Abstract
The treatment of advanced non-small cell lung cancer (NSCLC) is currently driven by the detection of targetable oncogenic drivers, i.e. epidermal growth factor receptor, echinoderm microtubule-associated protein-like 4–anaplastic lymphoma kinase, etc. Those patients who are wildtype for known and valuable oncogenes can receive standard chemotherapy as first-line treatment, with the possibility of adding bevacizumab. With regard to second-line treatment, nintedanib can improve the efficacy of docetaxel. Nintedanib is a tyrosine kinase inhibitor targeting three angiogenesis-related transmembrane receptors. The usefulness of nintedanib as an anticancer agent for NSCLC has been proved by both preclinical and clinical phase I and II trials; however, its approval for the use in clinical practice has been possible because of the positive results of the LUME-Lung 1 trial (nintedanib + docetaxel versus docetaxel alone) in terms of progression-free survival and overall survival, and a manageable tolerability profile. Therefore, the good results seen in the clinical trials with nintedanib in the second-line setting for NSCLC patients with adenocarcinoma subtype are encouraging enough to recommend it in clinical practice.
Keywords: angiogenesis, FGFR, nintedanib, NSCLC, PDGFR, targeted therapy, VEGFR
Introduction
We are experiencing very exciting days in thoracic oncology thanks to the advent of new promising compounds which will shortly lead to a radical change in the treatment algorithm of non-small cell lung cancer (NSCLC). Targeted therapies, including epidermal growth factor receptor (EGFR) and echinoderm microtubule-associated protein-like 4–anaplastic lymphoma kinase (EML-ALK4) tyrosine kinase inhibitors (TKIs), represent the standard first-line therapy for EGFR-mutated or EML-ALK translocated NSCLC, showing a clear superiority compared with platinum-based chemotherapy [Bronte et al. 2014; Rolfo et al. 2014]. Although targetable ‘oncogene addicted’ tumors affect only a minority of patients, accounting for about 15–20% of the overall NSCLC population, other oncogenic drivers such as ROS1, cMet, human epidermal growth factor receptor type 2 (HER-2) and BRAF have been recently identified and clinical trials are investigating the activity of new targeted agents in order to extend the number of patients who may benefit from tailored approaches [Passiglia et al. 2015a; Bronte et al. 2010]. However, ‘non-oncogene addicted’ tumors currently represent the majority of NSCLC cases, classified into squamous versus nonsquamous histotype. For them, histology-driven platinum-based combinations with gemcitabine, pemetrexed and/or bevacizumab remain the standard first-line treatment, reaching a survival plateau of 14–16 months, including maintenance strategy [Paz-Ares et al. 2013; Lopez-Chavez et al. 2012]. Docetaxel, pemetrexed and erlotinib are recommended as equal second-line options [Peters et al. 2012], while erlotinib represent the only drug approved as third-line therapy on the basis of the BR21 trial results [Shepherd et al. 2005].
The question of comparative efficacy between EGFR-TKI and single agent chemotherapy in wildtype NSCLC populations has been addressed by several trials and meta-analysis [Zhao et al. 2014; Li et al. 2014], producing controversial results. Therefore, the choice of the best second-line therapy remains a matter of discussion and ongoing research. Combining anti-angiogenic agents with chemotherapy has recently emerged as a very promising strategy in this setting of patients. Two phase III randomized studies, comparing the oral triple TKI, nintedanib, and the anti vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody, ramucirumab, both in combination with docetaxel versus docetaxel alone, have displayed a significant survival advantage in favor of the combination arms in patients who progressed to platinum-based chemotherapy [Reck et al. 2014; Garon et al. 2014], leading to their approval by regulatory agencies.
In this review we focus our attention on nintedanib, which has been recently approved by European Medicine Agency (EMA) for the second-line treatment of NSCLC patients with adenocarcinoma subtype. We briefly describe the angiogenesis process and preclinical activity of such compound in NSCLC models. All clinical evidence emerging from phase I–III trials are subsequently reported, with discussion of controversial aspects of competition with the other compounds and ultimately looking at its best place in NSCLC therapy.
Angiogenesis of NSCLC
Cancer growth and its metastatic capability are sustained by angiogenesis, which is their lifeblood [Folkman, 1990]. Genetic and epigenetic events induce the ‘angiogenic switch’, a shift from a vascular tumor phenotype to a neovascularization condition which lends strength to the mass, simplifying the migration of endothelial cells and the metastatic process [Pallis and Syrigos, 2013]. In this event, the balance of pro-angiogenetic vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), fibroblast growth factor α (TGF-α), platelet derived growth factor (PDGF), granulocyte-macrophage colony stimulating factor (GM-CSF), tumor necrosis factor α (TNF-α), placenta growth factor (PIGF), cytokines such as interleukin (IL-1, IL-6, IL-8) and monocyte chemotactic protein-1 (MCP-1), endothelin 1 (ET-1), endothelin 2 (ET-2) and protease matrix metalloproteinases (MMPs), cathepsins, gelatinase A and B, stromelysin and urokinase-type plasminogen activator (uPA), and anti-angiogenetic factors such as thrombospondin-1 (TSP-1), angiostatin moves in favor of the major secretion of pro-factors. Indeed, NSCLC tumor cells show the overexpression of VEGF, the most important vessel growth factor capable of controlling migration, proliferation, invasion and survival of endothelial cells, in both physiological and pathological ways [Jackson et al. 2010].
