Biochemical Characteristics of Study Cohort
| Characteristic | Value |
|---|---|
| No. of patients | 131 |
| Male-to-female ratio | 1:1.3 |
| Mean age ± SD, years (range) | 51 ± 15 (19-82) |
| Patients by symptom type, No. (%) | |
| Total* | 72 (55.0) |
| Flushing | 30 (22.9) |
| Diarrhea | 26 (19.8) |
| Wheezing/shortness of breath | 3 (2.3) |
| Abdominal pain | 10 (7.6) |
| Rash/itching | 2 (1.5) |
| Median fasting chromogranin A, ng/mL (range) | 87.5 (20-18,710) |
| Normal median fasting chromogranin A, ng/mL | < 93 |
| Median fasting gastrin, pg/mL (range) | 28 (10-17,290) |
| Normal median fasting gastrin, pg/mL | < 100 |
| Median fasting neuron-specific enolase, ng/mL (range) | 9.2 (5-45) |
| Normal median fasting neuron-specific enolase, ng/mL | ≤ 15 |
| Median fasting pancreatic polypeptide, pg/mL (range) | 108.5 (40-2,500) |
| Normal median fasting pancreatic polypeptide, pg/mL | < 291 |
| Median urinary 5-HIAA, mg/24 hours (range) | 4.9 (0.9-211) |
| Normal median urinary 5-HIAA, mg/24 hours | ≤ 8 |
| Mean SUVmax on 68Ga-DOTATATE PET/CT ± SD, range | 65.4 ± 47.2 (6.9-244) |
| Patients with prior histologically proven NET, No. (%) | 89 (68.5) |
| Instances of previous surgery by tumor type, No. (%) | |
| Total | 69 (77.5) |
| Pancreatic NET | 31 (44.9) |
| Gastro-enteric NET | 38 (55.1) |
| Type of prior proven NETs, No. (%)* | |
| Pancreatic NET | 36 (27.5) |
| Small/large bowel NET | 31/4 (23.7/3.0) |
| Insulinoma | 7 (5.3) |
| Gastric NET | 7 (5.3) |
| Thymic carcinoid† | 1 (0.8) |
| Vipoma | 2 (1.5) |
| Lung NET† | 1 (0.8) |
| 68Ga-DOTATATE PET/CT found primary, No. (%) | 4/14 (28.6) |
| Surgery recommended, No. (%)‡ | 19 (14.5) |
| PRRT recommended, No. (%) | 11 (8.4) |
Abbreviations: CT, computed tomography; 5-HIAA, 5-hydroxyindoleacetic acid; NET, neuroendocrine tumor; PET, positron emission tomography; PRRT, peptide receptor radionuclide therapy; SD, standard deviation; SUVmax, maximum standardized uptake value.
Some patients had more than one symptom or site of tumor manifestation. Sites were scored separately.
Patients with multiple endocrine neoplasia type 1 and gastro-entero-pancreatic NET that have additional manifestations, such as thymic carcinoid or lung NET.
On the basis of 68Ga-DOTATATE PET/CT.
Fig 1.
There was a significant correlation between (A) chromogranin A and (B) 24-hour urinary 5-hydroxyindoleacetic acid (5-HIAA) and the number of lesions per patient found by using 68Ga-DOTATATE positron emission tomography (PET)/computed tomography (CT) imaging (Spearman coefficient r = 0.46; P < .001; n = 128; and r = 0.43; P < .001; n = 119, respectively). Mean (C) chromogranin A and (D) 24-hour urinary 5-HIAA were significantly higher in patients with liver metastases present with 68Ga-DOTATATE PET/CT imaging (chromogranin A: liver positive [pos] 1083 ± 446 v liver negative [neg] 356 ± 104; Mann-Whitney P = .005; and 5-HIAA: liver positive 20 ± 5.8 v liver negative 4.7 ± 0.3; Mann-Whitney P < .001).
