Summary
Low-grade chronic inflammation underlies the development of the most dangerous cardiometabolic disorders including type 2 diabetes and its vascular complications. In contrast to acute inflammation induced by bacteria and viruses, chronic inflammation can be driven by abnormal reaction to endogenous factors, including Th2 cytokines, metabolic factors like advanced glycation end products (AGEs), modified lipoproteins, or hyperglycemia. The key innate immune cells that recognize these factors in blood circulation are monocytes. Inflammatory programming of monocytes which migrate into tissues can, in turn, result into generation of tissue macrophages with pathological functions. Therefore, determination of the molecular and functional phenotype of circulating monocytes is a very promising diagnostic tool for the identification of hidden inflammation, which can precede the development of the pathology. Here we propose a new test system for the identification of inflammatory programming of monocytes: surface biomarkers and ex vivo functional system. We summarize the current knowledge about surface biomarkers for monocyte subsets, including CD16, CCR2, CX3CR1, CD64, stabilin-1 and CD36, and their association with inflammatory human disorders. Furthermore, we present the design of an ex vivo monocyte-based test system with minimal set of parameters as a potential diagnostic tool for the identification of personalized inflammatory responses.
Keywords: Monocyte, Macrophage, Cytokine, Biomarker, Chitinase-like protein
Introduction
Low-grade chronic inflammation underlies the development of the most dangerous cardiometabolic disorders including type 2 diabetes and its vascular complications. A crucial role in the initiation of low-grade chronic inflammation is played by the immune cells of the adipose tissue [1]. In obesity the higher percentage of immune cells infiltrating the adipose tissue and their activation is associated with systemic inflammation, insulin resistance, and metabolic syndrome [2,3]. In type 2 diabetes additional inflammatory sites are found in muscle, liver and pancreas, with infiltrating immune cells secreting a range of pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6. These cytokines as well as C-reactive protein (CRP), fibrinogen, sialic acid, von Willebrand factor (vWF), D-dimer, and plasminogen activator inhibitor-1 (PAI-1) are also predictive of type 2 diabetes and its micro- and macrovascular complications [3,4].
Atherosclerosis is the most severe and life-threatening complication of diabetes which is characterized by the inflammation of the vascular wall and formation of atherosclerotic plaques caused by the infiltration and accumulation of lipid-laden macrophages [5,6]. Despite a long history of investigation of the inflammatory nature of atherosclerosis, the early events leading to the activation of endothelial cells and circulating monocytes are still questionable, and biomarkers for the initial stages of atherosclerosis are needed [5].
In contrast to the acute inflammatory reactions induced by bacteria and viruses, chronic inflammation can be driven by abnormal reaction to endogenous factors, including Th2 cytokines [7], metabolic factors like advanced glycation end products (AGEs) [8], modified lipoproteins [9], or hyperglycemia [10].
The key cells of the innate immune system that recognize these factors in blood circulation are monocytes. Inflammatory programming of monocytes which migrate into tissues can, in turn, result in a generation of tissue macrophages with pathological functions. Therefore, determination of the molecular and functional phenotype of circulating monocytes is a very promising diagnostic tool for the identification of hidden inflammation, which can precede the development of the pathology.
In this review we aim to summarize the current knowledge about surface biomarkers for monocyte subsets and present a design of an ex vivo monocyte-based test system with minimal set of parameters as a potential diagnostic tool for the identification of personalized inflammatory responses.
Monocyte Surface Biomarkers
The heterogeneity of circulating monocytes has been recognized for a long time, and various parameters can differ, including morphology, size, level of maturation, molecular profiles, and functional properties [11,12,13]. However, the primary parameter that is useful for clinical application and can be quantified by flow cytometry is the expression of surface biomarkers. The best known surface marker for the identification of subsets of human circulating CD14+ monocytes is CD16. Three major subpopulations of circulating monocytes can be distinguished according to the CD14 and CD16 ratio: CD14++ CD16- (classical), CD14++ CD16+ (intermediate), and CD14+ CD16++ (non-classical). The percentage of CD16+ monocytes is minor in healthy young individuals, not exceeding 5%, but is rising with age (reviewed in [14]).
CD16 (FcγR type III) had originally been described as an antigen that discriminates between natural killer (NK) subpopulations and was later found to be expressed on functionally distinct monocyte subsets (reviewed in [15]). Several studies suggested that CD16 can be an indicator for the specific inflammatory programming of monocytes. Thus, it was found that the population of CD16(+) monocytes is a major producer of inducible TNF in human blood, and the number of CD16(+) monocytes is increased during infections [16,17,18].
CD16+ monocytes are also increased in patients with metabolic disorders where low-grade inflammation is frequently found in fat tissue. Thus, the percentage of CD16+ monocytes positively correlates with the body mass index, insulin resistance/diabetes and intima-media-thickness, while weight loss after gastric bypass surgery in severely obese patients leads to a significant reduction of CD16+ monocytes [18,19].
There are also some indications that the amount of CD16+ is elevated in patients with familiar hypercholesterolemia (FH), a genetic disorder characterized by high cholesterol levels due to mutations in the LDLR gene which encodes the low-density lipoprotein (LDL) receptor that leads to early onset of atherosclerosis. A weak positive correlation of CD16+ monocytes with total plasma cholesterol and triglyceride levels was first described for FH patients with coronary heart disease [20]. It was recently demonstrated, that in patients with acute decompensated heart failure (ADHF) the admission proportion of CD14++ CD16- monocytes was lower, while the proportion of CD14++ CD16+ and CD14+ CD16++ monocytes was higher compared to healthy control individuals. Further, the proportion of CD14++ CD16- monocytes increased and the proportion of CD14+ CD16++ monocytes decreased between admission and discharge [21]. Therefore, the dynamics in the proportion of CD14++ CD16-, CD14++ CD16+ and CD14+ CD16++ monocyte subsets can be used as an indicator for resolution of an acute inflammatory state in patients with ADHF [21].
However, the reports about the increase of CD16+ monocytes in human inflammatory disorders are still controversial. And, since CD16 is expressed on a part of circulating monocytes in healthy individuals, it can also be considered as a differentiation marker for monocytes suggesting that CD14+ CD16+ monocytes are more mature than CD14+ CD16- ones [15].
The next surface marker that was proposed to distinguish between monocyte subsets is C-C chemokine receptor 2 (CCR2) (reviewed in [22]). CCR2 is a receptor for the major chemoattractant for monocytes, chemokine (C-C motif) ligand 2 (CCL2) / MCP-1, that is responsible for the monocyte recruitment to the sites of inflammation, including atherosclerotic plaques and tumors. Three monocyte subsets with distinct functions have been proposed based on the addition of CCR2 to CD14 and CD16 as a third surface biomarker. They include Mon1 (classical) CD14++ CD16- CCR2+, Mon2 (intermediate) CD14++ CD16+ CCR2+ and Mon3 (non-classical) CD14++ CD16+ CCR2-. The following primary functions were suggested for these subsets: phagocytosis and cytokine production for CD14++ CD16- CCR2+ monocytes, pro-angiogenic activity for CD14++ CD16+ CCR2+ monocytes, and collagen deposition and anti-inflammatory effects for CD14++ CD16+ CCR2- monocytes [22]. However, it remains to be identified how these 3 functional subsets correlate with the clinical manifestations of inflammatory disorders in humans.
Also CXC chemokine receptor 1 (CX3CR1), a receptor for the chemokine chemokine (C-X-C motif) ligand 1 (CX3CL1), can be used for the identification of monocytes with specific functional properties - patrolling vascular endothelium under homeostatic and inflammatory conditions [23]. The patrolling monocyte subset CX3CR1high CD14dim CD16+ in humans is distinct from the classical monocyte subset CCR2high CD14+ CD16- and exhibits unique functions in vasculature and inflammatory disease, including the removal of damaged cells and debris from the vasculature, regulation of wound healing, and the resolution of inflammation in damaged tissues.
A different system to define the subsets of the circulating human monocytes was suggested based on the expression of CD64, Fcγ-receptor-I. Grage-Griebenow et al. [11] have defined CD64- as ‘intermediate’ and CD64+ as ‘regular’. According to this system ‘intermediate’ monocytes are subdivided into the group of CD64- CD16- (below 10% of total monocytes) and a group that combines CD64-/CD16+ and CD14dim/CD16+ (below 10% of total monocytes). The CD64+ regular monocytes are subdivided into the groups of CD64+ CD16- (below 10% of total monocytes) and CD14+ CD16- (over 80% of total monocytes). The authors have provided a very comprehensive characterization of the subgroups by molecular profiles, including surface markers and cytokines, and functional properties, including phagocytosis, interaction with T cells, tumor cytotoxicity and others. However, further studies on human patient cohorts are needed in order to demonstrate that changes in the ratio of such groups associate clearly with pathophysiological processes in human disorders.
Potentially, also Toll-like receptors (TLRs) can be considered as markers for monocyte subsets in the circulation. Several studies reported changes in TLR expression on circulating monocytes in patients with metabolic, cardiovascular and infectious disorders. The overweight / metabolic syndrome group demonstrated a significant elevation in expression of TLR-2, TLR-4, TNF-α and IL-6 in peripheral monocytes, and increased circulating levels of TNF-α and IL-6 when compared with a overweight healthy group [24]. TLR-4 and TNF-α are potential sensitive diagnostic biomarkers for diabetic peripheral neuropathy in both general population and type 2 diabetic subjects [25]. Patients with acute coronary syndromes have an increased expression of TLR-2 and TLR-4 on circulating monocytes [26,27]. During sepsis in newborns, monocytes express higher levels of TLR-4 [28]. Monocytes from HIV-infected patients displayed enhanced expression of TLR-2 [29].
