Abstract
Background
Stent thrombosis (ST) is a rare but serious complication following percutaneous coronary intervention. Analysis of thrombus composition from patients undergoing catheter thrombectomy may provide important insights into the pathological processes leading to thrombus formation. We performed a large-scale multicentre study to evaluate thrombus specimens in patients with ST across Europe.
Methods
Patients presenting with ST and undergoing thrombus aspiration were eligible for inclusion. Thrombus collection was performed according to a standardized protocol and specimens were analysed histologically at a core laboratory. Serial tissue cross sections were stained with haematoxylin–eosin (H&E), Carstairs and Luna. Immunohistochemistry was performed to identify leukocyte subsets, prothrombotic neutrophil extracellular traps (NETs), erythrocytes, platelets, and fibrinogen.
Results
Overall 253 thrombus specimens were analysed; 79 (31.2%) from patients presenting with early ST, 174 (68.8%) from late ST; 79 (31.2%) were from bare metal stents, 166 (65.6%) from drug-eluting stents, 8 (3.2%) were from stents of unknown type. Thrombus specimens displayed heterogeneous morphology with platelet-rich thrombus and fibrin/fibrinogen fragments most abundant; mean platelet coverage was 57% of thrombus area. Leukocyte infiltrations were hallmarks of both early and late ST (early: 2260 ± 1550 per mm2 vs. late: 2485 ± 1778 per mm2; P = 0.44); neutrophils represented the most prominent subset (early: 1364 ± 923 per mm2 vs. late: 1428 ± 1023 per mm2; P = 0.81). Leukocyte counts were significantly higher compared with a control group of patients with thrombus aspiration in spontaneous myocardial infarction. Neutrophil extracellular traps were observed in 23% of samples. Eosinophils were present in all stent types, with higher numbers in patients with late ST in sirolimus-and everolimus-eluting stents.
Conclusion
In a large-scale study of histological thrombus analysis from patients presenting with ST, thrombus specimens displayed heterogeneous morphology. Recruitment of leukocytes, particularly neutrophils, appears to be a hallmark of ST. The presence of NETs supports their pathophysiological relevance. Eosinophil recruitment suggests an allergic component to the process of ST.
Keywords: Eosinophils, Histopathology, Neutrophils, Neutrophil extracellular traps, Platelets, Stent thrombosis, Thrombus aspiration
See page 1550 for the editorial comment on this article (doi:10.1093/eurheartj/ehw036)
Translational perspective.
Stent thrombosis is a rare but life-threatening complication of percutaneous coronary intervention. A detailed analysis of thrombus aspirated from patients during percutaneous revasularization may provide a deeper understanding of the pathological processes involved. Here, we report the results of the largest analysis of thrombus samples from patients with stent thrombosis performed so far. Besides the presence of platelets and fibrin, we found a significant number of immune cells, mainly neutrophils, some of which released prothrombotic fibres of DNA, and also eosinophil granulocytes. Our findings suggest that immune cells could represent a novel target for the prevention of stent thrombosis.
Introduction
Stent thrombosis (ST) is a life-threatening complication of percutaneous coronary intervention. Recent large-scale clinical registries reported an incidence of up to 0.4–0.6% per year though rates appear to be lower with newer-generation drug-eluting stents (DES) devices.1,2 The majority of ST patients present with acute myocardial infarction and rates of mortality following presentation are as high as 20–40%.3 In addition, patients treated with DES—the dominant devices used in contemporary practice—have been shown to be at higher risk of late ST and although this risk appears to be ameliorated with newer-generation DES devices, clinical practice guidelines continue to recommend a more prolonged duration of dual antiplatelet therapy after stenting with DES when compared with after bare metal stents.4
Analysis of thrombus specimens from patients presenting with ST can provide useful information regarding the pathophysiological process leading to thrombotic stent occlusion. Prior studies in small numbers of patients demonstrated that thrombus aspirates from patients with ST were comprised of fragments of fibrin and platelet-rich thrombus as well as trapped red blood cells.5 Moreover, the presence of leukocyte populations in significant numbers suggested an important role for inflammation in the pathogenesis of ST events. This latter observation is in keeping with increasing recognition of the importance of inflammation and immune response in atherothrombosis in general,6 as well as the known contribution of hypersensitivity reactions particularly following stenting with durable polymer DES.7–10 Although some pathological processes associated with ST have been identified, the triggering mechanisms remain incompletely understood, and the influence of factors such as timing of ST after the procedure, stent type or polymer coating is poorly characterized. Moreover existing studies did not include detailed characterization of immune cells and related extracellular components. In addition large-scale, multicentre studies with systematic analysis of thrombus from patients presenting with ST remains a notable scientific gap.
The Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort (PRESTIGE) consortium was established to investigate ST across Europe.11 A general description of the project is provided in Supplementary material online, Appendix. The central work of the clinical work package was a prospective multicentre registry of patients presenting with ST to participating centres across Europe. As part of this study, thrombus specimens retrieved from catheter thrombectomy were systematically collected and analysed in a central core laboratory. The present report details the main findings from the histopathological evaluation of thrombus specimens from these patients.
Methods
Study population and patient treatment
Patients presenting with stent thrombosis and undergoing catheter thrombectomy at participating centres were eligible for inclusion. Patients were prospectively enrolled using a centralized telephone registration system. A list of participating centres is provided in Supplementary material online, Appendix. Stent thrombosis was defined according to Academic Research Consortium (ARC) criteria for definite stent thrombosis. Data were collected according to a standardized protocol and was entered in a central electronic database (Open Clinica, Leuven Coordinating Centre, Leuven, Belgium). All patients provided written informed consent. Funding was provided by the European Commission under the Seventh Frame Work Programme (Grant agreement number 260309 PRESTIGE). As an additional control group, we also included analysis of thrombus aspirates from patients with spontaneous myocardial infarction undergoing thrombus aspiration in non-stented vessels segments. Recruitment of these patients occurred at a single centre (Deutsches Herzzentrum München, Munich, Germany) and did not comprise part of the PRESTIGE project.
Histopathological sampling and analysis
After crossing the lesion with a standard guide-wire, a thrombectomy catheter was advanced to the target lesion. Thrombus aspiration was performed using a manual suction device according to standard practice. The catheter type used was at the discretion of the cardiologist and was not recorded in the database. Thrombus was collected according to a standardized protocol, fixed in formalin 4%, and transported to the core laboratory for thrombus analysis (Deutsches Herzzentrum München, Munich, Germany). Here thrombus specimens were embedded in paraffin after 48 h of formalin fixation. Thrombus aspirates were processed and analysed wherever practicably possible. Some thrombi were received as small fragments and could not be processed for histological analysis. The smallest thrombus processed successfully had a cross-sectional area of 0.01 mm2 at the time of image acquisition. Serial cross sections of all thrombi were cut with 5 µm thickness. Paraffin-embedded tissue sections were deparaffinized by immersion in xylene and rehydrated in decreasing concentrations of ethanol. Sections were stained with H&E, Luna12 or Carstairs.13 Reagents were provided by EMS (Hatfield, USA). Slides were mounted with colourless mounting medium (Pertex).