The proportion of positive lesions in the study cohort is summarized in Table 3. 68Ga-DOTATATE PET/CT detected 95.1% of lesions (95% CI, 92.4% to 96.8%) with an average SUVmax of 65.4 ± 47 (range, 6.9 to 244), corresponding to 847 of 891 total lesions identified by the imaging denominator. This was significantly higher than anatomic imaging (CT and/or MRI; 404 of 891 lesions; 45.3% [95% CI, 37.9% to 52.9%]) and 111In-pentetreotide SPECT/CT (275 of 891 lesions; 30.9% [95% CI, 25.0% to 37.5%]). In 37 patients (28.2%), the imaging results were discordant: 111In-pentetreotide SPECT/CT (n = 36) and CT scan (n = 12) were negative and 68Ga-DOTATATE PET/CT was positive, and, in one patient, two duodenal lesions were seen using 111In-pentetreotide SPECT/CT only. Four hundred twenty-two lesions were detected by 68Ga-DOTATATE PET/CT imaging that were missed both by 111In-pentetreotide SPECT/CT and CT/MRI scans.
Table 3.
Proportion of Positive Lesions Detected by 68Ga-DOTATATE PET/CT, 111In-Pentetreotide SPECT/CT, and CT Scan on the Basis of Lesions Identified by Imaging Denominator
| Lesion by Compartment | 68Ga-DOTATATE PET/CT | 111In-Pentetreotide SPECT/CT | Anatomic Imaging (CT and/or MRI) | P (68Ga-DOTATATE v 111In-Pentetreotide) | P (68Ga-DOTATATE v CT/MRI) | |||
|---|---|---|---|---|---|---|---|---|
| No. of Lesions Found (imaging denominator) | Proportion of Positive Lesions, % (95% CI) | No. of Lesions Found (imaging denominator) | Proportion of Positive Lesions, % (95% CI) | No. of Lesions Found (imaging denominator) | Proportion of Positive Lesions, % (95% CI) | |||
| Total | 847 (891) | 95.1 (92.4 to 96.8) | 275 (891) | 30.9 (25.0 to 37.5) | 404 (891) | 45.3 (37.9 to 52.9) | < .001 | < .001 |
| Pancreas | 105 (110) | 95.5 (89.4 to 98.1) | 22 (110) | 20 (12.8 to 29.8) | 59 (110) | 53.6 (42.8 to 64.2) | < .001 | < .001 |
| Liver | 396 (408) | 97.1 (93.7 to 98.7) | 170 (408) | 41.7 (31.1 to 53.1) | 233 (408) | 57.1 (44.7 to 68.7) | < .001 | < .001 |
| Bowel | 49 (51) | 96.1 (75.9 to 99.5) | 7 (51) | 13.7 (5.4 to 30.7) | 6 (51) | 11.8 (3.6 to 32.6) | < .001 | < .001 |
| Lung and mediastinum | 30 (40) | 75 (54.4 to 88.3) | 16 (40) | 40 (25.3 to 56.7) | 30 (40) | 75 (53.0 to 88.9) | .003 | 1.0 |
| Abdomen and retroperitoneal lymph node | 144 (153) | 94.1 (87.1 to 97.4) | 41 (153) | 26.8 (16.6 to 40.2) | 60 (153) | 39.2 (29.7 to 49.6) | < .001 | < .001 |
| Bone | 123 (129) | 95.3 (82.5 to 98.9) | 19 (129) | 14.7 (6.4 to 30.6) | 16 (129) | 12.4 (7.3 to 20.3) | < .001 | < .001 |
NOTE. Imaging denominator represents the sum of functional and anatomic imaging.
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; SPECT, single-photon emission computed tomography.
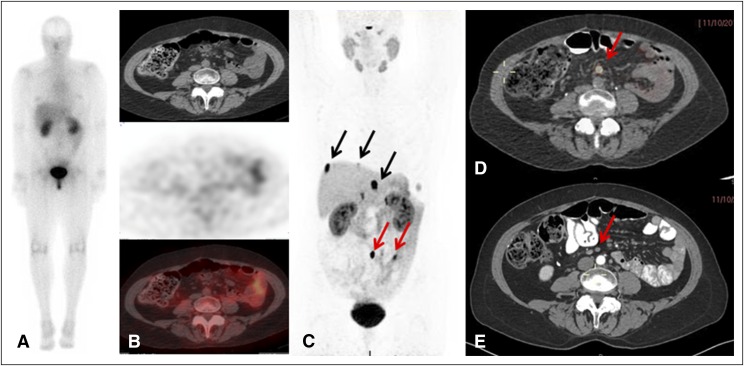
Fourteen patients had an unknown primary tumor, and in four patients the primary lesion was found by using 68Ga-DOTATATE PET/CT imaging, but was not seen with 111In-pentetreotide SPECT/CT, and in two of four patients it was not seen on CT/MRI. An example is shown in Figures 2A to 2E, in which 68Ga-DOTATATE PET/CT imaging confirmed known liver metastases and showed a small bowel primary tumor and a periaortic lymph node with a SUVmax of 75.
