Abstract
Immunotherapy has long played a role in urothelial cancers with the use of bacille Calmette Guérin (BCG) being a mainstay in the treatment of nonmuscle invasive bladder cancer. Novel therapeutic approaches have not significantly impacted mortality in this population and so a renaissance in immunotherapy has resulted. This includes recombinant BCG, oncolytic viruses, monoclonal antibodies, vaccines, and adoptive T-cell therapy. Herein, we provide a review of the current state of the art and future therapies regarding immunotherapeutic strategies for urothelial carcinoma.
Keywords: immunotherapy, NMIBC, urothelial carcinoma
Introduction
Urothelial cancer remains a challenge to treat, as evidenced by slowly declining yearly incidence rates, yet stable death rates for the last 15 years. Urothelial cancer is the fourth most common cause of cancer in men, with 74,000 new cases reported and 16,000 deaths in 2015 [Siegel et al. 2015]. In recent years, we have seen an escalation in the utilization of immune-based strategies for the treatment of all cancers, including urothelial cancer. Immunotherapy in fact has a substantial and relatively successful history in the treatment of urothelial cancer, with bacille Calmette–Guérin (BCG) currently accepted as the mainstay of treatment for nonmuscle-invasive bladder cancer (NMIBC) after transurethral resection. However, BCG has limited efficacy and the potential for side effects; high-risk NMIBC remains difficult to treat with high long-term rates of recurrence and disease progression. Therefore, there has been a concerted effort to develop new and innovative immunotherapy strategies. In this review, we provide a comprehensive assessment of current and potential future immune-based therapies for the treatment of urothelial cancer (Table 1).
Table 1.
Novel targeted therapies for urothelial cancer.
| Treatment modality | Targets | Stage of development |
|---|---|---|
| rBCG | ||
| Th1 cytokine based | IL-2, IL-12, IL-18, IFN-α, IFN-γ, S1PT | Preclinical/animal models |
| BCG subcomponent based | Cell wall, cell wall skeleton, subunit protein antigens | Phase II/III |
| Oncolytic viruses | ||
| rAd-IFN/Syn3 | IFN-α2b | Phase II |
| CG0070 | GM CSF | Phase II/III |
| Monoclonal antibodies | ||
| Tumor-associated antigens (TAAs) | hCG-β | Phase I |
| Checkpoint blockade inhibitors | CTLA-4, PD-1, PD-L1 | Phase I |
| Vaccines | ||
| DN24-02, AdHER dendritic cell vaccine | HER2 | Phase II |
| Cancer testis antigens (CTAs) | NY-ESO-1, MAGE-A3 | Phase II |
| PANVAC | MUC-1, CEA | Phase II |
| Adoptive T-cell therapy | CTAs (e.g. NY-ES0-1), TAAs | Preclinical/feasibility study |
CEA, carcinoembryonic antigen; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; GM-CSF, granulocyte macrophage colony stimulating factor; hCG-β, human chorionic gonadotropin β chain; HER2, human epidermal growth factor receptor 2; IFN, interferon; IL, interleukin; MUC-1, mucin-1; PD, programmed death; rAd, recombinant adenovirus; rBCG, recombinant bacille Calmette–Guérin; S1PT, pertussis toxin subunit S1.
BCG
BCG is a live, attenuated strain of Mycobacterium bovis initially developed by Calmette and Guérin for use as a vaccine to prevent tuberculosis. It has now become the foundation for the treatment of high-grade NMIBC with numerous studies demonstrating that administration of intravesical BCG to patients with superficial bladder tumors and carcinoma in situ (CIS) reduces recurrence rates and progression to muscle-invasive disease [Herr et al. 1988; Sylvester et al. 2005]. BCG acts as a nonspecific stimulant to the reticuloendothelial system and induces a local inflammatory response with subsequent infiltration of granulocytes, macrophages, natural killer cells, dendritic cells, and lymphocytes. This influx of immune cells leads to the local secretion of a wide array of cytokines, including interleukin (IL)-1, IL-2, IL-6, IL-8, IL-10, IL-12, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, granulocyte macrophage colony stimulating factor (GM-CSF), and soluble intercellular adhesion molecule 1 [Jackson et al. 1995]. T-cell-mediated immunity predominates and is associated with increased tumor destruction. Importantly, an anti-BCG-specific immune response that is induced via antigen presentation by dendritic cells to T cells amplifies the response elicited by BCG. Ratliff and colleagues demonstrated in well designed experiments that the absence of either CD4 or CD8 T-cell subsets eradicated the antitumor activity of intravesical BCG immunotherapy for bladder cancer [Ratliff et al. 1993].
Despite a defined benefit, 20–50% of patients will develop disease recurrence within 15 years of a successful induction cycle [Cookson et al. 1997], while only 25–45% of these patients will benefit from a second induction course [Haaff et al. 1986]. Lastly, availability of BCG has become a relevant issue as we are now experiencing unprecedented national shortages.
Recombinant BCG
Recent advances have focused on genetically engineered recombinant BCG (rBCG) strains that provide a novel tactic for modification of BCG to overcome some of the limitations of conventional BCG therapy. The two major rBCG strategies employed at this time are T helper 1 (Th1) cytokine-based rBCG and BCG-subcomponent-based rBCG.
Th1 cytokine-based rBCG
Since evidence has shown a Th1 immune response to be critical for successful intravesical BCG immunotherapy, genetically engineered rBCG constructs that allow for the ability to secrete Th1-stimulating cytokines have been developed. Common Th1 cytokine-based rBCG strategies include secretion of IL-2, IL-12, IL-18, IFN-α, and IFN-γ. IL-2 has been shown to enhance the proliferation of cytotoxic lymphocytes in vitro as well as augment the cytotoxic activity of natural killer (NK) cells and monocytes [Henney et al. 1981; Malkovsky et al. 1987]. Therefore, increased levels of IL-2 in the urine of patients shortly after treatment with BCG are indicative of a BCG-specific response [De Jong et al. 1990]. Studies looking at IL-2-based rBCG strains have shown that relative to BCG alone, IL-2 secreting rBCG strains can improve antigen-specific proliferation, induce a more favorable IFN-γ:IL-4 ratio, elicit higher levels of Th1 cytokines, and enhance antitumor cytotoxicity [Murray et al. 1996; Slobbe et al. 1999; Young et al. 2002]. As a result, IL-2 secreting rBCG may provide clinical benefit in the treatment of NMIBC and additional clinical trials are warranted.
