Abstract
Rheumatoid arthritis (RA) is associated with increased cardiovascular (CV) morbidity and mortality which cannot be fully explained by traditional CV risk factors; cumulative inflammatory burden and antirheumatic medication-related cardiotoxicity seem to be important contributors. Despite the acknowledgment and appreciation of CV disease burden in RA, optimal management of individuals with RA represents a challenging task which remains suboptimal. To address this need, the European League Against Rheumatism (EULAR) published recommendations suggesting the adaptation of traditional risk scores by using a multiplication factor of 1.5 if two of three specific criteria are fulfilled. Such guidance requires proper coordination of several medical specialties, including general practitioners, rheumatologists, cardiologists, exercise physiologists and psychologists to achieve a desirable result. Tight control of disease activity, management of traditional risk factors and lifestyle modification represent, amongst others, the most important steps in improving CV disease outcomes in RA patients. Rather than enumerating studies and guidelines, this review attempts to critically appraise current literature, highlighting future perspectives of CV risk management in RA.
Keywords: cardiovascular disease, cardiovascular management, cardiovascular risk, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is related with excess overall mortality [Dadoun et al. 2013] mainly due to increased cardiovascular (CV) disease, which accounts for over 50% of premature deaths in this population [Aviña-Zubieta et al. 2008]. The link between RA and CV morbidity has been unequivocally established in historical cohorts, as the disease effect on CV risk is considered comparable to that of diabetes [Van Halm et al. 2009; Lindhardsen et al. 2011]. RA patients appear to have about a twofold higher possibility for myocardial infarction than non-RA patients, similar with diabetes [Peters et al. 2009]. Other CV manifestations including valvular heart disease, arrhythmia, pericarditis and endocarditis as well as rheumatoid cardiac nodules have also been described but rarely cause clinically overt disease [Kitas et al. 2001]. On the contrary, myocarditis and microvascular disease are common, as suggested by newer imaging techniques, although their contribution to CV mortality remains unclear [Mavrogeni et al. 2009, 2014a]. Furthermore, RA is associated with a twofold higher possibility for heart failure with a worse prognosis than non-RA patients [Nicola et al. 2005]. Of note, diastolic heart failure with preserved ejection fraction seems to be more prevalent reflecting the influence of chronic inflammation on the myocardium [Yndestad et al. 2007; Davis et al. 2008; Mavrogeni et al. 2012; Mavrogeni et al. 2014b]. Accordingly, ventricular diastolic dysfunction and pulmonary hypertension represent frequent findings in long-term treated RA patients, even in the absence of clinically evident CV disease or traditional CV risk factors [Gonzalez-Juanatey et al. 2004]. In any case, atherothrombosis and especially coronary artery disease (CAD) hold the pivotal role to the increased mortality of the disease [Skeoch and Bruce, 2015] and are associated with more severe presentation and worse outcomes compared to the general population [Douglas et al. 2006; Mantel et al. 2015].
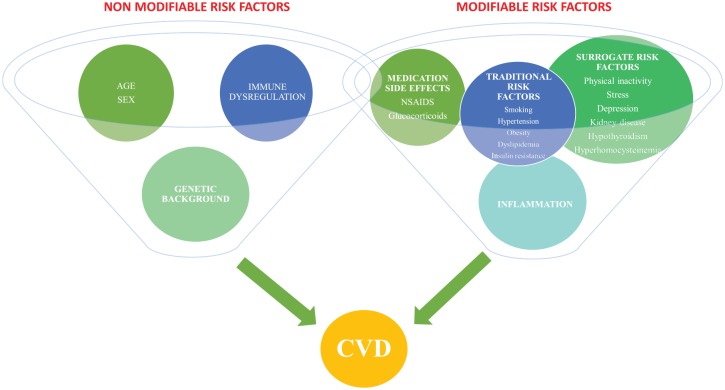
Traditional risk factors such as hypertension, smoking, dyslipidemia and obesity contribute to the endothelial dysfunction in RA but cannot fully explain the high magnitude of CV disease. Other RA-related factors, such as anti-inflammatory treatment side effects, extra-articular RA, and predominantly the chronic high-grade inflammatory state of the disease have been linked to the development of premature atherosclerosis (Figure 1) [Amaya-Amaya et al. 2013; Crowson et al. 2013; Beinsberger et al. 2014; Sandoo et al. 2015]. In addition, the inevitable sedentary lifestyle of RA patients confers an increased risk for CV disease [Naranjo et al. 2008].
Figure 1.
The complex interrelations between several risk factors in the development of premature atherosclerosis in RA. Modifiable risk factors represent a broad spectrum of heterogeneous parameters including traditional, surrogate and novel mainly RA-related risk factors. Age, sex, genetic basis of autoimmunity and atherosclerosis, as well as the presence of disease specific autoantibodies, are also drivers of vascular disease contributing to a lesser or greater extent to CV complications in this population.
CVD, cardiovascular disease; NSAIDs, nonsteroidal anti-inflammatory drugs.
Taken together, the atypical symptomatology that characterizes the occurrence of coronary syndromes in RA, the lack of large randomized-controlled trials (RCT), and the poor integration of prevention strategies in the management of patients, render CV risk assessment an important and challenging task among these individuals.
In this review rather than enumerating clinical studies and guidelines, we critically appraise current evidence about CV disease in RA, highlighting the existing controversies on the management of patients and providing future perspectives.
Traditional risk factors
Smoking
Current and exsmokers are more prevalent among RA patients. Specifically, the possibility of a RA patient being a current or an exsmoker is about 1.5 times higher than the general population [Boyer et al. 2011]. This is not unexpected as the causal link of smoking and the occurrence of RA has already been established [Bergstrom et al. 2011]. In addition, smoking has been associated with rheumatoid factor (RF) and anticitrullinated protein antibody (ACPA) positivity as well as more severe clinical presentation reflected by increased disability and radiographic damage [Rojas-Serrano et al. 2011]. Moreover, a recent meta-analysis confirmed the association of smoking with the CV risk in RA [Baghdadi et al. 2015].
Hypertension
The evidence about hypertension in RA appears conflicting. A meta-analysis that included seven case-control studies showed that no significant differences existed on the prevalence of hypertension amongst RA subjects and controls [Boyer et al. 2011]. In contrast, Panoulas and colleagues demonstrated a relatively higher prevalence [Panoulas et al. 2007] whilst the results of the international COMORA study reported that hypertension was prevalent in 40% of RA patients [Dougados et al. 2014]. Regardless of the occurrence of hypertension, the reported high rates of underdiagnosis and undertreatment of this condition within RA populations, particularly in younger and older overweight patients [Panoulas et al. 2007], as well as its association with end-organ damage [Panoulas et al. 2010] mandate a more aggressive strategy, targeting effective control of blood pressure and potential reduction of CV risk.
The diminished ability of the arterial system to adapt to alterations in the blood flow is a mechanism contributing to higher blood pressure in RA individuals. Several studies have showed that reduced arterial wall pliability and increased stiffness are involved in the pathogenesis of hypertension are also associated with the disease [Ben-Shlomo et al. 2014]. On the other hand, hypertension appears to cause more adverse effects to RA patients than the general population.
Despite the fact that disease modifying drugs have not been proven to increase blood pressure, the effect of other regimens such as nonsteroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids is substantial. Also, additional comorbidities including obesity, stress and physical inactivity may contribute to the development of hypertension. Proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) are associated with higher blood pressure illustrating the reciprocal link between hypertension and inflammation: the former causes vascular damage and thus induces inflammation via oxidative stress whereas the latter appear to increase arterial stiffness and wall pliability [Cohen Tervaert, 2011; McMaster et al. 2015].