Fig 2.
A case of a patient with known liver metastases and previously unknown primary lesion detected by 68Ga-DOTATATE positive emission tomography (PET)/computed tomography (CT). (A) 111In-Pentetreotide scan (planar) shows no pathologic uptake. (B, top) Axial CT, (middle) 111In-pentetreotide axial slice, and (bottom) fused single-photon emission CT/CT showing no pathologic uptake. (C) 68Ga-DOTATATE PET maximum-intensity projection image shows three liver metastases (black arrows) and two lesions in the abdomen (lymph node and enteric lesion; red arrows). (D) 68Ga-DOTATATE PET/CT image shows the mesenteric lymph node (maximum standardized uptake value, 75; red arrow). (E) Arterial phase CT shows corresponding small indeterminate lymph node (red arrow).
An analysis of 23 patients who had no biochemical evidence of GEPNETs but who had possible hormone-related symptoms, such as flushing and diarrhea (Table 1), showed that 68Ga-DOTATATE PET/CT imaging detected more lesions than did 111In-pentetreotide SPECT/CT (15 of 23 lesions [65.2%] v six of 23 lesions [26.1%]; P = .02) and CT/MRI (15 of 23 lesions [65.2%] v nine of 23 lesions [39.1%]; P = .14). CT/MRI missed liver lesions in four patients and duodenal and small bowel lesions in two patients, and 111In- pentetreotide SPECT/CT scanning missed liver lesions in three additional patients. Of 25 patients with hormonal-related symptoms and biochemical evidence of disease that had no prior proven diagnosis of NET, and in whom 68Ga-DOTATATE PET/CT imaging detected lesions, seven patients had negative findings with 111In-pentetreotide SPECT/CT imaging and three patients had negative findings using CT/MRI. In four of these patients, all three imaging modalities were negative.
Surgical resection of primary tumors and/or metastases was recommended in 34 of 131 patients (26%). Of these, 25 patients had undergone surgery at the time of data analysis. The histopathologic findings are summarized in Appendix Tables A1 and A2 (online only). On histopathology, 113 lesions were found to be positive for GEPNET, with 37 lesions (32.7%) being primary tumors and with 69 lymph nodes (61.1%) and seven distant metastases (6.2%). When we compared 68Ga-DOTATATE PET/CT imaging to surgical histopathology in a per-lesion analysis, we found true positive lesions in 72 of 113 lesions (63.7%), in 25 of 113 lesions (22.1%) with 111In-pentetreotide SPECT/CT imaging, and in 44 of 113 lesions (38.9%) with CT/MRI. All 38 lesions not detected by imaging studies but proven on histopathology represented lymph node metastases.
Impact of Imaging Results on Clinical Management
In 97 patients, we recommended nonsurgical management that included clinical and radiologic follow-up in a time period of 6 months to 1 year (63 patients), liver-directed therapy (four patients), and peptide receptor radionuclide therapy (PRRT; 11 patients) or systemic therapy with everolimus or sunitinib (19 patients).
