Abstract
Aims
The mechanistic basis of the symptoms and signs of myocardial ischaemia in patients without obstructive coronary artery disease (CAD) and evidence of coronary microvascular dysfunction (CMD) is unclear. The aim of this study was to mechanistically test short-term late sodium current inhibition (ranolazine) in such subjects on angina, myocardial perfusion reserve index, and diastolic filling.
Materials and results
Randomized, double-blind, placebo-controlled, crossover, mechanistic trial in subjects with evidence of CMD [invasive coronary reactivity testing or non-invasive cardiac magnetic resonance imaging myocardial perfusion reserve index (MPRI)]. Short-term oral ranolazine 500–1000 mg twice daily for 2 weeks vs. placebo. Angina measured by Seattle Angina Questionnaire (SAQ) and SAQ-7 (co-primaries), diary angina (secondary), stress MPRI, diastolic filling, quality of life (QoL). Of 128 (96% women) subjects, no treatment differences in the outcomes were observed. Peak heart rate was lower during pharmacological stress during ranolazine (−3.55 b.p.m., P < 0.001). The change in SAQ-7 directly correlated with the change in MPRI (correlation 0.25, P = 0.005). The change in MPRI predicted the change in SAQ QoL, adjusted for body mass index (BMI), prior myocardial infarction, and site (P = 0.0032). Low coronary flow reserve (CFR <2.5) subjects improved MPRI (P < 0.0137), SAQ angina frequency (P = 0.027), and SAQ-7 (P = 0.041).
Conclusions
In this mechanistic trial among symptomatic subjects, no obstructive CAD, short-term late sodium current inhibition was not generally effective for SAQ angina. Angina and myocardial perfusion reserve changes were related, supporting the notion that strategies to improve ischaemia should be tested in these subjects.
Trial registration
clinicaltrials.gov Identifier: NCT01342029.
Keywords: Coronary microvascular dysfunction, Angina
See page 1514 for the editorial comment on this article (doi:10.1093/eurheartj/ehw021)
Introduction
Patients with symptoms, evidence of ischaemia, and no obstructive coronary artery disease (CAD) are prevalent and increasing in frequency.1 Such patients have disability, healthcare resource consumption, and costs similar to those with obstructive CAD.2,3 Many have evidence for coronary microvascular dysfunction (CMD), which is associated with adverse cardiovascular events.4–7 Persistent symptoms are often atypical and a management challenge. Thus, there are many gaps in our knowledge about these patients related to their evaluation (invasive vs. non-invasive), mechanisms responsible for their symptoms and CMD, and treatment targets.
Late sodium current inhibition with ranolazine is effective for stable angina with obstructive CAD,8–15 and endorsed for such patients in the most recent ESC guidelines.16 Ranolazine inhibits the late sodium current in cardiomyocytes, decreasing Na+ and Ca2+ overload. During ischaemia, excess intracellular Ca2+ impairs myocyte relaxation facilitating increases in ventricular diastolic stiffness and further impaired perfusion. Ranolazine also promotes the switch from the inefficient fatty acid metabolism towards the oxygen-sparing glucose and lactate oxidation, further reducing oxygen consumption. Evidence to date, however, has not clearly demonstrated whether the anti-anginal effect is related to improved myocardial perfusion reserve. Furthermore, most CMD patients are women,17 yet pivotal trials with ranolazine8–15 contained few women and studied effort angina due to obstructive CAD.
Stress cardiac magnetic resonance perfusion imaging (CMRI) can non-invasively detect CMD,18–20 and is associated with disordered left ventricular (LV) diastolic function21 in CMD patients. A pilot trial suggested benefit of ranolazine in CMD subjects with typical angina.22
Accordingly, we conducted a trial in subjects with symptoms with no obstructive CAD, but evidence of CMD to mechanistically test whether symptoms are related to myocardial perfusion reserve index and can be influenced by late sodium current inhibition (www.clinicaltrials.gov, NCT01342029). The results should provide information to address some of the aforementioned knowledge gaps and direction for future studies in this enlarging population.