IL-12 has a synergistic effect with IL-2 in producing a cytotoxic T-cell response, and has been shown to increase IFN-γ production, specifically enhance the Th1 immune response, augment NK-cell activity, and induce tumor regression in animal models of cancer [Brunda et al. 1993; Nastala et al. 1994; O’Donnell et al. 2004]. IL-12 is instrumental in the BCG immune response, as BCG therapy was found to be ineffective for treatment of murine bladder cancer in IL-12 knockout mice [Riemensberger et al. 2002]. Unfortunately, a phase I study of the intravesical administration of human recombinant IL-12 alone in patients with recurrent superficial transitional cell carcinoma of the bladder proved to be ineffective as it showed no clinically relevant evidence of antitumor or immunologic effects [Weiss et al. 2003]. IL-12-based rBCG strains have been constructed for treatment of Mycobacterium tuberculosis infection and initial results in animal models are positive with strong evoked immunogenicity and Th1-cell responses [Deng et al. 2011; Lin et al. 2012]. Therefore, IL-12-based rBCG constructs may have the potential of enhancing intravesical BCG for treatment of NMIBC; further clinical trials in humans are necessary.
IL-18 is primarily secreted by activated macrophages and, along with IL-2 and IL-12, is thought to play a synergistic role in the induction of a Th1 immune response. Luo and colleagues developed an rBCG strain that functionally secretes mouse IL-18 [Luo et al. 2004]. This IL-18-secreting rBCG stain was shown to increase production of IFN-γ and GM-CSF, decrease production of IL-10, increase cellular proliferation, and stimulate higher differentiation of IFN-γ-secreting cells in mice splenocytes. Furthermore, IL-18 rBCG also enhanced BCG-induced macrophage cytotoxicity against murine bladder tumor cells (MBT-2) in a dose-dependent manner. Therefore, IL-18-based rBCG strains show promise as potential agents for NMIBC immunotherapy.
IFN-α, unlike the cytokines mentioned previously, has been extensively utilized in conjunction with BCG for the treatment of patients with superficial bladder cancers. IFN-α has been shown to have a wide array of antitumor properties, including enhancement of the Th1 immune response, triggering of apoptosis via increased TNF-related apoptosis-inducing ligand expression, increased antigen detection, and reduced angiogenesis in bladder tumors [Slaton et al. 1999; Papageorgiou et al. 2007]. A national multicenter phase II trial of combination BCG plus IFN-α-2B for treatment of recurring superficial bladder cancer displayed strong efficacy and minimal side effects [Joudi et al. 2006]. However, in one study in 236 patients with frequently recurring Ta/T1 grade 1–2 NMIBC, mitomycin C instillations followed by monthly BCG significantly reduced long-term disease recurrence in relation to alternating instillations of BCG and IFN-α2b [Jarvinen et al. 2015]. Therefore, it is unclear at this point in time if combination therapy of BCG with IFN-α offers any appreciable clinical benefit compared with BCG alone. IFN-α-secreting rBCG strains have been generated, and studies have shown these strains to be more effective than wild-type BCG in inducing IFN-γ production from peripheral blood mononuclear cells (PBMCs) and inducing PBMC cytotoxicity against bladder cancer cell lines in vitro [Luo et al. 2001; Liu et al. 2009; Ding et al. 2012].
IFN-γ is a critical cytokine in the BCG-mediated antitumor response and has been shown to effectively inhibit growth in bladder cancer cell lines [Hawkyard et al. 1992]. In one study, a murine IFN-γ-secreting rBCG strain was shown to upregulate major histocompatibility complex (MHC) class 1 expression on murine bladder cancer cell lines relative to rBCG control strain [Arnold et al. 2004]. In addition, intravesical instillation of IFN-γ-secreting rBCG led to enhanced recruitment of CD4+ T cells into the bladder as well as increased local expression of IL-2 and IL-4. Furthermore, in a murine orthotopic bladder cancer model, IFN-γ-secreting rBCG significantly improved survival relative to a control group that received wild-type control BCG.
Pertussis toxin-based rBCG has also been constructed. One study in mice revealed that rBCG expressing pertussis toxin subunit S1 (rBCG S1PT) can stimulate a strong antigen-specific Th1-dominant cellular response characterized by increased IFN-γ production and decreased IL-4 production [Nascimento et al. 2000]. A recent study conducted in orthotopic bladder cancer animal models demonstrated that rBCG-S1PT immunotherapy leads to bladder weight reduction as well as increased survival times relative to BCG treatment [Andrade et al. 2010].
BCG subcomponent-based rBCG
Another avenue of research has focused on non-live BCG subcomponents, including BCG cell wall and various BCG proteins and antigens to induce the same immune response as live BCG in the treatment of NMIBC while possibly avoiding serious side effects associated with live BCG infection. Zlotta and colleagues purified several BCG subcomponents, including the cell wall, various polysaccharides, and purified native proteins and found these subcomponents could enhance a Th1 immune response as well as increase lymphocyte-mediated cytotoxicity against bladder tumors in vitro [Zlotta et al. 2000]. The BCG cell wall has been found to be the most potent Th1 response inducer compared with the other subcomponents of BCG. In a 2009 multicenter study, a total of 55 patients with CIS of the bladder, 82% of whom had disease that failed to respond to previous BCG therapy, received induction plus maintenance therapy with intravesically administered mycobacterial cell wall-DNA complex (MCC) at two different doses (4 and 8 mg) [Morales et al. 2009]. The complete response rate at 26 weeks was 27.3% in the 4 mg group and 46.4% in the 8 mg group. In addition, the MCC was well tolerated by both dose groups with 90% of all adverse events being mild to moderate in nature. A phase II/III clinical trial [ClinicalTrials.gov identifier: NCT00406068] assessing MCC in the treatment of patients with NMIBC who were BCG refractory has been completed. Unfortunately, a phase III trial [ClinicalTrials.gov identifier: NCT01200992] evaluating MCC versus mitomycin C for the treatment of BCG refractory NMIBC closed early due to poor accrual.
Apart from MCC, the BCG cell wall skeleton (BCG CWS) is a potential substitute for live BCG. However, clinical use has been difficult due to unfavorable physiochemical characteristics preventing effective penetration of the urothelium, an essential step in the induction of the immune cascade. Delivery systems such as liposomal vectors and lipid nanoparticle packaging have been studied to improve delivery of BCG CWS and show promise for tumor growth inhibition in animal models of bladder cancer [Miyazaki et al. 2011; Nakamura et al. 2014].
