Abstract
Non-small cell lung cancer (NSCLC) is still the leading cause of cancer-related death, and the treatment of advanced NSCLC relies on systemic treatments. During the last decade, pemetrexed, an antifolate agent, gradually became a key component of the treatment for patients with advanced nonsquamous NSCLC. It has indeed been shown to be efficient for first-line, maintenance and second- or third-line treatment in this subgroup of NSCLC. Moreover, it is usually well tolerated, with few grade 3 and 4 toxicities. Several studies have tried to identify predictive biomarkers of pemetrexed efficacy. Due to pemetrexed’s mechanism of action, thymidilate synthase expression predictive value was investigated but could not be demonstrated. Currently, more than 400 trials of pemetrexed for the treatment of nonsquamous NSCLC are ongoing.
Keywords: lung cancer, nonsquamous, pemetrexed, survival, tolerability
Introduction
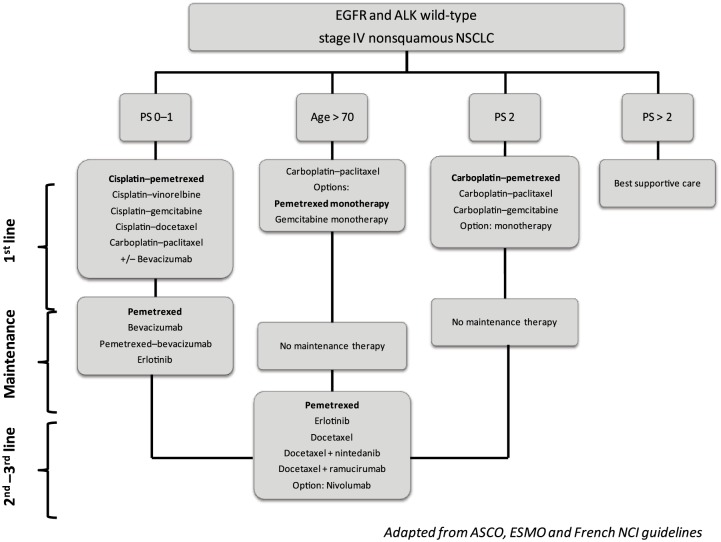
Lung cancer is still the leading cause of cancer-related deaths worldwide [Torre et al. 2015]. Efforts have been made during the last decades to improve advanced non-small cell lung cancer (NSCLC) outcomes. The most significant improvement for patients with lung cancer is the development of targeted therapies, prescribed on a personalized approach based on molecular profiling of the tumor and the identification of predictive biomarkers. More recently, immune checkpoint inhibitors (nivolumab) and new antiangiogenic agents (nintedanib, ramucirumab) emerged as new treatment options for pretreated lung cancer patients. However, standard chemotherapy remains a key component of advanced NSCLC treatment. Figure 1 summarizes guidelines for the treatment of Epidermal growth factor receptor (EGFR) and Anaplastic lymphoma kinase (ALK)-negative stage IV nonsquamous NSCLC. American Society of Clinical Oncology (ASCO) guidelines currently suggest that patients with stage IV nonsquamous NSCLC negative or unknown EGFR sensitizing mutation or ALK rearrangement and performance status (PS) 0 to 1 should receive a platinum-based combination of two cytotoxic drugs [Masters et al. 2015]. Pemetrexed, an antifolate agent, is one of the recommended drugs combined with cisplatin or carboplatin for first-line treatment of these patients.
Figure 1.
Treatment algorithm of EGFR and ALK wild-type nonsquamous stage IV non-small cell lung cancer.
EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; NSCLC, non-small cell lung cancer; PS, Eastern Cooperative Oncology Group performance status.
Pemetrexed was approved by the Food and Drug Association for several steps of nonsquamous NSCLC treatment (first line, maintenance therapy, and second and third lines). Based on results of the phase III studies described below, pemetrexed progressively became one of the most frequently used cytotoxic chemotherapy agents for treating stage IV nonsquamous NSCLC.
This review provides an overview of pemetrexed pharmacodynamics and pharmacokinetics, of the main studies leading to pemetrexed indications in nonsquamous NSCLC treatment, and of potential predictive biomarkers of pemetrexed efficacy.
Pharmacodynamics and pharmacokinetics
Pemetrexed belongs to the ‘folate antimetabolites’ class of chemotherapy agents. Pemetrexed inhibits cell replication and growth through the inhibition of three enzymes involved in purine and pyrimidine synthesis: thymidylate synthase (TS), dihydrofolate reductase (DHFR) and glycinamide ribonucleotide formyltransferase (GARFT) [McLeod et al. 2000]. Consequently, pemetrexed inhibits deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) synthesis, needed for cell growth and survival.
Pemetrexed undergoes limited hepatic metabolism and is mainly eliminated in the urine. Its half-life clearance is 3.5 hours for patients with normal renal function (glomerular filtration rate (GFR) = 90 ml/min). Phase I and pharmacokinetic studies of pemetrexed administered every 3 weeks to patients with advanced solid tumors suggested that pemetrexed was well tolerated at doses of 500 mg/m2 with vitamin supplementation [Mita et al. 2006]. Pemetrexed is not recommended for patients with a GFR of less than 40 ml/min. Pemetrexed pharmacokinetics are independent from concurrent administration of cisplatin or vitamins [Mita et al. 2006].
First-line treatment
Several phase II and III studies assessed the efficacy and safety of pemetrexed for first-line treatment of advanced nonsquamous NSCLC (Table 1). Based on the results of these studies, pemetrexed was approved in combination with cisplatin or carboplatin. In addition, the combination of pemetrexed with other cytotoxic chemotherapies, targeted therapies or antiangiogenic agents has also been studied in the first-line setting. Table 1 reports the results of the main phase III studies investigating pemetrexed in nonsquamous NSCLC treatment.
Table 1.
