Abstract
Purpose:
No standard salvage regimen has been established for patients with recurrent and metastatic nasopharyngeal carcinoma (NPC) and disease progression after prior platinum-based chemotherapy. This phase II study was designed to evaluate the efficacy and safety of gemcitabine plus S-1 (GS) chemotherapy as a remedial regimen in this setting.
Methods:
In this multicenter phase II study, 49 patients with recurrent and metastatic NPC who failed previous platinum-based chemotherapy received gemcitabine (1.0 g/m2 on days 1 and 8) plus oral S-1 chemotherapy (twice daily from day 1 to 14). Each cycle was repeated every 3 weeks for two cycles at least. The dose of S-1 was determined according to the body surface area (BSA): 40 mg twice a day for BSA <1.25 m2; 50 mg twice a day for 1.25 m2 ⩽ BSA <1.5 m2; and 60 mg twice a day for BSA ⩾1.5 m2.
Results:
Treatment was generally well-tolerated. A total of seven patients (14.3%) had grade 3 toxicities and the main toxicity was myelosuppression, whereas the nonhematology adverse events were minimal. There were 3 complete responses (6.4%), 17 partial responses (36.2%), and the overall response rate was 42.6% (95% confidence interval: 27.3–61.2). Median time to progression was 5.8 months and median survival was 14.8 months. The 1- and 2-year survival rates were 64% and 30%, respectively.
Conclusions:
Gemcitabine plus S-1 offers a satisfactory clinical activity and an acceptable safety profile for recurrent and metastatic NPC patients after failure of platinum-based chemotherapy.
Keywords: gemcitabine, nasopharyngeal carcinoma, platinum, S-1
Introduction
Nasopharyngeal carcinoma (NPC) is prevalent in South China, particularly in Guangdong Province [Cao et al. 2011; Chang and Adami, 2006]. Although many improvements in technology and equipment of radiotherapy have been achieved and concurrent chemoradiotherapy (CCRT) was used, the outcome of locoregionally advanced NPC patients is still unsatisfactory. The main reason of failure is locoregional relapse and distant metastasis [Zhang et al. 2013; Suarez et al. 2010]. NPC is also sensitive to chemotherapy and a treatment result of survival prolongation can often be achieved for recurrent or metastatic disease [Zhang et al. 2013; Suarez et al. 2010; Fandi et al. 2000; Ma and Chan, 2005]. Palliative chemotherapy has been used mostly with platinum-based regimens as a first line treatment [Fandi et al. 2000; Ma and Chan, 2005]. However, for those patients with disease progression after failure of prior platinum-based treatment, there is no standard salvage chemotherapy. Therefore, new active regimens with a favorable toxicity profile need to be explored.
Gemcitabine has shown significant activity in many kinds of tumors like pancreatic, non-small cell lung cancer, and breast cancer. Also, our previous phase II study suggested promising activity of gemcitabine as a single agent in patients with recurrent or metastatic NPC who were refractory to platinum-based treatment [Zhang et al. 2008].
S-1 is a promising new oral anticancer agent comprising of tegafur and two modulators. 5-chloro-2 4-dihydroxypyridine is one of the modulators which improves the pharmacological actions of fluorouracil (5-FU) by competitively inhibiting its degradation. The other modulator is potassium oxonate which localizes in gastrointestinal mucosa and selectively inhibits orotate phosphoribosyltransferase [Shirasaka et al. 1996; Saif et al. 2009]. S-1 has shown satisfactory clinical activity for many solid cancers, including head and neck cancer [Saif et al. 2009; Tsukuda et al. 2005]. Also, we have previously conducted a multi-institutional retrospective analysis to evaluate the efficacy and safety of S-1 chemotherapy for recurrent and metastatic NPC patients after failure of platinum-based chemotherapy and suggested that S-1 was a safe and effective therapeutic option [Peng et al. 2014].
Based on these promising results, we designed a multicenter, open-label, single-arm phase II trial to evaluate the efficacy and toxicity of gemcitabine plus S-1 (GS) for recurrent and metastatic NPC patients who were refractory to platinum-based treatment.
