Abstract
Myofibroblasts and extracellular matrix are important components in wound healing. Alpha-smooth muscle actin (α-SMA) is a marker of myofibroblasts. Fibrillin-1 is a major constituent of microfibrils and an extracellular-regulator of TGF-β1, an important cytokine in the transdifferentiation of resident fibroblasts into myofibroblasts. To study the correlation between changes in fibrillin-1 expression and myofibroblast differentiation, we examined alterations in fibrillin-1 and α-SMA expression in organotypic cultures of dental pulp in vitro. Extracted healthy human teeth were cut to 1-mm-thick slices and cultured for 7 days. In intact dental pulp, fibrillin-1 was broadly distributed, and α-SMA was observed in pericytes and vascular smooth muscle cells. After 7 days of culture, immunostaining for fibrillin-1 became faint concomitant with a downregulation in its mRNA levels. Furthermore, fibroblasts, odontoblasts and Schwann cells were immunoreactive for α-SMA with a significant increase in α-SMA mRNA expression. Double immunofluorescence staining was positive for pSmad2/3, central mediators of TGF-β signaling, and α-SMA. The administration of inhibitors for extracellular matrix proteases recovered fibrillin-1 immunostaining; moreover, fibroblasts lost their immunoreactivity for α-SMA along with a downregulation in α-SMA mRNA. These findings suggest that the expression of α-SMA is TGF-β1 dependent, and fibrillin-1 degradation and downregulation might be implicated in the differentiation of myofibroblasts in dental pulp wound healing.
Keywords: α-SMA, culture, fibrillin-1, human dental pulp, pSmad2/3
Introduction
Fibrillin-1 is the main structural component of extracellular microfibrils. It is ubiquitously distributed in a wide variety of tissues and plays a critical role in the maintenance of connective tissue architecture through its involvement in elastic fiber formation (Ramirez et al. 2004). Mutations in the fibrillin-1 gene cause Marfan syndrome, a systemic disorder of the connective tissue (Bolar et al. 2012) that is also associated with abnormal dental pulp chambers (De Coster et al. 2004) and severe periodontal disease (Suda et al. 2009). Although fibrillin-1 is a structural component, it contributes to the extracellular regulation of transforming growth factor-β1 (TGF-β1) storage, release, and activation (Ramirez and Sakai 2010; Massam-Wu et al. 2010). Fibrillin-1 is highly susceptible to proteolytic degradation by serine proteases (Kielty et al. 1994) and several matrix metalloproteinases (MMPs) (Ashworth et al. 1999). Moreover, the breakdown of fibrillin-1-containing microfibrils may be a common mechanism for the release of active TGF-β1 that is sequestered in microfibrils (Chaudhry et al. 2007).
Alpha-smooth muscle actin (α-SMA) is a cytoskeletal protein that is expressed in certain types of stem cells and precursor cells (Kinner et al. 2002), as well as in pericytes and smooth muscle cells of blood vessels (Morikawa et al. 2002). During tissue repair and regeneration following injury, α-SMA-positive fibroblasts, termed myofibroblasts, appear in various tissues (Chaponnier et al. 2004). The expression of α-SMA in myofibroblasts has been shown to enhance the contractile activity of the cells (Hinz et al. 2001) to contribute to closure of the wounded tissue. In addition, myofibroblasts are thought to play a central role in wound healing by secreting various cytokines and growth factors as well as extracellular matrix and its degradative enzymes (Powell et al. 1999). It is generally accepted that the major source of myofibroblasts is local connective tissue fibroblasts (Higashiyama et al. 2011). TGF-β1 is the most important cytokine in the transdifferentiation of resident fibroblastic cells into the contractile, wound-healing myofibroblasts that express α-SMA (Vaughan et al. 2000; Serini and Gabbiani 1999). When TGF-β binds to its cognate receptors, the intracellular mediators Smad2/3 become phosphorylated (pSmad2/3), and this activated complex then translocates into the nucleus to initiate target gene transcription (Abdollah et al. 1997). pSmad2/3 is then exported to the cytoplasm where it is finally degraded (Fukuchi et al. 2001).
Myofibroblasts and the extracellular matrix are important components in wound healing (Hinz 2007). Dental pulp is a soft connective tissue enclosed by mineralized dentin, and the pulp cells have a neural crest origin. The peripheral cells of the pulp are called odontoblasts, and these cells are directly associated with dentinogenesis. In intact human dental pulp, fibrillin-1 is broadly distributed (Yoshiba et al. 2012a), and α-SMA is expressed by pericytes and smooth muscle cells in blood vessels (Yoshiba et al. 2012b). The expression of fibrillin-1 and α-SMA is drastically altered during wound healing of human dental pulp tissue in vivo (Yoshiba et al. 2012a; Yoshiba et al. 2012b). At the wound edge at 2 weeks where α-SMA-positive myofibroblasts are transiently found, fibrillin-1 expression is absent, probably owing to protein degradation and mRNA downregulation (Yoshiba et al. 2012a; Yoshiba et al. 2012b). So far, fibrillin-1 is the only extracellular matrix component that disappears during wound healing of human dental pulp. The extracellular matrix components fibronectin, decorin, and latent TGF-β-binding protein (LTBP)-1 are known to act as TGF-β1 reservoirs (Doyle et al. 2012). These components are broadly distributed in human dental pulp and their localization patterns are similar to that of fibrillin-1 under normal conditions. However, during dental pulp tissue wound healing, fibronectin, decorin, LTBP-1, and two other components—the extracellular matrix glycoprotein tenascin-C and the fibrillin-1 isoform fibrillin-2—are all constantly expressed, even at the wound edge where fibrillin-1 expression is absent (Yoshiba et al. 1996, 2012a, 2013). Thus, the loss of fibrillin-1 is considerably specific.