Results of phase III trials of pemetrexed for nonsquamous non-small cell lung cancer treatment.
| Line | Ref | Treatment | n | PFS (months) | HR [95% CI] | OS (months) | HR [95% CI] |
|---|---|---|---|---|---|---|---|
| 1st line | [Scagliotti et al. 2008] | Cisplatin–pemetrexed | 862 | 4.8 | 1.04 [0.94–1.15] | 10.3 | 0.94 [0.84–1.05] |
| Cisplatin–gemcitabine | 863 | 5.1 | 10.3 | ||||
| [Zukin et al. 2013] | Pemetrexed | 102 | 2.8 | 0.46 [0.35–0.63] | 5.3 | 0.62 [0.46–0.83] | |
| Carboplatin–pemetrexed | 103 | 5.8 | 9.3 | ||||
| [Schmid-Bindert et al. 2013] | Cisplatin–pemetrexed–necitumumab | 315 | 5.6 | 0.96 [0.80–1.16] | 11.3 | 1.01 [0.84–1.21] | |
| Cisplatin–pemetrexed | 318 | 5.6 | 11.5 | ||||
| Maintenance | [Mubarak et al. 2012]; [Paz-Ares et al. 2012] | Pemetrexed | 359 | 4.1 | 0.62 [0.49–0.79] | 13.9 | 0.78 [0.64–0.96] |
| Placebo | 180 | 2.8 | 11 | ||||
| [Belani et al. 2012]; [Barlesi et al. 2013] | Bevacizumab | 125 | 3.7 | 0.57 [0.44–0.75] | 13.2 | 0.87 [0.63–1.21] | |
| Pemetrexed–bevacizumab | 128 | 7.4 | 17.6 | ||||
| [Rittmeyer et al. 2013] | Carbo–pemetrexed–bevacizumab | 472 | 6 | 0.83 [0.71–0.96] | 12.6 | 1.00 [0.86–1.16] | |
| Carbo–pemetrexed–bevacizumab | 467 | 5.6 | 13.4 | ||||
| 2nd line; 3rd line | [Patel et al. 2013] | Pemetrexed | 265 | 2.9 | 0.97 [0.82–1.16] | 8.3 | 0.99 [0.80–1.20] |
| Docetaxel | 276 | 2.9 | 7.9 | ||||
| [Hanna et al. 2004] | Pemetrexed 500 mg/m2 | 295 | 2.6 | 1.01 [0.84–1.23] | 6.7 | 0.97 [0.82–1.15] | |
| Pemetrexed 900 mg/m2 | 293 | 2.8 | 6.9 | ||||
| [Waller et al. 2015] | Pemetrexed–vandetanib | 256 | 17.6 w | 0.86 [0.69–1.06] | 10.5 | 0.86 [0.65–1.13] | |
| Pemetrexed | 278 | 11.9 w | 9.2 |
W, weeks; Ref, reference; n, number; PFS, progression-free survival; HR, hazard ratio; OS, overall survival; CI, confidence interval.
Platinum-based chemotherapy
Four phase II studies investigated the combination of pemetrexed with cisplatin or carboplatin for nonsquamous NSCLC first-line treatment [Zinner et al. 2005]. These assays showed good clinical efficacy with an overall survival (OS) ranging between 8.9 and 13.5 months. A pooled retrospective analysis of two of these phase II trials showed that the pemetrexed plus platinum combination tended to be more effective in nonsquamous histology subtypes in comparison with squamous cell carcinoma, in terms of objective response rates (ORRs) that were 30% versus 17%, respectively; progression-free survival (PFS) that was 5.6 versus 4.7 months, respectively; hazard ratio (HR), 0.72; 95% confidence interval (CI), 0.43–1.19, and OS (10.5 versus 9.8 months, respectively; HR, 0.95; 95% CI, 0.52–1.74) [Zinner et al. 2010]. Moreover, Schuette and colleagues demonstrated that pemetrexed plus cisplatin and pemetrexed plus carboplatin had a good efficacy profile, with an OS of 11.7 and 8.9 months, respectively, and a PFS of 6.0 and 4.7 months, respectively [Schuette et al. 2013].
Several phase II studies also investigated pemetrexed in combination with nonplatinum cytotoxic chemotherapy such as gemcitabine [Spigel et al. 2010], vinorelbine [Clarke et al. 2005] or paclitaxel [Stathopoulos et al. 2007]. The results of these studies are encouraging, with median OS ranging from 7.9 to 14.0 months. However, Spigel and colleagues suggested that platinum-based regimens were associated with a better time to progression (TTP) that was 10.2 versus 4.7 months without platinum and OS (14.8 versus 7.5 months) [Spigel et al. 2012].
Finally, a phase III study showed that the combination of cisplatin and pemetrexed was not inferior to cisplatin–gemcitabine, with a similar median survival of 10.3 months (HR = 0.94; 95% CI, 0.84–1.05) [Scagliotti et al. 2008]. OS was significantly better with cisplatin–pemetrexed in the subgroup of adenocarcinoma in comparison with cisplatin–gemcitabine (12.6 versus 10.9 months, respectively, p = 0.03), whereas in the subgroup of squamous cell carcinoma, OS was better with cisplatin–gemcitabine than with cisplatin–pemetrexed (10.4 versus 6.7 months, respectively, p = 0.05). These results were then confirmed by two meta-analyses of randomized clinical trials [Treat et al. 2012]. The combination of cisplatin and pemetrexed was thus approved for first-line treatment of performance status, PS1, nonsquamous NSCLC patients.
Pemetrexed has also been studied for first-line treatment of special subpopulations. For elderly patients, pemetrexed alone showed a good antitumor activity (response rate = 25%) [Kim et al. 2013], and the combination of pemetrexed with carboplatin induced an ORR of 28.6% (95% CI, 16.58–43.26) [Gervais et al. 2013]. For patients with poor performance status, pemetrexed alone was compared with the combination of pemetrexed with carboplatin and OS was improved with the combination regimen (5.3 versus 9.3 months respectively, HR = 0.62; 95% CI, 0.46–0.83; p = 0.001) [Zukin et al. 2013]. Furthermore, the combination of pemetrexed with cisplatin was shown to be effective for the treatment of patients with asymptomatic brain metastasis (ORR = 34.9%) [Ortuzar et al. 2012].
