Abstract
The B-cell receptor signaling pathway has emerged as an important therapeutic target in chronic lymphocytic leukemia and other B-cell malignancies. Novel agents have been developed targeting the signaling enzymes spleen tyrosine kinase (SYK), Bruton’s tyrosine kinase, and phosphoinositide 3-kinase delta. This review discusses the rationale for targeting these enzymes, as well as the preclinical and clinical evidence supporting their role as therapeutic targets, with a particular focus on SYK inhibition with entospletinib.
Keywords: B-cell receptor signaling inhibitors, chronic lymphocytic leukemia, entospletinib, idelalisib, lymphoid malignancies, phosphoinositide 3-kinase delta, spleen tyrosine kinase inhibitors
Entospletinib overview
B-cell receptor (BCR) signaling regulates many cell-fate decisions in normal B cells [Casola et al. 2004] and has emerged as an invaluable therapeutic pathway in numerous B-cell malignancies. The recent Food and Drug Administration (FDA) approvals of ibrutinib and idelalisib highlight the critical role of different kinase targets within the BCR signaling pathway. These drugs have unique spectrums of clinical activity with ibrutinib showing the greatest impact in chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), Waldenström’s macroglobulinemia (WM) or lymphoplasmacytic lymphoma [Byrd et al. 2013, 2014, 2015; Treon et al. 2015; Wang et al. 2013] and possibly the activated B-cell subtype of diffuse large B-cell lymphoma (DLBCL) [Mathews Griner et al. 2014]. In contrast, idelalisib has the greatest activity in CLL and follicular lymphoma (FL) [Furman et al. 2014; Gopal et al. 2014]. Although some of these distinctions may be attributable to the drugs themselves, other differences are likely due to the pathway-kinase target. While both drugs inhibit BCR signaling, spleen tyrosine kinase (SYK), Bruton’s tyrosine kinase (BTK), and phosphoinositide 3-kinase delta (PI3Kδ) have a variety of functions independent of BCR signaling, including transmission of signals from tumor necrosis factor (TNF) superfamily receptors, integrins, and cytokines. Further, there is anecdotal evidence that these drugs may work in sequence, even after resistance has developed to the alternative therapy [Mato et al. 2015]. For these reasons, the clinical developments of additional novel agents targeting BCR signaling will likely benefit patients with B-cell lymphoproliferative disorders.
B-cell receptor signaling
The BCR pathway includes kinases, adaptor proteins, and transcription factors. These proteins cooperate to initiate and transmit signaling from the BCR to complex downstream effectors through a variety of protein modifications and transcriptional changes [Rolli et al. 2002]. SYK, BTK, and PI3Kδ are critical signaling enzymes within the BCR signaling cascade that have emerged as important targets of novel agents [Fowler and Davis, 2013].
LYN
Signaling is initiated at the BCR, also known as a surface immunoglobulin (Ig). Although this molecule lacks any intrinsic signaling capability, it recruits LYN kinase to phosphorylate cluster of differentiation (CD)79, which in turn initiates the signaling cascade [Ackermann et al. 2015]. LYN functions to initiate signaling, but also has a negative feedback role that dampens overall signaling [Nishizumi et al. 1998]. Mice lacking LYN kinase manifest immune hyperactivity, suggesting that the negative feedback role for this enzyme plays a vital role in pathway balance [Lamagna et al. 2014]. Clinical trials of the pan-Src family inhibitor dasatinib have shown disappointing results in B-cell malignancies [Amrein et al. 2011].
Spleen tyrosine kinase
The dual CD79 sites phosphorylated by LYN kinase become a singular docking site for SYK. After binding to CD79, SYK undergoes autophosphorylation, resulting in enzyme activation [Kipps, 2007]. Thereafter, SYK activates a multitude of effectors, including the kinases BTK and PI3K [Gobessi et al. 2009]. Mice lacking SYK fail to develop B cells, and laboratory models of B cells modified to eliminate SYK have profound deficiencies in BCR signaling [Ackermann et al. 2015]. Because of its central role in BCR signaling, the SYK inhibitor R406 was studied preclinically for its therapeutic potential in B-cell malignancies [Sharman et al. 2007; Gobessi et al. 2009], and a clinical trial of fostamatinib (the oral prodrug of R406) pioneered the field of BCR signaling inhibition [Friedberg et al. 2010].
Fostamatinib demonstrated modest activity in DLBCL, but the more clinically meaningful benefit was identified in CLL, where 6 of 11 patients experienced a partial response to therapy. This was also the first demonstration of the unique pattern of clinical responses in which lymph node reduction was simultaneously associated with significant lymphocytosis [Friedberg et al. 2010]. This has also occurred with therapeutic inhibition of BTK and PI3Kδ [Byrd et al. 2013; Furman et al. 2014]. It has been subsequently recognized that therapy with BCR-signaling inhibitors results in significant alterations in cytokine signaling and egress of malignant lymphocytes from protective niches in the marrow, nodes and spleen [Herman et al. 2014]. Over time, these lymphocytes passively die because of the lack of BCR signaling.
Bruton’s tyrosine kinase
Another molecule downstream of SYK is BTK, a kinase that helps propagate downstream signaling from the BCR. The gene for BTK lies on the X chromosome, and loss of BTK results in Bruton’s agammaglobulinemia, in which patients lack B cells and fail to synthesize antibodies [Conley et al. 2009]. Therapeutic inhibition of BTK with the covalent inhibitor ibrutinib has resulted in profound clinical activity in a variety of B-cell malignancies [Byrd et al. 2013, 2014, 2015; Treon et al. 2015; Wang et al. 2013]. Several other BTK inhibitors have been evaluated, including CC-292 and GDC-0834; however, development of these molecules has been discontinued. Other BTK inhibitors, such as ONO-4059 (aka ONO/GS-4059) [ClinicalTrials.gov identifier: NCT01659255] and ACP-196 [ClinicalTrials.gov identifier: NCT02029443], are still in active development and may become available for the treatment of patients with B-cell malignancies.
In CLL, ibrutinib initially demonstrated substantial clinical activity in single-arm, phase II studies in both relapsed and refractory CLL, as well as in patients who were treatment naïve [Byrd et al. 2013; O’Brien et al. 2014]. Subsequent, randomized, phase III studies demonstrated the superiority of ibrutinib compared with ofatumumab in previously treated patients and compared with chlorambucil in treatment-naïve patients, as well as an additional benefit when added to bendamustine and rituximab (BR) [Byrd et al. 2014; Burger et al. 2015; Chanan-Khan et al. 2015]. Additional treatment indications include both MCL and WM based upon single-arm, phase II studies [Treon et al. 2015; Wang et al. 2013], and randomized studies are ongoing.
Phosphoinositide 3-kinase
PI3K propagates signals further downstream in the BCR pathway. PI3K activates the AKT and mechanistic target of rapamycin (mTOR) pathways, but also integrates signals from a variety of non-BCR cell-surface receptors [Puri and Gold, 2012]. The PI3K family has several isoforms, and the delta isoform is relatively specific to lymphocytes [Foster et al. 2012; Okkenhaug and Vanhaesebroeck, 2003]. Idelalisib was the first-in-class PI3Kδ inhibitor to achieve FDA approval in both FL and CLL.
