Abstract
Head and neck squamous cell cancer (HNSCC) is a malignancy with a rapidly changing demographic profile, given the recent epidemic of human papilloma virus related cancers. Most patients present with locally advanced disease and receive combination therapeutic approaches with curative potential, albeit with significant toxicity. Up to a third of patients, however, will eventually develop recurrent or metastatic disease. The prognosis of such patients is dismal, as palliative treatment options remain limited. Immune-directed therapies offer a novel therapeutic strategy beyond cytotoxic chemotherapy and are currently being evaluated in a wide variety of malignancies. HNSCC is a particularly favorable disease for immunotherapy, as immune evasion and dysregulation have been shown to play a key role in the initiation and progression of HNSCC. This review focuses on the latest developments in immunotherapy in HNSCC, with a particular focus on checkpoint inhibitors, adoptive cellular therapies, and vaccines.
Keywords: head and neck cancer, immunotherapy, PD-1 inhibitors, cancer vaccines, adoptive cellular therapies
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a relatively common cancer, with about 500,000 cases worldwide every year [Siegel et al. 2015]. A majority of the patients with HNSCC present with locally advanced disease and are managed in a multidisciplinary setting [Pignon et al. 2009]. Radiation therapy, surgery and chemotherapy are used in combination to maximize therapeutic benefit. For patients who experience disease recurrence not amenable to curative intent treatment and for those presenting with distant metastases, chemotherapy is the mainstay of therapy. Despite recent advances in treatment, including approval and use of epidermal growth factor receptor directed targeted therapies [Vermorken et al. 2008a, 2008b], overall survival in very advanced and recurrent or metastatic HNSCC remains very poor.
The immune system plays a key role in the development and progression of HNSCC. Recent advances in our understanding of the immune system and oncogenesis have accelerated development of the field of immunotherapy for cancer generally and for HNSCC in particular, with the genuine promise of expanding useful and potentially less toxic treatment options for patients. A variety of approaches are under evaluation, including immune checkpoint inhibitors, therapeutic vaccines, and adoptive T-cell therapies. We will summarize the latest developments in the emerging field of immunotherapy in HNSCC, with a particular focus on these modalities.
Immune checkpoint inhibitors
The human body utilizes a number of checkpoints to dampen the immune response and in this fashion limit damage to normal human tissues that would otherwise occur with an uncontrolled immune response to exogenous threats. By coopting these normal adaptive and protective pathways, tumor cells are thought to evade the immune system and grow uncontrolled in the host [Keir et al. 2008]. Checkpoint inhibitors block these normal immunoregulatory pathways, thereby enhancing immune surveillance and tumor cell clearance. While no checkpoint inhibitors have been approved to date for the treatment of HNSCC, multiple agents have been evaluated in clinical trials with preliminary and highly promising evidence of clinical efficacy.
Inhibition of the programmed death 1 (PD-1) pathway has been evaluated in multiple tumor types. PD-1 is a receptor frequently expressed on activated T cells. PD-L1 and PD-L2, the key ligands of PD-1, are expressed on normal tissues and lead to T-cell anergy when bound. Multiple tumors, including HNSCC, can express PD-L1, which contribute to the tumor’s ability to evade the immune system [Topalian et al. 2012; Lyford-Pike et al. 2013]. Pembrolizumab (Keytruda, Merck, Kenilworth, NJ) is a humanized immunoglobulin (Ig)-G4 monoclonal antibody with a high affinity for PD-1 [Hamid et al. 2013; Garon et al. 2015]. Pembrolizumab was studied in a phase I ‘basket study’ that included a cohort of patients with recurrent or metastatic HNSCC. In a heavily pretreated population of 150 patients (37.9% of patients had received at least three prior lines of therapy for metastatic disease), an overall response rate of 24.8% was observed, including one complete response. The median duration of response had not yet been reached at the time of presentation, with a range of 7.3–25.1 weeks. Response rate did not differ based upon p16 expression, a surrogate biomarker of human papilloma virus (HPV) status [Seiwert et al. 2015]. A phase II and phase III study are currently underway to more precisely characterize the efficacy (response rate, response duration and side effects) of pembrolizumab in recurrent or metastatic HNSCC [ClinicalTrials.gov identifiers: NCT02255097, NCT02252042]. Durvalumab (Medimmune, Gaithersburg, MD), an engineered IgG1 antibody to PD-L1, has also been evaluated in 62 patients with recurrent or metastatic HNSCC. The overall response rate was 12%, and the duration of response had not been reached at the time of presentation (range 4–43 weeks) [Segal et al. 2015]. Further studies are currently underway to evaluate this agent in HNSCC [ClinicalTrials.gov identifier: NCT02207530]. Nivolumab (Opdivo, Bristol-Myers Squibb, New York, NY) is another PD-1 inhibitor that is currently being evaluated in HNSCC, although no data have been pre-sented to date [ClinicalTrials.gov identifiers: NCT02105636, NCT02488759].