Combinations with targeted therapies
Several targeted therapies were investigated in combination with pemetrexed and platinum for first-line treatment of nonsquamous NSCLC. Bevacizumab is the only antiangiogenic treatment approved for first-line treatment of selected nonsquamous NSCLC patients in association with platinum and pemetrexed [Spigel et al. 2012; Patel et al. 2009], and showed a comparable efficacy and safety profile for elderly patients [Dy et al. 2014]. Other antiangiogenic therapies such as ramucirumab (ORR = 49.3%) [Doebele et al. 2015], pazopanib (ORR = 23%) [Scagliotti et al. 2013], aflibercept (ORR = 26%) [Chen et al. 2014] and axitinib (ORR = 45.5%) [Belani et al. 2014] showed some clinical activity in combination with cisplatin and pemetrexed for first-line treatment of nonsquamous NSCLC. However, pazopanib and aflibercept showed unacceptable levels of toxicities.
Phase II studies also investigated enzastaurin, a protein kinase Cβ inhibitor, in combination with cisplatin and pemetrexed. Enzastaurin did not improve survival (OS = 7.2 versus 12.7 months with cisplatin plus pemetrexed, p = 0.05) [Casey et al. 2010].
Finally, pemetrexed has been studied in combination with anti-EGFR targeted therapies. Cetuximab showed an ORR of 38.5%, a PFS of 5.8 months and a 1-year survival rate of 45% in a single-arm phase II study [Schmid-Bindert et al. 2013]. However, the phase III INSPIRE study did not show any additional survival improvement due to the addition of necitumumab to chemotherapy with cisplatin–pemetrexed in comparison with chemotherapy alone (OS = 11.3 and 11.5 months respectively; HR = 1·01; 95% CI, 0.84–1.21; p = 0·96) [Paz-Ares et al. 2015].
Maintenance therapy
Pemetrexed was studied as continuation maintenance after pemetrexed and platinum-based first-line chemotherapy. A randomized phase II study of pemetrexed versus placebo after first-line chemotherapy with cisplatin–pemetrexed indeed showed promising results in terms of PFS [Mubarak et al. 2012]. A phase III study was then conducted and showed an improvement with pemetrexed in comparison with placebo regarding PFS (4.1 versus 2.8 months respectively; HR = 0.62; 95% CI, 0.49–0.79; p < 0.0001) [Paz-Ares et al. 2012] and OS (13.9 versus 11.0 months respectively; HR = 0.78; 95% CI, 0.64–0.96; p = 0.0195) [Paz-Ares et al. 2013]. The quality of life during maintenance therapy was similar in both arms except for an increased loss of appetite and a delayed worsening of pain and hemoptysis in the pemetrexed arm [Belani et al. 2012].
Furthermore, for patients receiving first-line treatment with cisplatin, pemetrexed and bevacizumab, maintenance therapy with pemetrexed and bevacizumab was compared with bevacizumab alone. The combination increased PFS (7.4 versus 3.7 respectively; HR = 0.57; 95% CI, 0.44–0.75; p < 0.001) [Barlesi et al. 2013]. However, there was a trend but no significant difference for OS between the combination of pemetrexed plus bevacizumab and bevacizumab alone (17.6 versus 13.2 months respectively; HR = 0.87; 95% CI, 0.63–1.21; p = 0.29) [Barlesi et al. 2014]. Notably, the addition of pemetrexed did not deteriorate quality of life [Rittmeyer et al. 2013].
Finally, following first-line chemotherapy with carboplatin, pemetrexed and bevacizumab, a study compared maintenance by pemetrexed plus bevacizumab versus paclitaxel plus bevacizumab. The efficacy was similar in both arms in terms of PFS (6.0 versus 5.6 months respectively, p = 0.12) and OS (12.6 versus 13.4 months respectively, p = 0.949) [Patel et al. 2013].
Second- and third-line treatment
Pemetrexed monotherapy
Pemetrexed monotherapy was approved as second- or third-line treatment of nonsquamous NSCLC on the basis of a phase III study of pemetrexed alone in comparison with docetaxel alone [Hanna et al. 2004]. There was no significant difference in terms of median PFS (2.9 and 2.9 months, respectively; HR = 0.97; 95% CI, 0.82–1.16) or median survival time (8.3 and 7.9 months, respectively; HR = 0.99; 95% CI, 0.80–1.20; p = 0.226) [Hanna et al. 2004]. A few years later, Cullen and colleagues compared two different doses of pemetrexed (500 and 900 mg/m2). As the higher dose did not improve efficacy, 500 mg/m2 remains the recommended dose of pemetrexed [Cullen et al. 2008]. It has also been proven that second-line pemetrexed efficacy was not influenced by maintenance therapy with gemcitabine continuation or switch maintenance with erlotinib [Bylicki et al. 2013]. Pemetrexed was also compared with gefitinib in an Asian population of EGFR wild-type nonsquamous NSCLC with a significant improvement in PFS for pemetrexed (4.8 versus 1.6 months, p < 0.001) [Zhou et al. 2014]. However, in comparison with erlotinib there was no significant difference in terms of TTP (p = 0.195), ORR (p = 0.469) or OS (p = 0.986) [Karampeazis et al. 2013].
Combination of pemetrexed with chemotherapy
Pemetrexed was also investigated in combination with other drugs in pretreated patients with NSCLC. In this setting, the addition of carboplatin to pemetrexed was compared with pemetrexed alone in several phase II trials. Although this combination improved TTP (4.2 versus 2.8 months, respectively; p = 0.005), it did not improve survival (HR for OS = 0.90, 95% CI, 0.74–1.10) [Ardizzoni et al. 2012]. More recently, pemetrexed was studied in combination with other cytotoxic chemotherapies such as eribulin, but those kinds of combinations did not provide any therapeutic advantage: PFS was 21.4 weeks in the eribulin–cisplatin arm versus 23.4 weeks in the pemetrexed alone arm (HR = 1; 95% CI, 0.6–1.7) [Waller et al. 2015].