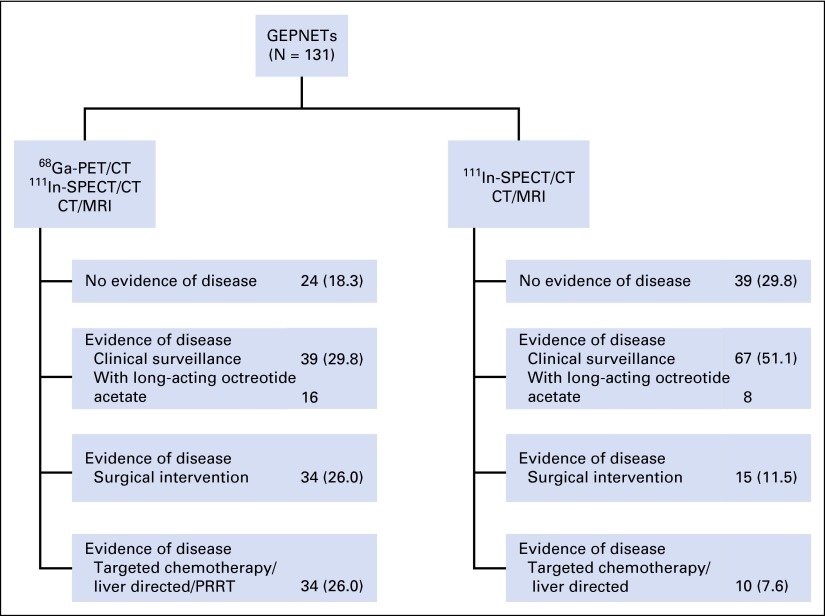
Upon comparing the different imaging findings and evaluating their impact on clinical management, we found that, compared with 111In-pentetreotide SPECT/CT, 68Ga-DOTATATE PET/CT imaging detected additional lesions in 93 of 131 patients (71.0%), and in 69 of 131 patients (52.7%) compared with CT scan. Of those, in 44 of 93 patients (47.3%) and in 34 of 69 patients (49.2%), these additional lesions consisted of metastatic lesions found by using 68Ga-DOTATATE PET/CT imaging. These additional findings led to a change in management in 43 of 131 patients (32.8%). An example of a patient with additional lesions that were found by using 68Ga-DOTATATE PET/CT but that were not seen on 111In-pentetreotide SPECT/CT and CT scan is shown in Appendix Figure A1 (online only), and the specific changes in management recommendation as a result of 68Ga-DOTATATE PET/CT imaging are shown in Figure 3.
Fig 3.
Clinical management diagram on the basis of the three imaging modalities. Data are given as No. (%). CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; PRRT, peptide receptor radionuclide therapy; SPECT, single-photon emission computed tomography.
DISCUSSION
Our results show that 68Ga-DOTATATE PET/CT scanning is superior to 111In-pentetreotide SPECT/CT and anatomic imaging in the detection of unknown primary tumors and primary and metastatic GEPNETs. 68Ga-DOTATATE PET/CT had a detection rate of 95.2% compared with detection rates of 45.6% and 30.9% for anatomic and 111In- pentetreotide SPECT/CT imaging, respectively. 68Ga-DOTATATE PET/CT identified primary tumors in 28.6% of patients with previously unknown primary tumors. Furthermore, this led to the alteration of patient management recommendations in 32.8% of patients. 68Ga-DOTATATE PET/CT also detected disease in patients without biochemical evidence of GEPNET and in those with symptoms and no biochemical evidence of disease.
We found that serum chromogranin A, urinary 5-HIAA, and serum neuron-specific enolase correlated with the number of tumors found by using 68Ga-DOTATATE PET/CT in patients with GEPNET, and that chromogranin A and 5-HIAA were elevated in patients with liver metastases. These findings are consistent with data in the literature, in which serum chromogranin A and urinary 5-HIAA have been associated with tumor burden and patient survival.20,21 Of interest, we show that 68Ga-DOTATATE PET/CT imaging was helpful in detecting disease in 15 of 23 symptomatic patients (65.2%) with biochemically negative workups, offering a rationale with treatment possibilities for their otherwise unexplained symptoms.
68Ga-DOTATATE PET/CT detected significantly more lesions in the bowel and bone when compared with 111In-pentetreotide SPECT/CT and CT/MRI scanning, with 111In-pentetreotide SPECT/CT detecting slightly more lesions than CT/MRI. This difference was significant again for the pancreas, liver, and lymph nodes in the abdomen and retroperitoneal compartment. Furthermore, we showed that 68Ga-DOTATATE PET/CT imaging performed as well as anatomic imaging (CT/MRI) in detecting lesions in the lung and mediastinal compartment, suggesting that these two imaging modalities could be complementary for staging patients with GEPNET and suspected lung metastases.
