Abstract
Purpose
Patients with acute myeloid leukemia (AML) who are in morphologic complete remission are typically considered separately from patients with active disease (ie, ≥ 5% marrow blasts by morphology) in treatment algorithms for allogeneic hematopoietic cell transplantation (HCT), which implies distinct outcomes for these two groups. It is well recognized that the presence of minimal residual disease (MRD) at the time of transplantation is associated with adverse post-HCT outcome for those patients in morphologic remission. This effect of pre-HCT MRD prompted us to compare outcomes in consecutive patients in MRD-positive remission with patients with active AML who underwent myeloablative allogeneic HCT at our institution.
Patients and Methods
We retrospectively studied 359 consecutive adults with AML who underwent myeloablative allogeneic HCT from a peripheral blood or bone marrow donor between 2006 and 2014. Pre-HCT disease staging included 10-color multiparametric flow cytometry on bone marrow aspirates in all patients. Any level of residual disease was considered to be MRD positive.
Results
Three-year relapse estimates were 67% in 76 patients in MRD-positive morphologic remission and 65% in 48 patients with active AML compared with 22% in 235 patients in MRD-negative remission. Three-year overall survival estimates were 26%, 23%, and 73% in these three groups, respectively. After multivariable adjustment, MRD-negative remission status remained statistically significantly associated with longer overall and progression-free survival as well as lower risk of relapse compared with MRD-positive morphologic remission status or having active disease, with similar outcomes between the latter two groups.
Conclusion
The similarities in outcomes between patients in MRD-positive morphologic remission and those with active disease at the time of HCT support the use of treatment algorithms that use MRD- rather than morphology-based disease assessments.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) is potentially curative in patients with acute myeloid leukemia (AML).1-5 Many studies indicate that allogeneic HCT reduces patient risk of experiencing relapse and improves relapse-free and overall survival (OS) in adverse-risk and intermediate-risk AML in first morphologic complete remission (CR).3 Current treatment algorithms and research protocol assignments as well as analyses of post-HCT outcomes typically separate patients in morphologic remission (ie, < 5% bone marrow blasts) from those with active disease (≥ 5% blasts in bone marrow) after either experiencing relapse or failure to enter initial remission with chemotherapy. This distinction reflects the poorer outcome in patients with active AML at the time of transplantation. Yet, outcomes vary widely in patients who undergo transplantation while in morphologic remission, with the presence of minimal residual disease (MRD) before HCT, as detected by multiparameter flow cytometry (MFC), indicating a high relapse risk and short survival.6-8 Here, we asked whether post-HCT outcomes in patients who are in MRD-positive CR at the time of transplantation more closely resembled outcomes in patients with active AML or those in MRD-negative remission pre-HCT. We therefore compared outcomes following transplantation in these groups using a cohort of consecutive patients who underwent myeloablative allogeneic HCT from a peripheral blood or bone marrow donor.
PATIENTS AND METHODS
Study Cohort
Patients with AML age ≥ 18 years were included if they underwent first allogeneic HCT with myeloablative conditioning using peripheral blood or bone marrow as the stem cell source from April 2006 until October 2014, with the former date corresponding to the introduction of a refined MFC-based MRD detection method. Results from 205 patients who underwent HCT while in morphologic remission were reported previously.9-11 We used the 2008 WHO criteria to define AML12 and Medical Research Council/National Cancer Research Institute criteria to assign cytogenetic risk.13 Secondary leukemia was defined as AML following an antecedent hematologic disorder, that is, myelodysplastic syndrome or myeloproliferative neoplasm, or treatment with systemic chemotherapy and/or radiotherapy.11 Treatment responses were categorized as proposed by International Working Groups.14,15 Criteria for diagnosis and grading of acute and chronic graft-versus-host disease (GVHD) have been reported previously.16,17 All patients were treated on institutional review board–approved protocols or standard treatment protocols and gave consent in accordance with the Declaration of Helsinki. Follow-up was current as of April 24, 2015.
MFC Detection of MRD
Ten-color MFC was performed routinely using bone marrow aspirates and a panel of three antibody combinations as part of the transplant arrival work-up.9-11 Up to 1 million events per tube were acquired on a custom-built LSR II flow cytometer (BD Biosciences; San Jose, CA), and data compensation and analysis was performed using in-house software (WoodList; B.L.W.). The same assay and largely the same group of hematopathologists were used during the time course of the study. Compared with either normal or regenerating marrow, MRD was identified by visual inspection as a cell population showing deviation from normal antigen-expression patterns seen in specific cell lineages at specific stages of maturation.10,11 The sensitivity of the MFC MRD assay varies with the type of phenotypic aberrancy and immunophenotypes of normal cells in background populations. Therefore, the MRD assay does not have uniform sensitivity across all patients but is able to detect MRD when present in the majority of patients down to a level of 0.1% and in progressively smaller subsets of patients as the level of residual disease continues to decrease. The abnormal cell population was quantified as a percentage of the total CD45+ white blood cell events. Any level of residual disease was considered MRD positive.9-11 Results from the MFC assessment of MRD were available to the transplant teams.