Combination of pemetrexed with targeted therapies
Pemetrexed was studied in combination with antiangiogenic therapies such as bevacizumab [Adjei et al. 2010], vandetanib [de Boer et al. 2011] or sunitinib [Heist et al. 2014]. None of these studies met their primary endpoint. A phase I trial also studied the combination pemetrexed and nintedanib, and showed one complete response and 50% stable disease among 26 patients treated [Ellis et al. 2010]. In the same way, several anti-EGFR-targeted therapies were studied in combination with pemetrexed in comparison with pemetrexed alone for second- or third-line treatment of nonsquamous NSCLC patients. The monoclonal antibodies cetuximab and matuzumab did not improve patients’ clinical outcomes, with a median survival of 42 weeks with cetuximab, 5.9 months with matuzumab every 3 weeks and 12.4 months with weekly matuzumab [Jalal et al. 2009; Schiller et al. 2010]. However, the addition of the tyrosine kinase inhibitor, erlotinib, to pemetrexed improved PFS in phase II trials in comparison with pemetrexed alone (3.2 versus 2.9 months, p = 0.005), but increased grade 3 and 4 toxicities [Dittrich et al. 2014]. In the subgroup of EGFR wild-type patients, the addition of erlotinib to pemetrexed did not improve its efficacy, with an ORR of 11.1% [Minami et al. 2013].
Several other drugs, such as gadolinium [Edelman et al. 2011], itraconazole [Rudin et al. 2013], enzastaurin [Chiappori et al. 2010] and bortezomib [Scagliotti et al. 2010] were studied in combination with pemetrexed for pretreated nonsquamous NSCLC patients. However, none of them improved efficacy in comparison with pemetrexed alone.
Safety and tolerability
Myelosuppression was the predominant dose-limiting toxicity of pemetrexed reported in phase I trials [McDonald et al. 1998]. As a correlation has been established between poor folate and increased toxicity to pemetrexed, folic acid and vitamin B12 supplementation is recommended during pemetrexed treatment [Niyikiza et al. 2002].
Furthermore, safety and tolerability were assessed in several phase III studies (Table 2). Scagliotti and colleagues showed that survival without any grade 3 or 4 drug-related adverse event was significantly higher with cisplatin–pemetrexed in comparison with cisplatin–gemcitabine (HR = 0.70; 95% CI, 0.63–0.78; p<0.001) [Scagliotti et al. 2009]. In the same way, Gronberg and colleagues found more grade 3 and 4 adverse events with carboplatin–gemcitabine than with carboplatin–pemetrexed [Grønberg et al. 2009]. However, health-related quality of life was not significantly different between the two arms (7.0 versus 7.3 months respectively, p = 0.63). In the second-line setting, Hanna and colleagues reported more neutropenia (40.2% versus 5.3%, p < 0.001), febrile neutropenia (12.7% versus 1.9%, p < 0.001) and hospital admission for other drug-related adverse events (10.5% versus 6.4%, p < 0.092) with docetaxel in comparison with pemetrexed [Hanna et al. 2004]. In addition, Pujol and colleagues demonstrated a significantly longer survival without grade 3 or 4 toxicity with pemetrexed in comparison with docetaxel (HR = 0.60; 95% CI, 0.50–0.72) [Pujol et al. 2007].
Table 2.
Grade 3 and 4 toxicities reported in phase III studies.
| Toxicity grade 3 or 4(%) | Cisplatin–pemetrexed [Scagliotti et al. 2008]1st line | Pemetrexed alone [Mubarak et al. 2012] maintenance | Pemetrexed alone [Patel et al. 2013] 2nd and 3rd line |
|---|---|---|---|
| Hematological: | |||
| Neutropenia | 15.1 | 4 | 5.3 |
| Febrile neutropenia | 1.3 | NA | 1.9 |
| Anemia | 5.6 | 4 | 4.2 |
| Thrombocytopenia | 4.1 | 1 | 1.9 |
| Leukopenia | 4.8 | 2 | NA |
| Nonhematological: | |||
| Alopecia | 11.9 | NA | 0 |
| Nausea | 7.2 | <1 | 2.6 |
| Vomiting | 6.1 | 0 | 1.5 |
| Fatigue | 6.7 | 4 | 5.3 |
NA, not applicable.
Predictive biomarkers
As mentioned above, the benefit of pemetrexed has been demonstrated in nonsquamous NSCLC and not in squamous NSCLC [Syrigos et al. 2010].
Several molecular biomarkers have been investigated for the prediction of response to pemetrexed but none has been approved. It has first been suggested that a low level of TS expression could be responsible for a better sensitivity to pemetrexed. Several retrospective and prospective studies showed that a low TS level was associated with a better PFS for patients treated with pemetrexed [Lee et al. 2013]. However, since all patients were treated with pemetrexed in these studies, it was not possible to assess if the role of the TS expression level was prognostic or predictive. Moreover, Gronberg and colleagues also found that a low TS expression level was associated with a longer OS (9.7 versus 6.2 months, p < 0.001), but this effect was the same for patients treated with carboplatin plus pemetrexed and carboplatin plus gemcitabine, suggesting a prognostic role of the TS level more than a predictive role [Grønberg et al. 2013]. More recently, Chamizo and colleagues found that a low TS expression level was associated with higher response rates to pemetrexed (29% versus 3% in patients with TS overexpression, p = 0.025) [Chamizo et al. 2015]. Sun and colleagues also showed that cisplatin plus pemetrexed was superior to cisplatin plus gemcitabine in TS-positive patients (ORR = 40% and 39%, respectively) but not in TS-negative patients (ORR = 47% and 21%, respectively, interaction p = 0.0084) [Sun et al. 2015], finally suggesting a predictive role of TS expression. As the results of these studies are inconsistent, there is a need for further predictive studies to confirm the predictive role of TS expression for response to pemetrexed therapy. Moreover, in a phase I study of pemetrexed and lapatinib for second-line treatment of advanced NSCLC, a high level of cell-free TS RNA was associated with poorer outcomes [Ramlau et al. 2015].
Furthermore, Fennell and colleagues conducted an exploratory study of gene-expression profiling and found nine genes related to TS expression and associated with PFS and OS of patients treated with pemetrexed in a phase II trial of pemetrexed maintenance [Fennell et al. 2014]. Other studies suggested that TS gene polymorphism could be associated with PFS of patients receiving pemetrexed [Krawczyk et al. 2014]. Finally, ALK fusions were investigated as a potential predictive biomarker of pemetrexed efficacy. EML4-ALK rearrangements were indeed shown to be associated with low TS-mRNA expression [Xu et al. 2015]. PFS was not statistically different in ALK-positive and ALK-negative patients [Shaw et al. 2013].
