Abstract
Two new phenone derivatives penicophenones A (1) and B (2), a new cyclic tetrapeptide penicopeptide A (3), and five known compounds were isolated from the culture broth of Penicillium commune, an endophytic fungus derived from Vitis vinifera. Compounds 1–3 were elucidated by extensive spectroscopic analyses including 1D and 2D NMR and HRESIMS. The absolute configurations of 1 and 3 were determined by comparing its ECD with related molecules and modified Marfey’s analysis, respectively. Penicophenone A (1) possesses a rare benzannulated 6,6-spiroketal moiety, which is a new member of the unusual structural class with peniphenone A as the representative. Compound 3 exhibited significant inhibition activities against 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) in vitro and showed strong binding affinity to 11β-HSD1. Moreover, compound 3 treatments decreased the lipid droplet accumulation associate with the inhibition of 11β-HSD1 expression in differentiate-induced 3T3-L1 preadipocytes. Furthermore, the molecular docking demonstrated that compound 3 coordinated in the active site of 11β-HSD1 is essential for the ability of diminishing the enzyme activity.
Glucocorticoids influence a wide variety of physiologic processes, including lipid and glucose metabolism, immune modulation, cell growth, and anti-inflammatory responses. Excessive activation of glucocorticoid hormones (GCs) can result in metabolic syndromes with multiple clinical features, including insulin resistant diabetes, obesity, dyslipidemia, and hypertension1. 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), which converts inactive glucocorticoid into active glucocorticoid, such as cortisol in humans can amplify the local levels and activities of GCs2. Given that 11β-HSD1 is abundantly expressed in metabolically important tissues which become resistant to insulin action in type 2 diabetes, such as adipose, muscle, and liver tissue, the inhibition of 11β-HSD1 offers the ability to restore the metabolic action of insulin in these tissues3. Consequently, the developments of 11β-HSD1 inhibitor drugs are urgently demanded.
Natural products (NPs) have represented a cornerstone of pharmaceutical research, as they offer a diverse range of bioactive substructures, chemical scaffolds, and potentially lower toxicity profiles4. As the literature reported, more than 50% of new medicines approved by the US Food and Drug Administration (FDA) between 1981 and 2014 were direct or indirect derived from NPs5. Encouraged by this, our research group has been dedicated to explore new NPs targeting 11β-HSD1 in recent years. Fungi are rich sources of novel secondary metabolites with diverse structures and various biological activities, which have attracted considerable attentions. In our search for new bioactive substances from fungi6,7,8,9,10,11, the fungus Penicillium commune was phytochemically investigated, which led to the isolation of two new phenone derivatives penicophenones A (1) and B (2), a new cyclic tetrapeptide penicopeptide A (3), and five known compounds 3-benzylidene-3,4-dihydro-4-methyl-lH-l,4-benzodiazepine-2,5-dione (4)12, (+)-cyclopenol (5)13, cyclopenin (6)13,14, emindole SB (7)15, and penilactones A (8)16 (Fig. 1). Compounds 1–8 were subjected to 11β-HSD1 inhibitory assays, and compound 3 exhibited significant inhibition activities against 11β-HSD1 in vitro and showed strong binding affinity to recombinant human 11β-HSD1 by microscale thermophoresis (MST) assays. Moreover, compound 3 treatments decreased the lipid droplet accumulation associate with the inhibition of 11β-HSD1 activity of 3T3-L1 cells in vivo. Furthermore, the molecular docking demonstrated that compound 3 is able to occupy the major active site and adopt a conformation similar to the known inhibitor, carbenoxolone.
Figure 1. Structures of 1–8.
Results and Discussion
Isolation and Structure Elucidation
The strain of P.commune was cultured on rice at 28 °C for 31 days, and then extracted with EtOAc to yield a crude extract. The extract was suspended in H2O and partitioned successively with petroleum ether and EtOAc to yield a petroleum ether-soluble extract and a EtOAc-soluble extract. The petroleum ether-soluble part was separated and purified to obtain compounds 1 (8.1 mg), 6 (7.5 mg), and 8 (7.9 mg). The EtOAc extract was also separated and purified to yield compounds 2 (9.6 mg), 3 (40.7 mg), 4 (36.0 mg), 5 (40 mg), and 7 (23 mg)
Penicophenone A (1) was isolated as colorless oil. The molecular formula of C18H24O5 was deduced by HRESIMS (m/z 321.1694 [M + H]+, calcd for C18H25O5, 321.1702) and 13C NMR data, suggesting seven degrees of unsaturation. The 1H NMR (Table 1) spectrum of 1 showed an aromatic proton (δH 7.28, s), two oxygenated methine protons (δH 4.00, m; 3.61, dqd, J = 12.5, 6.2, 2.1 Hz), six aliphatic protons (δH 2.69, dd, J = 16.8, 6.4 Hz; 2.20, dd, J = 16.8, 2.8 Hz; 2.13, ddd, J = 12.6, 4.8, 1.7 Hz; 1.81, m; 1.09, dd, J = 12.6, 11.3 Hz; and 0.99, dt, J = 12.1, 11.6 Hz), and four methyls (δH 2.33, s; 1.94, s; 0.87, d, J = 6.3 Hz; and 0.73, d, J = 7.1 Hz). The 13C NMR data (Table 1) revealed that 1 has a skeleton based on 18 carbons, including a carbonyl (δC 204.8), six aromatic carbons (δC 110.1–161.9), a hemiketal carbon (δC 102.9), two oxygenated methines (δC 67.7 and 65.3), four aliphatic carbons (δC 34.5–24.5), and four methyls (δC 15.4–26.3).