Following promising activity in a phase I study [Flinn et al. 2014], idelalisib was evaluated in a variety of indolent non-Hodgkin’s lymphomas (iNHLs) and demonstrated significant activity in marginal zone lymphoma (MZL), WM, small lymphocytic lymphoma (SLL), and FL [Gopal et al. 2014]. Based upon a single-arm, phase II study of patients with disease refractory to both rituximab and alkylating agents [Gopal et al. 2014], idelalisib was granted FDA approval in both FL and SLL. Although idelalisib demonstrated an approximately 50% response rate in these refractory FL patients [Gopal et al. 2014], ibrutinib only led to responses in 11% of patients considered rituximab refractory [Bartlett et al. 2014], highlighting some of the important differences in the biologic spectrum of activity of the respective kinases.
In CLL, idelalisib was evaluated in two randomized studies in which patients were assigned to a CD20 antibody versus the same CD20 antibody in combination with idelalisib. In the rituximab versus rituximab and idelalisib study, marked improvements of the combination in overall response rate, progression-free survival (PFS) and overall survival (OS) led to FDA approval in this indication [Furman et al. 2014]. The similarly designed study with ofatumumab was recently reported with substantial improvements in outcome for patients receiving combinations containing idelalisib [Jones et al. 2015a]. A study of BR plus idelalisib in the frontline setting has been conducted, and the results are pending [ClinicalTrials.gov identifier: NCT01980888]. A study in the relapsed setting found that the addition of idelalisib to BR was superior to BR alone in reducing the risk of progressive disease and death, as well as increasing PFS and OS, with a safety profile consistent with previously reported studies [Zelenetz et al. 2015].
Rationale for further exploration of spleen tyrosine kinase inhibitors
Although clinical validation of BCR signaling inhibition was first demonstrated with therapeutic inhibition of SYK, clinical development of therapeutics against this target stalled for several years while the development of fostamatinib focused on rheumatologic indications. In the meantime, both ibrutinib and idelalisib showed striking improvements in clinical response rates and OS compared with existing standard therapies in a variety of treatment settings. Despite the clinically meaningful improvement in outcomes for patients receiving ibrutinib and idelalisib, emergence of resistant disease remains problematic [Maddocks et al. 2015]. There is a clinical need for additional agents targeting this pathway that can be used to salvage patients with resistant disease or when simultaneously used in novel combinations to improve the efficacy of the existing agents. SYK remains an important, yet incompletely studied, target for additional drug development. Several agents, including fostamatinib, cerdulatinib, TAK-659, and entospletinib are currently being evaluated in this setting. Among SYK inhibitors in clinical development, entospletinib (also known as GS-9973) has reported the most comprehensive clinical experience to date. The following discussion will focus on the preclinical and clinical experience to date with this molecule.
Entospletinib
Entospletinib was designed following the initial clinical efficacy reports of the activity of fostamatinib in rheumatologic diseases and hematologic cancers [Weinblatt et al. 2008; Genovese et al. 2011; Friedberg et al. 2010]. Despite the demonstration of clinical efficacy, the development of fostamatinib was hampered by dose-limiting adverse effects (AEs), including a high incidence of hypertension, gastrointestinal effects, and neutropenia, which have been attributed to off-target kinase activities. To overcome this hurdle, entospletinib was synthesized and demonstrated substantially improved biochemical and cellular selectivity versus R406 [Currie et al. 2014; Burke et al. 2014]. Entospletinib modulates B-cell function by inhibiting BCR-stimulated, B-cell linker of NFκB (BLNK) phosphorylation, CD86 cell-surface activation-marker expression and proliferation, while showing enhanced cellular selectivity versus Janus kinase 2 (JAK2), cKIT, Fms-like tyrosine kinase 3 (FLT3), RET, and vascular endothelial growth factor receptor 2 (VEGFR2) versus R406 [Currie et al. 2014]. The improved selectivity increases the clinically achievable SYK target coverage [Ramanathan et al. 2013] without the concomitant dose-limiting AEs noted with fostamatinib, allowing for potentially improved efficacy and the ability to be safely combined with other targeted therapeutics in oncology.
Impact on B-cell receptor signaling
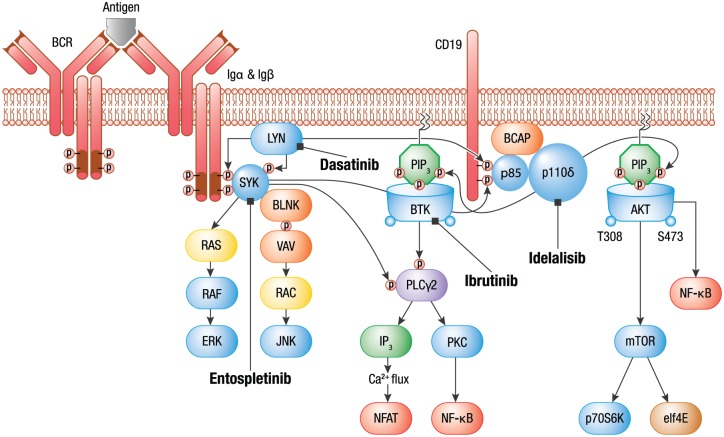
BCR signaling contributes to cell survival, proliferation and resistance to apoptosis in B-cell malignancies. Gene-expression profiling and immunoblotting in cellular assays of malignant B-cell lines and primary CLL samples demonstrate increased expression of BCR pathway components and pathway activation, including phosphorylation of SYK, PLCγ2, signal transducers and activators of transcription 3 (STAT3) and extracellular signal regulated kinases 1 and 2 (ERK 1/2) compared with healthy B cells [Buchner et al. 2009]. As constitutive BCR signaling is required for B-cell survival, inhibition of the BCR-signaling cascade by SYK, BTK, and PI3Kδ inhibition validates this pathway as a pathogenic driver in B-cell malignancies (Figure 1).
Figure 1.
BCR signaling.
Adapted from Puri, K., Di Paolo, J. and Gold, M. (2013) B-Cell receptor signaling inhibitors for treatment of autoimmune inflammatory diseases and B-Cell malignancies. Int Rev Immunol 32: 397–427. Reprinted by permission of Taylor & Francis Ltd.
Focusing on SYK and using a combination of mass spectroscopy and immunoblotting analysis, the phosphorylation of 13 signaling proteins were inhibited by entospletinib, and a subset of these phosphorylation sites were inhibited in basally activated, primary CLL cells [Di Paolo et al. 2014]. Inhibition of SYK by entospletinib in primary CLL cells resulted in cellular apoptosis [Burke et al. 2014; Jones et al. 2015b]. These observations confirm and extend earlier reports with structurally distinct SYK inhibitors (R406 and PRT06260) that showed reduction in AKT and ERK phosphorylation and induction of apoptosis in primary CLL cells [Gobessi et al. 2009; Buchner et al. 2009; Burke et al. 2014] and reduced PLCγ2 and AKT [Cheng et al. 2011] phosphorylation and proliferation of DLBCL cell lines [Chen et al. 2008; Spurgeon et al. 2013]. Importantly, in CLL cases with the adverse Ig heavy chain unmutated BCR or ZAP70(+) prognostic markers, SYK inhibitors exerted stronger cytotoxicity than in mutated BCR samples from in vitro studies [Buchner et al. 2009]. The effect of SYK inhibition in CLL predicted by the in vitro studies was confirmed in vivo in the Eμ-TCL1 transgenic CLL mouse model, in which R406 inhibited BCR signaling, induced a transient increase in circulating lymphocytes, reduced the proliferation and survival of the malignant B cells and prolonged survival of the treated CLL mice [Suljagic et al. 2010]. Together, these observations demonstrate the importance of SYK function in multiple BCR cancer cell-signaling cascades.