Dyslipidemia
Abnormal lipid metabolism and especially low density lipoprotein (LDL) hold a key role in the atherosclerotic procedure. Dyslipidemia is common in RA affecting between 55–65% of patients [Toms et al. 2010]. Paradoxically, lower lipid levels are associated with increased CV risk in RA [Semb et al. 2010] whereas levels of LDL, high density lipoprotein (HDL), and total cholesterol (TC) are inversely correlated with markers of chronic inflammation, forming a complex puzzle which results in premature atherosclerosis [Myasoedova et al. 2011]. Inflammation promotes consumption or reduces synthesis of lipoproteins whereas functional and structural changes of these molecules may also occur during periods of high disease activity [Carpentier and Scruel, 2002]. For example, HDL function is impaired and may even express proinflammatory characteristics in about 20% of RA patients [Hahn et al. 2008]. In line with this, the cholesterol efflux capacity of HDL is impaired in association with a reduced antioxidant activity [Charles-Schoeman et al. 2012]. To lend more support, high-grade inflammation in severe RA correlates with lower adiponectin concentration which seems to cluster with atherogenic dyslipidemia (TC/HDL ratio and triglycerides/HDL ratio) as well as with high plasma glucose levels [Gonzalez-Gay et al. 2008].
The apolipoprotein-related mortality risk (AMORIS) study evaluated the prognostic significance of TC and triglycerides for CV events among RA and non-RA patients. A statistically significant predictive value was demonstrated for non-RA patients whereas the results for RA patients were inconsistent [Semb et al. 2010]. In addition, impaired LDL, increased apolipoprotein (apo)-B/apo-A1 ratio and lipoprotein (a) levels seem to promote accelerated atherosclerotic procedure [Robertson et al. 2013]. The above controversies reveal the significance of the confounding effect of inflammation. In this context, the TC/HDL ratio (atherogenic index) has been recommended as a better marker for CV risk assessment because of the parallel decrement of their circulating levels during inflammation [Robertson et al. 2013]. Accordingly, the assessment of lipid profile during low-disease activity may produce more reliable estimations, but more research is required to confirm this approach.
The complexity of lipid metabolism, its effect on the CV risk and the unacceptable low rate of primary lipid screening in RA patients [Bartels et al. 2011] is reflected in the underutilization of lipid-lowering therapy even in RA patients who fulfill general population thresholds for treatment [Toms et al. 2010] and underlines the necessity for the incorporation of guidelines in the routine examination and follow up of people with RA. In this way, earlier identification of individuals at higher risk, implementation of prevention strategies and prompt introduction of appropriate treatment can be achieved.
Body mass index, obesity and physical inactivity
Alterations in body composition exhibit another paradoxical relationship in RA as lower body mass index (BMI) is associated with increased CV risk. Contrary to its protective effect in non-RA patients [Escalante et al. 2005], low BMI independently predicts CV death even after adjusting for the most important risk factors [Maradit-Kremers et al. 2005]. The underlying pathophysiological mechanism comprises of the effect of chronic uncontrolled systemic inflammation on adipose tissue which leads to muscle degradation and involuntary fat accumulation, a condition known as rheumatoid cachexia, characterized by stable or slightly increased body weight [Summers et al. 2010]. Of note, sarcopenia, overfat and sarcopenic obesity seem to be more prevalent in normal weight RA patients underlining the inaccuracy of BMI as a surrogate for body composition [Giles et al. 2008]. Obesity contributes to the development of inflammation via changes in metabolism and function of adipose tissue [Dessein and Solomon, 2013]. Furthermore, obesity appears to coexist with other CV risk factors such as hypertension, insulin resistance and dyslipidemia [Bray and Bellanger, 2006]. There is a complex interrelation between obesity, physical inactivity, RA and CV disease. Patients with RA adapt an inactive lifestyle with significantly reduced physical activity due to a number of reasons, including joint pain and stiffness, psychological disturbances or even fear of aggravating their disease, which apparently worsens the CV risk profile and leads to a higher BMI [Metsios et al. 2009]. Only a few cross-sectional studies have looked into factors that may influence obesity in RA and they have found a relationship between increased body weight and limited physical activity, low energy intake and underweight state [Stavropoulos-Kalinoglou et al. 2010] as well as a lowest prevalence of obesity amongst active smokers [Stavropoulos-Kalinoglou et al. 2008]. Despite the fact that obesity is considered to be a potent contributor to the inflammatory pathogenesis of atherosclerosis [Berg and Scherer, 2005] and observations indicating that it independently associates with the presence of traditional CV risk factors [Stavropoulos-Kalinoglou et al. 2009] and higher C-reactive protein (CRP) concentrations in RA [Dessein et al. 2007], a clear relationship between accumulation of excessive fat and heightened CV risk in this population is lacking [Stavropoulos-Kalinoglou et al. 2011]. For example, Wolfe and Michaud reported that classical CV risk factors but not obesity influenced the occurrence rates of myocardial infarction in RA patients [Wolfe and Michaud, 2012]. The paradoxical effects of obesity in this population appear to extend beyond CV disease as published data show that obese RA patients have a lower degree of joint involvement and damage suggesting an uncoupling of high CRP levels from synovial inflammation [Van Der Helm-Van Mil et al. 2008].
Finally, it is worth noting that BMI thresholds and definitions of obesity utilized in the general population may not capture these specific changes in body composition among RA patients [Stavropoulos-Kalinoglou et al. 2007] indicating the necessity for alternative tools such as waist to hip ratio, which may more accurately demonstrate lean and fat tissue changes in this population. Clearly, much research is required to elucidate the mechanisms and establish the biological basis of these phenomena.
Insulin resistance and metabolic syndrome
The prevalence of insulin resistance is increased in RA, underlying the role of several atherogenic processes such as endothelial dysfunction, lipid metabolism dysregulation and acute phase response which are common in both conditions [Dessein et al. 2003]. It represents the main mechanism of metabolic syndrome which is also more frequent among RA and is associated with increased CV morbidity and mortality [Da Cunha et al. 2012]. Furthermore, insulin resistance seems to provoke chronic low-grade inflammation, regardless of the disease’s activity whereas high-disease activity precipitates the adverse effects of metabolic syndrome in endothelial dysfunction [Ferraccioli and Gremese, 2011]. The use of glucocorticoids, antihypertensive treatment and abdominal obesity are considered additional contributors to the alteration of the glucose metabolism in RA [Dessein and Joffe, 2006], however, the exposure to oral steroids was not found to be associated with the prevalence of metabolic syndrome in a cross-sectional study including 398 RA subjects [Toms et al. 2008]. Finally, administration of methotrexate has demonstrated a significant negative correlation with the presence of metabolic syndrome [Toms et al. 2009], possibly due to its anti-inflammatory effects. A dramatic improvement of insulin resistance and insulin sensitivity was observed following a single infusion of the anti-TNF monoclonal antibody, infliximab. Long-term positive effects of TNF-α antagonists, infliximab and etanercept, on insulin resistance in RA patients with severe disease has also been reported [Gonzalez-Gay et al. 2010]. However, anti-TNF-α regimens seem to improve insulin sensitivity in normal weight but not obese RA patients [Stavropoulos-Kalinoglou et al. 2012].
RA-related risk factors
RA inflammation and immune dysregulation
The chronic high-grade inflammatory state of RA, the severity of the disease as reflected by joint erosions, extra-articular manifestations, and the ensuing physical disability are established risk factors for CV disease in this population [Kremers et al. 2008]. Accordingly, inflammatory and serological markers such as erythrocyte sedimentation rate, RF, ACPA and CRP have been positively associated with increased atherogenicity and CV events in several studies assessing RA patients [Farragher et al. 2008; Kremers et al. 2008; Liang et al. 2009]. as well as in the general population [Skeoch and Bruce, 2015].
It is now well-recognized that systemic inflammation contributes to the initiation and development of accelerated atherosclerosis since inflammatory processes in the rheumatoid synovium and atherosclerotic plaques are remarkably similar [Stevens et al. 2005]. The increased synthesis and release of proinflammatory cytokines such as TNF-α and IL-6 to the systemic circulation leads to endothelial dysfunction, the initial step to atherosclerosis. TNF-α induces cytokine release by activating the monocytes and IL-6 activates immune cells involved in the plaque formation and rupture [Choy, 2012]. To lend more support, RA patients without a history of CV disease had more frequent coronary plaques, which were more severe and more prone to rupture, compared to matched controls in a recent study [Karpouzas et al. 2014]. This is in line with previous observations indicating instability of atherosclerotic plaque, probably due to the high magnitude of vascular inflammatory load, as one of the most important factors of higher rates and worse outcomes of acute coronary events in RA patients [Aubry et al. 2007]. Last but not least, chronic high-grade systemic inflammation precipitates the adverse effects of traditional CV risk factors on vascular wall underlying the complexity of the links between RA-related factors, impairment of endothelial function and myocardial injury [Mavrogeni et al. 2009; Sandoo et al. 2012; Liao and Solomon, 2013].
