Abstract
Introduction:
Radical cystectomy (RC) is the gold standard treatment for muscle-invasive bladder cancer. This procedure has a high rate of perioperative complications, many of which are infectious in nature. The objective of our study was to evaluate demographic, intrinsic and extrinsic patient variables associated with developing readmission within 30 days due to infectious complications following RC.
Methods:
We acquired data available from the American College of Surgeons National Surgical Quality Improvement Program. We queried this dataset to identify all patients who underwent RC for muscle-invasive malignant disease (CPT 188.x) in 2012 based on CPT coding. Logistic regression analysis was used to investigate the relationship between preoperative variables and readmissions for infectious complications.
Results:
Of the 961 patients undergoing cystectomy for malignancy, 159 (17%) required readmission for any indications at a median of 16 days (interquartile range 13–22 days) postoperatively. We identified 71 of a total of 159 (45%) readmissions, which were due to infectious complications. Smoking was more prevalent in the patient population readmitted for an infectious complication compared with the patient population readmitted for a non-infectious complication (37% versus 25%; p = 0.03). Using logistic regression analysis smoking was associated with a significant risk for readmission due to an infectious cause (odds ratio 2.28, 95% confidence interval 1.82–2.97, p = 0.02). Readmission due to an infectious etiology was not associated with other perioperative factors including type of urinary diversion, sex, duration of operation, hypertension, or recent weight loss.
Conclusion:
Readmission following RC is a common occurrence and infectious complications drive readmission in almost half of the cases. Current smoking was the only independent risk factor for an infectious readmission. Counseling patients in smoking cessation prior to the procedure may provide an avenue for quality improvement to limit readmissions.
Keywords: bladder cancer, complications, cystectomy, National Surgical Quality Improvement Program, smoking
Introduction
Urinary bladder cancers are the sixth leading cause of cancer-related death in the United States [Siegel et al. 2014]. Radical cystectomy (RC) with urinary diversion remains the gold standard of treatment for muscle-invasive bladder cancer and select cases of high-risk nonmuscle-invasive bladder cancer [Siegel et al. 2014]. However, RC is met with high rates of perioperative complications and readmissions, which can turn into lengthy and expensive hospitalizations [Pietzak et al. 2014].
Following RC, infectious complications are a common occurrence [Pietzak et al. 2014]. While some of these may not necessitate inpatient management, approximately 25% of patients are readmitted for treatment [Skolarus et al. 2014]. Infectious complications are a significant source of financial burden to the hospital and to the patient [Wick et al. 2011].
Readmissions are also employed as a quality indicator used by state, federal and private payers to compare outcomes amongst medical centers. Given the economic climate and the competitive nature of healthcare systems, reducing hospital readmissions hence becomes the priority of hospital-based systems [Wick et al. 2011].
Our study aimed to examine the American College of Surgeons’ (ACS) National Surgical Quality Improvement Program (NSQIP) database to identify patients who underwent RC. We hypothesized that by using a single year of a large multi-institutional dataset we could elucidate statistically the major risk factors associated with developing a readmission secondary to an infectious complication that might not be found in a single institutional cohort.
Material and methods
Patient population
We acquired data available from the ACS NSQIP, a nationally validated, risk-adjusted, outcomes-based database that prospectively reports data on 135 variables including 30-day morbidity and mortality for major surgical procedures at over 450 institutions [Ingraham et al. 2010]. We queried this dataset to identify all patients who underwent RC for malignant disease [International Classification of Diseases ninth revision (ICD9) 188, 188.x] in 2012 based on Current Procedural Terminology (CPT) coding (51570, 51575, 51580, 51585, 51590, 51595, 51596, 51597). Patients who underwent RC for diagnoses other than bladder cancer were excluded. We elected to use the year 2012 as a pilot year for our analysis. We subdivided patients undergoing continent versus incontinent diversion based on CPT codes. We calculated body mass index (BMI) from variables in the dataset. Patients were defined as having poor nutrition if they had albumin less than 3.5 g/dl, recent weight loss of over 10% or BMI less than 18.5.