Statistical Analysis
OS and progression-free survival (PFS) were estimated using the Kaplan-Meier method. Probabilities of nonrelapse mortality (NRM), relapse, and acute and chronic GVHD were summarized using cumulative incidence estimates. For patients in morphologic remission undergoing transplantation, documented relapse was considered progression; for patients with active AML undergoing transplantation, progression was assumed when palliative or curative-intent, post-HCT AML chemotherapy was administered. NRM was defined as death without prior relapse and was considered a competing risk for relapse, whereas relapse was considered a competing risk for NRM. Death was considered a competing risk for acute and chronic GVHD. Associations with OS and PFS were assessed by using Cox proportional hazards regression, and associations with relapse and NRM were assessed by using proportional subdistribution hazard models that account for competing risks. Other than disease stage (remission v not in remission) and MRD, multivariable models included age at the time of HCT, cytogenetic risk group at time of AML diagnosis (adverse v favorable/intermediate), type of AML at diagnosis (secondary v de novo), karyotype at time of HCT (normalized v not normalized for patients presenting with abnormal karyotypes), and peripheral blood counts at the time of HCT (recovered [ie, absolute neutrophil count > 1,000/µL and platelet count > 100,000/µL] v not recovered). Missing cytogenetic risk and karyotype were accounted for as separate categories. Categorical patient characteristics were compared between individual patient groups by using Fisher’s exact tests, and continuous characteristics were compared by using one-way analysis of variance tests. No adjustments were made for multiple comparisons, and all two-sided P values from the regression models were derived by using the Wald test. Statistical analyses were performed using STATA software (STATA, College Station, TX) and R software (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics
We identified 376 patients who met our inclusion criteria. We excluded patients in third remission (n = 2); patients who met marrow criteria for remission but had circulating blasts of undetermined significance in the peripheral blood (n = 4); patients who did not undergo pre-HCT MRD assessment (n = 6); and patients for whom we had no detailed pretransplant information on disease characteristics and treatment (n = 4). The remaining 359 patients had myeloablative HCT from a related (n = 142) or unrelated (n = 217) donor and had detailed pretransplant pathologic studies that included MRD data available for retrospective analysis. Three-hundred eleven patients (86.6%) had < 5% bone marrow blasts and thus met the morphologic marrow criteria for leukemia-free state and remission.15 Of these, 248 patients (79.7%) had recovered neutrophil and platelet counts and met morphologic criteria for CR, whereas 63 patients (20.3%) had incomplete recovery of neutrophils and/or platelets and met morphologic criteria for CRi. Seventy-six of 311 remission patients (24.4%) had MRD by MFC, that is, these patients were MRD positive, whereas 235 patients (75.6%) had no flow cytometric evidence of MRD, that is, these patients were MRD negative. Among MRD-positive patients, 17 had MRD detectable at < 0.1% (the lowest two were 0.007%), 24 had MRD levels of 0.1% to 1%, and 35 had MRD levels greater than 1%. As previously reported, the outcomes for patients in first (n = 232) and second (n = 79) remission were similar (Appendix Fig A1, online only)10 and were combined for subsequent analyses. Forty-eight patients (13.4%) had ≥ 5% bone marrow blasts in their pre-HCT assessment and were thus classified as having active AML. Seven of these 48 patients had never received therapy for AML, that is, these seven patients had untreated, newly diagnosed AML, whereas 16 patients had experienced AML relapse for which they did not receive any salvage chemotherapy. Twenty-five patients either experienced failure of salvage chemotherapy for relapsed AML or had primary refractory AML and did not achieve remission with up to two cycles of induction chemotherapy. Although limited by the small sample size, a subset analysis suggested that OS was similar for these three subsets of patients with active AML at the time of HCT (median OS for patients with untreated, newly diagnosed AML, 4.9 months [95% CI, 1.8 to NA months]; median OS for patients with untreated, relapsed AML, 7.1 months [95% CI, 4.4 to NA months]; and median OS for patients with primary refractory AML or unsuccessfully treated, relapsed AML, 3.5 months [95% CI, 2.7 to 9.1 months]; Appendix Fig A2) and the subsets were combined for subsequent analyses. The characteristics of the study population, donors, and transplants stratified by disease status are summarized in Table 1. There were several statistically significant differences between patients in MRD-negative