The signaling pathway, arising from the relationship between VEGF and its receptors (VEGFR), acts alone or in synergy with platelet-derived growth factor (PDGF) receptors (PDGFRs), fibroblast growth factor (FGF) receptors (FGFR) and angiopoietin (Ang)/endothelial TEK tyrosine kinase (Tie2), and seems critical for the growth of new vessels and the tumor cell survival [Onimaru and Yonemitsu, 2011]. Several studies have indicated that the tissue overexpression of VEGF and of these three molecules appears to be associated with poor prognosis and probable increase of angiogenesis, suggesting a potential prognostic role in NSCLC patients [Villaruz and Socinski, 2015]. The expression of VEGF is also regulated by PDGF through autocrine mechanisms. A higher percentage of NSCLC patients with high PDGF tissue levels may have a major resistance to VEGF-targeted therapy. In order to confirm this, in vitro preclinical studies have shown a greater therapeutic efficacy by both VEGF and PDGF inhibition than inhibiting only VEGF. This evidence may also indicate the dependence of angiogenesis on the co-expression of VEGF and PDGF in NSCLC tumors. In addition, the concurrent overexpression of VEGF and FGF or Ang ligands seems to be likely involved in the regulation of angiogenesis and in a worse prognosis of NSCLC patients, instead of the high expression of VEGF alone [Piperdi et al. 2014]. Hypoxia, angiogenesis and the NSCLC development are part of a network of events that may be regulated by VEGF-targeted therapy.
Preclinical development of nintedanib
Nintedanib was identified during a program for small molecule inhibitors of angiogenesis and studies were extended to various solid tumors. Recent evidence shows that nintedanib is a potent endothelial cell proliferation inhibitor with a good safety profile – proven in both in vitro and in vivo studies.
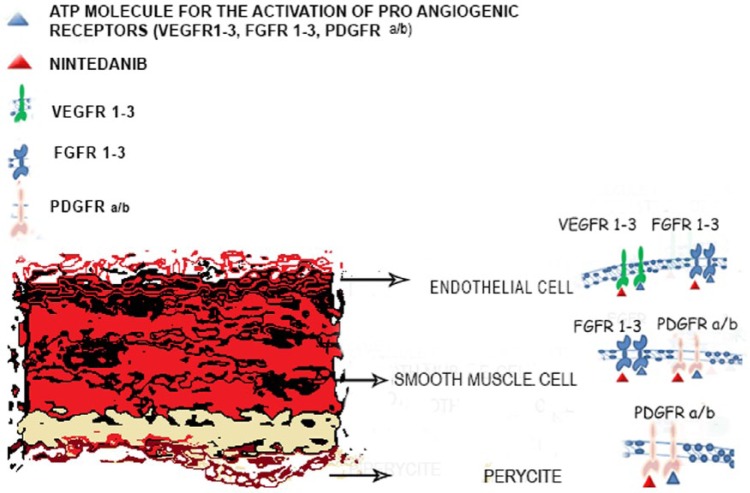
Nintedanib is an indolinone derivative, multiple TKI. This molecule occupies the adenosine triphosphate (ATP) binding sites in the kinase domain of pro-angiogenic receptors, including VEGFR-1, VEGFR-2, VEGFR-3, FGFR-1, FGFR-2, FGFR-3, PDGFR-α and PDGFR-β, inhibiting the downstream signaling pathways. Nintedanib also inhibits FMS-like tyrosine kinase 3 (FLT3) and SRC family members, but does not affect other kinases such as insulin-like growth factor receptor (IGFR) and the epidermal growth factor receptor (EGFR) (Figure 1) [Rolfo et al. 2013]. In vitro studies showed that treatment with nintedanib induced proliferation arrest and apoptosis in three cell types involved in angiogenesis (endothelial cells, smooth muscle cells and pericytes) through the inhibition of both AKT and mitogen-activated protein kinases (MAPK) signaling pathways, resulting in an overexpression of the apoptosis marker cleaved caspase-3 [Hilberg et al. 2008].
Figure 1.
Nintedanib mechanism of action.
ATP, adenosine triphosphate; FGFR, fibroblast growth factor receptor; PDGFR, platelet-derived growth factor; VEGFR, vascular endothelial growth factor receptor 2.
These data have been confirmed by in vivo studies performed in human NSCLC xenografts. One of these studies analyzed the effects of nintedanib both as a single agent and in combination with standard chemotherapy, showing a potent inhibition of proliferation and increased apoptosis of tumor cells in xenografts that did not respond to platinum-based chemotherapy and bevacizumab. In addition, nintedanib reduced vessel permeability and perfusion, microvessel density (CD31) and pericyte coverage (NG2), resulting in a potent antiangiogenic effect. The activity of nintedanib was not associated with an increased expression of the epithelial mesenchymal transition (EMT) markers, which is a common mechanism of resistance to anti-angiogenic therapies [Kutluk Cenik et al. 2013]. Another recent study evaluating the cotreatment of nintedanib with small interfering RNAs (siRNAs) against six specific genes involved in EMT has shown that this molecule is able to induce reversal of EMT by the regulation of two mesenchymal genes, SYDE1 and ZEB1 [Huang et al. 2015].
