Abstract
Adults with relapsed/refractory B-acute lymphoblastic leukemia (ALL) have a complete remission (CR) rate of 20–45% and median overall survival of 3–9 months, depending on the duration of the first remission and number of lines of salvage therapy. Allogeneic hematopoietic stem cell transplantation (alloHSCT) is the only curative option for adult patients with relapsed/refractory ALL, and achievement of CR is a crucial step before alloHSCT. Blinatumomab is a bispecific T-cell engager (BiTE®) antibody construct with dual specificity for CD19 and CD3, simultaneously binding CD3-positive cytotoxic T cells and CD19-positive B cells, resulting in T-cell-mediated serial lysis of normal and malignant B cells. It recently gained accelerated approval by the US Food and Drug Administration (FDA) for the treatment of relapsed/refractory Philadelphia chromosome-negative ALL, based on a large phase II trial of 189 adults with relapsed/refractory B-ALL, which showed a CR/CRh (CR with partial hematologic recovery) of 43% after two cycles of treatment. Toxicities include cytokine-release syndrome (CRS) and neurologic events (encephalopathy, aphasia, and seizure). CRS can be alleviated by step-up dosing and dexamethasone, without affecting the cytotoxic effect of blinatumomab. The cause of neurologic toxicity is unclear but is also observed with other T-cell therapies and may relate to variable expression of CD19 within the brain. This review encompasses the preclinical rationale of using the BITE® class of compounds (blinatumomab being the only one that is FDA approved), with clinical data using blinatumomab in the relapsed/refractory setting (pediatrics and adults), the minimal residual disease setting (adults), as well as Philadelphia chromosome-positive ALL. The review also examines the main adverse events: their prevention, recognition, and management; possible mechanisms of resistance; causes of relapse. It also summarizes future trials evaluating the drug earlier in the treatment course to improve activity.
Keywords: B-acute lymphoblastic leukemia, blinatumomab, relapsed/refractory
Background
Acute lymphoblastic leukemia (ALL) is a rare but often fatal disease, with 6020 new cases and 1440 deaths estimated to have occurred in the USA in 2014 [American Cancer Society, 2014]. Although ALL is most common in the first 5 years of life, approximately 40% of patients are diagnosed after age 20 years [Larson, 2006]. Around 90% of adult patients achieve a remission with current induction therapy; however, in contrast to childhood ALL, 40–50% will eventually relapse [Linker et al. 2002].
For adult patients with ALL who experience first relapse, salvage chemotherapy can induce a second complete remission (CR) in 30–45% of patients, with median overall survival (OS) of 5–9 months [Thomas et al. 1999; Fielding et al. 2007; Tavernier et al. 2007; Oriol et al. 2010]. For patients with primary refractory disease, a short duration of first remission (< 12 months), relapse after allogeneic hematopoietic stem cell transplantation (alloHSCT), or disease that has failed multiple lines of therapy, CRs occur in 20–30% of patients, with a median OS of 3–6 months. Treatment-related mortality is high (12–23%) [Thomas et al. 1999; Fielding et al. 2007; Tavernier et al. 2007; Oriol et al. 2010]. AlloHSCT is the only curative option for adult patients with relapsed or refractory ALL, and achievement of CR is a crucial step before alloHSCT. The 5-year OS estimate for patients receiving alloHSCT after a second CR is 33% versus 17% for patients receiving alloHSCT with active disease [Gökbuget et al. 2012b]. New therapies are therefore needed for patients with relapsed/refractory ALL.
T-cell-based therapies have received considerable attention in recent years as a promising immunological treatment for various malignancies, but they must account for the layered complexity of T-cell-antigen recognition and activation. One crucial factor is the specificity of the T-cell receptor (TCR), a heterodimeric protein generated by rearrangement of germline genomic segments [Wucherpfennig et al. 2010], which results in combinatorial diversity and a broad repertoire of specificities that are clonally distributed on T cells. Unlike immunoglobulins, which may recognize native proteins, TCRs recognize peptide fragments that are cleaved by cytoplasmic proteases, transported across lipid membranes, and ultimately bound in the cleft of major histocompatibility class (MHC) antigens. An individual TCR contacts residues in the extremely polymorphic MHC protein as well as the peptide fragment bound therein. Very few TCRs need to be triggered to activate a T cell, and signaling depends on the phosphorylation of tyrosine domains within the associated complex containing the CD3 antigen [Weiss et al. 1991; Irvine et al. 2002]. Depending on the developmental stage of the T cell, there are additional inputs that influence the outcome of a TCR-mediated signal. For instance, activation of a naïve T cell requires a costimulatory signal through CD28. In contrast, a T cell that is chronically exposed to antigen may not respond to TCR signals because of dampening signals through PD-1 [Intlekofer and Thompson, 2013].
The clinical successes of CTLA-4 and PD-1 antagonists demonstrate that, in some patients with advanced cancer, there is a population of T cells that recognize cancer cells [Tumeh et al. 2014]. The size of the cancer-reactive T-cell population is under investigation, as is the nature of its antigen specificity. Whereas checkpoint blockade immunotherapy and tumor vaccines seek to amplify endogenous T-cell specificities, another strategy is to bypass them. This is the approach of the chimeric antigen receptor (CAR) and bispecific T-cell engagers (BiTE®). The CAR and BiTE® molecules facilitate a polyclonal T-cell response to tumor-associated antigens (TAA) in their native forms, independently of MHC molecules, antigen presentation, and TCR recognition. To recognize TAAs, both CAR and BiTE® incorporate the antigen-binding specificity of monoclonal antibodies in the form of a single-chain variable fragment (scFv). The scFv is incorporated in the CAR as the extracellular domain of a transmembrane receptor. In its cytoplasmic domain, a CAR contains one or more activation domains from lymphocyte molecules, such as CD3zeta, CD28, and 4-1BB [Van der Stegen et al. 2015]. The CAR is virally transduced into T cells ex vivo, followed by expansion and reinfusion into patients. The unique modular system of CARs is such that individual domains may be substituted to allow potential variety in downstream effects.
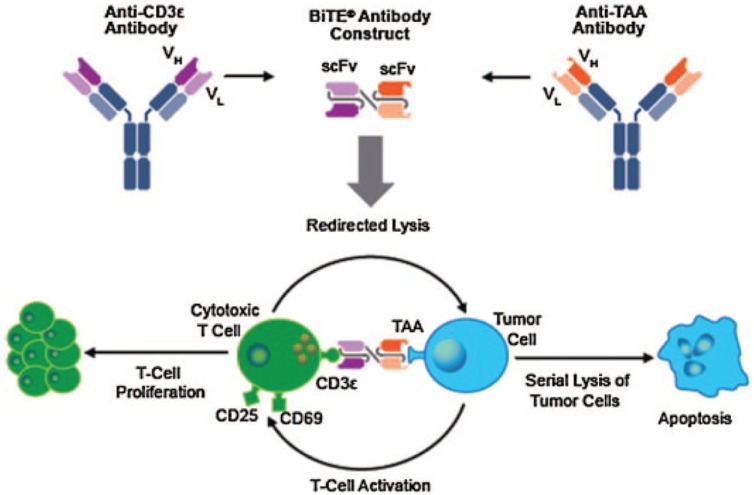
BiTE® molecules are a novel form of bispecific antibodies intended to create a short bridge between a patient’s T cells and malignant cells. A BiTE® molecule consists of two scFvs separated by a short and flexible linker (Figure 1). The N-terminal scFv recognizes the TAA. The C-terminal scFv binds the invariant CD3ε molecule on T cells. Extensive studies with full length anti-CD3ε monoclonal antibodies in vitro have demonstrated that, when immobilized, they can as a minimum qualitatively recapitulate TCR-based signaling [Ledbetter et al. 1986; Umetsu et al. 1987].