Selection of primary outcome
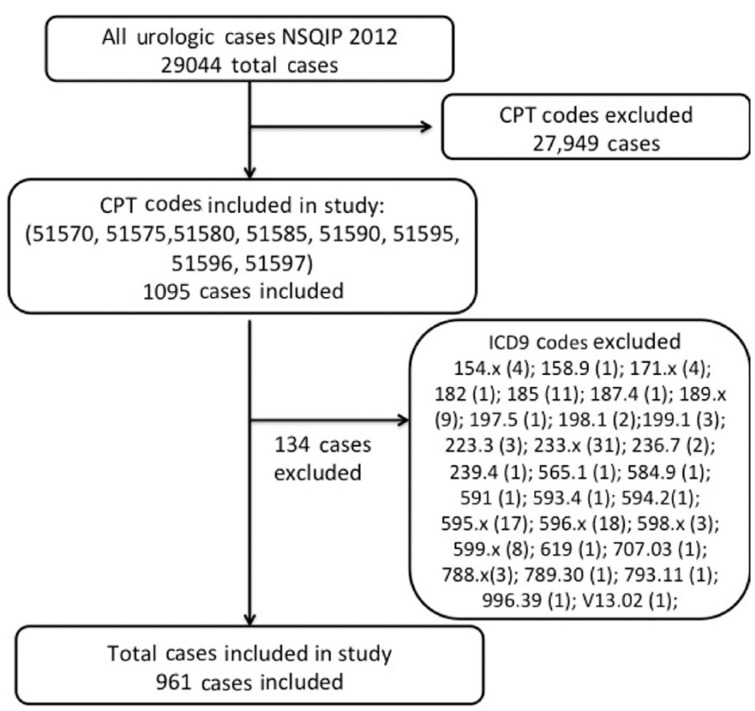
Patients were considered as having a readmission for infectious parameters if their primary indication for readmission was sepsis, septic shock, any organ system infection, or ICD codes 008.45, 038.x, 039.9, 569.61, 590.x, 599.x, 780.6x, 785.50, 998.5, and 999.32. ICD codes listed under the category ‘other’ in the NSQIP database were also assessed, and codes, which are based on an infectious etiology, were included in the final data analysis. Table 1 lists the ICD codes included in the study and their corresponding indication for readmission. While 159 patients were readmitted, based on the data coding in the NSQIP dataset, we were unable to determine the exact indication for readmission in 22 patients and therefore excluded them from the additional analyses. See Figure 1 for flow chart explaining identification of study patient population.
Table 1.
ICD coding for primary diagnosis for infectious readmissions after radical cystectomy.
| ICD code | Number of patients | Indication |
|---|---|---|
| 008.45 | 3 | Intestinal infection due to Clostridium difficile |
| 038.x | 1 | Septicemia |
| 039.9 | 1 | Actinomycotic infection of unspecified site |
| 569.61 | 1 | Infection of colostomy or enterostomy |
| 590.x | 3 | Infections of kidney |
| 599.x | 2 | Other disorders of urethra and urinary tract |
| 780.6x | 6 | Fever and other physiologic disturbances of temperature regulation |
| 785.50 | 1 | Shock unspecified |
| 998.5 | 1 | Postoperative infection not elsewhere classified |
| 999.32 | 1 | Bloodstream infection due to central venous catheter |
ICD, International Classification of Diseases.
Figure 1.
Flow chart explaining the identification of study patient population. ICD9, International Classification of Diseases ninth revision; NSQIP, National Surgical Quality Improvement Program; CPT, Current Procedural Terminology.
Statistical analysis
We initially performed a comparative univariable analysis to compare the demographic and treatment factors in this dataset. We included the following factors: sex, age, documented medical comorbidities, current smoking and alcohol usage, preoperative surgery or blood transfusion, the training level of resident in the operating room, history of prior radiation therapy, chronic steroid use, and American Society of Anesthesia (ASA) classification. Missing data were excluded.