Material and methods
Patients’ eligibility
Eligibility criteria included histologically confirmed recurrent or metastatic NPC, age from 18–75 years, Eastern Cooperative Oncology Group (ECOG) performance status between 0–2, a life expectancy ⩾12 weeks, and at least one measurable lesion according to the Response Evaluation Criteria In Solid Tumors (RECIST) version 1.0. Patients had to have received at least one palliative platinum-based chemotherapy followed by a disease progression. Adequate liver (bilirubin level ⩽1.5 mg/dl, aspartate aminotransferase or alanine aminotransferase levels ⩽2.5 times the upper limit of normal), renal (serum creatinine level ⩽1.5 mg/dl), and bone marrow function (hemoglobin level ⩾10 g/dl, white blood cell count ⩾4000/µl, neutrophil count ⩾1500/µl, and platelet count ⩾100,000/µl) were required. Informed consent was obtained from all individual participants included in the study, and the study was approved by the local ethical committees. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki declaration (and its later amendments) or comparable ethical standards.
Treatment
Gemcitabine 1.0 g/m2 was added to 100 ml normal saline and infused over 30 min given on days 1 and 8 following 2 weeks rest. S-1 chemotherapy was administered at a planned standard dose and schedule (40 mg twice a day for body surface area (BSA) <1.25 m2; 50 mg twice a day for 1.25 m2 ⩽ BSA <1.5 m2; and 60 mg twice a day for BSA ⩾1.5 m2). S-1 was given on days 1 through 14 of a 21-day cycle. This combination was repeated every 3 weeks. Chemotherapy was discontinued in case of disease progression, presence of unacceptable toxicity, or patient refusal.
Evaluation of treatment and dose modification
Tumor response was evaluated every two cycles during the chemotherapy and then every 3 months after the completion of the chemotherapy using RECIST, version 1.0. Toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Event (NCI-CTCAE), version 3.0, before each treatment cycle. Complete physical examination, serum chemistry analysis, chest X-ray and a computed tomography scan of target sites were performed before the start of this study. Before each treatment cycle, patients were required to have a neutrophil count ⩾1500/µl, a platelet count ⩾100,000/µl, and adequate hepatic and renal function. Chemotherapy was discontinued in the case of disease progression, presence of unacceptable toxicity, or patient refusal.
In the case of toxicities with grade 3 intensity or higher, the dose was modified according to the following criteria: the dose of GS for the subsequent cycle was reduced by 20% in the case of a repeat of any grade 3 toxicity, and reduced by 40% in the case of a repeat of any grade 4 toxicity. If a dose reduction exceeding 40% was required, the patients were excluded from the study. The use of growth factor was permitted.
Statistics
This trial was proposed according to Simon’s [Simon, 1989] two-stage phase II optimal trial design. Based on a hypothesis that a response rate (RR) of 40% was expected to detect when compared with a minimal, clinically meaningful RR of 20%, the total sample size of 48 patients was necessary. All recruited patients were included in the intention-to-treat analysis of efficacy and toxicity. The primary objective was a RR with a 95% confidence interval (CI) and secondary objectives were toxicity, time to progression (TTP) and overall survival (OS). TTP and OS were estimated by the Kaplan–Meier method. TTP was measured from the date of entry into the study until the date of progression and the OS was calculated from the date of entry to the date of last follow up or death. The statistical analyses were performed using an SPSS software package (SPSS 16.0 Inc., Chicago, IL, USA).
Results
Patients’ characteristics
Between April 2011 and March 2014, 49 patients with recurrent or metastatic NPC progressed on platinum-based chemotherapy received GS chemotherapy at the Fifth Affiliated Hospital of Sun Yat-Sen University, Cancer Center of Sun Yat-Sen University and the People’s Hospital of Zhongshan City, China. The characteristics of the patients are summarized in Table 1.
Table 1.
Patients’ characteristics.
| Characteristics | Number of patients (n = 49) | % |
|---|---|---|
| Age (years) | Median 48 (range, 30–69) | |
| Sex | ||
| Female | 13 | 26.5 |
| Male | 36 | 73.5 |
| ECOG performance status | ||
| 0 | 10 | 20.4 |
| 1 | 25 | 51.0 |
| 2 | 14 | 28.6 |
| Tumor-involved site | ||
| Nasopharyngeal | 5 | 10.2 |
| Lymph node | 12 | 24.5 |
| Lung | 27 | 55.1 |
| Liver | 14 | 28.6 |
| Bone | 7 | 14.3 |
| Number of involved sites | ||
| 1 | 28 | 57.1 |
| 2 | 14 | 28.6 |
| 3 | 7 | 14.3 |
| Initial received platinum-based chemotherapy for recurrence or metastases | ||
| Cisplatin + 5-FU | 6 | 12.2 |
| Cisplatin + gemcitabine | 18 | 36.7 |
| Cisplatin + docetaxel | 17 | 34.7 |
| Cisplatin + paclitaxel | 11 | 22.4 |
| Carboplatin + paclitaxel | 12 | 24.5 |
| Number of chemotherapy regimens received | ||
| One | 16 | 32.7 |
| Two | 23 | 46.9 |
| ⩾Three | 10 | 20.4 |
5-FU, 5-fluorouracil; ECOG, Eastern Cooperative Oncology Group.