Ongoing efforts to identify a predictive biomarker of response to PD-1 blockade have been very challenging. PD-L1 staining was initially thought to be an appealing and scientifically rational option, and many studies of PD-1 inhibitors have been designed around this biomarker [Garon et al. 2015]. The use of PD-L1 as a biomarker is quite controversial, however, as PD-L1 expression is dynamic over time and predictably also displays intratumor heterogeneity [Taube et al. 2014]. Given these limitations, it is not surprising that the utility of this biomarker has been inconsistent [Brahmer et al. 2015; Garon et al. 2015; Gettinger et al. 2015; Rizvi et al. 2015]. In the two reported studies of PD-1 inhibition in HNSCC, responses do seem to be higher among patients with PD-L1 staining cancers [Seiwert et al. 2014; Segal et al. 2015]. Pembrolizumab recently gained approval in non-small cell lung cancer with a companion diagnostic PD-L1 assay to guide its use, so there may at least be emerging standardization in measurement of this biomarker. Whether this biomarker assay will be a part of any eventual US Food and Drug Administration labeling in HNSCC remains to be seen.
In general, the side-effect profile associated with PD-1 and PD-L1 blockade has been favorable. Side effects typical of cytotoxic chemotherapy, such as nausea and cytopenias, are nearly absent. The most common side effect of these agents is fatigue, usually grade 1–2. While there is a mechanistic concern for significant immune-mediated toxicity in normal tissues, which in the case of other checkpoint inhibitors such as ipilimumab has included cases of severe colitis, pneumonitis, hepatitis, hypophysitis and dermatitis, these events occur in less than 5% of patients (all cancer types) receiving PD-1 or PDL-1 inhibitors [Brahmer et al. 2015; Garon et al. 2015; Rizvi et al. 2015; Seiwert et al. 2015].
Novel pharmacologic agents that target other immune checkpoints and costimulatory receptors, including CTLA4, OX40, 4-1BB, LAG-3 and HLA-E, are currently being evaluated in a wide variety of tumors, including HNSCC. In addition, there are significant efforts underway to evaluate combinations of immune checkpoint inhibitors with a variety of other anticancer therapies, including radiation, surgery, and cytotoxic chemotherapy.
Vaccines
HPV-related HNSCC represents an ideal target for development of preventive and therapeutic HPV vaccines [Gillison et al. 2008]. Preventive HPV vaccines generate neutralizing antibodies against HPV major capsid protein L1, which are expressed on the mucous membranes, neutralizing the presenting HPV virus immediately and preventing infection. Commercially available vaccines include a bivalent vaccine (Cervarix; GlaxoSmithKline, Brentford, Middlesex, UK) approved for the prevention of HPV infections from types 16 and 18 and a nine-valent HPV vaccine (Gardasil-9; Merck and Co, Kenilworth, NJ) for prevention of HPV types 16, 18, 31, 33, 45, 52, and 58. These vaccines do not exert therapeutic effects on existing HPV-related lesions and do not have any effect on HPV-associated malignancy because they do not induce a cytolytic T-cell response. A cytolytic T-cell response is required to eliminate virally infected cells.
HPV-specific immunotherapeutic approaches are being developed to generate cellular immunity against HPV-infected cells with the goal of clearing existing HPV infections, eliminating preexisting HPV-related lesions and preventing the formation of new lesions. An understanding of the molecular progression of HPV-associated cancer has led to the conclusions that the HPV E6 and E7 proteins are useful targets for the development of HPV therapeutic vaccines [Bagarazzi et al. 2012]. These viral oncoproteins are expressed early in the course of infection and have multifunctional roles in oncogenesis. E6 promotes degradation of p53, indirectly activates telomerase, and disrupts the function of the cellular phosphatase tumor suppressor PTPN13, resulting in invasive tumor growth [Spanos et al. 2008]. E7 inactivates pRb and activates Mi2beta, a component of a histone deacetylase complex essential for oncogenic transformation [Brehm et al. 1999].