The use of 68Ga-DOTATATE PET/CT has been recommended for staging22 and for detecting early recurrences after resection of NET,23 and its impact on medical and surgical management strategies has been shown in retrospective studies.18,24 On the basis of 68Ga-DOTATATE PET/CT imaging, Hofman et al18 reported a change in treatment plan in 47% of patients with NET, and Ilhan et al25 found additional information for surgical planning in 95% of cases with small bowel (ileum) or pancreatic NET, which resulted in changes in management in one fifth of patients.
To our knowledge, the current study is the largest prospective study of 68Ga-DOTATATE PET/CT in a homogenous cohort of patients with GEPNETs. There have been four prospective studies, one with a mixed cohort of 19 patients with NET using 68Ga-DOTATATE,26 and three other studies with different 68Ga-DOTA peptides: two using 68Ga-DOTATOC in a mixed cohort of 84 and 27 patients with NET, including lung NETs, neuroblastoma, and paraganglioma, and one using 68Ga-DOTANOC in 109 patients with GEPNET.All four prospected studies showed the superiority of 68Ga-DOTA peptide imaging compared with conventional scintigraphy and anatomic imaging.27-29 Our study results are consistent with these studies but provide additional new data. First, two thirds of patients with symptoms of carcinoid syndrome and no biochemical evidence of disease were found to have lesions by using 68Ga-DOTATATE PET/CT imaging. Second, our management recommendations changed on the basis of real-time assessment of the burden of disease in patients and a standardized management algorithm. Last, our study shows that 68Ga-DOTATATE was able to identify unknown primary GEPNET and disease in symptomatic patients without biochemical evidence of disease.
For progressive metastatic GEPNET, there are several therapeutic options, such as surgical resection and/or pharmacologic and liver-directed therapies. PRRT is a relatively new therapeutic modality that targets the positivity of SSTR in GEPNET as the treatment rationale, permitting targeted therapy with somatostatin analogs labeled with radionuclides. According to published guidelines, PRRT is indicated for the treatment of patients with positive expression of SSTR2, metastatic, or inoperable NET30; these are mainly patients with SSTR2-expressing GEPNETs and bronchial NETs. Large series have shown median progression-free survival of up to 40 months for PRRT with 177Lu-DOTA-octreotate.31 Therefore, it is important, when selecting treatment options, to use the 68Ga-DOTA peptide PET/CT to stage patients with progressive, metastatic, and/or inoperable GEPNETs and to determine the tumor somatostatin receptor status. Furthermore, 68Ga-DOTATATE PET/CT scanning has the advantage over 111In-pentetreotide SPECT/CT in that it requires a shorter imaging time and has a lower cost32 and radiation exposure (1.1 rem).33
Our study does have limitations. While most lesions detected by using 68Ga-DOTATATE PET/CT imaging are likely to be true positive findings on the basis of their high SUV and location, we do not have histopathologic proof for every lesion detected; thus, false positive results are possible. The study design—not a randomized controlled trial—and the lack of follow-up data does not allow us to definitively answer whether the therapeutic options selected on the basis of 68Ga-DOTATATE PET/CT imaging resulted in improved long-term patient outcome. The number of lesions per patient found by using 68Ga-DOTATATE PET/CT imaging is an approximation of tumor burden and is not a precise measurement of tumor mass/burden. In addition, the imaging denominator is not a perfect gold standard and could represent some false positive results. Furthermore, 68Ga-DOTATATE PET/CT imaging is known to show lower disease avidity for poorly differentiated NET,34 and it would therefore be beneficial for disease characterization to perform 18F-FDG PET/CT imaging in these situations, as this could show higher avidity. Our study design did not include 18F-FDG PET/CT imaging in every patient as part of the clinical research protocol, although we did perform it when clinically indicated. Furthermore, adding a diagnostic, contrast-enhanced CT scan might improve the sensitivity and specificity of SPECT/CT or PET/CT imaging to detect NET.
In conclusion, 68Ga-DOTATATE PET/CT imaging is more sensitive for staging and detecting unknown primary GEPNETs than is 111In-pentetreotide SPECT/CT and anatomic imaging with CT/MRI. Therefore, we believe it should be implemented in the initial management and follow-up of patients with GEPNETs as it can significantly optimize patient care decisions.