Treatment efficacy
A total of 47 of the 49 patients (95.9%) were assessable for efficacy. One patient was lost to follow up after two cycles of the treatment, and one patient with grade 3 thrombocytopenia after the first cycle withdrew the consents. All efficacy data were reported using the intention-to-treat principle. A total of 218 cycles of GS were delivered to 49 patients, with the median number of four cycles (range, 1–6 cycles) administered per patient. A total of 3 patients (6.4%) had complete response (CR), 17 patients (36.2%) had partial responses (PR), 19 patients (40.4%) had stable disease (SD) and 8 patients (17.0%) had progressive disease (PD). The overall RR (CR + PR) was 42.6% (95% CI: 27.3–61.2), and the disease control rate (CR + PR + SD) was 83.0%. With a median follow-up period of 12.2 months (range: 2.5–32 months), the median TTP was 5.8 months (95% CI: 3.2–7.6 months; Figure 1), median OS for all patients was 14.8 months (95% CI: 9.5–18.9 months; Figure 2), with a 1-year survival rate of 64% and a 2-year survival rate of 30%.
Figure 1.
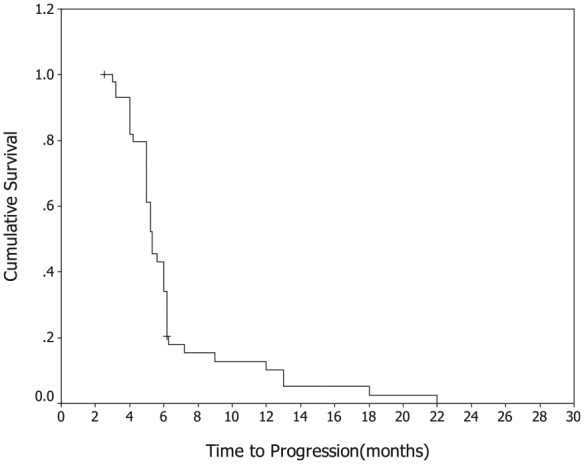
Kaplan–Meier Curve of progression-free survival.
Figure 2.
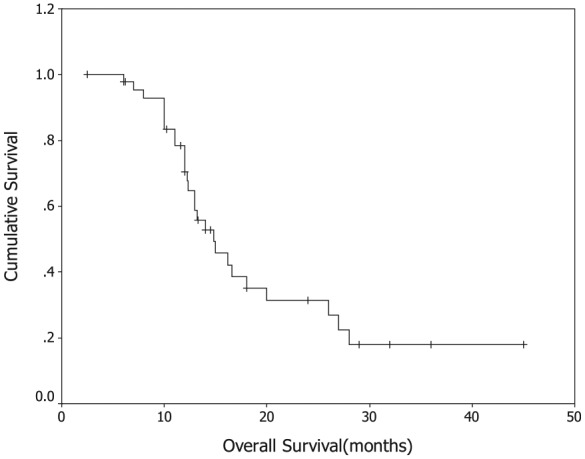
Kaplan–Meier Curve of overall survival.
Toxicity of treatment
Most patients had mild toxicity. Table 2 summarizes the toxicity of the GS regimen. The main hematological toxicity was neutropenia. There were four patients (8.2%) experiencing grade 3 neutropenia. Only one patient (2.0%) developed grade 3 thrombocytopenia and two (4.1%) patients developed grade 3 anemia. Although the patient with grade 3 thrombocytopenia successfully recovered after treatment, she withdrew from this trial after the first cycle of chemotherapy. No grade 4 hematological toxicities were observed. The main nonhematological toxicities were nausea (34.7%) and vomiting (18.4%). Hand–foot syndrome (HFS) was observed in three patients (6.1%) with grade 1 toxicity. Diarrhea was observed in six patients (12.3%). Hepatic, renal and other toxicities were mild. In general, no grade 3 or 4 nonhematological toxicities or treatment-related death were observed.