Together, these oncogenic alterations drive rapid cellular proliferation, suppress or downregulate key tumor suppressor proteins, and lead to cellular immortality. In addition, E6/E7 expression is required to maintain a malignant transformed phenotype. Persistence of malignant cells, in fact, requires the continuous expression of the human papillomavirus oncogenes [Scheffner et al. 1990]. Consequently, these oncoproteins provide ideal nonself antigenic targets for immune-based therapies.
Current treatment of HPV-positive HNSCC could potentially be improved across the spectrum of treatment settings with the addition of immunotherapy directed against HPV and its activated oncoproteins. Several experimental HPV vaccine strategies including vector-based, peptide-based, protein-based, nucleic-acid-based, dendritic vaccines and RNA replicon approaches have been shown to enhance HPV-specific immune cell activity and antitumor responses in murine model tumor systems. Clinical trials are currently underway, based on encouraging preclinical results from these therapeutic HPV vaccines.
The most advanced program involves VGX-3100, a novel and highly immunogenic HPV16/18-specific DNA vaccine, which consists of synthetic consensus DNA sequences from HPV16 and HPV18 E6 and E7. Therapeutic DNA vaccines represent a novel approach and one that offers several potential advantages. DNA vaccines do not elicit antivector immune responses, for example, and thus have the capacity for the repeated administrations that may be required to achieve and maintain target immune responses. DNA vaccines are also relatively easy to manufacture.
In a phase I study in women with cervical intraepithelial neoplasia (CIN), VGX-3100 was capable of stimulating robust immune responses to antigens from high-risk HPV serotypes (16 and 18) in the majority of patients [Bagarazzi et al. 2012]. In a subsequent phase II study the efficacy, safety, and immunogenicity of VGX 3100 was assessed in patients with HPV-16 and HPV-18 associated CIN2/3 [ClinicalTrials.gov identifier: NCT01304524]. A total of 167 patients received either VGX-3100 (n = 125) or placebo (n = 42) [Trimble et al. 2015]. About half of the patients, n = 53 (49.5%) who received vaccine and 11 (30.6%) of 36 placebo recipients showed histopathological regression (p = 0.034). Concomitant histopathological regression and viral clearance were significantly greater after therapeutic vaccination with VGX-3100 compared with placebo. In addition, in patients treated with VGX-3100 whose lesions regressed, the intensity of CD8-positive infiltrates increased in normal mucosa in the stromal compartment, whereas in recipients of the placebo whose lesions regressed, the intensity of CD8-positive infiltrates in normal mucosa did not change. This was the first trial to successfully demonstrate that a therapeutic vaccine inducing immunity to HPV oncoproteins can elicit therapeutically useful T-cell and antibody responses in patients with preinvasive cervical disease caused by HPV-16 and HPV-18 [Trimble et al. 2015].
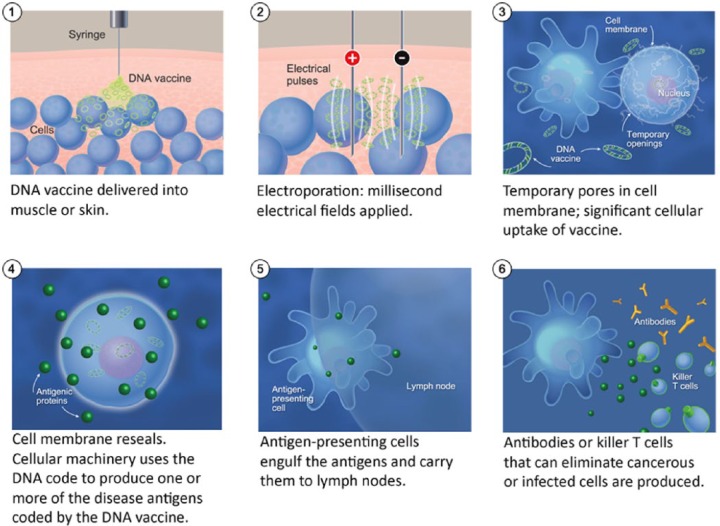
Based on safety and immunogenicity results in CIN, we designed a phase I/IIa open-label study to evaluate the safety, tolerability, and immunogenicity of INO-3112 [VGX 3100 plus INO 9112, adjuvant interleukin (IL)-12] delivered by electroporation to subjects with HPV-associated HNSCC [ClinicalTrials.gov identifier: NCT02163057] (Figure 1). INO-3112 (6 mg of VGX-3100 plus 1 mg of INO-9012) is delivered intramuscularly followed by electroporation with the CELLECTRA (Inovio, Plymouth Meeting, PA) device, once every 3 weeks for a total of four doses. Patients are enrolled in two cohorts (cohort I: pre and post definitive surgery; cohort II: patients post chemoradiation). Preliminary data presented at the 2015 SITC meeting [Aggarwal et al. 2015] showed that the vaccine administration is safe, with no grade 3 or higher treatment-emergent adverse effects (AEs).