Immune activation, as reflected by the presence of autoantibodies, is considered as an important nonmodifiable risk factor for CV disease in RA. RF and antinuclear antibody positivity have been associated with an increased risk for myocardial infarction, heart failure and vascular disease even after adjusting for the presence of rheumatic disease [Liang et al. 2009]. Moreover, ACPA are closely linked with endothelial dysfunction and seem to promote accelerated atherogenicity [Hjeltnes et al. 2011]. Considering all the above, systemic inflammation and immune dysregulation serve as major RA-related CV risk factors which may, at least partly, account for the excess of CV disease in RA.
Antirheumatic drug-related cardiotoxicity
The use of NSAIDs is associated with increased CV risk, hypertension and heart failure in the general population; however their effect in RA patients remains a subject of controversy. Goodson and colleagues showed that there was no correlation between the use of NSAIDs and increased CV risk in early undifferentiated inflammatory arthritis whereas a meta-analysis by Trelle and colleagues concluded that there is not enough evidence to guarantee the safety of these drugs [Goodson et al. 2009; Trelle et al. 2011]. A more recent, large scale longitudinal cohort study that used data from the Danish nationwide registry showed that the increased CV risk associated with the overall use of NSAIDs in RA was modest and significantly lower than in non-RA subjects [Lindhardsen et al. 2013]. This pattern remained true for the individual NSAIDs investigated with the exception of rofecoxib and diclofenac which were both associated with a significantly increased CV risk [Lindhardsen et al. 2013]. Current recommendations suggest the use of NSAIDs with caution in RA individuals, especially in the presence of additional CV risk factors or established CV disease [Marks et al. 2012]. Nonetheless, further research is needed in order to fully comprehend the complex impact of these regimens on the CV risk in RA populations.
The administration of glucocorticoids is generally associated with increased CV risk considering the drug’s effect on hypertension and lipid or glucose metabolism. In agreement, several studies confirm this adverse effect in RA which appears to be dose dependent [Avina-Zubieta et al. 2013; Del Rincon et al. 2014; Listing et al. 2015]. Daily doses of prednisone <5–7.5 mg are considered relatively well tolerated. Nonetheless, there are studies which demonstrate that the anti-inflammatory effect of glucocorticoids counteracts the increment of the CV risk and therefore their use may be beneficial [Naranjo et al. 2008]. This concurs with observations indicating a lack of association between steroid use and metabolic syndrome in RA individuals [Toms et al. 2008]. Of note, EULAR guidelines recommend the use of glucocorticoids at the lowest dose, for the shortest period of time [Peters et al. 2010].
Although biologic drugs have enormously improved clinical outcomes in RA patients conferring a potential beneficial effect on the CV risk, anti-TNF-α therapy should be administered with caution in patients with moderate or severe heart failure as it may lead to a deterioration of previously established cardiac disease [Listing et al. 2008]. TNF-α inhibitors, leflunomide and less commonly used cyclosporine have been linked with increased risk of developing high blood pressure in RA patients [Gasparyan et al. 2012; Zhao et al. 2015]. Disease modifying drugs, especially methotrexate, may exhibit adverse effects on lipid profile and similar observations have been recently reported for IL-6 inhibitors [Souto et al. 2015]. It is worth noting that even emerging treatments for RA including small molecules, such as spleen tyrosine kinase inhibitors, have been associated with high blood pressure in randomized controlled trials [Kitas et al. 2014]. Given the wide variety of CV side effects related to the administration of broadly used conventional and biologic disease modifying drugs, the appreciation of unfavorable effects of individual regimens on traditional and disease-related CV risk factors represents an important element of CV prevention and physicians treating RA patients should be aware of this additional risk.
Genetic risk factors
The genetic background is also related to the heightened CV disease risk in RA. Human leukocyte antigen (HLA)-DRB1*04 shared epitope alleles seem to predispose for CV events [Gonzalez-Gay et al. 2007]. Interestingly, TNF-α rs1800629 gene polymorphism was also associated with increased risk of CV disease in patients who carried the rheumatoid shared epitope [Rodriguez-Rodriguez et al. 2011]. In addition, there are polymorphisms outside the major histocompatibility complex (MHC) region that demonstrate increased prognostic value for atherosclerosis even after adjusting for traditional CV risk factors [Palomino-Morales et al. 2010; Lopez-Mejias et al. 2012]. Various polymorphisms have also been connected to an increased prevalence of hypertension, dyslipidemia, and higher levels of endothelial dysfunction markers such as asymmetric dimethylarginine [Panoulas et al. 2008; Toms et al. 2012; Dimitroulas et al. 2014].
Additional risk factors
The aggravated psychological state of the patients and especially stress may be important for the CV risk. Stress dysregulates immune system and may trigger autoimmune proinflammatory processes. In this regard, Solomon and colleagues observed an association between symptoms of tension and carotid plaque occurrence in RA in a study including black and white Africans [Solomon et al. 2012].
The relationship between depression and systemic inflammation is reciprocal. The former seems to promote inflammation through immune activation whereas the latter may induce depressive symptoms. In general the poor compliance to chronic therapies and the general difficulty in the management and persuasion of depressive patients may have an additional input to the increased comorbidities in this special subgroup of RA patients.
Finally, kidney disease, hypothyroidism, hyperhomocysteinemia and vitamin D deficiency, which are common in RA individuals, contribute to the increased CV risk and should receive the appropriate attention by clinicians [Daoussis et al. 2010; Gonzalez-Gay et al. 2010; Haque et al. 2012; Raterman et al. 2012]
Cardiovascular risk assessment: a Gordian knot in RA
Calculators used to assess the CV risk in the general population (Systematic Coronary Risk Evaluation [SCORE], Framingham) underestimate it in RA, although some of them incorporate high sensitivity CRP in the estimation of CV risk [Gonzalez et al. 2008; Crowson et al. 2012]. In addition, all the traditional calculators have been developed on the perception that men exhibit a greater risk for CV disease than women, who represent the major population in RA. For example, about one fourth of women with RA that are classified as very low risk according to the SCORE calculator, exhibit carotid plaques which reclassifies them to the high risk category [Corrales et al. 2015]. It is worth noting that QRISK2 which is used in the United Kingdom, includes RA as an independent risk factor and seems to improve the risk estimation [Hippisley-Cox et al. 2008]. However, Arts and colleagues demonstrated that QRISK2 overestimates the CV risk [Arts et al. 2015]. European Society of Cardiology (ESC) guidelines in 2012 also incorporate RA as a CV risk factor but without promoting significant alterations concerning the clinical management of the patients [Perk et al. 2012]. Finally, the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines increased the proportion of patients with RA who were eligible for lipid-lowering therapy although the CV risk estimation remained inaccurate [Kawai et al. 2015; Tournadre et al. 2015].
The need of including the strong confounding effect of inflammation in the CV risk stratification of patients along with the mounting evidence of the excess of CV disease in RA led EULAR to develop in 2010 specific recommendations for CV risk assessment and management of patients with RA and other inflammatory diseases (Table 1) [Peters et al. 2010]. These recommendations are based on evidence-based expert consensus opinion but data from large-scale prospective studies is lacking and, as the authors concurred, they should be reexamined in the light of new evidence.
Table 1.