Taken together, we hypothesized that fibrillin-1 degradation and mRNA downregulation may correlate with the transdifferentiation of fibroblasts into α-SMA-positive myofibroblasts during dental pulp wound healing. The tooth slice organ culture technique has been applied to investigate the factors regulating pulp tissue repair (Magloire et al. 1996; Sloan and Smith 1999; Sloan and Lynch 2012), as it allows for the maintenance of tissue morphology together with odontoblast phenotype. We thus designed the present study to address the hypothesis by examining alterations in the expression of fibrillin-1 and α-SMA using the tooth slice organ culture model. Moreover, double-labeling immunofluorescence was used to detect α-SMA and pSmad2/3 to evaluate the involvement of the TGF-β/Smad signaling pathway in myofibroblast differentiation in dental pulp tissue.
Materials & Methods
This study was approved by the Niigata University Ethics Committee (No. 21-R17-09-10). Human healthy permanent teeth were obtained from young patients (18–25 years old) undergoing orthodontic treatment, who had provided informed consent.
Tissue Culture of Dental Pulp
Twenty-eight intact third molars were used in this study. After extraction under local anesthesia, the whole tooth was cut with a low speed diamond wheel saw (South Bay Technology; San Clemente, CA) at a thickness of 1 mm. The explants were rinsed with Hank’s solution (Gibco; Paisley, Scotland) containing antibiotics and then placed on filter inserts (Millicell-CM; 0.4-µm pore size, 30-mm diameter; Millipore, Billerica, MA) in 12-well plates containing 1.5 ml culture medium per well. The culture medium consisted of Dulbecco’s modified Eagle’s medium (Gibco) containing 10 IU/ml penicillin, 10 µg/ml streptomycin (Gibco), and 50 µg/ml ascorbic acid (Sigma-Aldrich; St Louis, MO). The surfaces of the tissue slices were wet but not submerged. The explants were incubated at 37°C in a humidified incubator with 5% CO2. Some slices were treated with freshly prepared protease inhibitors including a matrix metalloproteinase inhibitor (4-Abz-Gly-Pro-D-Leu-D-Ala-NH-OH, which inhibits MMP-1, -3, -8, and -9; Merck Biosciences; Kenilworth, NJ) at 150 µM (Chaudhry et al. 2007) and a P1860 protease inhibitor cocktail (Sigma-Aldrich) with broad specificity, including serine and cysteine proteases. A total of 36 replicate samples were assayed per experiment (with or without protease inhibitors). Sixteen replicate samples were treated with 20 µM SB431542 (Cayman Chemical; Ann Arbor, MI), an inhibitor of the TGF-β type I receptor. The culture medium was replaced every 3 days, and the cultures were maintained for 7 days. Experiments were repeated more than five times.
Immunohistochemical Staining
Cultured teeth were fixed with 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4) at 4°C for 24 hr and then decalcified in 10% EDTA (pH 7.4) for 1 week at 4°C. The specimens were embedded in optimal cutting temperature (OCT) compound (Sakura; Tokyo, Japan), frozen in liquid nitrogen, and then cut as 10-µm-thick frozen sections that were mounted onto poly-L-lysine-coated slides (Matsunami; Tokyo, Japan). To detect pSmad2/3, the pulp tissue was separated from the dentin with a scalpel and embedded in OCT compound at 1, 2, 3, 4, 5 and 7 days of culture. Frozen sections (10-µm thick) were prepared and fixed in acetone for 10 min at 4°C.
Indirect immunoperoxidase staining was performed on cryosections. After treating the sections with 0.3% H2O2 in PBS for 30 min at room temperature, sections were incubated with primary antibodies for 2 hr followed by incubation with the corresponding horseradish peroxidase-conjugated secondary antibody for 1 hr. Immune complexes were visualized using 3,3’-diaminobenzidine (S3000; Dako, Glostrup, Denmark). Immunostained sections were then counterstained with methyl green. Negative control staining was performed by replacing the primary antibodies with nonimmune mouse, rabbit, or goat IgG (Santa Cruz Biotechnology; Dallas, TX). For double immunofluorescence staining, sections were incubated with a cocktail of antibodies against pSmad2/3 and α-SMA, followed by incubation with a mixture of secondary antibodies. The following antibodies were used: mouse anti-human fibrillin-1 (clone 26; MAB2502; Chemicon, Temecula, CA); mouse anti-α-SMA (clone 1A4; A5228; Sigma-Aldrich), goat anti-human TGF-β1 (AB246NA; R&D Systems, Minneapolis, MN), and rabbit anti-human pSmad2/3 (SC11769; Santa Cruz Biotechnology) primary antibodies, and horseradish peroxidase (HRP)-labeled swine anti-rabbit IgG (Dako), HRP-labeled rabbit anti-goat IgG (Dako), HRP-labeled rabbit anti-mouse IgG (Dako), and goat anti-rabbit IgG secondary antibodies conjugated to Alexa Fluor 488 (Invitrogen; Carlsbad, CA), and a goat anti-mouse IgG secondary antibody conjugated to Alexa Fluor 546 (Invitrogen). Control sections did not show any specific immunoreactivity.
Quantitative RT-PCR Analysis
Total RNA was isolated from cultured human dental pulp using an RNeasy Plus Micro kit (Qiagen; Hilden, Germany) according to the manufacturer’s instructions. Quantitative RT-PCR was carried out using a One Step SYBR PrimeScript PLUS RT-PCR kit (Takara Bio; Shiga, Japan) with the Opticon Real-Time PCR System (MJ Research, Inc., Waltham, MA). The primer sets used for quantitative RT-PCR were as follows: fibrillin-1 (sense: 5’-GGAACGTGAAGGAAACCAGA-3’, antisense: 5’-GGCAAATGGGGACAATACAC-3’), α-SMA (sense: 5’-TGTTCCAGCCATCCTTCATC-3’, antisense: 5’-TAGGGCCGTGATCTCCTTCT-3’), TGF-β1 (sense: 5’-CACGTGGAGCTGTACCAGAA-3’, antisense: 5’-CTA AGGCGAAAGCCCTCAA-3’), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (sense: 5’-ACACCCACTCCTCCACCTTT-3’, antisense: 5’-TTCCTCTTGTGCTCTTGC TG-3’). mRNA expression levels were normalized to the GAPDH mRNA levels. Data were expressed as the mean ± SD (n=3). Statistical significance between two groups was evaluated using the Student’s t-test.