For immunohistochemistry, antigen retrieval was performed using heat and citrate buffer. Specimens were washed in PBS and blocked with 5 µg/mL anti-mouse CD16/32 (eBioscience) and 1% BSA (PAA Laboratories) in PBS for 30 min. Primary antibodies were incubated for 1 h at room temperature, and sections were then washed in PBS containing 0.1% Tween. Secondary antibodies were incubated for 1 h at room temperature. Samples were mounted using an anti-fade mounting medium (DAKO) and sealed with a coverslip. Fibrin/fibrinogen was stained using a rabbit polyclonal antibody (DAKO #A0080). Neutrophil extracellular traps were identified by their expression of neutrophil elastase (NE) using a rabbit polyclonal neutrophil elastase antibody (Abcam #Ab68672).14 Platelets were identified using a rabbit polyclonal CD41 antibody (Acris #AP54811PU-N). Rabbit IgG was used for control stainings (Supplementary material online, Figure S1). Goat anti-rabbit Alexa Fluor 594 (Invitrogen) was used as secondary antibody. DNA was stained with Hoechst 33342 Solution (Invitrogen). Cells and neutrophil extracellular traps (NETs) were quantified in four visual fields using a 40× objective (176 × 131 µm). Data were normalized to square meter thrombus area. For the assessment of cell counts, coefficient of variation for inter- and intra-observer variability was 0.78 and 0.77%, respectively. The following preconditions had to be fulfilled for quantification of NET formation: (i) attendance of filamentary structured extracellular DNA, (ii) respective DNA had to originate from cells that stained positive for a neutrophil marker, and (iii) filamentary structures had to be decorated with a marker for neutrophil granule proteins such as NE. Eosinophils were counted on whole sections stained with Luna and the results were presented as cells/mm2 thrombus area. The percentage of fibrinogen, erythrocyte, and platelet area in relation to the whole thrombus area was investigated in overview images of the thrombi taken with the above-mentioned camera and analysed using Cap-Image 7.1 software (Dr Zeintl, Heidelberg, Germany) and Adobe Photoshop. Images were acquired using either a Zeiss Imager M2 Axio epifluorescence microscope and processed using AxioVision AxioVision SE64 Rel. 4.9 software, or a Leica DMRB epifluorescence microscope equipped with a Zeiss AxioCam and processed using AxioVision 4.6 software (Zeiss).
Histological features and comparisons of interest
Patients were categorized according to timing of ST—as early (<30days) and late (>30days) post stent implantation (on the basis that mechanistic factors tend to be different between early and late ST)—and type of stent at the index procedure (DES or bare metal stent). Further categorization of timing of ST was performed in accordance with ARC criteria. In a detailed analysis, we then compared the number of leukocytes, neutrophils, NETs, and eosinophils between (i) early and late ST, (ii) DES and bare metal stent, and (iii) DES types.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (IBM SPSS Statistics) version 23.0 (IBM, Ehningen, Germany) and Prism 5 (GraphPad Software, La Jolla, USA). Data were tested for normal distribution using the Kolmogorov–Smirnov test. For comparison of continuous data between two groups, we used Student's t-test or Mann–Whitney U-test, for three and more groups, we used analysis of variance ANOVA or Kruskal–Wallis test, according to the distribution of the data. For comparison of categorical data between two groups we used χ2 or Fisher's exact test as appropriate. For comparisons of multiple groups, post hoc testing was performed applying the methods of Bonferroni (Dunn's test). Significance level was set at a two-sided α of 0.05.
Results
Five hundred and forty-one patients presenting with ST to participating centres between December 2010 and February 2014 were included in the PRESTIGE registry. Thrombus samples were collected from 294 patients. A total of 41 patients were excluded because thrombus specimens were too small for analysis. Overall, thrombus from 253 patients was available for histological analysis. Baseline patient characteristics are shown in Table 1. Procedural characteristics at the time of the ST are shown in Table 2. Baseline laboratory values are shown in Table 3.
Table 1.
Baseline clinical and demographic characteristics of patients with analysable thrombus aspirates according to presentation as early and late stent thrombosis
| Early (<30 days), n = 79 | Late (>30 days), n = 174 | P-value | |
|---|---|---|---|
| Age | 66 [57,74] | 62 [54,72] | 0.94 |
| Sex | |||
| Male | 54 (68.4) | 143 (82.2) | 0.014 |
| Coronary artery disease, n (%) | |||
| 1-vessel | 36 (48.0) | 97 (59.1) | 0.24 |
| 2-vessel | 25 (33.3) | 46 (28.0) | |
| 3-vessel | 14 (18.7) | 21 (12.9) | |
| Multi-vessel disease | 39 (52.0) | 67 (40.9) | 0.11 |
| History of coronary bypass | 5 (6.4) | 16 (9.2) | 0.46 |
| Ejection fraction <30% | 2 (2.7) | 4 (2.4) | >0.99 |
| Risk factors | |||
| Diabetes | 30 (38.5) | 35 (20.2) | 0.002 |
| Hypertension | 42 (55.3) | 69 (41.3) | 0.043 |
| Ex-/smoker | 51 (65.4) | 123 (72.3) | 0.22 |
| Hypercholesterolaemia | 66 (83.5) | 157 (90.2) | 0.13 |
| Clinical presentation | |||
| Unstable angina pectoris | 3 (3.8) | 6 (3.5) | 0.629 |
| Non-ST-elevation MI | 9 (11.5) | 28 (16.2) | |
| ST-elevation MI | 66 (84.6) | 139 (80.3) | |
| Antiplatelet therapy | |||
| Aspirin | 69 (87.3) | 138 (80.7) | 0.20 |
| ADP-receptor antagonist | 65 (82.3) | 43 (25.0) | <0.001 |
| Clopidogrel | 43 (66.2) | 26 (60.5) | |
| Prasugrel | 7 (10.8) | 11 (25.6) | |
| Ticagrelor | 15 (23.1) | 6 (14) | |
| Dual antiplatelet therapy | 60 (75.9) | 36 (20.9) | <0.001 |
| Coexisting conditions | |||
| Renal failure (GFR < 30 mL/min) | 6 (7.8) | 10 (5.8) | 0.580 |
| Dialysis | 1 (1.3) | 2 (1.1) | >0.99 |
| Stroke | 6 (7.7) | 9 (5.2) | 0.57 |
| Autoimmune disease | 1 (1.4) | 5 (2.9) | 0.67 |
| Active malignancy | 3 (3.9) | 5 (3.0) | 0.71 |
| Stent type | |||
| Bare metal stent | 22 (27.8) | 57 (32.8) | <0.001 |
| First-generation DES | 4 (5.1) | 49 (28.2) | |
| Second-generation DES | 45 (57.0) | 47 (27.0) | |
| Unknown DES type | 8 (10.1) | 13 (7.5) | |
| Presentation at index intervention | 0.026 | ||
| Stable angina pectoris | 18 (24.0) | 49 (30.4) | |
| Unstable angina pectoris | 6 (8.0) | 21 (13.0) | |
| Non-ST-elevation MI | 23 (30.7) | 23 (14.3) | |
| ST-elevation MI | 28 (37.3) | 68 (42.2) | |
| EF < 30% at index PCI | 2 (2.7) | 3 (1.8) | 0.65 |
Data are shown as median [Q1, Q3] or n (%). Percentages were calculated on the basis of patients with available information.