The toxic potential of nintedanib has been thoroughly evaluated in a toxicological program including in vitro and in vivo studies. The majority of these studies showed a tolerable safety profile of this compound excluding any severe cardiovascular, respiratory, or neurological adverse effects as well as any mutagenic potential of nintedanib [Roth et al. 2015].
Evidence from clinical trials with nintedanib
Phase I and phase II trials
Nintedanib activity has been studied in different kinds of neoplasm such as ovarian cancer, NSCLC, breast cancer, glioblastoma, colorectal cancer, urothelial carcinoma, biliary tract carcinoma, and head and neck cancer, which provided substantial information about the tolerability of this drug [Mross et al. 2010]. With regard to its use in advanced NSCLC, Doebele and colleagues investigated nintedanib use in combination with paclitaxel and carboplatin in chemotherapy-naïve advanced NSCLC [Doebele et al. 2012]. In this trial, 26 patients received nintedanib at the starting dose of 50 mg twice daily on days 2–21 in association with paclitaxel at the dose of 200 mg/m2 and carboplatin at area under the curve (AUC) 5 on day 1 of each 21 day cycle. The treatment was well tolerated with only a few dose-limiting toxicities such as liver enzyme elevations, thrombocytopenia, abdominal pain and rash that did not affect the pharmacokinetic (PK) parameters of the nintedanib or chemotherapeutic backbone.
The scientific community is also waiting for the results of another phase I/II study in which nintedanib was added to gemcitabine/cisplatin as first-line treatment of advanced or recurrent NSCLC, specifically of squamous histology to evaluate its activity in this group of patients. In another dose escalation phase I trial, nintedanib in association with pemetrexed was investigated in a cohort of 26 patients with advanced NSCLC previously treated with a first-line platinum-based chemotherapy. The schedule of treatment was nintedanib given at staring dose of 100 mg twice daily on days 2–21 in association with a standard dose of pemetrexed (500 mg/m2) on day 1 of a 21 day cycle. The resultant maximum tolerated dose (MTD) of nintedanib was determined to be 200 mg twice daily; similar to the previous studies, nintedanib showed a good safety profile [17 dose limiting toxicities (DLT)] with interesting clinical efficacy (1 complete response, 2 partial responses and 8 stable disease of 20 evaluable patients) [Ellis et al. 2010].
The same MTD of nintedanib (200 mg twice daily) was established in another Japanese trial [Daga et al. 2013], where similar efficacy results as the previous studies was found and showing proof of efficacy of this drug in an Asiatic population. In another more recent Asiatic experience, Okamoto and colleagues evaluated the combination of nintedanib with docetaxel in advanced NSCLC patients who had been previously treated. They found promising and encouraging safety and efficacy results, obtaining a 73% of disease control rate and only 12 DLTs [Okamoto et al. 2015]. The time-to-progression and objective response rate found in this study provided the rationale to continue investigation about this drug within large randomized controlled trials.
Phase III trials
With regard to the evaluation of nintedanib in a wider population, its efficacy was tested in two randomized controlled phase III trials, with the aim of improving the clinical result of the standard cytotoxic agents in advanced NSCLC treatment. Reck and colleagues investigated the role of nintedanib (200 mg twice daily on days 2–21) in addition to docetaxel (75 mg/m2) on day 1 of 21 day cycle compared with docetaxel (75 mg/m2) plus placebo in the LUME-Lung 1 trial. The primary endpoint was progression-free survival (PFS). PFS was assessed by an independent central review, analyzed after 714 events in all patients. Overall survival (OS) was predefined as a key secondary outcome; other secondary outcomes were investigator-assessed PFS, tumor response by central review and investigator assessment, safety and tolerability. With regard to EGFR status evaluation, there was no preplanned subgroup analysis for this item because the test was not included in clinical practice at the time of the trial design.
The nintedanib plus docetaxel group showed a significantly longer PFS as determined by central independent review than the control group [median PFS 3.4 versus 2.7 months, respectively; hazard ratio (HR) 0.85; 95% confidence interval (CI) 0.75–0.96; p = 0.0019], with a more pronounced benefit in patients with adenocarcinoma histology (median PFS 4.2 versus 2.8 months, respectively; HR 0.84; 95% CI 0.71–1.00; p = 0.0485). OS showed only a trend in favor of the combination (median OS 10.1 versus 9.1 months; HR 0.94; 95% CI 0.83–1.05; p = 0.272) in all histologies, with more benefit in the adenocarcinoma subgroup (median OS 12.6 versus 10.3 months, respectively; HR 0.83; 95% CI 0.70–0.99; p = 0.0359) (Table 1). The tolerability profile was similar to that shown in phase I/II exploratory trials. Patients most commonly experienced diarrhea, increases of transaminases, nausea, decreased appetite and vomiting – all easily manageable by supportive treatment or dose reduction. Note that the combination of nintedanib plus docetaxel increased not only PFS in all NSCLC histologies, but also improved OS even in the adenocarcinoma subgroup, including those patients who experienced progression of disease within 9 months of start of first-line therapy [Reck et al. 2014].
Table 1.
