Abstract
AIM: To assess the safety of enhanced recovery after surgery (ERAS) program in gastrectomy and influences on nutrition state and insulin-resistance.
METHODS: Our ERAS program involved shortening the fasting periods and preoperative carbohydrate loading. Eighty gastrectomy patients were randomly assigned to either the conventional group (CG) or ERAS group (EG). We assessed the clinical characteristics and postoperative outcomes prospectively. The primary endpoint was noninferiority in timely discharge from the hospital within 12 d. Secondary endpoints were the incidence of aspiration at anesthesia induction, incidence of postoperative complications, health related quality of life (HRQOL) using the SF8 Health Survey questionnaire, nutrition state [e.g., albumin, transthyretin (TTR), retinal-binding protein (RBP), and transferrin (Tf)], the homeostasis model assessment-insulin resistance (HOMA-R) index, postoperative urine volume, postoperative weight change, and postoperative oral intake.
RESULTS: The ERAS program was noninferior to the conventional program in achieving discharge from the hospital within 12 d (95.0% vs 92.5% respectively; 95%CI: -10.0%-16.0%). There was no significant difference in postoperative morbidity between the two groups. Adverse events such as vomiting and aspiration associated with the induction of general anesthesia were not observed. There were no significant differences with respect to postoperative urine volume, weight change, and oral intake between the two groups. EG patients with preoperative HOMA-R scores above 2.5 experienced significant attenuation of their HOMA-R scores on postoperative day 1 compared to CG patients (P = 0.014). There were no significant differences with respect to rapid turnover proteins (TTR, RBP and Tf) or HRQOL scores using the SF8 method.
CONCLUSION: Applying the ERAS program to patients who undergo gastrectomy is safe, and improves insulin resistance with no deterioration in QOL.
Keywords: Gastrectomy, Carbon hydrogen oxygen, Insulin resistance, Enhanced recovery after surgery, Randomized controlled trial
Core tip: We conducted a prospective study in gastrectomy patients to evaluate the efficacy of enhanced recovery after surgery (ERAS) programs. ERAS was safe and improved insulin resistance in these patients.
INTRODUCTION
Gastrectomy is a high-risk procedure owing to the stress of surgery and resulting complications. In the past few decades, oral intake was not permitted for a long period of time after surgery because of the chance that intraluminal pressure on the anastomosis would induce leakage. To that end, enhanced recovery after surgery (ERAS) programs were introduced, and found to be safe and useful for patients undergoing colectomy[1]. Previous reports have shown the benefits of ERAS programs such as controlling preoperative carbohydrate loading to regulate blood sugar[2,3] and early rehabilitation to prevent pneumonia, bowel obstruction, and thrombosis[1]. Postoperative early feeding significantly reduces hospital stay yet does not increase postoperative complications[4,5]. Similar programs have recently been introduced for gastrectomy patients, with a number of reports having been published in the past few years[6-10]. Moreover, postoperative oral feeding is being steadily introduced at earlier time points. In 2014, a report on the safety of commencing oral intake on postoperative day (POD) 1 after gastrectomy was published[11]. Although this report proposed that introducing postoperative oral feeding sooner is safe, the overall consequences of early postoperative feeding are still unclear.
In April 2012, we revised our clinical pathway for gastrectomy, employing a modified ERAS protocol originally used for colorectal resection. In this study, we compared postoperative outcomes between patients who received perioperative care according to our modified ERAS protocol, which involved shorter fasting periods and increased carbohydrate loading, and those who received conventional perioperative care. We evaluated the clinical consequence of this protocol in gastric surgery, assessing safety as well as postoperative nutrition state, insulin-resistance, and quality of life (QOL).