We used χ2 analyses to compare readmissions between categorical variables. We then used the Mann–Whitney U test to assess readmission rates for non-normally distributed and Student’s t test for normally distributed comparisons of continuous variables.
We subsequently performed a logistic regression analysis to determine independent predictors of readmission for infectious complications within 30 days of the procedure. JMP 11.0 (SAS Institute, Cary, NC, USA) software was used for all statistical analyses.
The ACS NSQIP and the hospitals participating in the ACS NSQIP are the sources of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Results
We identified 961 patients who underwent cystectomy for malignancy, and of these, 159 (17%) required readmission for any indication within 30 days of their primary procedure. Readmission occurred at a median of 16 days (interquartile range 13–22 days) postoperatively. Demographic data are listed in Table 2 comparing patients who did and did not require readmission. The median age among patients requiring readmission was 69 years. Patients who were readmitted were more likely to have had recent weight loss (8.2%) compared with patients who were not readmitted (2.6%, p = 0.002). History of dyspnea on exertion (p = 0.58), history of severe chronic obstructive pulmonary disease (COPD) (p = 0.73), history of congestive heart failure (p = 0.75), history of hypertension (p = 0.73), dialysis requirement (p = 0.75), history of steroid usage (p = 0.95), or a history of bleeding disorder (p = 0.31) were similar between both groups of patients. All preoperative laboratory and hematologic findings were similar between groups as well (data not shown). Operative time was similar between the two cohorts (p = 0.29). In addition, the type of urinary diversion was not associated with the rate of readmission (p = 0.06).
Table 2.
Baseline characteristics of cohort stratified by readmission status.
| Parameter | Not readmitted, n (%)/mean (SD) | Readmitted,n (%)/mean (SD) | p value |
|---|---|---|---|
| Total patients | 802 | 159 | |
| Male | 623 (77.7%) | 123 (77.4%) | 0.93 |
| Age | 68.8 (10.0) | 69 (9.4) | 0.77 |
| Positive smoking history | 205 (25.6%) | 47 (29.6%) | 0.3 |
| Serum creatinine | 1.17 (0.53) | 1.19 (0.67) | 0.65 |
| Serum albumin | 3.91 (0.57) | 3.89 (0.52) | 0.69 |
| History of chronic steroid usage | 26 (3.2%) | 5 (3.14%) | 0.95 |
| Recent >10% weight loss | 21 (2.6%) | 13 (8.2%) | 0.002 |
| Chemotherapy for malignancy (⩽30 days preoperatively) | 50 (6.2%) | 11 (6.9%) | 0.91 |
| History of MI 6 months prior to surgery | 2 (0.25%) | 0 (0.0%) | 0.63 |
| History of severe COPD | 67 (8.35%) | 12 (7.5%) | 0.73 |
| Preoperative alkaline phosphatase (mg/dl) | 80.0 (27.2) | 85.6 (33.3) | 0.11 |
| Preoperative HCT (%) | 37.6 (5.4) | 37.2 (5.4) | 0.34 |
| ASA classification | 0.67 | ||
| Class 1 or 2 | 199 (25%) | 37 (23%) | |
| Class 3 or 4 | 605 (75%) | 122 (77%) |
ASA, American Society of Anesthesia; COPD, chronic obstructive pulmonary disease; HCT, hematocrit; MI, myocardial infarction; SD, standard deviation.
A total of 71 (45%) of the 159 patients who had cystectomy and required readmission were readmitted secondary to an infectious complication. Smoking was more common in patients readmitted for an infectious (38%) versus noninfectious related complication (21%, p = 0.03). Readmission due to an infectious etiology was not associated with diversion type, sex, race, transfusion requirement or duration of operative procedure (p > 0.05 for all). Furthermore, patients undergoing chemotherapy for malignancy in the 30-day preoperative period, those with greater than 10% loss of body weight in the last 6 months, those with poor nutritional status, or those with diabetes mellitus were variables not associated with increased readmission. Once again, hematologic and laboratory findings were similar between the patients readmitted for infectious and noninfectious indications (data not shown). Sepsis was documented in 18 (25%) of those admitted for infectious complications. While 159 patients were readmitted, based on the data coding in the NSQIP dataset, we were unable to determine the exact indication for readmission in 22 patients and therefore excluded them from the additional analyses. A comparison of demographic and perioperative variables for patients experiencing noninfectious versus infectious readmission is presented in Table 3 with the respective p values.