Figure 1.
Generation of humoral and cellular immunity with INO-3112 (VGX 3100 plus INO 9112, adjuvant interleukin 12) and electoporation [ClinicalTrials.gov identifier: NCT02163057]. Image courtesy of Dr Mark Bagarazzi, Inovio Pharmaceuticals, Plymouth Meeting, PA, USA.
The most common AEs were injection site pain (n = 11), local erythema (n = 4) and hematoma/swelling (n = 2, each). Among samples tested to date, four of five evaluable patients showed elevated anti HPV16 and 18 E6/E7 antibody titers compared with baseline. Nine of 10 evaluable patients exhibited increased HPV-specific cellular responses by interferon γ ELISpot. Seven of eight evaluable patients had HPV-specific CD8-positive T-cell activation concurrent with increased lytic proteins (granzymes and perforin) by flow cytometric analysis. All tested patients had positive cellular immune responses in at least one assay [Aggarwal et al. 2015].
There are other therapeutic vaccines moving into advanced stages of clinical investigation in HNSCC, including ADXS11, a live attenuated Listeria monocytogenes (Lm) based immunotherapy that secretes an antigen-adjuvant fusion (Lm-LLO) protein consisting of a truncated fragment of the Lm protein listeriolysin O (LLO) fused to HPV-16 E7. This vaccine has been shown to decrease regulatory T cells and cause complete regression of established, transplanted HPV-TC-1 tumors in mice [Wallecha et al. 2012; Chen et al. 2014]. A phase II trial of ADX11 [ClinicalTrials.gov identifier: NCT02002182] is accruing newly diagnosed patients with stage II–IV HPV16-positive squamous cell carcinoma of the oropharynx who are scheduled to receive ablative transoral robotic surgery. Patients on this trial will receive vaccine in the neoadjuvant setting, and immune responses will be assessed serially after surgery. The primary endpoint is change in HPV E6/E7-specific CD8-positive cytotoxic lymphocyte responses.
Overexpression of the cellular protein p16 is a hallmark of HPV-associated cancers. As this protein is preferentially expressed in tumors, p16 is an interesting and attractive vaccine target. Results from a phase I/IIa first-in-humans trial to evaluate the safety and immunogenicity of vaccination with 12 subcutaneous applications of a p16INK4peptide (P16_37-63) mixed with Montanide ISA-51 VG (Seppic, Fairfield, NJ) were reported recently. Twenty patients received at least four applications and were evaluable for induction of an immune response. Vaccination with P16_37-63 induced cellular and humoral immune responses against the P16_37-63 peptide without significant toxicities. CD4 T cells directed against p16 positive cells were induced in 11 of 20 patients, CD8 T cells in 4 of 20 and antibodies in 14 of 20 patients [Reuschenbach et al. 2015].