EULAR recommendations for cardiovascular risk assessment and management [Peters et al. 2010].
| 1. RA is associated with greater risk for CVD |
| 2. The control of the disease activity is imperative for lowering the CVR |
| 3. Cardiovascular risk assessment according to national guidelines, annually or whenever the antirheumatic treatment changes, is necessary for all patients with RA |
4. Risk score models should be adapted for RA patients after the multiplication by a factor of 1.5 if two of the three following criteria are fulfilled:
|
| 5. When the SCORE model is applied the TC/HDL ratio should be used |
| 6. Pharmacological treatments should follow the national guidelines |
| 7. Statins, ACE inhibitors/A-II blockers are the first treatment options |
| 8. The effect of NSAIDs and coxibs on the CVR is not well established. Caution is required when prescribing them, especially for patients with documented CVD or at high CVR |
| 9. The lowest dose possible of corticosteroids is advised |
| 10. Smoking cessation is recommended |
ACE, angiotensin converting enzyme; ACPA, anticitrullinated protein antibody; CVD, cardiovascular disease; CVR, cardiovascular risk; HDL, high density lipoprotein; NSAID, nonsteroidal anti-inflammatory drug; RA, rheumatoid arthritis; RF, rheumatoid factor; TC, total cholesterol.
EULAR recommendations suggest the adaptation of traditional calculators like SCORE and Framingham and attempt to adjust them for RA by the multiplication of the measured risk score by a factor of 1.5 if two of the three criteria below are fulfilled: (1) RA disease duration >10 years, (2) presence of RF or anti-CCP and (3) presence of severe extra-articular manifestations. In addition, EULAR proposes an annual CV reassessment excluding patients with low CV risk or low disease activity which may be followed up every two or three years [Peters et al. 2010].
Several studies highlight the inefficiency of the EULAR recommendations demonstrating that the CV risk score in RA seems to be underestimated even after the aforementioned multiplication. In a study of 327 patients by Corrales and colleagues, 96 individuals were classified as low risk according to SCORE, 201 at moderate risk, and 30 at high or very high risk [Corrales et al. 2014]. After the multiplication for RA, only 5 (2%) of patients were reclassified as high or very high risk. Another more recent study demonstrated that the percentage of patients’ reclassification after applying the EULAR adjustment for RA was relatively low, regardless of the risk score calculator used, with a maximum value of 12% [Karpouzas et al. 2013].
Other aspects of CV risk, that are not included in CV risk calculators for the general population, should be taken into account in RA patients. For example, the identification of subclinical atherosclerosis may alter the risk score calculations with classifying more patients at the very high risk category. In this regard, it has been demonstrated that carotid plaque or carotid intima media thickness (cIMT) value >0.9 mm was found in over 60% of RA patients in moderate risk category [Corrales et al. 2014].
On the basis of the above findings, EULAR introduced revised recommendations which have not been published yet. The integration of carotid ultrasonography in the screening of patients and the multiplication of the risk score by 1.5 irrespective of the presence of the aforementioned three criteria represent new additions [Nurmohamed, 2015]. The effectiveness of these new recommendations remains to be established by future studies.
Cardiovascular risk management in RA: from uniformity to individuality
It is well accepted that physicians with different sub-specialties, rheumatologists, cardiologists and general practitioners, should be involved in the management of patients with RA although there is still a confusion regarding the role of each health provider in evaluating and treating CV risk. The key point of optimal coordination is the awareness of the increased CV disease burden accompanied by appropriate determination of the CV risk profile. The referral of high-risk individuals to a primary care physician or a skilled cardiologist, followed by educational programs for the patients focusing on the importance of lifestyle modification, constitute further necessary actions [Semb et al. 2014]. Lessons taken by other conditions, and particularly diabetes mellitus, should be incorporated in RA populations, for example, the implementation of primary prevention therapy for the management of traditional CV risk factors achieving predetermined targets for blood pressure and cholesterol levels. On the top of these interventions tight control of inflammation is necessary for the decrease of CV risk (Figure 2). Due to the frequent atypical symptomatology of CV disease, a high index of suspicion for CV events is required predominantly in the presence of any suspicious signs or symptoms such as tiredness, chest pain or gradually deteriorated dyspnea. It is important, that any decision about patients’ management needs to be critically extrapolated from current evidence and recommendations but in the end it should be individualized.
Figure 2.
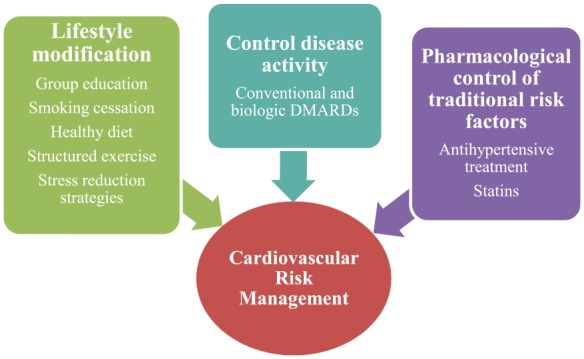
The main domains of CV risk management in RA. Communication of increased CV risk and education of RA patients followed by implementation of lifestyle changes is necessary for the better management of CV disease. Evaluation and treatment of classical risk factors combined with adequate control of disease activity and systemic inflammatory load represent important elements of CV risk prevention and management in RA patients.
CV, cardiovascular; DMARDS, disease modifying antirheumatic drugs; RA, rheumatoid arthritis.
Lifestyle modification
Patients’ awareness of their increased CV risk holds a key role for a successful lifestyle modification strategy. In this regard, John and colleagues developed an eight-week cognitive behavioral patient education program which resulted in significant changes in knowledge and intentions to modify adverse CV disease behaviors accompanied by clinical improvement in blood pressure. Although no actual behavioral changes were observed, such interventions, including group education and distribution of written and online material to RA patients and allied healthcare professionals, constitute modern strategies but their integration to routine clinical setting still remains a challenging task [John et al. 2011, 2013].
Patients with RA should be encouraged to quit smoking, adapt a healthy diet, avoid excessive alcohol intake, control weight and maintain a normal physical activity. Structured exercise demonstrated a protective effect for CV disease in RA patients [Stavropoulos-Kalinoglou et al. 2013; Metsios and Lahart, 2015]. Not surprisingly, physically active patients appear to have a substantial improvement of the symptoms representing the primary barriers for physical activity and exercise, such as pain and fatigue [Veldhuijzen Van Zanten et al. 2015]. Moreover, individualized or group counselling aiming to improve the psychological state of patients and reduce stress would be appropriate therapeutic actions and valuable additions to the traditional therapeutic strategies.
Control of the traditional risk factors
Blood pressure control in RA patients should follow guidelines applied to the general population as no specific targets have been suggested for RA [Perk et al. 2012; Innala et al. 2011]. EULAR recommend the use of angiotensin-2 receptor inhibitors or angiotensin-converting enzyme blockers as a first option for antihypertensive treatment because of their additional role on inflammation [Peters et al. 2010].
LDL’s indicated threshold in RA differs according to the grade of the CV risk. In very high risk category cholesterol targets are <1.8 mmol/l, in high risk <2.5 mmol/l and in low and moderate risk <3 mmol/l [Perk et al. 2012]. Diet modification represents the first line intervention in order to achieve these goals. When this measure fails, statins are the indicated lipid-lowering therapy even when administered for primary prevention in patients without dyslipidemia [Danninger et al. 2014; Ridker, 2014]. Their beneficial effect on endothelial dysfunction, plaque stabilization and chronic inflammation, independently of cholesterol levels, renders statin treatment a valuable therapeutic agent in RA both in the context of primary or secondary prevention [Maki-Petaja et al. 2007; Paraskevas, 2008].
Due to the high prevalence of insulin resistance in RA, glucose screening is essential and a glucose tolerance test should be considered even in the presence of minor indications. HbA1c should be maintained <7% with the appropriate pharmacological or lifestyle interventions [Perk et al. 2012].
Control of RA activity
The adequate suppression of the disease activity is necessary to counteract the effects of systemic inflammation on the CV risk in RA and it is considered the cornerstone of managing CV disease complications. In addition, the control of symptoms is important due to their role on the restriction of physical activity and deterioration of the psychosocial status of the patient. Unfortunately, all current knowledge derives from observational studies and, therefore, any conclusion should be interpreted with caution.
Methotrexate has been associated with CV risk reduction and improvement of CV disease outcomes. Specifically, in a large meta-analysis of 10 cohort studies, the administration of methotrexate decreased the risk for CV events by 21%, whereas the risk for myocardial infarction by 18% [Micha et al. 2011]. The exact mechanism of this suppressive effect remains unclear but the effective control of systemic inflammation appears to be the main moderator [Roubille et al. 2015]. TNF-α antagonists control systemic inflammation and may also exhibit a cardioprotective effect [Barnabe et al. 2011]. In line, these regimens appear to restore the antioxidant function of HDL [Van Sijl et al. 2011].
