Abstract
The treatment of metastatic castrate-resistant prostate cancer (mCRPC) has grown over the past decade. The majority of patients develop bone metastases, which pose a significant burden on morbidity and mortality, especially skeletal-related events. Whilst demonstrating a favourable safety profile and improving symptoms, radiopharmaceuticals have until recently failed to show a survival benefit. However, since the large phase III randomized ALSYMPCA trial, the calcium mimetic properties of radium-223 (Ra223) have improved patients’ quality of life and improved survival whilst keeping toxicities to a minimum. This review article summarizes the clinical data including our real life experience on the usage of the alpha emitter Ra223 in mCRPC, paying particular attention to how clinicians should best monitor response.
Keywords: prostate, castrate resistant, radium-223, bone metastases
Introduction
The incidence of metastatic castrate resistant prostate cancer (mCRPC) is increasing. The estimated incidence of prostate cancer in the UK is 40,000 new cases per annum, with 15–30% presenting with bone metastases at the time of diagnosis. Many of these men will progress to develop mCRPC. The optimal management of these men poses a significant challenge to the oncologist. Until 2004, chemotherapy was the mainstay of treatment for mCRPC. Since then different strategies have emerged with novel agents to manipulate the androgen receptor, targeting the immune system and treating the bone micro-environment. The development of novel agents has been of great benefit but there is uncertainty regarding the optimal use of these treatments in sequence and indeed combination. One of these promising new agents with a novel mode of action is radium-223 (Ra223), an alpha emitter which targets osseous metastases in mCRPC.
In mCRPC, the commonest metastases are bone, with >90% diagnosed radiologically [Cheetham and Petrylak, 2012]. Osteoblastic bone metastases occurring in areas of new bone formation are a common cause of morbidity and mortality, and have a secondary economic burden on healthcare. Traditionally bone pain has been managed with analgesics, external beam radiotherapy and beta-emitting radioisotopes. Whilst some of the current standard treatments such as bisphosphonates, rank ligand inhibitors and bone-seeking beta emitters such as strontium have been shown to lengthen time to symptomatic progression, none have demonstrated a survival advantage [Windsor, 2001; Iranikhah et al. 2014]. This may be the reason why physicians have been reluctant to use them at an earlier stage in a patient’s treatment. The development of Ra223, however, has the potential to challenge this mindset though it remains unclear where in the treatment pathway of mCRPC is Ra223 best placed.
Mechanism of action and clinical application
Ra223 is the sixth novel agent to be added to the treatment of mCRPC, having been approved by the US Food and Drug Administration (FDA) in May 2013. It is administered via an intravenous route over 1 minute and stabilizes in the circulatory system within a few hours. The standard dose is 50 kBq/kg.
Physics and radiobiological principles of Ra223
Ra223 is an isotope of radium with a short halflife of 11.4 days. Its calcium mimetic properties enable it to rapidly form complexes with the bone mineral hydroxyapatite, self-targeting the bone metastases. The short range of Ra223 (<100 μm) within tissue allows it to target new osteoblastic bone turnover whilst keeping collateral tissue damage to a minimum [Bruland et al. 2006]. The high energy alpha particles concentrated within areas of increased bone turnover induce DNA double-strand breaks, making it difficult to repair and hence resulting in a good cytotoxic effect.
Ra223, similar to other radioisotopes, decays to daughter products of which its decay series is very similar to Ra226, eventually forming lead-207. Of the total decay energy, 93.5% is emitted as four alpha particles; <4% as beta particles and <2% as gamma or X-rays.
Radiobiologically, alpha emitters have a much higher relative biological effectiveness (RBE) than beta particles or X-rays and y-rays, which ranges between 10 and 20. Their high charge and mass properties makes them useful for tumour cell kill. This, when combined with a high linear energy transfer (LET), means they deposit their ionizing dose within a small volume minimizing damage to surrounding normal tissue. Cell kill by Ra223 is also not reliant upon cells being in cycle, which is advantageous in prostate cancer of a low proliferative rate [Cheetham and Petrylak, 2012].