Methods
Patient population
Inclusion/exclusion criteria, study protocol, and list of investigators appear in Supplementary material online, Online Exhibit A. Briefly, we enrolled subjects with symptoms thought due to ischaemia, no obstructive CAD (<50% epicardial coronary stenosis in all epicardial coronary arteries), and preserved LV ejection fraction, who had abnormal coronary reactivity testing (CRT) [coronary flow reserve (CFR) <2.5, or no dilation (≤0% change) with acetylcholine (Ach) response], or abnormal stress CMRI <2.0. Institutional Review Boards approved the study at the two sites and subjects gave written informed consent.
Study design
To mechanistically test effects, we conducted a double-blind, placebo-controlled, crossover trial, with short-term (2 week) ranolazine–placebo exposure order randomly assigned. Obstructive CAD absence and CRT measures were verified [Women's Ischemia Syndrome Evaluation (WISE) Angiography and Coronary Reactivity Cores]. At baseline, subjects completed demographic/health history questionnaires, including the Seattle Angina Questionnaire (SAQ23 and SAQ-7),24 angina diary, Duke Activity Status Index (DASI),25 and general quality of life (QoL).26 Anti-anginal medications were unchanged. To maximize concurrent WISE recruitment, the study drug exposure was reduced from the pilot study22 4–2 weeks followed by 2-week washout, and crossover to 2 weeks (see Supplementary material online,Supplementary Data and Figure 1). Ranolazine (Gilead Sciences, Foster City, CA, USA) was administered as 500 mg orally twice daily for 1-week and increased to 1000 mg twice daily for 1-week as tolerated (subjects taking verapamil or diltiazem maintained 500 mg b.i.d. of ranolazine or placebo). Treatment compliance was measured by pill count. Data were collected at baseline (SAQ) and at the end of each treatment period (SAQ, CMRI).
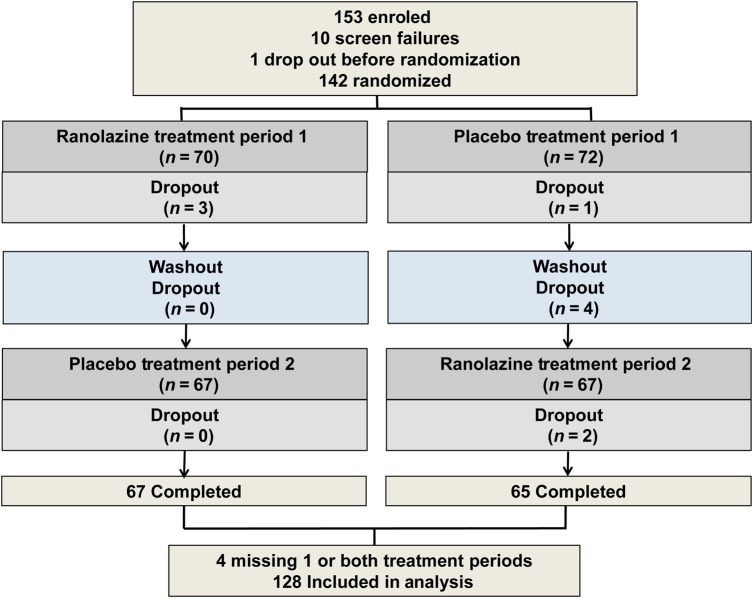
Figure 1.
Study subject enrolment, screening, randomization and completion flow diagram. Treatment period 1 and 2: randomized to sequence of ranolazine first followed by crossover to placebo, or vice-versa.