Control of other factors affecting the CV risk
Antidepressants are generally recommended to all RA patients suffering from depression, although their association with the reduction of CV risk has not been established. Accordingly, the proper screening and adequate therapy for renal failure, hypothyroidism, vitamin D deficiency and hyperhomocysteinemia, which may be induced by methotrexate, is advised for all patients with RA [Hollan et al. 2015].
CV risk in RA: next steps for a better future
The CV excess in RA patients has not been explained adequately. Future research could shed some light on the role of chronic inflammation on vasculature and the specific mechanisms which lead to the disturbance of the molecular equilibria that constitutes the substrate of accelerated atherogenicity. The ultimate goal remains the development of more effective therapeutic strategies for the prevention and treatment of CV disease. In addition, prospective studies could allow the integration of newer imaging techniques to the CV risk assessment of RA patients. Among these techniques cardiac magnetic resonance (CMR) seems more promising to provide early information and illustrate the pathophysiological background of the underlying heart disease. CMR makes the noninvasive distinction between ischemic and nonischemic heart failure feasible but the early recognition of myocardial inflammation is what really determinates its usefulness. Truthfully, higher disease activity was associated with more frequent abnormal myocardial CMR findings in patients with RA without known cardiac disease [Kobayashi et al. 2010]. The existing CV risk calculators are not supported by adequate evidence, lack the anticipated accuracy and their improvement is imperative but challenging for the scientific community. Prospective studies are needed to evaluate the true extent of the impact of the traditional risk factors on the CV risk in RA and to determine more appropriate management strategies, pharmacological or not.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Thomas Zegkos, First Cardiology Department, AHEPA University Hospital, Thessaloniki, Greece.
George Kitas, Arthritis Research UK Epidemiology Unit, School of Translational Medicine, University of Manchester, Manchester, UK.
Theodoros Dimitroulas, Fourth Department of Internal Medicine, Hippokratio Hospital, 49 Konstantinoupoleos Str, 54642 Thessaloniki, Greece.
References
- Amaya-Amaya J., Sarmiento-Monroy J., Mantilla R., Pineda-Tamayo R., Rojas-Villarraga A., Anaya J. (2013) Novel risk factors for cardiovascular disease in rheumatoid arthritis. Immunol Res 56: 267–286. [DOI] [PubMed] [Google Scholar]
- Arts E., Popa C., Den Broeder A., Semb A., Toms T., Kitas G., et al. (2015) Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis 74: 668–674. [DOI] [PubMed] [Google Scholar]
- Aubry M., Maradit-Kremers H., Reinalda M., Crowson C., Edwards W., Gabriel S. (2007) Differences in atherosclerotic coronary heart disease between subjects with and without rheumatoid arthritis. J Rheumatol 34: 937–942. [PubMed] [Google Scholar]
- Avina-Zubieta J., Abrahamowicz M., De Vera M., Choi H., Sayre E., Rahman M., et al. (2013) Immediate and past cumulative effects of oral glucocorticoids on the risk of acute myocardial infarction in rheumatoid arthritis: a population-based study. Rheumatology (Oxford) 52: 68–75. [DOI] [PubMed] [Google Scholar]
- Aviña-Zubieta J., Choi H., Sadatsafavi M., Etminan M., Esdaile J., Lacaille D. (2008) Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Care & Research 59: 1690–1697. [DOI] [PubMed] [Google Scholar]
- Baghdadi L., Woodman R., Shanahan E., Mangoni A. (2015) The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta-analysis. PLoS One 10: e0117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabe C., Martin B., Ghali W. (2011) Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 63: 522–529. [DOI] [PubMed] [Google Scholar]
- Bartels C., Kind A., Everett C., Mell M., McBride P., Smith M. (2011) Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum 63: 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinsberger J., Heemskerk J., Cosemans J. (2014) Chronic Arthritis and cardiovascular disease: altered blood parameters give rise to a prothrombotic propensity. Semin Arthritis Rheum 44: 345–352. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y., Spears M., Boustred C., May M., Anderson S., Benjamin E., et al. (2014) Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63: 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A., Scherer P. (2005) Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96: 939–949. [DOI] [PubMed] [Google Scholar]
- Bergstrom U., Jacobsson L., Nilsson J., Berglund G., Turesson C. (2011) Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford) 50: 2005–2013. [DOI] [PubMed] [Google Scholar]
- Boyer J., Gourraud P., Cantagrel A., Davignon J., Constantin A. (2011) Traditional cardiovascular risk factors in rheumatoid arthritis: a meta-analysis. Joint Bone Spine 78: 179–183. [DOI] [PubMed] [Google Scholar]
- Bray G., Bellanger T. (2006) Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine 29: 109–117. [DOI] [PubMed] [Google Scholar]
- Carpentier Y., Scruel O. (2002) Changes in the concentration and composition of plasma lipoproteins during the acute phase response. Curr Opin Clin Nutr Metab Care 5: 153–158. [DOI] [PubMed] [Google Scholar]
- Charles-Schoeman C., Lee Y., Grijalva V., Amjadi S., Fitzgerald J., Ranganath V., et al. (2012) Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis 71: 1157–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy E. (2012) Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 51: v3–11. [DOI] [PubMed] [Google Scholar]
- Cohen Tervaert J. (2011) Hypertension: an autoimmune disease? Hypertens Res 34: 443–444. [DOI] [PubMed] [Google Scholar]
- Corrales A., Dessein P., Tsang L., Pina T., Blanco R., Gonzalez-Juanatey C., et al. (2015) Carotid artery plaque in women with rheumatoid arthritis and low estimated cardiovascular disease risk: a cross-sectional study. Arthritis Res Ther 17: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales A., Gonzalez-Juanatey C., Peiro M., Blanco R., Llorca J., Gonzalez-Gay M. (2014) Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: results of a population-based study. Ann Rheum Dis 73: 722–727. [DOI] [PubMed] [Google Scholar]
- Crowson C., Liao K., Davis J., Solomon D., Matteson E., Knutson K., et al. (2013) Rheumatoid arthritis and cardiovascular disease. Am Heart J 166: 622–628, e621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowson C., Matteson E., Roger V., Therneau T., Gabriel S. (2012) Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol 110: 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cunha V., Brenol C., Brenol J., Fuchs S., Arlindo E., Melo I., et al. (2012) Metabolic syndrome prevalence is increased in rheumatoid arthritis patients and is associated with disease activity. Scand J Rheumatol 41: 186–191. [DOI] [PubMed] [Google Scholar]
- Dadoun S., Zeboulon-Ktorza N., Combescure C., Elhai M., Rozenberg S., Gossec L., et al. (2013) Mortality in rheumatoid arthritis over the last fifty years: systematic review and meta-analysis. Joint Bone Spine 80: 29–33. [DOI] [PubMed] [Google Scholar]
- Danninger K., Hoppe U., Pieringer H. (2014) Do statins reduce the cardiovascular risk in patients with rheumatoid arthritis? Int J Rheum Dis 17: 606–611. [DOI] [PubMed] [Google Scholar]
- Daoussis D., Panoulas V., Antonopoulos I., John H., Toms T., Wong P., et al. (2010) Cardiovascular risk factors and not disease activity, severity or therapy associate with renal dysfunction in patients with rheumatoid arthritis. Ann Rheum Dis 69: 517–521. [DOI] [PubMed] [Google Scholar]
- Davis J., Roger V., Crowson C., Kremers H., Therneau T., Gabriel S. (2008) The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum 58: 2603–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rincon I., Battafarano D., Restrepo J., Erikson J., Escalante A. (2014) Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol 66: 264–272. [DOI] [PubMed] [Google Scholar]