The amount absorbed by bone depends on the disease burden. After a few hours the amount of radium that has been taken up can be visibly observed by methylene diphosphonate (MDP) bone scan imaging or positron emission tomography computed tomography (PET CT) in the bone or gastrointestinal (GI) tract. Ra223 clears rapidly from the blood, concentrating within bone approximately 20–30 minutes postinjection leaving 2% activity within the blood 4 hours after injection. The major route of elimination is via the faecal route, which approximates to 50–60% with only 5% excreted by the kidneys [Carrasquillo et al. 2013].
Understanding the dosimetry to organs post Ra223 is contentious and lacking in published data. According to the International Commission on Radiological Protection (ICRP) Publication 67 (ICRP 67), 25% of Ra223 in an adult is transferred to the skeleton, with the uptake corresponding to bone metabolism. The majority of Ra223 is then quickly re-transferred to the blood with biological half times ranging between 0.1 and 1 day [Lassmann and Nosske, 2013]. The specific mode of action of Ra223 in bone has been evaluated in preclinical studies.
Clinical studies of Ra223
The specificity of Ra223 to bone and marrow as well as its redistribution was confirmed by Henriksen and colleagues. Within 24 hours, significant uptake was seen in the bone marrow compared with the bone matrix with the level decreasing rapidly by 14 days [Henriksen et al. 2002]. Ra223, like other alpha-emitting radioisotopes, shows little redistribution of its daughter products from bone, that is, <1% at 3 days compared with from soft tissues (<24 hours) implying that their activity is concentrated and stays within the bone itself [Henriksen et al. 2003]. Short-term toxicity studies in mice demonstrated a relatively high tolerance of the bone marrow to the damaging effects of the alpha particles [Larsen et al. 2006].
Early phase I and II studies addressed optimum dosage of Ra223 but also response to prostate-specific antigen (PSA) and alkaline phosphatase (ALP) markers. In phase II clinical studies of Ra223, a dose–response relationship was noted between the three doses of 25, 50 and 80 kBq/kg in terms of ⩾50% ALP decline. Interestingly the quantity of decline in ALP was marginal between the 50 and 80 kBq/kg dose of 67% and 66%, respectively, but overall ALP decline was much greater than PSA [Parker et al. 2013b]. This small decline or even PSA flare may be due to increased tumour lysis and hence release of PSA.
Toxicity profile of Ra223
The safety profile of Ra223 allows its administration as an outpatient treatment with practical advice focused on good hygiene to minimize exposure such as separating laundry and using special bathroom provisions. In general as the exposure rate is low, latex gloves are sufficient when handling the isotopes.
Ra223 is generally well tolerated. Alpha particles do not traverse the bone marrow and therefore haematological toxicity is uncommon. Due to its excretion route, nonhaematological side effects are mainly gastrointestinal, notably diarrhoea, nausea and constipation as shown in the ALSYMPCA trial. The incidence of GI toxicities was similar to the placebo group, although with <2% Grade 3 or 4. There was very little difference in Grade 3 or 4 haematological toxicities when compared with placebo. The commonest haematological event recorded was anaemia with an incidence of 11%, Grade 3 and 2% Grade 4. This showed no difference with placebo; 6% patients developed Grade 3 or 4 thrombocytopenia versus 2% in placebo. Grade 3 febrile neutropenia was rare, being reported in 2% versus 1% with placebo [Parker et al. 2013a].
Ra223 is currently dosed on weight. However, as its activity is mainly concentrated within the skeleton, acting primarily in bone, an alternative height adjusted model has been suggested. There is limited work on this to date apart from small retrospective observational studies. A recent retrospective study published for the American Society of Clinical Oncology Annual Meeting 20104 (ASCO 2014) by Longo and colleagues showed that, after recalculating the standard dose of 50 kBq/Kg for different heights, the average dose based on height administered was 27.4 kBq/cm (17.39–40.7 kBq/cm). The side effect profile, in particular haematological and GI toxicities, was similar across all height adjusted doses with higher doses shown to be well tolerated. Based on such a small study there is yet to be an optimal height dosing regimen, but future studies using height-based dosing escalation to evaluate safety and efficacy have been proposed [Longo et al. 2014].