We were able to demonstrate that also scavenger receptors (SRs) can be used as biomarkers for monocyte subsets in cardiometabolic disorders. SRs comprise a large group of heterogeneous transmembrane proteins expressed on the cell surface with one common major function: clearance of non-self and unwanted-self components by internalization and targeting for the degradation in lysosomes [5]. The complex interplay between various SRs affects the progression of atherosclerosis, since some SRs (e.g. CD36) respond to modified lipoproteins by amplification of inflammation, while some SRs seem to support tolerogenic reactions. Our long-term studies indicate that stabilin-1 is one of such tolerogenic SRs that mediates clearance of modified lipoproteins, apoptotic bodies, cytokines, extracellular matrix components, and growth factors without induction of pro-inflammatory reactions [30,31,32,33,34,35].
It was suggested that in cardiovascular disorders the outcome of different SR activities will result in the decision of monocytes and macrophages to maintain the homeostatic balance, to support chronic inflammation and plaque instability, or to induce rapid fibrotic processes stabilizing the plaque [5].
We demonstrated that SRs contribute also to the functional diversity of monocyte subpopulations in patients with FH [36]. We have examined whether CD14+ CD16+ and CD14++ CD16- monocytes show functional differences in response to hyperlipidemic conditions in FH patients. It was demonstrated that CD14+ CD16+ FH monocytes exhibit an increased uptake of oxidized LDL via CD36, while CD14++ CD16- FH monocytes preferentially internalize native LDL (nLDL). Increased expression of CD68, stabilin-1, and CD11c was identified on CD14+ CD16+ FH monocytes that correlated with higher adherence to activated endothelial cells in response to oxidized LDL (oxLDL) and nLDL. This study suggested that CD14+ CD16+ monocytes in FH patients have the specific function of oxLDL uptake at activated endothelial cell layers, which is critical for the clearance of oxLDL deposits from the vessel wall under hyperlipidemic conditions [36] (table 1).
Table 1.
Functions of surface markers for human monocytes subsets
| Marker | Function | Role in inflammation | References |
|---|---|---|---|
| CD14 | co-receptor for the detection of LPS | regulation of production of both pro-inflammatory cytokines and cytokine inhibitors | [105, 106] |
| CD16 | binds to the Fc portion of IgG antibodies | stimulation of production of pro-inflammatory cytokines and antigen presentation for T cells | [17] |
| CCR2 | receptor for MCP1/CCL2, CCL7, CCL8, CCL13 and CCL16 | induction of monocytes infiltration, involved in angiogenesis | [22, 107] |
| CX3CR1 | receptor for CX3CL1 | patrolling vascular endothelium, removal of debris from the vasculature, regulation of wound healing | [23] |
| CD64 | binds to the Fc portion of IgG antibodies | phagocytosis, chemotaxis, induction of pro-inflammatory cytokines | [108, 109] |
| TLR-2/TLR-4 | recognizes PAMPs and DAMPs | induction of pro-inflammatory cytokines | [110] |
| CD36 | clearance of non-self and unwanted components | uptake of oxLDL which leads to up-regulation of pro-atherosclerotic cytokines and ROS production. Critical for macrophage foam cell formation | [111, 112] |
| Stabilin-1 | clearance of non-self and unwanted components | silent clearance of unwanted-self components (modified lipoproteins, cytokines, SPARC, apoptotic bodies) | [30, 31, 32, 34] |
PAMPs = Pathogen-associated molecular patterns; DAMPs = damage-associated molecular patterns.
Diversity of Monocyte-Derived Macrophages
Analysis of surface biomarkers on circulating monocytes and identification of the proportion of specific monocyte subpopulations is a promising tool for the detection of chronic inflammation in the human body. However, low-grade inflammation does not necessarily have a manifestation at the level of differential expression of surface monocyte markers and can be detected only by using more advanced functional ex vivo systems, based on monocyte-derived macrophages.
Adult monocyte-derived macrophages together with yolk sac- and fetal monocyte-derived macrophages constitute a pull of resident cells in solid tissues that can initiate acute inflammation by secretion of cytokines, enzymes and other active molecules in response to pathogens or trauma [37]. Macrophages attract and activate other cells of the adaptive immune system, in particular T cells, to sites of chronic inflammation. Further, macrophages are able to sense the time point when an injury is terminated, to start the process of resolution of the inflammation, and to control the healing phase. We and others have demonstrated that macrophages orchestrate the healing phase by releasing tolerogenic cytokines and growth factors as well as extracellular matrix enzymes and structural components, and by clearance of waste molecules and apoptotic cells [31,33,38]. During the healing phase, macrophages suppress inflammatory activities of other immune cells, clear the tissue from apoptotic bodies and other unwanted-self components, induce and support angiogenesis to supply healing tissue with oxygen and nutrition, and stimulate somatic cells to reconstitute normal tissue composition [39].
Our long-term investigation of macrophage biology and recent work of other research groups have revealed that, to fulfill these multiple temporary and spatially coordinated tasks, macrophages have developed a great diversity in their subpopulations and plasticity for their functional phenotypes [39,40,41,42]. It is widely accepted that macrophage phenotypes represent a wide spectrum of activation states not restricted to M1 (classically IFN-γ-activated) and M2 (alternatively IL-4-activated) subtypes. In this review we will utilize M1 and M2 for simplicity to define acute inflammatory macrophages as M1 and healing/anti-inflammatory macrophages as M2 [37,42].
Pathological events that block the ability of macrophages to change the phenotype from M1 to M2 lead to chronic inflammation [43,44]. We and others have demonstrated that inflammatory macrophages (M1) can be converted to healing macrophages (M2) by stimulation with cytokines [41,45]. In tissues, macrophage subpopulations can be identified by immunohistology and by using double and triple immunofluorescent staining with advanced confocal microscopy visualization [46]. However, most of macrophages' histological biomarkers define tissue-specific homeostatic or alternatively activated macrophages (like CD206 and stabilin-1). Such markers can also be used for the identification of tumor-associated macrophages [46,47,48,49]. However, specific markers, which can define inflammatory macrophages in sites of acute or chronic inflammation in human tissue by immunohistological methods still have to be developed.
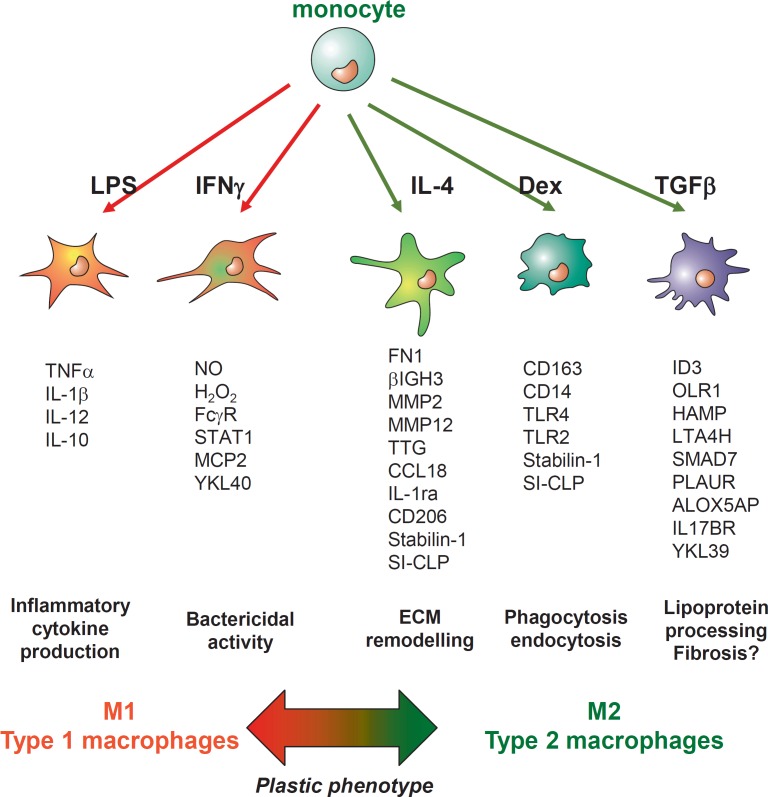
The task of our work for over 15 years was to analyze the response of human monocytes to distinct M1 and M2 stimuli. Using several systematic approaches including subtractive hybridization, Affymetrix chip technology, analysis of protein networks by yeast two-hybrid technology as well as number of functional assays, we have identified that not a single factor but a complex molecular signature reflects responses of monocytes to the specific factors during the monocyte-to-macrophage differentiation ex vivo. Molecular profiles of the macrophage subpopulations have been verified by various methods including RT-PCR, Western blotting, flow cytometry, immunofluorescence/confocal microscopy as well as ELISA. Figure 1 summarizes the molecular profiles of macrophages identified by us differentiated, from circulating primary human monocytes in the presence of M1 stimuli (lipopolysaccharides (LPS) or IFN-γ) or M2 stimuli (TGF-β, IL-4, or glucocorticoids). These molecular signatures are also indicative for the functional diversity of macrophage subpopulations we studied. We have developed and applied functional assays for endocytosis, phagocytosis, transcytosis, transmigration, and bacterial killing to connect specific molecular profiles with the functional diversity of macrophage subtypes. Thus, we identified that IL-4 is a major driver for the tissue remodeling activity of M2 macrophage by the induction of the release of matrix metalloproteinases (MMPs), tissue transglutaminase, and other extracellular matrix components [50]. We also demonstrated that the major impact of glucocorticoids on macrophage activity is the enhancement of endocytic and phagocytic activity toward unwanted-self products that is essential both for the resolution of inflammation and maintenance of homeostatic tissue balance [31,32,33,50]. We identified a complex response of primary macrophages to TGF-β. TGF-β is a pleiotropic growth factor for which both a protective and a detrimental role in atherogenesis was demonstrated [51]. We have shown that during the process of monocyte-to-macrophage differentiation, the presence of glucocorticoids is essential for the expression of TGF-βRII on the surface of macrophages that allows them to respond to TGF-β1 by activation of Smad2/3 signaling and induction of a complex gene expression program [40]. However, genes activated by TGF-β1 in primary human macrophages were not limited to the known Smad2/3-dependent ones (like Smad 7) (fig. 1). The next study we performed has demonstrated that TGF-β1 induces not only Smad2/3 signaling but also Smad1/5 signaling in primary mature human macrophages, where ALK5/ALK1 heterodimer was responsible for the induction of Smad1/5 signaling by TGF-β1, but not bone morphogenetic proteins (BMPs) [52]. Activation of Smad1/5 by TGF-β1 induces very interesting profiles of genes including HAMP (coding for the antimicrobial peptide hepcidin) and PLAUR (coding for the receptor for plasminogen urokinase activator), which contribute to atherosclerotic plaque vulnerability. Hepcidin alters iron metabolism in macrophages and reduces lipid efflux [53,54], while increased expression of the receptor for plasminogen urokinase activator PLAUR is indicative for plaques instability and rupture [55]. Therefore, for the first time we were able to do distinguish between tolerogenic/profibrotic effects and chronic inflammatory effects of TGF-β1 on macrophages. These identified molecular profiles can be also further used in diagnostics to predict the outcome of the effect of TGF-β in atherosclerotic patients, where Smad1/5 induced genes that are indicative for the pro-atherogenic effects, while Smad2/3 mediates atheroprotective effects of TGF-β.