remission, in MRD-positive remission, and with active disease, most notably with regard to cytogenetic risk at diagnosis (there were higher proportions of patients with adverse-risk cytogenetics in MRD-positive remission and with active AML compared with patients in MRD-negative remission; P = .001), prevalence of secondary leukemia (higher in patients in MRD-positive remission and active AML patients than in patients in MRD-negative remission; P < .001), and proportion of patients with fully recovered peripheral blood counts before HCT (higher in patients in remission than in those with active disease; P < .001) but also with regard to conditioning regimens, particularly the use of radiolabeled antibodies in patients with active AML (P < .001), and with regard to GVHD prophylaxis (P < .001).
Table 1.
Pretransplantation Demographics and Clinical Characteristics of Study Cohort Stratified by Disease Status
| Variable | Patients in MRD-Negative Remission (n = 235) | Patients in MRD-Positive Remission (n = 76) | Patients With Active AML (n = 48) | All Patients (N = 359) | P |
|---|---|---|---|---|---|
| Median age at HCT, years (range) | 47.5 (19.1-71.6) | 51.2 (18.2-72.6) | 55.0 (21.1-75.3) | 50.0 (18.2-75.3) | .681 |
| Male gender, % | 51.1 | 65.8 | 45.8 | 53.5 | .043 |
| Median WBC at diagnosis, × 103/µL (range) | 9.9 (0.2-297.2) | 3.9 (0.3-250.0) | 8.0 (0.9-268.0) | 8.1 (0.2-297.2) | .250 |
| Cytogenetics, No. (%) | .001 | ||||
| Favorable/intermediate | 189 (81.8) | 45 (60.8) | 30 (62.5) | 264 (74.8) | < .001 |
| Favorable | 23 (10.0) | 2 (2.7) | 1 (2.1) | 26 (7.4) | |
| Intermediate | 166 (71.9) | 43 (58.1) | 29 (60.4) | 238 (67.4) | |
| Adverse | 42 (18.2) | 29 (39.2) | 18 (37.5) | 89 (25.2) | |
| Missing | 4 | 2 | 0 | 6 | |
| Secondary AML, % | 22.1 | 42.1 | 52.1 | 30.4 | < .001 |
| Disease status, No. (%) | |||||
| Remission 1 | 181 (77.0) | 51 (67.1) | — | 232 (64.6) | |
| Remission 2 | 54 (23.0) | 25 (32.9) | — | 79 (22.0) | |
| Untreated | — | — | 7 (14.6) | 7 (1.9) | |
| Relapse (untreated) | — | — | 16 (33.3) | 16 (4.5) | |
| Relapse/refractory (treated) | — | — | 25 (52.1) | 25 (7.0) | |
| Median remission duration before HCT, days (range) | 106 (7-465) | 70.5 (11-485) | — | — | .677 |
| Recovered peripheral blood counts before HCT, No. (%)* | 192 (81.7) | 56 (73.7) | 10 (20.8) | 258 (71.9) | < .001 |
| Routine cytogenetics before HCT, No. (%) | < .001 | ||||
| Normalized karyotype | 208 (46.0) | 24 (31.6) | — | 132 (36.8) | |
| Abnormal karyotype | 21 (8.9) | 26 (34.2) | 38 (79.2) | 85 (23.7) | |
| Missing/noninformative data | 106 (45.1) | 26 (34.2) | 10 (20.8) | 142 (39.6) | |
| Median time between pre-HCT MRD assay and HCT, days (range) | 23 (7-68) | 23 (11-55) | 25.5 (10-55) | 23 (7-68) | .388 |
| Median abnormal blasts by MFC, % (range) | 0 (0) | 0.60 (0.007-19.4) | 15.5 (0.8-83.9) | 0 (0-83.9) | < .001 |
| Unrelated donor, % | 60.9 | 60.5 | 58.3 | 60.5 | .948 |
| Median donor age, years (range) | 37.1 (10.6-70.4) | 35.5 (12.7-77.8) | 33.2 (18.1-58.2) | 36.1 (10.6-77.8) | .624 |
| Male donor, % | 57.0 | 55.3 | 64.6 | 57.7 | .560 |
| Conditioning regimen, No. (%) | < .001 | ||||
| BU/CY ± L-TBI | 80 (34.0) | 26 (34.2) | 5 (10.4) | 111 (30.9) | |
| BU/FLU, BU/VP16, or BU/CLO | 64 (27.2) | 15 (19.7) | 11 (22.9) | 90 (25.1) | |
| H-TBI ± CY or FLU | 24 (10.2) | 8 (10.5) | 8 (16.7) | 40 (11.1) | |
| H-TBI/Tepa/FLU | 10 (4.3) | 1 (1.3) | 0 (0) | 11 (3.1) | |
| Treo/FLU ± L-TBI | 47 (20.0) | 14 (18.4) | 4 (8.3) | 65 (18.1) | |
| FLU/radiolabeled Ab/L-TBI ± CY | 10 (4.3) | 12 (15.8) | 20 (41.7) | 42 (11.7) | |
| Stem cell source, No. (%) | .246 | ||||
| PBSC | 187 (79.6) | 60 (78.9) | 43 (89.6) | 290 (80.8) | |
| BM | 48 (20.4) | 16 (21.1) | 5 (10.4) | 69 (19.2) | |
| GVHD prophylaxis, No. (%) | < .001 | ||||
| Calcineurin inhibitor + methotrexate | 183 (77.9) | 53 (69.7) | 26 (54.2) | 262 (73.0) | |
| Calcineurin inhibitor + MMF | 10 (4.3) | 10 (13.2) | 18 (37.5) | 38 (10.6) | |
| CY ± calcineurin inhibitor ± MMF | 32 (13.6) | 12 (15.8) | 3 (6.3) | 47 (13.1) | |
| Other | 10 (4.3) | 1 (1.3) | 1 (2.1) | 12 (3.3) |
Abbreviations: Ab, antibody; BM, bone marrow; BU, busulfan; CLO, clofarabine; CR1, first CR; CR2, second CR; CY, cyclophosphamide; FLU, fludarabine; HCT, hematopoietic cell transplantation; H-TBI, high-dose total body irradiation; L-TBI, low-dose total body irradiation; MFC, multiparameter flow cytometry; MMF, mycophenolate mofetil; MRD, minimal residual disease; neg, negative; PBSC, peripheral blood stem cells; pos, positive; Tepa, thiotepa; Treo, treosulfan VP16, etoposide.
Absolute neutrophil count ≥ 1,000/µL and platelets ≥ 100,000/µL.
Acute and Chronic GVHD
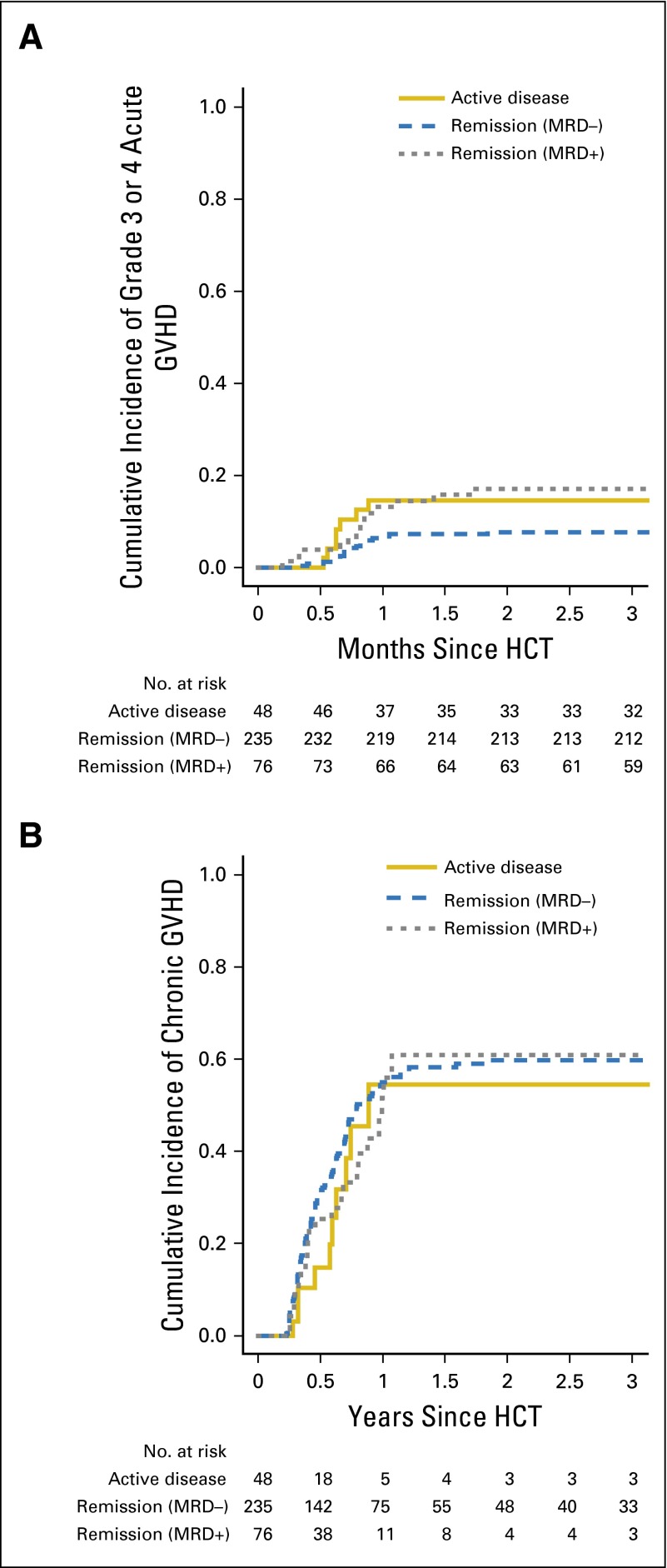
The 100-day cumulative incidences of grade 3 or 4 acute GVHD as well as the 180-day cumulative incidence of chronic GVHD were not statistically significantly different for patients in MRD-negative remission, in MRD-positive remission, and with active AML (Fig 1 and Table 2).