Figure 1.
Construction of a prototypical bispecific T-cell engager (BiTE®) antibody and mechanism of action.
(Reprinted with permission from Zugmaier et al. [2015].)
Blinatumomab: preclinical observations
Blinatumomab is the first BiTE® molecule to have been tested in clinical trials and to have been approved by the US Food and Drug Administration (FDA) for relapsed/refractory Philadelphia-negative B-ALL. (Approval is accelerated.) It has also received conditional approval from the European Medicines Agency. The target antigen is CD19, a cell-surface receptor expressed on B cells from the late pro-B-cell stage until plasma cell differentiation. Likewise, it is expressed on the malignant counterpart through most stages of normal B-cell ontogeny and nearly ubiquitously in B-cell leukemias and lymphomas [Raponi et al. 2011; Wang et al. 2012]. Coculture of CD19-expressing targets and effector T cells in the presence of blinatumomab results in the formation of an immune synapse between target and effector [Offner et al. 2006]. The T-cell releases the pore-forming protein perforin, permitting granzyme entry into the target cell and resulting in caspase cell activation, target-cell nuclear condensation, and membrane blebbing (Figure 1) [Gruen et al. 2004; d’Argouges et al. 2009; Haas et al. 2009].
Blinatumomab alone does not activate T cells, and lysis of CD19-negative cells is not observed. Conversely, expression of CD19 is sufficient to render Chinese hamster ovary cells susceptible to blinatumomab-mediated killing. However, CD28-mediated costimulation or T-cell preactivation is not strictly required for populations of healthy donor T cells. At high effector:target ratios (E:T), killing is rapid. Both CD8+ and CD4+ cells kill targets, the former having faster kinetics. Blinatumomab is also effective at extremely low concentrations, indicative of the T cell’s sensitivity to CD3-mediated signaling [Loffler et al. 2000], and reflecting a process of serial lysis as observed by video microscopy: a single T cell can latch on to, kill, and disengage from CD19+ cells within 30–200 min. The effector T cell can then latch on to another target cell [Hoffmann et al. 2005].
Engagement leads to T-cell proliferation, cytokine production, and upregulation of CD25 and CD69. CD8+ effector memory cells may demonstrate the most robust proliferative response, indicating that variability in T-cell populations among individuals may account for the 1–2 log range in blinatumomab potency observed with healthy donor T cells. In assays with Nalm-6 lymphoma targets, the half-maximal effective concentration (EC50) for blinatumomab was between 10 pg/ml and 80 pg/ml for approximately 75% of the T-cell donors; 20% of donors showed EC50 values greater than 130 pg/ml [Dreier et al. 2002]. Whether this is solely due to the activation status of the donor, or whether additional factors contribute, is unclear. The clinical significance is also unknown. Interestingly, in clinical trials, analysis of T-cell activation parameters has not yet revealed differences among responders and nonresponders. The T-cell activation profiles of responders and nonresponders were similar, and the target dose of 15 µg/m2/day leads to a steady-state serum concentration of 731 ± 163 pg/ml [Klinger et al. 2012], which should be above the EC50 of the least sensitive patients (500 pg/ml) [Dreier et al. 2002]. Therefore, rather than dose escalation, other approaches, such as overcoming the inherent resistant mechanisms of target cells, may be needed to address nonresponse.
In vivo proof-of-concept experiments were performed in mouse xenotransplantation models. Blinatumomab, along with human T cells, delayed tumor progression [Schlereth et al. 2006]. Pharmacokinetic data were generated in multiple animal species, and most importantly, demonstrated a terminal half-life of approximately 2 h. This is in contrast to a 21-day half-life of full-length antibodies. The difference is likely accounted for, in part, by the absence of the Fc portion on the BiTE® molecule, which is necessary for Fc receptor-mediated recycling. Blinatumomab exposure was proportional to dose and did not accumulate. Comprehensive pharmacokinetic information has been reported [Sanford, 2015].
Blinatumomab does not bind to lymphocytes from most commonly used animal species, with the exception of chimpanzees. (Blinatumomab is not cross-reactive with commonly used laboratory animal species. Therefore, a surrogate molecule, muS103new, has been generated and possesses binding affinities for murine CD3 and CD19 that are similar to those of blinatumomab for the human homologs, and it has been used to further analyze pharmacodynamic properties.)
Initial clinical experience
The initial clinical studies in patients with relapsed and refractory non-Hodgkin lymphoma (NHL), using short infusions of intravenously administered blinatumomab, did not show efficacy and was poorly tolerated [Nagorsen et al. 2012]. Not surprisingly, cytokine-release syndrome (CRS) and associated symptoms were frequently observed. Neurotoxicity, manifesting as encephalopathy, tremor, aphasia, and seizures, was also noted.
In light of its short serum half-life, blinatumomab was next tested as a continuous intravenous infusion among patients with relapsed and refractory NHL [Bargou et al. 2008]. Step-dose escalation was employed for most patients, with the goal of attenuating cytokine release according to the first-dose phenomenon [Chatenoud et al. 1990]. Depletion of circulating malignant cells was observed at doses of 5 µg/m2, whereas more efficacious clearance from bone marrow and lymph nodes was observed at doses of 15 µg/m2 and 60 µg/m2, respectively. Following initiation of blinatumomab infusions, T cells transiently disappeared from the circulation, but reappeared with an activated phenotype, including expression of CD25 and CD69. For many patients, T-cell counts returned to or exceeded baseline levels, driven by an expansion of CD4+ and CD8+ cells with the phenotype of effector memory cells. Of the initial 35 patients treated, 20 had elevation of cytokines, most commonly interleukin (IL)-10, IL-6, and/or interferon-γ. The maximum tolerated dose (MTD) was 60 µg/m2 because of a dose-limiting toxicity of neurotoxicity. Among subjects receiving a dose of 60 µg/m2/day, the overall response rate was 69%, including complete response rates of 37%. Responses were observed with follicular (80%), mantle cell (71%), and diffuse large B-cell lymphoma (55%). The median duration of response was 404 days [Goebeler et al. 2013]. Further analysis of the dose–exposure ratio showed no relationship between drug clearance and body-surface area or weight. There was also no effect on clearance by creatinine clearance, age, or sex.