- Dessein P., Joffe B. (2006) Insulin resistance and impaired beta cell function in rheumatoid arthritis. Arthritis Rheum 54: 2765–2775. [DOI] [PubMed] [Google Scholar]
- Dessein P., Joffe B., Stanwix A. (2003) Inflammation, insulin resistance, and aberrant lipid metabolism as cardiovascular risk factors in rheumatoid arthritis. J Rheumatol 30: 1403–1405. [PubMed] [Google Scholar]
- Dessein P., Norton G., Woodiwiss A., Joffe B., Solomon A. (2007) Independent role of conventional cardiovascular risk factors as predictors of C-reactive protein concentrations in rheumatoid arthritis. J Rheumatol 34: 681–688. [PubMed] [Google Scholar]
- Dessein P., Solomon A. (2013) Towards the elucidation of the true impact of adipocytokines on cardiovascular risk in rheumatoid arthritis. Arthritis Res Ther 15: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitroulas T., Sandoo A., Hodson J., Smith J., Panoulas V., Kitas G. (2014) Relationship between dimethylarginine dimethylaminohydrolase gene variants and asymmetric dimethylarginine in patients with rheumatoid arthritis. Atherosclerosis 237: 38–44. [DOI] [PubMed] [Google Scholar]
- Dougados M., Soubrier M., Antunez A., Balint P., Balsa A., Buch M., et al. (2014) Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis 73: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas K., Pace A., Treharne G., Saratzis A., Nightingale P., Erb N., et al. (2006) Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis 65: 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante A., Haas R., Del Rincon I. (2005) Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med 165: 1624–1629. [DOI] [PubMed] [Google Scholar]
- Farragher T., Goodson N., Naseem H., Silman A., Thomson W., Symmons D., et al. (2008) Association of the HLA-DRB1 gene with premature death, particularly from cardiovascular disease, in patients with rheumatoid arthritis and inflammatory polyarthritis. Arthritis Rheum 58: 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraccioli G., Gremese E. (2011) Adiposity, joint and systemic inflammation: the additional risk of having a metabolic syndrome in rheumatoid arthritis. Swiss Med Wkly 141: w13211. [DOI] [PubMed] [Google Scholar]
- Gasparyan A., Ayvazyan L., Cocco G., Kitas G. (2012) Adverse cardiovascular effects of antirheumatic drugs: implications for clinical practice and research. Curr Pharm Des 18: 1543–1555. [DOI] [PubMed] [Google Scholar]
- Giles J., Ling S., Ferrucci L., Bartlett S., Andersen R., Towns M., et al. (2008) Abnormal body composition phenotypes in older rheumatoid arthritis patients: association with disease characteristics and pharmacotherapies. Arthritis Rheum 59: 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A., Maradit Kremers H., Crowson C., Ballman K., Roger V., Jacobsen S., et al. (2008) Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis 67: 64–69. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay M., Gonzalez-Juanatey C., Lopez-Diaz M., Pineiro A., Garcia-Porrua C., Miranda-Filloy J., et al. (2007) HLA-DRB1 and persistent chronic inflammation contribute to cardiovascular events and cardiovascular mortality in patients with rheumatoid arthritis. Arthritis Rheum 57: 125–132. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay M., Gonzalez-Juanatey C., Vazquez-Rodriguez T., Miranda-Filloy J., Llorca J. (2010) Insulin resistance in rheumatoid arthritis: the impact of the anti-TNF-alpha therapy. Ann N Y Acad Sci 1193: 153–159. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay M., Llorca J., Garcia-Unzueta M., Gonzalez-Juanatey C., De Matias J., Martin J., et al. (2008) High-grade inflammation, circulating adiponectin concentrations and cardiovascular risk factors in severe rheumatoid arthritis. Clin Exp Rheumatol 26: 596–603. [PubMed] [Google Scholar]
- Gonzalez-Juanatey C., Testa A., Garcia-Castelo A., Garcia-Porrua C., Llorca J., Ollier W., et al. (2004) Echocardiographic and doppler findings in long-term treated rheumatoid arthritis patients without clinically evident cardiovascular disease. Semin Arthritis Rheum 33: 231–238. [DOI] [PubMed] [Google Scholar]
- Goodson N., Brookhart A., Symmons D., Silman A., Solomon D. (2009) Non-steroidal anti-inflammatory drug use does not appear to be associated with increased cardiovascular mortality in patients with inflammatory polyarthritis: results from a primary care based inception cohort of patients. Ann Rheum Dis 68: 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B., Grossman J., Ansell B., Skaggs B., McMahon M. (2008) Altered lipoprotein metabolism in chronic inflammatory states: proinflammatory high-density lipoprotein and accelerated atherosclerosis in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther 10: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque U., Bathon J., Giles J. (2012) Association of vitamin D with cardiometabolic risk factors in rheumatoid arthritis. Arthritis Care Res (Hoboken) 64: 1497–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippisley-Cox J., Coupland C., Vinogradova Y., Robson J., Minhas R., Sheikh A., et al. (2008) Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 336: 1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjeltnes G., Hollan I., Forre O., Wiik A., Mikkelsen K., Agewall S. (2011) Anti-CCP and RF Igm: predictors of impaired endothelial function in rheumatoid arthritis patients. Scand J Rheumatol 40: 422–427. [DOI] [PubMed] [Google Scholar]
- Hollan I., Dessein P., Ronda N., Wasko M., Svenungsson E., Agewall S., et al. (2015) Prevention of cardiovascular disease in rheumatoid arthritis. Autoimmun Rev 14: 952–969. [DOI] [PubMed] [Google Scholar]
- Innala L., Moller B., Ljung L., Magnusson S., Smedby T., Sodergren A., et al. (2011) Cardiovascular events in early ra are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 13: R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John H., Hale E., Bennett P., Treharne G., Carroll D., Kitas G. (2011) Translating patient education theory into practice: developing material to address the cardiovascular education needs of people with rheumatoid arthritis. Patient Educ Couns 84: 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John H., Hale E., Treharne G., Kitas G., Carroll D. (2013) A randomized controlled trial of a cognitive behavioural patient education intervention vs. a traditional information leaflet to address the cardiovascular aspects of rheumatoid disease. Rheumatology (Oxford) 52: 81–90. [DOI] [PubMed] [Google Scholar]
- Karpouzas G., Malpeso J., Choi T., Nightingale P., Budoff M., Kitas G. (2013) Differential performance of various cardiovascular risk calculators in predicting surrogate coronary outcomes in rheumatoid arthritis. Ann Rheum Dis 72: 413. [Google Scholar]
- Karpouzas G., Malpeso J., Choi T., Li D., Munoz S., Budoff M. (2014) Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis 73: 1797–1804. [DOI] [PubMed] [Google Scholar]
- Kawai V., Chung C., Solus J., Oeser A., Raggi P., Stein C. (2015) The ability of the 2013 American College of Cardiology/American Heart Association cardiovascular risk score to identify rheumatoid arthritis patients with high coronary artery calcification scores. Arthritis Rheumatol 67: 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitas G., Abreu G., Jedrychowicz-Rosiak K., Miller J., Nakov R., Panfilov S., et al. (2014) The effects of the spleen tyrosine kinase inhibitor fostamatinib on ambulatory blood pressure in patients with active rheumatoid arthritis: results of the Oskira-ABPM (ambulatory blood pressure monitoring) randomized trial. J Am Soc Hypertens 8: 780–790. [DOI] [PubMed] [Google Scholar]