MI, myocardial infarction; DES, drug-eluting stent.
Table 2.
Procedural characteristics of patients with analysable thrombus aspirates according to presentation as early and late stent thrombosis
| Early (<30 days), n = 79 | Late (>30 days), n = 174 | P-value | |
|---|---|---|---|
| Target vessel | |||
| Left anterior descending | 46 (63.0) | 70 (41.9) | 0.02 |
| Left circumflex | 7 (9.6) | 18 (10.8) | |
| Right coronary | 19 (26.0) | 74 (44.3) | |
| Bypass graft | 1 (1.4) | 5 (3.0) | |
| TIMI flow pre-intervention | |||
| 0 | 67 (89.3) | 140 (82.4) | 0.20 |
| I | 0 (0.0) | 7 (4.1) | |
| II | 4 (5.3) | 16 (9.4) | |
| III | 4 (5.3) | 7 (4.1) | |
| Procedure | |||
| Thrombus aspiration performed | 79 (100) | 174 (100) | |
| Balloon angioplasty | 70 (92.1) | 146 (85.4) | 0.14 |
| Additional stent implanted | 40 (51.3) | 99 (58.9) | 0.26 |
| Use of glycoprotein receptor antagonists | 11 (16.4) | 41 (28.1) | 0.07 |
Data are shown as n (%). Percentages were calculated on the basis of patients with available information.
TIMI, thrombolysis in myocardial infarction.
Table 3.
Laboratory parameters of patients with analysable thrombus aspirates according to presentation as early and late stent thrombosis
| Early (<30 days), n = 79 | Late (>30 days), n = 174 | ||
|---|---|---|---|
| CRP (mg/L) | 5.0 [2.1, 15.5] | 2.2 [0.8, 10.0] | 0.006 |
| Leukocytes (109/L) | 10.9 [8.6, 14.4] | 10.8 [8.2, 14.3] | 0.71 |
| Platelets (109/L) | 270.5 [224.5, 328.3] | 223.5 [178.0, 268.0] | <0.001 |
| CKmax (U/L) | 527.0 [178.8, 1629.0] | 628.5 [211.5, 1664.8] | 0.71 |
| CK-MBmax (U/L) | 78 [30.0, 200.0] | 95 [43.0, 191.0] | 0.62 |
| Troponin Tmax (ng/mL) | 3.2 [0.7, 31.4] | 4.74 [1.0, 24.2] | 0.32 |
Data are shown as median [Q1, Q3].
As an additional control group, we also included analysis of thrombus aspirates from 104 patients with spontaneous myocardial infarction undergoing thrombus aspiration in non-stented vessel segments. The baseline characteristics of these patients are shown in Supplementary material online, Table S1.
Frequency of stent types and timing of stent thrombosis
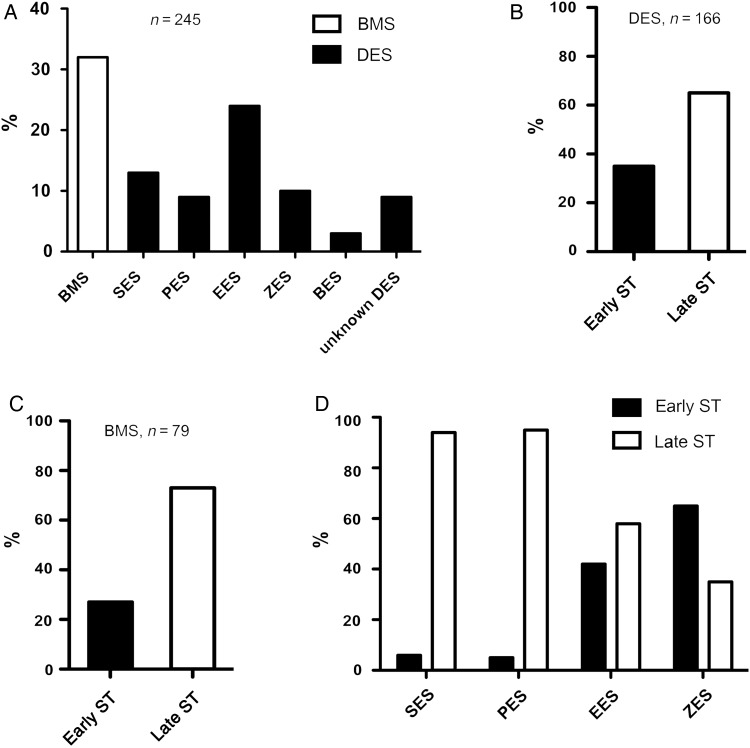
In terms of timing of ST, 79 (31.2%) thrombus specimens were from patients presenting with early ST and 174 (68.8%) from late ST. In terms of stent type, 166 (65.6%) thrombus samples were from DES and 79 (31.2%) from bare metal stents; 8 (3.2%) were of unknown stent type (Figure 1A). The proportion of early and late ST was broadly similar in samples from DES and bare metal stents (Figure 1B and C). The breakdown of stents according to the specific type of DES is shown in Figure 1A; overall, everolimus-eluting stents were most commonly represented. Early stent thrombosis cases in sirolimus- and paclitaxel-eluting stents were poorly represented; early and late ST were more equally represented in newer-generation DES (everolimus, zotarolimus eluting) (Figure 1D).
Figure 1.
Frequency of stent types and timing of stent thrombosis. (A) Percent distribution of thrombus aspirates retrieved from bare metal (white bar) and drug-eluting (black bars) stents (n = 245), sirolimus, paclitaxel, everolimus, zotarolimus, biolimus; (B) percent distribution of early vs. late stent thrombosis in drug-eluting stents (n = 166); (C) percent distribution of early vs. late stent thrombosis in bare metal stents (n = 79); (D) percent distribution of early vs. late stent thrombosis in first and second-generation drug-eluting stents. Sirolimus (n = 31), paclitaxel (n = 22), everolimus (n = 59), zotarolimus (n = 23).