Outcomes of nintedanib in the LUME-Lung 1 study.
| Outcomes | All histologies | Adenocarcinoma |
|---|---|---|
| (Nintedanib + docetaxel versus docetaxel) | (Nintedanib + docetaxel versus docetaxel) | |
| ORR (%) | 4.4 versus 3.3 | N.A |
| DCR (%) | 54 versus 41.3 | N.A |
| mPFS | 3.4 versus 2.7 months (HR 0.85, 95% CI 0.75–0.96, p = 0.0019) | 4.2 versus 2.8 months (HR 0.84, 95% CI 0.71–1.00, p = 0.0485) |
| mOS | 10.1 versus 9.1 (HR 0.94, 95% CI 0.83–1.05], p = 0.2720) | 12.6 versus 10.3 (HR 0.83, 95% CI 0.70–0.99, p = 0.0359) |
DCR, disease control rate; mOS, median overall survival; mPFS, median progression-free survival; N.A, not available; ORR, objective response rate.
Nintedanib, at the same dose, was also investigated in combination with pemetrexed (500 mg/m2) and compared with pemetrexed (500 mg/m2) plus placebo in the LUME-Lung 2 phase III controlled randomized trial [Hanna et al. 2013]. The primary endpoint was the same (PFS) based on independent central review and a preplanned futility analysis was performed to verify if the primary endpoint was unlikely to be met. The study met its primary endpoint for the combination arm (4.4 versus 3.6 months compared with placebo; HR 0.83; 95% CI 0.70–0.99; p = 0.0435), although the recruitment was stopped prematurely and this difference has not translated into an OS benefit (12.2 versus 12.7 months, respectively; HR 1.03; 95% CI 0.85–1.24; p = 0.7921). Also in this study, nintedanib showed relatively high increases in aspartate transaminase (AST) and alanine aminotransferase (ALT) liver enzymes and gastrointestinal events, but simple to resolve with standard medications.
Based on new knowledge in the field of molecular biology, interesting data could emerge from an ongoing phase III study [ClinicalTrials.gov identifier: NCT02299141] that is evaluating the effectiveness of nintedanib in molecularly selected NSCLC patients, with investigation of the potential role of some genes (VEGFR1-3, PDGFR-A, PDGFR-B and FGFR1-3) that could be involved in the regulation of mechanisms of acquired resistance to anti-angiogenic agents.
Place of nintedanib in the current treatment algorithm of NSCLC
The results of the LUME-Lung 1 trial interrupted the negative trend of anti-angiogenic agent efficacy in NSCLC. Since the therapeutic success obtained by bevacizumab in the first-line setting, several multi-TKIs, including sunitinib, sorafenib, vandetanib and motesanib, had been investigated in clinical trials, both as single agents or in combination with chemotherapy, but failing to demonstrate any impact on the OS of NSCLC patients [Scagliotti et al. 2010, 2012a, 2012b; Paz-Ares et al. 2012; de Boer et al. 2011; Herbst et al. 2010]. After several years of research, this is the first study showing a modest, but significant OS advantage in favor of a combination regimen over the standard single-agent therapy in NSCLC patients who progressed to first-line platinum chemotherapy [Reck et al. 2014]. Specifically, the addition of nintedanib to docetaxel seems to benefit the subgroup of patients with adenocarcinoma histology (median OS 12.6 versus 10.3 months; HR 0.83; 95% CI 0.70–0.99; p = 0.0359), including patients with poor prognosis who progressed within 9 months from beginning of single-line treatment (median OS 10.9 versus 7.9 months; HR 0.75; 95% CI: 0.60–0.92; p = 0.0073). Patient-reported outcomes for symptoms and quality of life (QoL) from the LUME-Lung 1 are also encouraging, showing no detrimental effect on patients’ QoL [Novello et al. 2015] and further supporting the use of such drug in this setting of patients.
At the same time, the REVEL phase III randomized study investigated the addition of another anti-angiogenic agent, the anti-VEGFR2 monoclonal antibody, ramucirumab, to docetaxel in NSCLC patients who failed prior platinum-based chemotherapy. The results of this trial have also shown a significant superiority of combination therapy versus docetaxel alone in the overall population, including both squamous and nonsquamous histotypes [Garon et al. 2014], thus confirming that combining angiogenesis inhibitors with chemotherapy is an effective treatment strategy in the second-line setting. A careful analysis of the phase III LUME-Lung 1 and REVEL trials suggests that the characteristics of included patients were similar in both studies. In particular, the percentage of patients who received prior anti-angiogenic therapies, such as bevacizumab, was very low as was the number of elderly patients, while patients with performance status of 2 were not admitted in both the studies. Interestingly, a recent subgroup analysis of the LUME-Lung 1 has shown that adenocarcinoma patients treated with nintedanib seem to maintain an OS benefit independently of prior first-line therapy with bevacizumab (median OS 14.9 versus 8.7 months; HR 0.61; 95% CI 0.31–1.20), even if it did not reach a statistical significance because of the small number of such patients [Heigener et al. 2015]. Information about EGFR mutation status was known in only 30% of patients in the REVEL study and in none of the patients in the LUME-Lung 1 study (similarly for EML-ALK status), thus limiting any definitive consideration on the efficacy of both drugs in the subgroup of oncogene-addicted patients.