Table 2.
Treatment-related adverse events (n = 49).
| Adverse events | NCI-CTCAE grade n (% of patients) |
||||
|---|---|---|---|---|---|
| Grade | 1 | 2 | 3 | 4 | |
| Hematological | |||||
| Leukopenia | 22 (44.9) | 20 (40.8) | 4 (8.2) | 0 (0.0) | |
| Anemia | 18 (36.7) | 12 (24.5) | 2 (4.1) | 0 (0.0) | |
| Thrombocytopenia | 13 (26.5) | 8 (16.3) | 1 (2.0) | 0 (0.0) | |
| Nonhematological | |||||
| Nausea | 12 (24.5) | 5 (10.2) | 0 (0.0) | 0 (0.0) | |
| Vomiting | 7 (14.3) | 2 (4.1) | 0 (0.0) | 0 (0.0) | |
| Diarrhea | 4 (8.2) | 2 (4.1) | 0 (0.0) | 0 (0.0) | |
| Stomatitis | 4 (8.2) | 1 (2.0) | 0 (0.0) | 0 (0.0) | |
| Hand-foot syndrome | 3 (6.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Fatigue | 5 (10.2) | 1 (2.0) | 0 (0.0) | 0 (0.0) | |
NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Event.
Discussion
Chemotherapy has played an important role both in combined modality of chemoradiotherapy for locoregionally advanced disease and palliative treatment for metastatic disease. Platinum-based combination regimens (typically cisplatin + 5-FU) are the most commonly used chemotherapy regimens [Suarez et al. 2010; Fandi et al. 2000; Ma and Chan, 2005; Ma et al. 2008; Au and Ang, 1994]. However, patients with disease progression usually miss the chance to receive further treatment due to severe toxicities of prior platinum-based chemotherapy. For these reasons, new regimens with safety profiles and improved efficacy are thus highly desirable [Lee et al. 2012].
Many prior trials had shown that gemcitabine in combination with cisplatin or alone were active against NPC [Zhang et al. 2008; Foo et al. 2002; Ngan et al. 2002]. Using as a single agent, Foo and colleagues reported an overall RR of 48% in 27 patients with chemotherapy-pretreated NPC and we reported an overall RR of 43.8% in 30 patients after platinum-based chemotherapy failed [Foo et al. 2002; Zhang et al. 2008]. Ngan and colleagues reported a phase II trial using gemcitabine plus cisplatin for the treatment of recurrent or metastatic NPC [Ngan et al. 2002]. A total of nine patients had CR and 23 patients had PR, achieving an overall RR of 73% and 1-year survival rate of 62%. A subgroup analysis indicated that patients pretreated with cisplatin had a RR similar to that of chemotherapy-naïve patients. These data confirm that gemcitabine in combination with cisplatin or alone is active for cisplatin-pretreated or cisplatin-resistant NPC. As a single agent, S-1 is active for the treatment of gastric, pancreatic, colorectal, breast, and head and neck cancers [Saif et al. 2009; Tsukuda et al. 2005; Schoffski, 2004; Koizumi et al. 2008]. Our previous study confirmed the efficacy and safety of S-1 chemotherapy for recurrent and metastatic NPC patients after failure of platinum-based chemotherapy [Peng et al. 2014]. So, a combination of gemcitabine and S-1 to treat NPC after failure of treatment with platinum-based regimen seemed to be very rational. To our knowledge, there is no report of this particular combination in the treatment of NPC.