MATERIALS AND METHODS
Patients
This study was a prospective, single center, randomized phase II clinical trial. We studied consecutive patients who underwent gastrectomy at the Department of Gastroenterological and Transplant Surgery, Hiroshima University, between September 2011 and February 2015. Eighty patients were randomly categorized into 2 groups; 40 patients were assigned to each of the conventional treatment group (CG) and the ERAS group (EG). Patients were assigned according to the stratified randomization method by age (< 70 vs ≥ 70) and surgical approach (abdominal vs laparoscopic surgery). All patients completed their treatment. This study was approved by the institutional review board of the study institution (Hiroshima University, Japan, No.Rin-269) and was conducted in accordance with the Declaration of Helsinki. The study was registered at University Hospital Medical Information Network Clinical Trial Registry (UMIN000020538). Voluntary written informed consent was obtained from all subjects enrolled in this study.
Perioperative care
Patients in both groups were managed perioperatively using equivalent standardized clinical pathway protocols except for perioperative nutrition and intravenous fluid. In the EG, intravenous fluid was restricted to a minimal daily requirement during the first 3 postoperative days. Additional intravenous fluid was administered when patient showed poor oral intake of water or food. The CG received intravenous fluid for 1 wk postoperatively.
Regarding perioperative oral nutrition, patients in the EG received 875 mL of carbohydrate-rich (157 g) fluid until 2 h before the surgery. On POD 1, the patients commenced oral intake with water and oral rehydration solution. On POD 2, patients began to consume a liquid diet. In the CG, patients were allowed to drink water until the day before the surgery. On POD 2, these patients commenced oral intake beginning with water; liquid diets were offered on POD 3 (Figure 1).
Figure 1.
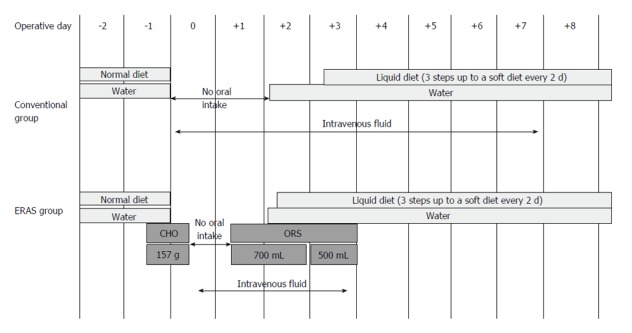
Schema of the study plan for patients participating in the enhanced recovery after surgery program and those subjected to conventional perioperative treatment. CHO: Carbon hydrogen oxygen; ERAS: Enhanced recovery after surgery; ORS: Oral rehydration solution.
Hospital discharge was recommended based on the following criteria: (1) No requirement for intravenous nutrition; (2) Tolerable pain with no or oral-only analgesics; (3) The ability to fully ambulate without assistance; and (4) A willingness to go home.
Data collection
All data were retrieved from the patients’ database and clinical records. The primary endpoint was noninferiority in achieving discharge from the hospital within 12 d. Secondary endpoints were the incidence of aspiration at the induction of anesthesia, incidence of postoperative complications, health related QOL (HRQOL) using the SF8 Health Survey questionnaire[12,13], nutrition state [e.g., albumin (ALB), transthyretin (TTR), retinal-binding protein (RBP), and transferrin (Tf)], the homeostasis model assessment-insulin resistance (HOMA-R) index, postoperative urine volume, postoperative weight change, and postoperative oral intake. HRQOL comprised of 2 components: the physical component summary (PCS) and the mental component summary (MCS). Complications were defined as being of grades ≥ 2 according to the Clavien-Dindo classification within 30 d after surgery. HOMA-R was calculated as immunoreactive insulin × fasting blood sugar/405.
Statistical analysis
All analyses were conducted according to a statistical analysis plan. We tested the noninferiority of ERAS with respect to the rate of achievement of discharge from the hospital within 12 d; the targeted noninferiority margin was 15 percentage points.
Assuming a success rate of 85% for the achievement of hospital discharge within 12 d for EG, and a success rate of 90% achievement of the same for CG, the number of patients required to establish noninferiority with 80% power was 33 for each study group. With a presumed dropout rate of 10%, we planned to enroll 80 patients in total.