Results
Changes in the Protein Localization of Fibrillin-1 and α-SMA in Cultured Dental Pulp Tissue
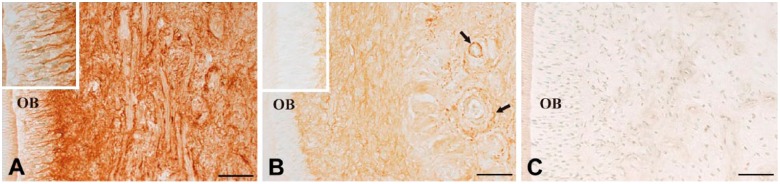
In uncultured normal dental pulp, fibrillin-1 was broadly and densely detected in the dental pulp (Fig. 1A), including within the interodontoblastic area (Fig. 1A, insert). The distribution of fibrillin-1 was altered after 7 days of culture. Fibrillin-1 staining became rather faint in the dental pulp, but it was partially maintained around the blood vessels (Fig. 1B, arrows). No staining for fibrillin-1 was detectable in the interodontoblastic area after 7 days of culture (Fig. 1B, insert). A negative control section did not show any specific immunoreactivity (Fig. 1C).
Figure 1.
Immunohistochemical staining for fibrillin-1 in uncultured normal pulp (A) and after 7 days of culture (B). In uncultured normal pulp, fibrillin-1 is densely detected in the dental pulp (A) and within the interodontoblastic area (A, inset). In contrast, after 7 days of culture, fibrillin-1 staining becomes rather faint (B), except around blood vessels (B, arrows) and the interodontoblastic area is devoid of fibrillin-1 (B, insert). A negative control section shows no specific immunoreactivity (C). OB, odontoblast. Scale, 50 µm.
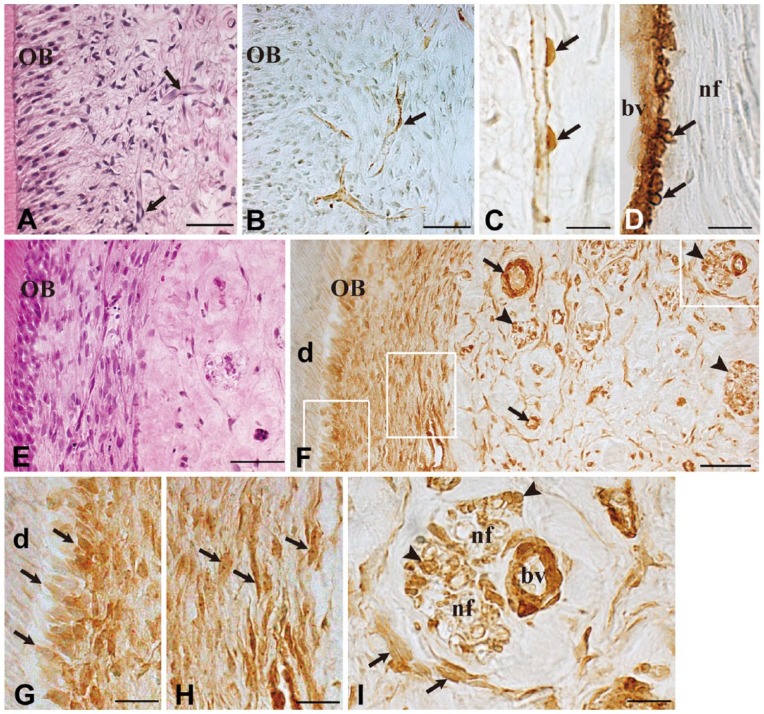
Capillaries were observed in the periphery of the uncultured normal dental pulp (Fig. 2A, arrows). Immunoreactions for α-SMA were obvious in pericytes along the capillaries (Fig. 2B, arrow). Pulp fibroblasts and odontoblasts showed no immunoreactions for α-SMA (Fig. 2B). At a higher magnification, α-SMA-reactive pericytes were evident along the capillary (Fig. 2C, arrows), and vascular smooth muscle cells were also immunoreactive to α-SMA (Fig. 2D, arrows). Nerve fibers were devoid of positive α-SMA immunoreaction (Fig. 2D). After 7 days of culture, numerous fibroblasts were seen in the subodontoblast layer (Fig. 2E). The distribution of α-SMA-positive cells was markedly altered after 7 days of culture. Fibroblasts (Fig. 2F–2I) and odontoblasts (Fig. 2F–2G) showed positive immunoreactions for α-SMA. In addition, α-SMA was detectable along nerve fibers (Fig. 2F, arrowheads). At a higher magnification, positive α-SMA staining was noticed in Schwann cells of nerve fibers (Fig. 2I, arrowheads) as well as in fibroblasts (Fig. 2I, arrows).
Figure 2.
Immunohistochemical staining for α-SMA in uncultured normal pulp and after 7 days of culture. Hematoxylin-eosin staining (A, E) and immunostaining for α-SMA (B–D, F–I) in uncultured normal tissue (A–D) and after 7 days of culture (E–I). Higher magnification views of the boxed areas in (F) are shown in (G), (H) and (I), respectively. In uncultured normal pulp, capillaries are observed in the periphery of the dental pulp (A, arrows), and immunoreactions for α-SMA are obvious in pericytes (B and C, arrows) and vascular smooth muscle cells (D, arrows). After 7 days of culture, α-SMA immunoreactivity can be recognized in various cell types (F, arrows: blood vessels; arrowheads: nerve fibers) (F) including odontoblasts (G, arrows), fibroblasts (H and I, arrows), and Schwann cells (I, arrowheads). OB, odontoblast; d, dentin; bv, blood vessel; nf, nerve fiber. Scale (A–B, E–F) 50 µm; (C–D, G–I) 20 µm.