Clinical experience with ALL and minimal residual disease
After these initial trials with NHL, focus intensified on blinatumomab for ALL and took into account the importance of continuous intravenous infusion (see Tables 1 and 2). The presence of minimal residual disease (MRD) following standard multiagent chemotherapy is assumed to represent chemoresistance: in the German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia (GMALL) experience, conventional CR was achieved by 89% of patients with B-cell ALL [Gökbuget et al. 2012a]. At 16 weeks following the initiation of therapy, the molecular CR rate was 66%. Disease-free survival at 5 years was substantially higher for subjects achieving molecular CR compared with those with persistent MRD. Stratification of patients based on MRD may allow more selective treatment intensification for patients with measurable residual disease, and potentially, treatment de-intensification for patients with molecular CR [Brüggemann et al. 2006]. Exploring de-intensification is preliminary, but investigators hope, especially for children, that potential long-term complications can be moderated by reducing intensity of treatment. MRD evaluation is likely to be part of this approach. In this disease setting, MRD is detected either from individual rearrangements of immunoglobulin/TCR genes, or recurrent chromosomal translocations and resultant fusion genes, including BCR-ABL and MLL-AF4; multicolor flow cytometry is sometimes used.
Table 1.
Blinatumomab clinical trials.
| Patient population, phase | Number of patients | Remission rate (CR/CRh) | MRD-negative rate among responders | Survival (median follow up) | Salvaged to transplant | Reference, ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|
| Adult MRD of B-precursor ALL, phase II | 21 | N/A | 80% | RFS 65% (33 months) | 50% | Topp et al. [2011, 2012], NCT00560794 |
| Adult MRD of B-precursor ALL, phase II | 116 | N/A | 80% | N/A | N/A | Goekbuget et al. [2014], NCT01207388 |
| Adult R/R B-precursor ALL, phase I/II | 36 | 69% | 88% | OS 9.8 months (12.1 months); RFS 7.6 months (9.7 months) | 52% | Topp et al. [2014], NCT01209286 |
| Adult Ph- R/R B-precursor ALL, phase II | 189 | 43% | 82% | OS 6.1 months (9.8 months); RFS 5.9 months (8.9 months) | 40% | Topp et al. [2015a], NCT01466179 |
| Pediatric and adolescent R/R B-precursor ALL, phase I | 41 | 32% | 77% | OS 5.7 months; RFS 8.3 months (12.4 months) | 69% | Von Stackelberg et al. [2014], NCT01471782 |
| Pediatric and adolescent R/R B-precursor ALL, phase II | 39 | 31% | 42% | OS 4.3 months (6 months); RFS 5.6 months | 50% | Gore et al. [2014], NCT01471782 |
ALL, acute lymphoblastic leukemia; CR, complete remission; CRh, complete remission with partial hematologic recovery; MRD, minimal residual disease; OS, overall survival; Ph, Philadelphia chromosome; RFS, relapse-free survival; R/R, relapsed/refractory.
Table 2.
Additional information from blinatumomab clinical trials.
| Patient population, phase | Blinatumomab dose (µg/m2/day) | Severe adverse events | Comment | Reference, ClinicalTrials.gov identifier |
|---|---|---|---|---|
| Adult MRD of B-precursor ALL, phase II | 15 | Lymphopenia, leukopenia, infections | Two relapses occurred in immunoprivileged sites | Topp et al. [2011, 2012], NCT00560794 |
| Adult MRD of B-precursor ALL, phase II | 15 | Pyrexia, tremor, aphasia, encephalopathy, overdose | 98% of patients had response in first cycle | Goekbuget et al. [2014], NCT01207388 |
| Adult R/R B-precursor ALL, phase II | 5, 15, 30 | Leukopenia, thrombocytopenia, infections, nervous system/psychiatric disorders | Stepwise dosing plus prophase treatment designed to reduce risk of CRS | Topp et al. [2014], NCT01209286 |
| Adult Ph- R/R B-precursor ALL, phase II | 9→28 | Febrile neutropenia, neutropenia, anemia | Dexamethasone prophase treatment did not affect response. Nearly half who interrupted treatment still achieved subsequent CR/CRh |
Topp et al. [2015a], NCT01466179 |
| Pediatric and adolescent R/R B-precursor ALL, phase I | 5, 15, 30 | N/A | Pharmacokinetic parameters similar to adults. | Von Stackelberg et al. [2014], NCT01471782 |
| Pediatric and adolescent R/R B-precursor ALL, phase II | 5→15 | Anemia, pyrexia, increased alanine and aspartate aminotransferases, febrile neutropenia | 3/39 patients had CRS | Gore et al. [2014], NCT01471782 |
ALL, acute lymphoblastic leukemia; CR, complete remission; CRh, complete remission with partial hematologic recovery; CRS, cytokine-release syndrome; MRD, minimal residual disease; Ph, Philadelphia chromosome; R/R, relapsed/refractory.
Blinatumomab was tested in a pilot phase II trial of 21 MRD-positive patients, 5 of whom had Ph+ disease [Topp et al. 2011, 2012]. Blinatumomab was administered for 28 days of a 42-day cycle at a dose of 15 µg/m2/day. In the MRD setting, because of low tumor burden and resultant low incidence of CRS, step-up dosing is not required. Around 80% of patients had complete MRD responses, all of which occurred in cycle 1. There were no cases of severe CRS. Grade 3/4 neurologic events were observed in four patients, including a seizure that required treatment discontinuation. Fortunately, the neurologic adverse events (AEs) were fully reversible. Lymphopenia was the most common grade ⩾ 3 adverse event, occurring in 33% of patients. Nine patients proceeded to alloHCT; with a median follow-up time of 32.9 months, the relapse free survival (RFS) rate was 65%. For the 11 subjects who did not receive alloHCT, the RFS was 60%. Among the six subjects who achieved an MRD response and did not receive additional therapy, four had not experienced MRD relapse.
An international confirmatory study was then conducted [Goekbuget et al. 2014]. A total of 116 patients with MRD levels of 10−3 or higher were eligible. Two thirds of patients were in first CR. Preliminary results were reported and demonstrated a 78% MRD complete response rate. Treatment-related serious AEs occurred in 60% of patients; those occurring in 5% or more of patients were tremor (7%), aphasia (5%), and encephalopathy (5%). In a recently reported long-term follow-up analysis, investigators reported that MRD complete response induced by blinatumomab was associated with improved OS, RFS, and duration of response in comparison with a lack of MRD complete response from blinatumomab treatment [Gökbuget et al. 2015].