Fig. 1.
Molecular signatures and functional subtypes of human ex vivo generated monocyte-derived macrophages. LPS and IFN-γ are grouped as M1-driving stimuli. IL-4, dexamethasone (Dex) and TGF-β are grouped as M2-driving stimuli. The list of specifically activated genes is indicated for each macrophage subtype. The most pronounced functional activities for each macrophage subtype are defined according to the molecular profile and functional tests.
Chitinase-Like Proteins
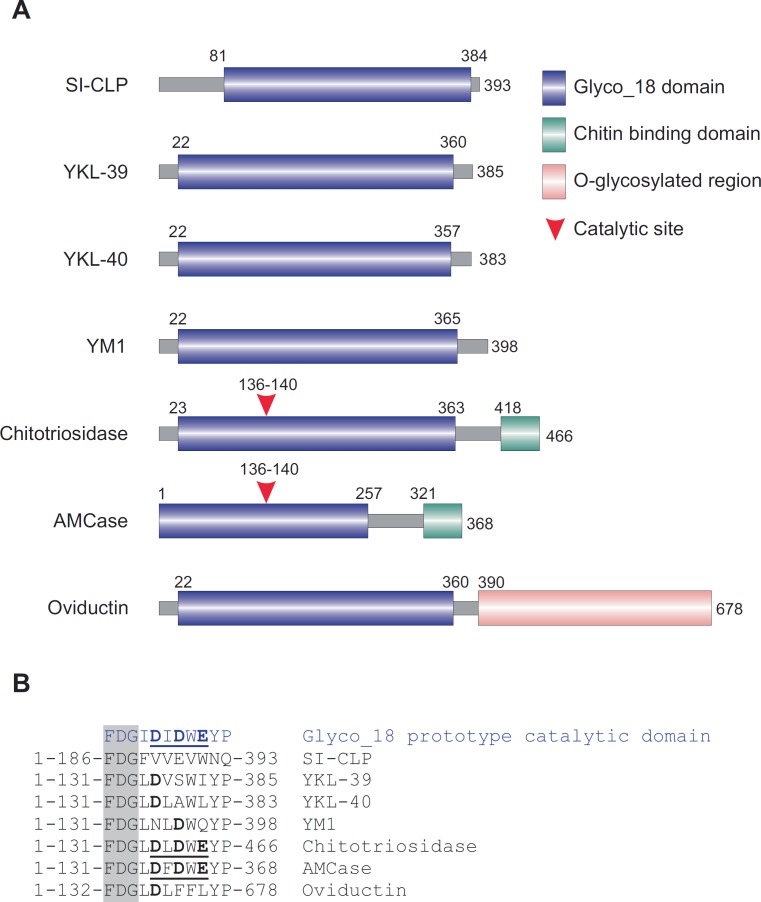
The challenging issue for distinguishing macrophage subpopulations is to identify the minimal amount of parameters indicative for the response to some specific stimuli that can be also linked to the specific function. In this review we would like to attract the attention to a new set of biomarkers that belong to the family of chitinase-like proteins (CLPs). Mammalian CLPs are induced at sites of inflammation and in tumors. The common structural feature of mammalian chitinases and CLPs is the presence of highly conserved Glyco_18 domain. The Glyco_18 domain is characteristic for the evolutionary conservative chitinases which belong to the family 18 glycosyl hydrolases. Enzymatically active chitinases catalyze the hydrolysis of chitin, and their evolutionary conserved function in lower life forms is host defense against chitin-containing organisms [56]. There are 6 Glyco_18 domain-containing proteins identified in human up to date and one additional in rodents (see fig. 2[57]). The last member of mammalian cCLP is SI-CLP, secretion of which is regulated by the receptor stabilin-1 in macrophages [57,58]. Only two mammalian Glyco_18-containing proteins are true chitinases and possess enzymatic activity: chitotriosidase and acidic mammalian chitinase AMCase [59,60]. Both chitotriosidase and AMCase, in addition to the Glyco_18 domain, contain the functional chitin-binding domain on their C-terminus. Oviductin/MUC9 contains a Glyco_18 domain and a long fragment with numerous sites for O-glycosylation, typical for mucins. YKL-39, YKL-40, SI-CLP, and YM1/YM2 (only in rodents) contain a Glyco_18 domain only, lack critical amino acids within the catalytic site (fig. 2), and therefore do not exhibit enzymatic activity [61].
Fig. 2.
Chitinases and chitinase-like proteins. A Schematic presentation of mammalian Glyco_18 domain-containing proteins. B Critical amino acid in catalytic sites. The FDG sequence preceding catalytic motif is shown in the shadowed box. Catalytic amino acids are shown in bold. Complete active catalytic motifs are underlined. This research was originally published in Blood[57]: Kzhyshkowska J, et al: Novel stabilin-1 interacting chitinase-like protein (SI-CLP) is up-regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood 2006;107:3221-3228. © the American Society of Hematology.
The feature which makes human CLPs very attractive as precise biomarkers for macrophage activation in response to specific stimuli is that these proteins are differentially activated in primary human macrophages: YKL-40 is induced by IFN-γ and suppressed by dexamethasone, SI-CLP is induced by IL-4 in combination with dexamethasone and suppressed by IFN-γ, and YKL-39 is highly specifically upregulated by TGF-β [57,62].
Three human CLPs are secreted into the extracellular space and can be detected in tissues and in blood circulation. However, up to date reliable tools for the detection of the CLPs in the circulation are developed only for YKL-40 and include radioimmunoassay (RIA) [63] and ELISA developed by Quidel and R&D systems.
Elevated levels of YKL-40 (CHI3L1) were first discovered in patients with inflammatory joint injuries and afterwards were found in other inflammatory diseases. Currently YKL-40 is being suggested as a biomarker in inflammatory pulmonary, cardiovascular, metabolic, joint-related, hepatic and neurologic disorders [64,65]. High levels of YKL-40 in serum, bronchoalveolar lavage fluid, or sputum were found in various pulmonary disorders. In asthma, YKL-40 levels correlated with some clinical signs such as exacerbation, level of control, and obesity [66,67,68,69]. In chronic obstructive pulmonary disease, high levels of YKL-40 were shown to correlate with disease severity and overall survival [70,71]. Similar findings were obtained in patients with cystic fibrosis lung disease [72,73]. Additionally, YKL-40 has been suggested as a biomarker for pulmonary sarcoidosis [63], although some studies did not find any correlation with clinical signs on follow-up [74].
Higher concentrations of YKL-40 in serum and plasma were also reported in patients with cardiovascular disorders. In patients with acute myocardial infarction [75], coronary artery disease [76,77] and carotid atherosclerosis [78], YKL-40 levels correlated with the degree of vascular obstruction, symptoms and severity of the disease. Additionally, it was shown that high serum YKL-40 is associated with vascular complications in patients with type I and type II diabetes [79,80]. Furthermore, analysis of YKL-40 levels in plasma or serum could predict long-term mortality in patients with coronary disease and type II diabetes [81,82].
Increased YKL-40 levels in serum, plasma, or synovial fluid (SF) were found to correlate with disease activity and response to treatment in patients with rheumatoid arthritis and osteoarthritis [83,84]. In rheumatoid arthritis, YKL-40 serum levels were also found to correlate with erythrocyte sedimentation rate and IgM rheumatoid factor [85]. Patients with osteoarthritic lesions had a significantly higher concentration of YKL-40 in SF as compared to plasma (1,027.9 ± 78.3 ng/ml vs. 67.2 ± 4.5 ng/ml) and this level correlated to the levels of some catabolic factors (MMP-1, MMP-3, IL-6 and IL-17) associated with the disease [86].
Furthermore, elevated levels of YKL-40 were shown in patients with various other disorders, ranging from periodontal disease [87] over diseases associated with liver fibrosis [88], to neurodegenerative disorders like Alzheimer's disease [89], inflammatory bowel disease [90] and various forms of cancer [61]. Thus YKL-40 is elevated in virtually all forms of chronic inflammation.