Perspectives
During the last decade, the place of pemetrexed for the treatment of nonsquamous NSCLC became established. More recently, the development of new therapeutic options such as targeted therapies or immunotherapy modified the use of pemetrexed. However, pemetrexed remains a key drug for the treatment of patients with advanced nonsquamous NSCLC because of its good efficacy and tolerability profile. Further development of the drug relies on combinations of pemetrexed with other drugs or on the extension of pemetrexed use to early-stage or locally advanced diseases. More than 400 clinical trials are currently investigating pemetrexed for the treatment of advanced lung cancer patients (www.clinicaltrials.gov). Among these trials, 132 are still recruiting patients. Most of them are studying the combination of pemetrexed with other chemotherapies, targeted therapies, or immunotherapies.
However, it was shown that incremental costs per life-year gained for first-line treatment were US$148,994 for cisplatin plus pemetrexed induction in comparison with cisplatin plus gemcitabine followed by erlotinib maintenance, and US$191,270 for cisplatin plus pemetrexed followed by pemetrexed maintenance in comparison with cisplatin plus pemetrexed induction only [Kumar et al. 2015]. Further comparative cost-effectiveness studies are required to efficiently use the pemetrexed. To date, these data by themselves should not limit the use of pemetrexed and must be integrated into the cost-effectiveness assessment of all new treatment options, such as targeted therapies and immunotherapies.
Conclusion
Pemetrexed has become one of the most frequently prescribed chemotherapeutic agents for advanced nonsquamous NSCLC treatment. It is now approved for first-line, maintenance and second or third-line treatment of nonsquamous NSCLC and is generally well tolerated, with few grade 3 and 4 toxicities. Several biomarkers, such as TS expression, have been investigated as predictive biomarkers of pemetrexed efficacy. However, all biomarkers to date have failed to demonstrate any predictive role. Finally, hundreds of clinical trials of pemetrexed for NSCLC treatment are still ongoing in order to extend the development of the drug.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Pascale Tomasini, Aix Marseille University, Assistance Publique Hôpitaux de Marseille, Department of Multidisciplinary Oncology & Therapeutic Innovations, Marseille, France.
Fabrice Barlesi, Aix Marseille University, Assistance Publique Hôpitaux de Marseille, Department of Multidisciplinary Oncology & Therapeutic Innovations, Marseille, France.
Celine Mascaux, Aix Marseille University, Assistance Publique Hôpitaux de Marseille, Department of Multidisciplinary Oncology & Therapeutic Innovations, Marseille, France.
Laurent Greillier, Aix Marseille University, Assistance Publique Hôpitaux de Marseille, Department of Multidisciplinary Oncology & Therapeutic Innovations, Marseille, France.
References
- Adjei A., Mandrekar S., Dy G., Molina J., Adjei A., Gandara D., et al. (2010) Phase II trial of pemetrexed plus bevacizumab for second-line therapy of patients with advanced non-small-cell lung cancer: NCCTG and SWOG study N0426. J Clin Oncol 28: 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardizzoni A., Tiseo M., Boni L., Vincent A., Passalacqua R., Buti S., et al. (2012) Pemetrexed versus pemetrexed and carboplatin as second-line chemotherapy in advanced non-small-cell lung cancer: results of the GOIRC 02-2006 randomized phase II study and pooled analysis with the NVALT7 trial. J Clin Oncol 30: 4501–4507. [DOI] [PubMed] [Google Scholar]
- Barlesi F., Scherpereel A., Gorbunova V., Gervais R., Vikström A., Chouaid C., et al. (2014) Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol 25: 1044–1052. [DOI] [PubMed] [Google Scholar]
- Barlesi F., Scherpereel A., Rittmeyer A., Pazzola A., Ferrer Tur N., Kim J., et al. (2013) Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol 31: 3004–3011. [DOI] [PubMed] [Google Scholar]
- Belani C., Brodowicz T., Ciuleanu T., Krzakowski M., Yang S., Franke F., et al. (2012) Quality of life in patients with advanced non-small-cell lung cancer given maintenance treatment with pemetrexed versus placebo (H3E-MC-JMEN): results from a randomised, double-blind, phase III study. Lancet Oncol 13: 292–299. [DOI] [PubMed] [Google Scholar]
- Belani C., Yamamoto N., Bondarenko I., Poltoratskiy A., Novello S., Tang J., et al. (2014) Randomized phase II study of pemetrexed/cisplatin with or without axitinib for non-squamous non-small-cell lung cancer. BMC Cancer 14: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylicki O., Ferlay C., Chouaid C., Lavolé A., Barlési F., Dubos C., et al. (2013) Efficacy of pemetrexed as second-line therapy in advanced NSCLC after either treatment-free interval or maintenance therapy with gemcitabine or erlotinib in IFCT-GFPC 05-02 phase III study. J Thorac Oncol 8: 906–914. [DOI] [PubMed] [Google Scholar]
- Casey E., Harb W., Bradford D., Bufill J., Nattam S., Patel J., et al. (2010) Randomized, double-blinded, multicenter, phase II study of pemetrexed, carboplatin, and bevacizumab with enzastaurin or placebo in chemonaïve patients with stage IIIB/IV non-small cell lung cancer: Hoosier Oncology Group LUN06-116. J Thorac Oncol 5: 1815–1820. [DOI] [PubMed] [Google Scholar]