Table 3.
Demographic and perioperative variables for patients with noninfectious versus infectious readmissions.
| Noninfectious readmission, n (%)/mean (SD) | Infectious readmission, n (%)/mean (SD) | p value | |
|---|---|---|---|
| Total patients | 66 | 71 | |
| Male | 56 (84.8%) | 52 (73.2%) | 0.09 |
| Age | 69.5 (8.1) | 70 (10.2 | 0.85 |
| Smoking | 14 (21.2%) | 27 (38%) | 0.03 |
| Serum creatinine (mg/dl) | 1.23 (0.07) | 1.19 (0.81) | 0.79 |
| Serum albumin (g/dl) | 3.94 (0.53) | 3.85 (0.47) | 0.43 |
| Chemotherapy for malignancy (⩽30 days preoperatively) | 5 (7.6%) | 5 (7.04%) | 0.44 |
| >10% loss of body weight in last 6 months | 5 (7.6%) | 7 (9.9%) | 0.64 |
| Total operating time (min) | 366 (121) | 369 (134) | 0.89 |
| Poor nutritional status | 14 (21.2%) | 15 (21.2%) | 0.99 |
| Race: white | 58 (87.9%) | 63 (88.7%) | 0.88 |
| Diabetic status: yes | 15 (22.7%) | 13 (18.3%) | 0.52 |
| Dyspnea: yes | 7 (10.6%) | 8 (11.3%) | 0.9 |
| Independent: yes | 64 (97%) | 70 (98.6%) | 0.51 |
| BMI (kg/m2) | 29 (6) | 28.6 (6.5) | 0.67 |
| Discharge to home: yes | 56 (84.8%) | 60 (84.5%) | 0.96 |
BMI, body mass index.
We performed univariable logistic regression among the entire cohort of 961 patients to identify risk factors for readmission for an infectious complication (Table 4). Current smokers had twofold increased odds of readmission due to infection [odds ratio (OR) 2.28, 95% confidence interval (CI) 1.08–4.98, p = 0.03). There was a trend in increased risk of readmission due to infection in women (OR 2.05, 95% CI 0.89–4.96) and in patients with continent urinary diversion (OR 2.73, 95% CI 0.97–8.96). However, variables such as poor nutrition, age, chronic steroid use, preoperative albumin, preoperative hematocrit, operative duration, diabetic status, and BMI were not significantly associated with readmission due to infection. Multivariable regression analysis was not performed due to only finding one variable with statistical significance on the univariable analysis as well as the risk of over modeling due to lack of enough events (infectious readmissions).
Table 4.
Univariable logistic regression assessing independent predictors for 30-day readmission for infectious complication.
| Variable | OR | 95% CI | p value |
|---|---|---|---|
| Poor nutrition | 1.01 | 0.44–2.29 | 0.99 |
| Current smoker (ref: nonsmoker) | 2.28 | 1.08–4.98 | 0.03 |
| Age (per year increase) | 1.01 | 0.97–1.05 | 0.5 |
| Sex (ref: male) | 2.05 | 0.89–4.96 | 0.09 |
| Chronic steroid usage (ref: no) | 1.41 | 0.22–11.0 | 0.71 |
| Albumin (continuous) | 1.4 | 0.61–3.28 | 0.42 |
| Preoperative hematocrit (continuous) | 1.02 | 0.96–1.09 | 0.52 |
| Operative time (continuous minutes) | 1 | 0.99–1.00 | 0.89 |
| Continent diversion (ref: incontinent) | 2.73 | 0.97–8.96 | 0.06 |
| Diabetes | 0.76 | 0.33–1.75 | 0.52 |
| BMI (continuous) | 1.01 | 0.96–1.07 | 0.66 |
BMI, body mass index; CI, confidence interval; OR, odds ratio; ref, reference.