Patients’ backgrounds were analyzed using the Fisher’s exact test to compare categorical data (Table 1). Logistic regression analysis was used to compare outcomes between groups (Table 2). Statistical analyses were conducted using the SPSS statistical software (version 19; SPSS Inc., Chicago, IL, United States). Statistical comparisons of parameters were performed using Student’s t-test. A P value of < 0.05 was considered statistically significant.
Table 1.
Comparison of characteristics between conventional group and enhanced recovery after surgery group
| Characteristics | Conventional group (n = 40) | ERAS group (n = 40) | P value |
| Gender | 1.000 | ||
| Male | 24 | 20 | |
| Female | 16 | 20 | |
| Age (yr) | 1.000 | ||
| < 70 | 28 | 29 | |
| ≥ 70 | 12 | 11 | |
| BMI (kg/m2) | 1.000 | ||
| < 22 | 18 | 18 | |
| ≥ 22 | 22 | 22 | |
| ASA-PS | 1.000 | ||
| 1 | 10 | 11 | |
| 2 | 30 | 29 | |
| Method | 0.679 | ||
| Partial gastrectomy | 9 | 6 | |
| Distal gastrectomy | 27 | 30 | |
| Total gastrectomy | 4 | 4 | |
| Approach | 0.809 | ||
| Abdominal surgery | 12 | 13 | |
| Laparoscopic surgery | 28 | 27 | |
| Tumor characteristics | 0.568 | ||
| Gastric cancer | 31 | 34 | |
| Submucosal tumor (GIST or schwannoma) | 9 | 6 |
BMI: Body mass index; ASA-PS: American Society of Anesthesiologists - physical status; ERAS: Enhanced recovery after surgery; GIST: Gastrointestinal mesenchymal tumor.
Table 2.
Comparison of postoperative outcomes between conventional group and enhanced recovery after surgery group
| Conventional group (n = 40) | ERAS group (n = 40) | Difference (95%CI) | P value | |
| Accomplishment of the discharge from the hospital within 12 d | 92.5% (37/40) | 95.0% (38/40) | 2.5% (-10.0%-16.0%) | 0.646 |
| Postoperative hospital stay (d) | 10.4 (7-23) | 9.8 (6-20) | -0.58 (-1.63-0.48) | 0.280 |
| Postoperative morbidity (Clavien-Dindo grade ≥ 2) | 12.5% (5/40) | 10.0% (4/40) | -2.5% (-19.0%-14.0%) | 0.724 |
ERAS: Enhanced recovery after surgery.
RESULTS
Clinical characteristics
The clinical characteristics of the patients in both the CG and EG are described in Table 1. There were no significant differences with respect to gender, age, body mass index), American Society of Anesthesiologists - physical status, surgical method, surgical approach, or tumor characteristics.
Postoperative outcomes
Table 2 summarizes postoperative outcomes in both groups. For the primary endpoint (rate of achievement of discharge from the hospital within 12 d), a successful outcome was achieved in 38 of 40 patients (95.0%) in the EG compared to 37 of 40 (92.5%) patients in the CG (Table 2). The lower limit of the two-sided 95%CI for the difference between the study arms (10%) was within the protocol-specified noninferiority margin of 15%. Therefore, ERAS treatment met our criteria for noninferiority to conventional treatment. Mean postoperative hospital stay was also similar between the two groups, as was postoperative morbidity.