- Chamizo C., Zazo S., Dómine M., Cristóbal I., García-Foncillas J., Rojo F., et al. (2015) Thymidylate synthase expression as a predictive biomarker of pemetrexed sensitivity in advanced non-small cell lung cancer. BMC Pulm Med 15: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Modiano M., Neal J., Brahmer J., Rigas J., Jotte R., et al. (2014) A phase II multicentre study of ziv-aflibercept in combination with cisplatin and pemetrexed in patients with previously untreated advanced/metastatic non-squamous non-small cell lung cancer. Br J Cancer 110: 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappori A., Bepler G., Barlesi F., Soria J., Reck M., Bearz A., et al. (2010) Phase II, double-blinded, randomized study of enzastaurin plus pemetrexed as second-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol 5: 369–375. [DOI] [PubMed] [Google Scholar]
- Clarke S., Boyer M., Millward M., Underhill C., Moylan E., Yip D., et al. (2005) A phase I/II study of pemetrexed and vinorelbine in patients with non-small cell lung cancer. Lung Cancer 49: 401–412. [DOI] [PubMed] [Google Scholar]
- Cullen M., Zatloukal P., Sörenson S., Novello S., Fischer J., Joy A., et al. (2008) A randomized phase III trial comparing standard and high-dose pemetrexed as second-line treatment in patients with locally advanced or metastatic non-small-cell lung cancer. Ann Oncol 19: 939–945. [DOI] [PubMed] [Google Scholar]
- De Boer R., Arrieta Ó., Yang C., Gottfried M., Chan V., Raats J., et al. (2011) Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol 29: 1067–1074. [DOI] [PubMed] [Google Scholar]
- Dittrich C., Papai-Szekely Z., Vinolas N., Sederholm C., Hartmann J., Behringer D., et al. (2014) A randomised phase II study of pemetrexed versus pemetrexed+erlotinib as second-line treatment for locally advanced or metastatic non-squamous non-small cell lung cancer. Eur J Cancer 50: 1571–1580. [DOI] [PubMed] [Google Scholar]
- Doebele R., Spigel D., Tehfe M., Thomas S., Reck M., Verma S., et al. (2015) Phase II, randomized, open-label study of ramucirumab in combination with first-line pemetrexed and platinum chemotherapy in patients with nonsquamous, advanced/metastatic non-small cell lung cancer. Cancer 121: 883–892. [DOI] [PubMed] [Google Scholar]
- Dy G., Molina J., Qi Y., Ansari R., Thomas S., Ross H., et al. (2014) NCCTG N0821 (Alliance): A phase II first-line study of pemetrexed, carboplatin, and bevacizumab in elderly patients with advanced nonsquamous non-small-cell lung cancer with good performance status. J Thorac Oncol 9: 1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman M., Otterson G., Leach J., Malpass T., Salgia R., Jones D., et al. (2011) Multicenter phase II trial of Motexafin gadolinium and pemetrexed for second-line treatment in patients with non-small cell lung cancer. J Thorac Oncol 6: 786–789. [DOI] [PubMed] [Google Scholar]
- Ellis P., Kaiser R., Zhao Y., Stopfer P., Gyorffy S., Hanna N. (2010) Phase I open-label study of continuous treatment with BIBF 1120, a triple angiokinase inhibitor, and pemetrexed in pretreated non-small cell lung cancer patients. Clin Cancer Res 16: 2881–2889. [DOI] [PubMed] [Google Scholar]
- Fennell D., Myrand S., Nguyen T., Ferry D., Kerr K., Maxwell P., et al. (2014) Association between gene expression profiles and clinical outcome of pemetrexed-based treatment in patients with advanced non-squamous non-small cell lung cancer: exploratory results from a phase II study. PloS One 9: e107455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais R., Robinet G., Clément-Duchêne C., Denis F., El Kouri C., Martin P., et al. (2013) Pemetrexed and carboplatin, an active option in first-line treatment of elderly patients with advanced non-small cell lung cancer (NSCLC): a phase II trial. Lung Cancer 80: 185–190. [DOI] [PubMed] [Google Scholar]
- Grønberg B., Bremnes R., Fløtten O., Amundsen T., Brunsvig P., Hjelde H., et al. (2009) Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 27: 3217–3224. [DOI] [PubMed] [Google Scholar]
- Grønberg B., Lund-Iversen M., Strøm E., Brustugun O., Scott H. (2013) Associations between TS, TTF-1, FR-α, FPGS, and overall survival in patients with advanced non-small-cell lung cancer receiving pemetrexed plus carboplatin or gemcitabine plus carboplatin as first-line chemotherapy. J Thorac Oncol 8: 1255–1264. [DOI] [PubMed] [Google Scholar]
- Hanna N., Shepherd F., Fossella F., Pereira J., De Marinis F., von Pawel J., et al. (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22:1589–1597. [DOI] [PubMed] [Google Scholar]
- Heist R., Wang X., Hodgson L., Otterson G., Stinchcombe T., Gandhi L., et al. (2014) CALGB 30704 (Alliance): A randomized phase II study to assess the efficacy of pemetrexed or sunitinib or pemetrexed plus sunitinib in the second-line treatment of advanced non-small-cell lung cancer. J Thorac Oncol 9: 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal S., Waterhouse D., Edelman M. J., Nattam S., Ansari R., Koneru K., et al. (2009) Pemetrexed plus cetuximab in patients with recurrent non-small cell lung cancer (NSCLC): a phase I/II study from the Hoosier oncology group. J Thorac Oncol 4: 1420–1424. [DOI] [PubMed] [Google Scholar]
- Karampeazis A., Voutsina A., Souglakos J., Kentepozidis N., Giassas S., Christofillakis C., et al. (2013) Pemetrexed versus erlotinib in pretreated patients with advanced non-small cell lung cancer: a Hellenic Oncology Research Group (HORG) randomized phase III study. Cancer 119: 2754–2764. [DOI] [PubMed] [Google Scholar]
- Kim Y., Hirabayashi M., Kosaka S., Nikaidoh J., Yamamoto Y., Shimada M., et al. (2013) Phase II study of pemetrexed as first-line treatment in elderly (⩾75) non-squamous non-small-cell lung cancer: Kyoto Thoracic Oncology Research Group Trial 0901. Cancer Chemother Pharmacol 71: 1445–1451. [DOI] [PubMed] [Google Scholar]