In addition to directly affecting cellular signaling and apoptosis, BCR signaling facilitates attraction and retention of malignant B cells within the protective secondary lymphoid niches by altering the cytokine environment within these tissues [Quiroga et al. 2009; Buchner et al. 2009]. The negative CLL prognostic indicator, ZAP70 expression, results in enhanced BCR signaling and elevated expression of the chemokines CCL3 and CCL4 and chemokine receptors, including CCR7, which are required for increased migration and homing to the protective lymph node microenvironment [Calpe et al. 2011]. SYK inhibition in CLL cells with entospletinib inhibited secretion of these cytokines, confirming the earlier results of R406 inhibition of CCL3 and CCL4 secretion [Burger et al. 2009; Hoellenriegel et al. 2012; Burke et al. 2014]. These results likely explain the CLL cell redistribution that results in rapid lymph-node shrinkage and lymphocytosis following inhibition with entospletinib and other SYK inhibitors in patients with CLL.
BCR signaling via SYK activity is known to affect other B-cell functions with poorly characterized roles in malignant B-cell survival, including regulation of B-cell activation markers necessary for T-cell activation [Currie et al. 2014], alteration of actin dynamics [Le Roux et al. 2007] and other cell-surface proteins.
Beyond the B-cell receptor: mechanism for future combinations
Independent of BCR signaling, SYK has been shown to be required for signaling downstream of Fc receptors, integrins and other receptors on immune cells [Mócsai et al. 2010; Tan et al. 2013; Currie et al. 2014]. As more is understood about SYK’s role in integrating various signals, the potential for applying combination approaches to treat B-cell malignancies will undoubtedly emerge. Combinations with other BCR-signaling molecules to increase the depth of inhibition or combinations with distinct pathway inhibitors, such as BCL-2 inhibitors, could have the potential to improve and extend the durability of clinical responses.
CLL cells grow and proliferate in a microenvironmental tissue niche surrounded by mesenchymal stromal cells, nurse-like cells and lymphoma-associated macrophages in concert with T cells, natural killer cells and extracellular matrix components that interact and activate tumor B cells via BCR, TNF family members (i.e., BAFF, APRIL) and tissue-homing chemokine receptors and adhesion molecules [Herishanu et al. 2011]. This stromal interaction with CLL cells occurs independently of the BCR. In coculture experiments, entospletinib inhibited pSYK, pAKT, pMEK, pERK, pJNK2, pPKCδ and pS6 kinase in primary CLL cells incubated in the presence of HS-5 stromal cell coculture [Di Paolo et al. 2014], expanding the findings that R406 inhibited SYK and AKT phosphorylations, as well as F-actin formation following chemokine and integrin stimulation in primary CLL cells [Buchner et al. 2010]. Stromal cell activation in the entospletinib studies resulted in a similar, although not identical, cellular phosphorylation profile that was inhibited by entospletinib treatment [Di Paolo et al. 2014], highlighting the modulation of BCR-independent signaling by entospletinib. Given the strong stromal cell effects on CLL survival, these results open the possibility of combining entospletinib with other pathway inhibitors to more completely suppress the pathologic signaling emanating from the stromal cell coculture.
Clinical trial combinations of entospletinib with idelalisib were initiated partially on the preclinical observation that the combination of idelalisib and entospletinib could more completely inhibit pAKT signaling in primary CLL cells with HS-5 stimulation [Burke et al. 2014]. Further justification for the combination was based upon the relatively distinct patterns of clinical responses in which idelalisib showed a more profound effect in iNHL [Gopal et al. 2014], whereas entospletinib showed a more substantial clinical benefit in CLL [Sharman et al. 2015a]. The results of the clinical study will be described later in the review.
Alternatively, combinations with other BCR inhibitors can be used to circumvent the development of resistance to a single inhibitor. For instance, resistance to ibrutinib has been characterized and arises from BTK-active site mutations and PLCγ2 activating mutations and other signaling-pathway activations, such as CARD11 [Woyach et al. 2014; Liu et al. 2015; Improgo et al. 2013; Chang et al. 2015]. In vitro experiments showed that the addition of entospletinib to ibrutinib-resistant cell lines harboring BTK active site mutations or PLCγ2 mutations insensitive to ibrutinib were sensitive to entospletinib, demonstrating the potential for an increased duration of response (DOR) with the dual combination of the two inhibitors [Liu et al. 2015; Chang et al. 2015]. Clinical studies looking at entospletinib treatment following ibrutinib or idelalisib failures are in progress [Clinicaltrials.gov identifier: NCT01799889; Sharman et al. 2015d].
SYK inhibition has been shown to alter actin dynamics in response to both BCR [Le Roux et al. 2007] and non-BCR-mediated (integrin) signaling [Pearce et al. 2011]. B-cell-microtubule assembly is also regulated in part by SYK [Faruki et al. 2000], and pharmacologic SYK inhibition resulted in the block of the G1/S phase transition, resulting in cell-cycle arrest in DLBCL lines by preventing SYK-dependent activation of PLCγ2 and AKT [Cheng et al. 2011]. More recently, evidence that SYK inhibition may synergize with existing antimicrotubule cytotoxic agents [Yu et al. 2015] in epithelial tissues was reported. These reports highlight the potential for interplay between SYK and other microtubule agents with activity in hematologic neoplasms. Importantly, entospletinib synergized with the microtubule inhibitor, vincristine, and induced cytotoxicity via apoptosis in a wide range of malignant hematologic cell lines and demonstrated in vivo efficacy in a DLBCL cell xenograft [Axelrod et al. 2015].
SYK activation has been implicated as a mechanism for chemotherapy-induced drug resistance by upregulating antiapoptotic factors, such as MCL-1 in CLL cells, and inhibition of SYK with R406 in combination with fludarabine-abrogated stroma-mediated drug resistance by preventing upregulation of MCL-1 [Buchner et al. 2010]. Additional reports demonstrate that SYK inhibition can stabilize mRNA for prosurvival signaling proteins, such as BCL-XL [Wang et al. 2014]. These observations lead to the attractive hypothesis that SYK combinations with chemotherapeutics or prosurvival protein inhibitors, such as BCL-2 inhibitors, could reduce the incidence of chemotherapy-induced resistance and enhance the efficacy of prosurvival protein inhibitors, respectively. In a panel of primary CLL samples, the addition of entospletinib to a BCL-2 inhibitor, ABT-199, increased the level of apoptosis achieved in both the presence and absence of stromal-cell coculture, suggesting that an enhanced activity of this combination could have clinical benefit [Jones et al. 2015b; Bojarczuk et al. 2015].