Lastly, rBCG strategies utilizing various other BCG subunit proteins and antigens such as PstS1, MPT-64, and Ag85B have been evaluated and show efficacy in enhancing cytotoxicity on superficial bladder cancers in both in vitro and orthotopic murine bladder models [Sanger et al. 2004; Yu et al. 2007; Begnini et al. 2013]. Interestingly, poly-rBCG DNA vaccines that specify multiple BCG antigens such as Ag85A, Ag85B, Mpt64, and PstS3 have been developed and shown to elicit Th1-predominant immune responses, inhibit tumor growth, and prolong the survival of bladder tumor bearing mice [Lee et al. 2004].
Oncolytic virus therapy
Oncolytic virus therapy takes advantage of an altered environment within a tumor cell, such that intravesically administered viruses replicate only in tumor cells and not in normal cells. Oncolytic viruses act via direct destruction of tumor cells and through stimulation of host antitumor immune responses. Several different viruses, such as adenovirus, herpes simplex virus, reovirus, and oncolytic vaccina virus, have been studied for oncolytic properties in the preclinical setting, with a few moving into early phase clinical trials [Potts et al. 2012]. Here, we focus on the following two recombinant adenovirus-based therapies: rAd-IFNα/Syn3 and CG0070. RAd-IFNα/Syn3 is a nonreplicating recombinant adenovirus vector encoding IFN-α2b. Syn3 is a clinical surfactant excipient which enhances the adenoviral mediated transduction (process by which foreign DNA is introduced into the tumor cells via the viral vector) of urothelial cancer cells [Dinney et al. 2013]. In a phase I trial in 17 patients with recurrent NMIBC, intravesical rAd-IFNα/Syn3 was well tolerated and 6 of 14 patients (43%) with detectable urine IFNα had a complete response at 3 months, which lasted an average of 31 months [Dinney et al. 2013]. There is currently an ongoing phase II study examining intravesical administration of rAd-IFN/Syn3 in patients with BCG-refractory or relapsed bladder cancer [ClinicalTrials.gov identifier: NCT01687244].
CG0070 is a selectively replicating oncolytic adenovirus that replicates in cancer cells with defective retinoblastoma tumor suppressor protein (Rb), which is commonly mutated in many bladder cancers. Furthermore, CG0070 is designed to encode human GM CSF [Ramesh et al. 2006]. In a phase 1 study of CG0070 in 35 patients with NMIBC, high urine GM CSF levels were detected in all patients and the complete response rate across all cohorts was 48.6% with a median duration of 10.4 months [Burke et al. 2012]. A phase II/III study examining the efficacy of CG0070 in patients with NMIBC whose condition has failed to respond to prior BCG is currently ongoing [ClinicalTrials.gov identifier: NCT01438112].
Monoclonal antibodies
An exciting area of research in the field of immunotherapy for urothelial cancer focuses on monoclonal antibodies directed against tumor-associated antigens. One such example is human chorionic gonadotropin-β chain (hCG-β), which is found in 30–40% of bladder cancers and whose expression correlates with more aggressive cancers, more advanced disease, higher recurrence rates, and decreased survival rates [Iles, 2007]. CDX-1307 is a novel monoclonal antibody under study for the treatment of bladder cancer [Morse et al. 2011a]. CDX-1307 consists of a fusion between B11, a human monoclonal antibody specific for the mannose receptor on antigen-presenting cells (APCs), and hCG-β. This structure allows CDX-1307 to attach to APCs and become internalized, with subsequent presentation of hCG-β to T cells and antigen-specific activation. A phase I study in patients with advanced epithelial malignancies revealed that CDX-1307 was well tolerated and induced hCG-β-specific humoral and T-cell responses when coadministered with toll-like receptor (TLR) agonists [Morse et al. 2011b]. Unfortunately, a study of CDX-1307 vaccine for treatment of patients with newly diagnosed muscle-invasive bladder cancer [ClinicalTrials.gov identifier: NCT01094496] was terminated early due to slow enrollment.
Checkpoint inhibitors
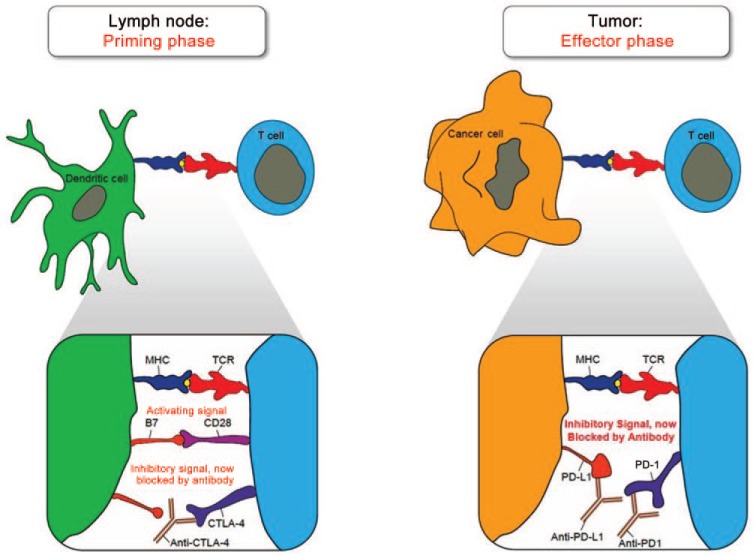
Checkpoint blockade inhibitors offer another potential strategy for the treatment of urothelial cancers. Immune checkpoints are inhibitory pathways that physiologically counterbalance costimulatory signals to modulate the immune response and keep T-cell proliferation in check. These inhibitory mechanisms are critical for maintaining self-tolerance, controlling the overall duration and strength of the immune response, and minimizing damage to healthy tissues. Cancer cells can potentially take advantage of this mechanism by overexpressing immune checkpoint molecules, which effectively blunts the immune response in the immediate tumor microenvironment. Therefore, monoclonal antibodies capable of disrupting the inhibitory receptor–ligand interactions involved in immune checkpoint mechanisms have been developed as potential strategies to treat cancers (Figure 1).
Figure 1.
Mechanism of action for checkpoint blockade inhibitors. CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; MHC, major histocompatibility complex; PD, programmed death; TCR, T-cell receptor (Courtesy of Wkly 2015; 145: w14066).
Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) is an immune checkpoint protein receptor that antagonizes the interaction between CD28 on T cells and B7 on APCs, and therefore inhibits the secondary costimulatory signal that APCs provide to T cells. This process is a normal physiologic mechanism that works to modulate the immune response as appropriate and inhibit excessive T-cell proliferation. However, in certain cancers, T-regulatory cells in the tumor microenvironment can constitutively express CTLA-4, which suppresses an effective antitumor immune response [Rothschild et al. 2015]. Stemming from this concept, CTLA-4 blocking antibodies have been produced as immunotherapy for cancer. The antibody ipilimumab, an anti-CTLA-4 agent, demonstrated survival benefit in a landmark phase III randomized clinical trial conducted in patients with previously treated metastatic melanoma [Hodi et al. 2010]. Patients receiving ipilimumab plus glycoprotein 100 (gp100) had a median overall survival of 10 months, as compared with 6.4 months for patients receiving gp100 alone. However, grade 3 or 4 immune-related adverse events occurred in 10–15% of patients treated with ipilimumab in this study. There are currently several ongoing trials assessing ipilimumab in urologic malignancies, mainly for prostate cancer and kidney cancer. Liakou and colleagues conducted a presurgical clinical trial with anti-CTLA-4 antibody in six patients with localized bladder cancer demonstrating increased expression of inducible costimulator (ICOS) in CD4 T cells from peripheral blood and tumor tissue [Liakou et al. 2008]. Increase in these CD4 (+) ICOS (hi) cells led to an increase in the effector: regulatory T-cell ratio and increased production of IFN-γ. Subsequently, a phase I trial assessing ipilimumab treatment in 12 patients with localized urothelial carcinoma of the bladder demonstrated a tolerable safety profile with most drug-related adverse events consisting of grade 1/2 toxicities [Carthon et al. 2010]. Furthermore, all 12 patients had increased presence of CD4 (+) ICOS (hi) T cells in both tumor tissue and systemic circulation. Further trials are warranted to assess the efficacy of anti-CTLA-4 agents for the treatment of urothelial cancers.
Another critical immune checkpoint mechanism involves interactions between programmed death 1 (PD-1) receptor found mainly on T cells and its ligands PD-L1 (B7-H1) and PD-L2 (B7-DC). PD-1 is a 288 amino acid type 1 transmembrane protein that is expressed on activated T cells and acts via its downstream pathways to limit the proliferation and activity of T cells to suppress autoimmunity and tissue damage during an inflammatory response [Keir et al. 2008]. Both type 1 and type II IFNs upregulate PD-L1 and PD-L2 expression as part of the inflammatory response in peripheral tissues [Eppihimer et al. 2002]. Several different cancer types, including urothelial cancers, take advantage of this checkpoint blockade inhibition by overexpressing PD-L1 in order to evade immune-mediated destruction [Zou and Chen, 2008]. In urothelial cancers, PD-L1 expression by tumor cells is correlated with higher levels of BCG unresponsiveness, advanced stage, higher postoperative recurrence, and poor survival [Inman et al. 2007; Nakanishi et al. 2007]. Stemming from these results, various forms of PD-L1 manipulation have been developed as potential immunotherapy for aggressive muscle-invasive cancers. A recent phase I clinical trial assessed the anti-PD-L1 antibody MPDL3280A, which inhibits the interaction of PD-L1 with PD-1 for the treatment of metastatic urothelial bladder cancer [Powles et al. 2014]. Of the 67 patients assessed for efficacy of treatment, 50 patients (75%) had visceral metastases at baseline and 48 patients (72%) had already received and failed to respond to two or more previous systemic treatments. For patients with a minimum of 6 weeks of follow up, the objective response rate (ORR) in patients with strong PD-L1 expression by immunohistochemistry (IHC) staining of tumor-infiltrating immune cells (⩾5% PD-L1 positive) was relatively high at 43%, whereas the ORR in patients with weak PD-L1 expression (⩽5% PD-L1 positive) was 11%. Furthermore, treatment with MPDL3280A had a favorable toxicity profile with most adverse events being grade 1 or 2 and transient in nature. Overall, decreased appetite and fatigue were the most commonly reported toxicities. Preliminary findings from the phase Ib KEYNOTE-012 [ClinicalTrials.gov identifier: NCT01848834] study assessing the PD-1 inhibitor pembrolizumab also revealed promising results with an ORR of 25% in 33 patients with advanced urothelial cancer (51.5% failing at least two previous systemic therapies) [Gupta et al. 2015]. There are currently several ongoing clinical trials assessing checkpoint blockade inhibitors, mainly anti-CTLA-4 agents and PD/PD-L1 inhibitors, in various combinations as well as in conjunction with other cytotoxic therapies [Wu et al. 2015]. These trials are now proposed in NMIBC given the encouraging preliminary results in metastatic urothelial cancer.
In addition, there are various other immune checkpoint proteins that are currently being studied in basic science laboratories, and are on the verge of clinical evaluation in urothelial cancer. They include HLA-G, B7-H3, B7-H4, LAG3, and TIM3, among others [Carosella et al. 2015]. Development of monoclonal antibodies to these immune checkpoints is an active field and may demonstrate a future role in the treatment of urothelial cancers.
Vaccines
An additional avenue of immunotherapy research for urothelial cancer has focused on vaccine development. Potential targets include oncoproteins such as human epidermal growth factor receptor 2 (HER2), cancer testis antigens (CTAs), and tumor-associated antigens such as carcinoembryonic antigen (CEA) and Mucin-1 (MUC-1). A meta-analysis that looked into the prognostic role of HER2 in bladder cancer revealed that HER2 is expressed in 27.8–85.2% of bladder cancers and that HER2 expression is correlated with higher tumor grade, lymph node metastasis, and poor disease-specific survival [Zhao et al. 2015]. DN24-02 is an autologous cellular immunotherapy vaccine designed to target the HER2 receptor that is currently being investigated in a randomized phase II study in patients with high-risk HER2+ urothelial carcinoma [ClinicalTrials.gov identifier: NCT01353222]. It consists of autologous PBMCs and APCs that are activated ex vivo with a recombinant fusion protein and then subsequently infused back into the patient. Preliminary results revealed that in 30 patients who had completed three infusions, DN24-02 had the ability to activate APCs, increase HER2-specific antibody responses, and increase expression of T-cell cytokines consistent with immunological prime-boost response. In addition, most adverse events (92.3%) were grade 1–2 in severity [Bajorin et al. 2014]. Another HER2 vaccine, AdHER2/neu dendritic cell vaccine, is currently being investigated at the National Institutes of Health Clinical Center as part of a phase I trial [ClinicalTrials.gov identifier: NCT01730118] in patients with HER2+ metastatic solid tumors and bladder cancer. In a mouse model of breast cancer, vaccination with an adenoviral ErbB-2/neu vaccine demonstrated the ability to cure large established subcutaneous HER2-expressing breast cancers as well as lung metastatic disease [Park et al. 2008]. Furthermore, it was shown that the antitumor activity involved antibody-mediated blockade of HER2 function.