In our current study, this regimen indicated a RR of 42.6% with the median TTP and OS of 5.8 and 14.8 months, and 1- and 2-year survival rates were 64% and 30%, respectively. In contrast to other clinical trials, the combination of GS seems to be a promising regimen in platinum-pretreated refractory NPC. In addition, based on our previous phase II study result and clinical experience [Zhang et al. 2008], we recruited eighteen patients who failed previous gemcitabine plus cisplatin chemotherapy and found that GS regimen was still effective in these patients with an overall RR of 44.4% (8 out of 18 patients). In a study treating cisplatin-resistant NPC patients with gemcitabine plus vinorelbine, Wang and colleagues found that this regimen was safe and effective with an overall RR of 36%, a median progression-free survival (PFS) of 5.6 months and a median OS of 11.9 months [Wang et al. 2006]. Yau and colleagues reported a phase II trial about pemetrexed plus cisplatin for the treatment of recurrent or metastatic NPC after platinum-based chemotherapy failed [Yau et al. 2012]. One patient (7%) achieved CR, two (13%) achieved partial response, and eight (53%) had SDs. The median TTP was 30 weeks. Chua and colleagues reported a phase II study in which 49 Chinese patients with platinum-refractory advanced NPC received capecitabine (at a dose of 1000–1250 mg/m2 twice daily for 14 days) in a 3-week cycle [Chua et al. 2008]. The overall RR was 37% with a median TTP of 5 months and median OS of 14 months. Ngeow and colleagues confirmed the single-agent activity of docetaxel in the setting of heavily pretreated metastatic NPC [Ngeow et al. 2011]. The median PFS of 5.8 months and OS of 12.8 months are encouraging. In a prior study investigating the efficacy of capecitabine plus nedaplatin for recurrent and metastatic NPC after failure of platinum-based chemotherapy, we reported similar survival data (an overall RR of 41.7%, a median PFS of 5.8 months and a median OS of 12.4 months) [Peng et al. 2013]. However, whether GS therapy is indeed comparable or superior to other regimens as a salvage treatment in term of efficacy has not yet been fully demonstrated by our current study and requires further prospective randomized study.
We also confirmed the safety of GS, especially the favorable nonhematologic toxicity profile. Only one patient was discontinued from the trial because of treatment-related toxicities and there were no treatment-related deaths. Compared with capecitabine or 5-FU-based regimens, GS showed lower hand–foot syndrome toxicity. Myelosuppression is the only main toxicity observed in the current trial. Four cases of grade 3 leukopenia were seen and were reversed with granulocyte colony-stimulating factor (G-CSF) treatment. Owing to the relatively mild toxicity, many of the patients could receive their treatment in an outpatient setting. Thus, this combination regimen balances the anticancer efficacy and the maintenance of quality of life for recurrent or metastatic NPC patients after front line failure.
In conclusion, GS is a safe and effective salvage regimen for platinum-pretreated refractory recurrent and metastatic NPC. GS seems to be not only efficient but also favorable in maintaining quality of life, which makes it a reasonable and promising choice for patients with platinum-resistant NPC.
Acknowledgments
We thank all of the patients and their families for their willingness to take part in this study.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
PeiJian Peng, Department of Medical Oncology, The Fifth Affiliated Hospital of Sun Yat-Sen University, Zhuhai, People’s Republic of China.
XueQing Ou, Department of Radiation Oncology, The Fifth Affiliated Hospital of Sun Yat-Sen University, Zhuhai, Guangdong Province, People’s Republic of China.
Hai Liao, Department of Medical Oncology, Cancer Center, Sun Yat-Sen University, Guangzhou, People’s Republic of China.
YuMeng Liu, Department of Oncology, the People’s Hospital of Zhongshan City, Zhongshan, People’s Republic of China.
SiYang Wang, Department of Radiation Oncology, The Fifth Affiliated Hospital of Sun Yat-Sen University, Zhuhai, Guangdong Province, People’s Republic of China.
ZhiBin Cheng, Department of Radiation Oncology, The Fifth Affiliated Hospital of Sun Yat-Sen University, Zhuhai, Guangdong Province, People’s Republic of China.
Zhong Lin, Department of Medical Oncology, The Fifth Affiliated Hospital of Sun Yat-Sen University, 52 Mei Hua Road East, Zhuhai 519000, Guangdong Province, People’s Republic of China.