Clinical experience with entospletinib
The safety and efficacy of entospletinib was evaluated in an open-label, phase II trial that enrolled five separate cohorts of patients with relapsed or refractory CLL, FL, other iNHLs (WM, SLL, MZL), MCL or DLBCL [Sharman et al. 2015a]. The safety analysis included all treated subjects across the five cohorts (n = 186). The most common nonhematologic AEs (>20%) of any grade included fatigue, nausea, diarrhea, decreased appetite, constipation, cough and headache. Grade 3 nonhematologic AEs (>2%) included fatigue, nausea and dyspnea. Treatment-emergent serious AEs occurring in 2% or more of patients included dyspnea, pneumonia, febrile neutropenia, dehydration, and pyrexia. Treatment-emergent AEs occurring in 2% or more of patients and leading to study-drug discontinuation included fatigue, increased alanine transaminase (ALT), and headache. Grade 3 or more laboratory abnormalities occurring in greater than 10% of patients included neutropenia and increased ALT/aspartate transaminase (AST) [Sharman et al. 2015a].
Efficacy data have been reported for the patients with CLL (n = 41) [Sharman et al. 2015a]. Of 39 evaluable patients who had at least one postbaseline assessment, 94.9% achieved a reduction in adenopathy; a 50% or more decrease in the sum of products of the diameters (SPD) from baseline was achieved by 61.5% (95% CI, 44.6–76.6%) of subjects, and 33% had less than a 50% decrease in SPD from baseline. A total of 25 of 41 patients achieved a partial response (PR), whereas none achieved a complete response (CR). Three of the patients (7.3%) achieved a nodal response (>50% reduction in SPD) with persistent lymphocytosis. Of the 25 responding patients in the CLL cohort, the median DOR had not yet been reached (95% CI: 6.5 months to not reached) [Sharman et al. 2015a]. PFS at 24 weeks in the CLL cohort was 70.1% (95% CI: 51.3–82.7%). Median PFS was 13.8 months (95% CI: 7.7 months to not reached), based upon a median follow up of 7.7 months. Early results in a small cohort of entospletinib-treated patients either intolerant of, or resistant to, ibrutinib or idelalisib show reductions in lymph node volume in the majority of evaluated patients with low rates of early progression [Sharman et al. 2015d]. Longer follow up and a larger sample size of patients will be required before this strategy can be thoroughly evaluated.
Data in the FL cohort (n = 41) have also been reported [Sharman et al. 2015c]. A total of 37 of the 41 patients were evaluable for efficacy. Seven of 37 patients (19%) had a 50% or more decrease in SPD from baseline, and 19 of 37 patients (51%) had less than a 50% decrease in SPD from baseline. Seven of 41 patients (17%) achieved a PR, and one of 41 patients (2%) achieved a CR for an overall response rate of 20% (95% CI: 9–35%). Of the remaining patients, 20 (49%) had stable disease, 11 (27%) had progressive disease, and 2 discontinued the study prior to the first scheduled tumor assessment (one due to adverse events, and one withdrew consent). The 24-week PFS in the FL cohort was 52% (95% CI: 33–67%), and the median PFS of 5.7 months was demonstrated with a median follow up of 3.6 months. Of the eight responding patients in the FL cohort, one patient was ongoing with a DOR of 11 months; three patients discontinued the study due to progressive disease and had DOR of 2.2, 4.5 and 10.9 months at the time of discontinuation; and four patients had no follow-up tumor assessment after responding (two were ongoing, and two discontinued the study due to AEs and were lost to follow up).
Several other indolent lymphoma studies have reported results. In a group of 15 patients with SLL, all patients evaluated experienced a reduction of adenopathy with an overall response rate of 67% and similar PFS to CLL patients [Sharman et al. 2015b]. Patients with lymphoplasmacytic lymphoma (n = 12) and MZL (n = 17) experienced response rates of 17% and 12% with 24-week PFS rates of 49% and 46%, respectively [Sharman et al. 2015c]. Clinical activity in DLBCL has not been reported. A summary of the clinical trials evaluating entospletinib monotherapy or combination therapy can be found in Table 1.
Table 1.
Clinical trials evaluating entospletinib as monotherapy or in combination therapy.
| Entospletinib: monotherapy or combination therapy | Condition | Title | Reference/ClinicalTrials.gov identifier |
|---|---|---|---|
| Monotherapy | Relapsed or refractory CLL | An open-label phase II trial of entospletinib (GS-9973), a selective SYK inhibitor, in CLL | Sharman et al. [2015a] |
| NCT01799889 | |||
| Monotherapy | CLL refractory to prior therapy with ibrutinib or idelalisib | Clinical activity of entospletinib (GS-9973), a selective SYK inhibitor, in patients with CLL previously treated with an inhibitor of BCR pathway signaling | Sharman et al. [2015d] |
| NCT01799889 | |||
| Monotherapy | Relapsed or refractory iNHL (FL, LPL/WM, SLL, MZL) | Phase II trial of entospletinib (GS-9973), a selective SYK inhibitor, in CLL and SLL | Sharman et al. [2015b] |
| NCT01799889 | |||
| Phase II trial of entospletinib (GS-9973), a selective SYK inhibitor, in iNHL | Sharman et al. [2015c] | ||
| Combination therapy with idelalisib | Relapsed or refractory CLL | Phase II study of idelalisib and entospletinib; pneumonitis limits combination therapy in relapsed refractory CLL and NHL | Study halted due to excessive immune-related toxicities Barr et al. [2015] |
| NCT01796470 | |||
| Combination therapy with vincristine and dexamethasone | Relapsed or refractory ALL | Safety and efficacy of entospletinib with vincristine and dexamethasone in adults with relapsed or refractory ALL |
Minden et al. [2015] NCT02404220 |
| Combination with vincristine and vincristine-based combination chemotherapy | Relapsed or refractory NHL | Safety and efficacy of entospletinib (GS-9973) combined with VCR and VCR-based combination chemotherapy in adult participants with NHL | NCT02568683 |
ALL, acute lymphoid leukemia; BCR, B-cell receptor; CLL, chronic lymphocytic leukemia; FL, follicular lymphoma; LPL, lymphoplasmacytic lymphoma; MZL, marginal zone lymphoma; iNHL, indolent non-Hodgkin’s lymphoma; NHL, non-Hodgkin’s lymphoma; SLL, small lymphocytic lymphoma; SYK, spleen tyrosine kinase; VCR, vincristine; WM, Waldenström’s macroglobulinemia.
In separate studies, cerdulatinib demonstrated anecdotal responses, but because of the dose-finding nature of the study, evaluation of response rates in different disease states has not been reported [Hamlin et al. 2015]. A preliminary report of the activity of TAK-659 showed responses in three of seven evaluable patients with DLBCL (all PR) and in two of two evaluable patients with FL (one PR and one CR) [Petrich et al. 2015].
Combination clinical studies
Eradication of disease and prevention of relapse remain a challenge in CLL. The success of BCR-targeted therapies with BTK, PI3Kδ and SYK demonstrate significant efficacy but few cases of disease eradication. Combinations of these agents, as well as others, have been proposed and initiated based on the rationale of providing enhanced clinical benefit in the diverse genetic background of CLL, targeting pathways emanating from microenvironmental signals or to increase the DOR by overcoming potential resistance to single-target inhibition.