Fig 1.
Cumulative incidences of acute graft-versus-host disease (GVHD) and chronic GVHD stratified by disease status. Estimates of (A) grade 3 or 4 acute GVHD and (B) chronic GVHD after myeloablative allogeneic hematopoietic cell transplantation (HCT) for adults with acute myeloid leukemia (AML), shown individually for patients in minimal residual disease (MRD) –negative (n = 235) and MRD-positive (n = 76) morphologic remission as well as those with active AML (n = 48).
Table 2.
Outcome Probabilities Stratified by Disease Status
| Outcome | Patients in MRD-Negative Remission (n = 235) | Patients in MRD-Positive Remission (n = 76) | Patients With Active AML (n = 48) |
|---|---|---|---|
| OS at 3 years | 73 (66-78) | 26 (17-37) | 23 (12-35) |
| PFS at 3 years | 67 (61-73) | 12 (5-21) | 13 (5-23) |
| Cumulative incidence of relapse at 3 years | 22 (16-28) | 67 (55-77) | 65 (49-76) |
| Cumulative incidence of NRM at 3 years | 11 (7-15) | 21 (13-31) | 23 (12-35) |
| Grade 3or 4 acute GVHD at 100 days | 8 (5-12) | 17 (10-27) | 15 (7-27) |
| Chronic GVHD at 18 months | 60 (49-71) | 61 (54-67) | 54 (40-68) |
NOTE. All data are given as percent (range).
Abbreviations: GVHD, graft-versus-host disease; MRD, minimal residual disease; neg, negative; NRM, nonrelapse mortality; OS, overall survival; PFS, progression-free survival; pos, positive.
Relationship Between Pre-HCT Disease Status and Post-HCT Outcome
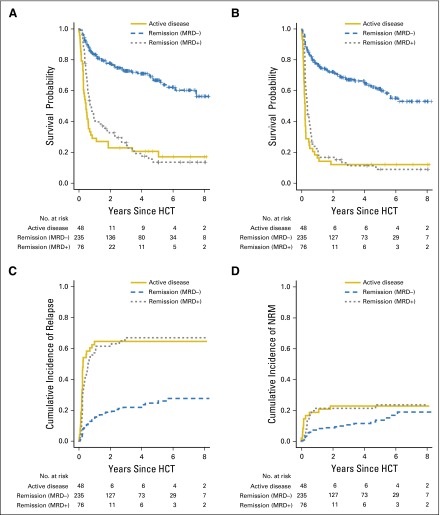
After transplantation, 132 patients experienced relapse or progression, 111 of whom died, and 57 patients died as a result of NRM for a total of 168 patient deaths. The median follow-up time after HCT in the 91 patients alive at last contact was 36.3 months (range, 3.1 to 99.1 months) for patients in MRD-negative remission, 49.9 months (range, 4.8 to 96.7) for patients in MRD-positive remission, and 63.8 months (range, 31.2 to 97.3 months) for patients with active AML. Estimates for OS and PFS and cumulative incidences of relapse and NRM were similar for patients in MRD-positive remission and in those with active AML and substantially inferior to those for patients in MRD-negative remission (Fig 2 and Table 2). Specifically, 3-year OS and PFS estimates were 26% (17% to 37%) and 12% (5% to 21%), respectively, for patients in MRD-positive remission, 23% (12% to 35%) and 13% (5% to 23%), respectively, for patients with active AML, and 73% (66% to 78%) and 67% (61% to 73%), respectively, for patients in MRD-negative remission. These differences were largely a result of differences in the cumulative risk of relapse, estimated at 67% (55% to 77%) for patients in MRD-positive remission and 65% (49% to 76%) for patients with active AML, but 22% (16% to 28%) for patients in MRD-negative remission. There was also an increased risk of NRM for patients with evidence of AML at the time of HCT, with 3-year estimates for NRM of 21% (13% to 31%) for patients in MRD-positive remission and 23% (12% to 35%) for patients with active AML compared with 11% (7% to 15%) for patients in MRD-negative remission.
Fig 2.
Association between pretransplant disease status and outcome for patients with acute myeloid leukemia (AML) after myeloablative hematopoietic cell transplantation (HCT). Estimates of (A) overall survival, (B) progression-free survival, (C) cumulative incidence of relapse, and (D) cumulative incidence of nonrelapse mortality (NRM) after myeloablative allogeneic HCT for adults with AML, shown individually for patients in minimal residual disease (MRD) –negative (n = 235) and MRD-positive (n = 76) morphologic remission as well as those with active AML (n = 48).
We fit regression models to evaluate OS, PFS, relapse, and NRM according to whether patients were in MRD-negative remission or MRD-positive remission, or had active AML or four levels of disease burden on the basis of log-increases in the percentage of abnormal bone marrow blasts as assessed by MFC (MRD negative v > 0 but < 0.5%, ≥ 0.5 but < 5%, and ≥ 5%). Table 3 summarizes the results from univariable models for these covariates and other factors. Being in MRD-negative remission at the time of HCT was significantly associated with better OS and PFS and with lower risk of relapse compared with patients in MRD-positive remission or those with active AML (all P < .001). Among patients with MFC evidence of leukemia, we found no statistically significant differences in outcomes when stratified on the basis of MRD levels (< 0.5% v 0.5% to 5% v > 5% abnormal blasts). A sensitivity analysis using different cut points, including < 0.1%, 0.1% to 1%, and > 1% abnormal blasts, was performed with similar findings.
Table 3.