The angina and nitroglycerin diary,12 SF-36,26 MOS-116,27 and HIS-GWB Mental Health Battery28 were used as previously described.29
Cardiac magnetic resonance imaging
The CMRI protocol (see Supplementary material online, Exhibit B) was performed (1.5 Tesla magnet, Siemens Sonata, Erlangen, Germany) with ECG-gating and phased array coil with 0.05 mmol/kg gadolinium first-pass perfusion three slice at rest, during pharmacological and cold-pressor stress.30 Myocardial scar was determined by late gadolinium enhancement (LGE) in a subset. Adenosine (140 μg/kg/min) or regadenoson (0.4 mg) if intolerant was consistent for periods. Cardiac magnetic resonance imaging was conducted under identical conditions and timing, dosing, and settings, ∼4-h after the morning study drug dose.
First-pass perfusion images were analysed using CAAS MRV CMRI analysis software Version 3.3 (Pie Medical Imaging B.V., Maastricht, the Netherlands). Software determined epicardial and endocardial contours of LV myocardium were manually corrected for MPRI intensity–time curves. Global sub-endocardial and sub-epicardial MPRI were calculated as the ratio of stress/rest relative perfusion upslope, corrected for LV cavity upslope (higher is better myocardial perfusion reserve). The sub-endocardial and sub-epicardial layers were software determined as the inner and outer 50% wall thickness. These methods have high inter-study and observer reproducibility,31,32 with best reproducibility in the mid-ventricle.18 An MPRI ≤1.8 is considered abnormal,20 and correlates with CRT20 and risk factors.33 Left ventricular mass and volumes were evaluated by manual tracing; papillary muscles were included in the LV mass/excluded from the LV volume. Volumetric diastolic filling was used to calculate early peak filling rate (PFR) and time to PFR (tPFR).21
Invasive coronary reactivity testing
Clinically indicated invasive CRT measured coronary micro- and macro-vascular endothelial and non-endothelial-dependent function6,34 was available in 87 subjects (62.5%). Coronary flow reserve was measured by Doppler flow-wire (FloWire® Volcano, San Diego, CA, USA) following intracoronary (IC) adenosine injections, while graded IC Ach assessed endothelial function.
All measurements were made in the WISE Angiographic, CMRI, and CRT Core Labs masked to treatment period.
Statistical analysis
Subjects were randomized at a 1:1 ratio and blocked by clinical site.35 Using a 2 × 2 crossover design, a sample size of 116 would achieve 90% power to detect a mean difference of 15 in SAQ score22 using a two-sided t-test at the 0.017 Holm–Bonferroni-corrected level of significance (HB), and standard deviation of 43.4 for paired difference in SAQ angina stability scores.22 Given the smaller standard deviations of the difference for the other two co-primary endpoints, the current trial was well powered for all the co-primary endpoints. A 10% attrition was assumed and target sample size was 134.
The analytic approach was a within-subjects comparison (paired) of the difference between baseline-treatment periods (SAQ, QoL) (a total of four measurements per subject, including two baselines and two treatment periods) or treatment periods (CMRI) (a total of two measurements per subject, including two treatment periods). The distribution of within-subjects differences was assessed to deploy appropriate statistical techniques. The primary approach was a standard paired t-test. Linear regression models were tested using treatment differences as the outcome. A stepwise procedure was used to choose the variables that were significantly associated with the outcomes. The overall type I error rate for the three co-primary outcome measures was controlled at 5% by the HB36 sequential procedure. Carry-over effects tested the interaction between treatment and period by comparing the mean within subject with means between the arms.37 Sub-group analyses included relevant clinical variables,15 prior ranolazine exposure, randomization sequence, site, adenosine vs. regadenoson, full vs. reduced ranolazine dose, prior myocardial infarction by history or CMRI-LGE, and qualifying CRT and CMRI variables. The significance level for outcomes other than the three co-primary endpoints was set to 0.05. A subject was included if they completed SAQ at baseline, SAQ, and CMRI for both treatment periods, and ≥50% of drug for both periods. Analyses were performed using SAS v9.3 (SAS Institute, Inc., Cary, NC, USA).
Study oversight
The study was investigator-initiated as an ancillary trial to the NHLBI-sponsored WISE, funded in part by Gilead Sciences, and used the WISE Data Safety Monitoring Committee. Statistical analysis was performed by the investigators independent of NHLBI and Gilead. The decision to submit for publication was made by the principal investigators who had access to all data after the last subject completed the study.