Discussion
Infectious complications were the largest contributor (45%, n = 71/159) to readmissions following RC with urinary diversion in this national multicenter surgical database. We identified active smoking as a risk factor for readmission (OR 2.2, 95% CI 1.82–4.98) secondary to an infectious etiology, and we suspect targeting this risk factor for intervention may reduce readmission rates due to infection.
RC remains the standard treatment for patients with muscle-invasive bladder cancer. Despite improvements in surgical technique, anesthesia and perioperative care, RC is still associated with a significant morbidity and a high rate of readmission. Previous reports have noted approximately 25% of complications following RC are infectious in nature [Shabsigh et al. 2009]. Lavallée and colleagues conducted a study in 2014 on 2303 patients with RC and reported that the leading infectious complication was urinary tract infections (9.5%), followed by sepsis events (9.7%) and surgical site infections (8.4%) [Lavallée et al. 2014]. In addition, approximately 3.2% patients had fascial dehiscence and 4.0% developed a deep vein thrombosis. They found several factors independently associated with the occurrence of any post-operative complication including: age, female gender, ASA class, pre-operative sepsis, COPD, low serum albumin concentration, pre-operative radiotherapy, pre-operative transfusion >4 units, and operative time >6 hours (all p < 0.05) [Lavallée et al 2014]. Although we did not examine the risk factors associated with developing a complication, our study looked at similar variables, which could contribute towards developing a readmission. We did not find a statistically significant association between sex, ASA class, COPD, low serum albumin (poor nutritional status), preoperative chemotherapy and preoperative transfusion, and developing a readmission. The small sample size of our patient subset contributed towards a lower statistical power and henceforth could explain the lack of statistical significance of certain variables in our study. This is particularly true for both female sex and diversion type, which trended towards but did not reach statistical significance following our statistical modeling.
In our study, we noted that infectious complications contribute to a greater proportion of readmissions following RC. We found that 45% of readmissions were infectious in nature. Infectious complications were the largest contributor to readmissions in this multicenter nationalized surgical database. This includes a comprehensive collection of infectious complications, including wound infections but also pneumonia or sepsis. Due to the extremely high morbidity and cost of readmission, it is critical to identify risk factors for developing readmission.
The demonstration of a high prevalence of postoperative infections following RC emphasizes the importance of identifying modifiable risk factors for the prevention of postoperative infections. Our findings suggest that being a current smoker is associated with developing a readmission secondary to an infectious etiology. To generalize our findings further, we may offer aggressive smoking cessation to candidate patients for mitigating the risk of readmission due to an infectious complication. Smoking is an important modifiable perioperative variable that may predispose patients not only to developing infectious complications, but it also increases patient risk of requiring an intensive care unit admission [Sudarshan et al. 2015]. Thus, addressing this variable preoperatively by offering smoking cessation may not only help to minimize the risk of developing postoperative infectious complications but also decrease the incidence of smokers undergoing major surgery in general. Hence, this is a primary form of prevention of infectious complications.
Our results suggest that smoking was the strongest risk factor associated with readmission for an infectious indication. Cigarette smoking has been shown to be associated with poor wound healing. Current hypotheses suggest that an immunologic mediated inflammatory response and ischemia are the primary mechanisms contributing to this phenomenon. The ischemia is thought to be from vasoconstriction induced hypoxia, which is exacerbated by increased blood viscosity from erythrocytosis (due to increased erythropoietin production), both of which lead to tissue microthrombosis. In addition to poor wound healing, smoking increases the risk of developing surgical site infections, along with decreasing the quality of scar formation following surgery [Pluvy et al. 2015]. Another study examined the detrimental effects of smoking on wound healing suggested that toxins in tobacco such as nicotine, carbon monoxide, and hydrogen cyanide are responsible for decreased tissue oxygenation, decreased fibroblast activity and collagen synthesis [McDaniel and Browning, 2014]. Furthermore, these toxins seem to impact the immune system by decreasing proliferation of white blood cells responsible for clearing pathogens, decreasing lymphocyte function, decreasing cytotoxity of natural killer cells and diminished bacteriocidal activity of neutrophils, and finally impaired epithelialization [McDaniel and Browning, 2014; Torres et al. 2013].