A similarly manageable tolerability profile has emerged from both studies, and data on QoL showed that it did not deteriorate with the addition of anti-angiogenic agents to chemotherapy. However, there are also differences in the safety of these two compounds. A significantly higher incidence of gastrointestinal events, including diarrhea, nausea, vomiting and increases in liver enzymes was reported in the nintedanib plus docetaxel arm compared with the control arm in the LUME-Lung 1 study. Adverse events usually associated to anti-angiogenic therapies, including bleeding/hemorrhage and hypertension occurred more frequently with the ramucirumab plus docetaxel combination, while they did not significantly increase with nintedanib [Reck et al. 2015]. The two anti-angiogenic drugs are different in terms of extension of OS benefit. In the LUME-Lung 1 study, this benefit was limited to patients with adenocarcinoma histology, whereas in the REVEL study it was extended to the overall NSCLC population. However the percentage of patients with squamous histology and treated with ramucirumab were very low (25%) to demonstrate the clinical efficacy of such agent in this subset of patients. On the basis of such scientific evidence, nintedanib plus docetaxel has been recently approved by the EMA as a second-line treatment for patients with lung adenocarcinoma while ramucirumab plus docetaxel received US Food and Drug Administration (FDA) approval as a second-line option for the overall NSCLC population.
We recently proposed a new treatment algorithm describing the sequences of therapy from first to third-line for non-oncogene addicted adenocarcinoma patients, suggesting the combination of both nintedanib and ramucirumab with docetaxel as a new standard second-line option in non elderly and Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) PS 0–1 patients who progressed to first-line platinum regimens [Bronte et al. 2015]. To date, there are no clinical trials comparing both nintedanib and/or ramucirumab plus docetaxel combinations with standard second-line options other than docetaxel. The comparisons between single-agent chemotherapy and the EGFR-TKI, erlotinib, suggested a potential survival benefit in favor of chemotherapy in EGFR wildtype NSCLC patients [Vale et al. 2015; Zhao et al. 2014; Osarogiagbon et al. 2015], thus providing indirect evidence about the superiority of combination regimens over EGFR-TKI.
Even if the addition of nintedanib to pemetrexed in the LUME-Lung 2 study has led to controversial results [Hanna et al. 2013], the fact remains that pemetrexed is currently used in combination with platinum as first-line treatment in most European patients with EGFR/ALK-WT lung adenocarcinoma. Therefore, it may be left out of the debate about the best second-line option in Europe, where the combination of nintedanib plus docetaxel may be considered as a new standard of care. For US patients, a direct comparison between single-agent pemetrexed and ramucirumab plus docetaxel combination is needed to establish the best therapy for patients with nonsquamous histology, who often do not receive pemetrexed first line. Currently, there are no studies which make a direct comparison between nintedanib and ramucirumab in NSCLC. However, nintedanib was registered by EMA for European patients, whereas ramucirumab was registered by FDA in the US. Hence, these two drugs can currently be prescribed in different populations.
Place of nintedanib in the future treatment algorithm of NSCLC
As well as angiogenesis inhibition, immunotherapy is emerging as another promising strategy in NSCLC. Several immune-checkpoint inhibitors, including both anti programmed death 1 (PD1) and anti programmed death-ligand 1 (PD-L1) monoclonal antibodies, have shown great activity in heavily pretreated NSCLC patients included in early phase I studies [Gettinger et al. 2015; Garon et al. 2015], reaching an objective response rate (ORR) close to 20%. Two randomized phase III studies (CheckMate 017 and 057) recently compared the anti-PD1 monoclonal antibody, nivolumab, versus docetaxel in pretreated NSCLC patients with squamous and nonsquamous histology respectively, showing a significant survival improvement in favor of nivolumab in both studies [Brahmer et al. 2015; Borghaei et al. 2015]. On the basis of these positive results, nivolumab recently received FDA approval for the treatment of patients with squamous NSCLC after prior platinum-based chemotherapy and will probably receive approval for nonsquamous histology soon. Furthermore, pembrolizumab has also been approved by the FDA for treatment of advanced, PD-L1-positive NSCLC after other treatments, leading to an interesting debate regarding the best second-line option for non-oncogene addicted NSCLC patients in the near future.
The lack of a direct comparison between the immune-checkpoint inhibitors and the new standard second-line therapy, represented by nintedanib and ramucirumab plus docetaxel combinations along with the absence of long-term survival analysis from published studies, currently limits any evidence-based recommendation. In the meantime, a careful evaluation of the characteristics of patients candidate to receive a second-line therapy, including general health conditions, comorbidities, disease burden and tumor-related symptoms, responses and tolerability to the first-line therapy and concomitant medications, together with the drug’s activity and safety profiles emerging from clinical studies could help to choice the best option to each of them. Indeed adenocarcinoma patients ‘fit for chemotherapy’, including non-elderly patients with PS 0–1, without significant comorbidities, who have tolerated first-line treatment well, or who have progressed within 9 months from beginning of first-line treatment could be the best candidates for nintedanib plus docetaxel combination in second-line. Conversely, older/PS2 people, current/former smokers or patients who experienced hematological toxicities from first-line therapy should receive immunotherapy as preferred option, excluding those with viral hepatitis or autoimmune disease or CNS symptomatic metastasis in treatment with steroids who are not eligible for such kind of treatment. However, strong evidence about the efficacy of immunotherapy in these subsets of patients have not been available yet.