A phase I/II study of 36 adult patients with relapsed/refractory ALL included a dose-finding and extension stage. Inclusion criteria were greater than 5% blasts in bone marrow, in patients with primary refractory disease or relapse after induction and consolidation chemotherapy or after alloHSCT. Exclusion criteria were Philadelphia-positive ALL eligible for tyrosine kinase (TK) inhibitors, relevant central nervous system (CNS) pathology, active CNS disease, or active graft versus host disease. Around 69% of patients achieved a CR or CRh (CR with partial hematologic recovery) (defined as platelets >50,000/µl, hemoglobin >70 g/l, and absolute neturophil count >500/µl). Around 88% of responders became MRD-negative. Of the 25 patients (52%) who achieved a CR/CRh, 13 were able to receive an alloHSCT. However, relapse was observed with 8 of 12 patients who did not receive transplantation and 2 of 13 who did. In contrast to the first-line setting, MRD-negativity therefore may not be as strongly tied to leukemia control in the relapsed/refractory setting. The median OS and RFS were 9.8 and 7.6 months, respectively. Three patients discontinued therapy, two of those for re-occurring neurologic AEs and one for grade 4 CRS [Topp et al. 2014]. A note on dose: the MTD for adults with NHL was determined to be 60 µg/m2/day, but doses as low as 15 µg/m2/day were effective for NHL with bone-marrow involvement [Bargou et al. 2008]. In this phase I/II trial for ALL, the optimal dose was found to be 5 µg/m2 stepped up to 15 µg/m2. There was no added benefit/toxicity ratio by raising the dose higher than 15 µg/m2 in ALL patients. The optimal doses decided upon here were lower than the doses administered for NHL, in order to minimize toxicity.
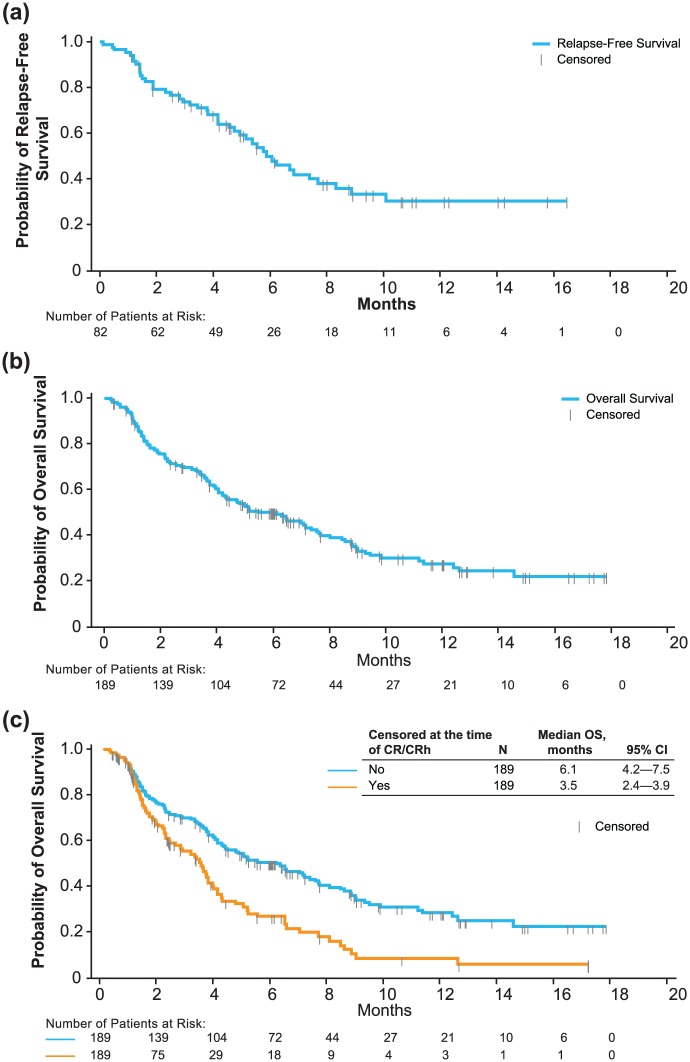
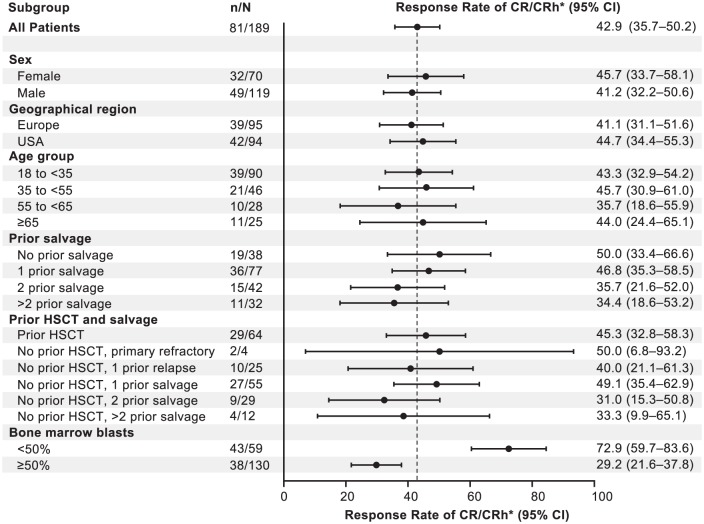
Subsequently, a confirmatory phase II trial examined blinatumomab for 189 adult patients with aggressive Philadelphia chromosome-negative relapsed/refractory ALL; those in first salvage with a first remission duration of more than 1 year were excluded [Topp et al. 2015a]. Blinatumomab was given at a dose of 9 µg/day for the first 7 days, followed by 28 µg/day, over 4 weeks for up to five cycles. Around 43% of patients achieved a CR or CRh after two cycles; the median OS and RFS were 6.1 and 5.9 months, respectively (Figure 2). Of the 18 patients achieving a CRh, 3 were ultimately able to obtain a CR with additional cycles of blinatumomab. Around 52% of CR/CRh responders without previous transplantation proceeded to alloHSCT. A total of 43 patients continued treatment beyond cycle 2; 22 and 12 individuals received cycles 4 and 5, respectively. Grades 3 and 4 neurologic events occurred in 13% of patients. Three patients experienced grade 3 CRS. With a median follow up of 8.9 months, 45% of those who achieved a response were still in remission. The Forest plot in Figure 3 displays CR and CRh among predefined subgroups; one conspicuous finding is that patients with less than 50% bone-marrow blasts at baseline experienced substantially higher response rates compared with patients with bone-marrow blasts 50% or higher (73% of the former versus 29% of the latter). Response rates were similar among patients with or without previous HSCT. Patients with MRD-negative CR or CRh had a longer OS and RFS than those with MRD-positive CR or CRh. After these findings, blinatumomab received accelerated approval from the FDA for the treatment of Philadelphia-negative, relapsed/refractory B-cell precursor ALL. Specifically, CR rate, indicating an improvement over existing therapies, the median RFS rate for patients who achieved CR, suggesting responses were reasonably durable, and the MRD response in a high proportion of responders informed the regulatory decision-making process [Przepiorka et al. 2015].
Figure 2.
Relapse-free survival (a), overall survival (b), and overall survival with censoring upon reaching a complete remission (c) from the study reported in Topp et al. [2015a].
(Reprinted with permission from Topp et al. [2015a].)
Figure 3.
Complete remission (CR) or CR with partial hematologic recovery (CRh) among prespecified patient subgroups after two treatment cycles. The dashed line marks the point estimate for CR/CRh for the entire patient population. (Reprinted with permission from Topp et al. [2015a].)