Histological analysis of thrombus specimens
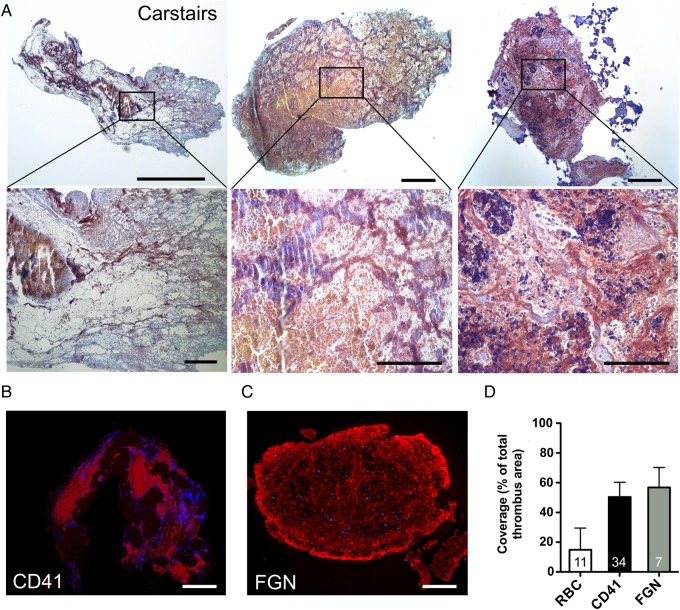
Thrombus specimens from patients with ST were subjected to systematic histological analysis; representative samples are displayed in Figure 2. Thrombus specimens displayed a compact, heterogeneous morphology. Specimens were rich in platelets (grey/blue to navy staining in Carstairs and red staining in CD41-specific immunohistochemistry) (Figure 2A and B). Mean platelet coverage was 57% of thrombus area. Fibrin/fibrinogen (FGN) represented a major component (red staining in Carstairs and in FGN-specific immunohistochemistry) (Figure 2A and C). Clusters of erythrocytes were present; however, the distribution was heterogeneous (Figure 2A). Comparison of the erythrocyte, platelet, and fibrin/fibrinogen components of thrombus area is shown in Figure 2D.
Figure 2.
Histological analysis of thrombus aspirated from intracoronary stents. (A) Representative Carstairs stainings of thrombus aspirates (n = 11). Upper row: overview image (left bar, 50 µm; other bars 100 µm). Bottom row: insets of the overview images (left bar, 25 µm; other bars 50 µm); platelets are stained in grey blue to navy, fibrin/fibrinogen in red and erythrocytes (RBC) in yellow; (B) representative image of platelet aggregation area (CD41 positive) in thrombus aspirates (n = 7). Nuclei were counterstained with Hoechst. Bar, 100 µm; (C) fibrin/fibrinogen immunofluorescence staining (n = 34). Nuclei were counterstained with Hoechst. Bar, 100 µm; (D) Coverage of whole thrombus area (%) with RBC, platelet (CD41) and fibrin/fibrinogen rich areas. Due to co-localization, overall coverage exceeds 100%; data are shown as mean + SD.
Leukocytes and neutrophil extracellular traps in thrombus specimens
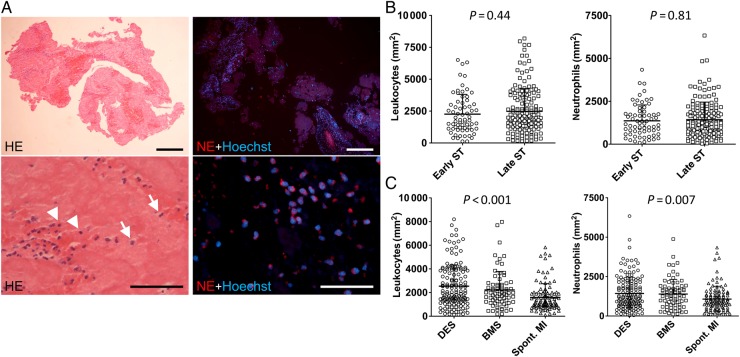
Overall in histological and immunohistochemical analyses of ST specimens, inflammatory cells were prominently represented. Cells were present in significant numbers and were mostly found in clusters or layers (Figure 3, Supplementary material online, Table S2). The majority of leukocytes were neutrophils as identified by staining for NE (Figure 3A). Regarding the timing of ST, there was no difference in the numbers of leukocytes (early: 2260 ± 1550 per mm2 vs. late: 2486 ± 1778 per mm2; P = 0.44) and neutrophils (early: 1364 ± 923 per mm2 vs. late: 1428 ± 1023 per mm2; P = 0.81) (Figure 3B). Overall, the number of leukocytes and neutrophils was not significantly different according to presentation with acute, subacute, late or very late ST (Supplementary material online, Figure S2); there was no correlation between leukocyte counts and time interval from index stenting (Supplementary material online, Figure S3). Neither were there significant differences according to antiplatelet therapy at the time of presentation (Supplementary material online, Figure S4). Regarding stent type, there was no significant difference in the numbers of leukocytes (DES: 2538 ± 1798 per mm2 vs. bare metal stents: 2218 ± 1567 per mm2; P = 0.33) and neutrophils (DES: 1429 ± 1041 per mm2 vs. bare metal stents: 1393 ± 931 per mm2; P = 0.97) (Figure 3C, Supplementary material online, Table S2). However, patients with spontaneous myocardial infarction (MI) had smaller numbers of leukocytes and neutrophils in comparison with ST patients (Figure 3B and C). In patients with very late ST, there were numerically more leukocytes with DES vs. bare metal stents but this was not statistically significant (Supplementary material online, Figure S5). Drug-eluting stents type had no significant influence on the overall number of inflammatory cells observed (Supplementary material online, Figure S6 and Supplementary Data).
Figure 3.
Leukocyte accumulation in stent thrombus specimens. (A) Leukocyte accumulation in human stent thrombus specimens. Left images: Haematoxylin–eosin staining (n = 253). Arrows indicate granulocytes, arrowheads indicate mononuclear cells. Right images: immunofluorescence staining of neutrophil elastase to identify neutrophils (n = 229). Nuclei are counterstained with Hoechst. Bars, 200 µm (upper row) and 50 µm (bottom row); (B) Quantification of leukocytes and neutrophils in early (n = 67) vs. late (n = 162) stent thrombosis (leukocytes: P = 0.44; neutrophils: P = 0.81); (C) Leukocytes and neutrophils in stent thrombosis from drug-eluting stents (n = 149) and bare metal stents (n = 73) and in thrombi aspirated from patients with spontaneous myocardial infarction (spont. myocardial infarction; n = 104) (P < 0.05 for drug-eluting stents vs. spont. myocardial infarction and bare metal stents vs. spont. myocardial infarction). Shown are mean + SD, each symbol in (B) and (C) represents one individual patient.