Table 1. NMR data for penicophenones A (1) and B (2)a.
| no. |
1 |
2 |
||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 157.3 | 111.2 | ||
| 2 | 118.2 | 160.9 | ||
| 3 | 7.28 s | 130.9 | 113.8 | |
| 4 | 114.1 | 160.6 | ||
| 5 | 161.9 | 116.3 | ||
| 6 | 110.1 | 7.58 s | 131.1 | |
| 7 | α 2.69 dd (16.8, 6.4) β 2.20 dd (16.8, 2.8) | 24.5 | 3.75 s | 21.54 |
| 8 | 1.88 m | 34.5 | 117.4 | |
| 9 | 102.9 | 114.9 | ||
| 10 | α 1.09 dd (12.6, 11.3) β 2.13 ddd (12.6, 4.8, 1.7) | 40.8 | 6.46 s | 100.8 |
| 11 | 4.00 m | 65.3 | 148.1 | |
| 12 | α 0.99 dt (12.1, 11.6) β 1.81 m | 43.1 | 141.8 | |
| 13 | 3.61 dqd (12.5, 6.3, 2.1) | 67.7 | 6.51 s | 114.9 |
| 14 | 1.94 s | 15.4 | 203.2 | |
| 15 | 204.8 | 2.50 s | 26.3 | |
| 16 | 2.33 s | 26.3 | 2.10 s | 16.1 |
| 17 | 0.73 d (7.1) | 15.4 | 3.52 s | 56.74 |
| 18 | 0.87 d (6.3) | 21.7 | 3.66 s | 55.5 |
a400 MHz for 1H NMR and 100 MHz for 13C NMR (1 in CD3OD and 2 in “DMSO-d6”).
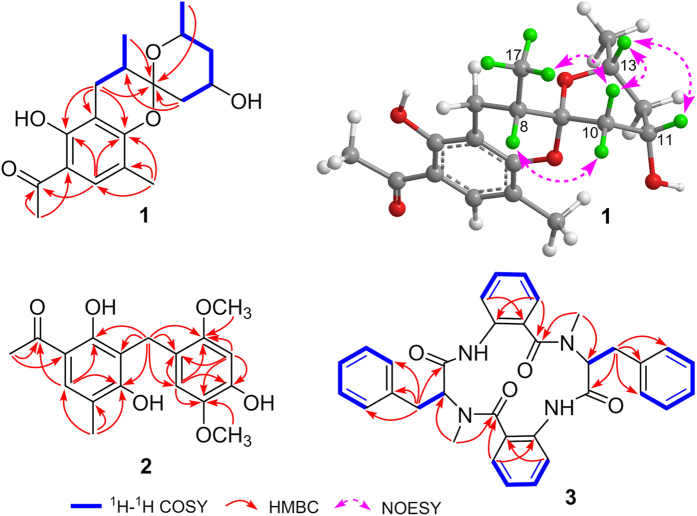
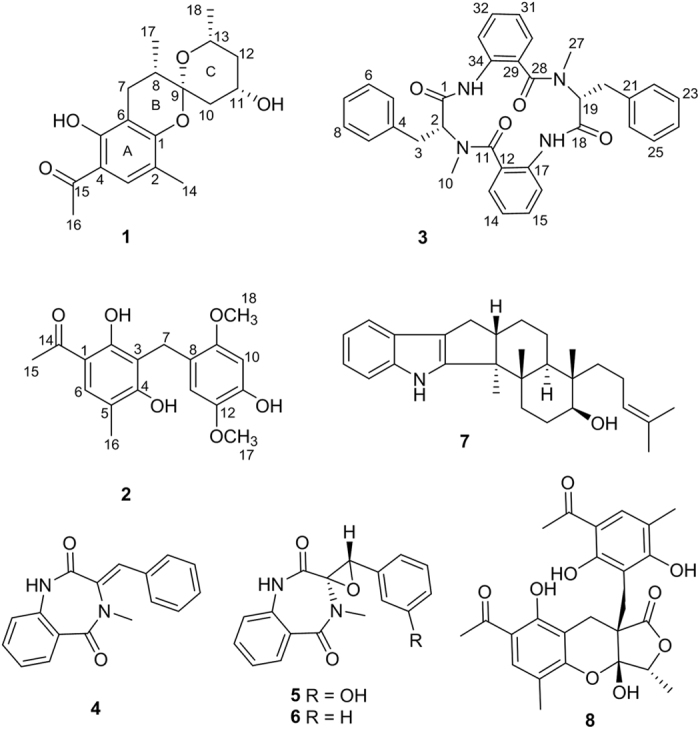
The planar structure was established by analyses of its 1H–1H COSY and HMBC spectra (Fig. 2). The pentasubstituted benzene ring (ring A) was constructed by HMBC correlations from H-7 to C-1, C-5, and C-6, from Me-14 to C-1, C-2, and C-3, and from H-3 to C-1 and C-5 based on the former mentioned six aromatic carbons. Additional HMBC cross-peaks from Me-16 to C-4 and C-15 and from H-3 to C-15 supported the location of the acetyl at C-4. The 1H–1H COSY spectrum showed the presence of two spin systems of H-7/H-8/Me-17 and H-10/H-11/H-12/H-13/Me-18, which were connected to the hemiketal carbon by HMBC interactions from H-7, H-10, Me-17, and Me-18 to C-9 and from H-10 to C-8. The chemical shifts of C-1 (δC 157.3) and C-5 (δC 161.9), together with degrees of unsaturation requirement, indicated the presence of an additional pyran ring (ring B) between C-1 and C-9 via an oxygen atom. Therefore, the planar structure of 1, with a rare benzannulated 6,6-spiroketal moiety was determined.
Figure 2. 1H–1H COSY and selected HMBC of 1–3 and key NOESY of 1.
The relative configuration of 1 was determined by a NOESY experiment (Fig. 2). NOESY cross-peak observed between H-10β and H-13 established a boat conformation of ring C. The α-orientation of the hydroxyl at C-11 was revealed by NOESY cross-peak of H-11/H-13. In addition, NOESY correlations of H-10β/Me-17 and H-10α/H-8 not only determined the configuration of the spiroketal carbon (C-9) but also clarified the α-orientation of Me-17. The absolute configuration of 1 was finally determined as 8S,9R,11S,13R by comparing its ECD spectrum (see Supplementary Information) with that of peniphenone A17, because both of them exhibit negative cotton effects around 230 and 280 nm. Thus, compound 1 determined to be a novel compound possessing a rare benzannulated 6,6-spiroketal core structure, which is a new member of the unusual structural class with peniphenone A as the representative. The biosynthetic pathway of 1 may be similar to that of peniphenone A with a different precursor of pyrone derivative (5-hydroxy-6-methyl-pyrone in 1 rather than 5-hydroxy-3,6-dimethyl-pyrone in peniphenone A), which lead to compound 1 with the absence of methyl group at C-10 compared with that of peniphenone A.