Protease Inhibitors Suppress Fibrillin-1 Degradation and α-SMA Expression
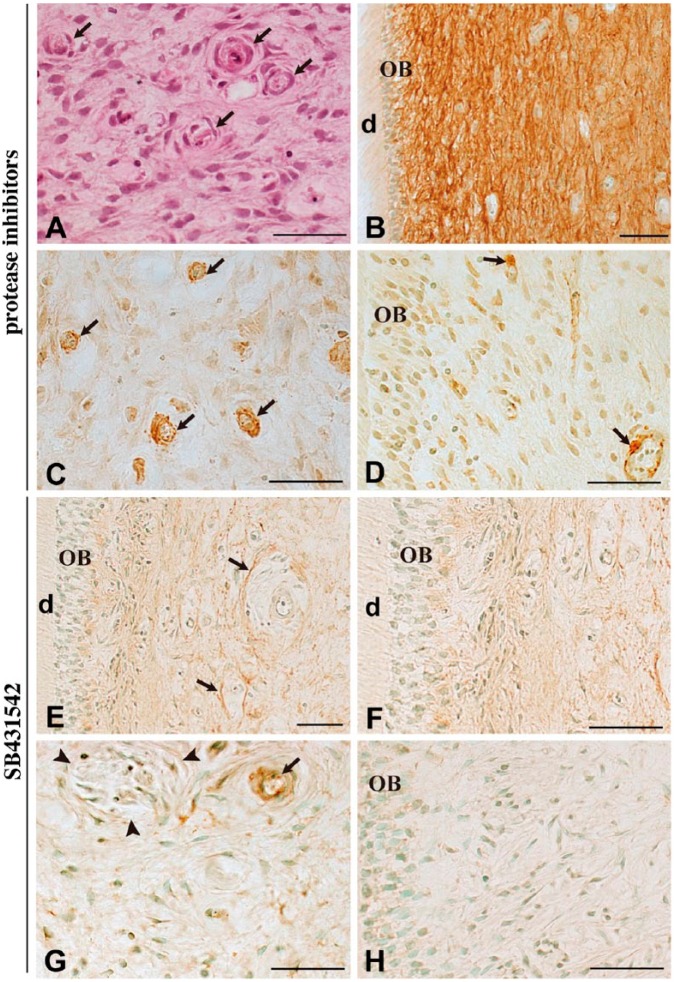
Next, we investigated the possible relationship between the decrease in fibrillin-1 immunoreactivity and the emergence of α-SMA-positive myofibroblasts by treatment with protease inhibitors. Using hematoxylin-eosin staining, we observed that the tissue morphology seemed to be maintained at 7 days when cultured with protease inhibitors (Fig. 3A). The administration of protease inhibitors recovered fibrillin-1 immunoreactivity (Fig. 3B). The protease inhibitors also induced changes in α-SMA expression, with little or no immunoreactivity detected in fibroblasts (Fig. 3C) and odontoblasts (Fig. 3D). Cells along the blood vessels remained positive for α-SMA irrespective of the addition of protease inhibitors (Fig. 3C–3D, arrows).
Figure 3.
Immunohistochemical staining for fibrillin-1 and α-SMA after 7 days of culture in the presence of protease inhibitors (4-Abz-Gly-Pro-D-Leu-D-Ala-NH-OH and P1860) or TGF-β type I receptor inhibitor (SB431542). Hematoxylin-eosin staining (A) and immunostaining for fibrillin-1 (B, E–F) and α-SMA (C, D). The tissue morphology in the presence of protease inhibitors appears to be maintained, even in blood vessels (A, arrows). Administration of protease inhibitors prevents the loss of fibrillin-1 immunoreactivity (B) and little immunoreactivity for α-SMA is noted in fibroblasts (C) and odontoblasts (D). Cells along the blood vessels remain positive for α-SMA (C–D, arrows). In the presence of SB431542, fibrillin-1 staining becomes rather faint except around the blood vessels (E, arrows), and the interodontoblastic area is devoid of fibrillin-1 (F). Most dental pulp cells are negative for α-SMA (G–H, arrowheads in G: nerve fiber), except along the blood vessel (G, arrow). OB, odontoblast; d, dentin. Scale, 50 µm.
SB431542 Suppresses α-SMA Expression
SB431542 is a potent and specific inhibitor of TGF-β type I receptor and is subsequently effective in inhibiting the phosphorylation of Smad2 (Inman et al. 2002). SB431542 did not affect the staining pattern of fibrillin-1 after 7 days of culture, where fibrillin-1 staining became rather faint, but it was partially maintained around the blood vessels (Fig. 3E, arrows). No staining for fibrillin-1 was detectable in the interodontoblastic area (Fig. 3F). In the presence of SB431542, most dental pulp cells, including Schwann cells (Fig. 3G) and odontoblasts (Fig. 3H), were negative for α-SMA. Cells along the blood vessels remained positive for α-SMA (Fig. 3G).
Changes in the Gene Expression of Fibrillin-1 and α-SMA in Cultured Pulp Tissue
The mRNA expression levels of fibrillin-1 were significantly decreased at 3 and 7 days of culture as compared with those in uncultured tissues (Fig. 4). After 7 days of culture in the presence of protease inhibitors or SB431542, fibrillin-1 mRNA levels were significantly decreased in comparison to untreated control cultures (Fig. 4). α-SMA levels at 3 days were not significantly different from that of uncultured tissue, irrespective of the addition of protease inhibitors (Fig. 4). The mRNA expression levels of α-SMA were increased by almost 3.5-fold at 7 days of culture as compared with those in uncultured tissues (Fig. 4), which is consistent with the increased immunoreactivity for α-SMA. Treatment of the dental pulp tissue with protease inhibitors led to a decrease in α-SMA mRNA levels in comparison to untreated control cultures at 7 days (Fig. 4). Furthermore, in the presence of SB431542, α-SMA mRNA levels were decreased to nearly one-eighth that of untreated control cultures at 7 days (Fig. 4).
Figure 4.