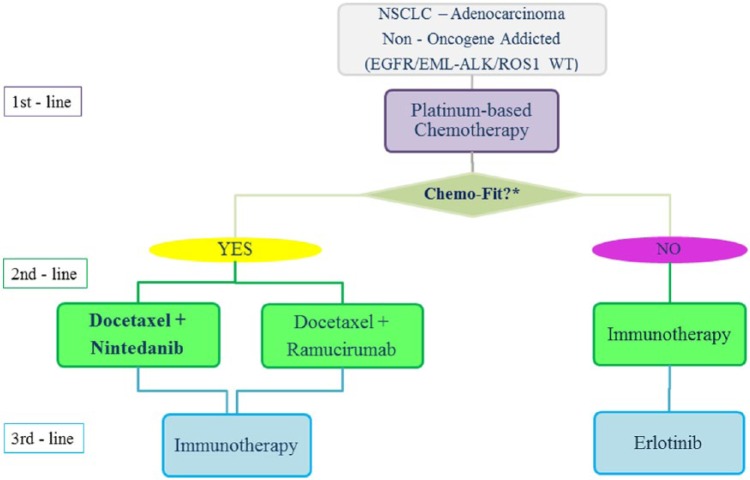
Furthermore, we have evidence about the activity of immune-checkpoint inhibitors in NSCLC after second-line treatment [Besse et al. 2015], but we do not have that data for nintedanib. This should also be taken into account in the design of a potential treatment algorithm in order to offer all the treatment options available to our patients and ultimately try to extend their survival outcomes. On the basis of such available evidence, we propose here a new treatment algorithm describing the sequence of therapy from first-line to third-line treatment for non-oncogene addicted adenocarcinoma patients, including also the new immune-checkpoint inhibitors which will be approved early for this setting of patients (Figure 2). Based on studies mostly on immune-checkpoint inhibitors in NSCLC, a recent pooled analysis performed by our group has recently confirmed that tumor PD-L1 expression is significantly associated with higher ORR in pretreated NSCLC receiving different anti-PD1 or anti-PD-L1 monoclonal antibodies [Passiglia et al. 2015b]. The identification and clinical validation of reliable predictive biomarkers for both anti-angiogenic treatments and immunotherapies is likely to help oncologists to select the best therapy for each patient in the near future.
Figure 2.
Treatment algorithm in non-oncogene addicted NSCLC adenocarcinoma including new emerging options.
*See text for definition of ‘Chemo-Fit’ criteria.
ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; EML, Echinoderm microtubule-associated protein-like; NSCLC, non-small cell lung cancer; WT, wildtype.
Conclusion
For a long time, a slight improvement of NSCLC prognosis has been achieved by standard chemotherapy. In this context, platinum-based regimens represented the backbone for first-line treatment. The findings about the impact of pro-angiogenic markers on NSCLC natural history yielded the addition of bevacizumab to standard first-line chemotherapy for patients with nonsquamous histology. However, a striking antitumor effect and a sizeable outcome improvement have been observed only with the introduction of TKIs for targetable oncogenic drivers.
In this landscape of novel treatments for NSCLC, nintedanib provides the opportunity to improve outcomes for docetaxel-based second-line therapy for patients with adenocarcinoma. Fortunately, this achievement has been obtained at the expense of manageable higher rates of hematological and gastrointestinal toxicity. For this reason, nintedanib should be considered for patients with adenocarcinoma who can undergo second-line chemotherapy.
Acknowledgments
Dr Chiara Drago contributed to the revision of the English language of this manuscript.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Giuseppe Bronte, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Italy.
Francesco Passiglia, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Italy.
Antonio Galvano, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Italy.
Nadia Barraco, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Italy.
Angela Listì, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Italy.
Marta Castiglia, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Italy.
Sergio Rizzo, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Italy.
Eugenio Fiorentino, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Italy.
Viviana Bazan, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Italy.
Antonio Russo, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Via del Vespro 129, 90127 Palermo, Italy.
References
- Besse B., Johnson M., Janne P., Garassino M., Eberhardt W., Peters S., et al. (2015) Phase II, single-arm trial (BIRCH) of atezolizumab as first-line or subsequent therapy for locally advanced or metastatic PD-L1-selected non-small cell lung cancer (NSCLC). Ann Oncol 26 (Suppl. 6): abstract 16LBA. [Google Scholar]
- Borghaei H., Paz-Ares L., Horn L., Spigel D., Steins M., Ready N., et al. (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer, N Engl J Med 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Reckamp K., Baas P., Crinò L., Eberhardt W., Poddubskaya E., et al. (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer, N Engl J Med 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte G., Passiglia F., Galvano A., Russo A. (2015) Anti-angiogenic drugs for second-line treatment of NSCLC patients: just new pawns on the chessboard?, Expert Opin Biol Ther 3 August 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Bronte G., Rizzo S., La Paglia L., Adamo V., Siragusa S., Ficorella C., et al. (2010) Driver mutations and differential sensitivity to targeted therapies: a new approach to the treatment of lung adenocarcinoma, Cancer Treat Rev 36(Suppl. 3): S21–S29. [DOI] [PubMed] [Google Scholar]
- Bronte G., Rolfo C., Giovannetti E., Cicero G., Pauwels P., Passiglia F., et al. (2014) Are erlotinib and gefitinib interchangeable, opposite or complementary for non-small cell lung cancer treatment? Biological, pharmacological and clinical aspects, Crit Rev Oncol Hematol 89: 300–313. [DOI] [PubMed] [Google Scholar]