Penicophenone B (2) was obtained as yellowish oil, with the molecular formula of C18H20O6 suggesting by HRESIMS data ([M]+ m/z 332.1254, calcd for C18H20O6, 332.1260). The 1H and 13C NMR spectra of 2 showed resonances of a carbonyl, 12 aromatic carbons (including three methines), a methylene, two methyls, and two methoxyls. Extensive analysis of the HMBC (Fig. 2) spectrum of 2 revealed that it had the same 2,4-diol-5-methyl acetophenone as that of 1. The remained tetrasubstituted benzene ring, including the location of the methoxyls, were elucidated by HMBC correlations from H-10 and H-13 to C-8, C-9, C-11, and C-12, from OMe-17 to C-12, and from OMe-18 to C-9. The linkage of the former established two rings via the methylene were further confirmed by HMBC correlations from H-7 to C-2, C-3, C-4, C-8, C-9, and C-13. Herein, the structure of 2 was elucidated as shown in Fig. 1.
Penicopeptide A (3) was assigned the molecular formula of C34H32N4O4 on the basis of its HRESIMS data ([M + Na]+ m/z 583.2310, calcd for C34H32N4O4Na, 583.2321). The peptide nature of 3 was inferred from the presence of signals of the amide N-Me (δH 2.90, s; 3.07, s and δC 39.8; 29.6) and amino acid protons (δH 4.33, dd, J = 10.6, 7.0 Hz and 4.43, t, J = 7.6 Hz) (Table 2). Careful interpretation of the 2D NMR data revealed the presence of two phenylalanine (Phe) and two 2-aminobenzoic acid residues. The connections of the above mentioned residues were established by HMBC (Fig. 2) interactions from Me-10 to C-2 and C-11, H-2 to C-11, Me-27 to C-19 and C-28, and H-19 to C-28 aided by the HRESIMS data. The absolute configurations of C-2 and C-19 in the Phe units were determined to be R by modified Marfey’s analysis (see Supplementary Information). Compound 3 is a symmetrical tetrapeptide, and normally its 1H and 13C NMR data of two units should typically overlaped; however this is not observed in this case. The main reason may be that the conformations of 3 are asymmetrical, which is supported by the conformational analysis (see Supplementary Information).
Table 2. NMR data for Penicopeptide A (3)a.
| no. | δH | δC | no. | δH | δC |
|---|---|---|---|---|---|
| 1 | 172.3 | 18 | 171.1 | ||
| 2 | 4.33 dd (10.6, 7.0) | 69.7 | 19 | 4.43 t (7.6) | 57.9 |
| 3 | 2.79 dd (13.5, 6.9) 2.66 dd (13.5, 10.8) | 35.2 | 20 | 3.40 dd (14.5, 7.9) 3.25 dd (14.5, 7.3) | 32.9 |
| 4 | 137.1 | 21 | 138.1 | ||
| 5 | 7.02 d (7.0) | 130.0 | 22 | 7.22 m | 130.0 |
| 6 | 7.22 m | 129.8 | 23 | 7.22 m | 129.6 |
| 7 | 7.22 m | 128.3 | 24 | 7.17 m | 127.8 |
| 8 | 7.22 m | 129.8 | 25 | 7.22 m | 129.6 |
| 9 | 7.02 d | 130.0 | 26 | 7.22 m | 130.0 |
| 10 | 2.90 s | 39.8 | 27 | 3.07 s | 29.6 |
| 11 | 168.2 | 28 | 170.7 | ||
| 12 | 127.8 | 29 | 128.3 | ||
| 13 | 7.96 brd (7.9) | 132.3 | 30 | 7.82 brd (7.8) | 131.8 |
| 14 | 7.34 t (7.5) | 125.9 | 31 | 7.28 m | 126.0 |
| 15 | 7.59 brd (7.8) | 134.2 | 32 | 7.51 brt (7.1) | 133.8 |
| 16 | 7.17 d (8.2) | 121.6 | 33 | 7.07 d (8.2) | 122.0 |
| 17 | 137.0 | 34 | 138.0 |
a400 MHz for 1H NMR and 100 MHz for 13C NMR in CD3OD.
The structure of 3-benzylidene-3,4-dihydro-4-methyl-lH-l,4-benzodiazepine-2,5-dione (4) was firstly determined by single crystal X-ray analysis (CCDC 1058389) in this study. In addition, its 1H and 13C NMR data were also provided.
Inhibition of compounds 1-8 on 11β-HSD1 enzyme activity
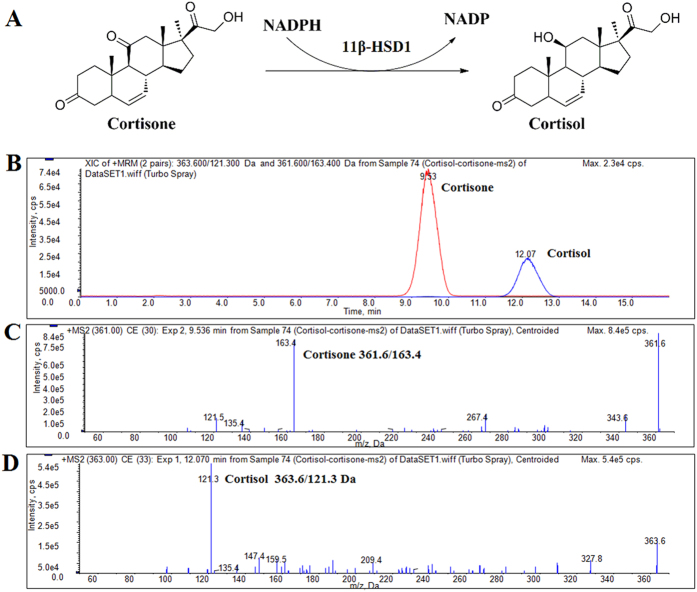
To explore the 11β-HSD1 inhibitory activities of compounds 1–8 in vitro, recombinant human 11β-HSD1 protein was incubated with various concentrations of compounds and the enzymatic activity (cortisone to cortisol) was tested using HPLC-MS/MS (Fig. 3). Among these compounds tested, compound 3 showed strong inhibitory effect against human 11β-HSD1, with an IC50 value of 9.07 ± 0.61 μM (Table 3).