A pioneering study focused on two BCR-targeted inhibitors, idelalisib and entospletinib, that were clinically investigated based on the laboratory observation that these synergistically induced apoptosis in CLL and further disrupted chemokine signaling from clinical samples [Burke et al. 2014]. A rapid, robust median objective response rate of 60% at 10 weeks was observed with the combination in CLL. Despite the encouraging efficacy, this study was terminated early due to pneumonitis in 18% of patients, with two fatalities that were directly attributed to treatment-related pneumonitis [Barr et al. 2015]. Pharmacodynamic biomarkers in this study indicated increases of interferon γ, and interleukins 6, 7 and 8 occurred over time in patients who ultimately developed pneumonitis, without similar trends observed in other patients. Importantly, this study highlights the need for clinical caution when evaluating novel combinations of targeted agents.
Based upon laboratory evidence of SYK regulation of microtubule dynamics, treatment of B-cell lineage acute lymphoid leukemia (B-ALL) with the combination of entospletinib and vincristine is currently being clinically evaluated [ClinicalTrials.gov identifier: NCT02404220; Minden et al. 2015]. Additional phase I studies for patients with iNHL or aggressive NHL are evaluating entospletinib in combination with vincristine-containing regimens, such as R-CHOP, dose-adjusted R-EPOCH, and R-CVP [ClinicalTrials.gov identifier: NCT02568683]. Other pathways for which combination strategies with entospletinib may be therapeutically beneficial include the use of BCL-2 inhibitors or other BCR signal proteins, such as BTK. Many novel combinations have been supported by preclinical studies or have reached clinical development (Table 2). This trend for chemotherapy-free, combination therapeutic evaluations will continue as efforts to eradicate hematologic malignancies are pursued. As initial clinical results are reported for the selected combinations, the safety and tolerability of given combinations will be informative for learning how to best combine these novel agents to achieve clinical effect in the future.
Table 2.
Novel combination therapies in development.
| Sponsor | Combination | Condition | Title | Study design | Reference/ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| AbbVie | Duvelisib (PI3Kδ,γ) + ABT-199 (BCL2) | CLL | Elevated level of BCL-2 is the primary target for inhibition during duvelisib (IPI-145) therapy: ABT-199 neutralizes the resistance mechanism in CLL | Preclinical evaluation of PBMCs collected from CLL patients who participated in phase I study of duvelisib treatment | Patel et al. [2015] |
| AbbVie | Duvelisib (PI3Kδ,γ) + ibrutinib (BTK) | 20 cell lines, including DLBCL, FL, T-cell and MCL, MM | High throughput, in vitro combination sensitivity screen in hematologic malignancies with the PI3K-δ,γ inhibitor, duvelisib | Preclinical evaluation measuring growth inhibition in a 6 × 6 or 9 × 9 dose combination matrix | Faia et al. [2015] |
| AbbVie | ABT-199 (BCL2) + ibrutinib (BTK) | MCL | Multi-institution, phase I/Ib study of ibrutinib with ABT-199 in relapsed/refractory MCL | Phase I, nonrandomized, open-label, single group | NCT02419560 |
| Acerta | ACP-196 (BTK) + ACP-319 (PI3K) | CLL | A multicenter, open-label, phase I pilot study of ACP 196 in combination with ACP-319 in subjects with relapsed/refractory CLL | Phase I, nonrandomized, open-label, single group | NCT02157324 |
| Acerta | ACP-196 (BTK) + pembrolizumab (anti-PD-1) | NHL, MM, Hodgkin’s lymphoma, CLL, Richter’s syndrome, WM | A phase Ib/II proof-of-concept study of the combination of ACP-196 and pembrolizumab in subjects with B-cell malignancies | Phase I/II nonrandomized, open-label, single group | NCT02362035 |
| Gilead | Lenalidomide (immunomodulator) + idelalisib (PI3Kδ) | Relapsed FL | A phase I trial of lenalidomide and idelalisib in recurrent FL | Phase I, open-label, single group | Study halted due to excessive immune-related toxicities |
| NCT01644799 | |||||
| Gilead | Entospletinib (SYK) + vincristine (antineoplastic) | ALL | Phase Ib, open-label, dose escalation and expansion study evaluating the safety and efficacy of entospletinib (GS-9973) with vincristine and dexamethasone in adult subjects with relapsed or refractory ALL | Phase I, open-label, dose escalation expansion | NCT02404220 |
| Gilead | Ibrutinib (BTK) + idelalisib (PI3Kδ) | B-cell malignancies | A phase Ib dose escalation and dose expansion study of ONO/GS-4059 combined with idelalisib in subjects with B-cell malignancies | Phase I, open-label, single group | NCT02457598 |
| Gilead | Lenalidomide (immunomodulator) + idelalisib (PI3Kδ) | MCL | A phase I/randomized phase II trial of idelalisib and lenalidomide in patients with relapsed/refractory MCL | Phase II, randomized, open-label, parallel group | NCT01838434 |
| Gilead | Entospletinib (SYK) + idelalisib (PI3Kδ) | CLL, MCL, DLBCL, iNHL | A phase II, open-label study evaluating the efficacy, safety, tolerability, and pharmacodynamics of GS-9973 in combination with idelalisib in subjects with relapsed or refractory hematologic malignancies | Phase II, open-label, single group | Manuscript in progress; study halted |
| NCT01796470 | |||||
| TG Therapeutics | TGR-1202 (PI3Kδ) + TG-1101 (CD20) | CLL, BCL | Ublituximab, a novel glycoengineered anti-CD20 mAb, in combination with TGR-1202, a next generation once daily PI3Kδ inhibitor, demonstrates activity in heavily pre-treated and high-risk CLL and BCL | Phase I, nonrandomized, CLL and NHL cohorts | Lunning et al. [2014] |
| TG Therapeutics | TGR-1202 (PI3Kδ) + TG-1101 (CD20) + ibrutinib (BTK) | CLL, NHL | A phase I/Ib study evaluating the efficacy and safety of ublituximab, a third-generation anti-CD20 mAb, in combination with TGR-1202, a novel PI3Kδ inhibitor; and ibrutinib, in patients with B-cell malignancies | Phase I/Ib, nonrandomized, open-label, single group | Lunning et al. [2015] |
| NCT02006485 | |||||
| TG Therapeutics | Ibrutinib (BTK) + ublituximab (CD20) | CLL, MCL | A multi-center phase II study with safety run-in evaluating the efficacy and safety of ublituximab in combination with ibrutinib in patients with select B-cell malignancies | Phase I/II, open-label, single group | Sharman et al. [2014] Full publication in progress |
| NCT02013128 | |||||
| TG Therapeutics | Ibrutinib (BTK) + ublituximab (CD20) | High-risk relapsed or refractory CLL | A phase III, randomized study to assess the efficacy and safety of ublituximab in combination with ibrutinib compared to ibrutinib alone, in patients with previously treated high-risk CLL | Phase III, randomized, open-label, parallel group | NCT02301156 |
ALL, acute lymphoid leukemia; BCL, B-cell lymphoma; BTK, bruton’s tyrosine kinase; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; iNHL, indolent non-Hodgkin’s lymphoma; mAb, monocloncal antibody; MCL, mantle cell lymphoma; MM, multiple myeloma; NHL, non-Hodgkin’s lymphoma; PBMC, peripheral blood mononuclear cell; PI3K, phosphoinositide-3 kinase; SYK, spleen tyrosine kinase; WM, waldenström’s macroglobulinemia.