Postoperative nutrition state and insulin resistance
Figure 2A shows the results of rapid turnover proteins analysis. Mean serum ALB values on the preoperative day and PODs 1, 3 and 7 in the CG vs EG were 4.40 g/dL vs 4.41 g/dL, 3.11 g/dL vs 3.0 g/dL, 2.86 g/dL vs 2.85 g/dL, and 3.42 g/dL vs 3.44 g/dL, respectively. Mean serum TTR values on the preoperative day and PODs 1, 3 and 7 in the CG vs EG were 24.7 mg/dL vs 24.4 mg/dL, 20.4 mg/dL vs 19.0 mg/dL, 14.6 mg/dL vs 13.1 mg/dL, and 20.0 mg/dL vs 18.4 mg/dL, respectively. Mean serum RBP values on the preoperative day and PODs 1, 3 and 7 in the CG vs EG were 4.18 mg/dL vs 3.82 mg/dL, 2.46 mg/dL vs 1.87 mg/dL, 1.34 mg/dL vs 0.88 mg/dL, and 3.36 mg/dL vs 3.09 mg/dL, respectively. Mean serum Tf values on the preoperative day and POD 7 in the CG vs EG were 201.6 mg/dL vs 205.6 mg/dL and 192.7 mg/dL vs 186.4 mg/dL, respectively. There were no significant differences with respect to ALB, TTR, RBP, and Tf between the two groups. Figure 2B shows the results of HOMA-R analysis. Mean HOMA-R scores on the preoperative day and PODs 1, 3 and 7 in the CG vs EG were 2.46 vs 3.21, 8.83 vs 6.54, 4.04 vs 2.72, and 2.23 vs 3.17, respectively. There was no significant difference between the two groups at any point. Insulin resistance is defined as the HOMA-R score being over 2.5. In a subgroup analysis of patients with insulin resistance (CG: n = 11, EG: n = 20), the mean HOMA-R scores on the preoperative day and PODs 1, 3 and 7 in the CG vs EG were 2.95 vs 2.39, 15.05 vs 6.64, 4.19 vs 3.12, and 2.23 vs 3.17, respectively. There was a significant attenuation of the HOMA-R score on POD 1 in the EG compared to the CG (P = 0.014).
Figure 2.
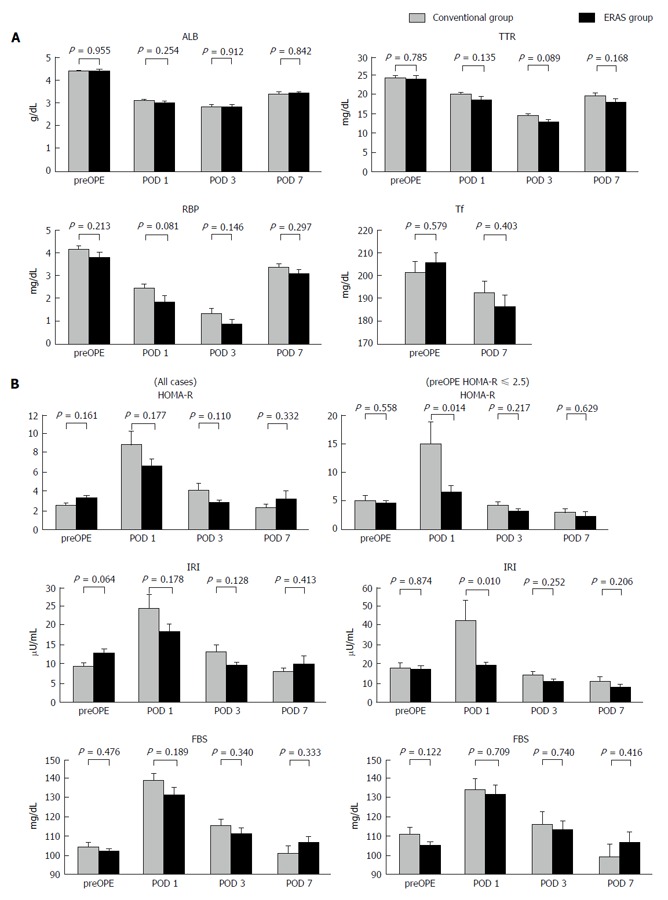
Changes in various serum factors during the perioperative period. A: Rapid turnover proteins; B: Insulin resistance index for all cases (left panel) and for the subgroup of patients in which the preOPE HOMA-R score was over 2.5 (right panel). ALB: Albumin; TTR: Transthyretin; RBP: Retinal-binding protein; Tf: Transferrin; HOMA-R: Homeostatic model analysis ratio-insulin resistance; IRI: Immunoreactive insulin; FBS: Fasting blood sugar; preOPE: Preoperative; POD: Postoperative day.