Cancer testis antigens
CTAs are a group of tumor-associated antigens that have little to no expression in normal adult tissues, variably increased expression in many different cancers, and the ability to induce a strong immune response. In a study examining the expression patterns of nine CTAs in a panel of high-grade urothelial carcinomas of the bladder, Sharma and colleagues found that at least one CTA was expressed in 77% of the tumor samples and that 61% of these samples expressed more than one CTA [Sharma et al. 2006]. Two CTAs studied in relation to urothelial cancer include NY-ESO-1 and MAGE-A3. Coadministration of a recombinant NY-ESO-1 protein vaccine with BCG and GM CSF in six patients with localized bladder cancer post cystectomy resulted in NY-ESO-1-specific antibody responses in five of six patients [Sharma et al. 2008]. A phase I study assessing NY-ESO-1 vaccine with or without sirolimus in the treatment of patients with NY-ESO-1-expressing solid tumors is currently underway and has completed recruitment [ClinicalTrials.gov identifier: NCT01522820]. Another phase I study [ClinicalTrials.gov identifier: NCT01498172] examining the coadministration of BCG with recMAGE-A3 + adjuvant AS15 (recMAGE-A3 + AS15 ASCI) for the treatment of NMIBC has been completed and the results are pending. A phase II trial evaluating the efficacy of recMAGE-A3 + AS-15 in patients with muscle-invasive bladder cancer after cystectomy is ongoing and has completed recruitment [ClinicalTrials.gov identifier: NCT01435356].
Pox-viral-based vaccine therapy
PANVAC is a cancer vaccine therapy that contains transgenes for the tumor-associated antigens MUC-1 and CEA as well as three human T-cell costimulatory molecules (B7.1, intracellular adhesion molecule 1, and leukocyte function-associated antigen 3). These costimulatory molecules can augment an immune response in the setting of weakly immunogenic cancer antigens such as MUC-1 and CEA [Madan et al. 2007]. In various malignancies including bladder cancer, cell surface expression of MUC-1 can be significantly increased, and increased MUC-1 expression patterns have been shown to correlate with higher stage and grade of bladder cancer [Ahmad et al. 2015]. CEA levels are also increased in certain bladder cancers and are found in up to 76% of high-grade bladder tumors [Cardillo et al. 2000]. A phase II study assessing the use of PANVAC + BCG therapy versus BCG alone in patients with high-grade NMIBC whose condition has failed to respond to prior BCG therapy is currently under accrual [ClinicalTrials.gov identifier: NCT02015104].
Adoptive T-cell therapy
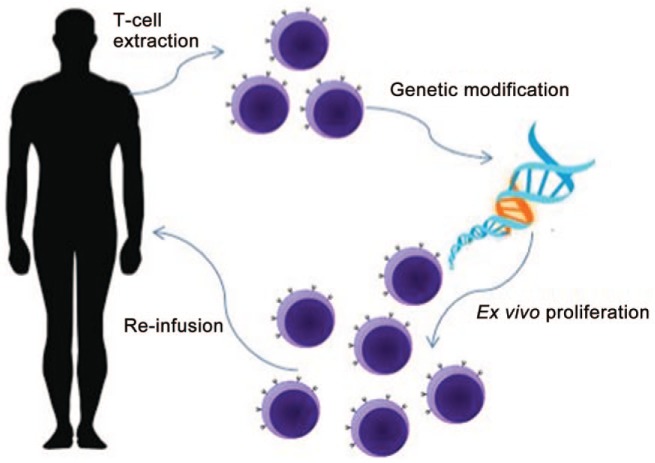
Adoptive T-cell therapy is an innovative and highly personalized cancer therapy that involves extraction of human T cells, expansion and manipulation of these cells ex vivo, and subsequent reinfusion of these proliferated immune cells back into the patient. This therapeutic avenue began with the discovery of tumor-infiltrating lymphocytes (TILs). It was found that the presence of immune infiltrate in tumors correlated with better prognosis following therapy [Duong et al. 2015]. As a potential therapeutic strategy, researchers have isolated tumor-infiltrating cells from various cancers and reinfused these cells back into patients after ex vivo proliferation. Further studies demonstrated that better clinical responses are observed when patients received a lymphodepleting preparative regimen before cell reinfusion [Rosenberg and Restifo, 2015]. Recently, this strategy was tested as a feasibility study in patients with advanced urothelial urinary bladder cancer [Sherif et al. 2010]. Tumor reactive lymphocytes were extracted from sentinel nodes draining human bladder cancer, expanded, and then subsequently reinfused back into patients. In 6 of 12 patients, it was feasible to administer the treatment without any major adverse effects, while technical failures were encountered in the other six patients.
Despite initial success, TIL extraction with subsequent reinfusion is not without its challenges. TILs are difficult to isolate from cancers other than melanoma. In addition, TIL therapy requires invasive procedures for extraction as well as the ability to grow ex vivo. In an attempt to broaden the scope of adoptive T-cell therapy to other cancers, techniques have been developed to modify host T cells with genetically engineered antitumor T-cell receptors (TCRs). This process involves isolation of T cells from peripheral blood and viral transduction with vectors in these cells to express a recombinant TCR specific for a chosen tumor antigen. These cells are then reinfused back into the patient (Figure 2). CTAs, as described earlier, are appropriate targets for this therapy because they have low expression in normal tissue and high expression in certain cancers. Genetically engineered TCRs directed against CTAs seem to be a viable option for urothelial cancers, given the high expression of CTAs in many urothelial tumors.
Figure 2.
Adoptive T-cell therapy.
Therapy with genetically modified TCRs requires TCR binding to antigen processed by APCs and presented on MHC receptors. Therefore, certain tumors can potentially escape detection by immune cells by downregulating molecules such as MHC class 1. To overcome this limitation, recombinant receptors called chimeric antigen receptors (CARs) have been developed [Barrett et al. 2015]. CARs consist of Ab-binding domains fused to T-cell signaling domains, allowing the direct binding of T cells to antigen on cancer cell surfaces that is non-MHC restricted. This, in effect, arms T cells with antitumor activity capable of targeting specific tumor-associated antigens regardless of MHC class 1 expression in the tumor cells [Maude et al. 2014]. The future application of adoptive T-cell therapies to urothelial cancers is very promising.