Univariable Regression Models for Relationship Between Individual Covariates and Post-HCT Outcome
| Regression Model | No. of Patients | Overall Mortality | Failure for PFS | Relapse | NRM | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| Disease status | |||||||||
| MRD-negative remission | 235 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| MRD-positive remission | 76 | 4.06 (2.86 to 5.75) | < .001 | 4.62 (3.31 to 6.44) | < .001 | 4.16 (2.68 to 6.44) | < .001 | 1.72 (0.93 to 3.17) | .084 |
| Active disease | 48 | 5.20 (3.50 to 7.72) | < .001 | 6.09 (4.17 to 8.88) | < .001 | 4.86 (2.49 to 9.49) | < .001 | 1.37 (0.52 to 3.65) | .530 |
| Percentage of abnormal blasts | |||||||||
| 0% | 235 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| > 0% but < 0.5% | 37 | 3.43 (2.22 to 5.31) | < .001 | 3.75 (2.47 to 5.70) | < .001 | 3.72 (2.25 to 6.15) | < .001 | 1.47 (0.65 to 3.34) | .360 |
| ≥ 0.5% but < 5% | 38 | 5.65 (3.70 to 8.61) | < .001 | 6.06 (4.03 to 9.09) | < .001 | 4.93 (2.87 to 8.47) | < .001 | 1.89 (0.83 to 4.34) | .130 |
| ≥ 5% | 49 | 4.65 (3.12 to 6.93) | < .001 | 5.97 (4.09 to 8.71) | < .001 | 4.57 (2.40 to 8.70) | < .001 | 1.52 (0.59 to 3.92) | .390 |
| Age (per 10 years) | 1.24 (1.09 to 1.42) | .001 | 1.14 (1.01 to 1.28) | .031 | 0.87 (0.75 to 1.00) | .047 | 1.27 (0.98 to 1.65) | .069 | |
| Cytogenetic risk group | |||||||||
| Intermediate/favorable | 264 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Adverse | 89 | 1.50 (1.08 to 2.09) | .016 | 1.59 (1.16 to 2.18) | .004 | 1.19 (0.77 to 1.85) | .440 | 0.66 (0.34 to 1.31) | .240 |
| Type of AML | |||||||||
| De novo | 144 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Secondary | 97 | 1.54 (1.12 to 2.10) | .007 | 1.45 (1.08 to 1.96) | .014 | 0.92 (0.62 to 1.36) | .670 | 1.04 (0.54 to 1.95) | .880 |
| Pre-HCT karyotype | |||||||||
| Normalized | 132 | 1 (reference) | < .001 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Not normalized | 85 | 3.16 (2.16 to 4.62) | 3.30 (2.30 to 4.73) | .040 | 1.26 (0.75 to 2.10) | .390 | 1.43 (0.66 to 3.10) | .360 | |
| Pre-HCT blood counts* | |||||||||
| Recovered | 258 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Not recovered | 101 | 1.85 (1.35 to 2.54) | < .001 | 1.93 (1.43 to 2.59) | < .001 | 0.97 (0.60 to 1.56) | .910 | 1.08 (0.58 to 2.22) | .810 |
NOTE. No. of events: overall mortality, n = 168 deaths; failure for PFS, n = 189 events; relapse, n = 132 relapses/disease progressions; NRM, n = 57 deaths without prior experience of relapse/disease progression.
Abbreviations: AML, acute myeloid leukemia; HCT, hematopoietic cell transplantation; HR, hazard ratio; MRD, minimal residual disease; neg, negative; NRM, nonrelapse mortality; PFS, progression-free survival; pos, positive.
Recovered: absolute neutrophil count ≥ 1,000/µL and platelets ≥ 100,000/µL; not recovered: absolute neutrophil count < 1,000/µL and/or platelets < 100,000/µL.
To assess the effect of disease status (MRD-negative remission v MRD-positive remission v active AML) after accounting for the covariates noted in Patients and Methods, we developed multivariable models for OS, PFS, relapse, and NRM. These models indicated that, independent of these covariates, MRD-negative remission was associated with the best outcomes (longer OS and PFS as well as lower risk of relapse), whereas MRD-positive remission and active AML were similar in their association with poorer outcomes (Table 4). There were no significant associations between disease status and NRM. In models in which the disease status was categorized on the basis of the percentage of MFC-quantified abnormal bone marrow blasts, the hazards for overall mortality and failure for PFS, relapse, and NRM were very similar between patients presenting with greater than 0 but < 0.5%, ≥ 0.5 but < 5%, or ≥ 5% abnormal blasts (Appendix Table A1). Again, a sensitivity analysis using different cut points (including < 0.1%, 0.1% to 1%, and > 1%) was performed with comparable findings.
Table 4.
Multivariable Regression Models for Disease Status
| Regression Model | No. of Patients | Overall Mortality | Failure for PFS | Relapse | NRM | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| Disease status | |||||||||
| MRD-negative remission | 235 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| MRD-positive remission | 76 | 3.69 (2.51 to 5.42) | < .001 | 4.38 (3.04 to 6.33) | < .001 | 4.17 (2.69 to 6.47) | < .001 | 1.72 (0.93 to 3.18) | .083 |
| Active disease | 48 | 4.40 (2.56 to 7.55) | < .001 | 5.30 (3.18 to 8.81) | < .001 | 4.87 (2.50 to 9.72) | < .001 | 1.37 (0.52 to 3.65) | .530 |
| Age (per 10 years) | 1.12 (0.98 to 1.29) | .089 | 0.98 (0.87 to 1.10) | .704 | 0.87 (0.75 to 1.00) | .050 | 1.27 (0.99 to 1.65) | .065 | |
| Cytogenetic risk group | |||||||||
| Intermediate/favorable | 264 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Adverse | 89 | 0.90 (0.61 to 1.32) | .583 | 1.00 (0.70 to 1.45) | .979 | 1.19 (0.77 to 1.86) | .430 | 0.66 (0.34 to 1.32) | .240 |
| Type of AML | |||||||||
| De novo | 144 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Secondary | 97 | 1.01 (0.73 to 1.41) | .953 | 0.99 (0.72 to 1.35) | .945 | 0.91 (0.62 to 1.35) | .640 | 1.03 (0.55 to 1.93) | .880 |
| Pre-HCT karyotype | |||||||||
| Normalized | 132 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Not normalized | 85 | 1.48 (0.94 to 2.33) | .088 | 1.58 (1.02 to 2.43) | .039 | 1.26 (0.75 to 2.10) | .380 | 1.43 (0.70 to 3.12) | .360 |
| Pre-HCT blood counts* | |||||||||
| Recovered | 258 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Not recovered | 101 | 0.87 (0.59 to 1.29) | .490 | 0.86 (0.59 to 1.24) | .418 | 0.97 (0.61 to 1.57) | .910 | 1.08 (0.58 to 2.03) | .800 |
NOTE. No. of events: overall mortality, n = 168 deaths; failure for PFS, n = 189 events; relapse, n = 132 relapses/disease progressions; NRM, n = 57 deaths without prior experience of relapse/disease progression.
Abbreviations: AML, acute myeloid leukemia; HCT, hematopoietic cell transplantation; HR, hazard ratio; MRD, minimal residual disease; neg, negative; NRM, nonrelapse mortality; PFS, progression-free survival; pos, positive.