Quality of life analyses
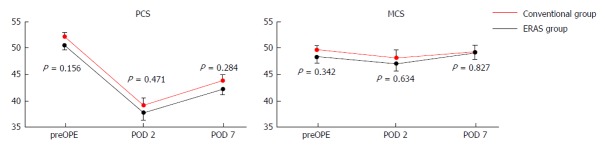
Figure 3 shows the HRQOL scores obtained using the SF8 Health Survey questionnaire. The mean PCS scores on the preoperative day and PODs 2 and 7 in the CG vs EG were 52.5 vs 50.8, 39.2 vs 37.7, and 44.0 vs 42.4 (P = 0.284), respectively. The mean MCS scores on the preoperative day and PODs 2 and 7 in the CG vs EG were 49.9 vs 48.6, 48.4 vs 47.3, and 49.7 vs 49.3, respectively. There were no significant differences with respect to PCS and MCS between the two groups.
Figure 3.
Scores obtained with the SF8 questionnaire during the perioperative period. PCS: Physical component summary; MCS: Mental component summary; preOPE: Preoperative; POD: Postoperative day; ERAS: Enhanced recovery after surgery.
Adverse events and other factors
Adverse events such as vomiting and aspiration associated with the induction of general anesthesia were not observed. There were no significant differences with respect to postoperative urine volume, weight change, and oral intake between the two groups (data not shown).
DISCUSSION
There are some reports on the usefulness of ERAS programs for gastrectomy[6-11]; however, the data are not yet conclusive. Almost every report concerning gastrectomy described the safety of ERAS but not its influence on nutrition state, insulin resistance, QOL, and other such parameters. In this study, we evaluated the effect of postoperative early oral nutrition and preoperative carbohydrate loading.
Our data showed that postoperative early oral nutrition did not increase morbidity, and the rate of achieving discharge from the hospital within 12 d was not compromised. Thus, our ERAS program was deemed to be safe. In fact, the period of hospital stay tended to be shorter in the EG compared to the CG, although the difference was not significant. Most patients do not wish to be discharged until they can ingest soft foods. We surmise that hospital stay can be further shortened if we inform the patients prior to their surgeries that they can be eligible for discharge once they no longer require intravenous nutrition, in order to incentivize them.
It was reported that marked insulin resistance was present after upper abdominal surgery[14]. Studies in diabetics undergoing surgery have shown that postoperative hyperglycemia has negative effects on wound infection[15]. This observation is not surprising, because poor glucose control in diabetes is associated with complications. There are two main reasons for experiencing postoperative insulin resistance. One is that the perioperative starvation induces the accumulation of lipid products in skeletal muscles and interferes with insulin signaling to produce insulin resistance[16]. The other is that the reaction of a living body to the surgical stress induces stress hormones (such as catecholamines, cortisol, and glucagon) as well as the release of the inflammatory cytokine interleukin-6[2,17,18]. To reduce postoperative insulin resistance, preoperative carbohydrate loading may be useful to counter preoperative starvation[2,3]. In this study, therefore, patients in the EG were administered carbohydrate-rich liquids until 2 h before surgery. There were no significant differences in insulin resistance between the two groups in the overall analysis. However, in subgroup analysis of patients with high preoperative HOMA-R, there was significant improvement in postoperative insulin resistance in the EG (Figure 2), presumably due to preoperative carbohydrate loading. This study is the first to suggest that preoperative carbohydrate loading in gastrectomy patients attenuates postoperative insulin resistance and thus lowers the risk of additional complications. It is reported that preoperative carbohydrate-rich beverages also reduce preoperative thirst, hunger and anxiety[19], and also carry postoperative benefits such as a small reduction in the length of hospital stay when compared with placebo-administered or fasting adult patients undergoing elective surgery[20]. However, the clinical consequences of improving postoperative insulin resistance are still unclear. In our study, the morbidity rate and the length of hospital stay were similar between EG and CG; however, further investigations are required to clarify the clinical influence of improving postoperative insulin resistance.
