Abstract
IMPORTANCE
Conclusive intraoperative pathologic confirmation of diffuse infiltrative glioma guides the decision to pursue definitive neurosurgical resection. Establishing the intraoperative diagnosis by histologic analysis can be difficult in low-cellularity infiltrative gliomas. Therefore, we developed a rapid and sensitive genotyping assay to detect somatic single-nucleotide variants in the telomerase reverse transcriptase (TERT) promoter and isocitrate dehydrogenase 1 (IDH1).
OBSERVATIONS
This assay was applied to tissue samples from 190 patients with diffuse gliomas, including archived fixed and frozen specimens and tissue obtained intraoperatively. Results demonstrated 96% sensitivity (95% CI, 90%–99%) and 100% specificity (95% CI, 95%–100%) for World Health Organization grades II and III gliomas. In a series of live cases, glioma-defining mutations could be identified within 60 minutes, which could facilitate the diagnosis in an intraoperative timeframe.
CONCLUSIONS AND RELEVANCE
The genotyping method described herein can establish the diagnosis of low-cellularity tumors like glioma and could be adapted to the point-of-care diagnosis of other lesions that are similarly defined by highly recurrent somatic mutations.
World Health Organization (WHO) grade II diffuse gliomas are a group of slow-growing primary central nervous system tumors1 for which progression-free and overall patient survival correlate with maximal up-front resection.2,3 As a consequence of tumor location, infiltrative growth, low cellularity, and often small stereotactic biopsy specimens, intraoperative histologic confirmation of diffuse glioma can be challenging.4 This diagnostic dilemma often involves multiple surgical samplings or clarification by final pathologic analysis several days after the diagnostic biopsy, requiring some patients to undergo a second neurosurgical procedure to achieve therapeutic resection. The ability to confirm a lesion as glioma during surgery could therefore facilitate intraoperative neurosurgical decisions with the potential to decrease risk from additional biopsy samplings or staged resections. We therefore developed a platform to simultaneously genotype the highly recurrent variants in IDH1 and the TERT promoter, which characterize over 80% of gliomas,5 within 60 minutes of tissue acquisition.
Methods
Tumor Specimens
Case records were reviewed, and glioma specimens were obtained under approval of the institutional review boards at Dana-Farber Cancer Institute and Massachusetts General Hospital. Samples were collected following informed, written consent. The records were reviewed under a protocol that allowed analysis of anonymized patient data collected following informed, written consent. There was no sex preference when obtaining specimens. For specimens with available sex information, the cohort was made up of 49% male and 51% female patients.
Rapid Genotyping Assay
We designed a quantitative polymerase chain reaction (PCR)-based method to detect cancer-specific mutations in specimens with low tumor density through (1) the inclusion of peptide nucleic acid (PNA) oligonucleotides that block amplification of wild-type alleles and (2) the incorporation of locked nucleic acid (LNA) into the detection probes to increase specific binding to the mutant allele (eMethods and eFigures 1 and 2 in the Supplement).
Specifically, this assay was designed to detect IDH1 R132H/C/G/L/S and TERT promoter mutations on chromosome 5 at positions 1 295 228 and 1 295 250 based on human genome reference version 19, referred to as TERT C228T or TERT C250T (eTable 1 in the Supplement). We could detect serial dilutions representing 0.1% to 10.0% of positive control genomic extracts diluted in negative control extracts, allowing for estimation of tumor purity (eFigures 3 and 4 in the Supplement).
Statistical Analysis
A 2-tailed, nonpaired t test was used to calculate the significance of the difference between the number of biopsy attempts required of diagnostic and nondiagnostic cases, with the estimated half-width of the 95% CI calculated using a t test distribution in Microsoft Excel. The 95% CIs for the reported sensitivity and specificity of the assay were calculated using the binomial test in the R statistical package (version 0.98.1091).