Conclusion
The field of immunotherapy offers exciting and potentially effective strategies for the treatment of urothelial cancers. Limited progress in survival outcomes for patients compels us to search for novel therapeutic options. BCG therapy has a storied and successful history in the treatment of superficial urothelial cancers and will continue to play an integral role. Other avenues such as rBCG, oncolytic viruses, monoclonal antibodies, vaccines, and adoptive T-cell therapy show promise as treatment for cancers of the urothelial tract. Combination therapies involving immunotherapy in addition to other therapeutic modalities such as cytotoxic therapy, radiation, ablation, or surgery may be the future for treatment of many different types of urothelial cancers. Additional clinical trials are warranted in this area. Future directions should focus on finding neoantigens specific for urothelial cancer, utilizing next-generation sequencing to predict responsiveness to immunotherapy, and formulating personalized therapeutic plans using adoptive T-cell therapy.
Footnotes
Funding: This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute.
This research was made possible through the National Institutes of Health (NIH) Medical Research Scholars Program, a public–private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, American Association for Dental Research, Howard Hughes Medical Institute, and the Colgate-Palmolive Company, as well as alumni of student research programs and other individual supporters.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Akhil Muthigi, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Arvin K. George, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
Sam J. Brancato, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
Piyush K. Agarwal, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Building 10- Hatfield CRC, Room 2-5952, Bethesda, MD 20892, USA.
References
- Ahmad S., Lam T., N’Dow J. (2015) Significance of MUC1 in bladder cancer. BJU Int 115: 161–162. [DOI] [PubMed] [Google Scholar]
- Andrade P., Chade D., Borra R., Nascimento I., Villanova F., Leite L., et al. (2010) The therapeutic potential of recombinant BCG expressing the antigen S1PT in the intravesical treatment of bladder cancer. Urol Oncol 28: 520–525. [DOI] [PubMed] [Google Scholar]
- Arnold J., De Boer E., O’Donnell M., Bohle A., Brandau S. (2004) Immunotherapy of experimental bladder cancer with recombinant BCG expressing interferon-gamma. J Immunother 27: 116–123. [DOI] [PubMed] [Google Scholar]
- Bajorin F., Sharma P., Gomella G., Plimack R., O’Donnell H., Hoffman-Censits H., et al. (2014) NeuACT, a phase II randomized, open-label trial of DN24-02: updated analysis of HER2 expression, immune responses, product parameters, and safety in patients with surgically resected HER2+ urothelial cancer. J Clin Oncol 32(Suppl. 4): abstr 296. [Google Scholar]
- Barrett D., Grupp S., June C. (2015) Chimeric antigen receptor- and TCR-modified T cells enter main street and wall street. J Immunol 195: 755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begnini K., Rizzi C., Campos V., Borsuk S., Schultze E., Yurgel V., et al. (2013) Auxotrophic recombinant mycobacterium bovis BCG overexpressing Ag85B enhances cytotoxicity on superficial bladder cancer cells in vitro. Appl Microbiol Biotechnol 97: 1543–1552. [DOI] [PubMed] [Google Scholar]
- Brunda M., Luistro L., Warrier R., Wright R., Hubbard B., Murphy M., et al. (1993) Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med 178: 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J., Lamm D., Meng M., Nemunaitis J., Stephenson J., Arseneau J., et al. (2012) A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol 188: 2391–2397. [DOI] [PubMed] [Google Scholar]
- Cardillo M., Castagna G., Memeo L., De Bernardinis E., Di Silverio F. (2000) Epidermal growth factor receptor, MUC-1 and MUC-2 in bladder cancer. J Exp Clin Cancer Res 19: 225–233. [PubMed] [Google Scholar]
- Carosella E., Ploussard G., Lemaoult J., Desgrandchamps F. (2015) A systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur Urol 68: 267–279. [DOI] [PubMed] [Google Scholar]
- Carthon B., Wolchok J., Yuan J., Kamat A., Ng Tang D., Sun J., et al. (2010) Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 16: 2861–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson M., Herr H., Zhang Z., Soloway S., Sogani P., Fair W. (1997) The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol 158: 62–67. [DOI] [PubMed] [Google Scholar]
- De Jong W., De Boer E., Van Der Meijden A., Vegt P., Steerenberg P., Debruyne F., et al. (1990) Presence of interleukin-2 in urine of superficial bladder cancer patients after intravesical treatment with bacillus Calmette-Guerin. Cancer Immunol Immunother 31: 182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Bao L., Yang X. (2011) Evaluation of immunogenicity and protective efficacy against mycobacterium tuberculosis infection elicited by recombinant mycobacterium bovis BCG expressing human interleukin-12P70 and early secretory antigen target-6 fusion protein. Microbiol Immunol 55: 798–808. [DOI] [PubMed] [Google Scholar]
- Ding G., Yu Y., Shen Z., Zhou X., Chen S., Liao G., et al. (2012) Antitumor effects of human interferon-alpha 2b secreted by recombinant bacillus Calmette-Guerin vaccine on bladder cancer cells. J Zhejiang Univ Sci B 13: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinney C., Fisher M., Navai N., O’Donnell M., Cutler D., Abraham A., et al. (2013) Phase I trial of intravesical recombinant adenovirus mediated interferon-alpha2b formulated in Syn3 for bacillus Calmette-Guerin failures in nonmuscle invasive bladder cancer. J Urol 190: 850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong C., Yong C., Kershaw M., Slaney C., Darcy P. (2015) Cancer immunotherapy utilizing gene-modified T cells: from the bench to the clinic. Mol Immunol 67: 46–57. [DOI] [PubMed] [Google Scholar]
- Eppihimer M., Gunn J., Freeman G., Greenfield E., Chernova T., Erickson J., et al. (2002) Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation 9: 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., O’Donnell P., Plimack E., Berger R., Montgomery B., Heath K., et al. (2015) A Phase 1b Study of Pembrolizumab (Pembro; MK-3475) for Advanced Urothelial Cancer. Presented at 2015 AUA Annual Meeting, 15–19 May 2015, New Orleans, LA. Abstract MP68-11. [Google Scholar]