Our study was not able to demonstrate any benefit of the ERAS program on nutritional state. In the CG, oral intake began on POD 2, whereas it commenced on POD 1 in the EG. This interval is too short to allow assessment of benefits on nutrition states. In terms of HRQOL, there was no significant difference between the two groups. We expected that a shorter period of fasting would have decreased mental stress in EG; however, data obtained through the SF8 Health Survey questionnaire did not reveal any such benefits. A more thorough questionnaire might better reflect early postoperative HRQOL status.
In conclusion, we demonstrated the non-inferiority of the ERAS program with respect to the rate of achieving discharge from the hospital within 12 d post-gastrectomy. Moreover, our results suggest that our ERAS program for gastrectomy is safe and improves postoperative insulin resistance in those patients who were originally insulin resistant. To our knowledge, this study is also the first to demonstrate that preoperative carbohydrate loading in gastrectomy patients attenuates postoperative insulin resistance. However, we did not observe any advantage in postoperative nutrition state and HRQOL due to our ERAS program. It is still uncertain whether the ERAS program is superior to conventional programs because the clinical benefits attributed to this program were not significant.
COMMENTS
Background
There are some reports on the safety of enhanced recovery after surgery (ERAS) programs for gastrectomy. In this study, the authors evaluated the effect of postoperative early oral nutrition and preoperative carbohydrate loading.
Research frontiers
Almost every report concerning gastrectomy described the safety of ERAS but not its influence on nutrition state, insulin resistance, quality of life, and other such parameters.
Innovations and breakthroughs
The authors demonstrated that their ERAS program for gastrectomy is safe and improves postoperative insulin resistance in those patients who were originally insulin resistant.
Applications
It is still uncertain whether the ERAS program is superior to conventional programs because the clinical benefits attributed to this program were not significant.
Terminology
ERAS program consist of many elements, including preoperative education, preoperative carbohydrate loading, omission of bowel preparation, epidural analgesia without opioids, early postoperative enteral feeding, early mobilization of patients, and thromboprophylaxis.
Peer-review
The authors proposed a good trial of ERAS in patients after gastrectomy for gastric cancer. It’s a promising idea to improve the recovery efficiency of patients. This manuscript was designed well, written fluently, and deduced credibly.
Footnotes
Institutional review board statement: The study was reviewed and approved by the institutional review board of the study institution (Hiroshima University, Japan).
Clinical trial registration statement: This study is registered at UMIN Clinical Trials Registry. The registration identification number is UMIN000020538.
Informed consent statement: All study participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Data sharing statement: Dataset is available from the corresponding author at ktanabe2@hiroshima-u.ac.jp.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 27, 2015
First decision: January 30, 2016
Article in press: March 16, 2016
P- Reviewer: Deng JY, Matsuda T, Mori H S- Editor: Gong ZM L- Editor: A E- Editor: Liu SQ
References
- 1.Fearon KC, Ljungqvist O, Von Meyenfeldt M, Revhaug A, Dejong CH, Lassen K, Nygren J, Hausel J, Soop M, Andersen J, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24:466–477. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Ljungqvist O, Nygren J, Thorell A. Insulin resistance and elective surgery. Surgery. 2000;128:757–760. doi: 10.1067/msy.2000.107166. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Wang Z, Ying X, Tian J, Sun T, Yi K, Zhang P, Jing Z, Yang K. Preoperative carbohydrate loading for elective surgery: a systematic review and meta-analysis. Surg Today. 2012;42:613–624. doi: 10.1007/s00595-012-0188-7. [DOI] [PubMed] [Google Scholar]