- Daga H., Takeda K., Okada H., Miyazaki M., Ueda S., Kaneda H., et al. (2013) Safety and efficacy of nintedanib (BIBF 1120) plus pemetrexed in Japanese patients with advanced or recurrent non-small cell lung cancer (NSCLC): a phase I study.J Clin Oncol 31(Suppl.): abstract 8056. [Google Scholar]
- De Boer R., Arrieta Ó., Yang C., Gottfried M., Chan V., Raats J., et al. (2011) Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial, J Clin Oncol 29: 1067–1074. [DOI] [PubMed] [Google Scholar]
- Doebele R., Conkling P., Traynor A., Otterson G., Zhao Y., Wind S., et al. (2012) A phase I, open-label dose-escalation study of continuous treatment with BIBF 1120 in combination with paclitaxel and carboplatin as first-line treatment in patients with advanced non-small-cell lung cancer, Ann Oncol 23: 2094–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis P., Kaiser R., Zhao Y., Stopfer P., Gyorffy S., Hanna N. (2010) Phase I open-label study of continuous treatment with BIBF 1120, a triple angiokinase inhibitor, and pemetrexed in pretreated non-small cell lung cancer patients, Clin Cancer Res 16: 2881–2889. [DOI] [PubMed] [Google Scholar]
- Folkman J. (1990) What is the evidence that tumors are angiogenesis dependent?, J Natl Cancer Inst 82: 4–6. [DOI] [PubMed] [Google Scholar]
- Garon E., Ciuleanu T., Arrieta O., Prabhash K., Syrigos K., Goksel T., et al. (2014) Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial, Lancet 384: 665–673. [DOI] [PubMed] [Google Scholar]
- Garon E., Rizvi N., Hui R., Leighl N., Balmanoukian A., Eder J., et al. (2015) Pembrolizumab for the treatment of non-small-cell lung cancer, N Engl J Med 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- Gettinger S., Horn L., Gandhi L., Spigel D., Antonia S., Rizvi N., et al. (2015) Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer, J Clin Oncol 33: 2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna N., Kaiser R., Sullivan R., Aren O., Ahn M., Tiangco B., et al. (2013) LUME-lung 2: a multicenter, randomized, double-blind, phase III study of nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after failure of first-line chemotherapy. J Clin Oncol 31(Suppl.): abstract 8034. [Google Scholar]
- Heigener D., Reck M., Mellemgaard A., Orlov S., Krzakowski M., von Pawel J., et al. (2015) Efficacy of nintedanib plus docetaxel after bevacizumab, pemetrexed or taxane therapy. Presented at 16th World Conference on Lung Cancer (WCLC 2015), mini-oral 17.07. http://library.iaslc.org/search-speaker?search_speaker=32414 [Google Scholar]
- Herbst R., Sun Y., Eberhardt W., Germonpré P., Saijo N., Zhou C., et al. (2010) Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial, Lancet Oncol 11: 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilberg F., Roth G., Krssak M., Kautschitsch S., Sommergruber W., Tontsch-Grunt U., et al. (2008) BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy, Cancer Res 68: 4774–4782. [DOI] [PubMed] [Google Scholar]
- Huang R., Kuay K., Tan T., Asad M., Tang H., Ng A., et al. (2015) Functional relevance of a six mesenchymal gene signature in epithelial-mesenchymal transition (EMT) reversal by the triple angiokinase inhibitor, nintedanib (BIBF1120), Oncotarget 6: 22098–22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A., Zhou B., Kim W. (2010) HIF, hypoxia and the role of angiogenesis in non-small cell lung cancer, Expert Opin Ther Targets 14: 1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutluk Cenik B., Ostapoff K., Gerber D., Brekken R. (2013) BIBF 1120 (nintedanib), a triple angiokinase inhibitor, induces hypoxia but not EMT and blocks progression of preclinical models of lung and pancreatic cancer, Mol Cancer Ther 12: 992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Yang L., Ou W., Zhang L., Zhang S., Wang S. (2014) Meta-analysis of EGFR tyrosine kinase inhibitors compared with chemotherapy as second-line treatment in pretreated advanced non-small cell lung cancer, PLoS One 9: e102777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Chavez A., Young T., Fages S., Leon L., Schiller J., Dowlati A., et al. (2012) Bevacizumab maintenance in patients with advanced non-small-cell lung cancer, clinical patterns, and outcomes in the Eastern Cooperative Oncology Group 4599 Study: results of an exploratory analysis, J Thorac Oncol 7: 1707–1712. [DOI] [PubMed] [Google Scholar]
- Mross K., Stefanic M., Gmehling D., Frost A., Baas F., Unger C., et al. (2010) Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors, Clin Cancer Res 16: 311–319. [DOI] [PubMed] [Google Scholar]
- Novello S., Kaiser R., Mellemgaard A., Douillard J., Orlov S., Krzakowski M., et al. (2015) Analysis of patient-reported outcomes from the LUME-Lung 1 trial: a randomised, double-blind, placebo-controlled, Phase III study of second-line nintedanib in patients with advanced non-small cell lung cancer, Eur J Cancer 51: 317–326. [DOI] [PubMed] [Google Scholar]
- Okamoto I., Miyazaki M., Takeda M., Terashima M., Azuma K., Hayashi H., et al. (2015) Tolerability of nintedanib (BIBF 1120) in combination with docetaxel: a phase 1 study in Japanese patients with previously treated non-small-cell lung cancer, J Thorac Oncol 10: 346–352. [DOI] [PubMed] [Google Scholar]