YKL-39, also known as chitinase 3-like-2 (CHI3L2), is a less studied member of the CLP family and is expressed by chondrocytes and synoviocytes [91,92]. On mRNA level, it was shown to be up-regulated in cartilage and chondrocytes of patients with osteoarthritis [92,93]. In contrast to YKL-40, YKL-39 was found to play a role in the autoimmune response in patients with rheumatoid arthritis and osteoarthritis, with anti-YKL-39 antibodies found in serum of approximately 11% of patients [94,95].
The association of circulating levels of SI-CLP with human inflammatory disorders still remains to be identified.
Ex vivo Monocyte/Macrophage-Based Test System as a Promising Diagnostic Approach
A part of resident tissue macrophages originate from monocytes that circulate in the blood stream and obtain their first differentiation and activation signal in the circulation [37]. Monocytes constitutively migrate in healthy tissues and are actively recruited at sites of inflammation. In tissues, monocytes differentiate into specific macrophage subtypes under the control of local soluble factors, where cytokines and growth factors have major effects.
However, already in the circulation monocytes can be exposed to the drivers of chronic inflammation that affect their potential to give origin to healthy tissue homeostatic macrophages, instead of giving origin to pro-inflammatory macrophages that will affect local tissue microenvironment. The factors in circulation that can affect pro-inflammatory programming of macrophages include hyperglycemia, modified lipoproteins, heat shock proteins, hormones, cytokines, growth factors as well as the previously described family of CLPs.
Nonetheless, this pro-inflammatory programming of monocytes in the circulation does not necessarily have a manifestation on the level of known surface biomarkers that can be detected by flow cytometry. In order to evaluate whether monocytes have been programmed in the circulation, the next level of complexity in the diagnostic system is needed that includes the isolation of monocytes and ex vivo examination of their responses to cytokines or other challenges. Such response can be detected on the level of inducible molecular biomarkers where quantifications by RT-PCR, ELISA and flow cytometry are the most precise methods. RT-PCR is a precise methodology to quantify expression of genes coding for the biomarkers the processing, transport or release of which have no additional control points regulated by cytokines or other tissue/disease-specific factors. One of such factors is CCL18, the most abundant M2-produced cytokine; mRNA levels and protein release in the intracellular space correspond to each other, and both RT-PCR and ELISA can be used for its quantification. However, release of several cytokines including TNF-α, the most frequently used biomarker for acute inflammation secreted by M1 macrophages, have a very complex mode of release regulation, and their secreted levels do not directly correspond to the level of mRNA synthesis. Therefore, ELISA has to be used for TNF-α quantification. The next level of test system complexity is needed if functional activity of the secreted factors has to be identified. For example, in case of MMPs, their release by macrophages do not necessarily correspond to the enzymatic activity, that can be controlled by activating and inhibiting factors produced not only by macrophages but also other cell types [96].
Quantification of the surface-expressed biomarkers or transmembrane proteins located in the intracellular vesicles is usually performed by flow cytometry on the intact or permeabilized cells, correspondingly. In order to access their functional activity a specific test has to be selected for each specific biomarker. For example, functional activity for the multifunctional receptor stabilin-1, an M2 marker, can include examination of endocytosis of modified lipoproteins and cytokines, phagocytosis of apoptotic bodies, or transcytosis of growth factors. The decision which function is examined has to be related to the specific pathology or medical question. For instance, for the examination of monocyte programming of patients with metabolic syndrome, the internalization, intracellular transporter and lysosomal degradation of modified lipoproteins is the most useful parameter to examine.
Based on our ex vivo experimental system for monocyte-to-macrophage differentiation, we have started to elaborate a diagnostically applicable test system. For the research purposes the best source of the functionally active monocytes are buffy coats. Using monocytes isolated out of buffy coats, we could demonstrate in over 15 years of experience that high level of standardization can be achieved in the isolation and cultivation procedures that allows to compare both molecular profiling and functional activity of monocytes for a number of individual healthy donors [41,50].
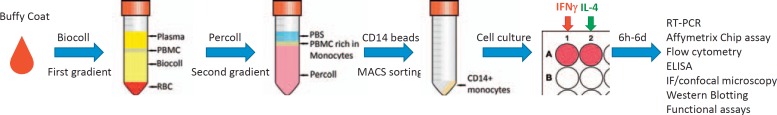
In our system we used a two-step gradient centrifugation (first gradient: Biocoll - to select for peripheral blood mononuclear cells; second gradient: Percoll - to enrich for monocytes) followed by a CD14+ positive selection and cultured the monocytes in serum-free medium, supplemented with 5 mmol/l glucose and 10−8 mol/l dexamethasone (fig. 3) [97]. In order to test whether monocytes of a healthy donor or a patient have normal reactions or are pre-programmed for the inflammatory, fibrotic or tolerogenic reactions, we cultivate monocytes in these conditions for 6 days to generate M0 (no cytokines), M1 (IFN-γ) and M2 (IL-4) macrophages. The release of the secreted cytokines can be monitored starting from 6 h of monocyte cultivation until day 6 and requires minimal amount of cells (1 × 106 cells for each subtype of macrophages). However, if flow cytometry, RT-PCR, or functional tests are applied, a separate sample (a well in a 6/12/24-well plate) is needed for each time point increasing the required number of primary monocytes. The amount of monocytes that can be isolated from buffy coats is usually between 4 × 107 and 6 × 107 for each healthy donor, which is sufficient for a number of stimulations and analysis of responses by various methods. When the test system has to be optimized for clinical use, the challenging issues are that the amount of patients' blood is restricted and the test system has to be adjusted for 5-6 × 106 primary monocytes for each patient. Using such a monocytes/macrophage differentiation system, more complex functions also can be examined, e.g. adhesion to the specific surface, including extracellular matrix or implant coating materials as well as the interaction with other cell types like adhesion to endothelium or transmigration along the endothelium layers. In order to define the range of healthy and disease-specific reactions, such methods require a high level of standardization.
Fig. 3.
Schematic representation of monocyte isolation out of buffy coats for their subsequent analysis. 30 ml of diluted buffy coat (1:1 in PBS) is added on top of 15 ml of Biocoll solution. After a centrifugation step (30 min at 420 × g, without break) 4 layers are obtained from top to bottom: plasma, peripheral blood mononuclear cell (PBMC), Biocoll and red blood cells (RBC). PBMCs are collected from the serum interphase and washed twice with PBS and then are layered on top of a freshly prepared Percoll gradient (13.5 ml Percoll + 15 ml of Minimal Essential Medium Eagle + 1.5 ml 10× Earle's Balanced Salt Solution). After another centrifugation step (30 min at 420 × g, without break) 3 layers are obtained from top to bottom: PBS, PMBCs rich in monocytes and Percoll. The first two layers are collected and washed with PBS and then are incubated with CD14 beads. After a magnetic cell sorting procedure CD14+ monocytes are collected (92-98% purity) and cultured in serum free medium. To generate M0, M1 or M2 macrophages no cytokines, IFN-γ or IL-4 are added respectively. Results can be obtained after 6h to 6 days by using the techniques listed in the figure.
Application of ex vivo Monocyte-Based Test System
Nowadays a series of medical issues in the field of dental, orthopedic, cardiovascular, and reconstructive surgery are solved with the use of implantable biomaterials. They can be used to support a biological structure, as in the case of bone screws and plates, to enhance as in the case of left ventricular assist devices, or to replace a biological structure as in the case of teeth implants. In order to protect the organism against foreign bodies, in some cases unfortunately our immune system launches a cascade of reactions with dramatic outcomes: from intense pain and chronic inflammation up to implant rejection. Macrophages are the immune cells responsible for the initiation of the foreign body response [98]. This is characterized by a release of pro-inflammatory cytokines and chemokines which attract other immune cells to the peri-implantation site and initiate acute inflammation. If it is not successfully resolved, it leads to chronic inflammation with the formation of foreign body giant cells and fibrous encapsulation of the implant. The chronic inflammation associated with the foreign body response leads to a change of microenvironment around the implants that can significantly affect the longevity of the implanted devices, the end result being additional costs related with an additional surgical intervention and a decrease in patient quality of life [99].
To solve these issues, different strategies have been adopted. One strategy is to select a more biocompatible, preferably a biodegradable implant material to minimize adverse immune reactions. However, in cases in which the mechanical properties of the implant are the primary concern and metals need to be used, a different strategy is applied. Thin biomaterial coatings are used to cover the main implant material, thus providing a more biocompatible surface without significantly modifying implant mechanical properties [100]. Moreover, in order to maximize the biocompatibility of the implant, there is a need of personalized selection of the material, based on the host's immune reaction at an early pre-implantation stage [99].
In our laboratory, we addressed this problem by analyzing macrophage responses to a biodegradable material [97] and to an implant coating [101]. For this purpose, we used our model system based on monocytes isolated out of buffy coats, as described earlier, and culturedthem with different implant materials. High-molecular polylactic acid (PLA) and its modifications were used as biodegradable materials, while the coatings were a multilayered film of polyarginine and hyaluronic acid (PAR/HA) with the ability to release an antimicrobial peptide: catestatin (CAT). We analyzed the release of key inflammatory cytokines at days 1, 3 and 6 of incubation by ELISA and the expression of key surface markers by immunofluorescence and confocal microscopy. As key inflammatory cytokines we took TNF-α (marker of M1 response) and CCL18 (marker of M2 response) which are shown to take part in chronic inflammation [102,103]. For the study of surface markers, we analyzed the expression of CD206 (marker of M2 response, strongly up-regulated in macrophages during chronic inflammation [104]) and stabilin-1 (marker of M2 response with tolerogenic functions [38]).