Conclusion
Targeted inhibition of BCR signaling has become a validated strategy across a variety of lymphoid malignancies. The FDA approvals of ibrutinib and idelalisib highlight the exciting potential of drugs targeting this pathway, yet also expose the need for more understanding of how BCR signaling proteins interact with one another and mediate crosstalk with other pathways regulating cytokine signaling, microtubule dynamics, microenvironment signaling and cell-survival proteins. As a key molecule in BCR signaling, SYK regulates many critical functions, yet the clinical development of SYK inhibitors has lagged behind the development of BTK and PI3Kδ inhibitors. Although the single-agent activity of entospletinib in CLL is impressive, regulatory-approval strategies for the molecule are considerably more difficult now than they might have been only several years ago. Whether entospletinib can find a route to approval as a single agent for patients either resistant to or intolerant of approved novel agents remains speculative. Alternatively, strategies to enhance activity or prevent resistance when given in combination with approved agents will likely be required. The efficacy and relative tolerability of entospletinib provides the potential for it to be a backbone for future therapeutic combinations.
In contrast, the activity in iNHL is likely insufficient to gain FDA approval as a single agent, yet emerging preclinical data and ongoing clinical trials focusing on the interaction of entospletinib with microtubule agents could open several regulatory strategies for the molecule in a variety of lymphoid malignancies. If SYK inhibition enhances the activity of agents such as vincristine, or the auristatins used in antibody drug conjugates, several routes to approval could emerge.
There is little question that SYK plays a central role in B-cell biology, and entospletinib has demonstrated intriguing potential as a therapy. Demonstrating that SYK inhibitors can play a central role in the treatment of B-cell malignancies remains an area of intense laboratory and clinical investigation.
Acknowledgments
Editorial assistance was provided by Impact Communication Partners, Inc.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosures: Gilead Sciences, Inc. provided funding for editorial assistance by Impact Communications, Inc.
Conflict of interest statement: J. Sharman received research funding and honoraria from Gilead Sciences, Inc., Pharmacyclics Inc., and Genentech, Inc., and received research funding and consulting fees from Celgene Corporation.
J. Di Paolo is employed at Gilead Sciences, Inc., and owns stock in the company.
Contributor Information
Jeff Sharman, Willamette Valley Cancer Institute and Research Center, US Oncology Research, 3377 Riverbend Drive, Suite 500, Springfield, OR 97477, USA.
Julie Di Paolo, Department of Biology, Gilead Sciences, Inc., Foster City, CA, USA.
References
- Ackermann J., Nys J., Schweighoffer E., McCleary S., Smithers N., Tybulewicz V. (2015) SYK tyrosine kinase is critical for B-cell antibody responses and memory B-cell survival. J Immunol 194: 4650–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein P., Attar E., Takvorian T., Hochberg E., Ballen K., Leahy K., et al. (2011) Phase II study of dasatinib in relapsed or refractory chronic lymphocytic leukemia. Clin Cancer Res 17: 2977–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod M., Fowles P., Silverman J., Clarke A., Tang J., Rousseau E., et al. (2015) The combination of entospletinib and vincristine demonstrates synergistic activity in a broad panel of hematological cancer cell lines and anti-tumor efficacy in a DLBCL xenograft model. Blood (ASH Annual Meeting) 126: 5123. [Google Scholar]
- Barr P., Saylors G., Spurgeon S., Cheson B., Greenwald D., O’Brien S., et al. (2015) SYK and PI3Κδ pathway inhibition resulted in increased rates of pneumonitis: implications for developing future small molecule combinations. Hematol Oncol (ICML Annual Meeting) 33: 121. [Google Scholar]
- Bartlett N., LaPlant B., Qi J., Ansell S., Kuruvilla J., Reeder C., et al. (2014) Ibrutinib monotherapy in relapsed/refractory follicular lymphoma (FL): preliminary results of a phase II consortium (P2C) trial. Blood (ASH Annual Meeting) 124: 800. [Google Scholar]
- Bojarczuk K., Sasi B., Gobessi S., Innocenti I., Laurenti L., Efremov D. (2015) Inhibition of SYK more effectively overcomes MCL-1 mediated ABT-199 resistance of BCR-stimulated CLL cells than inhibition of BTK or PI3Kdelta. Blood (ASH Annual Meeting) 126: 489. [Google Scholar]
- Buchner M., Baer C., Prinz G., Dierks C., Burger M., Zenz T., et al. (2010) Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood 115: 4497–4506. [DOI] [PubMed] [Google Scholar]
- Buchner M., Fuchs S., Prinz G., Pfeifer D., Bartholomé K., Burger M., et al. (2009) Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res 69: 5424–5432. [DOI] [PubMed] [Google Scholar]
- Burger J., Quiroga M., Hartmann E., Bürkle A., Wierda W., Keating M., et al. (2009) High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood 113: 3050–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J., Tedeschi A., Barr P., Robak T., Owen C., Ghia P., et al. (2015) Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 373: 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R., Meadows S., Loriaux M., Currie K., Mitchell S., Maciejewski P., et al. (2014) A potential therapeutic strategy for chronic lymphocytic leukemia by combining idelalisib and GS-9973, a novel spleen tyrosine kinase (SYK) inhibitor. Oncotarget 5: 908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J., Brown J., O’Brien S., Barrientos J., Kay N., Reddy N., et al. (2014) Ibrutinib versus ofatumumab in previously treated chronic lymphocytic leukemia. N Engl J Med 371(3): 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J., Furman R., Coutre S., Burger J., Blum K., Coleman M., et al. (2015) Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 125: 2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J., Furman R., Coutre S., Flinn I., Burger J., Blum K., et al. (2013) Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 369: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calpe E., Codony C., Baptista M., Abrisqueta P., Carpio C., Purroy N., et al. (2011) ZAP-70 enhances migration of malignant B lymphocytes toward CCL21 by inducing CCR7 expression via IgM-ERK1/2 activation. Blood 118: 4401–4410. [DOI] [PubMed] [Google Scholar]
- Casola S., Otipoby K., Alimzhanov M., Humme S., Uyttersprot N., Kutok J., et al. (2004) B cell receptor signal strength determines B-cell fate. Nat Immunol 5: 317–327. [DOI] [PubMed] [Google Scholar]
- Chanan-Khan A., Cramer P., Demirkan F., Fraser G., Silva R., Pylypenko H., et al. (2015) Ibrutinib combined with bendamustine and rituximab (BR) in previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): first results from a randomized, double-blind, placebo-controlled, phase III study. J Clin Oncol (ASCO Annual Meeting) 33: LBA7005. [Google Scholar]
- Chang B., Buggy J., Steggerda S. (2015) Mutations associated with resistance to inhibitors of Bruton’s tyrosine kinase (BTK). Pharmacyclics, Inc. Patent number 20150184249. [Google Scholar]