- Krawczyk P., Kucharczyk T., Kowalski D., Powrózek T., Ramlau R., Kalinka-Warzocha E., et al. (2014) Polymorphisms in TS, MTHFR and ERCC1 genes as predictive markers in first-line platinum and pemetrexed therapy in NSCLC patients. J Cancer Res Clin Oncol 140: 2047–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G., Woods B., Hess L., Treat J., Boye M., Bryden P., et al. (2015) Cost-effectiveness of first-line induction and maintenance treatment sequences in non-squamous non-small cell lung cancer (NSCLC) in the U.S. Lung Cancer 89: 294–300. [DOI] [PubMed] [Google Scholar]
- Lee S., Noh K., Lee J., Lee E., Min K., Hur G., et al. (2013) Thymidylate synthase and ERCC1 as predictive markers in patients with pulmonary adenocarcinoma treated with pemetrexed and cisplatin. Lung Cancer 81: 102–108. [DOI] [PubMed] [Google Scholar]
- Masters G., Temin S., Azzoli C., Giaccone G., Baker S., Brahmer J., et al. (2015) Systemic therapy for stage IV non-small-cell lung cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol 33: 3488–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A., Vasey P., Adams L., Walling J., Woodworth J., Abrahams T., et al. (1998) A phase I and pharmacokinetic study of LY231514, the multitargeted antifolate. Clin Cancer Res 4: 605–610. [PubMed] [Google Scholar]
- McLeod H., Cassidy J., Powrie R., Priest D., Zorbas M., Synold T., et al. (2000) Pharmacokinetic and pharmacodynamic evaluation of the glycinamide ribonucleotide formyltransferase inhibitor AG2034. Clin Cancer Res 6: 2677–2684. [PubMed] [Google Scholar]
- Minami S., Kijima T., Hamaguchi M., Nakatani T., Koba T., Takahashi R., et al. (2013) Phase II study of pemetrexed plus intermittent erlotinib combination therapy for pretreated advanced non-squamous non-small cell lung cancer with documentation of epidermal growth factor receptor mutation status. Lung Cancer 82: 271–275. [DOI] [PubMed] [Google Scholar]
- Mita A., Hammond L., Bonate P., Weiss G., McCreery H., Syed S., et al. (2006) Phase I and pharmacokinetic study of tasidotin hydrochloride (ILX651), a third-generation dolastatin-15 analogue, administered weekly for 3 weeks every 28 days in patients with advanced solid tumors. Clin Cancer Res 12: 5207–5215. [DOI] [PubMed] [Google Scholar]
- Mubarak N., Gaafar R., Shehata S., Hashem T., Abigeres D., Azim H., et al. (2012) A randomized, phase II study comparing pemetrexed plus best supportive care versus best supportive care as maintenance therapy after first-line treatment with pemetrexed and cisplatin for advanced, non-squamous, non-small cell lung cancer. BMC Cancer 12: 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyikiza C., Baker S., Seitz D., Walling J., Nelson K., Rusthoven J., et al. (2002) Homocysteine and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther 1: 545–552. [PubMed] [Google Scholar]
- Ortuzar W., Hanna N., Pennella E., Peng G., Langer C., Monberg M., et al. (2012) Brain metastases as the primary site of relapse in two randomized phase III pemetrexed trials in advanced non-small-cell lung cancer. Clin Lung Cancer 13: 24–30. [DOI] [PubMed] [Google Scholar]
- Patel J., Hensing T., Rademaker A., Hart E., Blum M., Milton D., et al. (2009) Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol 27: 3284–3289. [DOI] [PubMed] [Google Scholar]
- Patel J., Socinski M., Garon E., Reynolds C., Spigel D., Olsen M., et al. (2013) PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol 31: 4349–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares L., de Marinis F., Dediu M., Thomas M., Pujol J., Bidoli P., et al. (2012) Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase III, randomised controlled trial. Lancet Oncol 13: 247–255. [DOI] [PubMed] [Google Scholar]
- Paz-Ares L., de Marinis F., Dediu M., Thomas M., Pujol J., Bidoli P., et al. (2013) PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 31: 2895–2902. [DOI] [PubMed] [Google Scholar]
- Paz-Ares L., Mezger J., Ciuleanu T., Fischer J., von Pawel J., Provencio M., et al. (2015) Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non-small-cell lung cancer (INSPIRE): an open-label, randomised, controlled phase III study. Lancet Oncol 16: 328–337. [DOI] [PubMed] [Google Scholar]
- Pujol J., Paul S., Chouaki N., Peterson P., Moore P., Berry D., et al. (2007) Survival without common toxicity criteria grade 3/4 toxicity for pemetrexed compared with docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC): a risk-benefit analysis. J Thorac Oncol 2: 397–401. [DOI] [PubMed] [Google Scholar]
- Ramlau R., Thomas M., Novello S., Plummer R., Reck M., Kaneko T., et al. (2015) Phase I study of lapatinib and pemetrexed in the second-line treatment of advanced or metastatic non-small-cell lung cancer with assessment of circulating cell free thymidylate synthase RNA as a potential biomarker. Clin Lung Cancer 16: 348–357. [DOI] [PubMed] [Google Scholar]
- Rittmeyer A., Gorbunova V., Vikström A., Scherpereel A., Kim J., Ahn M., et al. (2013) Health-related quality of life in patients with advanced nonsquamous non-small-cell lung cancer receiving bevacizumab or bevacizumab-plus-pemetrexed maintenance therapy in AVAPERL (MO22089). J Thorac Oncol 8: 1409–1416. [DOI] [PubMed] [Google Scholar]
- Rudin C., Brahmer J., Juergens R., Hann C., Ettinger D., Sebree R., et al. (2013) Phase II study of pemetrexed and itraconazole as second-line therapy for metastatic nonsquamous non-small-cell lung cancer. J Thorac Oncol 8: 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scagliotti G., Felip E., Besse B., von Pawel J., Mellemgaard A., Reck M., et al. (2013) An open-label, multicenter, randomized, phase II study of pazopanib in combination with pemetrexed in first-line treatment of patients with advanced-stage non-small-cell lung cancer. J Thorac Oncol 8: 1529–1537. [DOI] [PubMed] [Google Scholar]