In addition to contributing to wound infections, smoking is an independent risk factor for developing pneumonia due to its harmful effects on the respiratory epithelium and impairment of the mucociliary clearance of bacteria from the respiratory tract [Torres et al. 2013]. In addition, it predisposes patients to developing COPD, which can further render patients susceptible to pneumonia [Torres et al. 2013].
Ultimately, as physicians we can offer smoking cessation options to our patient population such as pharmacotherapy and counseling to reduce the financial and socioeconomic burden of readmissions imposed to healthcare systems following surgery. There is significant evidence supporting the use of short-term smoking cessation strategies such as nicotine replacement therapy to decrease postoperative morbidity, however the optimal timeline of smoking cessation prior to surgery still remains unknown. Preoperative smoking cessation interventions should begin at least 6 weeks prior to surgery to decrease the risk of perioperative complications and postoperative morbidity [Thomsen et al. 2014]. It is important to recognize that there is no proof from our data that counseling patients in smoking cessation prior to the procedure may limit readmissions due to infectious complications. It seems logical that patients should be counseled on smoking cessation based on extrapolation of our data findings and from elucidation of the results presented in the Cochrane review [Thomsen et al. 2014]; however, our data do not explicitly support this conclusion. Our data do reveal that current smokers are at higher risk for readmission due to an infectious cause. It remains to be seen if smoking cessation intervention preoperatively would result in lower infectious rates following RC and is certainly an objective worth analyzing in prospective studies.
There were several limitations to our study. We elected to focus on the 2012 dataset based on having reviewed institutional data from that calendar year with hopes to improve outcomes at our local institution. We are currently performing further studies including all NSQIP years and for other urologic surgeries to validate the reliability and global applicability of our findings. Our decision to focus on the 2012 dataset led to a decreased sample size for our study, ultimately yielding less statistical power to the study. As more hospitals are included in the NSQIP database, we also hope to expand our study to include a greater sample size and patient population to obtain more statistical power. We note that the NSQIP dataset does not include several other potential modifiable variables, including usage and duration of ureteral stents, antibiotic choice and duration, case volume, final pathologic staging, and bowel preparation, which may limit the applicability of this analysis. Another major limitation of our study was that we failed to classify the infectious complications into hospital acquired versus nonhospital acquired or by organ systems. There were too few overall infectious complications to substratify the data, and there were other institutional (or surgeon) variables which are not included in these datasets. These variables include duration of ureteral stents, types of perioperative antibiotic usage, specific type of urinary diversion performed, pathology of the specimen, chlorohexidine gluconate or betadine skin preparation, patient temperature at the conclusion of the procedure, utility of early mobilization and incentive spirometry as these variables may all have an impact on perioperative infectious complications. It is also important to note that as infectious complications are likely higher following RC, consequently, some of these wound infections are treated on an outpatient basis. Another potential limitation to this study is that due to lack of pathology included in the dataset, we are unable to differentiate if hypoalbuminemia is due to poor nutrition or an advanced or aggressive primary lesion. Finally, while the NSQIP dataset does provide broad complication-based information on a spectrum of surgical procedures, these data may not be directly applicable to cystectomy alone. In fact, other groups have advocated surgery-specific datasets to provide the most robust data in complex procedures [Epelboym et al. 2014]. Prospective studies should further delineate the exact microbiologic infections and potentially the speciation of bacteria leading to infectious complications following RC. However, the NSQIP dataset is not equipped to provide this information and carries no detail concerning the bacterial species from the entered data. Further research from this multi-institutional collaboration of authors should focus on providing follow-up data to this initial study, and these may include speciation of bacteria and full susceptibility analysis.