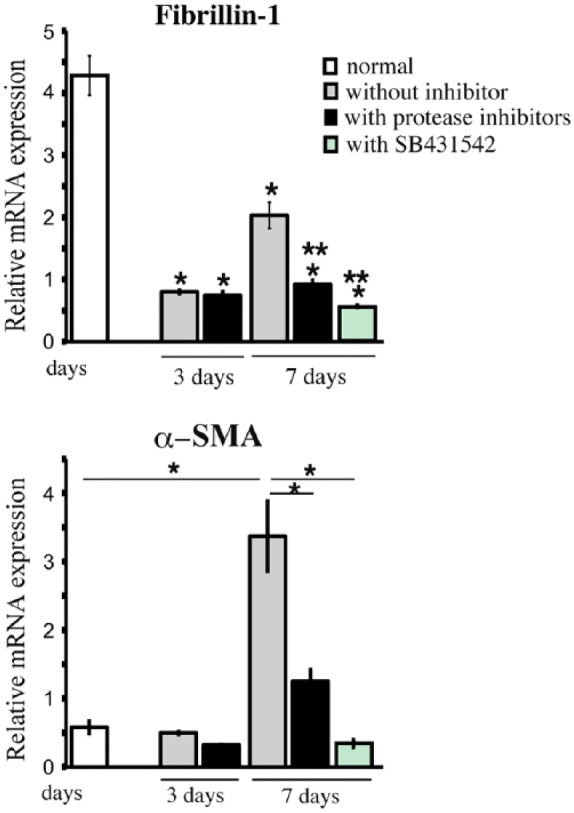
Quantitative RT-PCR analysis of fibrillin-1 and α-SMA. Fibrillin-1 mRNA levels decrease by almost half at 7 days of culture as compared with that in uncultured tissues. In contrast, α-SMA mRNA levels are significantly increased at 7 days of culture as compared with that in uncultured tissues, and this increase is significantly attenuated by protease inhibitors (4-Abz-Gly-Pro-D-Leu-D-Ala-NH-OH and P1860) and by the inhibitor of TGF-β type I receptor (SB431542). mRNA expression levels were normalized to GAPDH mRNA levels. Significant difference against uncultured normal (*) and day 7 (**) without inhibitors in fibrillin-1 mRNA expression. *p<0.05.
Immunohistochemical Staining and mRNA Expression of TGF-β1
Immunoreactivity for TGF-β1 was broadly detected in uncultured normal dental pulp (Fig. 5A). TGF-β1 mRNA levels were significantly decreased after 2 and 3 days of culture as compared with those in uncultured dental pulp (Fig. 5B). After 7 days, TGF-β1 mRNA levels were not significantly different in cultured tissues as compared with those in uncultured tissues (Fig. 5B). Protease inhibitors did not significantly affect the expression levels of TGF-β1 mRNA.
Figure 5.
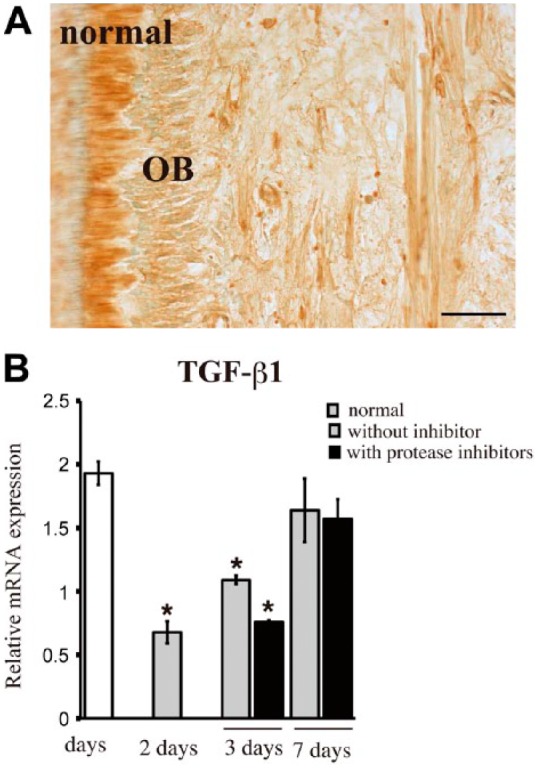
Immunohistochemical staining and quantitative RT-PCR analysis of TGF-β1. TGF-β1 is broadly observed in uncultured normal pulp (A). TGF-β1 mRNA levels are significantly decreased at 2 and 3 days (B). Protease inhibitors (4-Abz-Gly-Pro-D-Leu-D-Ala-NH-OH and P1860) do not affect TGF-β1 mRNA levels significantly (B). TGF-β1 mRNA expression levels normalized to GAPDH mRNA levels. OB, odontoblast. *p<0.05 compared with uncultured normal pulp. Scale, 50 µm.
Immunohistochemical Detection of pSmad2/3 in Cultured Dental Pulp
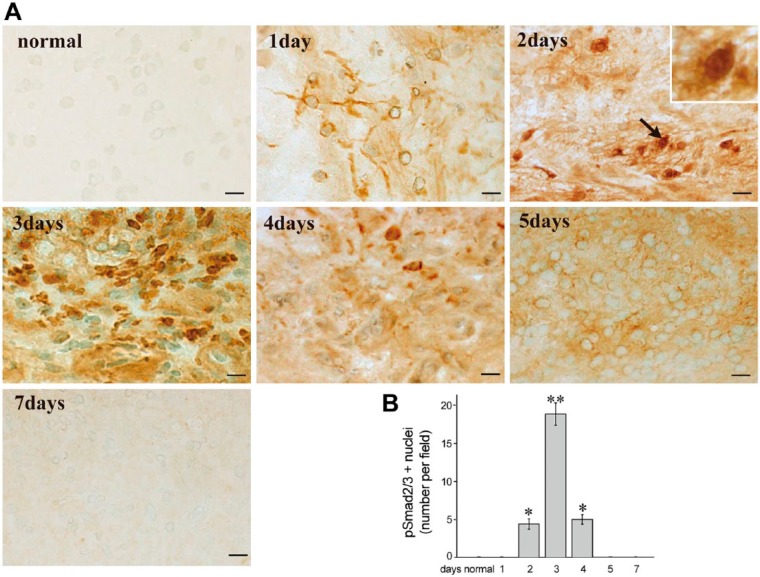
Immunocytochemistry showed no staining for pSmad2/3 in uncultured normal pulp (Fig. 6A). Time-dependent translocation of pSmad2/3 was detectable in the cytoplasm at 1 day and in the cell nucleus at 2, 3, and 4 days of culture (Fig. 6A). At 5 days, pSmad2/3 was diffusely localized in the cytoplasm (Fig. 6A), and no immunoreactivity was observed for pSmad2/3 at 7 days (Fig. 6A). The number of pSmad2/3-positive nuclei was the highest at 3 days of culture (Fig. 6B).