Results
Subject characteristics
Between 12 May 2011 and 10 August 2015, 435 subjects were screened, 393 eligible, 153 (96% women) enrolled, 10 (7%) subjects failed CMRI screening and 1 dropped out before randomization (Table 1 and Figure 1). Among the 142 randomized, 8 (6%) dropped out (1 before receiving treatment, 5 while receiving ranolazine, 1 during placebo washout, and 1 while receiving placebo), and an additional 3 (2%) missed one or both treatment periods. This yielded 128 subjects with data from both periods available for analysis.
Table 1.
Baseline demographic and clinical variables
| Variables | Mean ± SD, or absolute frequency (%) |
|---|---|
| Age (years) | 55.2 ± 9.8 |
| Female | 96% |
| BMI | 29.3 ± 7.6 |
| >30 | 50 (39.7%) |
| Race (non-Caucasian) | 31 (24.2%) |
| Tobacco use | |
| Current | 2 (1.6%) |
| Former | 38 (29.7%) |
| Never | 88 (68.8%) |
| History of hypertension | 69 (53.9%) |
| History of diabetes | 23 (18.0%) |
| History of hyperlipidaemia | 70 (54.7%) |
| Family history of premature coronary artery disease | 83 (64.8%) |
| Post-menopausal (n = 123) | 100 (81.3%) |
| Symptoms | |
| Typical Angina | 40 (31.3%) |
| Shortness of Breath | 88 (68.8%) |
| Palpitations | 53 (41.4%) |
| Nausea | 40 (31.3%) |
| Angina frequency (baseline SAQ angina frequency domain) | 59.6 ± 26.9 |
| LV Ejection Fraction (%) | 67.8 ± 7.7 |
| Qualifying CMRI (n = 86)a | |
| Global myocardial perfusion reserve index (MPRI) <2 (n = 67)b | 1.6 ± 0.3 |
| Qualifying CRT (n = 87)a | |
| LV end-diastolic filling pressure (LVEDP) (mmHg) (n = 74) | 14.8 ± 5.1 |
| Qualifying CFR <2.5 (n = 35)b | 2.2 ± 0.2 |
| Qualifying Ach response <0%b (n = 36) | −0.6 ± 15.6 |
| Beta-blockers | 54 (42.2%) |
| Calcium current blockers; non-dihydropyridine | 29 (22.7%); 7 (24%) |
| Angiotensin-converting enzyme inhibitors | 27 (21.1%) |
| Angiotensin receptor vlockers | 13 (10.2%) |
| Nitrates | 50 (39.1%) |
| Statins | 74 (57.8%) |
| Hormone replacement therapy | 16 (12.5%) |
Ach, acetylcholine; BSA, body surface area; BMI, body mass index; CFR, coronary flow reserve; CMRI, cardiac magnetic resonance imaging; CRT, coronary reactivity testing; MPRI, myocardial perfusion reserve index; LV, left ventricular; SAQ, Seattle Angina Questionnaire.
aSubjects could have both CRT and CMRI.
bSubjects could have CMRI and CFR and Ach qualifiers.
Many subjects were receiving beta-blockers, angiotensin-converting enzyme inhibitors, and statins. Despite anti-anginal therapy, all subjects had symptoms thought by their physicians to represent ischaemia although only 31% had typical angina. Among the 98 subjects with WISE baseline CMRI, 11% had myocardial scar by LGE (7-sub-endocardial or transmural, 3-mid-myocardial or epicardial or mixed, and 1-indeterminate), 69% had MPRI ≤1.8. Among the 87 subjects with baseline CRT, 38% had a CFR of <2.5. Systolic and diastolic blood pressures were 123 ± 18 and 72 ± 12 mmHg, respectively.