- Kitas G., Banks M., Bacon P. (2001) Cardiac involvement in rheumatoid disease. Clin Med 1: 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Giles J., Hirano M., Yokoe I., Nakajima Y., Bathon J., et al. (2010) Assessment of myocardial abnormalities in rheumatoid arthritis using a comprehensive cardiac magnetic resonance approach: a pilot study. Arthritis Res Ther 12: R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremers H., Crowson C., Therneau T., Roger V., Gabriel S. (2008) High ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients: a population-based cohort study. Arthritis Rheum 58: 2268–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K., Kremers H., Crowson C., Snyder M., Therneau T., Roger V., et al. (2009) Autoantibodies and the risk of cardiovascular events. J Rheumatol 36: 2462–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao K., Solomon D. (2013) Traditional cardiovascular risk factors, inflammation and cardiovascular risk in rheumatoid arthritis. Rheumatology (Oxford) 52: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhardsen J., Ahlehoff O., Gislason G., Madsen O., Olesen J., Torp-Pedersen C., et al. (2011) The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis 70: 929–934. [DOI] [PubMed] [Google Scholar]
- Lindhardsen J., Gislason G., Jacobsen S., Ahlehoff O., Olsen A., Madsen O., et al. (2013) Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: a nationwide cohort study. Ann Rheum Dis 73: 1515–1521. [DOI] [PubMed] [Google Scholar]
- Listing J., Kekow J., Manger B., Burmester G., Pattloch D., Zink A., et al. (2015) Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, tnfalpha inhibitors and rituximab. Ann Rheum Dis 74: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listing J., Strangfeld A., Kekow J., Schneider M., Kapelle A., Wassenberg S., et al. (2008) Does tumor necrosis factor alpha inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum 58: 667–677. [DOI] [PubMed] [Google Scholar]
- Lopez-Mejias R., Garcia-Bermudez M., Gonzalez-Juanatey C., Castaneda S., Miranda-Filloy J., Gomez-Vaquero C., et al. (2012) NFKB1–94ATTG INS/DEL polymorphism (rs28362491) is associated with cardiovascular disease in patients with rheumatoid arthritis. Atherosclerosis 224: 426–429. [DOI] [PubMed] [Google Scholar]
- Maki-Petaja K., Booth A., Hall F., Wallace S., Brown J., McEniery C., et al. (2007) Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol 50: 852–858. [DOI] [PubMed] [Google Scholar]
- Mantel A., Holmqvist M., Jernberg T., Wallberg-Jonsson S., Askling J. (2015) Rheumatoid arthritis is associated with a more severe presentation of acute coronary syndrome and worse short-term outcome. Eur Heart J 36: 3413–3422. [DOI] [PubMed] [Google Scholar]
- Maradit-Kremers H., Crowson C., Nicola P., Ballman K., Roger V., Jacobsen S., et al. (2005) Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 52: 402–411. [DOI] [PubMed] [Google Scholar]
- Marks J., Van Der Heijde D., Colebatch A., Buchbinder R., Edwards C. (2012) Pain pharmacotherapy in patients with inflammatory arthritis and concurrent cardiovascular or renal disease: a Cochrane systematic review. J Rheumatol Suppl 90: 81–84. [DOI] [PubMed] [Google Scholar]
- Mavrogeni S., Dimitroulas T., Bucciarelli-Ducci C., Ardoin S., Sfikakis P., Kolovou G., et al. (2014a) Rheumatoid arthritis: an autoimmune disease with female preponderance and cardiovascular risk equivalent to diabetes mellitus: role of cardiovascular magnetic resonance. Inflamm Allergy Drug Targets 13: 81–93. [DOI] [PubMed] [Google Scholar]
- Mavrogeni S., Dimitroulas T., Gabriel S., Sfikakis P., Pohost G., Kitas G. (2014b) Why currently used diagnostic techniques for heart failure in rheumatoid arthritis are not enough: the challenge of cardiovascular magnetic resonance imaging. Rev Cardiovasc Med 15: 320–331. [DOI] [PubMed] [Google Scholar]
- Mavrogeni S., Dimitroulas T., Kitas G. (2012) Multimodality imaging and the emerging role of cardiac magnetic resonance in autoimmune myocarditis. Autoimmun Rev 12: 305–312. [DOI] [PubMed] [Google Scholar]
- Mavrogeni S., Spargias K., Markussis V., Kolovou G., Demerouti E., Papadopoulou E., et al. (2009) Myocardial inflammation in autoimmune diseases: investigation by cardiovascular magnetic resonance and endomyocardial biopsy. Inflamm Allergy Drug Targets 8: 390–397. [DOI] [PubMed] [Google Scholar]
- McMaster W., Kirabo A., Madhur M., Harrison D. (2015) Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116: 1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsios G., Lahart I. (2015) Exercise as medicine in rheumatoid arthritis. Mediterr J Rheumatol 26: 54–61. [Google Scholar]
- Metsios G., Stavropoulos-Kalinoglou A., Panoulas V., Wilson M., Nevill A., Koutedakis Y., et al. (2009) Association of physical inactivity with increased cardiovascular risk in patients with rheumatoid arthritis. Eur J Cardiovasc Prev Rehabil 16: 188–194. [DOI] [PubMed] [Google Scholar]
- Micha R., Imamura F., Wyler Von Ballmoos M., Solomon D., Hernan M., Ridker P., et al. (2011) Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol 108: 1362–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myasoedova E., Crowson C., Kremers H., Roger V., Fitz-Gibbon P., Therneau T., et al. (2011) Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis 70: 482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo A., Sokka T., Descalzo M., Calvo-Alen J., Horslev-Petersen K., Luukkainen R., et al. (2008) Cardiovascular disease in patients with rheumatoid arthritis: results from the Quest-RA study. Arthritis Res Ther 10: R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola P., Maradit-Kremers H., Roger V., Jacobsen S., Crowson C., Ballman K., et al. (2005) The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum 52: 412–420. [DOI] [PubMed] [Google Scholar]
- Nurmohamed M. (2015) SP0033 EULAR recommendation update on cardiovascular disease in RA. Ann Rheum Dis 74: 9. [Google Scholar]
- Palomino-Morales R., Gonzalez-Juanatey C., Vazquez-Rodriguez T., Rodriguez L., Miranda-Filloy J., Fernandez-Gutierrez B., et al. (2010) A1298c polymorphism in the MTHFR gene predisposes to cardiovascular risk in rheumatoid arthritis. Arthritis Res Ther 12: R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panoulas V., Douglas K., Milionis H., Stavropoulos-Kalinglou A., Nightingale P., Kita M., et al. (2007) Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 46: 1477–1482. [DOI] [PubMed] [Google Scholar]
- Panoulas V., Douglas K., Smith J., Taffe P., Stavropoulos-Kalinoglou A., Toms T., et al. (2008) Polymorphisms of the endothelin-1 gene associate with hypertension in patients with rheumatoid arthritis. Endothelium 15: 203–212. [DOI] [PubMed] [Google Scholar]
- Panoulas V., Toms T., Metsios G., Stavropoulos-Kalinoglou A., Kosovitsas A., Milionis H., et al. (2010) Target organ damage in patients with rheumatoid arthritis: the role of blood pressure and heart rate. Atherosclerosis 209: 255–260. [DOI] [PubMed] [Google Scholar]
- Paraskevas K. (2008) Statin treatment for rheumatoid arthritis: a promising novel indication. Clin Rheumatol 27: 281–287. [DOI] [PubMed] [Google Scholar]
- Perk J., De Backer G., Gohlke H., Graham I., Reiner Z., Verschuren W., et al. (2012) European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the Fifth Joint Task Force of The European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Int J Behav Med 19: 403–488. [DOI] [PubMed] [Google Scholar]
- Peters M., Symmons D., Mccarey D., Dijkmans B., Nicola P., Kvien T., et al. (2010) EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 69: 325–331. [DOI] [PubMed] [Google Scholar]
- Peters M., Van Halm V., Voskuyl A., Smulders Y., Boers M., Lems W., et al. (2009) Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A Prospective Study. Arthritis Rheum 61: 1571–1579. [DOI] [PubMed] [Google Scholar]
- Raterman H., Nielen M., Peters M., Verheij R., Nurmohamed M., Schellevis F. (2012) Coexistence of hypothyroidism with inflammatory arthritis is associated with cardiovascular disease in women. Ann Rheum Dis 71: 1216–1218. [DOI] [PubMed] [Google Scholar]
- Ridker P. (2014) LDL cholesterol: controversies and future therapeutic directions. Lancet 384: 607–617. [DOI] [PubMed] [Google Scholar]
- Robertson J., Peters M., McInnes I., Sattar N. (2013) Changes in lipid levels with inflammation and therapy in ra: a maturing paradigm. Nat Rev Rheumatol 9: 513–523. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rodriguez L., Gonzalez-Juanatey C., Palomino-Morales R., Vazquez-Rodriguez T., Miranda-Filloy J., Fernandez-Gutierrez B., et al. (2011) TNF-a-308 (rs1800629) polymorphism is associated with a higher risk of cardiovascular disease in patients with rheumatoid arthritis. Atherosclerosis 216: 125–130. [DOI] [PubMed] [Google Scholar]