- Chen L., Monti S., Juszczynski P., Daley J., Chen W., Witzig T., et al. (2008) SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood 111: 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Coffey G., Zhang X., Shaknovich R., Song Z., Lu P., et al. (2011) SYK inhibition and response prediction in diffuse large B-cell lymphoma. Blood 118: 6342–6352. [DOI] [PubMed] [Google Scholar]
- Conley M., Dobbs A., Farmer D., Kilic S., Paris K., Grigoriadou S., et al. (2009) Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol 27: 199–227. [DOI] [PubMed] [Google Scholar]
- Currie K., Kropf J., Lee T., Blomgren P., Xu J., Zhao Z., et al. (2014) Discovery of GS-9973, a selective and orally efficacious inhibitor of spleen tyrosine kinase. J Med Chem 57: 3856–3873. [DOI] [PubMed] [Google Scholar]
- Di Paolo J., Clarke A., Rong H., Rousseau E. (2014) Mass-spectroscopy phosphorylation profiling identified differential activation of signaling molecules via spleen tyrosine kinase in B-cell receptor signaling and stromal cell stimulation in DLBCL and CLL cells that are inhibited by GS-9973. Cancer Res (AACR Annual Meeting) 74(Suppl. 19): 4205. [Google Scholar]
- Faia K., White K., Proctor J., Andrade P., Pink M., Rickles R., et al. (2015) High throughput in vitro combination sensitivity screen in hematologic malignancies with the phosphoinositide-3 kinase (PI3K)-δ,γ inhibitor, duvelisib. J Clin Oncol (ASCO Annual Meeting) 33: 8559. [Google Scholar]
- Faruki S., Geahlen R., Asai D. (2000) SYK-dependent phosphorylation of microtubules in activated B-lymphocytes. J Cell Sci 113: 2557–2565. [DOI] [PubMed] [Google Scholar]
- Flinn I., Kahl B., Leonard J., Furman R., Brown J., Byrd J., et al. (2014) Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-δ, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood 123: 3406–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J., Blunt M., Carter E., Ward S. (2012) Inhibition of PI3K signaling spurs new therapeutic opportunities in inflammatory/autoimmune diseases and hematological malignancies. Pharmacol Rev 64: 1027–1054. [DOI] [PubMed] [Google Scholar]
- Fowler N., Davis E. (2013) Targeting B-cell receptor signaling: changing the paradigm. Hematology Am Soc Hematol Educ Program 1: 553–560. [DOI] [PubMed] [Google Scholar]
- Friedberg J., Sharman J., Sweetenham J., Johnston P., Vose J., Lacasce A., et al. (2010) Inhibition of SYK with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 115: 2578–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman R., Sharman J., Coutre S., Cheson B., Pagel J., Hillmen P., et al. (2014) Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 370: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese M., Kavanaugh A., Weinblatt M., Peterfy C., DiCarlo J., White M., et al. (2011) An oral SYK kinase inhibitor in the treatment of rheumatoid arthritis: a three-month randomized, placebo-controlled, phase II study in patients with active rheumatoid arthritis that did not respond to biologic agents. Arthritis Rheum 63: 337–345. [DOI] [PubMed] [Google Scholar]
- Gobessi S., Laurenti L., Longo P., Carsetti L., Berno V., Sica S., et al. (2009) Inhibition of constitutive and BCR-induced SYK activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia 23: 686–697. [DOI] [PubMed] [Google Scholar]
- Gopal A., Kahl B., de Vos S., Wagner-Johnston N., Schuster S., Jurczak W., et al. (2014) PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 370: 1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin P., Flinn I., Wagner-Johnston N., Burger J., Michelson G., Pandey A., et al. (2015) Clinical and correlative results of a phase I study of cerdulatinib (PRT062070) a dual SYK/JAK inhibitor in patients with relapsed/refractory B cell malignancies. Blood (ASH Annual Meeting) 126: 3929. [Google Scholar]
- Herishanu Y., Pérez-Galán P., Liu D., Biancotto A., Pittaluga S., Vire B., et al. (2011) The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 117: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman S., Niemann C., Farooqui M., Jones J., Mustafa R., Lipsky A., et al. (2014) Ibrutinib-induced lymphocytosis in patients with chronic lymphocytic leukemia: correlative analyses from a phase II study. Leukemia 28: 2188–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoellenriegel J., Coffey G., Sinha U., Pandey A., Sivina M., Ferrajoli A., et al. (2012) Selective, novel spleen tyrosine kinase (SYK) inhibitors suppress chronic lymphocytic leukemia B-cell activation and migration. Leukemia 26: 1576–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Improgo R., Tiao G., Kiezun A., Wang Y., Werner L., Sougnez C., et al. (2013) NF-κB pathway mutations modulate cell survival and ibrutinib response in chronic lymphocytic leukemia. Blood 122: 670. [Google Scholar]
- Jones J., Wach M., Robak T., Brown J., Menter A., Vandenberghe E., et al. (2015a) Results of a phase III randomized, controlled study evaluating the efficacy and safety of idelalisib (IDELA) in combination with ofatumumab (OFA) for previously treated chronic lymphocytic leukemia (CLL). J Clin Oncol (ASCO Annual Meeting) 33: 7023. [Google Scholar]
- Jones R., Axelrod M., Tumas D., Quéva C., Di Paolo J. (2015b) Combination effects of B-cell receptor pathway inhibitors (entospletinib, ONO/GS-4059, and idelalisib) and a BCL-2 inhibitor in primary CLL cells. Blood (ASH Annual Meeting) 126: 1749. [Google Scholar]
- Kipps T. (2007) The B-cell receptor and ZAP-70 in chronic lymphocytic leukemia. Best Pract Res Clin Haematol 20: 415–424. [DOI] [PubMed] [Google Scholar]
- Lamagna C., Hu Y., DeFranco A., Lowell C. (2014) B-cell-specific loss of Lyn kinase leads to autoimmunity. J Immunol 192: 919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux D., Lankar D., Yuseff M., Vascotto F., Yokozeki T., Faure-André G., et al. (2007) SYK-dependent actin dynamics regulate endocytic trafficking and processing of antigens internalized through the B-cell receptor. Mol Biol Cell 18: 3451–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Woyach J., Zhong Y., Lozanski A., Lozanski G., Dong S., et al. (2015) Hypermorphic mutation of phospholipase C, γ2 acquired in ibrutinib-resistant CLL confers BTK independency upon B-cell receptor activation. Blood 126: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunning M., Vose J., Fowler N., Nastoupil L., Schreeder M., Siddiqi T., et al. (2015) Ublituximab plus TGR-1202 activity and safety profile in relapsed/refractory B-cell NHL and high-risk CLL. J Clin Oncol (ASCO Annual Meeting) 33: 8548. [Google Scholar]
- Lunning M., Vose J., Schreeder M., Fowler N., Nastoupil L., Siddiqi T., et al. (2014) Ublituximab, a novel glycoengineered anti-CD20 monoclonal antibody (mAb), in combination with TGR-1202, a next generation once daily PI3kδ inhibitor, demonstrates activity in heavily pre-treated and high-risk chronic lymphocytic leukemia (CLL) and B-cell lymphoma. Blood (ASH Annual Meeting) 124: 801. [Google Scholar]
- Maddocks K., Ruppert A., Lozanski G., Heerema N., Zhao W., Abruzzo L., et al. (2015) Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol 1: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews Griner L., Guha R., Shinn P., Young R., Keller J., Liu D., et al. (2014) High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci USA 111: 2340–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato A., Nabhan C., Barr P., Ujjani C., Hill B., Lamanna N., et al. (2015) Favorable outcomes in CLL pts with alternate kinase inhibitors following ibrutinib or idelalisib discontinuation: results from a large multi-center study. Blood (ASH Annual Meeting) 126: 719. [Google Scholar]