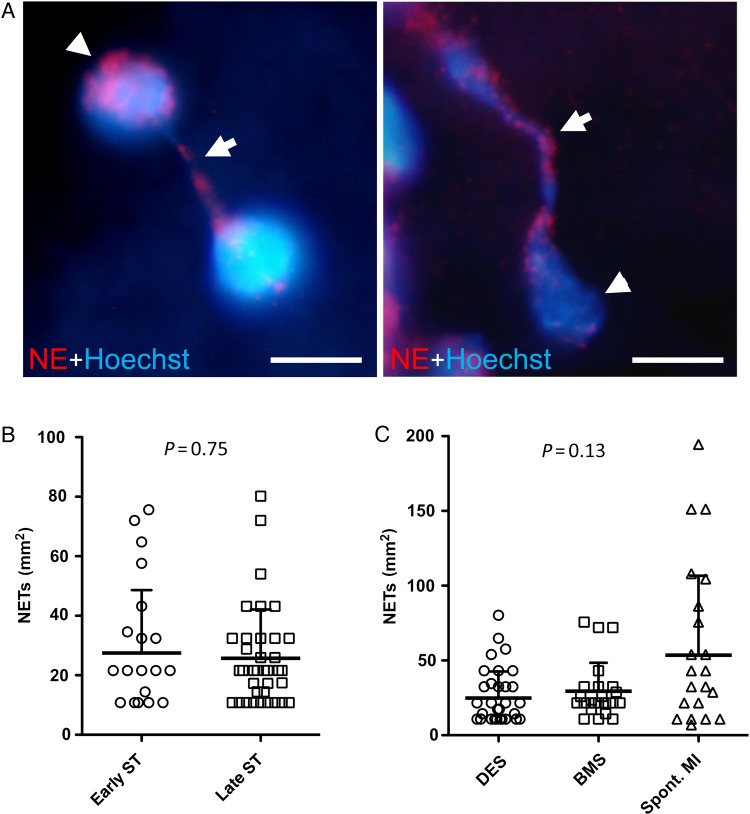
Neutrophil extracellular traps were also frequently observed in the thrombus specimens (Figure 4). Overall, 59 (23.3%) thrombus specimens were found to have extracellular DNA originating from neutrophils. There were no significant differences in the numbers of NETs according to timing of ST (early: 27 ± 21 per mm2 vs. late: 26 ± 16 per mm2; P = 0.75) (Figure 4B) or stent type (DES: 25 ± 18 per mm2 vs. bare metal stents: 29 ± 19 per mm2; P = 0.15) (Figure 4C), or compared with patients with spontaneous myocardial infarction (54 ± 53 per mm2; P = 0.13) (Figure 4C).
Figure 4.
Detection of neutrophil extracellular traps in stent thrombus specimens. (A) Immunofluorescence images of neutrophil extracellular traps stained for neutrophil elastase and DNA (Hoechst). Extracellular DNA originates from neutrophil elastase positive neutrophils. Arrowheads, nuclei; arrows, neutrophil extracellular trap fibres. Bars, 5 µm; (B) number of neutrophil extracellular traps in early (n = 23) vs. late (n = 37) stent thrombosis (P = 0.75); (C) quantification of neutrophil extracellular traps in thrombi derived from drug-eluting stents (n = 36), bare metal stents (n = 23), and spontaneous myocardial infarction (spont. myocardial infarction) (n = 25) (P = 0.13); data are shown as mean + SD, each symbol in (B) and (C) represents one individual patient.
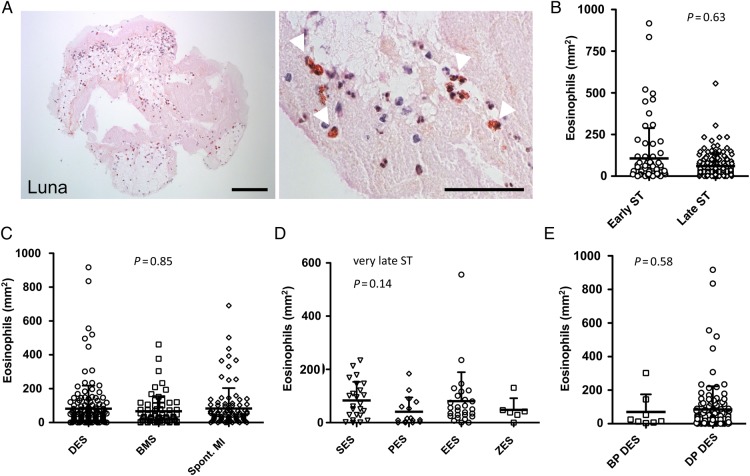
Eosinophil accumulation in thrombus specimens
Using Luna staining, we identified eosinophils in thrombus specimens (Figure 5A). There was no significant difference in the number of eosinophils according to the timing of ST (early 107 ± 181 per mm2; late 61 ± 73 per mm2; P = 0.63) (Figure 5B; Supplementary material online, Figure S3C and Supplementary Data). Neither was there an overall difference in the number of eosinophils according to the stent type (DES: 82 ± 136 per mm2 vs. bare metal stents: 67 ± 89 per mm2; P = 0.69) (Figure 5C; Supplementary material online, Figure S3C and Supplementary Data). Patients with spontaneous MI had similar numbers of eosinophils in comparison with ST patients (Figure 5C). In patients with very late ST, there were numerically more eosinophils in DES vs. bare metal stents but this was not statistically significant (Supplementary material online, Figure S5); however, in terms of DES stent type, we found numerically more eosinophils in aspirates from sirolimus-eluting (83 ± 70 per mm2) and everolimus-eluting stents (80 ± 109 per mm2) compared with paclitaxel- (41 ± 54 per mm2) and zotarolimus-eluting stents (48 ± 44 per mm2) (P = 0.14, Figure 5D; Supplementary material online, Figure S3D and Supplementary Data). There was no difference between durable polymer DES compared with bioabsorbable polymer DES (Figure 5E, P = 0.58).
Figure 5.
Eosinophil accumulation in stent thrombus specimens. (A) Eosinophils in human thrombi were identified by Luna staining. Arrowheads indicate eosinophils (red-brown colour). Bars, 100 µm (left) and 50 µm (right); (B) Number of eosinophils in early (n = 71) vs. late (n = 146) stent thrombosis (P = 0.63); (C) Quantification of eosinophils in thrombi derived from drug-eluting stents (n = 143), bare metal stents (n = 66) and spont. myocardial infarction (n = 93) (P = 0.85); (D) Number of eosinophils in very late ST according to drug-eluting stents type; SES (n = 24), PES (n = 16), EES (n = 27), ZES (n = 6) (P = 0.14); (E) Number of eosinophils according to drug-eluting stents polymer type; bioabsorbable polymer (BP drug-eluting stents): n = 8, durable polymer (DP drug-eluting stents): n = 120 (P = 0.58); data are shown as mean + SD, each symbol in (B–E) represents one individual patient.