Figure 6.
Immunohistochemical staining of pSmad2/3 in cultured pulp tissue without protease inhibitors and the mean number of pSmad2/3-positive nuclei. (A) Time-dependent translocation of pSmad2/3 is observed in the cytoplasm at 1 day and into the nucleus at 2, 3, and 4 days. At 5 days, pSmad2/3 is diffusely noted in the cytoplasm but no immunoreactivity for pSmad2/3 is observed at 7 days. The number of pSmad2/3-positive nuclei peaks at 3 days of culture. (B) The mean number of pSmad2/3-positive nuclei per field (×400) ± SE. n=4. *p<0.05 and **p<0.01 compared with uncultured normal pulp. Scale, 10 µm.
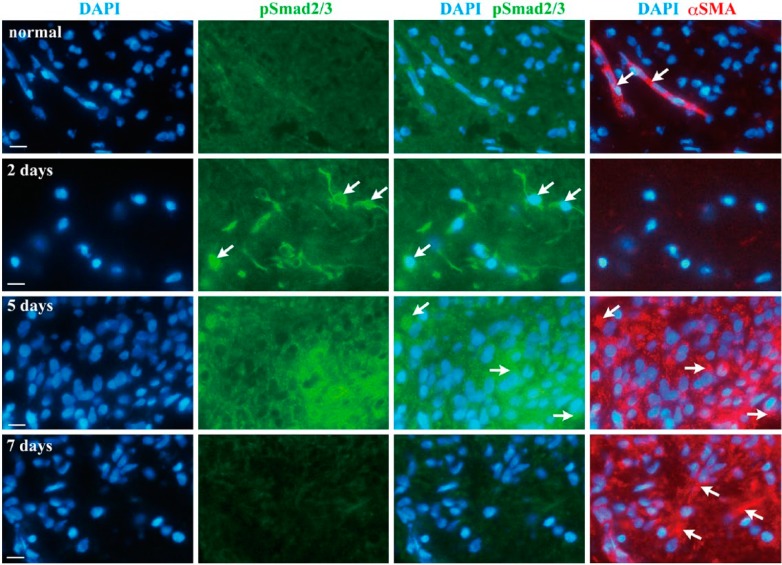
Double-labeling Immunofluorescence for pSmad2/3 and α-SMA
As shown by immunohistochemical analysis, specific immunofluorescence reactivity of pSmad2/3 was not found in uncultured normal pulp (Fig. 7), and α-SMA was detected only along blood vessels (Fig. 7, arrows in normal). At 2 days of culture, a nuclear accumulation of pSmad2/3 was detectable (Fig. 7, arrows in 2 days), yet the pSmad2/3-positive cells were negative for α-SMA (Fig. 7). pSmad2/3 and α-SMA double-positive cells were evident at 5 days (Fig. 7, arrows in 5 days). Thereafter, immunofluorescence staining for pSamd2/3 was decreased and α-SMA single-positive cells were abundantly observed at 7 days (Fig. 7, arrows in 7 days).
Figure 7.
Double-labeling immunofluorescence for pSmad2/3 and α-SMA in cultured pulp tissue without protease inhibitors. Nuclei (DAPI, blue), pSmad2/3 (green) and α-SMA (red) are shown in uncultured normal pulp at 2, 5 and 7 days of culture. Nuclear accumulation of pSmad2/3 is detectable at 2 days (arrows); thereafter, pSmad2/3 and α-SMA double-positive cells are evident at 5 days (arrows). α-SMA single-positive cells are abundantly observed at 7 days (arrows). Scale, 10 µm.
Discussion
Our data shows that fibroblasts and odontoblasts, as well as Schwann cells, in organotypic cultures exhibit immunoreactivity for α-SMA and a significant increase of α-SMA mRNA. These cells in dental pulp (Sloan et al. 2001) as well as Schwann cells (Stark et al. 2001) have been shown to express TGF-β receptors I and II. TGF-β1 is a direct inducer of the transdifferentiation of resident fibroblastic cells (Serini and Gabbiani 1999) and Schwann cells (Real et al. 2005) into α-SMA-expressing myofibroblasts. A highly significant increase in the number of pSmad2/3-positive nuclei was detected at 3 days of culture and, thereafter, an emergence of pSmad2/3 and α-SMA double-positive cells. Moreover, the increased expression of α-SMA was abolished by SB431542, a specific inhibitor of TGF-β type I receptor. Taken together, these observations suggest the activation of Smad-dependent TGF-β signaling pathways in the transdifferentiation of dental pulp cells into α-SMA-positive myofibroblasts in cultured dental pulp tissue.
There was a significantly higher number of cells with pSmad2/3 nuclear translocation at 3 days of culture, but not at 1, 5 or 7 days. TGF-β1 treatment significantly stimulates the nuclear translocation of pSmad2/3 at 60 min in rat pulmonary arterial smooth muscle cells, but such translocation decreases by 6 hr (Li et al. 2007). The rapid translocation of Smad proteins into the nucleus upon TGF-β1 treatment has also been shown in the mouse odontoblast cell line MDPC-23 (He et al. 2004). Therefore, in the present study, TGF-β1 stimulation may have been evoked between 2 and 4 days, with the highest stimulation on day 3. Meanwhile, TGF-β1 mRNA levels were significantly downregulated at 2 and 3 days.