Conclusion
Readmissions following major urologic surgery are a significant health burden. Smoking may be a potential risk factor for infection-related readmission. Preoperative counseling on smoking cessation should be encouraged in perioperative care pathways designed to achieve early recovery after surgical procedures and prevent readmission.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: We declare that this study does not represent any conflict of interest for any of the authors. We reaffirm that no intramural or extramural funding sources were involved in this study.
Contributor Information
Sij Hemal, Wake Forest School of Medicine, Department of Urology, Medical Center Boulevard, Winston Salem, NC 27106, USA.
Louis S. Krane, Department of Urology, Wake Forest Baptist Health, Winston Salem, NC, USA
Kyle A. Richards, Department of Urology, University of Wisconsin School of Medicine, Madison, WI, USA
Michael Liss, Department of Urology, University of Texas Health Science Center, San Antonio, TX, USA.
A. Karim Kader, Department of Urology, University of California at San Diego, La Jolla, CA, USA.
Ronald L. Davis, III, Department of Urology, Wake Forest Baptist Health, Winston Salem, NC, USA.
References
- Epelboym I., Gawlas I., Lee J., Schrope B., Chabot J., Allendorf J. (2014) Limitations of ACS-NSQIP in reporting complications for patients undergoing pancreatectomy: underscoring the need for a pancreas-specific module. World J Surg 38: 1461–1467. [DOI] [PubMed] [Google Scholar]
- Ingraham A., Richards K., Hall B., Ko C. (2010) Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg 44: 251–267. [DOI] [PubMed] [Google Scholar]
- Lavallée L., Schramm D., Witiuk K., Mallick R., Fergusson D., Morash C., et al. (2014) Peri-operative morbidity associated with radical cystectomy in a multicenter database of community and academic hospitals. PLoS One 9: e111281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel J., Browning K. (2014) Smoking, chronic wound healing, and implications for evidence-based practice. J Wound Ostomy Continence Nurs 41: 415–423; quiz E1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietzak E., Hwang W., Malkowicz S., Guzzo T. (2014) Factors influencing the length of stay after radical cystectomy: implications for cancer care and perioperative management. Ann Surg Oncol 21: 4383–4389. [DOI] [PubMed] [Google Scholar]
- Pluvy I., Garrido I., Pauchot J., Saboye J., Chavoin J., Tropet Y., et al. (2015) Smoking and plastic surgery, part I. Pathophysiological aspects: update and proposed recommendations. Ann Chir Plast Esthétique 60: e3–e13. [DOI] [PubMed] [Google Scholar]
- Shabsigh A., Korets R., Vora K., Brooks C., Cronin A., Savage C., et al. (2009) Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 55: 164–174. [DOI] [PubMed] [Google Scholar]
- Siegel R., Ma J., Zou Z., Jemal A. (2014) Cancer statistics, 2014. CA Cancer J Clin 64: 9–29. [DOI] [PubMed] [Google Scholar]
- Skolarus T., Jacobs B., Schroeck F., He C., Helfand A., Helm J., et al. (2014) Understanding readmission intensity after radical cystectomy. J Urol 193: 1500–1506. [DOI] [PubMed] [Google Scholar]
- Sudarshan M., Feldman L., St Louis E., Al-Habboubi M., Hassan M., Fata P., et al. (2015) Predictors of mortality and morbidity for acute care surgery patients. J Surg Res 193: 868–873. [DOI] [PubMed] [Google Scholar]
- Thomsen T., Villebro N., Møller A. (2014) Interventions for preoperative smoking cessation. Cochrane Database Syst Rev 3: CD002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A., Peetermans W., Viegi G., Blasi F. (2013) Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax 68: 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick E., Shore A., Hirose K., Ibrahim A., Gearhart S., Efron J., et al. (2011) Readmission rates and cost following colorectal surgery. Dis Colon Rectum 54: 1475–1479. [DOI] [PubMed] [Google Scholar]