Discussion
With analysis of samples from 253 patients, the present report represents the largest analysis of thrombus specimens from patients presenting with ST following percutaneous coronary intervention. The main findings are as follows: (i) thrombus aspirates were heterogeneous in composition containing platelet-rich thrombus, fibrin/fibrinogen fragments, erythrocytes, and inflammatory cells; (ii) the composition of thrombus from patients with early and late ST as well as with thrombosis in bare metal and drug-eluting stents was broadly similar; (iii) leukocytes were present in thrombus samples in substantial numbers, with neutrophil subpopulations accounting for the majority of cells, highlighting the important role of inflammatory cell recruitment in ST; (iv) for the first time, we could demonstrate that, as is the case in native vessel thrombosis, NETs are important components of stent thrombus aspirates with these structures present in approximately 1-in-4 samples; and (v) eosinophils are typically present in both bare metal and drug-eluting stent thrombus specimens, with higher numbers in patients with very late ST thrombosis in sirolimus- and everolimus-eluting stents.
The PRESTIGE consortium was established to investigate and reduce the incidence of ST after coronary intervention across Europe.11 The central work of the clinical work package was based around a prospective multicentre registry of patients presenting with ST to participating centres across Europe. In the setting of this registry, thrombus specimens retrieved from catheter thrombectomy were systematically collected and analysed in a central core laboratory. Through a Europe-wide multicentre collaboration involving the cooperation of a large network of participating clinical centres it was possible to collect 253 analysable ST thrombus aspirates—by far the largest number of aspirates from patients suffering a ST reported in the literature.
A number of issues should be considered in more detail when interpreting the data. First, the heterogeneous nature of the thrombus specimens is consistent with prior reports involving smaller numbers of patients. The central components were platelet-rich thrombus, fibrin/fibrinogen fragments, trapped erythrocytes, and inflammatory cells of leukocyte lineage. In terms of thrombus area, the greatest contribution was from platelets and fibrin/fibrinogen. This is not surprising as it is well known that activated platelets directly promote blood coagulation leading to subsequent fibrin formation, which plays a central role in thrombus growth and stabilization in human atherothrombosis.15–17
Secondly, it is increasingly recognized that immune cells participate in thrombosis, including native human coronary arteries.18,19 Leukocytes can induce local thrombosis as an intravascular defence strategy to pathogen infection.6 Innate immune cells activate procoagulant pathways to compartmentalize, retain and kill pathogens and trigger coagulation in models of vessel thrombosis.20 However, whether immunothrombosis-related pathways are also involved in human ST has been a poorly defined issue. In our analysis leukocytes, and specifically neutrophils, represented a hallmark of thrombus aspirates from both bare metal stents and DES. Moreover, analysing leukocyte counts according to timing of ST and type of underlying stent showed no significant difference in cell counts between the different groups. This suggests that leukocyte recruitment is likely a component of the final common pathway in ST irrespective of the initial anatomopathological trigger. In addition, in our analysis the numbers of leukocytes and neutrophils was significantly higher in patients with ST compared with spontaneous MI. This suggests that the immune response may play a relatively more important role in ST when compared with spontaneous MI.
Interestingly, we could demonstrate for the first time that NETs are important components of ST aspirates. Recent research has shown that neutrophils are able to build NETs through a specific type of cell death termed NETosis. These procoagulant extracellular DNA matrices represent a catalytic platform able to bind and activate platelets and other procoagulant effectors like FXII, thereby contributing to both venous and arterial thrombosis.14,20–23 Mangold et al. correlated high NET burden in aspirated thrombus from patients with acute myocardial infarction with larger infarct size and lesser ST-segment resolution underlining the potential clinical relevance of these structures.24 Overall, NETs were observed in approximately 1-in-4 thrombus samples in our study. The finding that the majority of aspirates did not contain NET-positive thrombi might be explained by concomitant early administration of heparin in patients with ST undergoing angioplasty in the setting of myocardial infarction. Heparin anticoagulant is known for its direct interaction with NETs, causing their degradation both in vitro25 and in vivo.14 Interpatient differences in DNase activity within the circulatory system might also account for the variability of this finding. DNase is able to cleave extracellular DNA and Mangold et al. showed that a higher DNase activity correlated negatively with coronary thrombus NET burden.24 Taken together, these observations suggest that pharmacological targeting of immunothrombosis may represent a realistic target for novel therapies. Inhibition of triggers of immunothrombosis, such as extracellular nucleic acids activating the contact phase, may not only result in efficient anticoagulation in the setting of ST but might also yield less therapy-associated bleeding. Future studies should evaluate whether inhibition of immunothrombosis pathways is effective and safe in clinical practice.
Thirdly, observations in relation to the eosinophil subpopulation of leukocytes were interesting for a number of reasons. Eosinophils were observed at some level across the spectrum of ST cases examined demonstrating a role of these cells in ST in general. Moreover, there were no significant differences in the number of eosinophils observed in early vs. late ST or in bare metal vs. drug-eluting stents. However, interestingly, examination of eosinophil counts according to DES subtypes in patients with very late ST showed numerically higher counts in ST occurring within sirolimus- and everolimus-eluting stents. Indeed, hypersensitivity reactions (e.g. type IVb delayed hypersensitivity reactions) with vasculitis of the vessel wall and lymphocytic and eosinophilic infiltration has previously been observed in autopsy specimens and preclinical studies after DES implantation leading to late ST.9,26,27 Moreover, a prior thrombus aspirate study by Cook et al. showed higher levels of eosinophils in patients with ST in sirolimus-eluting stents. A possible explanation is that both sirolimus- and everolimus-eluting stents utilize polymer coatings with methacrylate components, and this might represent a stimulus for hypersensitivity reactions.7,9,10
It is important to interpret the results of our analysis in the context in which the samples were obtained. For example, overall more samples were available from patients with late ST when compared with early ST. Although it is well documented in clinical studies that the majority of ST events occur during the initial 30 days after implantation,1,2 this distribution depends to some extent on the duration of follow-up available, with late ST increasingly better represented with increasing overall duration of follow-up. In addition, it might be hypothesized that patients with acute ST and clear mechanical risk factors were less likely to be represented in the registry. In addition, important differences were observed in relation to stent type. In this respect, the high representation of thrombus from everolimus-eluting stents most likely reflects the high usage of these stents in clinical practice. Similarly, the relative over-representation of late ST samples in patients treated with sirolimus- or paclitaxel-eluting stents reflects the low usage of these devices during the enrolment period of the study when newer-generation DES were the dominant devices in clinical use.