Figure 3. Determination of cortisone and cortisol by HPLC-MS/MS.
(A) Biological activity of 11β-HSD1. (B) HPLC-MS/MS Chromatogram of cortisone and cortisol, (C,D) Positive ESI MS/MS spectra acquired on an API4000 mass spectrometer for cortisol with collision energy of 33 eV and cortisone with collision energy of 30 eV.
Table 3. Binding affinity and inhibitory activities of compounds 1–8.
| Compound | Inhibitory activities against 11β-HSD1 | Dissociation constant with 11β-HSD1 |
|---|---|---|
| Kd a (μM) | IC50 (μM) | |
| 1 | n.b.b | n.i. c |
| 2 | n.b. | n.i. |
| 3 | 82.1 ± 5.01 | 9.07 ± 0.61 |
| 4 | n.b. | n.i. |
| 5 | 420.51 ± 12.11 | 45.51 ± 7.82 |
| 6 | 513.14 ± 10.61 | 68.05 ± 7. 84 |
| 7 | n.b. | n.i. |
| 8 | n.b. | n.i. |
a The Kd value is automatic calculated by the curve fitting, and presents as means ± SD for three experiments.
b n.b. is no clear binding detected in the MST measurement.
c n.i. is no inhibition detected in the experiments (IC50 > 200 μM).
Specific binding with 11β-HSD1
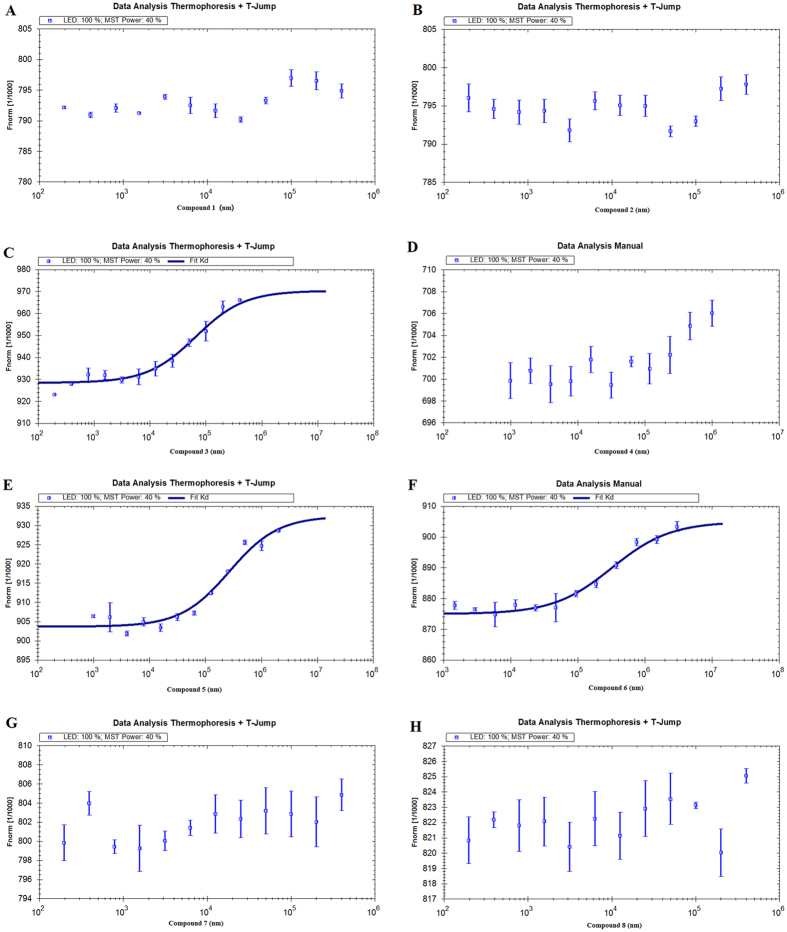
Measuring the thermophoretic behaviour of a protein in the presence of differing ligand concentrations by MST allows quantitative analysis of molecular interactions in solution. The MST technique has previously been used to investigate protein-protein, small organic molecule-protein and antibody-protein interactions. In this study, MST was utilized as an independent confirmation of the dissociation constant (Kd) between 1–8 and 11β-HSD1. As shown in Table 3, it is not surprising that the Kd value of compound 3 (82.1 ± 5.01 μM) was much lower than that of 5 and 6, and similarly, either no binding or no statistically significant binding was detected when 11β-HSD1 was titrated with compounds 1, 2, 4, 7, and 8 (Fig. 4). These results confirmed the specific binding of compound 3 to 11β-HSD1.
Figure 4. Measurement of affinity between compounds 1–8 with 11β-HSD1 by MST.
The resulting binding curve was shown. From the resulting binding curve, Kd of 82.1 ± 5.01 μM for 3 (C), 420.51 ± 12.11 μM for 5 (E), 513.14 ± 10.61 μM for 6 (F) were calculated.
Effect of compound 3 on lipid accumulation and 11β-HSD11 expression in 3T3-L1 cells
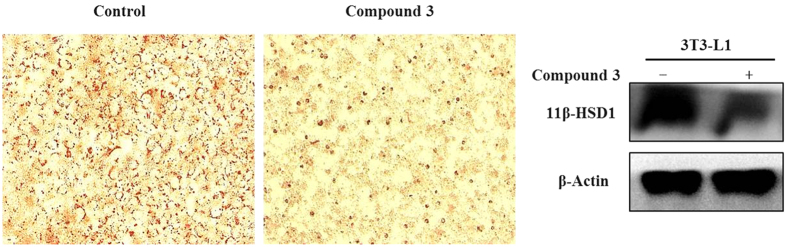
It was reported that 11β-HSD1 is highly expressed in normal liver18 and stimulates pre-adipocyte differentiation. As a result, many 11β-HSD1 inhibitors were investigated to prevent adipogenesis19. To further examine the 11β-HSD1 inhibitory effects of compound 3, we examined the anti-adipogenic effect of compound 3 during the differentiation of 3T3-L1 cells. As the results shown in Fig. 5, compound 3 treatments decreased the lipid droplet accumulation associate with the inhibition of 11β-HSD1 activities.