In the analysis of PAR/HA or PAR/HA + CAT coatings, we determined that they had a strong inhibitory effect on the production of both TNF-α and CCL18 as well as on the expression of CD206, suggesting that these types of coatings would be suitable for use in implantation to avoid chronic immune reactions [101]. When we tested the macrophage reactions to PLA and its modifications, we observed a donor-specific immune response to each type of material. By using the 4 proposed biomarkers, we were able to select the most biocompatible material for each individual donor [97]. Together these studies show that an in vitro test system based on CD14+ monocytes is a promising tool to rapidly identify a patient-specific immune response and select the appropriate biomaterial for each individual patient. By increasing the number of markers, we can raise the specificity of the assay (table 2).
Table 2.
Biomarkers for ex vivo diagnostics of macrophage immune responses
| Marker | Type of biomarker | Induced by (type of response) | Role in inflammation or tissue remodeling | References |
|---|---|---|---|---|
| Applied by us | ||||
| TNF-α | cytokine | IFN-γ, LPS (M1) | stimulation of production of pro-inflammatory cytokines, induction of endothelial dysfunction | [113, 114] |
| CCL18 | cytokine | IL-4 (M2) | directing the migration of regulatory T cells, regulation of angiogenesis, hematopoiesis | [115, 116] |
| CD206 | SR | IL-4 (M2) | phagocytosis of pathogenic microorganisms, internalization of lysosomal hydrolases, neutrophil-derived myeloperoxidase, activation of anti-inflammatory functions, involved in antigen presentation for T cells | [117, 118, 119] |
| Stabilin-1 | SR | IL-4 + dexamethasone (M2) | silent clearance of unwanted-self components (modified lipoproteins, SPARC, apoptotic bodies) | [31, 120] |
| Planned | ||||
| IL-1ß | cytokine | IFN-γ, LPS (M1) | induces pro-inflammatory state and endothelial dysfunction | [121] |
| IL-1Ra | cytokine | IL-4 (M2) | natural inhibitor of IL-1ß, reduces systemic inflammation | [122] |
| IL-6 | cytokine | IFN-γ, LPS (M1), immune complex + Toll-like receptor ligands (M2b) | induces production of pro-inflammatory cytokines and also anti-inflammatory cytokines, profibrogenic cytokine, angiogenesis | [123, 124] |
| IL-8 | cytokine | IFN-γ, LPS (M1) | promotor of angiogenesis, activates neutrophil granulocytes, chemotactic for migratory immune cells | [1, 125] |
| YKL-40 | cytokine | IFN-γ (M2) | induction of angiogenesis, stimulates bronchial smooth muscle cell proliferation and migration, inhibitor of apoptosis | [2, 61, 126] |
| YKL-39 | cytokine | TGF-β (M2) | unknown | |
Conclusions
Circulating monocytes in human blood are highly informative cells in order to detect early stage chronic/low-grade inflammation and predict the development of cardiometabolic disorders and their complications. Both the detection of surface biomarkers and an ex vivo test system are promising approaches to identify pro-inflammatory programming of monocytes. Despite identification of several surface biomarkers that can be used to distinguish between monocytes subsets, the information about the association of specific monocytes subsets with type and stage of inflammation is still limited and larger patient cohort studies are needed. Moreover, isolation of specific monocyte subsets and their functional analysis ex vivo is also required to predict their pathological functions after differentiation in the tissue or disease-specific environment. We have initiated the optimization of the ex vivo monocyte/macrophage-based test system, and were able to examine inflammatory versus tolerogenic reactions to materials used in implantation using a minimal set of parameters. However, this is only the first step towards the design of clinically applicable system to identify individual responses to a spectrum of endogenous drivers of cardiometabolic disorders, which can be used as a predictive tool for disease progression and for choosing a personalized therapeutic approach.
Disclosure Statement
The authors declared no conflict of interest.
Acknowledgements
This research was supported by BMBF/FASIE grant BIOIN (JK, AO), DFG GRK1874 DIAMICOM (JK, AG), Russian Foundation for Fundamental Research grant agreement 14-04-01920, EuropeanUnion's Seventh Framework Programme for research, technological development and demonstration under grant agreement no 602694 (JK).
References
- 1.Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. 2014;222:R113–127. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- 2.Emanuela F, Grazia M, Marco de R, Maria Paola L, Giorgio F, Marco B. Inflammation as a link between obesity and metabolic syndrome. J Nutr Metab. 2012;2012:476380. doi: 10.1155/2012/476380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Domingueti CP, Dusse LM, Carvalho MD, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2015 doi: 10.1016/j.jdiacomp.2015.12.018. doi: 10.1016/j.jdiacomp.2015. 12.018. [DOI] [PubMed] [Google Scholar]
- 5.Kzhyshkowska J, Neyen C, Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology. 2012;217:492–502. doi: 10.1016/j.imbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Orekhov AN, Sobenin IA, Gavrilin MA, Gratchev A, Kotyashova SY, Nikiforov NG, Kzhyshkowska J. Macrophages in immunopathology of atherosclerosis: a target for diagnostics and therapy. Curr Pharm Des. 2015;21:1172–1179. doi: 10.2174/1381612820666141013120459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jovanovic K, Siebeck M, Gropp R. The route to pathologies in chronic inflammatory diseases characterized by T helper type 2 immune cells. Clin Exp Immunol. 2014;178:201–211. doi: 10.1111/cei.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Puyvelde K, Mets T, Njemini R, Beyer I, Bautmans I. Effect of advanced glycation end product intake on inflammation and aging: a systematic review. Nutr Rev. 2014;72:638–650. doi: 10.1111/nure.12141. [DOI] [PubMed] [Google Scholar]
- 9.Ramji DP, Davies TS. Cytokines in atherosclerosis: key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015;26:673–685. doi: 10.1016/j.cytogfr.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angeli F, Reboldi G, Poltronieri C, Lazzari L, Sordi M, Garofoli M, Bartolini C, Verdecchia P. Hyperglycemia in acute coronary syndromes: from mechanisms to prognostic implications. Ther Adv Cardiovasc Dis. 2015;9:412–424. doi: 10.1177/1753944715594528. [DOI] [PubMed] [Google Scholar]
- 11.Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 12.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler-Heitbrock L. Blood monocytes and their subsets: established features and open questions. Front Immunol. 2015;6:423. doi: 10.3389/fimmu.2015.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glezeva N, Horgan S, Baugh JA. Monocyte and macrophage subsets along the continuum to heart failure: Misguided heroes or targetable villains? J Mol Cell Cardiol. 2015;89:136–145. doi: 10.1016/j.yjmcc.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Gratchev A, Ovsiy I, Manousaridis I, Riabov V, Orekhov A, Kzhyshkowska J. Novel monocyte biomarkers of atherogenic conditions. Curr Pharm Des. 2013;19:5859–5864. doi: 10.2174/1381612811319330004. [DOI] [PubMed] [Google Scholar]
- 16.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 18.Hilgendorf I, Swirski FK. Making a difference: monocyte heterogeneity in cardiovascular disease. Curr Atheroscler Rep. 2012;14:450–459. doi: 10.1007/s11883-012-0274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poitou C, Dalmas E, Renovato M, Benhamo V, Hajduch F, Abdennour M, Kahn JF, Veyrie N, Rizkalla S, Fridman WH, Sautes-Fridman C, Clement K, Cremer I. CD14dimcd16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2322–2330. doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- 20.Rothe G, Herr AS, Stohr J, Abletshauser C, Weidinger G, Schmitz G. A more mature phenotype of blood mononuclear phagocytes is induced by fluvastatin treatment in hypercholesterolemic patients with coronary heart disease. Atherosclerosis. 1999;144:251–261. doi: 10.1016/s0021-9150(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 21.Goonewardena SN, Stein AB, Tsuchida RE, Rattan R, Shah D, Hummel SL. Monocyte subsets and inflammatory cytokines in acute decompensated heart failure. J Card Fail. 2015 doi: 10.1016/j.cardfail.2015.12.014. doi: 10.1016/j.cardfail.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaipersad AS, Lip GY, Silverman S, Shantsila E. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol. 2014;63:1–11. doi: 10.1016/j.jacc.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Thomas G, Tacke R, Hedrick CC, Hanna RN. Nonclassical patrolling monocyte function in the vasculature. Arterioscler Thromb Vasc Bioly. 2015;35:1306–1316. doi: 10.1161/ATVBAHA.114.304650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy OT, Kim A, Ciccarelli C, Hayman LL, Wiecha J. Increased toll-like receptor (TLR) mRNA expression in monocytes is a feature of metabolic syndrome in adolescents. Pediatr Obes. 2013;8:e19–23. doi: 10.1111/j.2047-6310.2012.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu T, Meng Q, Ji J, Lou X, Zhang L. Toll-like receptor 4 and tumor necrosis factor-alpha as diagnostic biomarkers for diabetic peripheral neuropathy. Neurosci Lett. 2015;585:28–32. doi: 10.1016/j.neulet.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Ashida K, Miyazaki K, Takayama E, Tsujimoto H, Ayaori M, Yakushiji T, Iwamoto N, Yonemura A, Isoda K, Mochizuki H. Characterization of the expression of TLR2 (toll-like receptor 2) and TLR4 on circulating monocytes in coronary artery disease. J Atheroscler Thromb. 2005;12:53–60. doi: 10.5551/jat.12.53. [DOI] [PubMed] [Google Scholar]