Compliance and safety
Overall compliance was 97%, while 21% (ranolazine) and 14% (placebo) subjects reduced to the 500 mg twice daily dosing for side-effects. Serious adverse events during the ranolazine period occurred in five patients [hospitalization for NSTEMI (1); bronchospasm (1); chest pain, dizziness, and pre-syncope (2); and syncope (1)], during the washout periods were hospitalization for chest pain (ranolazine washout, 1 patient) and bradycardia (placebo washout, 1 patient), and 0 during the placebo. Non-serious adverse events during ranolazine occurred in seven subjects—nausea and dizziness (3), arm shaking (1), back pain (1), renal abnormality (1), and throat swelling (1); in six subjects during placebo—chest pain (3), throat swelling (1), cough (1), and sinus infection (1); and in two subjects during washout—chest pain (1) and rectocele (1).
Angina, QoL, haemodynamics, and CMRI results
None of the primary outcomes, other SAQ subscales, or angina or nitroglycerin use diary improved during ranolazine vs.. placebo (Table 2). Due to a higher variance in the baseline-treatment comparison, we also directly compared treatment periods14 and observed improved SAQ angina stability (7.23 ± 37.54, P = 0.03) and SAQ-7 (1.94 ± 12.90, P = 0.09) (neither significant at the 0.017 level). QoL depression improved during ranolazine (P = 0.0091) (Table 2). Results did not differ by menopausal status, or by excluding men. Mixed modelling in the entire cohort with treatment data demonstrated similar results.
Table 2.
Seattle Angina Questionnaire (SAQ), angina diary, functional capacity and quality of life (QoL), haemodynamic, myocardial perfusion reserve and diastolic filling treatment effect
| Ranolazine |
Placebo |
Treatment changea |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | Mean | 95% CI | N | P-value | |
| SAQ | ||||||||||
| Physical limitation | 68.09 | 23.34 | 123 | 66.70 | 23.34 | 120 | −0.22 | (−3.23, 2.79) | 114 | 0.89 |
| Angina stabilityb | 58.40 | 26.11 | 128 | 51.17 | 27.68 | 128 | 5.12 | (−3.51, 13.75) | 127 | 0.24 |
| Angina frequency b | 63.91 | 26.09 | 128 | 62.73 | 25.95 | 128 | 0.08 | (−4.18, 4.34) | 128 | 0.97 |
| Treatment satisfaction | 74.16 | 21.23 | 127 | 74.17 | 21.08 | 128 | −0.48 | (−3.98, 3.03) | 127 | 0.79 |
| QoL | 56.05 | 23.09 | 128 | 54.17 | 23.31 | 128 | 0.91 | (−2.44, 4.27) | 128 | 0.59 |
| SAQ overall | 62.49 | 19.32 | 128 | 60.97 | 20.11 | 128 | ||||
| SAQ-7 | ||||||||||
| Physical limitation | 71.67 | 24.18 | 124 | 70.15 | 25.03 | 122 | ||||
| Angina frequency | 63.91 | 26.09 | 128 | 62.73 | 25.95 | 128 | ||||
| QoL | 55.76 | 27.97 | 128 | 52.83 | 28.59 | 128 | ||||
| SAQ-7 overallb | 63.54 | 21.09 | 128 | 61.60 | 22.32 | 128 | 1.31 | (−1.56, 4.17) | 128 | 0.37 |
| Angina diary | ||||||||||
| Angina episode | 4.78 | 8.20 | 128 | 4.88 | 7.75 | 128 | −0.10 | (−0.94, 0.73) | 128 | 0.81 |
| NTG usage | 2.48 | 10.16 | 128 | 2.62 | 11.18 | 128 | −0.13 | (−1.0, 0.74) | 128 | 0.76 |