- Onimaru M., Yonemitsu Y. (2011) Angiogenic and lymphangiogenic cascades in the tumor microenvironment, Front Biosci (Schol Ed) 3: 216–225. [DOI] [PubMed] [Google Scholar]
- Osarogiagbon R., Cappuzzo F., Ciuleanu T., Leon L., Klughammer B. (2015) Erlotinib therapy after initial platinum doublet therapy in patients with EGFR wild type non-small cell lung cancer: results of a combined patient-level analysis of the NCIC CTG BR.21 and SATURN trials, Transl Lung Cancer Res 4: 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallis A., Syrigos K. (2013) Targeting tumor neovasculature in non-small-cell lung cancer, Crit Rev Oncol Hematol 86: 130–142. [DOI] [PubMed] [Google Scholar]
- Passiglia F., Bronte G., Castiglia M., Listì A., Calò V., Toia F., et al. (2015a) Prognostic and predictive biomarkers for targeted therapy in NSCLC: for whom the bell tolls?, Expert Opin Biol Ther 15: 1553–1566. [DOI] [PubMed] [Google Scholar]
- Passiglia F., Bronte G., Rizzo S., Galvano A., Sortino G., Musso E., et al. (2015b) PD-L1 expression as predictive biomarker in patients with NSCLC: a pooled analysis. Presented at 16th World Conference on Lung Cancer (WCLC 2015), mini-oral 31.01. http://library.iaslc.org/search-speaker?search_speaker=35078 [Google Scholar]
- Paz-Ares L., Biesma B., Heigener D., von Pawel J., Eisen T., Bennouna J., et al. (2012) Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer, J Clin Oncol 30: 3084–3092. [DOI] [PubMed] [Google Scholar]
- Paz-Ares L., de Marinis F., Dediu M., Thomas M., Pujol J., Bidoli P., et al. (2013) PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer, J Clin Oncol 31: 2895–2902. [DOI] [PubMed] [Google Scholar]
- Peters S., Adjei A., Gridelli C., Reck M., Kerr K., Felip E., et al. (2012) Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Ann Oncol 23(Suppl. 7): vii56–vii64. [DOI] [PubMed] [Google Scholar]
- Piperdi B., Merla A., Perez-Soler R. (2014) Targeting angiogenesis in squamous non-small cell lung cancer, Drugs 74: 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M., Kaiser R., Mellemgaard A., Douillard J., Orlov S., Krzakowski M., et al. (2014) Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial, Lancet Oncol 15: 143–155. [DOI] [PubMed] [Google Scholar]
- Reck M., Mellemgaard A., von Pawel J., Gottfried M., Bondarenko I., Cheng Y., et al. (2015) Anti-angiogenic-specific adverse events in patients with non-small cell lung cancer treated with nintedanib and docetaxel, Lung Cancer 90: 267–273. [DOI] [PubMed] [Google Scholar]
- Rolfo C., Passiglia F., Castiglia M., Raez L., Germonpre P., Gil-Bazo I., et al. (2014) ALK and crizotinib: after the honeymoon…what else? Resistance mechanisms and new therapies to overcome it, Transl Lung Cancer Res, 3(4),pp. 250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfo C., Raez L., Bronte G., Santos E., Papadimitriou K., Buffoni L., et al. (2013) BIBF 1120/ nintedanib : a new triple angiokinase inhibitor-directed therapy in patients with non-small cell lung cancer, Expert Opin Investig Drugs 22: 1081–1088. [DOI] [PubMed] [Google Scholar]
- Roth G., Binder R., Colbatzky F., Dallinger C., Schlenker-Herceg R., Hilberg F., et al. (2015) Nintedanib: from discovery to the clinic, J Med Chem 58: 1053–1063. [DOI] [PubMed] [Google Scholar]
- Scagliotti G., Novello S., von Pawel J., Reck M., Pereira J., Thomas M., et al. (2010) Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer, J Clin Oncol 28: 1835–1842. [DOI] [PubMed] [Google Scholar]
- Scagliotti G., Krzakowski M., Szczesna A., Strausz J., Makhson A., Reck M., et al. (2012a) Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase III trial, J Clin Oncol 30: 2070–2078. [DOI] [PubMed] [Google Scholar]
- Scagliotti G., Vynnychenko I., Park K., Ichinose Y., Kubota K., Blackhall F., et al. (2012b) International, randomized, placebo-controlled, double-blind phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1, J Clin Oncol 30: 2829–2836. [DOI] [PubMed] [Google Scholar]
- Shepherd F., Rodrigues Pereira J., Ciuleanu T., Tan E., Hirsh V., Thongprasert S., et al. (2005) Erlotinib in previously treated non-small-cell lung cancer, N Engl J Med 353: 123–132. [DOI] [PubMed] [Google Scholar]
- Vale C., Burdett S., Fisher D., Navani N., Parmar M., Copas A., et al. (2015) Should tyrosine kinase inhibitors be considered for advanced non-small-cell lung cancer patients with wild type EGFR? Two systematic reviews and meta-analyses of randomized trials, Clin Lung Cancer 16: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaruz L., Socinski M. (2015) The role of anti-angiogenesis in non-small-cell lung cancer: an update, Curr Oncol Rep 17: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N., Zhang X., Yan H., Yang J., Wu Y. (2014) Efficacy of epidermal growth factor receptor inhibitors versus chemotherapy as second-line treatment in advanced non-small-cell lung cancer with wild-type EGFR: a meta-analysis of randomized controlled clinical trials, Lung Cancer 85: 66–73. [DOI] [PubMed] [Google Scholar]