Recovered: absolute neutrophil count ≥ 1,000/µL and platelets ≥ 100,000/µL; not recovered: absolute neutrophil count < 1,000/µL and/or platelets < 100,000/µL
DISCUSSION
Data from this report support three major conclusions. First, as shown previously,9-11 patients with AML who are in MRDneg remission have favorable outcomes, with 3-year OS estimates exceeding 70% and a cumulative risk of relapse of no more than 20% to 25% after myeloablative HCT. Patients undergoing HCT while in morphologic remission with MFC-detectable MRD have a substantially increased relapse risk, approaching 65% to 70% after 3 years, and 3-year OS estimates of only approximately 25%, with MRD being the dominant risk factor for adverse outcome. Second, in line with several previous studies,1,2,4 a small but significant subset of patients with active leukemia at the time of transplantation can achieve long-term disease control with myeloablative conditioning. Third, we now show that outcomes for adults with morphologically detectable disease who have undergone transplantation closely resemble those for patients in MRD-positive remission, with a cumulative relapse risk of approximately 65% and survival estimates of 20% to 25% at 3 years. This similarity remained after accounting for other prognostic covariates. These data provide further evidence for the clinical relevance of MRD and indicate that an MRD-based definition of remission would lead to a better estimate of expected treatment outcomes. Such information would not only be of prognostic value but could facilitate future clinical trials. For example, patients with active AML could be combined with patients in MRD-positive remission who have a similarly high disease risk in trials aimed primarily at reducing the risk of post-transplant relapse. In contrast, patients in MRD-negative remission would be suitable for prospective, controlled studies evaluating whether lower-intensity allogeneic HCT or other treatment modalities, such as autologous HCT or non-HCT therapies, could further improve outcomes in these lower-risk patients.
There remains debate about which patient with AML should undergo allogeneic HCT. By highlighting differences in outcomes between patients in MRD-positive and MRD-negative morphologic remission at the time of HCT, many studies have pointed to MRD as a potential marker to guide treatment decisions. Indeed, emerging data from patients with core-binding factor leukemias suggest that adding MRD measurements to the disease response assessment may optimize long-term outcomes.18-20 Additional studies, however, are required to further validate this concept and to test whether it could apply to broader subsets of patients.
A particular strength of our analyses is that a bone marrow assessment with flow cytometric analysis of MRD is considered a standard of care at our institution during pretransplant work-up; therefore, our study includes all adults with AML who underwent myeloablative allogeneic HCT at our institution over an 8.5-year period. During that period, patients with AML were routinely assigned to myeloablative conditioning unless significant comorbidities were present or the patients were enrolled onto randomized studies comparing conditioning intensities. The presence of MRD, although perceived as a marker for increased risk of disease recurrence after transplantation, typically played no major role in choosing the type of preparative regimen. We would generally not perform transplantations in patients with active disease who are receiving reduced intensity or nonmyeloablative regimens; however, preparative regimens that were preferentially used for patients with AML in MRD-positive remission differed considerably from those used for patients with active AML. Whereas the latter group was most frequently administered radiolabeled antibodies or high-dose, total body irradiation, the former group was more frequently given a high-dose, busulfan-containing regimen. It is conceivable that such differences could lead to different degrees of antileukemic control, but better-controlled studies are required to test this possibility. Therefore, our results cannot be taken to demonstrate that post-transplant outcomes for patients in MRD-positive remission and for patients with active AML are identical. Another important limitation is that the number of patients with active AML who underwent transplantation was relatively small and somewhat heterogeneous, and it is therefore likely that these patients are not representative of all patients with active disease. Combining these patients into one category is an oversimplification, and our findings should not be used to suggest that all patients with active AML have similar post-transplant outcomes. However, at the minimum, our data indicate that the outcomes for patients in MRD-positive morphologic remission and for patients with active AML are similar and quite distinct from those results for patients in MRD-negative morphologic remission.
Several groups have proposed that, instead of using the technical detection limit of the MRD assay as threshold, different thresholds above the minimal detection limit may be optimal cutoffs for the best segregation of patients into categories of post-HCT relapse risk.6,7 Consistent with our previous analysis,10 our current study indicates that even patients with the lowest detectable amount of MRD have significantly worse outcomes than patients without MRD. Although the outcomes for patients with the lowest amount of MRD (< 0.5% in the current study) were slightly better than those for patients with higher amounts of MRD, there was no statistically significant evidence that increasing levels of MRD were associated with increasing risk of any outcome. It is possible that a larger cohort study could yield statistically significant outcome differences between patients with various levels of MFC-quantifiable residual leukemia. Nonetheless, MRD-positive patients, regardless of the level of MRD, are more similar to each other than are MRD-negative patients to MRD-positive patients with the lowest detectable levels of MRD—an observation that supports our approach of using the MRD assay detection limit as a threshold to distinguish MRD-negative patients from MRD-positive patients.
The goal of AML therapy has long been to achieve CR on the basis of the assumption that patients in CR live longer.21,22 CR requires both normal blood counts and < 5% marrow blasts by light microscopy.14,15 Subsequently, many studies have demonstrated the value of MRD, detected either molecularly or by MFC, as a biomarker for the intrinsic resistance of leukemia to therapy and as a predictor for an increased risk of relapse and worse outcome after chemotherapy as well as after autologous and allogeneic transplantation.6-8,23-25 Is it time to move toward an MRD-based definition of CR? We believe so. Our data show similar post-HCT outcomes for patients in MRD-positive morphologic remission and for patients with active disease that are distinctly worse than outcomes for patients in MRD-negative remission before transplantation. This suggests that decision algorithms that are based on the classic morphologic remission definition are not ideal. Our data support treatment algorithms that use MRD-based (ie, patients in MRD-negative CR v all other patients), rather than morphology-based, disease assessments.
Acknowledgment
We thank the physicians and nurses of the hematopoietic cell transplantation teams, the staff in the Long Term Follow-up Program at the Fred Hutchinson Cancer Research Center, the Hematopathology Laboratory at the University of Washington, and our patients for assisting with our research protocols.
Appendix
Fig A1.
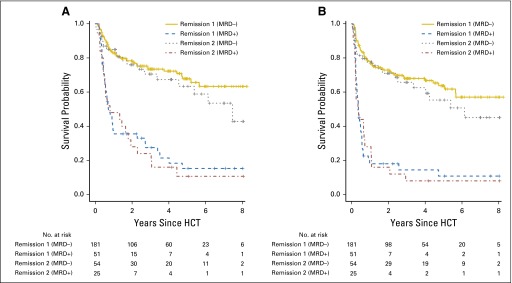
Association between pretransplant disease status and outcome for patients with acute myeloid leukemia (AML) undergoing myeloablative hematopoietic cell transplantation (HCT) while in morphologic remission. Estimates of (A) overall survival and (B) progression-free survival after myeloablative allogeneic HCT for adults with AML, shown individually for patients in minimal residual disease (MRD)–negative remission 1 (n = 181), MRD-negative remission 2 (n = 54), MRD-positive remission 1 (n = 51), and MRD-positive remission 2 (n = 25). neg, negative; pos, positive.