- 4.King PM, Blazeby JM, Ewings P, Longman RJ, Kipling RM, Franks PJ, Sheffield JP, Evans LB, Soulsby M, Bulley SH, et al. The influence of an enhanced recovery programme on clinical outcomes, costs and quality of life after surgery for colorectal cancer. Colorectal Dis. 2006;8:506–513. doi: 10.1111/j.1463-1318.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- 5.Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189–198. doi: 10.1097/SLA.0b013e31817f2c1a. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi H, Sasaki T, Fujita H. Preoperative management of surgical patients by “shortened fasting time”: a study on the amount of total body water by multi-frequency impedance method. Int J Med Sci. 2012;9:567–574. doi: 10.7150/ijms.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada T, Hayashi T, Cho H, Yoshikawa T, Taniguchi H, Fukushima R, Tsuburaya A. Usefulness of enhanced recovery after surgery protocol as compared with conventional perioperative care in gastric surgery. Gastric Cancer. 2012;15:34–41. doi: 10.1007/s10120-011-0057-x. [DOI] [PubMed] [Google Scholar]
- 8.Dorcaratto D, Grande L, Pera M. Enhanced recovery in gastrointestinal surgery: upper gastrointestinal surgery. Dig Surg. 2013;30:70–78. doi: 10.1159/000350701. [DOI] [PubMed] [Google Scholar]
- 9.Pędziwiatr M, Matłok M, Kisialeuski M, Migaczewski M, Major P, Winiarski M, Budzyński P, Zub-Pokrowiecka A, Budzyński A. Short hospital stays after laparoscopic gastric surgery under an Enhanced Recovery After Surgery (ERAS) pathway: experience at a single center. Eur Surg. 2014;46:128–132. doi: 10.1007/s10353-014-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada T, Hayashi T, Aoyama T, Shirai J, Fujikawa H, Cho H, Yoshikawa T, Rino Y, Masuda M, Taniguchi H, et al. Feasibility of enhanced recovery after surgery in gastric surgery: a retrospective study. BMC Surg. 2014;14:41. doi: 10.1186/1471-2482-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong O, Ryu SY, Jung MR, Choi WW, Park YK. The safety and feasibility of early postoperative oral nutrition on the first postoperative day after gastrectomy for gastric carcinoma. Gastric Cancer. 2014;17:324–331. doi: 10.1007/s10120-013-0275-5. [DOI] [PubMed] [Google Scholar]
- 12.Hashine K, Nakashima T, Iio H, Ueno Y, Shimizu S, Ninomiya I. Health-related quality of life in the first year after laparoscopic radical prostatectomy compared with open radical prostatectomy. Jpn J Clin Oncol. 2014;44:686–691. doi: 10.1093/jjco/hyu052. [DOI] [PubMed] [Google Scholar]
- 13.Tani M, Kawai M, Okada K, Hirono S, Hotta T, Takifuji K, Yamaue H. Evaluation of the health-related quality of life for patients following laparoscopic cholecystectomy. Surg Today. 2015;45:564–568. doi: 10.1007/s00595-014-0938-9. [DOI] [PubMed] [Google Scholar]
- 14.Thorell A, Efendic S, Gutniak M, Häggmark T, Ljungqvist O. Insulin resistance after abdominal surgery. Br J Surg. 1994;81:59–63. doi: 10.1002/bjs.1800810120. [DOI] [PubMed] [Google Scholar]
- 15.Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63:356–361. doi: 10.1016/s0003-4975(96)01044-2. [DOI] [PubMed] [Google Scholar]
- 16.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 17.Thorell A, Loftenius A, Andersson B, Ljungqvist O. Postoperative insulin resistance and circulating concentrations of stress hormones and cytokines. Clin Nutr. 1996;15:75–79. doi: 10.1016/s0261-5614(96)80023-9. [DOI] [PubMed] [Google Scholar]
- 18.Thorell A, Nygren J, Ljungqvist O. Insulin resistance: a marker of surgical stress. Curr Opin Clin Nutr Metab Care. 1999;2:69–78. doi: 10.1097/00075197-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Hausel J, Nygren J, Lagerkranser M, Hellström PM, Hammarqvist F, Almström C, Lindh A, Thorell A, Ljungqvist O. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93:1344–1350. doi: 10.1097/00000539-200111000-00063. [DOI] [PubMed] [Google Scholar]
- 20.Smith MD, McCall J, Plank L, Herbison GP, Soop M, Nygren J. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev. 2014;8:CD009161. doi: 10.1002/14651858.CD009161.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]