- Minden M., Stock W., Crosswell H., Siegel R., Chun P, Abella S., et al. (2015) A phase 1b, open-label, dose escalation and expansion study evaluating the safety and efficacy of entospletinib (GS-9973) with vincristine and dexamethasone in adult subjects with relapsed or refractory acute lymphoid leukemia (ALL). Blood (ASH Annual Meeting) 126: 23. [Google Scholar]
- Mócsai A., Ruland J., Tybulewicz V. (2010) The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol 10: 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizumi H., Horikawa K., Mlinaric-Rascan I., Yamamoto T. (1998) A double-edged kinase LYN: a positive and negative regulator for antigen receptor-mediated signals. J Exp Med 187: 1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien S., Furman R., Coutre S., Sharman J., Burger J., Blum K., et al. (2014) Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: an open-label, multicentre, phase Ib/II trial. Lancet Oncol 15: 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkenhaug K., Vanhaesebroeck B. (2003) PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol 3: 317–330. [DOI] [PubMed] [Google Scholar]
- Patel V., Balakrishnan K., Guerrieri R., Wierda W., O’Brien S., Gandhi V. (2015) Elevated level of BCL-2 is the primary target for inhibition during duvelisib (IPI145) therapy: ABT199 neutralizes the resistance mechanism in chronic lymphocytic leukemia. Cancer Res (AACR Annual Meeting) 2657. [Google Scholar]
- Pearce G., Audzevich T., Jessberger R. (2011) SYK regulates B-cell migration by phosphorylation of the F-actin interacting protein SWAP-70. Blood 117: 1574–1584. [DOI] [PubMed] [Google Scholar]
- Petrich A., Gordon L., Infante J., Madan S., Giles F., Nimeiri H., et al. (2015) Phase I dose-escalation study of TAK-659, an investigational SYK inhibitor, in patients (pts) with advanced solid tumor or lymphoma malignancies. Blood (ASH Annual Meeting) 126: 2693. [Google Scholar]
- Puri K., Di Paolo J., Gold M. (2013) B-cell receptor signaling inhibitors for treatment of autoimmune inflammatory diseases and B-cell malignancies. Int Rev Immunol 32: 397–427. [DOI] [PubMed] [Google Scholar]
- Puri K., Gold M. (2012) Selective inhibitors of phosphoinositide 3-kinase delta: modulators of B-cell function with potential for treating autoimmune inflammatory diseases and B-cell malignancies. Front Immunol 3: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga M., Balakrishnan K., Kurtova A., Sivina M., Keating M., Wierda W., et al. (2009) B-cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: specific targeting with a novel spleen tyrosine kinase inhibitor, R406. Blood 114: 1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S., DiPaolo J., Doan T., Burge D. (2013) Single and multiple dose-ranging evaluation of safety, pharmacokinetics, and pharmacodynamics of GS-9973, a novel pSYK inhibitor. Cancer Res (AACR Annual Meeting) 73: 32. [Google Scholar]
- Rolli V., Gallwitz M., Wossning T., Flemming A., Schamel W., Zürn C., et al. (2002) Amplification of B cell antigen receptor signaling by a SYK/ITAM positive feedback loop. Mol Cell 10: 1057–1069. [DOI] [PubMed] [Google Scholar]
- Sharman J., Hawkins M., Kolibaba K., Boxer M., Klein L., Wu M., et al. (2015a) An open-label phase II trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in chronic lymphocytic leukemia. Blood 125: 2336–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman J., Irish J., Coffey G., Czerwinski D., Hitoshi Y., Levy R. (2007) Targeting SYK kinase for the treatment of B-cell lymphoma. J Clin Oncol (ASCO Annual Meeting) 25: 3600. [Google Scholar]
- Sharman J., Klein L., Boxer M., Kolibaba K., Abella S., Di Paolo J., et al. (2015b) Phase II trial of entospletinib (GS-9973), a selective SYK inhibitor, in chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL). Blood (ASH Annual Meeting) 126: 4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman J., Klein L., Boxer M., Kolibaba K., Abella S., Eng C., et al. (2015c) Phase II trial of entospletinib (GS-9973), a selective SYK inhibitor, in indolent non-Hodgkin’s lymphoma (iNHL). Blood (ASH Annual Meeting) 126: 1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman J., Klein L., Boxer M., Kolibaba K., Hawkins M., Abella S., et al. (2014) A multi-center phase II study with safety run-in evaluating the efficacy and safety of ublituximab in combination with ibrutinib in patients with select B-cell malignancies. Blood (ASH Annual Meeting) 124: 4679. [Google Scholar]
- Sharman J., Shustov A., Smith M., Boyd T., Hagenstad C., Kolibaba K., et al. (2015d) Clinical activity of entospletinib (GS-9973), a selective SYK inhibitor, in patients with CLL previously treated with an inhibitor of B-cell receptor pathway signaling. Blood (ASH Annual Meeting) 126: 1744. [Google Scholar]
- Spurgeon S., Coffey G., Fletcher L., Burke R., Tyner J., Druker B., et al. (2013) The selective SYK inhibitor P505-15 (PRT062607) inhibits B cell signaling and function in vitro and in vivo and augments the activity of fludarabine in chronic lymphocytic leukemia. J Pharmacol Exp Ther 344: 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suljagic M., Longo P., Bennardo S., Perlas E., Leone G., Laurenti L., et al. (2010) The SYK inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Eµ- TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood 116: 4894–4905. [DOI] [PubMed] [Google Scholar]
- Tan S., Liao C, Lucas M., Stevenson C., DeMartino J. (2013) Targeting the SYK-BTK axis for the treatment of immunological and hematological disorders: recent progress and therapeutic perspectives. Pharmacol Ther 138: 294–309. [DOI] [PubMed] [Google Scholar]
- Treon S., Tripsas C., Meid K., Warren D., Varma G., Green R., et al. (2015) Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med 372: 1430–1440. [DOI] [PubMed] [Google Scholar]
- Wang M., Rule S., Martin P., Goy A., Auer R., Kahl B., et al. (2013) Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 369: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Childress M., Geahlen R. (2014) SYK interacts with and phosphorylates nucleolin to stabilize Bcl-x(L) mRNA and promote cell survival. Mol Cell Biol 34: 3788–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinblatt M., Kavanaugh A., Burgos-Vargas R., Dikranian A., Medrano-Ramirez G., Morales-Torres J., et al. (2008) Treatment of rheumatoid arthritis with a SYK kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum 58: 3309–3318. [DOI] [PubMed] [Google Scholar]
- Woyach J., Furman R., Liu T., Ozer H., Zapatka M., Ruppert A., et al. (2014) Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med 370: 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Gaillard S., Phillip J., Huang T., Pinto S., Tessarollo N., et al. (2015) Inhibition of spleen tyrosine kinase potentiates paclitaxel-induced cytotoxicity in ovarian cancer cells by stabilizing microtubules. Cancer Cell 28: 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenetz A., Robak T., Coiffier B., Delgado J., Marlton P., Adewoye A., et al. (2015) Idelalisib plus bendamustine and rituximab (BR) is superior to BR alone in patients with relapsed/refractory chronic lymphocytic leukemia: results of a phase III randomized double-blind placebo-controlled study. Blood (ASH Annual Meeting) 126: LBA-5. [Google Scholar]