Where does the target of Ra223 lie?
The correct target for Ra223 is a contentious issue partly due to the dissociation between clinical and PSA response. A significant PSA decline was demonstrated with enzalutamide and abiraterone, but such a decline was not seen in either the ALSYMPCA trial using Ra223 versus placebo or the IMPACT trial using sipuleucel-T versus placebo. In both trials, PSA assessment was measured as a secondary endpoint. Within the ALSYMPCA trial, 16% of patients given Ra223 had a ⩾30% reduction in PSA by 12 weeks versus placebo. This response was even lower in the IMPACT trial, with 2.6% having a ⩽50% PSA complete response versus placebo [Parker et al. 2013a].
In general, Ra223 response is not reflected in PSA levels, which poses a challenge as to how to best assess response. PSA is an androgen receptor targeted protein and therefore should fall in treatments acting via this pathway. Ra223, however, appears to target the tumour micro-environment, rather than the tumour itself, resulting in the bone becoming more resistant and able to cope with the increased burden of disease. PSA therefore becomes less reliable and as such patient education is of paramount importance. PSA response cannot be used in isolation and needs to be evaluated in conjunction with imaging and clinical review. ALP, however, has been shown to fall in response to Ra223 and thereby is a proposed alternative marker of response in patients with bone metastases in mCRPC.
Evidence in the treatment of bone metastases in CRPC
Within the past 5 years, treatment options such as abiraterone, enzalutamide and cabazitaxel have shown a survival advantage in mCRPC, although these have all been in the post-docetaxel setting. Prior to the ALSYMPCA trial, phase I and II clinical trials evaluated the safety and clinical benefit with Ra223.
The ALSYMPCA trial led by Parker and colleagues enrolled 614 patients with symptomatic bone metastases either pre- or post-docetaxel to receive Ra223 or placebo. Patients randomized to the Ra223 arm received 6 × 4 weekly cycles of 50 kBq/kg Ra223 with a primary endpoint of overall survival (OS). Ra223 showed an OS advantage irrespective of prior docetaxel use; for example, with prior docetaxel the median improvement was 3.1 months versus 4.6 months with none. There was also a greater fall in ALP in those receiving Ra223 than placebo; 47% had a ⩾30% reduction versus placebo and in those whose level was raised at baseline, 34% of patients returned to a normal ALP [Parker et al. 2013a]. The incidence of myelosuppression was low and there was little difference found in Grade 3 and 4 events between Ra223 and placebo.
Time to skeletal related events, defined as pathological fracture (vertebral or nonvertebral), use of external beam radiotherapy, new spinal cord compression or tumour-related surgical intervention, was longer with Ra223 than placebo (15.6 versus 9.8 months). In the subgroup analysis, there was a suggestion of a positive additive effect between bisphosphonates and Ra223 postulating that bisphosphonates may prepare a favourable environment for Ra223, to act on within the bone matrix [Parker et al. 2011]. This would seem a reasonable suggestion as, whilst bisphosphonates inhibit osteoclasts, osteoblastic activity can be increased which is the primary site of action of Ra223. Pain-related symptoms and subsequent poor quality of life also significantly improved after Ra223; however, a discordance was noted between PSA response and clinical benefit supporting the hypothesis regarding a bone micro-environment described earlier [Sartor et al. 2014].
Whilst the ALSYMPCA trial is the first phase III randomized trial, there are limitations. One needs to be careful when interpreting these results due to confounding factors such as co-administration of standard anti-androgen therapies of the ‘physician’s choice’ alongside Ra223 which may lead to significant bias. A further notable factor is the exclusion of patients with visceral metastases of which the incidence is at least 25% in mCRPC patients.