Acknowledgment
We thank Candice Cottle-Delisle and Roxanne Merkel for their help coordinating the clinical protocol and study patients, Lily A. Yang for data management, and the staff at the National Institutes of Health Positron Emission Tomography Department. We also thank the study patients and their families for participating in the clinical protocol.
Appendix
Fig A1.
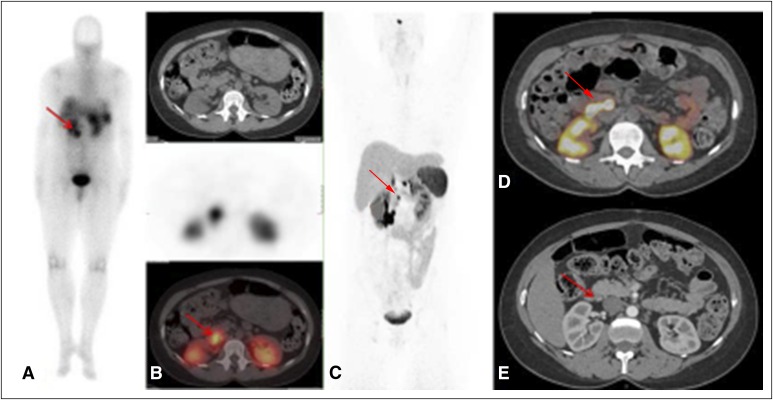
A 46-year-old patient with multiple endocrine neoplasia type 1 and known pancreatic and duodenal lesions who had lymph nodes not previously known that were detected by using 68Ga-DOTATATE positron emission tomography (PET)/computed tomography (CT) imaging. (A) 111In-Pentetreotide scan (planar) shows a unique unclear prerenal uptake. (B, top) Axial CT, (middle) 111In-pentetreotide axial slice, and (bottom) fused single-photo emission CT/CT showing unclear pathologic uptake (red arrow). (C) 68Ga-DOTATATE PET maximum-intensity projection image shows retropancreatic lymph node (red arrow) and duodenal and pancreatic lesions. (D) 68Ga-DOTATATE Q:17 PET/CT image shows the retropancreatic and periduodenal lymph node (maximum standardized uptake value, 96; red arrow). (E) Arterial phase CT shows corresponding lymph node (red arrow) that was read as a subcentimeter indeterminate lymph node.
Table A1.
Characteristics of Patients Who Underwent Surgery
| Characteristic | Value |
|---|---|
| No. of patients | 25 |
| Mean age, years (range) | 42 (21-73) |
| Male-to-female ratio | 1.5:1 |
| No. of sporadic tumors | 11 |
| No. of syndromic tumors | 14 |
| MEN1 | 10 |
| VHL | 4 |
Abbreviations: MEN1, multiple endocrine neoplasia type 1; VHL, von Hippel-Lindau.
Table A2.
TNM Staging for Patients Who Underwent Surgery
| Stage | Pancreatic NET (n = 16)* | Gastro-Entero-NET (n = 9)† |
|---|---|---|
| Tx | 1 | 3 |
| T0 | 2 | 1 |
| T1 | 3 | 1 |
| T2 | 7 | 3 |
| T3 | 3 | 1 |
| T4 | 0 | 0 |
| N0 | 12 | 2 |
| N1 | 4 | 7 |
| M0 | 14 | 7 |
| M1 | 2 | 2 |
| WHO grade | ||
| G1 | 6 | 5 |
| G2 | 6 | 2 |
| G3 | 1 | 0 |
Bosman et al: Lyon, France, International Agency for Research on Cancer, 2010.
Pape et al: Neuroendocrinology 95:135-156, 2012.