A phase II, single-arm multicenter trial to evaluate the efficacy of blinatumomab in adult subjects with relapsed/refractory Philadelphia-positive B-ALL has recently been completed. The eligibility criteria included patients who had relapsed or refractory disease to at least one second-generation TK inhibitor or were intolerant to second-generation TK inhibitors and intolerant or refractory to imatinib mesylate. Patients had to be at least 18 years old, have an Eastern Cooperative Oncology Group (ECOG) performance of 2 or less, and have more than 5% blasts in the bone marrow. Exclusion criteria included active ALL in the CNS, clinically relevant CNS pathology, and normal liver and kidney function. Blinatumomab was dosed as previously described. This study has been completed (see Table 3), and it was recently announced that 16 of 45 (36%) patients achieved a CR/CRh, and of those, 14 achieved a complete MRD response. AEs paralleled the history of Philadelphia-negative B-ALL trials [Martinelli et al. 2015].
Table 3.
Recently initiated blinatumomb clinical trials.
| Patient population | Phase, study type | Study status | ClinicalTrials.gov identifier |
|---|---|---|---|
| Adult R/R B-precursor ALL | Phase II, randomized, open label | Completed | NCT02013167 |
| Adult R/R Ph+ B-precursor ALL (41 patients) | Phase II, open label | Completed | NCT02000427 |
| Patients (1–30 years) with relapsed B-cell ALL | Phase III, risk stratified, randomized | Recruiting | NCT02101853 |
| Newly diagnosed breakpoint cluster region-ABL-negative B- lineage ALL | Phase III, randomized, open label | Recruiting | NCT02003222 |
| Elderly newly diagnosed ALL | Phase II, open label | Recruiting | NCT02143414 |
ALL, acute lymphoblastic leukemia; Ph, Philadelphia chromosome; R/R, relapsed/refractory.
Finally, a phase I/II pediatric study has aimed to determine blinatumomab dosing and to assess antileukemia activity and the safety profile, enrolling pediatric patients in second or greater relapse or those who experienced relapse after alloHSCT. In the phase I dose-escalation part, 41 patients with relapsed/refractory ALL were evaluated [Von Stackelberg et al. 2014]. The MTD was 15 µg/m2/day. CRS was dose-limiting, but step-wise dosing of 5–15 µg/m2/day was successful in ameliorating this condition. Around 32% of patients achieved a CR; of those, 77% were MRD negative. For patients who achieved a CR, the median RFS was 8.3 months. The median OS was 5.7 months. In preliminary results from the ensuing phase II trial, 12 of the 39 patients (31%) achieved a complete response; 42% in that group were MRD negative [Gore et al. 2014]. The median RFS for the CR responders was 5.6 months; the median OS was 4.3 months within the 6-month follow-up period.
Pharmacodynamic responses to blinatumomab have been reported for a number of trials, and they are generally consistent [Klinger et al. 2012]. There is rapid B- and T-cell loss from the circulation, but the reappearance of T cells is suggestive of redistribution. Interestingly, this observation has also been noted with OKT3 treatment, which was widely used as a means of immune suppression for renal allografting [Norman, 1995]. The pharmacodynamic findings are similar to those seen in the initial NHL trial [Bargou et al. 2008]. T-cell counts in the peripheral blood are driven primarily by an expansion of effector memory CD8+ cells. Cytokine secretion is observed but resolves quickly.
Safety and treatment considerations
The most common clinically significant toxicities reported in trials of relapsed/refractory ALL and MRD+ ALL have been CRS as well as CNS-related events, which are also observed in other T-cell therapies [Stone et al. 1996; Kochenderfer et al. 2012; Grupp et al. 2013; Davila et al. 2014]. CNS toxicities may manifest as tremor, aphasia, encephalopathy, and seizure. In the clinical trials, these toxicities have required immediate discontinuation of blinatumomab and additional administration of corticosteroids. There are indications that symptoms are reversible in nearly all cases and most subjects will be able to resume therapy; for example, all grade 3/4 AEs were resolved to grade ⩽ 1 in one investigation except for 1 instance of leukopenia and 5 instances of decreased blood immunoglobulin [Topp et al. 2011], and in another, 4 of 15 discontinuations from nervous system and psychiatric disorder AEs were permanent [Topp et al. 2014]. The mechanism of neurotoxicity remains unclear, but it does not appear to be related to active CNS involvement by disease, since those with cerebral spinal fluid abnormalities or other evidence of CNS disease were excluded from clinical trials. Those with prior CNS disease and treated with intrathecal chemotherapy and/or cranial radiation, however, were included; these patients did not experience increased CNS toxicity. An extra 36 patients were part of an additional evaluation cohort for specifically investigating the causes of CNS AEs, using pretreatment magnetic resonance imaging scans and spinal fluid analysis. This analysis is still pending. Neurotoxicity has also been observed with CD19-targeting CAR T cells but not with other BiTE® antibody constructs in clinical development, indicating that on-target toxicity has likely occurred. In a study of data available for the expression of two CD19 probes in six donor human brains from the Allen Brain Atlas, one brain displayed elevated CD19 expression in the left inferior frontal gyrus [Kranick et al. 2014]. The variability of CD19 expression in the brain suggests a potential explanation for neurotoxicity in a subset of patients, as patients with a certain pattern of CD19 expression in neurons may be susceptible to a neuroinflammatory response from CD19-engaging T cells.
Various features of CRS have been observed with immune therapies, and strict definitions and grading systems are emerging [Lee et al. 2014]. Cytokine elevation was observed and was proportional to the initial dose. Cytokine levels peaked at this hour but declined within 48 h. Cytokine elevation was not observed in subsequent cycles. This ‘first-dose effect’ has been observed with other immune therapies and provides the basis for step dosing. Corticosteroids are effective in the prevention or treatment of CRS, but with a potential negative impact on T-cell cytolysis. Brandl and colleagues demonstrated that, in vitro, physiologically relevant concentrations of dexamethasone attenuated cytokine release with no apparent effect on cytotoxicity [Brandl et al. 2007]. In contrast, corticosteroids are thought to result in the rapid disappearance of CAR T cells [Davila et al. 2014]. Given a putative role of IL-6 in CRS, the IL-6R-targeted antibody tocilizumab is gaining preference for the treatment of CRS in CAR T-cell recipients. Tocilizumab has also been used for the treatment of CRS-associated hemophagocytic lymphohistiocytosis in a patient treated with blinatumomab [Teachey et al. 2013]. Dexamethasone as prophylaxis is administered prior to starting blinatumomab when the dose is increased, or following interruptions of more than 4 h. Prophylactic antiseizure medications are not given; however, for patients who experience a seizure, secondary prophylaxis is recommended before resuming blinatumomab therapy.