Clinical benefit of Ra223
A small audit was conducted locally within our institution to evaluate first-hand experience with Ra223. A retrospective analysis of 58 patients’ case notes was performed of which the median follow up was 11.6 (1.0–18.1) months. Table 1 highlights the patients’ demographics.
Table 1.
Demographics.
| Demographic | Radium 223 (n = 58) |
|---|---|
| Age (years) median (range) | 71 (54–84) |
| ECOG performance status | |
| 0 | 18 (31%) |
| 1 | 35 (60%) |
| 2 | 5 (9%) |
| Number of cycles, median (range) | 5 (1–6) |
| Previous lines of treatment, median (range) | 3 (1–5) |
| Prior docetaxel (%) | |
| Yes | 30 (52%) |
| No | 28 (48%) |
| Prior bisphosphonates, n (%) | |
| Yes | 10 (17%) |
| No | 48 (83%) |
| Prior strontium, n (%) | |
| Yes | 3 (5%) |
| No | 55 (95%) |
ECOG, Eastern Cooperative Oncology Group.
Of the 58 patients, 50% had a documented clinical benefit defined by improvement in pain, mobility and reduction in analgesic requirements; 5% developed a skeletal related event similar to the incidence in the ALSYMPCA trial with a mean time of 218 days.
Median PSA increased post-treatment (225 versus 418), whilst median ALP fell (292 versus 138) as shown in Figures 1 and 2 below.
Figure 1.
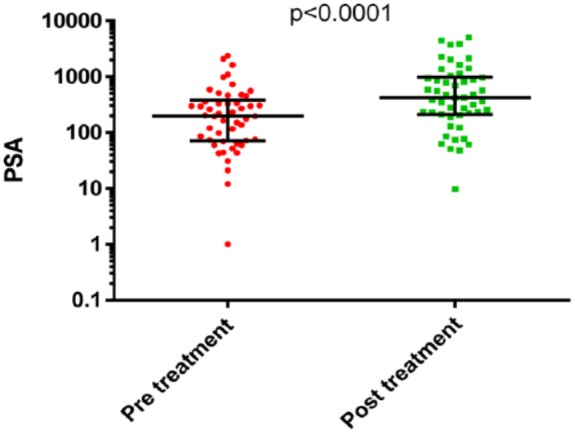
Comparison of PSA measurements pre- and post-treatment with Ra223.
Figure 2.
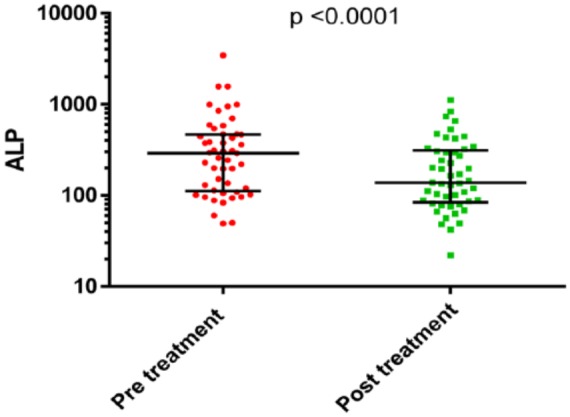
Comparison of ALP measurements pre- and post-treatment with Ra223.
There was no significant effect seen with pre- and post-haemoglobin (Hb). The incidence of Grade 3 or 4 events was low and there were no treatment related deaths. The incidence of haematological events was 2% Grade 3 anaemia, 3% Grade 3 neutropenia and no G3 thrombocytopenia. There were no documented episodes of neutropenic sepsis.
Just over two-thirds (62%) remain alive with a median progression-free survival (PFS) 7.23 months [95% confidence interval (CI) 5.73–7.93] and median OS of 8.33 months (95% CI 5.65–13.30). The majority of patients in this analysis had been heavily pretreated, receiving Ra223 as third line or higher. Despite this Ra223 was still well tolerated with a safe toxicity profile. The optimum sequence, however, is yet to be defined but we propose its earlier use in better selected patients with bone only disease.