Figure 5. Effects of compound 3 on lipid accumulation and 11β-HSD1 expression in 3T3-L1 cells.
Preadipocytes were induced to differentiate in the presence or absence of compound 3, which was then stained with Oil Red O and photographed (40×). 11β-HSD1 expressions were determined by western blot. β-Actin was used as a loading control.
Molecular docking analysis
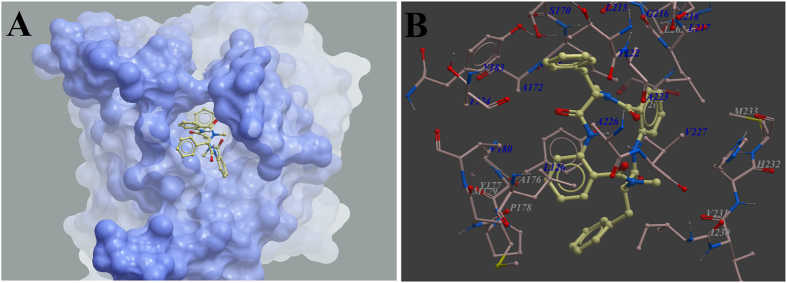
11β-HSD1 as the target of compound 3 was further evaluated by 3D molecular docking, utilizing the X-ray crystal structure of 11β-HSD1 (PDB code: 2BEL). Using the Molsoft ICM method (ICM-Pro 3.8 molecular docking software), we optimized the geometry of compound 3 and determined the binding site with the lowest-energy and the most favorable orientation of the compound. Compound 3 was observed to occupy the major active site with significant scores of ICM docking score and IMC docking mfScore, and adopted a conformation similar to that of known inhibitor (Fig. 6A). Compound 3 had the ability to form key hydrophobic interactions with residues Leu126, Tyr177, Tyr183, and Leu171 (Fig. 6B).
Figure 6. Low-energy binding conformations of compound 3 bound to 11β-HSD1 generated by virtual ligand docking. Compound 3 as the ball-and-stick model showing carbon (yellow), hydrogen (grey), oxygen (red) and Nitrogen (blue) atoms.
(A) Compound 3 was observed to occupy the active site with significant scores of ICM docking score and IMC docking mfScore, and adopted a conformation similar to that of known inhibitors. (B) Compound 3 had the ability to form key hydrophobic interaction with residues Leu126, Tyr177, Tyr183, and Leu171.
To date, several small molecule inhibitors of 11β-HSD1 were discovered from NPs, such as glycyrrhetinic acid, carbenoxolone, gossypol, and estradiol. However, these NPs often showed low specificity in the inhibition of 11β-HSD1 with serious side effects. Thus, more efforts to find new 11β-HSD1-targeting candidates are urgently demanded20,21. In this study, a new cyclic tetrapeptide penicopeptide A (3) was isolated from the fungus of Penicillium commune, which showed the most potent and concentration-dependent inhibitions activity with an IC50 value of 9.07 ± 0.61 μM. The possible mechanism may be that compound 3 is more accessible to the active site of 11β-HSD1 to exert selective inhibition effects, which is indicated by MST assay (Kd = 82.1 ± 5.01 μM). Furthermore, the molecular docking demonstrates that compound 3 coordinating in the active site of 11β-HSD1 is essential for the ability of diminishing the enzyme activity. In summary, the novel structure of compound 3 combined with its significant inhibitory activities of 11β-HSD1 reported in this study may greatly promote the studies of 11β-HSD1 inhibitors from natural products, and further investigations on the mechanism and structure-function relationships for developing more excellent agents are necessary.
Methods
General experimental procedures
Optical rotations were determined with a Perkin-Elmer 41 polarimeter equipped with a sodium lamp (589 nm). UV, CD, and FT-IR spectra were performed on a Varian Cary 50, a JASCO-810 CD spectrometer, and a Bruker Vertex 70 instruments, respectively. 1D and 2D NMR spectra were recorded on a Bruker AM-400 spectrometer, and the 1H and 13C NMR chemical shifts were referenced with respect to the solvent or solvent impurity peaks. HRESIMS were carried out in the positive ion mode on a Thermo Fisher LC-LTQ-Orbitrap XL spectrometer. X-ray data were collected using a Bruker APEX DUO instrument. Column chromatography was conducted with silica gel (200–300 and 300–400 mesh; Qingdao Marine Chemical Inc., China), Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Sweden), and MCI gel (75–150 μm, Mitsubishi Chemical Corporation, Tokyo, Japan). Semi-preparative HPLC was carried out on a Dionex quaternary system with a diode array detector at a flow rate of 2.5 mL/min using a reversed-phased C18 column (5 μm, 10 × 250 mm, YMC-pack ODS-A).Thin-layer chromatography (TLC) was performed with silica gel 60 F254 (Yantai Chemical Industry Research Institute) and RP-C18 F254 plates (Merck, Germany).
Fungal material
The fungal strain P.commune was isolated as an endophytic fungus from the plant Vitis vinifera, which was collected at Qingshui County in Gansu province of China, in July 2007. A voucher specimen (No. 20140301) has been deposited in the culture collection center of Tongji Medical College, Huazhong University of Science and Technology.