Combining immunotherapy with traditional treatments such as chemotherapy may be another option for improving the prognosis and quality of life of those with HPV-associated cancers. One clinical trial is evaluating cisplatin-based chemotherapy combined with p16_37-63 peptide vaccination in patients with HPV-positive cancers [ClinicalTrials.gov identifier: NCT02526316]. Combination checkpoint inhibition and vaccine-based approaches have been evaluated in the preclinical setting. In a TC-1 mouse tumor model, combination of Lm-LLO immunotherapy with anti-PD-1 antibody was associated with significant increase in antigen-specific immune responses in periphery and CD8 T-cell infiltration into the tumor. In vitro infection with Lm results in significant upregulation of surface PD-L1 expression on human monocyte-derived dendritic cells. This combination treatment also led to significant inhibition of tumor growth and prolonged survival and complete regression of tumors in treated animals [Mkrtichyan et al. 2013]. Based on synergistic data, clinical trials evaluating combinations of vaccine and check point inhibition in the metastatic, recurrent, refractory setting are underway [Cohen et al. 2015]. As we gain a greater understanding of the interaction of complex immune pathways, these combination therapies will be critical as a multipronged approach to address the tumor microenvironment. As data mature, application of these results in the earlier disease setting (adjuvant and postdefinitive chemoradiation setting) would be the logical next step.
With continued progress in HPV vaccine development, we may soon be able to incorporate a variety of safe and effective preventive and therapeutic vaccine strategies for the control of HPV-associated cancers, including those of the head and neck.
Adoptive T-cell therapy
Adoptive immunotherapy involves the administration of T cells that have been artificially manipulated to target tumor cells. Such therapies have already shown striking efficacy in the treatment of hematologic malignancies and certain solid tumors such as melanoma [Rosenberg, 2004; Porter et al. 2011; Garfall et al. 2015]. In the case of a virally mediated cancer such as Epstein-Barr virus (EBV) or HPV related HNSCC this is a potentially appealing approach, as there is a clear nonhost target at which to direct T cells.
Adoptive immunotherapy targeting EBV has been evaluated in multiple studies of patients with nasopharyngeal cancer. In a phase I study, 24 patients with recurrent or metastatic EBV-associated nasopharyngeal cancer received ex vivo expanded autologous T cells. These cells were directed towards EBV using a ADE1-LMPpoly adenoviral vector, and administered without any preceding lymphodepleting chemotherapy [Smith et al. 2012]. EBV-activated T-cell persistence in peripheral blood was brief, and no radiologic responses were seen. In spite of this, patients who received T-cell infusions had significantly longer overall survival than those patients whose T cells failed to expand ex vivo (523 versus 220 days). In another phase I study of patients with recurrent or metastatic EBV-associated nasopharyngeal cancer, 38 patients received a protocol of four cycles of gemcitabine and carboplatin followed by six infusions of autologous T cells. The cells were isolated from samples of peripheral blood mononuclear cells, and then EBV transformed using an EBV lymphoblastoid cell line [Chia et al. 2014]. While it is difficult to parse out the relative benefits of immunotherapy from chemotherapy in this single-arm study, it is notable that after adoptive immunotherapy five patients did not require any further chemotherapy for more than 34 months. Adoptive immunotherapy has also been explored in post-transplant lymphoproliferative disease (PTLD), another condition associated with EBV. EBV-directed allogeneic T cells were generated through enrichment of peripheral blood mononuclear cells. Once T cells were enriched, they were sensitized with irradiated EBV-transformed B cells. In this fashion, investigators were able to create a bank of EBV-directed cytotoxic lymphocytes to be used for the treatment of patients. Both HLA compatible and incompatible transfusions have been explored [Doubrovina et al. 2012]. Using this technique, investigators were able to achieve 1-year overall survival of approximately 50% in patient with rituximab-refractory PTLD, a disease usually associated with a survival measured in days to weeks [Prockop et al. 2015].
No study to date has reported the results of adoptive immunotherapy for HPV-related HNSCC. HPV-directed adoptive immunotherapy has been reported in a cohort of nine patients with metastatic cervical cancer. Tumor-infiltrating lymphocytes were obtained from resected tumors and then expanded with IL-2 in culture. Lymphocyte colonies with activity against E6/E7 were selected for further expansion and reinfusion into patients following lymphodepleting chemotherapy. An overall response rate of 33% was seen, including two complete responses, both continuing at 15 and 22 months at the time of publication. HPV reactivity of the autologous T-cell infusion and the persistence of HPV-reactive T cells 1 month after infusion were predictive of response [Stevanovic et al. 2015]. Further study of HPV-directed adoptive T-cell therapy is underway in other cohorts of HPV-related cancers, including HNSCC [ClinicalTrials.gov identifier: NCT02280811]. In this ongoing trial, patients undergo an autologous stem cell transplant using T cells transduced with a E6 T-cell receptor. The field of adoptive T-cell therapy has been transformed by the emerging technology of chimeric antigen receptor T cells (CAR-T), which allow for more precision in T-cell targeting [Porter et al. 2011; Maude et al. 2014; Garfall et al. 2015]. HPV and EBV are both likely targets for future CAR-T trials.