Footnotes
Listen to the podcast by Dr Strosberg at www.jco.org/podcasts
Supported by the Intramural Research Programs of the Center for Cancer Research, National Cancer Institute, and the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Samira M. Sadowski, Vladimir Neychev, Corina Millo, Peter Herscovitch, Karel Pacak, Stephen J. Marx, Electron Kebebew
Financial support: Electron Kebebew
Administrative support: Electron Kebebew
Provision of study materials or patients: Naris Nilubol, Electron Kebebew
Collection and assembly of data: Samira M. Sadowski, Vladimir Neychev, Corina Millo, Peter Herscovitch, Karel Pacak, Stephen J. Marx, Electron Kebebew
Data analysis and interpretation: Samira Sadowski, Vladimir Neychev, Corina Millo, Joanna Shih, Naris Nilubol, Karel Pacak, Stephen J. Marx, Electron Kebebew
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Samira M. Sadowski
No relationship to disclose
Vladimir Neychev
No relationship to disclose
Corina Millo
No relationship to disclose
Joanna Shih
No relationship to disclose
Naris Nilubol
No relationship to disclose
Peter Herscovitch
No relationship to disclose
Karel Pacak
No relationship to disclose
Stephen J. Marx
No relationship to disclose
Electron Kebebew
No relationship to disclose
REFERENCES
- 1.Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: A SEER analysis. J Cancer. 2012;3:292–302. doi: 10.7150/jca.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1–18. doi: 10.1016/j.ecl.2010.12.005. doi:10.1016/j.ecl.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Duh QY, Hybarger CP, Geist R, et al. Carcinoids associated with multiple endocrine neoplasia syndromes. Am J Surg. 1987;154:142–148. doi: 10.1016/0002-9610(87)90305-9. [DOI] [PubMed] [Google Scholar]
- 4.Charlesworth M, Verbeke CS, Falk GA, et al. Pancreatic lesions in von Hippel-Lindau disease? A systematic review and meta-synthesis of the literature. J Gastrointest Surg. 2012;16:1422–1428. doi: 10.1007/s11605-012-1847-0. [DOI] [PubMed] [Google Scholar]
- 5.Larson AM, Hedgire SS, Deshpande V, et al. Pancreatic neuroendocrine tumors in patients with tuberous sclerosis complex. Clin Genet. 2012;82:558–563. doi: 10.1111/j.1399-0004.2011.01805.x. [DOI] [PubMed] [Google Scholar]
- 6.Plöckinger U, Rindi G, Arnold R, et al. European Neuroendocrine Tumour Society Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours: A consensus statement on behalf of the European Neuroendocrine Tumour Society (ENETS) Neuroendocrinology. 2004;80:394–424. doi: 10.1159/000085237. [DOI] [PubMed] [Google Scholar]
- 7.Saxena A, Chua TC, Perera M, et al. Surgical resection of hepatic metastases from neuroendocrine neoplasms: A systematic review. Surg Oncol. 2012;21:e131–e141. doi: 10.1016/j.suronc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Sutcliffe R, Maguire D, Ramage J, et al. Management of neuroendocrine liver metastases. Am J Surg. 2004;187:39–46. doi: 10.1016/j.amjsurg.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 10.Ramage JK, Davies AH, Ardill J, et al. UKNETwork for Neuroendocrine Tumours Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut. 2005;54:iv1–iv16. doi: 10.1136/gut.2004.053314. (suppl 4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reubi JC, Kvols L, Krenning E, et al. Distribution of somatostatin receptors in normal and tumor tissue. Metabolism. 1990;39:78–81. doi: 10.1016/0026-0495(90)90217-z. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 12.de Herder WW, Hofland LJ, van der Lely AJ, et al. Somatostatin receptors in gastroentero-pancreatic neuroendocrine tumours. Endocr Relat Cancer. 2003;10:451–458. doi: 10.1677/erc.0.0100451. [DOI] [PubMed] [Google Scholar]
- 13.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 14.Rufini V, Calcagni ML, Baum RP. Imaging of neuroendocrine tumors. Semin Nucl Med. 2006;36:228–247. doi: 10.1053/j.semnuclmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Kwekkeboom DJ, Krenning EP. Somatostatin receptor imaging. Semin Nucl Med. 2002;32:84–91. doi: 10.1053/snuc.2002.31022. [DOI] [PubMed] [Google Scholar]