Results
To evaluate how intraoperative histologic analysis of low-cellularity tumors affects surgical decision making, we reviewed 72 cases of newly diagnosed WHO grade II diffuse gliomas treated at Brigham and Women’s Hospital from 2009 through 2014. In this series, biopsy specimens obtained in 28 (39%) of 72 cases could not be conclusively diagnosed as glioma on the intraoperative frozen specimen (eFigure 5 in the Supplement). These inconclusive stereotactic biopsies required additional surgical sampling compared with cases with diagnostic biopsies (mean [SD] number of biopsies for conclusive glioma diagnosis, 3.08 [1.20] vs 1.13 [0.30]; P = .01) (eTable 3 in the Supplement). Because of the anticipated difficulty in establishing an intraoperative glioma diagnosis, surgeons may elect to perform a stereotactic biopsy before proceeding with a definitive resection in a separate procedure following final histologic confirmation.
To augment intraoperative diagnosis, we developed an assay, OperaGen (for “operative genotyping”) to simultaneously genotype IDH1 and TERT promoter variants. We first evaluated OperaGen across a range of clinically annotated gliomas by comparing its molecular characterization with the histologic diagnosis of 80 archived formalin-fixed, paraffin-embedded glioma samples. OperaGen was able to detect every tumor with an IDH1 R132H mutation (58 of 58 samples, Figure 1A). Concurrent IDH1 and TERT promoter mutations detected by OperaGen accurately identified oligodendrogliomas in 38 (95%) of 40 cases with known 1p/19q codeletion.5 In addition, isolated TERT promoter mutations were detected in 17 (77%) of 22 glioblastomas (GBMs), a frequency consistent with prior reports.6 The TERT promoter status was confirmed by next-generation sequencing (NGS) with coverage greater than 180 000× (eFigure 6 in the Supplement). In addition, patient survival correlated with a retrospective analysis of IDH1 and TERT promoter genotype status by OperaGen (eFigure 7 in the Supplement).
Figure 1. Operative Genotyping (OperaGen) for Molecular Characterization of Glioma.
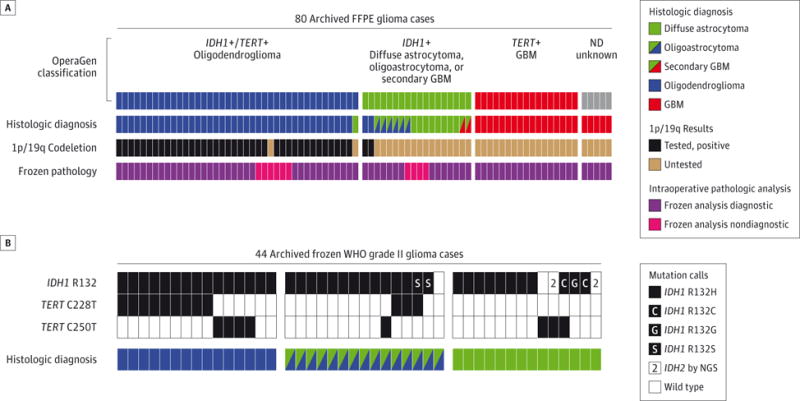
A, The top row of segmented colored bars represents detection of mutations in IDH1 and TERT by OperaGen in 80 archived glioma cases (1 case for each bar segment), with variants not detected (ND) in 5 specimens (gray segments). The second row represents final conventional neuropathologic diagnosis of each case, demonstrating concordance with OperaGen. The third row represents the clinical assessment of chromosome 1p/19p deletion by FISH or array CGH and is represented in black if the test result was positive and brown if it was untested. The bottom row illustrates that intraoperative frozen section analysis was diagnostic (purple) in 22 of 22 GBMs but inconclusive (pink) in 10 of 58 WHO grade II and III gliomas. B, Validation of OperaGen on archived frozen WHO grade II oligodendrogliomas, oligoastrocytomas, and astrocytomas revealed presence of IDH1 or TERT promoter mutations in 42 of 44 specimens. Five samples were noted to have noncanonical IDH1 mutations by specific VIC-conjugated oligonucleotide probes. Results were validated by targeted high-depth sequencing of these genes. CGH Indicates array comparative genomic hybridization; FFPE, formalin-fixed, paraffin-embedded; FISH, fluorescence in situ hybridization; GBM, glioblastoma; NGS, next-generation sequencing; WHO, World Health Organization.