References
- Au E., Ang P. (1994) A phase II trial of 5-fluorouracil and cisplatinum in recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol 5: 87–89. [DOI] [PubMed] [Google Scholar]
- Cao S., Simons M., Qian C. (2011) The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 30: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E., Adami H. (2006) The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15: 1765–1777. [DOI] [PubMed] [Google Scholar]
- Chua D., Wei W., Sham J., Au G. (2008) Capecitabine monotherapy for recurrent and metastatic nasopharyngeal cancer. Jpn J Clin Oncol 38: 244–249. [DOI] [PubMed] [Google Scholar]
- Fandi A., Bachouchi M., Azli N., Taamma A., Boussen H., Wibault P., et al. (2000) Long-term disease-free survivors in metastatic undifferentiated carcinoma of the nasopharyngeal type. J Clin Oncol 18: 1324–1330. [DOI] [PubMed] [Google Scholar]
- Foo K., Tan E., Leong S., Wee J., Tan T., Fong K., et al. (2002) Gemcitabine in metastatic nasopharyngeal carcinoma of the undifferentiated type. Ann Oncol 13: 150–156. [DOI] [PubMed] [Google Scholar]
- Koizumi W., Narahara H., Hara T., Takagane A., Akiya T., Takagi M., et al. (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9: 215–221. [DOI] [PubMed] [Google Scholar]
- Lee A., Ng W., Chan Y., Sze H., Chan C., Lam C. (2012) The battle against nasopharyngeal cancer. Radiother Oncol 104: 272–278. [DOI] [PubMed] [Google Scholar]
- Ma B., Chan A. (2005) Recent perspectives in the role of chemotherapy in the management of advanced nasopharyngeal carcinoma. Cancer 103: 22–31. [DOI] [PubMed] [Google Scholar]
- Ma B., Hui E., Chan A. (2008) Systemic approach to improving treatment outcome in nasopharyngeal carcinoma: current and future directions. Cancer Sci 99: 1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngan R., Yiu H., Lau W., Yau S., Cheung F., Chan T., et al. (2002) Combination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II study. Ann Oncol 13: 1252–1258. [DOI] [PubMed] [Google Scholar]
- Ngeow J., Lim W., Leong S., Ang M., Toh C., Gao F., et al. (2011) Docetaxel is effective in heavily pretreated patients with disseminated nasopharyngeal carcinoma. Ann Oncol 22: 718–722. [DOI] [PubMed] [Google Scholar]
- Peng P., Chen H., Ou X., Zeng L., Wu X., Liu Y., et al. (2014) Safety and efficacy of S-1 chemotherapy in recurrent and metastatic nasopharyngeal carcinoma patients after failure of platinum-based chemotherapy: multi-institutional retrospective analysis. Drug Des Devel Ther 8: 1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng P., Ou X., Chen Z., Liao H., Peng Y., Wang S., et al. (2013) Multicenter phase II study of capecitabine combined with nedaplatin for recurrent and metastatic nasopharyngeal carcinoma patients after failure of cisplatin-based chemotherapy. Cancer Chemother Pharmacol 72: 323–328. [DOI] [PubMed] [Google Scholar]
- Saif M., Syrigos K., Katirtzoglou N. (2009) S-1: a promising new oral fluoropyrimidine derivative. Expert Opin Investig Drugs 18: 335–348. [DOI] [PubMed] [Google Scholar]
- Schoffski P. (2004) The modulated oral fluoropyrimidine prodrug S-1, and its use in gastrointestinal cancer and other solid tumors. Anticancer Drugs 15: 85–106. [DOI] [PubMed] [Google Scholar]
- Shirasaka T., Shimamoto Y., Oshima H., Yamaguchi M., Kato T., Yonekura K., et al. (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 7: 548–557. [DOI] [PubMed] [Google Scholar]
- Simon R. (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10: 1–10. [DOI] [PubMed] [Google Scholar]
- Suarez C., Rodrigo J., Rinaldo A., Langendijk J., Shaha A., Ferlito A. (2010) Current treatment options for recurrent nasopharyngeal cancer. Eur Arch Otorhinolaryngol 267: 1811–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukuda M., Kida A., Fujii M., Kono N., Yoshihara T., Hasegawa Y., et al. (2005) Chemotherapy Study Group of Head and Neck Cancer: Randomized scheduling feasibility study of S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br J Cancer 93: 884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Chang J., Liu T., Lin C., Yu Y., Hong R. (2006) Phase II study of gemcitabine plus vinorelbine in the treatment of cisplatin-resistant nasopharyngeal carcinoma. Head Neck 28: 74–80. [DOI] [PubMed] [Google Scholar]
- Yau T., Shum T., Lee A., Yeung M., Ng W., Chan L. (2012) A phase II study of pemetrexed combined with cisplatin in patients with recurrent or metastatic nanopharyngeal carcinoma. Oral Oncol 48: 441–444. [DOI] [PubMed] [Google Scholar]
- Zhang L., Chen Q., Liu H., Tang L., Mai H. (2013) Emerging treatment options for nasopharyngeal carcinoma. Drug Des Devel Ther 7: 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhang Y., Huang P., Xu F., Peng P., Guan Z. (2008) Phase II clinical study of gemcitabine in the treatment of patients with advanced nasopharyngeal carcinoma after the failure of platinum-based chemotherapy. Cancer Chemother Pharmacol 61: 33–38. [DOI] [PubMed] [Google Scholar]