- 27.Methe H, Kim J-O, Kofler S, Weis M, Nabauer M, Koglin J. Expansion of circulating toll-like receptor 4-positive monocytes in patients with acute coronary syndrome. Circulation. 2005;111:2654–2661. doi: 10.1161/CIRCULATIONAHA.104.498865. [DOI] [PubMed] [Google Scholar]
- 28.Redondo AC, Ceccon ME, Silveira-Lessa AL, Quinello C, Palmeira P, Carvalho WB, Carneiro-Sampaio M. TLR-2 and TLR-4 expression in monocytes of newborns with late-onset sepsis. J Pediatr (Rio J) 2014;90:472–478. doi: 10.1016/j.jped.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Heggelund L, Müller F, Lien E, Yndestad A, Ueland T, Kristiansen KI, Espevik T, Aukrust P, Frøland SS. Increased expression of toll-like receptor 2 on monocytes in HIV infection: possible roles in inflammation and viral replication. Clin Infect Dis. 2004;39:264–269. doi: 10.1086/421780. [DOI] [PubMed] [Google Scholar]
- 30.Kzhyshkowska J, Gratchev A, Brundiers H, Mamidi S, Krusell L, Goerdt S. Phosphatidylinositide 3-kinase activity is required for stabilin-1-mediated endosomal transport of acLDL. Immunobiology. 2005;210:161–173. doi: 10.1016/j.imbio.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Kzhyshkowska J, Workman G, Cardo-Vila M, Arap W, Pasqualini R, Gratchev A, Krusell L, Goerdt S, Sage EH. Novel function of alternatively activated macrophages: stabilin-1-mediated clearance of SPARC. J Immunol. 2006;176:5825–5832. doi: 10.4049/jimmunol.176.10.5825. [DOI] [PubMed] [Google Scholar]
- 32.Kzhyshkowska J, Gratchev A, Schmuttermaier C, Brundiers H, Krusell L, Mamidi S, Zhang J, Workman G, Sage EH, Anderle C, Sedlmayr P, Goerdt S. Alternatively activated macrophages regulate extracellular levels of the hormone placental lactogen via receptor-mediated uptake and transcytosis. J Immunol. 2008;180:3028–3037. doi: 10.4049/jimmunol.180.5.3028. [DOI] [PubMed] [Google Scholar]
- 33.Park SY, Jung MY, Lee SJ, Kang KB, Gratchev A, Riabov V, Kzhyshkowska J, Kim IS. Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. J Cell Sci. 2009;122:3365–3373. doi: 10.1242/jcs.049569. [DOI] [PubMed] [Google Scholar]
- 34.Schledzewski K, Geraud C, Arnold B, Wang S, Grone HJ, Kempf T, Wollert KC, Straub BK, Schirmacher P, Demory A, Schonhaber H, Gratchev A, Dietz L, Thierse HJ, Kzhyshkowska J, Goerdt S. Deficiency of liver sinusoidal scavenger receptors stabilin-1 and −2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors. J Clin Invest. 2011;121:703–714. doi: 10.1172/JCI44740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kzhyshkowska J. Multifunctional receptor stabilin-1 in homeostasis and disease. Sci World J. 2010;10:2039–2053. doi: 10.1100/tsw.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosig S, Rennert K, Krause S, Kzhyshkowska J, Neunubel K, Heller R, Funke H. Different functions of monocyte subsets in familial hypercholesterolemia: Potential function of CD14+ CD16+ monocytes in detoxification of oxidized LDL. FASEB J. 2009;23:866–874. doi: 10.1096/fj.08-118240. [DOI] [PubMed] [Google Scholar]
- 37.Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2015;17:34–40. doi: 10.1038/ni.3324. [DOI] [PubMed] [Google Scholar]
- 38.Kzhyshkowska J, Krusell L. Cross-talk between endocytic clearance and secretion in macrophages. Immunobiology. 2009;214:576–593. doi: 10.1016/j.imbio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Gratchev A, Kzhyshkowska J, Kannookadan S, Ochsenreiter M, Popova A, Yu X, Mamidi S, Stonehouse-Usselmann E, Muller-Molinet I, Gooi L, Goerdt S. Activation of a TGF-beta-specific multistep gene expression program in mature macrophages requires glucocorticoid-mediated surface expression of TGF-beta receptor II. J Immunol. 2008;180:6553–6565. doi: 10.4049/jimmunol.180.10.6553. [DOI] [PubMed] [Google Scholar]
- 41.Gratchev A, Kzhyshkowska J, Kothe K, Muller-Molinet I, Kannookadan S, Utikal J, Goerdt S. Mphi1 and Mphi2 can be re-polarized by Th2 or Th1 cytokines, respectively, and respond to exogenous danger signals. Immunobiology. 2006;211:473–486. doi: 10.1016/j.imbio.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultze JL. Reprogramming of macrophages-new opportunities for therapeutic targeting. Curr Opin Pharmacol. 2015;26:10–15. doi: 10.1016/j.coph.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Martens JH, Kzhyshkowska J, Falkowski-Hansen M, Schledzewski K, Gratchev A, Mansmann U, Schmuttermaier C, Dippel E, Koenen W, Riedel F, Sankala M, Tryggvason K, Kobzik L, Moldenhauer G, Arnold B, Goerdt S. Differential expression of a gene signature for scavenger/lectin receptors by endothelial cells and macrophages in human lymph node sinuses, the primary sites of regional metastasis. J Pathol. 2006;208:574–589. doi: 10.1002/path.1921. [DOI] [PubMed] [Google Scholar]
- 47.Buldakov M, Zavyalova M, Krakhmal N, Telegina N, Vtorushin S, Mitrofanova I, Riabov V, Yin S, Song B, Cherdyntseva N, Kzhyshkowska J. CD68+, but not stabilin-1+ tumor associated macrophages in gaps of ductal tumor structures negatively correlate with the lymphatic metastasis in human breast cancer. Immunobiology. 2015 doi: 10.1016/j.imbio.2015.09.011. doi: 10.1016/j.imbio.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Laoui D, Movahedi K, Van Overmeire E, Van den Bossche J, Schouppe E, Mommer C, Nikolaou A, Morias Y, De Baetselier P, Van Ginderachter JA. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol. 2011;55:861–867. doi: 10.1387/ijdb.113371dl. [DOI] [PubMed] [Google Scholar]
- 49.Schonhaar K, Schledzewski K, Michel J, Dollt C, Gkaniatsou C, Geraud C, Kzhyshkowska J, Goerdt S, Schmieder A. Expression of stabilin-1 in M2 macrophages in human granulomatous disease and melanocytic lesions. Int J Clin Exp Pathol. 2014;7:1625–1634. [PMC free article] [PubMed] [Google Scholar]
- 50.Gratchev A, Kzhyshkowska J, Utikal J, Goerdt S. Interleukin-4 and dexamethasone counterregulate extracellular matrix remodelling and phagocytosis in type-2 macrophages. Scand J Immunol. 2005;61:10–17. doi: 10.1111/j.0300-9475.2005.01524.x. [DOI] [PubMed] [Google Scholar]
- 51.Toma I, McCaffrey TA. Transforming growth factor-beta and atherosclerosis: interwoven atherogenic and atheroprotective aspects. Cell Tissue Res. 2012;347:155–175. doi: 10.1007/s00441-011-1189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nurgazieva D, Mickley A, Moganti K, Ming W, Ovsyi I, Popova A, Sachindra, Awad K, Wang N, Bieback K, Goerdt S, Kzhyshkowska J, Gratchev A. TGF-beta1, but not bone morphogenetic proteins, activates SMAD1/5 pathway in primary human macrophages and induces expression of proatherogenic genes. J Immunol. 2015;194:709–718. doi: 10.4049/jimmunol.1300272. [DOI] [PubMed] [Google Scholar]
- 53.Saeed O, Otsuka F, Polavarapu R, Karmali V, Weiss D, Davis T, Rostad B, Pachura K, Adams L, Elliott J, Taylor WR, Narula J, Kolodgie F, Virmani R, Hong CC, Finn AV. Pharmacological suppression of hepcidin increases macrophage cholesterol efflux and reduces foam cell formation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:299–307. doi: 10.1161/ATVBAHA.111.240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu X, Yung LM, Cheng WH, Yu PB, Babitt JL, Lin HY, Xia Y. Hepcidin regulation by BMP signaling in macrophages is lipopolysaccharide dependent. PloS One. 2012;7:e44622. doi: 10.1371/journal.pone.0044622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Svensson PA, Olson FJ, Hagg DA, Ryndel M, Wiklund O, Karlstrom L, Hulthe J, Carlsson LM, Fagerberg B. Urokinase-type plasminogen activator receptor is associated with macrophages and plaque rupture in symptomatic carotid atherosclerosis. Int J Mol Med. 2008;22:459–464. [PubMed] [Google Scholar]