We next evaluated OperaGen in a cohort of frozen WHO grade II diffuse gliomas to determine whether this assay could clarify intraoperative diagnosis in these lower-cellularity tumors. Because of the high frequency of IDH1 mutations in these glioma subtypes, we designed VIC-conjugated oligonucleotide detection probes to capture additional IDH1 mutations otherwise not detected by immunohistochemical analysis found in up to 12% of IDH1-mutated gliomas.7 When multiplexed with the fluorescein-conjugated IDH1 R132H probe, these reactions allowed for simultaneous detection of glioma-specific IDH1 variants, discriminating the less frequent mutations from IDH1 R132H (eFigure 8 in the Supplement). With this design we were able to detect either IDH1 or TERT promoter mutations in 42 (95%) of 44 frozen diffuse glioma specimens (Figure 1B).
To explore the possible false-positive rate associated with OperaGen, we analyzed 50 frozen GBM specimens (eFigure 9 in the Supplement) and validated the results by high-depth sequencing. Separately, 14 non-glioma brain biopsy specimens tested negative for IDH1 and TERT promoter variants by OperaGen (eTable 2 in the Supplement). Both specificity and sensitivity of diagnosis were high for oligodendrogliomas, diffuse astrocytomas, oligoastrocytomas, and IDH1-mutant GBMs, where the strong relationship to IDH1 mutation in this aggregated series of 174 cases revealed 96% sensitivity (95% CI, 90%–99%) and 100% specificity (95% CI, 95%–100%) (eFigure 9 in the Supplement). In contrast, sensitivity was lower for the diagnosis of GBM with isolated TERT promoter mutations, consistent with reported rates in this tumor type and likely a reflection of alternative mechanisms of telomere maintenance.8
We observed a higher rate of inconclusive intraoperative histology with stereotactic biopsies compared with resections (62% vs 23%, Figure 2A). Because of its sensitivity, we asked whether OperaGen could identify glioma-specific mutations in limited tissue obtained from stereotactic biopsies. We identified 14 cases of glioma for which intraoperative pathology was inconclusive in spite of repeated biopsy sampling (eTable 3 in the Supplement). In 93% of these cases (13 of 14), we were able to identify TERT promoter or IDH1 variants in the first available specimen (first or second biopsy core, Figure 2B). As illustrated by these results, OperaGen could help establish a glioma diagnosis while minimizing the number of biopsies and the risks associated with additional passes.9
Figure 2. Molecular Characterization of Stereotactic Biopsy Specimens Facilitates the Diagnosis of Glioma.
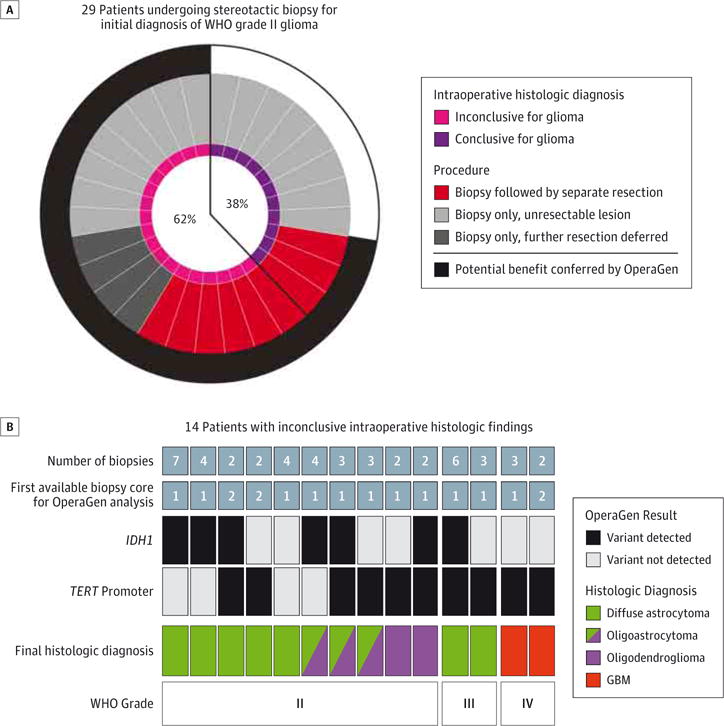
A, Analysis of WHO grade II gliomas newly diagnosed by stereotactic biopsy revealed that 18 of 29 cases had inconclusive intraoperative histologic findings (each radial circle segment represents 1 case). B, Tissue from 14 glioma cases with inconclusive intraoperative histologic findings were assessed by OperaGen. The total number of biopsy specimens obtained for each case is listed in the top row, while the first available stereotactic biopsy specimen used for OperaGen testing is listed in the second row. OperaGen detected either an IDH1 mutation (third row) or TERT promoter mutation (fourth row) in 13 of 14 cases. Final histologic diagnosis and WHO grade obtained by conventional neuropathologic analysis are listed in bottom 2 rows. GBM, glioblastoma; WHO, World Health Organization.