- Haaff E., Dresner S., Ratliff T., Catalona W. (1986) Two courses of intravesical bacillus Calmette-Guerin for transitional cell carcinoma of the bladder. J Urol 136: 820–824. [DOI] [PubMed] [Google Scholar]
- Hawkyard S., Jackson A., James K., Prescott S., Smyth J., Chisholm G. (1992) The inhibitory effects of interferon gamma on the growth of bladder cancer cells. J Urol 147: 1399–1403. [DOI] [PubMed] [Google Scholar]
- Henney C., Kuribayashi K., Kern D., Gillis S. (1981) Interleukin-2 augments natural killer cell activity. Nature 291: 335–338. [DOI] [PubMed] [Google Scholar]
- Herr H., Laudone V., Badalament R., Oettgen H., Sogani P., Freedman B., et al. (1988) Bacillus Calmette-Guerin therapy alters the progression of superficial bladder cancer. J Clin Oncol 6: 1450–1455. [DOI] [PubMed] [Google Scholar]
- Hodi F., O’Day S., McDermott D., Weber R., Sosman J., Haanen J., et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles R. (2007) Ectopic hCGbeta expression by epithelial cancer: malignant behaviour, metastasis and inhibition of tumor cell apoptosis. Mol Cell Endocrinol 260–262: 264–270. [DOI] [PubMed] [Google Scholar]
- Inman B., Sebo T., Frigola X., Dong H., Bergstralh E., Frank I., et al. (2007) PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer 109: 1499–1505. [DOI] [PubMed] [Google Scholar]
- Jackson A., Alexandroff A., Kelly R., Skibinska A., Esuvaranathan K., Prescott S., et al. (1995) Changes in urinary cytokines and soluble intercellular adhesion molecule-1 (ICAM-1) in bladder cancer patients after bacillus Calmette-Guerin (BCG) immunotherapy. Clin Exp Immunol 99: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen R., Marttila T., Kaasinen E., Rintala E., Aaltomaa S., Kallio J., et al. (2015) Long-term outcome of patients with frequently recurrent non-muscle-invasive bladder carcinoma treated with one perioperative plus four weekly instillations of mitomycin C followed by monthly bacillus Calmette-Guerin (BCG) or alternating BCG and interferon-alpha2b instillations: prospective randomised finnbladder-4 study. Eur Urol 68: 611–617. [DOI] [PubMed] [Google Scholar]
- Joudi F., Smith B., O’Donnell M. (2006) Final results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alpha-2b for reducing recurrence of superficial bladder cancer. Urol Oncol 24: 344–348. [DOI] [PubMed] [Google Scholar]
- Keir M., Butte M., Freeman G., Sharpe A. (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26: 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Chang S., Hsieh D., Yu D. (2004) Immunotherapy for bladder cancer using recombinant bacillus Calmette-Guerin DNA vaccines and interleukin-12 DNA vaccine. J Urol 171: 1343–1347. [DOI] [PubMed] [Google Scholar]
- Liakou C., Kamat A., Tang D., Chen H., Sun J., Troncoso P., et al. (2008) CTLA-4 blockade Increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci USA 105: 14987–14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Su I., Chang J., Chen Y., Lu J., Dou H. (2012) Recombinant BCG coexpressing AG85B, CFP10, and interleukin-12 induces multifunctional Th1 and memory T cells in mice. Apmis 120: 72–82. [DOI] [PubMed] [Google Scholar]
- Liu W., O’Donnell M., Chen X., Han R., Luo Y. (2009) Recombinant bacillus Calmette-Guerin (BCG) expressing interferon-alpha 2b enhances human mononuclear cell cytotoxicity against bladder cancer cell lines in vitro. Cancer Immunol Immunother 58: 1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Chen X., Han R., O’Donnell M. (2001) Recombinant bacille Calmette-Guerin (BCG) expressing human interferon-alpha 2b demonstrates enhanced immunogenicity. Clin Exp Immunol 123: 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Yamada H., Chen X., Ryan A., Evanoff D., Triccas J., et al. (2004) Recombinant mycobacterium bovis bacillus Calmette-Guerin (BCG) expressing mouse IL-18 augments Th1 immunity and macrophage cytotoxicity. Clin Exp Immunol 137: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan R., Arlen P., Gulley J. (2007) PANVAC-VF: poxviral-based vaccine therapy targeting CEA and MUC1 in carcinoma. Expert Opin Biol Ther 7: 543–554. [DOI] [PubMed] [Google Scholar]
- Malkovsky M., Loveland B., North M., Asherson G., Gao L., Ward P., et al. (1987) Recombinant interleukin-2 directly augments the cytotoxicity of human monocytes. Nature 325: 262–265. [DOI] [PubMed] [Google Scholar]
- Maude S., Frey N., Shaw P., Aplenc R., Barrett D., Bunin N., et al. (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J., Nishiyama H., Yano I., Nakaya A., Kohama H., Kawai K., et al. (2011) The therapeutic effects of R8-liposome-BCG-CWS on BBN-induced rat urinary bladder carcinoma. Anticancer Res 31: 2065–2071. [PubMed] [Google Scholar]
- Morales A., Phadke K., Steinhoff G. (2009) Intravesical mycobacterial cell wall-DNA complex in the treatment of carcinoma in situ of the bladder after standard intravesical therapy has failed. J Urol 181: 1040–1045. [DOI] [PubMed] [Google Scholar]
- Morse M., Bradley D., Keler T., Laliberte R., Green J., Davis T., et al. (2011a) CDX-1307: a novel vaccine under study as treatment for muscle-invasive bladder cancer. Expert Rev Vaccines 10: 733–742. [DOI] [PubMed] [Google Scholar]
- Morse M., Chapman R., Powderly J., Blackwell K., Keler T., Green J., et al. (2011b) Phase I study utilizing a novel antigen-presenting cell-targeted vaccine with toll-like receptor stimulation to induce immunity to self-antigens in cancer patients. Clin Cancer Res 17: 4844–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P., Aldovini A., Young R. (1996) Manipulation and potentiation of antimycobacterial immunity using recombinant bacille calmette-guerin strains that secrete cytokines. Proc Natl Acad Sci USA 93: 934–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Fukiage M., Higuchi M., Nakaya A., Yano I., Miyazaki J., et al. (2014) Nanoparticulation of BCG-CWS for application to bladder cancer therapy. J Control Release 176: 44–53. [DOI] [PubMed] [Google Scholar]