Our study has some additional important limitations. First, only patients with successful thrombus aspiration were eligible for inclusion. This impacts on the generalizability of the data. Second, analysis of thrombus is restricted to retrievable pieces of thrombus and theoretically differences might exist between thrombus fragments that are removed vs. those that are retained or displaced. Third, findings in relation to thrombus components remain observational in nature and caution must be used in interpreting comparative findings between different timings and stent types. Fourth, analysis is limited to the description of aspirated cell types, which are interpreted in isolation from information regarding the underlying pathology of the vessel wall; this might have been derived from intravascular imaging data. Fifth, although flow cytometry analysis of thrombus could have provided additional useful data, due to logistical considerations related to multicentre recruitment such an analysis was not planned as part of the current study. Finally, our analysis did not report thrombus age at the time of aspiration. This is because methods for adjudication of thrombus age are not standardized and robustness of these observations is unclear.
In conclusion, we present a comprehensive analysis of the largest series of thrombus samples from patients presenting with ST in the literature to date. The main finding was that thrombus samples were heterogeneous in composition with platelet-rich thrombus, fibrin/fibrinogen fragments, and erythrocytes accounting for the largest volume of the thrombus samples. Moreover, leukocyte recruitment was a hallmark of human ST and NETs, central effectors of immunothrombosis, could be detected in human ST for the first time, supporting their relevance in the pathophysiology of this condition. Eosinophils are also recruited in ST, indicating that allergic reactions could contribute to ST, with differential eosinophilic counts according to DES type, suggesting that hypersensitivity reactions might be more important with certain types of polymer-based stents. Notwithstanding the multifactorial nature of the process of ST, the findings of the present study suggest that immune cells could represent an important target for the prevention of ST in future experimental research and clinical trials.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The research leading to these results has received funding from the European Union Seventh Framework Programme FP7/2007-2013 under grant agreement n° HEALTH-F2-2010-260309 (PRESTIGE). Funding to pay the Open Access publication charges for this article was provided by the European Commission under the Seventh Frame Work Programme (Grant agreement number 260309 PRESTIGE).
Conflict of interest: R.A.B. reports receiving lectures fees from B. Braun Melsungen AG, Biotronik, and Boston Scientific. A.G. reports advisory board membership, lecture fees, travel bursary, research support from Abbott Vascular, Boston Scientific, The Medicines Company, Eli Lilly, Daiichi-Sankyo, and Medtronic. D.T. reports personal fees from AstraZeneca, Bayer, BMS, Boehringer-lngelheim, Daiichi-Sankyo, Eli Lilly, MSD, Otsuka, and Pfizer. L.J.F. reports grants from Sanofi and Bristol-Myers-Squibb. P.G.S. reports personal fees from Amarin, AstraZeneca, Bayer, Boehringer-lngelheim, Bristol-Myers-Squibb, Daiichi-Sankyo, GlaxoSmithKiine, Lilly, Merck-Sharpe-Dohme, Novartis, Otsuka, Pfizer, Roche, Medtronic, Vivus, Janssen, Orexigen and Regado, grants and personal fees from Sanofi and Servier, personal fees and non-financial support from The Medicines Company. C.V. reports personal fees from Lilly and Novartis. A.K. reports submission of patent applications in relation to drug-eluting stent technology.
Supplementary Material
Appendix
PRESTIGE consortium members
Partners
Deutsches Herzzentrum München (DHM), Azienda Ospedaliera Papa Giovanni XXIII (BER), Samodzielny Publiczny Zaklad Opieki Zdrowotnej Szpital Uniwersytecki W Krakowie (KRAK), St. Antonius Ziekenhuis Nieuwegein (NIE), University of Leicester (ULEIC), Universitäts-Herzzentrum Freiburg-Bad Krozingen GmbH (UHZ), Institut national de la santé et de la recherche médicale (INSERM), Rigas Tehniska Universitate (RTU), Kitozyme S.A. (KIZ), Helmholtz Zentrum München, Deutsches Forschungszentrum für Gesundheit und Umwelt GmbH (HMGU), Katholieke Universiteit Leuven (K.U.LEUVEN), Servicio Madrileño de Salud: Hospital Universitario Clinico San Carlos (SC) and Hospital Universitario de La Princesa (HULP), BIOTRONIK SE & Co. KG (BIO), neoplas GmbH (NEO).
Investigators
Belgium: Tom Adriaenssens (K.U.LEUVEN), Ian Buysschaert (ZNA Middelheim), Mickaël Chausson (initially KIZ, now Synolyne Pharma), Dries De Cock (K.U.LEUVEN), Jo Dens (Oost-Limburg Hospital, Genk), Emanuele Barbato (Cardiovascular Center, OLV Hospital Aalst), Walter Desmet (K.U.Leuven), Sandrine Gautier (initially KIZ, now Synolyne Pharma), Paul Vermeersch (ZNA Middelheim), Peter Sinnaeve (KU LEUVEN); Czech Republic: Ota Hlinomaz (St Anne University Hospital, Brno), France: Helene Abergel (INSERM), Laurent Feldman (INSERM), Martine Jandrot-Perrus (INSERM), Didier Letourneur (INSERM), Pierre Mangin (INSERM); Véronique Olivier (INSERM), Caroline Roques (INSERM); Germany: Robert A. Byrne (DHM), Sue Chandraratne (initially DHM, now Klinikum der Universität München), Matthias Gratz (BIO); Michael Joner (DHM), Adnan Kastrati (DHM), Elisabeth Kennerknecht (DHM), Ildiko Konrad (DHM), Tobias Koppara (DHM), Steffen Massberg (initially DHM, now Klinikum der Universität München), Franz-Josef Neumann (UHZ), Vasilis Ntziachristos (HMGU), Sheryl Opinaldo (initially DHM, now Klinikum der Universität München), Vanessa Philippi (initially DHM, now Klinikum der Universität München), Julia Riegger (initially DHM, now Klinikum der Universität München), Amir Rosenthal (HMGU), Alexander Rzany (BIO), Christian Schulz (initially DHM, now Klinikum der Universität München), Kristin Steigerwald (DHM), Tomohiso Tada (DHM), Anna Titova (initially DHM, now Klinikum der Universität München), Dietmar Trenk (UHZ), Christian Valina (UHZ), Andreas Vogelsang (NEO), Erion Xhepa (DHM); Italy: Chiara Bernelli (BER); Micol Coccato (BER), Giulio Guagliumi (BER), Kenichi Komukai (BER), Vasile Sirbu (BER); Latvia: Garry Kerch (RTU); The Netherlands: Giovanni Amoroso (Onze Lieve Vrouwe Gasthuis, Amsterdam), Jurriën ten Berg (NIE), Willem J.M. Dewilde (Amphia Ziekenhuis, Breda), Thea C. Godschalk (NIE), Antonius A.C.M. Heestermans (Medisch Centrum Alkmaar), Darshni A. Jhagroe (NIE), Joanne J. Wykrzykowska (Academisch Medisch Centrum, Amsterdam), Mark H.M. Winkens (TweeSteden ziekenhuis, Tilburg); Poland: Dariusz Dudek (KRAK), Łukasz Rzeszutko (KRAK), Roman Wojdyla (KRAK), Wojciech Zasada (KRAK); Spain: Fernando Alfonso (HULP, SC), Javier Cuesta (HULP), Miguel Medina (SC); United Kingdom: Colin Berry (University of Glasgow; Golden Jubilee National Hospital, Glasgow), James Cotton (The Royal Wolverhampton Hospitals NHS Trust), Nick Curzen (University Hospital Southampton NHS Foundation Trust), Margaret McEntegart (Golden Jubilee National Hospital, Glasgow), Robert Gerber (East Sussex Healthcare NHS Trust), Anthony Gershlick (ULEIC), Alison H. Goodall (ULEIC), Simon Hetherington (Kettering General Hospital NHS Foundation Trust), Jonathan Hill (King's College Hospital NHS Foundation Trust), Damian Kelly (Derby Hospitals NHS Foundation Trust), Nikesh Malik (ULEIC), Keith Oldroyd (Golden Jubilee National Hospital, Glasgow), Helen Routledge (Worcestershire Acute Hospitals NHS Trust), Joanne Shannon (Frimley Health Foundation Trust), Venkatesan Suresh (Plymouth Hospitals NHS Trust), Azfar Zahman (Newcastle Upon Tyne Hospitals NHS Foundation Trust).