We next demonstrated that this assay could be carried out during glioma resection, ruling out the presence of 0.1% mutant allele fraction within 60 minutes (eFigures 3 and 10 in the Supplement). We present 2 cases to demonstrate how this workflow could be applied in a clinical setting. The first case illustrates the ability to detect TERT C228T in GBM (Figure 3A). The second patient had a frozen section diagnosis of GBM; however, OperaGen detected an IDH1 R132H mutation and no TERT promoter mutation, which raised the possibility of a WHO grade II or III glioma (Figure 3B). Indeed, the pathologic assessment from the permanent specimen ultimately established the tumor as an IDH1-mutant WHO grade III anaplastic oligoastrocytoma. While intraoperative histologic analysis confirmed the lesion as glioma, further molecular classification as an IDH1-mutant tumor could guide the decision to extend the surgical margin into adjacent nonenhancing tumor.2,3
Figure 3. Sensitive Detection of Glioma-Specific Somatic Variants Within an Intraoperative Timeframe Using Operative Genotyping (OperaGen).
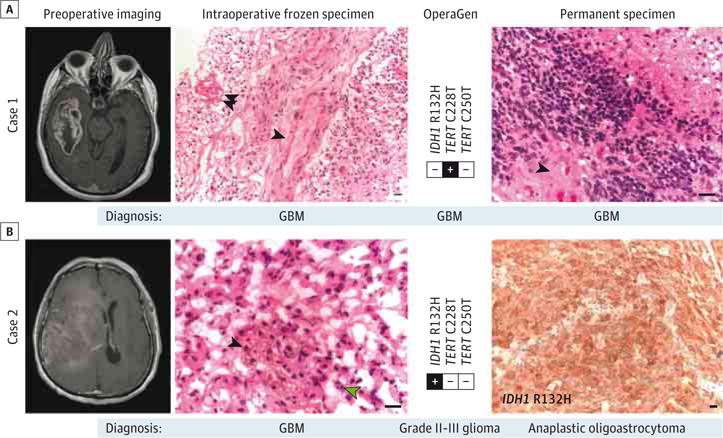
A, Case 1 demonstrates the detection of TERT promoter mutation from a specimen that was diagnosed as high-grade glioma on intraoperative frozen specimen analysis (single arrowhead indicating microvascular proliferation; double arrowhead, necrosis) and GBM on permanent specimen analysis (single arrowhead indicating microvascular proliferation). Both specimens stained with hematoxylin-eosin. B, Case 2 frozen specimen (hematoxylin-eosin) was diagnosed as GBM based on the presence of microvascular proliferation (single black arrowhead) and mitotic figures (single green arrowhead). The OperaGen detection of IDH1 R132H would alternatively have been consistent with WHO grade II or III glioma, which was in agreement with findings of the permanent specimen analysis of IDH1-mutant anaplastic oligoastrocytoma (immunohistochemically stained). All scale bars represent 10 μm. GBM indicates glioblastoma; WHO, World Health Organization.