Fermentation, extraction, and isolation
The strain of P.commune was cultured with rice at 28 °C for 31 days, and then extracted with EtOAc to yield a crude extract (172 g). The extract was suspended in H2O and partitioned successively with petroleum ether and EtOAc to yield a petroleum ether-soluble extract (94 g) and a EtOAc-soluble extract (48 g). The petroleum ether-soluble part (94 g) was separated into five fractions (Fr.1−Fr.5) via silica gel column chromatography (CC, 1.5 kg, 10 × 100 cm) eluted with gradient petroleum ether/acetone (50:1 → 1:1). Compounds 1 (8.1 mg), 6 (7.5 mg), and 8 (7.9 mg) were purified from Fr.4 by repeated silica gel and ODS CC (MeOH/H2O, 30:70 → 90:10). The EtOAc extract (48 g) was subjected to silica gel CC (800 g, 10 × 100 cm) with gradient petroleum ether/acetone (20:1 → 1:2) to give seven fractions (Fr.A–Fr.G). Fr.C was further purified by repeated silica gel CC and semi-preparative HPLC (MeOH/H2O, 70:30) to give compounds 2 (9.6 mg) and 7 (23 mg). Purification of Fr.D by MPLC (MeOH/H2O, 30:70 → 70:30) followed by semi-preparative HPLC (MeOH/H2O, 65:35) led to the isolation of 3 (40.7 mg) and 4 (36.0 mg). Fr.F was also purified by MPLC (MeOH/H2O, 30:70 → 70:30) followed by semi-preparative HPLC to give 5 (40 mg).
Penicophenone A (1) Colorless oil; [α]20D + 6.0 (c = 0.44, MeOH); UV (MeOH) λmax (log ε) = 216 (4.01), 282 (3.87), 328 (3.47) nm; IR νmax = 3392, 1627, 1478, 1447, 1382, 1179, 1136, 1072 cm–1; CD (MeOH) λmax (Δε) 229 (−3.0), 280 (−1.5) nm; For 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Table 1; HRESIMS [M + H] + m/z 321.1694 (calcd for C18H25O5, 321.1702).
Penicophenone B (2) Yellowish oil; UV (MeOH) λmax (log ε) = 205 (4.72), 288 (4.05) nm; IR νmax = 3216, 1689, 1623, 1483, 1453, 1395, 1199 cm–1; For 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Table 1; HRESIMS [M]+ m/z 332.1254 (calcd for C18H20O6, 332.1260).
Penicopeptide A (3) Colorless gum; [α]20D −48.8 (c = 1.11, MeOH); UV (MeOH) λmax (log ε) = 214 (4.63), 292 (3.48) nm; IR νmax = 3455, 1687, 1625, 1482, 1396 cm–1; CD (MeOH) λmax (Δε) 222 (−16.4), 256 (+5.09), 288 (−4.0) nm; For 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Table 2; HRESIMS [M + Na]+ m/z 583.2310 (calcd for C34H32O4Na, 583.2321).
3-benzylidene-3,4-dihydro-4-methyl-lH-l,4-benzodiazepine-2,5-dione (4)1 H NMR: δH 7.89, dd, 1 H (7.9, 1.5); 7.54, ddd, 1H (8.1, 7.4, 1.6); 7.34–7.44, overlapped, 5 H; 7.28, ddd, 1 H (7.9, 7.4, 1.1); 7.13, dd, 1 H (8.1, 0.7); 3.11, s, 3 H. 13C NMR: δC 172.4, 169.0, 137.9, 135.3, 134.1, 133.6, 131.8, 130.9, 130.2, 130.2, 126.8, 125.9, 122.0, 36.1.
Hydrolysis and modified Marfey’s Analysis of 3 Compound 3 (0.8 mg) was dissolved in 1.0 mL of 6 N HCl and heated to 110 °C for 1 h. The solution was evaporated to remove the trace HCl under vacuum. The divided hydrolysate (0.4 mg each) was treated with 100 μL of 1 N NaHCO3 followed by 50 μL of 1% L- or D-FDAA in acetone. The mixture was stirred at 80 °C for 15 min. After quenching by the addition of 50 μL of 2 N HCl, the mixture was analyzed by HPLC to assign the chirality of the amino acid (Linear gradient: 10−40% acetonitrile in 60 minutes). Separately, the standard amino acids L-Phe and D-Phe were derivatized with FDAA in the same manner as 3.
Protein expression and purification
The gene encoding 11β-HSD1 (Genebank: NC_018912.2) was cloned into the pET-28a vector (Novagen). After the recombinant plasmids were verified by sequencing, the plasmid was transformed into E. coli BL21(DE3) (Invitrogen) which were grown in LB medium at 37 oC to an OD600 (0.8–1.0) and induced by 0.4 mM isopropyl -D-thiogalactopyranoside (IPTG) at 20 oC for 16 hours. Bacterial cells were lysed by ultrasonification on ice in a buffer containing 20 mM Tris, pH 8.5, 200 mM NaCl, 5 mM mercaptoethanol, 0.1% TritonX-100, and 5% glycerol. Soluble C-terminally hexa-histidine tagged 11β-HSD1 was bound to Ni-agarose affinity resin (Qiagen), washed with a buffer containing 20 mM Tris, pH 8.5, 200 mM NaCl, and 10 mM imidazole, and eluted with a buffer containing 20 mM Tris, pH 8.8, 250 mM NaCl, and 150 mM imidazole. Thrombin (Roche) was added at one unit per 4 mg protein to the elute, which was then dialyzed against 20 mM Tris pH 8.5 and 100 mM NaCl at room temperature for overnight digestion. After thrombin digestion to remove the hexahistidine tag, contaminant proteins were removed by loading the elute onto a second cobalt affinity column equilibrated in the dialyzing buffer and collecting the flow-through. The protein was further purified with anion exchange chromatography, using a linear gradient of 10 mM to 1 M NaCl concentration and size exclusion chromatography at 20 mM Tris pH 8.5 and 200 mM NaCl22,23.