- Scagliotti G., Germonpré P., Bosquée L., Vansteenkiste J., Gervais R., Planchard D., et al. (2010) A randomized phase II study of bortezomib and pemetrexed, in combination or alone, in patients with previously treated advanced non-small-cell lung cancer. Lung Cancer 68: 420–426. [DOI] [PubMed] [Google Scholar]
- Scagliotti G., Parikh P., von Pawel J., Biesma B., Vansteenkiste J., Manegold C., et al. (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26: 3543–3551. [DOI] [PubMed] [Google Scholar]
- Scagliotti G., Park K., Patil S., Rolski J., Goksel T., Martins R., et al. (2009) Survival without toxicity for cisplatin plus pemetrexed versus cisplatin plus gemcitabine in chemonaïve patients with advanced non-small cell lung cancer: a risk-benefit analysis of a large phase III study. Eur J Cancer 45: 2298–2303. [DOI] [PubMed] [Google Scholar]
- Schiller J. H., von Pawel J., Schütt P., Ansari R. H., Thomas M., Saleh M., et al. (2010) Pemetrexed with or without matuzumab as second-line treatment for patients with stage IIIB/IV non-small cell lung cancer. J Thorac Oncol 5: 1977–1985. [DOI] [PubMed] [Google Scholar]
- Schmid-Bindert G., Gebbia V., Mayer F., Arriola E., Márquez-Medina D., Syrigos K., et al. (2013) Phase II study of pemetrexed and cisplatin plus cetuximab followed by pemetrexed and cetuximab maintenance therapy in patients with advanced nonsquamous non-small cell lung cancer. Lung Cancer 81: 428–434. [DOI] [PubMed] [Google Scholar]
- Schuette W., Gröschel A., Sebastian M., Andreas S., Müller T., Schneller F., et al. (2013) A randomized phase II study of pemetrexed in combination with cisplatin or carboplatin as first-line therapy for patients with locally advanced or metastatic non-small-cell lung cancer. Clin Lung Cancer 14: 215–223. [DOI] [PubMed] [Google Scholar]
- Shaw A., Varghese A., Solomon B., Costa D., Novello S., Mino-Kenudson M., et al. (2013) Pemetrexed-based chemotherapy in patients with advanced, ALK-positive non-small cell lung cancer. Ann Oncol 24: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigel D., Hainsworth J., Barton J., Patton J., Zubkus J., Simons L., et al. (2010) Phase II study of biweekly pemetrexed and gemcitabine in patients with previously untreated advanced non-small cell lung cancer. J Thorac Oncol 5: 841–845. [DOI] [PubMed] [Google Scholar]
- Spigel D., Hainsworth J., Shipley D., Ervin T., Kohler P., Lubiner E., et al. (2012) A randomized phase II trial of pemetrexed/gemcitabine/bevacizumab or pemetrexed/carboplatin/bevacizumab in the first-line treatment of elderly patients with advanced non-small cell lung cancer. J Thorac Oncol 7: 196–202. [DOI] [PubMed] [Google Scholar]
- Stathopoulos G., Dimitroulis J., Toubis M., Katis C., Karaindros D., Stathopoulos J., et al. (2007) Pemetrexed combined with paclitaxel in patients with advanced or metastatic non-small-cell lung cancer: a phase I-II trial. Lung Cancer 57: 66–71. [DOI] [PubMed] [Google Scholar]
- Sun J., Ahn J., Jung S., Sun J., Ha S., Han J., et al. (2015) Pemetrexed plus cisplatin versus gemcitabine plus cisplatin according to thymidylate synthase expression in nonsquamous non-small-cell lung cancer: a biomarker-stratified randomized phase II trial. J Clin Oncol 33: 2450–2456. [DOI] [PubMed] [Google Scholar]
- Syrigos K., Vansteenkiste J., Parikh P., von Pawel J., Manegold C., Martins R., et al. (2010) Prognostic and predictive factors in a randomized phase III trial comparing cisplatin-pemetrexed versus cisplatin-gemcitabine in advanced non-small-cell lung cancer. Ann Oncol 21: 556–561. [DOI] [PubMed] [Google Scholar]
- Torre L., Bray F., Siegel R., Ferlay J., Lortet-Tieulent J., Jemal A. (2015) Global cancer statistics, 2012. CA A Cancer J Clin 65: 87–108. [DOI] [PubMed] [Google Scholar]
- Treat J., Scagliotti G., Peng G., Monberg M., Obasaju C., Socinski M. (2012) Comparison of pemetrexed plus cisplatin with other first-line doublets in advanced non-small cell lung cancer (NSCLC): a combined analysis of three phase III trials. Lung Cancer 76: 222–227. [DOI] [PubMed] [Google Scholar]
- Waller C., Vynnychenko I., Bondarenko I., Shparyk Y., Hodge J., Freeman A., et al. (2015) An open-label, multicenter, randomized phase Ib/II study of eribulin mesylate administered in combination with pemetrexed versus pemetrexed alone as second-line therapy in patients with advanced nonsquamous non-small-cell lung cancer. Clin Lung Cancer 16: 92–99. [DOI] [PubMed] [Google Scholar]
- Xu C., Wang G., Wang W., Gao W., Han C., Gao J., et al. (2015) Association between EML4-ALK fusion gene and thymidylate synthase mRNA expression in non-small cell lung cancer tissues. Exp Ther Med 9: 2151–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Cheng Y., Yang J., Zhao M., Zhang L., Zhang X., et al. (2014) Pemetrexed versus gefitinib as a second-line treatment in advanced nonsquamous nonsmall-cell lung cancer patients harboring wild-type EGFR (CTONG0806): a multicenter randomized trial. Ann Oncol 25: 2385–2391. [DOI] [PubMed] [Google Scholar]
- Zinner R., Fossella F., Gladish G., Glisson B., Blumenschein G., Papadimitrakopoulou V., et al. (2005) Phase II study of pemetrexed in combination with carboplatin in the first-line treatment of advanced nonsmall cell lung cancer. Cancer 104: 2449–2456. [DOI] [PubMed] [Google Scholar]
- Zinner R., Novello S., Peng G., Herbst R., Obasaju C., Scagliotti G. (2010) Comparison of patient outcomes according to histology among pemetrexed-treated patients with stage IIIB/IV non-small-cell lung cancer in two phase II trials. Clin Lung Cancer 11: 126–131. [DOI] [PubMed] [Google Scholar]
- Zukin M., Barrios C., Pereira J., Ribeiro R., Beato C., de M., do Nascimento Y., et al. (2013) Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 31: 2849–2853. [DOI] [PubMed] [Google Scholar]