Discussion
Histologic assessment combined with molecular testing can provide a more accurate integrated diagnosis of brain tumors.10 In accord, research techniques have applied real-time PCR detection of IDH1 mutation11 or mass spectrometry to the intraoperative detection of 2-hydroxyglutarate, the metabolite of mutant IDH1.12 OperaGen was designed to simultaneously detect distinguishing genomic features in both the TERT promoter and IDH1 to sensitively identify diffuse glioma in an intraoperative timeframe. Optimal surgical intervention is shaped by the glioma subtype, since unlike primary GBM, IDH1-mutant gliomas appear to benefit from complete resection of enhancing and nonenhancing disease.2,3
While alternative techniques such as NGS can also detect somatic variants in low-purity tumors, the equipment, time, and analytical methods required for NGS preclude widespread clinical use and intraoperative application. Herein we have demonstrated an approach that can identify tumor-specific alterations to a sensitivity of 0.1% mutant allele fraction within 60 minutes. We envision running this assay in parallel with frozen section analysis to augment cases where pathologic findings may be inconclusive. Though this testing may add time to these inconclusive cases, a definitive molecular diagnosis could reduce the instances of repeated biopsies, as highlighted in Figure 2, limiting the potential hazards associated with multiple sampling. Furthermore, the availability of intraoperative molecular information could obviate the need for staged craniotomies, thus reducing the risks associated with a second neurosurgical procedure. Before provisioning Opera-Gen for clinical use, larger cohort studies will be needed to address false-positive and false-negative rates resulting from issues related to tissue quality or surgical sampling error. The ability to obtain intraoperative molecular information also expands the possibility of administering direct intratumoral therapy or performing interstitial thermal ablation of glioma in a single procedure.13
Conclusions
The molecular genotyping approach described herein has potential application beyond the diagnosis of diffuse gliomas. TERT promoter mutations have been identified in a large number of other cancers5 and appear to distinguish tumor from non-neoplastic tissue. The principles of this assay could also be applied to other conditions associated with well-defined variants, such as KRAS, BRAF, and EGFR mutants to facilitate precise intraoperative marginal analysis.14,15 Ultimately, such translational genomic approaches are critical for improving point-of-care diagnosis in the era of precision cancer medicine.
Supplementary Material
At a Glance.
Intraoperative diagnosis of diffuse glioma is critical for distinguishing tumor from nonneoplastic mimics and defining the goals of neurosurgical resection.
Intraoperative genotyping for recurrent mutations in IDH1 and the TERT promoter could characterize over 80% of diffuse gliomas.
This assay was validated on 174 glioma specimens and was able to correctly identify WHO grades II and III glioma with 96% sensitivity (95%CI, 90%–99%) and 100% specificity (95%CI, 95%–100%).
Glioma-defining mutations were identified in 13 of 14 previously inconclusive intraoperative stereotactic biopsy samples.
It is possible to characterize glioma-defining mutations within 60 minutes during a live neurosurgical resection.
Acknowledgments
Funding/Support: Dr Shankar is supported by grant R25NS065743 from the National Institutes of Health (NIH) National Institutes of Neurologic Disorders and Stroke, the American Brain Tumor Association Basic Research Fellowship supported by the Humor to Fight the Tumor Event Committee. Dr Rinne is supported by a National Cancer Institute (NCI) K12 Training Fellowship and the Claudia Adams Barr Program in Basic Cancer Research. Dr Cahill is supported by the Burroughs Wellcome Career Award and the NIH-NCI Specialized Programs of Research Excellence (SPORE) grant P50CA165962.
Role of the Funder/Sponsor: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Matthew Meyerson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The following authors contributed equally to this work: Ganesh M. Shankar, MD, PhD, Joshua M. Francis, PhD, and Mikael L. Rinne, MD, PhD.
Study concept and design: Shankar, Francis, Rinne, Ramkissoon, Garraway, Brastianos, Ligon, Louis, Cahill, Meyerson.
Acquisition, analysis, or interpretation of data: Shankar, Francis, Rinne, Ramkissoon, Huang, Venteicher, Akama-Garren, Kang, Lelic, Kim, Brown, Charbonneau, Golby, Sekhar Pedamallu, Hoang, Sullivan, Cherniack, Stemmer-Rachamimov, Reardon, Wen, Brastianos, Curry, Barker, Hahn, Nahed, Ligon, Louis.
Drafting of the manuscript: Shankar, Francis, Rinne, Sekhar Pedamallu, Reardon, Brastianos, Nahed, Louis, Meyerson.
Critical revision of the manuscript for important intellectual content: Shankar, Francis, Rinne, Ramkissoon, Huang, Venteicher, Akama-Garren, Kang, Lelic, Kim, Brown, Charbonneau, Golby, Hoang, Sullivan, Cherniack, Garraway, Stemmer-Rachamimov, Reardon, Wen, Curry, Barker, Hahn, Ligon, Louis, Cahill, Meyerson.
Statistical analysis: Shankar, Francis, Rinne, Akama-Garren, Sekhar Pedamallu, Louis,
Obtained funding: Cahill.