Conclusion
There have been significant recent advances in the use of immunotherapy for the treatment of patients with advanced HNSCC. New insights into our understanding of the tumor microenvironment and its relationship to the immune system, mechanisms of immune evasion and oncogenesis are already changing the therapeutic landscape and improving patient outcomes for previously intractable diseases. Immune checkpoint inhibitors have firmly secured their place in the treatment paradigm of patients with melanoma and non-small cell lung cancer, with emerging efficacy data in advanced HNSCC. Several new approaches, including therapeutic vaccines with potential applications for the curative therapy of HNSCC and adoptive T-cell therapy for recurrent or metastatic disease, are under active development. Because of the complex biology and diversity of tumor evasion strategies, combination approaches will be crucial for the broadest application and utility of immune-directed therapies.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Joshua M. Bauml has received research funding from Merck, payable to the University of Pennsylvania. Dr. Roger B. Cohen has received research funding from Merck and Macrogenics payable to the University of Pennsylvania. Dr. Charu Aggarwal has received research funding from Inovio payable to the University of Pennsylvania.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Joshua M. Bauml, Division of Hematology–Oncology, University of Pennsylvania, Philadelphia, PA, USA
Roger B. Cohen, Division of Hematology–Oncology, University of Pennsylvania, Philadelphia, PA, USA
Charu Aggarwal, Department of Medicine, Division of Hematology–Oncology, University of Pennsylvania, 624 South Pavilion, Perelman Center for Advanced Medicine, 3400 Civic Center Boulevard, Philadelphia, PA 19104, USA.
References
- Aggarwal C., Cohen R., Morrow M. (2015) Immunotherapy with VGX-3100 (HPV16 and HPV18 plasmids) + INO-9012 (DNA encoding IL-12) in human papillomavirus (HPV) associated head and neck squamous cell carcinoma (HNSCCa): interim safety and immunogenicity results. J Immunother Cancer 3(Suppl. 2): P426. [Google Scholar]
- Bagarazzi M., Yan J., Morrow M., Shen X., Parker R., Lee J., et al. (2012) Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med 4: 155ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Reckamp K., Baas P., Crino L., Eberhardt W., Poddubskaya E., et al. (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm A., Nielsen S., Miska E., McCance D., Reid J., Bannister A., et al. (1999) The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. EMBO J 18: 2449–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Ozbun L., Chong N., Wallecha A., Berzofsky J., Khleif S. (2014) Episomal expression of truncated listeriolysin O in LmddA-LLO-E7 vaccine enhances antitumor efficacy by preferentially inducing expansions of CD4+FoxP3- and CD8+ T cells. Cancer Immunol Res 2: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia W., Teo M., Wang W., Lee B., Ang S., Tai W., et al. (2014) Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther 22: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E., Moore K., Slomovitz B., Chung C., Anderson M., Morris S., et al. (2015) Phase I/II study of ADXS11–001 or MEDI4736 immunotherapies alone and in combination, in patients with recurrent/metastatic cervical or human papillomavirus (HPV)-positive head and neck cancer. J Immunother Cancer 3(Suppl. 2): P147. [Google Scholar]
- Doubrovina E., Oflaz-Sozmen B., Prockop S., Kernan N., Abramson S., Teruya-Feldstein J., et al. (2012) Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood 119: 2644–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfall A., Maus M., Hwang W., Lacey S., Mahnke Y., Melenhorst J., et al. (2015) Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med 373: 1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon E., Rizvi N., Hui R., Leighl N., Balmanoukian A., Eder J., et al. (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- Gettinger S., Horn L., Gandhi L., Spigel D., Antonia S., Rizvi N., et al. (2015) Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 33: 2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillison M., D’Souza G., Westra W., Sugar E., Xiao W., Begum S., et al. (2008) Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 100: 407–420. [DOI] [PubMed] [Google Scholar]