Fig A2.
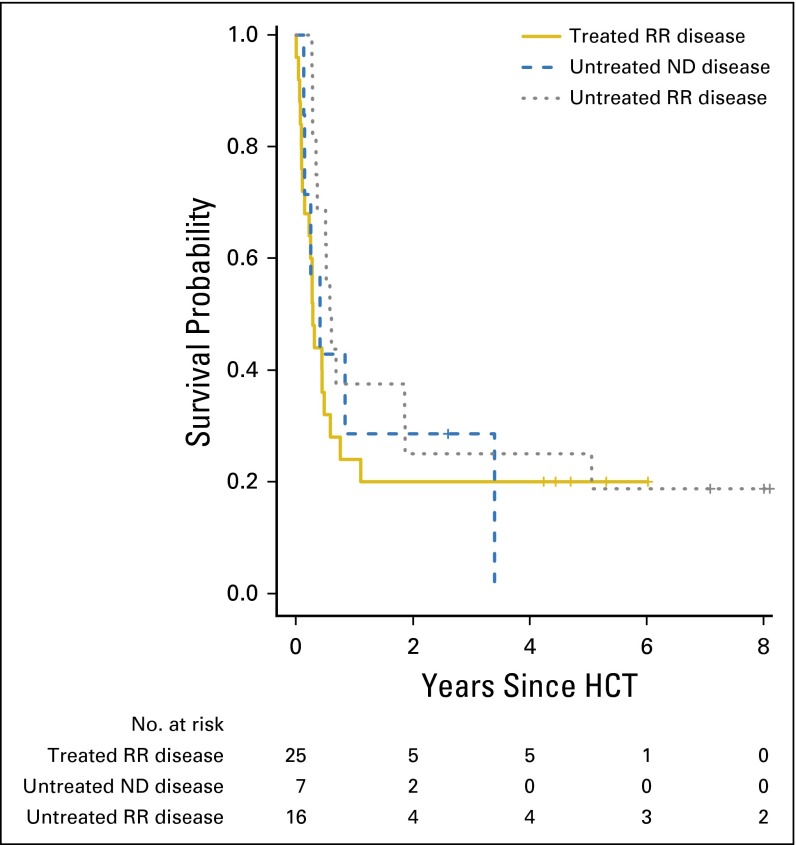
Association between pretransplant treatment history and outcome for patients with acute myeloid leukemia (AML) undergoing myeloablative hematopoietic cell transplantation (HCT) while having active disease. Estimate of overall survival after myeloablative allogeneic HCT for adults with AML, shown individually for patients with untreated newly diagnosed (ND) disease (n = 7), patients with relapsed AML who did not undergo pre-HCT salvage chemotherapy (n = 16), and patients who experienced failure of salvage chemotherapy for either relapsed or refractory (RR) AML (n = 25).
Table A1.
Multivariable Regression Models for the Percentage of Abnormal Bone Marrow Blasts
| Regression Model | No. of Patients | Overall Mortality | Failure for PFS | Relapse | NRM | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| Percentage of abnormal blasts | |||||||||
| 0% | 235 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| > 0% but < 0.5% | 37 | 3.17 (1.99 to 5.05) | < .001 | 3.61 (2.32 to 5.63) | < .001 | 3.74 (2.26 to 6.19) | < .001 | 1.47 (0.65 to 3.35) | .360 |
| ≥ 0.5% but < 5% | 38 | 4.75 (2.98 to 7.59) | < .001 | 5.71 (3.65 to 8.95) | < .001 | 4.95 (2.88 to 8.51) | < .001 | 1.90 (0.83 to 4.35) | .130 |
| ≥ 5% | 49 | 3.83 (2.25 to 6.52) | < .001 | 5.18 (3.12 to 8.60) | < .001 | 4.58 (2.40 to 8.72) | < .001 | 1.52 (0.59 to 3.92) | |
| Age (per 10 years) | 1.12 (0.98 to 1.28) | .107 | 0.97 (0.86 to 1.09) | .618 | 0.86 (0.74 to 0.99) | .040 | 1.27 (0.98 to 1.64) | .070 | |
| Cytogenetic risk group | |||||||||
| Intermediate/favorable | 264 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Adverse | 89 | 0.96 (0.64 to 1.44) | .841 | 1.03 (0.71 to 1.50) | .873 | 1.21 (0.77 to 1.88) | .410 | 0.69 (0.40 to 1.41) | .310 |
| Type of AML | |||||||||
| De novo | 144 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Secondary | 97 | 1.01 (0.73 to 1.41) | .947 | 0.99 (0.73 to 1.36) | .971 | 0.92 (0.62 to 1.35) | .660 | 1.02 (0.54 to 1.93) | .940 |
| Pre-HCT karyotype | |||||||||
| Normalized | 132 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Not normalized | 85 | 1.43 (0.90 to 2.27) | .134 | 1.50 (0.96 to 2.34) | .073 | 1.25 (0.74 to 2.11) | .400 | 1.34 (0.58 to 3.09) | .500 |
| Pre-HCT blood counts* | |||||||||
| Recovered | 258 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Not recovered | 101 | 0.94 (0.64 to 1.36) | .730 | 0.88 (0.62 to 1.27) | .503 | 1.01 (0.64 to 1.59) | .960 | 1.06 (0.58 to 1.94) | .860 |
NOTE. No. of events: overall mortality, n = 168 deaths; failure for PFS, n = 189 events; relapse, n = 132 relapses/disease progressions; NRM, n = 57 deaths without prior experience of relapse/disease progression.
Abbreviations: AML, acute myeloid leukemia; HCT, hematopoietic cell transplantation; HR, hazard ratio; NRM, nonrelapse mortality; PFS, progression-free survival.
Recovered: absolute neutrophil count ≥ 1,000/µL and platelets ≥ 100,000/µL; not recovered: absolute neutrophil count < 1,000/µL and/or platelets < 100,000/µL
Footnotes
See accompanying editorial on page 300
Supported by training Grant No. T32-HL007093 from the National Heart, Lung, and Blood Institute of the National Institutes of Health (A.B.H.). R.B.W. is a Leukemia & Lymphoma Society Scholar in Clinical Research.