- 56.Arakane Y, Muthukrishnan S. Insect chitinase and chitinase-like proteins. Cell Molr Life Sci. 2010;67:201–216. doi: 10.1007/s00018-009-0161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kzhyshkowska J, Mamidi S, Gratchev A, Kremmer E, Schmuttermaier C, Krusell L, Haus G, Utikal J, Schledzewski K, Scholtze J, Goerdt S. Novel stabilin-1 interacting chitinase-like protein (SI-CLP) is up-regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood. 2006;107:3221–3228. doi: 10.1182/blood-2005-07-2843. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Gratchev A, Riabov V, Mamidi S, Schmuttermaier C, Krusell L, Kremmer E, Workman G, Sage EH, Jalkanen S, Goerdt S, Kzhyshkowska J. A novel GGA-binding site is required for intracellular sorting mediated by stabilin-1. Mol Cell Biol. 2009;29:6097–6105. doi: 10.1128/MCB.00505-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boot RG, Blommaart EF, Swart E, Ghauharali-van der Vlugt K, Bijl N, Moe C, Place A, Aerts JM. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J Biol Chem. 2001;276:6770–6778. doi: 10.1074/jbc.M009886200. [DOI] [PubMed] [Google Scholar]
- 60.Renkema GH, Boot RG, Muijsers AO, Donker-Koopman WE, Aerts JM. Purification and characterization of human chitotriosidase, a novel member of the chitinase family of proteins. J Biol Chem. 1995;270:2198–2202. doi: 10.1074/jbc.270.5.2198. [DOI] [PubMed] [Google Scholar]
- 61.Kzhyshkowska J, Yin S, Liu T, Riabov V, Mitrofanova I. Role of chitinase-like proteins in cancer. Biol Chem. 2016;397:231–247. doi: 10.1515/hsz-2015-0269. [DOI] [PubMed] [Google Scholar]
- 62.Gratchev A, Schmuttermaier C, Mamidi S, Gooi L, Goerdt S, Kzhyshkowska J. Expression of osteoarthritis marker YKL-39 is stimulated by transforming growth factor beta (TGF-beta) and IL-4 in differentiating macrophages. Biomark Insights. 2008;3:39–44. [PMC free article] [PubMed] [Google Scholar]
- 63.Johansen JS, Milman N, Hansen M, Garbarsch C, Price PA, Graudal N. Increased serum YKL-40 in patients with pulmonary sarcoidosis - a potential marker of disease activity? Respir Med. 2005;99:396–402. doi: 10.1016/j.rmed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 64.Johansen JS, Jensen HS, Price PA. A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br J Rheumatol. 1993;32:949–955. doi: 10.1093/rheumatology/32.11.949. [DOI] [PubMed] [Google Scholar]
- 65.Prakash M, Bodas M, Prakash D, Nawani N, Khetmalas M, Mandal A, Eriksson C. Diverse pathological implications of YKL-40: answers may lie in ‘outside-in’ signaling. Cell Signal. 2013;25:1567–1573. doi: 10.1016/j.cellsig.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 66.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, Cullen M, Grandsaigne M, Dombret MC, Aubier M, Pretolani M, Elias JA. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 67.Gavala ML, Kelly EA, Esnault S, Kukreja S, Evans MD, Bertics PJ, Chupp GL, Jarjour NN. Segmental allergen challenge enhances chitinase activity and levels of CCL18 in mild atopic asthma. Clin Exp Allergy. 2013;43:187–197. doi: 10.1111/cea.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hansen JW, Thomsen SF, Porsbjerg C, Rasmussen LM, Harmsen L, Johansen JS, Backer V. YKL-40 and genetic status of CHI3L1 in a large group of asthmatics. Eur Clin Respir J. 2015;2:25117. doi: 10.3402/ecrj.v2.25117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Specjalski K, Chelminska M, Jassem E. YKL-40 protein correlates with the phenotype of asthma. Lung. 2015;193:189–194. doi: 10.1007/s00408-015-9693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holmgaard DB, Mygind LH, Titlestad IL, Madsen H, Pedersen SS, Johansen JS, Pedersen C. Plasma YKL-40 and all-cause mortality in patients with chronic obstructive pulmonary disease. BMC Pulm Med. 2013;13:77. doi: 10.1186/1471-2466-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Letuve S, Kozhich A, Arouche N, Grandsaigne M, Reed J, Dombret MC, Kiener PA, Aubier M, Coyle AJ, Pretolani M. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol. 2008;181:5167–5173. doi: 10.4049/jimmunol.181.7.5167. [DOI] [PubMed] [Google Scholar]
- 72.Bouvet GF, Maignan M, Arslanian E, Coriati A, Rabasa-Lhoret R, Berthiaume Y. Association between serum YKL-40 level and dysglycemia in cystic fibrosis. Cytokine. 2015;71:296–301. doi: 10.1016/j.cyto.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 73.Hector A, Kormann MS, Mack I, Latzin P, Casaulta C, Kieninger E, Zhou Z, Yildirim AO, Bohla A, Rieber N, Kappler M, Koller B, Eber E, Eickmeier O, Zielen S, Eickelberg O, Griese M, Mall MA, Hartl D. The chitinase-like protein YKL-40 modulates cystic fibrosis lung disease. PloS One. 2011;6:e24399. doi: 10.1371/journal.pone.0024399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kruit A, Grutters JC, Ruven HJ, van Moorsel CC, van den Bosch JM. A CHI3L1 gene polymorphism is associated with serum levels of YKL-40, a novel sarcoidosis marker. Respir Med. 2007;101:1563–1571. doi: 10.1016/j.rmed.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 75.Nojgaard C, Host NB, Christensen IJ, Poulsen SH, Egstrup K, Price PA, Johansen JS. Serum levels of YKL-40 increases in patients with acute myocardial infarction. Coron Artery Dis. 2008;19:257–263. doi: 10.1097/MCA.0b013e3282f40dd5. [DOI] [PubMed] [Google Scholar]
- 76.Kucur M, Isman FK, Karadag B, Vural VA, Tavsanoglu S. Serum YKL-40 levels in patients with coronary artery disease. Coron Artery Dis. 2007;18:391–396. doi: 10.1097/MCA.0b013e328241d991. [DOI] [PubMed] [Google Scholar]
- 77.Zheng JL, Lu L, Hu J, Zhang RY, Zhang Q, Chen QJ, Shen WF. Increased serum YKL-40 and C-reactive protein levels are associated with angiographic lesion progression in patients with coronary artery disease. Atherosclerosis. 2010;210:590–595. doi: 10.1016/j.atherosclerosis.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 78.Michelsen AE, Rathcke CN, Skjelland M, Holm S, Ranheim T, Krohg-Sorensen K, Klingvall MF, Brosstad F, Oie E, Vestergaard H, Aukrust P, Halvorsen B. Increased YKL-40 expression in patients with carotid atherosclerosis. Atherosclerosis. 2010;211:589–595. doi: 10.1016/j.atherosclerosis.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 79.Batinic K, Hobaus C, Grujicic M, Steffan A, Jelic F, Lorant D, Hortenhuber T, Hoellerl F, Brix JM, Schernthaner G, Koppensteiner R, Schernthaner GH. YKL-40 is elevated in patients with peripheral arterial disease and diabetes or pre-diabetes. Atherosclerosis. 2012;222:557–563. doi: 10.1016/j.atherosclerosis.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 80.Rathcke CN, Persson F, Tarnow L, Rossing P, Vestergaard H. YKL-40, a marker of inflammation and endothelial dysfunction, is elevated in patients with type 1 diabetes and increases with levels of albuminuria. Diabetes Care. 2009;32:323–328. doi: 10.2337/dc08-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harutyunyan M, Gotze JP, Winkel P, Johansen JS, Hansen JF, Jensen GB, Hilden J, Kjoller E, Kolmos HJ, Gluud C, Kastrup J. Serum YKL-40 predicts long-term mortality in patients with stable coronary disease: a prognostic study within the claricor trial. Immunobiology. 2013;218:945–951. doi: 10.1016/j.imbio.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 82.Lin CH, Li HY, Jiang YD, Chang TJ, Chuang LM. Plasma YKL-40 predicts 10-year cardiovascular and all-cause mortality in individuals with type 2 diabetes. Clin Endocrinol. 2013;79:185–191. doi: 10.1111/cen.12015. [DOI] [PubMed] [Google Scholar]
- 83.Johansen JS, Stoltenberg M, Hansen M, Florescu A, Horslev-Petersen K, Lorenzen I, Price PA. Serum YKL-40 concentrations in patients with rheumatoid arthritis: relation to disease activity. Rheumatology (Oxford) 1999;38:618–626. doi: 10.1093/rheumatology/38.7.618. [DOI] [PubMed] [Google Scholar]
- 84.Volck B, Johansen JS, Stoltenberg M, Garbarsch C, Price PA, Ostergaard M, Ostergaard K, Lovgreen-Nielsen P, Sonne-Holm S, Lorenzen I. Studies on YKL-40 in knee joints of patients with rheumatoid arthritis and osteoarthritis. Involvement of YKL-40 in the joint pathology. Osteoarthritis Cartilage. 2001;9:203–214. doi: 10.1053/joca.2000.0377. [DOI] [PubMed] [Google Scholar]
- 85.Vos K, Steenbakkers P, Miltenburg AM, Bos E, van Den Heuvel MW, van Hogezand RA, de Vries RR, Breedveld FC, Boots AM. Raised human cartilage glycoprotein-39 plasma levels in patients with rheumatoid arthritis and other inflammatory conditions. Ann Rheum Dis. 2000;59:544–548. doi: 10.1136/ard.59.7.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaananen T, Koskinen A, Paukkeri EL, Hamalainen M, Moilanen T, Moilanen E, Vuolteenaho K. YKL-40 as a novel factor associated with inflammation and catabolic mechanisms in osteoarthritic joints. Mediators Inflamm. 2014;2014:215140. doi: 10.1155/2014/215140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Keles ZP, Keles GC, Avci B, Cetinkaya BO, Emingil G. Analysis of YKL-40 acute-phase protein and interleukin-6 levels in periodontal disease. JPeriodontol. 2014;85:1240–1246. doi: 10.1902/jop.2014.130631. [DOI] [PubMed] [Google Scholar]
- 88.Tao H, Yang JJ, Shi KH, Huang C, Zhang L, Lv XW, Li J. The significance of YKL-40 protein in liver fibrosis. Inflamm Res. 2014;63:249–254. doi: 10.1007/s00011-013-0698-9. [DOI] [PubMed] [Google Scholar]