References
- 1. Tada T, Byrne RA, Simunovic I, King LA, Cassese S, Joner M, Fusaro M, Schneider S, Schulz S, Ibrahim T, Ott I, Massberg S, Laugwitz KL, Kastrati A. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv 2013;6:1267–1274. [DOI] [PubMed] [Google Scholar]
- 2. Raber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y, Wenaweser P, Daemen J, Meier B, Juni P, Serruys PW, Windecker S. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation 2012;125:1110–1121. [DOI] [PubMed] [Google Scholar]
- 3. Schulz S, Schuster T, Mehilli J, Byrne RA, Ellert J, Massberg S, Goedel J, Bruskina O, Ulm K, Schomig A, Kastrati A. Stent thrombosis after drug-eluting stent implantation: incidence, timing, and relation to discontinuation of clopidogrel therapy over a 4-year period. Eur Heart J 2009;30:2714–2721. [DOI] [PubMed] [Google Scholar]
- 4. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A, Authors/Task Force m. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 5. Cook S, Ladich E, Nakazawa G, Eshtehardi P, Neidhart M, Vogel R, Togni M, Wenaweser P, Billinger M, Seiler C, Gay S, Meier B, Pichler WJ, Juni P, Virmani R, Windecker S. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation 2009;120:391–399. [DOI] [PubMed] [Google Scholar]
- 6. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 2013;13:34–45. [DOI] [PubMed] [Google Scholar]
- 7. Otsuka F, Yahagi K, Ladich E, Kutys R, Alexander R, Fowler D, Virmani R, Joner M. Hypersensitivity reaction in the US Food and Drug Administration-approved second-generation drug-eluting stents: histopathological assessment with ex vivo optical coherence tomography. Circulation 2015;131:322–324. [DOI] [PubMed] [Google Scholar]
- 8. Otsuka F, Byrne RA, Yahagi K, Mori H, Ladich E, Fowler DR, Kutys R, Xhepa E, Kastrati A, Virmani R, Joner M. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J 2015; 10.1093/eurheartj/ehv205. [DOI] [PubMed] [Google Scholar]
- 9. Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 2007;115:2435–2441. [DOI] [PubMed] [Google Scholar]
- 10. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006;48:193–202. [DOI] [PubMed] [Google Scholar]
- 11. PRESTIGE Consortium Adriaenssens T, Byrne R. Prevention of late stent thrombosis by an interdisciplinary global European effort: PRESTIGE. Eur Heart J 2014;35:2128–2129. [PubMed] [Google Scholar]
- 12. Luna L. Manual of HistologicStaining Methods of the Armed Forces Institute of Pathology, 3rd ed.New York: Blakiston Division, McGraw-Hill, 1968, p114–115. [Google Scholar]
- 13. Carstairs KC. The identification of platelets and platelet antigens in histological sections. J Pathol Bacteriol 1965;90:225–231. [DOI] [PubMed] [Google Scholar]
- 14. von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 2012;209:819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crea F, Liuzzo G. Pathogenesis of acute coronary syndromes. J Am Coll Cardiol 2013;61:1–11. [DOI] [PubMed] [Google Scholar]
- 16. Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med 2008;359:938–949. [DOI] [PubMed] [Google Scholar]
- 17. Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest 2008;118:1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wyss CA, Neidhart M, Altwegg L, Spanaus KS, Yonekawa K, Wischnewsky MB, Corti R, Kucher N, Roffi M, Eberli FR, Amann-Vesti B, Gay S, von Eckardstein A, Luscher TF, Maier W. Cellular actors, Toll-like receptors, and local cytokine profile in acute coronary syndromes. Eur Heart J 2010;31:1457–1469. [DOI] [PubMed] [Google Scholar]
- 19. Klingenberg R, Brokopp CE, Grives A, Courtier A, Jaguszewski M, Pasqual N, Vlaskou Badra E, Lewandowski A, Gaemperli O, Hoerstrup SP, Maier W, Landmesser U, Luscher TF, Matter CM. Clonal restriction and predominance of regulatory T cells in coronary thrombi of patients with acute coronary syndromes. Eur Heart J 2015;36:1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 2010;16:887–896. [DOI] [PubMed] [Google Scholar]
- 21. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–1535. [DOI] [PubMed] [Google Scholar]
- 22. Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood 2014;123:2768–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schulz C, Engelmann B, Massberg S. Crossroads of coagulation and innate immunity: the case of deep vein thrombosis. J Thromb Haemost 2013;11(Suppl. 1):233–241. [DOI] [PubMed] [Google Scholar]
- 24. Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenbock A, Simon D, Laimer D, Bangert C, Kammerlander A, Mascherbauer J, Winter MP, Distelmaier K, Adlbrecht C, Preissner KT, Lang IM. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res 2015;116:1182–1192. [DOI] [PubMed] [Google Scholar]
- 25. Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA 2010;107:15880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, Mihalcsik L, Tespili M, Valsecchi O, Kolodgie FD. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 2004;109:701–705. [DOI] [PubMed] [Google Scholar]
- 27. Wilson GJ, Nakazawa G, Schwartz RS, Huibregtse B, Poff B, Herbst TJ, Baim DS, Virmani R. Comparison of inflammatory response after implantation of sirolimus- and paclitaxel-eluting stents in porcine coronary arteries. Circulation 2009;120:141–149, 1, 2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.