Long-term toxicity
The ALSYMPCA trial has reported long-term toxicities of 571 patients treated with Ra223 over a 3 year follow-up period. The commonest adverse events at the interim analysis at 1.5 years were hematologic; however, myelosuppression incidence was low at <3% and there were no Grade 3 or 4 nonhaematological treatment related adverse events except for one pathological fracture (<1%). To date there have been no reports of acute myelogenous leukaemia, myelodysplastic syndrome or primary bone cancers after 3 years of follow up. Such results are currently in abstract form [Parker et al. 2015]. The observational REASSURE study aims to assist further in the evaluation of long-term toxicities over a 10-year period, paying particular attention to secondary related malignancies [ClinicalTrials.gov identifier: NCT02141438].
Future developments
Whilst Ra223 has been proven to improve symptoms and survival in patients with mCRPC, many have already completed several lines of treatment. The hypothesis that micro metastatic bone disease could be eradicated by the early adjuvant use of Ra223 is currently being addressed. There are phase I/II trials investigating the benefits of combination therapy with docetaxel and Ra223 [ClinicalTrials.gov identifier: NCT01106352].
Targeting the immune system with sipuleucel-T has been used since 2010, whereby it demonstrated a survival advantage of 4.1 months [Sheikh et al. 2013]. Since then, further immune targeted strategies including vaccines and monoclonal antibodies have been investigated. A current phase II study is investigating the combination of immune targeted therapies with sipuleucel-T and Ra223 to determine if the immune systems response can be enhanced by the production of antigens by radiopharmaceuticals [ClinicalTrials.gov identifier: NCT02463799].
Clear guidance, however, is still lacking regarding the optimal sequence of treatments for patients with mCRPC and the correct time to switch therapies. Due to patient related PSA anxiety, treatments are often discontinued too early on PSA progression alone rather than a combination of radiological and biochemical progression. Good clinical acumen is of paramount importance here, particularly in patients with bone pain or rapidly rising PSA levels. Attention needs to be focused on the common complications of mCRPC in order that pain, spinal cord compression and nutritional status can all be managed appropriately.
Preclinical data have suggested cross-resistance between androgen receptor targeted treatments and taxane based chemotherapy, which makes optimal sequencing even more important [van Soest et al. 2013]. However, as the majority of patients present with bone only disease, a logical suggestion would be earlier use of Ra223 in this patient group. It seems likely that as more agents are produced there will be considerable overlay between treatments all acting via different mechanisms of action in mCRPC.
Over the past decade, physicians’ understanding of the pathophysiology of the disease has improved leading to the development of newer drugs to individualize treatment. Within prostate cancer, there is significant heterogeneity, both between and within the patients themselves. Methods to individualize treatment such as genetic profiling and molecular analysis of metastatic lesions may improve our understanding of the complexity of the tumour cell. This could lead to a predictive biomarker model to optimize agent selection in the management of this heterogeneous disease.
Conclusion
Ra223 is a novel agent with a unique mode of action. Trials and clinical experience have demonstrated its excellent safety profile. It has been shown to provide a survival benefit in mCRPC with predominantly osseous metastases. Its adoption into clinical practice has provided another weapon in the management of mCRPC and the potential for combination therapy with other novel agents with differing modes of action. There is also emerging evidence that the greatest benefit of Ra223 will be achieved in its earlier use in patients with mCRPC. In addition there may be scope for future trials addressing the potential of its adjuvant use in the hormone sensitive scenario.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Christina Hague, Department of Clinical Oncology, The Christie NHS Foundation Trust, Wimslow Road, Manchester M20 4BX, UK.