The transdifferentiation of dental pulp cells into α-SMA-positive myofibroblasts was associated with fibrillin-1 degradation and mRNA downregulation. Matrix sequestration of cytokines is crucial for their regulated activation and signaling. TGF-β is latent when sequestered in the extracellular matrix and its activation, for example, by specific integrins, is a key step in the transdifferentiation process (Buscemi et al. 2011; Shi et al. 2011). Numerous studies have also highlighted the role of fibrillin-1 in the regulation of TGF-β bioavailability. Mice deficient in fibrillin-1 show increased TGF-β activity (Neptune et al. 2003) and active Smad2/3 signaling (Carta et al. 2009). The breakdown of fibrillin-1-containing microfibrils is proposed to be a common mechanism for the release of active TGF-β1 stored in microfibrils (Chaudhry et al. 2007). Fibrillin-1 is highly susceptible to proteolytic degradation. MMP-2, -3, -9, -12 and -13 cleave fibrillin-1 (Ashworth et al. 1999), and these MMPs are expressed in human dental pulp tissue (Palosaari et al. 2003; Wisithphrom and Windsor 2006). We found that protease inhibitors prevented the degradation of fibrillin-1 and, concomitantly, the downregulation of α-SMA expression. Meanwhile, the inhibitors did not significantly affect the expression levels of TGF-β1. An MMP knockout mouse model has suggested a crucial role of MMPs in the organization of the cellular actin network by liberation of cytokines from the extracellular matrix (Bullard et al. 1999). It thus can be speculated that TGF-β1 pooled in the extracellular matrix might have contributed to the increased expression of α-SMA in the present study. Moreover, TGF-β1 has been shown to increase fibrillin-1 expression (Lorena et al. 2004), which would explain the increase in fibrillin-1 mRNA between days 3 and 7 in the context of extracellular matrix degradation. On the other hand, MMPs have been suggested to have direct and/or indirect signaling mechanisms involved in regulation of cyto-differentiation (Chen and Li 2009); therefore, we cannot exclude the unknown effects behind MMPs on myofibroblast differentiation.
The dentine matrix is secreted by odontoblasts, which are highly differentiated cells of the pulp. During wound healing of dental pulp in vivo, newly differentiating and differentiated odontoblast-like cells transiently express α-SMA, some of which co-expressed nestin, a neurogenic cytoskeletal protein expressed in odontoblasts (Yoshiba et al. 2012b). In the present organ culture model, odontoblasts were showed α-SMA positivity at 7 days of culture, which were also stained with nestin (data not shown). Currently, it is not clear how the α-SMA-positive odontoblasts are involved in the dental pulp healing process. However, considering the multiple roles of myofibroblasts in wound healing (Powell et al. 1999), they may secret extracellular matrix proteases that degrade the dentine matrix to result in the release of growth factors and cytokines from the matrix, thus yielding repair responses.
In summary, we here showed that fibroblasts and odontoblasts as well as Schwann cells express α-SMA via the pSmad2/3 signaling pathway in organotypic cultures, which involves degradation of fibrillin-1 protein and mRNA downregulation. We showed that the administration of protease inhibitors could suppress fibrillin-1 degradation and significantly attenuate the expression of α-SMA. These alterations to expression observed in the present study were well in accord with those of our previous in vivo studies, which analyzed the expression patterns of fibrillin-1 and α-SMA during wound healing processes in the dental pulp (Yoshiba et al. 2012a, 2012b). These findings therefore raise the possibility that fibrillin-1 degradation and downregulation might be implicated in the transdifferentiation of dental pulp cells into α-SMA-positive myofibroblasts in dental pulp wound healing.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant-in-aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan (no. 22592119, 25462952, and 24592863).
References
- Abdollah S, Macías-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana JL. (1997). TbetaRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. J Biol Chem 272:27678-27685. [DOI] [PubMed] [Google Scholar]
- Ashworth JL, Murphy G, Rock MJ, Sherratt MJ, Shapiro SD, Shuttleworth CA, Kielty CM. (1999). Fibrillin degradation by matrix metalloproteinases: implications for connective tissue remodelling. Biochem J 340:171-181. [PMC free article] [PubMed] [Google Scholar]
- Bolar N, Van Laer L, Loeys BL. (2012). Marfan syndrome: from gene to therapy. Curr Opin Pediatr 24:498-504. [DOI] [PubMed] [Google Scholar]
- Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, Ronan J, Werb Z, Banda MJ. (1999). Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg 230:260-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B. (2011). The single-molecule mechanics of the latent TGF-β1 complex. Curr Biol 21:2046-2054. [DOI] [PubMed] [Google Scholar]
- Carta L, Smaldone S, Zilberberg L, Loch D, Dietz HC, Rifkin DB, Ramirez F. (2009). p38 MAPK is an early determinant of promiscuous Smad2/3 signaling in the aortas of fibrillin-1 (Fbn1)-null mice. J Biol Chem 284:5630-5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaponnier C, Gabbiani G. (2004). Pathological situations characterized by altered actin isoform expression. J Pathol 204:386-395. [DOI] [PubMed] [Google Scholar]
- Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM. (2007). Fibrillin-1 regulates the bioavailability of TGF-β1. J Cell Biol 176:355-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li Y. (2009). Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adh Migr 3:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coster PJ, Martens LC, De Paepe A. (2004). Orofacial manifestations of congenital fibrillin deficiency: pathogenesis and clinical diagnostics. Pediatr Dent 26:535-537. [PubMed] [Google Scholar]
- Doyle JJ, Gerber EE, Dietz HC. (2012). Matrix-dependent perturbation of TGFβ signaling and disease. FEBS Lett 586:2003-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi M, Imamura T, Chiba T, Ebisawa T, Kawabata M, Tanaka K, Miyazono K. (2001). Ligand-dependent degradation of Smad3 by a ubiquitin ligase complex of ROC1 and associated proteins. Mol Biol Cell 12:1431-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He WX, Niu ZY, Zhao SL, Jin WL, Gao J, Smith AJ. (2004). TGF-beta activated Smad signalling leads to a Smad3-mediated down-regulation of DSPP in an odontoblast cell line. Arch Oral Biol 49:911-918. [DOI] [PubMed] [Google Scholar]