- 89.Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, Mintun MA, Peskind ER, Li G, Galasko DR, Clark CM, Quinn JF, D'Angelo G, Malone JP, Townsend RR, Morris JC, Fagan AM, Holtzman DM. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biol Psychiatry. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Erzin Y, Uzun H, Karatas A, Celik AF. Serum YKL-40 as a marker of disease activity and stricture formation in patients with Crohn's disease. J Gastroenterol Hepatol. 2008;23:e357–362. doi: 10.1111/j.1440-1746.2007.05121.x. [DOI] [PubMed] [Google Scholar]
- 91.Hu B, Trinh K, Figueira WF, Price PA. Isolation and sequence of a novel human chondrocyte protein related to mammalian members of the chitinase protein family. J Biol Chem. 1996;271:19415–19420. doi: 10.1074/jbc.271.32.19415. [DOI] [PubMed] [Google Scholar]
- 92.Knorr T, Obermayr F, Bartnik E, Zien A, Aigner T. YKL-39 (chitinase 3-like protein 2), but not YKL-40 (chitinase 3-like protein 1), is up regulated in osteoarthritic chondrocytes. Ann Rheum Dis. 2003;62:995–998. doi: 10.1136/ard.62.10.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steck E, Breit S, Breusch SJ, Axt M, Richter W. Enhanced expression of the human chitinase 3-like 2 gene (YKL-39) but not chitinase 3-like 1 gene (YKL-40) in osteoarthritic cartilage. Biochem Biophys Res Commun. 2002;299:109–115. doi: 10.1016/s0006-291x(02)02585-8. [DOI] [PubMed] [Google Scholar]
- 94.Sekine T, Masuko-Hongo K, Matsui T, Asahara H, Takigawa M, Nishioka K, Kato T. Recognition of YKL-39, a human cartilage related protein, as a target antigen in patients with rheumatoid arthritis. Ann Rheum Dis. 2001;60:49–54. doi: 10.1136/ard.60.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsuruha J, Masuko-Hongo K, Kato T, Sakata M, Nakamura H, Sekine T, Takigawa M, Nishioka K. Autoimmunity against YKL-39, a human cartilage derived protein, in patients with osteoarthritis. J Rheumatol. 2002;29:1459–1466. [PubMed] [Google Scholar]
- 96.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in APOE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stankevich KS, Gudima A, Filimonov VD, Kluter H, Mamontova EM, Tverdokhlebov SI, Kzhyshkowska J. Surface modification of biomaterials based on high-molecular polylactic acid and their effect on inflammatory reactions of primary human monocyte-derived macrophages: perspective for personalized therapy. Mater Sci Eng C Mater Biol Appl. 2015;51:117–126. doi: 10.1016/j.msec.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 98.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kzhyshkowska J, Gudima A, Riabov V, Dollinger C, Lavalle P, Vrana NE. Macrophage responses to implants: prospects for personalized medicine. J Leukoc Biol. 2015;98:953–962. doi: 10.1189/jlb.5VMR0415-166R. [DOI] [PubMed] [Google Scholar]
- 100.Nilsson B, Korsgren O, Lambris JD, Ekdahl KN. Can cells and biomaterials in therapeutic medicine be shielded from innate immune recognition? Trends Immunol. 2010;31:32–38. doi: 10.1016/j.it.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ozcelik H, Vrana NE, Gudima A, Riabov V, Gratchev A, Haikel Y, Metz-Boutigue MH, Carrado A, Faerber J, Roland T, Kluter H, Kzhyshkowska J, Schaaf P, Lavalle P. Harnessing the multifunctionality in nature: a bioactive agent release system with self-antimicrobial and immunomodulatory properties. Adv Healthc Mater. 2015;4:2026–2036. doi: 10.1002/adhm.201500546. [DOI] [PubMed] [Google Scholar]
- 102.Kollert F, Binder M, Probst C, Uhl M, Zirlik A, Kayser G, Voll RE, Peter HH, Zissel G, Prasse A, Warnatz K. CCL18 - potential biomarker of fibroinflammatory activity in chronic periaortitis. J Rheumatol. 2012;39:1407–1412. doi: 10.3899/jrheum.111143. [DOI] [PubMed] [Google Scholar]
- 103.Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48:751–762. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- 104.Melgert BN, ten Hacken NH, Rutgers B, Timens W, Postma DS, Hylkema MN. More alternative activation of macrophages in lungs of asthmatic patients. J Allergy Clin Immunol. 2011;127:831–833. doi: 10.1016/j.jaci.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 105.Ebong SJ, Goyert SM, Nemzek JA, Kim J, Bolgos GL, Remick DG. Critical role of CD14 for production of proinflammatory cytokines and cytokine inhibitors during sepsis with failure to alter morbidity or mortality. Infect Immun. 2001;69:2099–2106. doi: 10.1128/IAI.69.4.2099-2106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tapping RI, Tobias PS. Soluble CD14-mediated cellular responses to lipopolysaccharide. Chem Immunol. 2000;74:108–121. doi: 10.1159/000058751. [DOI] [PubMed] [Google Scholar]
- 107.Chu HX, Arumugam TV, Gelderblom M, Magnus T, Drummond GR, Sobey CG. Role of CCR2 in inflammatory conditions of the central nervous system. J Cereb Blood Flow Metab. 2014;34:1425–1429. doi: 10.1038/jcbfm.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grage-Griebenow E, Flad HD, Ernst M, Bzowska M, Skrzeczynska J, Pryjma J. Human MO subsets as defined by expression of CD64 and CD16 differ in phagocytic activity and generation of oxygen intermediates. Immunobiology. 2000;202:42–50. doi: 10.1016/S0171-2985(00)80051-0. [DOI] [PubMed] [Google Scholar]
- 109.Li Y, Lee PY, Sobel ES, Narain S, Satoh M, Segal MS, Reeves WH, Richards HB. Increased expression of FcgammaRI/CD64 on circulating monocytes parallels ongoing inflammation and nephritis in lupus. Arthritis Res Ther. 2009;11:R6. doi: 10.1186/ar2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Y, Yin H, Zhao M, Lu Q. TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clin Rev Allergy Immunol. 2014;47:136–147. doi: 10.1007/s12016-013-8402-y. [DOI] [PubMed] [Google Scholar]
- 111.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Makino N, Maeda T, Sugano M, Satoh S, Watanabe R, Abe N. High serum TNF-α level in type 2 diabetic patients with microangiopathy is associated with eNOS down-regulation and apoptosis in endothelial cells. J Diabetes Complications. 2005;19:347–355. doi: 10.1016/j.jdiacomp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 114.Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, Dellsperger K, Zhang C. Role of TNF-alpha in vascular dysfunction. Clin Sci. 2009;116:219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schutyser E, Richmond A, Van Damme J. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J Leukoc Biol. 2005;78:14–26. doi: 10.1189/jlb.1204712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chenivesse C, Chang Y, Azzaoui I, Yahia SA, Morales O, Plé C, Foussat A, Tonnel A-B, Delhem N, Yssel H. Pulmonary CCL18 recruits human regulatory T cells. J Immunol. 2012;189:128–137. doi: 10.4049/jimmunol.1003616. [DOI] [PubMed] [Google Scholar]
- 117.Gazi U, Martinez-Pomares L. Influence of the mannose receptor in host immune responses. Immunobiology. 2009;214:554–561. doi: 10.1016/j.imbio.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 118.Keler T, Ramakrishna V, Fanger MW. Mannose receptor-targeted vaccines. Expert Opin Biol Ther. 2004;4:1953–1962. doi: 10.1517/14712598.4.12.1953. [DOI] [PubMed] [Google Scholar]
- 119.Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92:1177–1186. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- 120.Kzhyshkowska J, Gratchev A, Goerdt S. Stabilin-1, a homeostatic scavenger receptor with multiple functions. J Cell Mol Med. 2006;10:635–649. doi: 10.1111/j.1582-4934.2006.tb00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rigor RR, Beard RS, Litovka OP, Yuan SY. Interleukin-1β-induced barrier dysfunction is signaled through PKC-θ in human brain microvascular endothelium. Am J Physiol Cell Physiol. 2012;302:C1513–C1522. doi: 10.1152/ajpcell.00371.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Böni‐Schnetzler M, Donath MY. How biologics targeting the IL‐1 system are being considered for the treatment of type 2 diabetes. Br J Clin Pharmacol. 2013;76:263–268. doi: 10.1111/j.1365-2125.2012.04297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barnes TC, Anderson ME, Moots RJ. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int J Rheumatol. 2011;2011:721608. doi: 10.1155/2011/721608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mendieta D, De la Cruz-Aguilera DL, Barrera-Villalpando MI, Becerril-Villanueva E, Arreola R, Hernández-Ferreira E, Pérez-Tapia SM, Pérez-Sánchez G, Garcés-Alvarez ME, Aguirre-Cruz L. IL-8 and IL-6 primarily mediate the inflammatory response in fibromyalgia patients. J Neuroimmunol. 2016;290:22–25. doi: 10.1016/j.jneuroim.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 125.Shi J, Wei PK. Interleukin-8: a potent promoter of angiogenesis in gastric cancer. Oncol Lett. 2016;11:1043–1050. doi: 10.3892/ol.2015.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Francescone RA, Scully S, Faibish M, Taylor SL, Oh D, Moral L, Yan W, Bentley B, Shao R. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J Biol Chem. 2011;286:15332–15343. doi: 10.1074/jbc.M110.212514. [DOI] [PMC free article] [PubMed] [Google Scholar]