- 16.Falconi M, Bartsch DK, Eriksson B, et al. Barcelona Consensus Conference participants ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: Well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012;95:120–134. doi: 10.1159/000335587. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Kan Y, Ge BH, et al. Diagnostic role of Gallium-68 DOTATOC and Gallium-68 DOTATATE PET in patients with neuroendocrine tumors: A meta-analysis. Acta Radiol. 2014;55:389–398. doi: 10.1177/0284185113496679. [DOI] [PubMed] [Google Scholar]
- 18.Hofman MS, Kong G, Neels OC, et al. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56:40–47. doi: 10.1111/j.1754-9485.2011.02327.x. [DOI] [PubMed] [Google Scholar]
- 19.Haug AR, Rominger A, Mustafa M, et al. Treatment with octreotide does not reduce tumor uptake of (68)Ga-DOTATATE as measured by PET/CT in patients with neuroendocrine tumors. J Nucl Med. 2011;52:1679–1683. doi: 10.2967/jnumed.111.089276. [DOI] [PubMed] [Google Scholar]
- 20.van Adrichem RC, Hofland LJ, Feelders RA, et al. Chromogranin A, Ki-67 index and IGF-related genes in patients with neuroendocrine tumors. Endocr Connect. 2013;2:172–177. doi: 10.1530/EC-13-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nölting S, Kuttner A, Lauseker M, et al. Chromogranin a as serum marker for gastroenteropancreatic neuroendocrine tumors: A single center experience and literature review. Cancers (Basel) 2012;4:141–155. doi: 10.3390/cancers4010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virgolini I, Ambrosini V, Bomanji JB, et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging. 2010;37:2004–2010. doi: 10.1007/s00259-010-1512-3. [DOI] [PubMed] [Google Scholar]
- 23.Haug AR, Cindea-Drimus R, Auernhammer CJ, et al. Neuroendocrine tumor recurrence: Diagnosis with 68Ga-DOTATATE PET/CT. Radiology. 2014;270:517–525. doi: 10.1148/radiol.13122501. [DOI] [PubMed] [Google Scholar]
- 24.Frilling A, Sotiropoulos GC, Radtke A, et al. The impact of 68Ga-DOTATOC positron emission tomography/computed tomography on the multimodal management of patients with neuroendocrine tumors. Ann Surg. 2010;252:850–856. doi: 10.1097/SLA.0b013e3181fd37e8. [DOI] [PubMed] [Google Scholar]
- 25.Ilhan H, Fendler WP, Cyran CC, et al. Impact of (68)Ga-DOTATATE PET/CT on the surgical management of primary neuroendocrine tumors of the pancreas or ileum. Ann Surg Oncol. 2015;22:164–171. doi: 10.1245/s10434-014-3981-2. [DOI] [PubMed] [Google Scholar]
- 26.Etchebehere EC, de Oliveira Santos A, Gumz B, et al. 68Ga-DOTATATE PET/CT, 99mTc-HYNIC-octreotide SPECT/CT, and whole-body MR imaging in detection of neuroendocrine tumors: A prospective trial. J Nucl Med. 2014;55:1598–1604. doi: 10.2967/jnumed.114.144543. [DOI] [PubMed] [Google Scholar]
- 27.Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 28.Naswa N, Sharma P, Kumar A, et al. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: A prospective single-center study. AJR Am J Roentgenol. 2011;197:1221–1228. doi: 10.2214/AJR.11.7298. [DOI] [PubMed] [Google Scholar]
- 29.Buchmann I, Henze M, Engelbrecht S, et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007;34:1617–1626. doi: 10.1007/s00259-007-0450-1. [DOI] [PubMed] [Google Scholar]
- 30.Bodei L, Mueller-Brand J, Baum RP, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–816. doi: 10.1007/s00259-012-2330-6. [Erratum: Eur J Med Mol Imaging 41:584, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 32.Schreiter NF, Brenner W, Nogami M, et al. Cost comparison of 111In-DTPA-octreotide scintigraphy and 68Ga-DOTATOC PET/CT for staging enteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39:72–82. doi: 10.1007/s00259-011-1935-5. [DOI] [PubMed] [Google Scholar]
- 33.Walker RC, Smith GT, Liu E, et al. Measured human dosimetry of 68Ga-DOTATATE. J Nucl Med. 2013;54:855–860. doi: 10.2967/jnumed.112.114165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kayani I, Bomanji JB, Groves A, et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112:2447–2455. doi: 10.1002/cncr.23469. [DOI] [PubMed] [Google Scholar]