- Higashiyama R, Nakao S, Shibusawa Y, Ishikawa O, Moro T, Mikami K, Fukumitsu H, Ueda Y, Minakawa K, Tabata Y, Bou-Gharios G, Inagaki Y. (2011). Differential contribution of dermal resident and bone marrow-derived cells to collagen production during wound healing and fibrogenesis in mice. J Invest Dermatol 131:529-536. [DOI] [PubMed] [Google Scholar]
- Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. (2001). Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 12:2730-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. (2007). Formation and function of the myofibroblast during tissue repair. J Invest Dermatol 127:526-537. [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolás FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. (2002). SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 62:65-74. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Woolley DE, Whittaker SP, Shuttleworth CA. (1994). Catabolism of intact fibrillin microfibrils by neutrophil elastase, chymotrypsin and trypsin. FEBS Lett 351:85-89. [DOI] [PubMed] [Google Scholar]
- Kinner B, Zaleskas JM, Spector M. (2002). Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp Cell Res 278:72-83. [DOI] [PubMed] [Google Scholar]
- Li P, Oparil S, Novak L, Cao X, Shi W, Lucas J, Chen YF. (2007). ANP signaling inhibits TGF-beta-induced Smad2 and Smad3 nuclear translocation and extracellular matrix expression in rat pulmonary arterial smooth muscle cells. J Appl Physiol 102: 390-398. [DOI] [PubMed] [Google Scholar]
- Lorena D, Darby IA, Reinhardt DP, Sapin V, Rosenbaum J, Desmoulière A. (2004). Fibrillin-1 expression in normal and fibrotic rat liver and in cultured hepatic fibroblastic cells: modulation by mechanical stress and role in cell adhesion. Lab Invest 84:203-212. [DOI] [PubMed] [Google Scholar]
- Magloire H, Joffre A, Bleicher F. (1996). An in vitro model of human dental pulp repair. J Dent Res 75:1971-1978. [DOI] [PubMed] [Google Scholar]
- Massam-Wu T, Chiu M, Choudhury R, Chaudhry SS, Baldwin AK, McGovern A, Baldock C, Shuttleworth CA, Kielty CM. (2010). Assembly of fibrillin microfibrils governs extracellular deposition of latent TGF beta. J Cell Sci 123:3006-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. (2002). Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 160:985-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. (2003). Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33:407-411. [DOI] [PubMed] [Google Scholar]
- Palosaari H, Pennington CJ, Larmas M, Edwards DR, Tjäderhane L, Salo T. (2003). Expression profile of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in mature human odontoblasts and pulp tissue. Eur J Oral Sci 111:117-127. [DOI] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. (1999). Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 277:C1-C9. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Sakai LY, Dietz HC, Rifkin DB. (2004). Fibrillin microfibrils: multipurpose extracellular networks in organismal physiology. Physiol Genomics 19:151-154. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Sakai LY. (2010). Biogenesis and function of fibrillin assemblies. Cell Tissue Res 339:71-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real C, Glavieux-Pardanaud C, Vaigot P, Le-Douarin N, Dupin E. (2005). The instability of the neural crest phenotypes: Schwann cells can differentiate into myofibroblasts. Int J Dev Biol 49:151-159. [DOI] [PubMed] [Google Scholar]
- Serini G, Gabbiani G. (1999). Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res 250:273-283. [DOI] [PubMed] [Google Scholar]
- Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. (2011). Latent TGF-β structure and activation. Nature 474:343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan AJ, Smith AJ. (1999). Stimulation of the dentine-pulp complex of rat incisor teeth by transforming growth factor-beta isoforms 1-3 in vitro. Arch Oral Biol 44:149-156. [DOI] [PubMed] [Google Scholar]
- Sloan AJ, Couble ML, Bleicher F, Magloire H, Smith AJ, Farges JC. (2001). Expression of TGF-beta receptors I and II in the human dental pulp by in situ hybridization. Adv Dent Res 15:63-67. [DOI] [PubMed] [Google Scholar]
- Sloan AJ, Lynch CD. (2012). Dental tissue repair: novel models for tissue regeneration strategies. Open Dent J 6:214-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark B, Carlstedt T, Risling M. (2001). Distribution of TGF-beta, the TGF-beta type I receptor and the R-II receptor in peripheral nerves and mechanoreceptors; observations on changes after traumatic injury. Brain Res 913:47-56. [DOI] [PubMed] [Google Scholar]
- Suda N, Shiga M, Ganburged G, Moriyama K. (2009). Marfan syndrome and its disorder in periodontal tissues. J Exp Zool B Mol Dev Evol 503-509. [DOI] [PubMed] [Google Scholar]
- Vaughan MB, Howard EW, Tomasek JJ. (2000). Transforming growth factor-β1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res 257:180-189. [DOI] [PubMed] [Google Scholar]
- Wisithphrom K, Windsor LJ. (2006). The effects of tumor necrosis factor-alpha, interleukin-1beta, interleukin-6, and transforming growth factor-beta1 on pulp fibroblast mediated collagen degradation. J Endod 32:853-861. [DOI] [PubMed] [Google Scholar]
- Yoshiba K, Yoshiba N, Nakamura H, Iwaku M, Ozawa H. (1996). Immunolocalization of fibronectin during reparative dentinogenesis in human teeth after pulp capping with calcium hydroxide. J Dent Res 75:1590-1597. [DOI] [PubMed] [Google Scholar]
- Yoshiba N, Yoshiba K, Ohkura N, Hosoya A, Shigetani Y, Yamanaka Y, Izumi N, Nakamura H, Okiji T. (2012a). Expressional alterations of fibrillin-1 during wound healing of human dental pulp. J Endod 38:177-184. [DOI] [PubMed] [Google Scholar]
- Yoshiba N, Yoshiba K, Ohkura N, Shigetani Y, Takei E, Hosoya A, Nakamura H, Okiji T. (2012b). Immunohistochemical analysis of two stem cell markers of α-smooth muscle actin and STRO-1 during wound healing of human dental pulp. Histochem Cell Biol 138:583-592. [DOI] [PubMed] [Google Scholar]
- Yoshiba N, Yoshiba K, Ohkura N, Shigetani Y, Takei E, Hosoya A, Nakamura H, Okiji T. (2013). Distributional Alteration of Extracellular Matrix during Wound Healing of Human Dental Pulp: Dynamic Remodeling of Fibrillin-1 Matrix. Jpn J Conserv Dent 56:161-168. [Google Scholar]