The incidence and severity of side effects may be dependent on the cycle and disease status. CRS is usually seen in the first cycle of treatment and is related to disease burden. The risk of CRS is lower for those in MRD; dose setup is not necessary. Significant cytopenias do not usually occur after attaining CR/CRh, however, CNS events can occur during first and subsequent cycles. As blinatumomab is believed to target all CD19+ cells, normal cells expressing CD19 are also affected. An increase in infections is therefore a concern. In the trial of 21 patients with MRD-positive ALL, 4 patients had infections (2 catheter-related events, 1 case of Gram-negative sepsis, and 1 instance of pneumonia). In the study of 36 patients with refractory/relapsed ALL, 1 death related to fungal sepsis was reported [Topp et al. 2014]. The long-term effects of depleting CD19+ B cells are yet to be determined. However, because CD19 is absent from pluripotent bone-marrow stem cells, reconstitution of B cells is achievable upon discontinuation of therapy. Hypogammaglobulinemia is present throughout treatment; intravenous immunoglobin replacement therapy is recommended for patients who develop this immunodeficiency. Finally, individuals with renal impairment have also been excluded from trials, and no formal pharmacokinetic studies have been performed in these patients.
Successful treatment with blinatumomab has implications for its role as a bridge to alloHSCT. Allogeneic transplantation is the only curative option for adults with relapsed/refractory ALL, and achieving a prior CR is critical. Substantial proportions (around 40–50%) of patients achieving CR/CRh were able to proceed to transplant [Topp et al. 2014, 2015a].
Older patients are frequently ineligible for transplantation, and treatment options are limited for those with refractory/relapsed ALL. Researchers pooled data for older patients from two studies, finding that 56% achieved CR or CRh within two cycles of treatment. Around 60% of responding patients had a complete MRD response, and four other patients had a MRD of lower than10−4. In all, the older patients responded to and tolerated the treatment to the same extent as the overall study populations [Kantarjian et al. 2015]. A study recruiting elderly patients with newly diagnosed ALL is underway (see Table 3).
Stability and infusion methods
As mentioned, short infusions of blinatumomab failed to demonstrate efficacy and were poorly tolerated. This result is due to the agent’s short half-life and high clearance. Blinatumomab is now administered as a continuous infusion for at least 4 weeks. By this method, drug levels in serum are sustained, predictable throughout the infusion period, and show dose linearity. Continuous infusion reduces toxicity in that AEs linked to an initial polyclonal activation of T cells are mostly constrained to the limited starts of infusion. Blinatumomab is given by means of a Hickman/peripherally inserted central catheter. Patients are usually observed in the hospital for the first 9 days, and if there are no complications, they can be transitioned to the outpatient setting. A bag change every 2 days is the approved method in the USA.
Continuous infusion was a crucial decision that changed the course of blinatumomab development. After pharmacokinetic analysis of the first trial using continuous infusion showed a continued presence of blinatumomab in serum over the entire infusion period, with dose linearity, all subsequent trials have used this mode of infusion, which has received favorable appraisals by patients and physicians [Nagorsen et al. 2012]. A short half-life might actually pose an advantage in that drug levels in patients may be precisely controlled [Baeuerle and Reinhardt, 2009], and in turn sustain T-cell activity against CD19+ targets. Nevertheless, research has also shown that, at least in animal models, many BiTE® molecules are bioavailable through subcutaneous injection, either by repeated bolus injection or an insulin minipump device [Baeuerle et al. 2009].
Resistance and relapse
The mechanisms of resistance to blinatumomab remain to be fully elucidated. Resistance may not derive solely from intrinsic target-cell factors but from functional inadequacies of effector T cells, suboptimal E:T ratios, or both. Comparable response rates between the MRD-positive setting and in patients with less than 50% bone-marrow blasts lends support to the idea that unfavorable E:T ratios are influential. T-cell exhaustion, in addition, may be promoted by tumor cell burden. The prospect of T-cell exhaustion opens up the possibility of combining blinatumomab with immune modulators to increase potency. Owing to the novel mechanism of action of BiTE® antibody constructs, traditional modes of drug resistance are not predicted. Indeed, comparable efficacy is observed irrespective of prior lines of therapy. Cytogenetic abnormalities in the leukemic cells do not appear to be related to efficacy in relapsed ALL. No difference in T-cell and cytokine profiles has been observed between responding and resisting disease, nor has any relationship between drug exposure and response been determined.
It has recently been found that the percentage of regulatory T cells (Tregs) prior to blinatumomab therapy may predict response in relapsed/refractory ALL patients [Duell et al. 2014]. In an analysis of 31 relapsed/refractory ALL patients, those who were refractory to blinatumomab treatment had a median of 16.1% (8.4–73%) Tregs, whereas responders had a median of 8.55% Tregs (3.8–14.2%) (p = 0.00013). The authors also reported upregulation of CD69, CD25, and PD-1 by regulatory T cells incubated with blinatumomab and primary ALL blasts, suggesting a potential role of immune-inhibiting molecules, such as PD-1, in resistance to blinatumomab.
Similarly to the state of knowledge of blinatumomab resistance, relapse mechanisms are unclear. Antigen escape through loss of CD19 on target cells has been observed, but instances have been relatively rare. Extramedullary disease, likely reflecting occult disease in sanctuary sites that are not penetrated by blinatumomab, T cells, or both at the commonly administered doses, has occurred. A relapse does not necessarily imply resistance, as 36% of patients with relapsed disease at least 3 months after a remission responded to retreatment [Topp et al. 2015b].
Future outlook
Blinatumomab, the most clinically advanced drug in the BiTE® category of antibodies, has led to promising results for patients with MRD or relapsed/refractory disease. Toxicities are common and can be severe, but in many cases they are manageable. On the basis of these outcomes, phase III randomized trials are underway (see Table 3). Using blinatumomab at relatively early stages of disease will be examined. Other immunotherapies, including the previously mentioned CAR T cells, as well as CD22-directed agents, such as inotuzumab ozogamicin, are also under intense investigation and may see widespread future use.
Although impressive response rates have been observed with blinatumomab, many patients did not experience benefit. Future efforts focusing on combination therapies, higher doses, optimizing drug delivery, and controlling AEs, may improve performance. Under certain circumstances, increasing the dose of blinatumomab may be beneficial, recognizing that the MTD observed in NHL trials was higher than the dose used in the ALL trials. Similarly, to attempt to achieve an improved response rate for patients with extramedullary disease, the doses administered in NHL should be investigated. Lastly, because blinatumomab is directed toward CD19, any B-cell malignancy is potentially a target for this antibody: moreover, it is possible (but not certain) that blinatumomab, similarly to the anti-CD20 antibody rituximab, is applicable for nonmalignant conditions including autoimmune disease.
Current therapies for adult ALL have shown improved outcomes using adolescent and young adult regimens for patients up to the age of 60 years, however, there is high treatment-related morbidity and mortality in patients greater than 60 years of age. The Southwest Oncology Group has initiated a clinical trial for newly diagnosed ALL patients who are 65 years or older. Blinatumomab as upfront therapy will be given to Ph-negative patients, and blinatumomab in combination with dasatinib will be administered to those who are Ph-positive. The ECOG-American College of Radiology Imaging Network Cancer Research Group is investigating the role of blinatumomab given as consolidation therapy compared with standard chemotherapy consolidation in a phase III randomized trial. Lastly, the Children’s Oncology Group has a randomized phase III trial for patients under the age of 30 years with relapsed ALL, comparing the role of blinatumomab as consolidation therapy with the chemotherapy consolidation given in the R3 ALL regimen. By employing these new therapies in the upfront setting, this will hopefully change the paradigm of how we treat ALL in the future with improved outcomes and decreased toxicity.