Enzyme inhibition assay
The inhibitory effects of 1–8 on 11β-HSD1 were measured using recombinant human 11β-HSD1 protein through HPLC-MS/MS method24,25. Briefly, 2.5 μL recombinant 11β-HSD1 protein (0.512 mg protein/ml) was added to 100 μL buffer (100 mM KCl, 20 mM NaCl, 20 mM Hepes, pH 7.5) containing NADPH (1 mM) and cortisone (50 μM) with or without various concentrations of compounds. The reactants were incubated at 37 oC for 30 min. After extraction with dichloromethane, evaporation and resolvation with 200 μL methanol, cortisone and cortisol were measured by HPLC-MS/MS and multi-reactions monitoring (MRM) technology. Detection was performed using a Shimadzu LC-20AD HPLC system equipped with an SHIMADZU VP-ODS 150 L × 4.6 mm column connected to a API 4000 LC/MS/MS system. Samples were analyzed after injection and equilibrated with 45% solution A (0.1% formic acid in water) and 55% solution B (Methanol) under isocratic elution with the flow rate of 0.8 mL/min. The HPLC eluent were tested by HPLC-MS/MS using collision energy 33 eV (cortisol) and 30 eV (collision) in positive ion mode. Analyst quantitation optimization wizard was used to optimize the collision energies and other electric lens settings for MRM detections. The inhibition rates were calculated using the concentration ratios of cortisone and cortisol in tubes with or without various compounds.
Binding affinity assay using microscale thermophoresis
The ability of the purified 11β-HSD1 to bind with potential ligands was analyzed using MST. The protein was labeled with the Monolith NT™ Protein Labeling Kit RED (Cat#L001) according to the supplied labeling protocol26,27. Labelled 11β-HSD1 was kept constant at 50 nM, while all samples tested were diluted in a 20 mM HEPES (pH 7.5) and 0.05 (v/v)% Tween-20. Compounds were diluted in 12 dilution steps covering the range of appropriate concentrations. After 10 min incubation at room temperature, samples were loaded into MonolithTM standard-treated capillaries and the thermophoresis was measured at 22 °C after 30 min incubation on a Monolith NT.115 instrument (NanoTemper Technologies, München, Germany). Laser power was set to 40% using 30 seconds on-time and the LED power was set to 100%. The dissociation constrant Kd values were fitted by using the NTAnalysis software (NanoTemper Technologies, München, Germany).
Differentiation Induction of 3T3-L1
3T3-L1 preadipocytes were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences. Both of them were cultured in DMEM medium (Gibco, USA) containing 10% FBS, penicillin (100 U/mL), streptomycin (100 g/mL) in 5% CO2 at 37 °C. 3T3-L1 preadipocytes were induced to differentiate as previously described. In short, differentiation was induced by adding 0.5 mmol/L 3-isobutyl-1-methyxanthine (Sigma), 10 μg/mL insulin (Sigma), and 1 mmol/L dexamethasone (Sigma) to the medium. After 48 h incubation (d2), the medium was replaced with DMEM containing 10 μg/mL insulin and 50 μM compound 3. On d4, the medium was replaced with DMEM containing compound 3, and then changed back to the same medium every on d6.
Oil red O staining
Preadipocytes 3T3-L1 were induced to differentiate with or without compounds 3 for 6 days, and then washed twice with PBS, fixed in 3.7% formaldehyde for 1 h and stained with Oil Red O for another 1 h at room temperature. Cells were then washed several times with PBS, and photographed by a light microscope (Olympus, Japan).
Western Blot Analysis
Cells were treated and then lysed in a radio immune-precipitation assay buffer. Protein concentrations were determined using a BCA protein assay kit (Byontime, Beijing, China) and equalized before loading. Samples were denatured and subjected to electrophoresis in 10% SDS-PAGE gels followed by transfer to PVDF membrane and probed with 11β-HSD1 (sc-20175) antibody and β-Actin. Blots bands were visualized using the horseradish peroxidase conjugated secondary antibodies and chemiluminescent substrate.
Molecular Docking
Crystal structures of huaman 11β-HSD1 (PDB code: 2BEL) was obtained from the Protein Data Bank (http://www.rcsb.org)28. The docking was performed by using ICM 3.8.2 modeling software on an Intel i7 4960 processor (MolSoft LLC, San Diego, CA)29. Ligand binding pocket residues were selected by using graphical tools in the ICM software, to create the boundaries of the docking search. In the docking calculation, potential energy maps of the receptor were calculated using default parameters. Compounds were imported into ICM and an index file was created. Conformational sampling was based on the Monte Carlo procedure30, and finally the lowest-energy and the most favorable orientation of the ligand were selected.
Additional Information
How to cite this article: Sun, W. et al. Novel small molecule 11β-HSD1 inhibitor from the endophytic fungus Penicillium commune. Sci. Rep. 6, 26418; doi: 10.1038/srep26418 (2016).
Supplementary Material
Acknowledgments
The authors would like to thank the Analytical and Testing Center at Huazhong University of Science and Technology for assistance in conducting ECD and IR analyses. This work was financially supported by the Program for New Century Excellent Talents in University, State Education Ministry of China (NCET-2008-0224), the National Natural Science Foundation of China (Nos. 31370372, 81573316, 31270395, 31570361, and 81202423), and the National Science and Technology Project of China (Nos. 2011ZX09102-004 and 2013ZX09103001-020).
Footnotes
Author Contributions W.S., X.C., Q.T. and H.Z. contributed equally to this work. They performed the main experiments, analyzed the data, and wrote the manuscript; Y.H. and L.L. provided many useful suggestions about the fermentation and isolation; Y.X., G.Y. and Z.L. edited and polished this manuscript; J.W., H.L. and Y.Z. designed the experiments and commented the manuscript. All authors reviewed the manuscript.