- Rojas-Serrano J., Perez L., Garcia C., Moctezuma F., Alvarez-Hernandez E., Vazquez-Mellado J., et al. (2011) Current smoking status is associated to a non-ACR 50 response in early rheumatoid arthritis. A cohort study. Clin Rheumatol 30: 1589–1593. [DOI] [PubMed] [Google Scholar]
- Roubille C., Richer V., Starnino T., McCourt C., McFarlane A., Fleming P., et al. (2015) The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 74: 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoo A., Dimitroulas T., Hodson J., Smith J., Douglas K., Kitas G. (2015) Cumulative inflammation associates with asymmetric dimethylarginine in rheumatoid arthritis: a 6 year follow-up study. Rheumatology (Oxford) 54: 1145–1152. [DOI] [PubMed] [Google Scholar]
- Sandoo A., Kitas G., Carroll D., Veldhuijzen Van Zanten J. (2012) The role of inflammation and cardiovascular disease risk on microvascular and macrovascular endothelial function in patients with rheumatoid arthritis: a cross-sectional and longitudinal study. Arthritis Res Ther 14: R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semb A., Kvien T., Aastveit A., Jungner I., Pedersen T., Walldius G., et al. (2010) Lipids, myocardial infarction and ischaemic stroke in patients with rheumatoid arthritis in the apolipoprotein-related mortality risk (Amoris) study. Ann Rheum Dis 69: 1996–2001. [DOI] [PubMed] [Google Scholar]
- Semb A., Rollefstad S., Van Riel P., Kitas G., Matteson E., Gabriel S. (2014) Cardiovascular disease assessment in rheumatoid arthritis: a guide to translating knowledge of cardiovascular risk into clinical practice. Ann Rheum Dis 73: 1284–1288. [DOI] [PubMed] [Google Scholar]
- Skeoch S., Bruce I. (2015) Atherosclerosis in rheumatoid arthritis: is it all about inflammation? Nat Rev Rheumatol 11: 390–400. [DOI] [PubMed] [Google Scholar]
- Solomon A., Woodiwiss A., Abdool-Carrim A., Stevens B., Norton G., Dessein P. (2012) The carotid artery atherosclerosis burden and its relation to cardiovascular risk factors in black and white Africans with established rheumatoid arthritis: a cross-sectional study. J Rheumatol 39: 1798–1806. [DOI] [PubMed] [Google Scholar]
- Souto A., Salgado E., Maneiro J., Mera A., Carmona L., Gomez-Reino J. (2015) Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: a systematic review and meta-analysis. Arthritis Rheumatol 67: 117–127. [DOI] [PubMed] [Google Scholar]
- Stavropoulos-Kalinoglou A., Metsios G., Koutedakis Y., Kitas G. (2011) Obesity in rheumatoid arthritis. Rheumatology (Oxford) 50: 450–462. [DOI] [PubMed] [Google Scholar]
- Stavropoulos-Kalinoglou A., Metsios G., Koutedakis Y., Nevill A., Douglas K., Jamurtas A., et al. (2007) Redefining overweight and obesity in rheumatoid arthritis patients. Ann Rheum Dis 66: 1316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavropoulos-Kalinoglou A., Metsios G., Panoulas V., Douglas K., Nevill A., Jamurtas A., et al. (2008) Cigarette smoking associates with body weight and muscle mass of patients with rheumatoid arthritis: a cross-sectional, observational study. Arthritis Res Ther 10: R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavropoulos-Kalinoglou A., Metsios G., Panoulas V., Douglas K., Nevill A., Jamurtas A., et al. (2009) Associations of obesity with modifiable risk factors for the development of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis 68: 242–245. [DOI] [PubMed] [Google Scholar]
- Stavropoulos-Kalinoglou A., Metsios G., Panoulas V., Nightingale P., Koutedakis Y., Kitas G. (2012) Anti-tumour necrosis factor alpha therapy improves insulin sensitivity in normal-weight but not in obese patients with rheumatoid arthritis. Arthritis Res Ther 14: R160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavropoulos-Kalinoglou A., Metsios G., Smith J., Panoulas V., Douglas K., Jamurtas A., et al. (2010) What predicts obesity in patients with rheumatoid arthritis? an investigation of the interactions between lifestyle and inflammation. Int J Obes (Lond) 34: 295–301. [DOI] [PubMed] [Google Scholar]
- Stavropoulos-Kalinoglou A., Metsios G., Veldhuijzen Van Zanten J., Nightingale P., Kitas G., Koutedakis Y. (2013) Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis 72: 1819–1825. [DOI] [PubMed] [Google Scholar]
- Stevens R., Douglas K., Saratzis A., Kitas G. (2005) Inflammation and atherosclerosis in rheumatoid arthritis. Expert Rev Mol Med 7: 1–24. [DOI] [PubMed] [Google Scholar]
- Summers G., Metsios G., Stavropoulos-Kalinoglou A., Kitas G. (2010) Rheumatoid cachexia and cardiovascular disease. Nat Rev Rheumatol 6: 445–451. [DOI] [PubMed] [Google Scholar]
- Toms T., Panoulas V., Douglas K., Griffiths H., Kitas G. (2008) Lack of association between glucocorticoid use and presence of the metabolic syndrome in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther 10: R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms T., Panoulas V., John H., Douglas K., Kitas G. (2009) Methotrexate therapy associates with reduced prevalence of the metabolic syndrome in rheumatoid arthritis patients over the age of 60- more than just an anti-inflammatory effect? A Cross Sectional Study. Arthritis Res Ther 11: R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms T., Smith J., Panoulas V., Blackmore H., Douglas K., Kitas G. (2012) Apolipoprotein E gene polymorphisms are strong predictors of inflammation and dyslipidemia in rheumatoid arthritis. J Rheumatol 39: 218–225. [DOI] [PubMed] [Google Scholar]
- Toms T., Symmons D., Kitas G. (2010) Dyslipidaemia in rheumatoid arthritis: the role of inflammation, drugs, lifestyle and genetic factors. Curr Vasc Pharmacol 8: 301–326. [DOI] [PubMed] [Google Scholar]
- Tournadre A., Tatar Z., Pereira B., Chevreau M., Gossec L., Gaudin P., et al. (2015) Application of the European Society of Cardiology, Adult Treatment Panel III and American College of Cardiology/American Heart Association Guidelines for cardiovascular risk management in a French cohort of rheumatoid arthritis. Int J Cardiol 183: 149–154. [DOI] [PubMed] [Google Scholar]
- Trelle S., Reichenbach S., Wandel S., Hildebrand P., Tschannen B., Villiger P., et al. (2011) Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 342: c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Helm-Van Mil A., Van Der Kooij S., Allaart C., Toes R., Huizinga T. (2008) A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis 67: 769–774. [DOI] [PubMed] [Google Scholar]
- Van Halm V., Peters M., Voskuyl A., Boers M., Lems W., Visser M., et al. (2009) Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: a cross-sectional study, the Carré Investigation. Annals of the Rheumatic Diseases 68: 1395–1400. [DOI] [PubMed] [Google Scholar]
- Van Sijl A., Peters M., Knol D., De Vet R., Sattar N., Dijkmans B., et al. (2011) The effect of TNF-alpha blocking therapy on lipid levels in rheumatoid arthritis: a meta-analysis. Semin Arthritis Rheum 41: 393–400. [DOI] [PubMed] [Google Scholar]
- Veldhuijzen Van Zanten J., Rouse P., Hale E., Ntoumanis N., Metsios G., Duda J., et al. (2015) Perceived barriers, facilitators and benefits for regular physical activity and exercise in patients with rheumatoid arthritis: a review of the literature. Sports Med 45: 1401–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe F., Michaud K. (2012) Effect of Body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken) 64: 1471–1479. [DOI] [PubMed] [Google Scholar]
- Yndestad A., Damas J., Oie E., Ueland T., Gullestad L., Aukrust P. (2007) Role of inflammation in the progression of heart failure. Curr Cardiol Rep 9: 236–241. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Hong D., Zhang Y., Sang Y., Yang Z., Zhang X. (2015) Association between anti-TNF therapy for rheumatoid arthritis and hypertension: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 94: e731. [DOI] [PMC free article] [PubMed] [Google Scholar]