Acknowledgments
The authors would like to thank James Sanchez (City of Hope) for editorial support. Amgen, Inc. did not provide technical writing services or other services in the preparation of the manuscript.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: JEB is an employee of Amgen, Inc. and a shareholder in Amgen, Inc. ASS provides consulting for Amgen, Inc. and serves on the speakers’ bureau. Amgen’s employment of JEB was disclosed at the onset of the preparation of the manuscript.
Contributor Information
Jonathan E. Benjamin, Amgen Inc., Global Development, Oncology, Thousand Oaks, CA, USA
Anthony S. Stein, Department of Hematology and Hematopoietic Cell Transplantation, Gehr Family Center for Leukemia Research, City of Hope, 1500 East Duarte Road, Duarte, CA 91010-3000, USA.
References
- American Cancer Society (2014) Cancer Facts and Figures. Atlanta, GA: American Cancer Society; Available at: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/index (accessed 25 August 2015). [Google Scholar]
- Baeuerle P., Kufer P., Bargou R. (2009) BiTE: teaching antibodies to engage T-cells for cancer therapy. Curr Opin Mol Ther 11: 22–30. [PubMed] [Google Scholar]
- Baeuerle P., Reinhardt C. (2009) Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res 69: 4941–4944. [DOI] [PubMed] [Google Scholar]
- Bargou R., Leo E., Zugmaier G., Klinger M., Goebeler M., Knop S., et al. (2008) Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321: 974–977. [DOI] [PubMed] [Google Scholar]
- Brandl C., Haas C., d’Argouges S., Fisch T., Kufer P., Brischwein K., et al. (2007) The effect of dexamethasone on polyclonal T cell activation and redirected target cell lysis as induced by a CD19/CD3-bispecific single-chain antibody construct. Cancer Immunol Immunother 56: 1551–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann M., Raff T., Flohr T., Gokbuget N., Nakao M., Droese J., et al. (2006) Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood 107: 1116–1123. [DOI] [PubMed] [Google Scholar]
- Chatenoud L., Ferran C., Legendre C., Thouard I., Merite S., Reuter A., et al. (1990) In vivo cell activation following OKT3 administration. Systemic cytokine release and modulation by corticosteroids. Transplantation 49: 697–702. [DOI] [PubMed] [Google Scholar]
- d’Argouges S., Wissing S., Brandl C., Prang N., Lutterbuese R., Kozhich A., et al. (2009) Combination of rituximab with blinatumomab (MT103/MEDI-538), a T cell-engaging CD19-/CD3-bispecific antibody, for highly efficient lysis of human B lymphoma cells. Leuk Res 33: 465–473. [DOI] [PubMed] [Google Scholar]
- Davila M., Riviere I., Wang X., Bartido S., Park J., Curran K., et al. (2014) Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6: 224ra225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier T., Lorenczewski G., Brandl C., Hoffmann P., Syring U., Hanakam F., et al. (2002) Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer 100: 690–697. [DOI] [PubMed] [Google Scholar]
- Duell J., Dittrich M., Bedke T., Mueller T., Rasche L., Dandekar T., et al. (2014) Crucial role of regulatory T cells in predicting the outcome of the T cell engaging antibody blinatumomab in relapsed and refractory B precursor ALL patients. Blood 124: 2291. [Google Scholar]
- Fielding A., Richards S., Chopra R., Lazarus H., Litzow M., Buck G., et al. (2007) Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 109: 944–950. [DOI] [PubMed] [Google Scholar]
- Goebeler M., Viardot A., Kufer P., Topp M., Knop S., Mackensen A., et al. (2013) Final results from a phase 1 study of blinatumomab in patients with relapsed/refractory non-Hodgkin’s lymphoma. Hematol Oncol 31: 302. [Google Scholar]
- Goekbuget N., Dombret H., Bonifacio M., Reichle A., Graux C., Havelange V., et al. (2014) BLAST: a confirmatory, single-arm, phase 2 study of blinatumomab, a bispecific T-cell engager (BiTE (R)) antibody construct, in patients with minimal residual disease B-precursor acute lymphoblastic leukemia (ALL). Blood 124: 379. [Google Scholar]
- Gökbuget N., Dombret H., Bonifacio M., Reichle A., Graux C., Faul C., et al. (2015) Long-term outcomes after blinatumomab treatment: follow-up of a phase 2 study in patients (Pts) with minimal residual disease (MRD) positive B-cell precursor acute lymphoblastic leukemia (ALL). Blood 126: 680. [Google Scholar]
- Gökbuget N., Kneba M., Raff T., Trautmann H., Bartram C.-R., Arnold R., et al. (2012a) Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood 120: 1868–1876. [DOI] [PubMed] [Google Scholar]
- Gökbuget N., Stanze D., Beck J., Diedrich H., Horst H., Huttmann A., et al. (2012b) Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 120: 2032–2041. [DOI] [PubMed] [Google Scholar]
- Gore L., Locatelli F., Zugmaier G., Zwaan C., Bhojwani D., Handgretinger R., et al. (2014) Initial results from a phase 2 study of blinatumomab in pediatric patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Blood 124: 3703. [Google Scholar]
- Gruen M., Bommert K., Bargou R. (2004) T-cell-mediated lysis of B cells induced by a CD19xCD3 bispecific single-chain antibody is perforin dependent and death receptor independent. Cancer Immunol Immunother 53: 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp S., Kalos M., Barrett D., Aplenc R., Porter D., Rheingold S., et al. (2013) Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C., Krinner E., Brischwein K., Hoffmann P., Lutterbuse R., Schlereth B., et al. (2009) Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology 214: 441–453. [DOI] [PubMed] [Google Scholar]
- Hoffmann P., Hofmeister R., Brischwein K., Brandl C., Crommer S., Bargou R., et al. (2005) Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer 115: 98–104. [DOI] [PubMed] [Google Scholar]
- Intlekofer A., Thompson C. (2013) At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol 94: 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine D., Purbhoo M., Krogsgaard M., Davis M. (2002) Direct observation of ligand recognition by T cells. Nature 419: 845–849. [DOI] [PubMed] [Google Scholar]
- Kantarjian H., Stein A., Bargou R., Grande C., Larson R., Stelljes M., et al. (2015) Safety and activity of blinatumomab for older patients (Pts) with relapsed/refractory (R/R) B-precursor acute lymphoblastic leukemia (ALL) in two phase 2 studies. J Clin Oncol 33: 7043. [Google Scholar]
- Klinger M., Brandl C., Zugmaier G., Hijazi Y., Bargou R., Topp M., et al. (2012) Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/Cd3-bispecific BiTE antibody blinatumomab. Blood 119: 6226–6233. [DOI] [PubMed] [Google Scholar]