- Nakanishi J., Wada Y., Matsumoto K., Azuma M., Kikuchi K., Ueda S. (2007) Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother 56: 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento I., Dias W., Mazzantini R., Miyaji E., Gamberini M., Quintilio W., et al. (2000) Recombinant mycobacterium bovis BCG expressing pertussis toxin subunit S1 induces protection against an intracerebral challenge with live Bordetella pertussis in mice. Infect Immun 68: 4877–4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastala C., Edington H., McKinney T., Tahara H., Nalesnik M., Brunda M., et al. (1994) Recombinant Il-12 administration induces tumor regression in association with IFN-gamma production. J Immunol 153: 1697–1706. [PubMed] [Google Scholar]
- O’Donnell M., Luo Y., Hunter S., Chen X., Hayes L., Clinton S. (2004) Interleukin-12 Immunotherapy of murine transitional cell carcinoma of the bladder: dose dependent tumor eradication and generation of protective immunity. J Urol 171: 1330–1335. [DOI] [PubMed] [Google Scholar]
- Papageorgiou A., Dinney C., McConkey D. (2007) Interferon-alpha induces TRAIL expression and cell death via an IRF-1-dependent mechanism in human bladder cancer cells. Cancer Biol Ther 6: 872–879. [DOI] [PubMed] [Google Scholar]
- Park J., Terabe M., Steel J., Forni G., Sakai Y., Morris J., et al. (2008) Therapy of advanced established murine breast cancer with a recombinant adenoviral ERBB-2/NEU vaccine. Cancer Res 68: 1979–1987. [DOI] [PubMed] [Google Scholar]
- Potts K., Hitt M., Moore R. (2012) Oncolytic viruses in the treatment of bladder cancer. Adv Urol 2012: 404581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T., Eder J., Fine G., Braiteh F., Loriot Y., Cruz C., et al. (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515: 558–562. [DOI] [PubMed] [Google Scholar]
- Ramesh N., Ge Y., Ennist D., Zhu M., Mina M., Ganesh S., et al. (2006) CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor – armed oncolytic adenovirus for the treatment of bladder cancer. Clin Cancer Res 12: 305–313. [DOI] [PubMed] [Google Scholar]
- Ratliff T., Ritchey J., Yuan J., Andriole G., Catalona W. (1993) T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol 150: 1018–1023. [DOI] [PubMed] [Google Scholar]
- Riemensberger J., Bohle A., Brandau S. (2002) IFN-gamma and IL-12 but not IL-10 are required for local tumour surveillance in a syngeneic model of orthotopic bladder cancer. Clin Exp Immunol 127: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S., Restifo N. (2015) Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild S., Thommen D., Moersig W., Muller P., Zippelius A. (2015) Cancer immunology – development of novel anti-cancer therapies. Swiss Med Wkly 145: w14066. [DOI] [PubMed] [Google Scholar]
- Sanger C., Busche A., Bentien G., Spallek R., Jonas F., Bohle A., et al. (2004) Immunodominant PSTS1 antigen of mycobacterium tuberculosis is a potent biological response modifier for the treatment of bladder cancer. BMC Cancer 4: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Bajorin D., Jungbluth A., Herr H., Old L., Gnjatic S. (2008) Immune responses detected in urothelial carcinoma patients after vaccination with NY-ESO-1 protein plus BCG and GM-CSF. J Immunother 31: 849–857. [DOI] [PubMed] [Google Scholar]
- Sharma P., Shen Y., Wen S., Bajorin D., Reuter V., Old L., et al. (2006) Cancer-testis antigens: expression and correlation with survival in human urothelial carcinoma. Clin Cancer Res 12: 5442–5447. [DOI] [PubMed] [Google Scholar]
- Sherif A., Hasan M., Marits P., Karlsson M., Winqvist O., Thorn M. (2010) Feasibility of T-cell-based adoptive immunotherapy in the first 12 patients with advanced urothelial urinary bladder cancer. Preliminary data on a new immunologic treatment based on the sentinel node concept. Eur Urol 58: 105–111. [DOI] [PubMed] [Google Scholar]
- Siegel R., Miller K., Jemal A. (2015) Cancer statistics, 2015. CA Cancer J Clin 65: 5–29. [DOI] [PubMed] [Google Scholar]
- Slaton J., Perrotte P., Inoue K., Dinney C., Fidler I. (1999) Interferon-alpha-mediated down-regulation of angiogenesis-related genes and therapy of bladder cancer are dependent on optimization of biological dose and schedule. Clin Cancer Res 5: 2726–2734. [PubMed] [Google Scholar]
- Slobbe L., Lockhart E., O’Donnell M., Mackintosh C., De Lisle G., Buchan G. (1999) An in vivo comparison of bacillus Calmette-Guerin (BCG) and cytokine-secreting BCG vaccines. Immunology 96: 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester R., Van Der Meijden A., Witjes J., Kurth K. (2005) Bacillus Calmette-Guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol 174: 86–91; discussion 91–82. [DOI] [PubMed] [Google Scholar]
- Weiss G., O’Donnell M., Loughlin K., Zonno K., Laliberte R., Sherman M. (2003) Phase 1 study of the intravesical administration of recombinant human interleukin-12 in patients with recurrent superficial transitional cell carcinoma of the bladder. J Immunother 26: 343–348. [DOI] [PubMed] [Google Scholar]
- Wu Y., Enting D., Rudman S., Chowdhury S. (2015) Immunotherapy for urothelial cancer: from BCG to checkpoint inhibitors and beyond. Expert Rev Anticancer Ther 15: 509–523. [DOI] [PubMed] [Google Scholar]
- Young S., O’Donnell M., Lockhart E., Buddle B., Slobbe L., Luo Y., et al. (2002) Manipulation of immune responses to mycobacterium bovis by vaccination with IL-2- and IL-18-secreting recombinant bacillus Calmette Guerin. Immunol Cell Biol 80: 209–215. [DOI] [PubMed] [Google Scholar]
- Yu D., Lee C., Chang S. (2007) Immunotherapy for orthotopic murine bladder cancer using bacillus Calmette-Guerin recombinant protein MPT-64. J Urol 177: 738–742. [DOI] [PubMed] [Google Scholar]
- Zhao J., Xu W., Zhang Z., Song R., Zeng S., Sun Y., et al. (2015) Prognostic Role of HER2 expression in bladder cancer: a systematic review and meta-analysis. Int Urol Nephrol 47: 87–94. [DOI] [PubMed] [Google Scholar]
- Zlotta A., Van Vooren J., Denis O., Drowart A., Daffe M., Lefevre P., et al. (2000) What are the immunologically active components of bacille Calmette-Guerin in therapy of superficial bladder cancer? Int J Cancer 87: 844–852. [DOI] [PubMed] [Google Scholar]
- Zou W., Chen L. (2008) Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 8: 467–477. [DOI] [PubMed] [Google Scholar]