References
- Macfarlane D. P., Forbes S. & Walker B. R. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J. Endocrinol. 197, 189–204 (2008). [DOI] [PubMed] [Google Scholar]
- Chapman K., Holmes M. & Seckl J. 11-Hydroxysteroid Dehydrogenases: Intracellular Gate-Keepers of Tissue Glucocorticoid Action. Physiol Rev. 93, 1139–1206 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton B. S. et al. Pharmacological characterization of the selective 11β-hydroxysteroid dehydrogenase 1 inhibitor, BI 135585, a clinical candidate for the treatment of type 2 diabetes. Eur. J. Pharmacol. 746, 50–55 (2015). [DOI] [PubMed] [Google Scholar]
- Chan D. S. et al. Structure-based discovery of natural-product-like TNF-α inhibitors. Angew. Chem. Int. Ed. 49, 2922–2926 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D. J. & Cragg G. M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. doi: 10.1021/acs.jnatprod.5b01055 (2016). [DOI] [PubMed] [Google Scholar]
- Chen C. et al. Armochaetoglobins K-R, Anti-HIV Pyrrole-Based Cytochalasans from Chaetomium globosumTW1-1. Eur. J. Org. Chem. 2015, 3086–3094 (2015). [Google Scholar]
- Chen C. et al. Nine new cytochalasan alkaloids from Chaetomium globosum TW1-1 (Ascomycota, Sordariales). Sci. Rep. 6, 18711 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. et al. Armochaeglobines A and B, Two New Indole-Based Alkaloids from the Arthropod-Derived Fungus Chaetomium globosum. Org. Lett. 17, 644–647 (2015). [DOI] [PubMed] [Google Scholar]
- Zhu H. et al. Asperchalasine A, a Cytochalasan Dimer with an Unprecedented Decacyclic Ring System, from Aspergillus flavipes. Angew. Chem. Int. Ed. 54, 13374–13378 (2015). [DOI] [PubMed] [Google Scholar]
- Chen C. et al. Armochaetoglobins A-J: Cytochalasan Alkaloids from Chaetomium globosum TW1-1, a Fungus Derived from the Terrestrial Arthropod Armadillidium vulgare. J. Nat. Prod. 78, 1193–1201 (2015). [DOI] [PubMed] [Google Scholar]
- Zhu H. et al. Epicochalasines A and B, Two Bioactive Merocytochalasans Bearing Caged Epicoccine Dimer Units from Aspergillus flavipes. Angew. Chem. Int. Ed. doi: and . [DOI] [PubMed] [Google Scholar]
- Smith H., Wegfahrt P. & Rapoport H. The synthesis of cyclopenin. Journal of the American Chemical Society. J. Am. Chem. Soc. 90, 1668–1669. (1968). [Google Scholar]
- Li J., Wang J., Jiang C. S., Li G. & Guo Y. W. (+)-Cyclopenol, a new naturally occurring 7-membered 2,5-dioxopiperazine alkaloid from the fungus Penicillium sclerotiorum endogenous with the Chinese mangrove Bruguiera gymnorrhiza. J. Asian Nat. Prod. Res. 16, 542–548 (2014). [DOI] [PubMed] [Google Scholar]
- Liu J. L. et al. Purifications and structure elucidations of cyclopenol and cyclopenin. J. Xiamen Univ. (Nat. Sci.) 51, 386–390 (2012). [Google Scholar]
- Nozawa K., Nakajima S., Kawai K. I. & Udagawa S. I. Isolation and structures of indoloditerpenes, possible biosynthetic intermediates to the tremorgenic mycotoxin, paxilline, from Emericella striata. J. Chem. Soc. Perk. T. 1, 2607–2610 (1988). [Google Scholar]
- Wu G. et al. Penilactones A and B, two novel polyketides from Antarctic deep-sea derived fungus Penicillium crustosum PRB-2. Tetrahedron 68, 9745–9749 (2012). [Google Scholar]
- Li H. et al. Peniphenones A-D from the mangrove fungus Penicillium dipodomyicola HN4-3 A as inhibitors of Mycobacterium tuberculosis phosphatase MptpB. J. Nat. Prod. 77, 800–806 (2014). [DOI] [PubMed] [Google Scholar]
- Ma R. et al. Switch of glycolysis to gluconeogenesis by dexamethasone for treatment of hepatocarcinoma. Nat. Commun. 4, 2508 (2013). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. 11 beta-hydroxysteroid dehydrogenase type 1 promotes differentiation of 3T3-L1 preadipocyte. Acta pharmacol. sin. 28, 1198–1204 (2007). [DOI] [PubMed] [Google Scholar]
- Sakamuri S. S. V. P. et al. Carbenoxolone treatment ameliorated metabolic syndrome in WNIN/Ob obese rats, but induced severe fat loss and glucose intolerance in lean rats. PloS One 7, e50216 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M. Z. et al. Inhibition of 11β–hydroxysteroid dehydrogenase type II selectively blocks the tumor COX-2 pathway and suppresses colon carcinogenesis in mice and humans. J. Clin. Invest. 119, 876–885 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. S. et al. Novel acidic 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitor with reduced acyl glucuronide liability: The discovery of 4-[4-(2-adamantylcarbamoyl)-5-tert-butyl-pyrazol-1-yl] benzoic acid (AZD8329). J. Med. Chem. 55, 10136–10147 (2012). [DOI] [PubMed] [Google Scholar]
- Julian L. D. et al. Discovery of Novel, Potent Benzamide Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1 (11β-HSD1) Exhibiting Oral Activity in an Enzyme Inhibition ex Vivo Model. J. Med. Chem. 51, 3953–3960 (2008). [DOI] [PubMed] [Google Scholar]
- Goldberg F. W. et al. Free-Wilson and Structural Approaches to Co-optimizing Human and Rodent Isoform Potency for 11β-Hydroxysteroid Dehydrogenase Type 1 (11β-HSD1) Inhibitors. J. Med. Chem. 55, 10652–10661 (2012). [DOI] [PubMed] [Google Scholar]
- Gao L., Chiou W. J., Camp H. S., Burns D. J. & Cheng X. Quantitative measurements of corticosteroids in ex vivo samples using on-line SPE-LC/MS/MS. J. Chromatogr. B 877, 303–310 (2009). [DOI] [PubMed] [Google Scholar]
- Khavrutskii L. et al. Protein Purification-free Method of Binding Affinity Determination by Microscale Thermophoresis. JOVE-J. Vis. Exp. e50541 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. L. & Simon N. Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature 507, 68–72 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. et al. The high resolution structures of human, murine and guinea pig 11-beta-hydroxysteroid dehydrogenase type 1 reveal critical differences in active site architecture. doi: 10.2210/pdb2bel/pdb (2004). [DOI]
- Abagyan R., Totrov M. & Kuznetsov D. Docking and Structure Prediction from the Distorted Native Conformation. J. Comput. Chem. 15, 488–506 (1994). [Google Scholar]
- Li Z. & Scheraga H. A. Monte Carlo-minimization approach to the multiple-minima problem in protein folding. Proc. Natl. Acad. Sci. 84, 6611–6615 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.