- Kochenderfer J., Dudley M., Feldman S., Wilson W., Spaner D., Maric I., et al. (2012) B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119: 2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranick S., Phan G., Kochenderfer J., Rosenberg S., Nath A. (2014) Aphasia as a complication of CD19-targeted chimeric antigen receptor immunotherapy (S52.006). Neurology 82(Suppl.): S52.006. [Google Scholar]
- Larson R. (2006) Management of acute lymphoblastic leukemia in older patients. Semin Hematol 43: 126–133. [DOI] [PubMed] [Google Scholar]
- Ledbetter J., June C., Martin P., Spooner C., Hansen J., Meier K. (1986) Valency of CD3 binding and internalization of the CD3 cell-surface complex control T cell responses to second signals: distinction between effects on protein kinase C, cytoplasmic free calcium, and proliferation. J Immunol 136: 3945–3952. [PubMed] [Google Scholar]
- Lee D., Gardner R., Porter D., Louis C., Ahmed N., Jensen M., et al. (2014) Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker C., Damon L., Ries C., Navarro W. (2002) Intensified and shortened cyclical chemotherapy for adult acute lymphoblastic leukemia. J Clin Oncol 20: 2464–2471. [DOI] [PubMed] [Google Scholar]
- Loffler A., Kufer P., Lutterbuse R., Zettl F., Daniel P., Schwenkenbecher J., et al. (2000) A recombinant bispecific single-chain antibody, CD19 X CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 95: 2098–2103. [PubMed] [Google Scholar]
- Martinelli G., Dombret H., Chevallier P., Ottmann O., Gokbuget N., Topp M. (2015) Complete molecular and hematologic response in adult patients with relapsed/refractory (R/R) Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia (ALL) following treatment with blinatumomab: results from a phase 2 single-arm, multicenter study (Alcantara). Blood 126: 679. [DOI] [PubMed] [Google Scholar]
- Nagorsen D., Kufer P., Baeuerle P., Bargou R. (2012) Blinatumomab: a historical perspective. Pharmacol Ther 136: 334–342. [DOI] [PubMed] [Google Scholar]
- Norman D. (1995) Mechanisms of action and overview of OKT3. Ther Drug Monit 17: 615–620. [DOI] [PubMed] [Google Scholar]
- Offner S., Hofmeister R., Romaniuk A., Kufer P., Baeuerle P. (2006) Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol 43: 763–771. [DOI] [PubMed] [Google Scholar]
- Oriol A., Vives S., Hernandez-Rivas J., Tormo M., Heras I., Rivas C., et al. (2010) Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA study group. Haematologica 95: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przepiorka D., Ko C., Deisseroth A., Yancey C., Candau-Chacon R., Chiu H., et al. (2015) FDA approval: blinatumomab. Clin Cancer Res 21: 4035–4039. [DOI] [PubMed] [Google Scholar]
- Raponi S., De Propris M., Intoppa S., Milani M., Vitale A., Elia L., et al. (2011) Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: analysis of 552 cases. Leuk Lymphoma 52: 1098–1107. [DOI] [PubMed] [Google Scholar]
- Sanford M. (2015) Blinatumomab: first global approval. Drugs 75: 321–327. [DOI] [PubMed] [Google Scholar]
- Schlereth B., Kleindienst P., Fichtner I., Lorenczewski G., Brischwein K., Lippold S., et al. (2006) Potent inhibition of local and disseminated tumor growth in immunocompetent mouse models by a bispecific antibody construct specific for murine CD3. Cancer Immunol Immunother 55: 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M., Sausville E., Fay J., Headlee D., Collins R., Figg W., et al. (1996) A phase I study of bolus versus continuous infusion of the anti-CD19 immunotoxin, IGG-HD37-dgA, in patients with B-cell lymphoma. Blood 88: 1188–1197. [PubMed] [Google Scholar]
- Tavernier E., Boiron J., Huguet F., Bradstock K., Vey N., Kovacsovics T., et al. (2007) Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia 21: 1907–1914. [DOI] [PubMed] [Google Scholar]
- Teachey D., Rheingold S., Maude S., Zugmaier G., Barrett D., Seif A., et al. (2013) Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 121: 5154–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Kantarjian H., Smith T., Koller C., Cortes J., O’Brien S., et al. (1999) Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer 86: 1216–1230. [DOI] [PubMed] [Google Scholar]
- Topp M., Gokbuget N., Stein A., Zugmaier G., O’Brien S., Bargou R., et al. (2015a) Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 16: 57–66. [DOI] [PubMed] [Google Scholar]
- Topp M., Gokbuget N., Zugmaier G., Degenhard E., Goebeler M., Klinger M., et al. (2012) Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood 120: 5185–5187. [DOI] [PubMed] [Google Scholar]
- Topp M., Gokbuget N., Zugmaier G., Klappers P., Stelljes M., Neumann S., et al. (2014) Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol 32: 4134–4363. [DOI] [PubMed] [Google Scholar]
- Topp M., Kufer P., Gokbuget N., Goebeler M., Klinger M., Neumann S., et al. (2011) Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 29: 2493–2498. [DOI] [PubMed] [Google Scholar]
- Topp M., Stelljes M., Zugmaier G., Barnette P., Heffner L., Trippett T., et al. (2015b) Re-exposure to blinatumomab after CD19-positive relapse: experience from three trials in patients (Pts) with relapsed/refractory B-precursor acute lymphoblastic leukemia (R/R ALL). J Clin Oncol 33: 7051. [Google Scholar]
- Tumeh P., Harview C., Yearley J., Shintaku I., Taylor E., Robert L., et al. (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetsu D., Katzen D., Chatila T., Miller R., Jabara H., Maher M., et al. (1987) Requirements for activation of human peripheral blood T cells by mouse monoclonal antibodies to CD3. Clin Immunol Immunopathol 43: 48–64. [DOI] [PubMed] [Google Scholar]
- Van der Stegen S., Hamieh M., Sadelain M. (2015) The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov 14: 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Stackelberg A., Locatelli F., Zugmaier G., Handgretinger R., Trippett T., Rizzari C., et al. (2014) Phase 1/2 study in pediatric patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia (BCP-ALL) receiving blinatumomab treatment. Blood 124: 2292. [Google Scholar]
- Wang K., Wei G., Liu D. (2012) CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol 1: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Irving B., Tan L., Koretzky G. (1991) Signal transduction by the T cell antigen receptor. Semin Immunol 3: 313–324. [PubMed] [Google Scholar]
- Wucherpfennig K., Gagnon E., Call M., Huseby E., Call M. (2010) Structural biology of the T-cell receptor: insights into receptor assembly, ligand recognition, and initiation of signaling. Cold Spring Harb Perspect Biol 2: a005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugmaier G., Klinger M., Schmidt M., Subklewe M. (2015) Clinical overview of anti-CD19 BiTE(®) and ex vivo data from anti-CD33 BiTE(®) as examples for retargeting T cells in hematologic malignancies. Mol Immunol 67: 58–66. [DOI] [PubMed] [Google Scholar]