Presented in part at the 2015 Society of Hematologic Oncology Annual Meeting, Houston, TX, September 16-19, 2015, and the 2015 Annual Meeting of the American Society of Hematology, Orlando, FL, December 5-8, 2015.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Frederick R. Appelbaum, Roland B. Walter
Provision of study materials or patients: Brent L. Wood, Jerald P. Radich, Marco Mielcarek, Frederick R. Appelbaum
Collection and assembly of data: Daisuke Araki, Anna B. Halpern, Yi Zhou, Roland B. Walter
Data analysis and interpretation: Brent L. Wood, Megan Othus, Jerald P. Radich, Marco Mielcarek, Elihu H. Estey, Frederick R. Appelbaum, Roland B. Walter
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia: Time to Move Toward a Minimal Residual Disease–Based Definition of Complete Remission?
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Daisuke Araki
No relationship to disclose
Brent L. Wood
Honoraria: Seattle Genetics, Abbott/AbbVie
Megan Othus
No relationship to disclose
Jerald P. Radich
No relationship to disclose
Anna B. Halpern
No relationship to disclose
Yi Zhou
No relationship to disclose
Marco Mielcarek
No relationship to disclose
Elihu H. Estey
No relationship to disclose
Frederick R. Appelbaum
Honoraria: Amgen, Celator, National Marrow Donor Program, Neumedicines
Consulting or Advisory Role: Amgen, National Marrow Donor Program, Neumedicines, Pfizer
Roland B. Walter
No relationship to disclose
REFERENCES
- 1.Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood. 2011;117:2307–2318. doi: 10.1182/blood-2010-10-265603. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton BK, Copelan EA. Concise review: The role of hematopoietic stem cell transplantation in the treatment of acute myeloid leukemia. Stem Cells. 2012;30:1581–1586. doi: 10.1002/stem.1140. [DOI] [PubMed] [Google Scholar]
- 3.Cornelissen JJ, Gratwohl A, Schlenk RF, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: An integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9:579–590. doi: 10.1038/nrclinonc.2012.150. [DOI] [PubMed] [Google Scholar]
- 4.Kanate AS, Pasquini MC, Hari PN, et al. Allogeneic hematopoietic cell transplant for acute myeloid leukemia: Current state in 2013 and future directions. World J Stem Cells. 2014;6:69–81. doi: 10.4252/wjsc.v6.i2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vyas P, Appelbaum FR, Craddock C. Reprint of: Allogeneic hematopoietic cell transplantation for acute myeloid leukemia. Biol Blood Marrow Transplant. 2015;21:S3–S10. doi: 10.1016/j.bbmt.2014.12.032. 2015 (suppl 2) [DOI] [PubMed] [Google Scholar]
- 6.Buccisano F, Maurillo L, Del Principe MI, et al. Prognostic and therapeutic implications of minimal residual disease detection in acute myeloid leukemia. Blood. 2012;119:332–341. doi: 10.1182/blood-2011-08-363291. [DOI] [PubMed] [Google Scholar]
- 7.Buckley SA, Appelbaum FR, Walter RB. Prognostic and therapeutic implications of minimal residual disease at the time of transplantation in acute leukemia. Bone Marrow Transplant. 2013;48:630–641. doi: 10.1038/bmt.2012.139. [DOI] [PubMed] [Google Scholar]
- 8.Campana D, Leung W. Clinical significance of minimal residual disease in patients with acute leukaemia undergoing haematopoietic stem cell transplantation. Br J Haematol. 2013;162:147–161. doi: 10.1111/bjh.12358. [DOI] [PubMed] [Google Scholar]
- 9.Walter RB, Gooley TA, Wood BL, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29:1190–1197. doi: 10.1200/JCO.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter RB, Buckley SA, Pagel JM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122:1813–1821. doi: 10.1182/blood-2013-06-506725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter RB, Gyurkocza B, Storer BE, et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2015;29:137–144. doi: 10.1038/leu.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 13.Grimwade D, Hills RK, Moorman AV, et al. National Cancer Research Institute Adult Leukaemia Working Group Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Bennett JM, Kopecky KJ, et al. International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Döhner H, Estey EH, Amadori S, et al. European LeukemiaNet Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 17.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Zhu HH, Zhang XH, Qin YZ, et al. MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: Results from the AML05 multicenter trial. Blood. 2013;121:4056–4062. doi: 10.1182/blood-2012-11-468348. [DOI] [PubMed] [Google Scholar]
- 19.Qin YZ, Xu LP, Chen H, et al. Allogeneic stem cell transplant may improve the outcome of adult patients with inv(16) acute myeloid leukemia in first complete remission with poor molecular responses to chemotherapy. Leuk Lymphoma. 2015:1–8. doi: 10.3109/10428194.2015.1032964. [DOI] [PubMed] [Google Scholar]
- 20.Kayser S, Schlenk RF, Grimwade D, et al. Minimal residual disease-directed therapy in acute myeloid leukemia. Blood. 2015;125:2331–2335. doi: 10.1182/blood-2014-11-578815. [DOI] [PubMed] [Google Scholar]
- 21.Freireich EJ, Gehan EA, Sulman D, et al. The effect of chemotherapy on acute leukemia in the human. J Chronic Dis. 1961;14:593–608. doi: 10.1016/0021-9681(61)90118-7. [DOI] [PubMed] [Google Scholar]
- 22.Walter RB, Kantarjian HM, Huang X, et al. Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: A combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M.D. Anderson Cancer Center Study. J Clin Oncol. 2010;28:1766–1771. doi: 10.1200/JCO.2009.25.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiNardo CD, Luger SM. Beyond morphology: Minimal residual disease detection in acute myeloid leukemia. Curr Opin Hematol. 2012;19:82–88. doi: 10.1097/MOH.0b013e3283501325. [DOI] [PubMed] [Google Scholar]
- 24.Ravandi F, Jorgensen JL. Monitoring minimal residual disease in acute myeloid leukemia: Ready for prime time? J Natl Compr Canc Netw. 2012;10:1029–1036. doi: 10.6004/jnccn.2012.0105. [DOI] [PubMed] [Google Scholar]
- 25.Paietta E. Minimal residual disease in acute myeloid leukemia: Coming of age. Hematology (Am Soc Hematol Educ Program) 2012;2012:35–42. doi: 10.1182/asheducation-2012.1.35. [DOI] [PubMed] [Google Scholar]