Administrative, technical, or material support: Shankar, Francis, Rinne, Ramkissoon, Huang, Venteicher, Kang, Lelic, Kim, Brown, Charbonneau, Golby, Hoang, Sullivan, Stemmer-Rachamimov, Hahn, Nahed, Ligon, Cahill.
Study supervision: Ramkissoon, Golby, Reardon, Brastianos, Curry, Barker, Hahn, Louis, Cahill, Meyerson.
Review of results and analysis at several stages: Garraway.
Conflict of Interest Disclosures: Dr Rinne is a consultant for N-of-One Inc. Drs Cherniack and Meyerson have received a commercial research grant from Bayer. Dr Garraway received a commercial research grant from Novartis and is a consultant and advisory board member for Novartis, Foundation Medicine, and Boehringer Ingelheim; he also has equity interest in Foundation Medicine. Dr Reardon is an advisory board member for Abbvie, Cavion, Genentech/Roche, Merck, Midatech, Novartis, Stemline Therapeutics, Amgen and Momenta Pharmaceuticals, and is a speaker for Roche/Genentech and Merck. Dr Wen is an advisory board member for AbbVie, Cavion, Celldex, Cubist, Genentech/Roche, Midatech, Momenta, Novartis, Novocure, SigmaTau, and Vascular Biogenics and is a speaker for Merck. Dr Meyerson has ownership interest in and is a consultant and advisory board member for Foundation Medicine. A provisional patent application for this technology has been filed. No other disclosures are reported.
Additional Contributions: The authors thank the staff members of the Department of Pathology tissue banks of the Brigham and Women’s Hospital, the Dana-Farber Cancer Institute, and the Massachusetts General Hospital for their help and dedication.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. World Health Organization Histological Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer; 2007. [Google Scholar]
- 2.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 3.Beiko J, Suki D, Hess KR, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16(1):81–91. doi: 10.1093/neuonc/not159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol. 2001;3(3):193–200. doi: 10.1093/neuonc/3.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nonoguchi N, Ohta T, Oh J-E, Kim Y-H, Kleihues P, Ohgaki H. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol. 2013;126(6):931–937. doi: 10.1007/s00401-013-1163-0. [DOI] [PubMed] [Google Scholar]
- 7.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis JM, Zhang C-Z, Maire CL, et al. EGFR variant heterogeneity in glioblastoma resolved through single-nucleus sequencing. Cancer Discov. 2014;4(8):956–971. doi: 10.1158/2159-8290.CD-13-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGirt MJ, Woodworth GF, Coon AL, et al. Independent predictors of morbidity after image-guided stereotactic brain biopsy: a risk assessment of 270 cases. J Neurosurg. 2005;102(5):897–901. doi: 10.3171/jns.2005.102.5.0897. [DOI] [PubMed] [Google Scholar]
- 10.Louis DN, Perry A, Burger P, et al. International Society Of Neuropathology–Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24(5):429–435. doi: 10.1111/bpa.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanamori M, Kikuchi A, Watanabe M, et al. Rapid and sensitive intraoperative detection of mutations in the isocitrate dehydrogenase 1 and 2 genes during surgery for glioma. J Neurosurg. 2014;120(6):1288–1297. doi: 10.3171/2014.3.JNS131505. [DOI] [PubMed] [Google Scholar]
- 12.Santagata S, Eberlin LS, Norton I, et al. Intraoperative mass spectrometry mapping of an onco-metabolite to guide brain tumor surgery. Proc Natl Acad Sci U S A. 2014;111(30):11121–11126. doi: 10.1073/pnas.1404724111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sloan AE, Ahluwalia MS, Valerio-Pascua J, et al. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma: clinical article. J Neurosurg. 2013;118(6):1202–1219. doi: 10.3171/2013.1.JNS1291. [DOI] [PubMed] [Google Scholar]
- 14.Masasyesva BG, Tong BC, Brock MV, et al. Molecular margin analysis predicts local recurrence after sublobar resection of lung cancer. Int J Cancer. 2005;113(6):1022–1025. doi: 10.1002/ijc.20683. [DOI] [PubMed] [Google Scholar]
- 15.van Loo E, Mosterd K, Krekels GAM, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: A randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50(17):3011–3020. doi: 10.1016/j.ejca.2014.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