- Hamid O., Robert C., Daud A., Hodi F., Hwu W., Kefford R., et al. (2013) Safety and tumor responses with lambrolizumab (Anti-PD-1) in melanoma. N Engl J Med 369: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir M., Butte M., Freeman G., Sharpe A. (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26: 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford-Pike S., Peng S., Young G., Taube J., Westra W., Akpeng B., et al. (2013) Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 73: 1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude S., Frey N., Shaw P., Aplenc R., Barrett D., Bunin N., et al. (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkrtichyan M., Chong N., Abu Eid R., Wallecha A., Singh R., Rothman J., et al. (2013) Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy. J Immunother Cancer 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignon J., Le Maitre A., Maillard E., Bourhis J. (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92: 4–14. [DOI] [PubMed] [Google Scholar]
- Porter D., Levine B., Kalos M., Bagg A., June C. (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop S., Doubrovina E., Baroudy K., Boulad F., Khalaf R., Papadopoulos E., et al. (2015) Epstein-Barr virus-specific cytotoxic T lymphocytes for treatment of rituximab-refractory Epstein-Barr virus-associated lymphoproliferative disorder. Cancer Research 75: CT107. [Google Scholar]
- Reuschenbach M., Rafiyan M., Pauligk C., Karbach J., Kloor M., Prigge E., et al. (2015) Phase I/IIa trial targeting p16INK4a by peptide vaccination in patients with human papillomavirus-associated cancer. J Clin Oncol 33(Suppl. 15): e14030. [Google Scholar]
- Rizvi N., Mazieres J., Planchard D., Stinchcombe T., Dy G., Antonia S., et al. (2015) Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 16: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. (2004) Shedding light on immunotherapy for cancer. N Engl J Med 350: 1461–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Werness B., Huibregtse J., Levine A., Howley P. (1990) The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63: 1129–1136. [DOI] [PubMed] [Google Scholar]
- Segal N., Ou S., Balmanoukian A., Fury M., Massarelli E., Brahmer J., et al. (2015) Safety and efficacy of MEDI4736, an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. J Clin Oncol 33: 3011. [Google Scholar]
- Seiwert T., Burtness B., Weiss J., Gluck I., Eder J., Pai S., et al. (2014) A phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV-associated head and neck (H/N) cancer. ASCO Meeting Abstracts 32: 6011. [Google Scholar]
- Seiwert T., Haddad R., Gupta S., Mehra R., Tahara M., Berger R., et al. (2015) Antitumor activity and safety of pembrolizumab in patients (pts) with advanced squamous cell carcinoma of the head and neck (SCCHN): preliminary results from KEYNOTE-012 expansion cohort. J Clin Oncol 33: LBA6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R., Miller K., Jemal A. (2015) Cancer statistics, 2015. CA Cancer J Clin 65: 5–29. [DOI] [PubMed] [Google Scholar]
- Smith C., Tsang J., Beagley L., Chua D., Lee V., Li V., et al. (2012) Effective treatment of metastatic forms of Epstein-Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy. Cancer Res 72: 1116–1125. [DOI] [PubMed] [Google Scholar]
- Spanos W., Hoover A., Harris G., Wu S., Strand G., Anderson M., et al. (2008) The PDZ binding motif of human papillomavirus type 16 E6 induces PTPN13 loss, which allows anchorage-independent growth and synergizes with ras for invasive growth. J Virol 82: 2493–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanovic S., Draper L., Langhan M., Campbell T., Kwong M., Wunderlich J., et al. (2015) Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol 33: 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube J., Klein A., Brahmer J., Xu H., Pan X., Kim J., et al. (2014) Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 20: 5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S., Drake C., Pardoll D. (2012) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble C., Morrow M., Kraynyak K., Shen X., Dallas M., Yan J., et al. (2015) Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 386: 2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermorken J., Herbst R., Leon X., Amellal N., Baselga J. (2008a) Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer 112: 2710–2719. [DOI] [PubMed] [Google Scholar]
- Vermorken J., Mesia R., Rivera F., Remenar E., Kawecki A., Rottey S., et al. (2008b) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359: 1116–1127. [DOI] [PubMed] [Google Scholar]
- Wallecha A., French C., Petit R., Singh R., Amin A., Rothman J. (2012) Lm-LLO-based immunotherapies and HPV-associated disease. J Oncol 2012: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]