John P. Logue, Department of Clinical Oncology, The Christie NHS Foundation Trust, Manchester, UK
References
- Bruland Ø., Nilsson S., Fisher D., Larsen R. (2006) High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res 12: 6250s–6257s. [DOI] [PubMed] [Google Scholar]
- Carrasquillo J., O’Donoghue J., Pandit-Taskar N., Humm J., Rathkopf D., Slovin S., et al. (2013) Phase I pharmacokinetic and biodistribution study with escalating doses of 223Ra-dichloride in men with castration-resistant metastatic prostate cancer. Eur J Nucl Med Mol Imaging 40: 1384–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham P., Petrylak D. (2012) Alpha particles as radiopharmaceuticals in the treatment of bone metastases: mechanism of action of radium-223 chloride (Alpharadin) and radiation protection. Oncology 26: 330–337, 341. [PubMed] [Google Scholar]
- Henriksen G., Breistø K., Bruland Ø., Fodstad Ø., Larsen R. (2002) Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res 62: 3120–3125. [PubMed] [Google Scholar]
- Henriksen G., Fisher D., Roeske J., Bruland Ø., Larsen R. (2003) Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J Nucl Med 44: 252–259. [PubMed] [Google Scholar]
- Iranikhah M., Stricker S., Freeman M. (2014) Future of bisphosphonates and denosumab for men with advanced prostate cancer. Cancer Manag Res 6: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R., Saxtorph H., Skydsgaard M., Borrebaek J., Jonasdottir T., Bruland O. (2006) Radiotoxicity of the alpha-emitting bone-seeker 223Ra injected intravenously into mice: histology, clinical chemistry and hematology. In Vivo 20: 325–332. [PubMed] [Google Scholar]
- Lassmann M., Nosske D. (2013) Dosimetry of 223Ra-chloride: dose to normal organs and tissues. Eur J Nucl Med Mol Imaging 40: 207–212. [DOI] [PubMed] [Google Scholar]
- Longo T., Nordquist L., Mehr S., Mcdonald M. (2014) Consideration of height-based rather than weight-based dosing as a more appropriate method of treating with radium-223. J Clin Oncol 32: abstr e16075. [Google Scholar]
- Parker C., Heinrich D., O’Sullivan J.M., Fossa S., Chodacki A., Demkow T., et al. (2011) Overall survival benefit of radium-223 chloride (Alpharadin™) in the treatment of patients with symptomatic bone metastases in Castration-resistant Prostate Cancer (CRPC): a phase III randomized trial (ALSYMPCA). Eur J Cancer 47(Suppl. 2): 3. [Google Scholar]
- Parker C., Nilsson S., Heinrich D., Helle S., O’Sullivan J., Fosså S., et al. (2013a) Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369: 213–223. [DOI] [PubMed] [Google Scholar]
- Parker C., Pascoe S., Chodacki A., O’Sullivan J., Germá J., O’Bryan-Tear C., et al. (2013b) A randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur Urol 63: 189–197. [DOI] [PubMed] [Google Scholar]
- Parker C., Vogelzang N., Sartor A., Coleman R., Fang F., Skjorestad I., et al. (2015) 3-year safety follow-up of radium-223 dichloride (Ra-223) in patients (Pts) with castration-resistant prostate cancer (CRPC) and symptomatic bone metastases (Mets) from ALSYMPCA. J Clin Oncol 33(Suppl. 7): abstract 195. [Google Scholar]
- Sartor O., Coleman R., Nilsson S., Heinrich D., Helle S., O’Sullivan J., et al. (2014) Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 15: 738–746. [DOI] [PubMed] [Google Scholar]
- Sheikh N., Petrylak D., Kantoff P., Dela Rosa C., Stewart F., Kuan L., et al. (2013) Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother 62: 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Soest R., van Royen M., de Morrée E., Moll J., Teubel W., Wiemer E., et al. (2013) Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer. Eur J Cancer 49: 3821–3830. [DOI] [PubMed] [Google Scholar]
- Windsor P. (2001) Predictors of response to strontium-89 (Metastron) in skeletal metastases from prostate cancer: report of a single centre’s 10-year experience. Clin Oncol 13: 219–227. [DOI] [PubMed] [Google Scholar]