| SF-36 | ||||||||||
| Energy/fatigue | 47.49 | 20.93 | 128 | 46.45 | 20.43 | 128 | 1.40 | (−2.27, 5.07) | 119 | 0.45 |
| Emotional | 70.12 | 17.14 | 128 | 68.28 | 16.97 | 128 | 2.82 | (−0.56, 6.19) | 119 | 0.10 |
| MOS-116 | ||||||||||
| Moody | 5.09 | 1.05 | 127 | 4.92 | 1.19 | 128 | 0.14 | (−0.12, 0.41) | 118 | 0.28 |
| Low spirits | 5.12 | 1.08 | 127 | 5.05 | 1.13 | 128 | 0.10 | (−0.12, 0.32) | 118 | 0.36 |
| HIS-GWB | ||||||||||
| Depressed | 4.39 | 0.74 | 127 | 4.27 | 0.87 | 128 | 0.20 | (0.05, 0.36) | 118 | 0.009 |
| Strain | 4.28 | 1.41 | 127 | 4.20 | 1.44 | 128 | 0.11 | (−0.20, 0.42) | 118 | 0.49 |
| DASI score | 6.35 | 4.83 | 128 | 6.20 | 5.05 | 128 | 0.31 | (−0.58, 1.21) | 128 | 0.49 |
| Pharmacological stress | ||||||||||
| HR (b.p.m.) | 95.17 | 13.50 | 128 | 98.73 | 14.15 | 128 | −3.55 | (−4.99, −2.12) | 128 | <0.001 |
| SBP (mmHg) | 127.2 | 21.60 | 129 | 127.3 | 20.9 | 128 | −0.56 | (−3.93, 3.10) | 129 | 0.75 |
| DBP (mmHg) | 60.17 | 16.52 | 129 | 60.32 | 16.57 | 128 | −0.49 | (−3.23, 2.52) | 129 | 0.73 |
| Stress RPP | 12 082 | 2707 | 128 | 12 611 | 2796 | 128 | −523 | (−901, −97) | 128 | 0.010 |
| Global MPRI | 1.98 | 0.46 | 128 | 1.96 | 0.42 | 125 | 0.01 | (−0.08, 0.09) | 125 | 0.88 |
| MPRI mid–sub-endocardial | 1.83 | 0.48 | 127 | 1.77 | 0.38 | 124 | 0.06 | (−0.04, 0.15) | 124 | 0.23 |
| Diastolic filling | ||||||||||
| PFR (mL/s) | 333.3 | 105.9 | 127 | 328.8 | 97.1 | 128 | 4.3 | (−9.05, 17.71) | 127 | 0.52 |
| tPFR (ms) | 163.9 | 45.3 | 127 | 157.4 | 37.7 | 128 | 6.6 | (−1.13, 14.33) | 127 | 0.09 |
DASI, Duke Activity Status Index [in metabolic equivalents (METS)]; HIS-GWB Mental Health Battery; HR, heart rate; MOS 116, Medical Outcomes Study; MPRI, myocardial perfusion reserve index; MPRI-mid, mid-ventricular MPRI; mL, milliliter; NTG, nitroglycerin; PFR, peak filling rate; Pharmacologic Stress, adenosine or regadenoson infusion; QoL, quality of life; RPP, rate-pressure product; SAQ, Seattle Angina Questionnaire; ms, milliseconds; SF-36, MOS-36-Item Short-Form Health Survey; tPFR, time to peak filling rate.
aThe SAQ, QoL, and DASI were measured pre- and post-treatment for both periods. All other outcomes were measured only post-treatment. Treatment change for SAQ, QoL, and DASI are the difference ranolazine-placebo in post- to pre-treatment changes.
bThe HB critical levels were used for testing the three co-primary endpoints.
Overall, CMRI was interpretable in 127 pharmacological and 126 cold-pressor stress tests. We observed significantly lower pharmacological stress mean heart rate (HR) and rate-pressure product (RPP) during ranolazine vs. placebo (Table 2), but otherwise no haemodynamic differences. Pharmacological stress MPRI did not significantly improve with ranolozine vs. placebo (Table 2), nor did cold pressor (data not shown), or HR-corrected pharmacological stress MPRI (mid-ventricular global 2.06 ± 0.53 vs. 2.0 ± 0.42, P = 0.15) or mid-ventricular sub-endocardial (1.83 ± 0.48 vs. 1.77 ± 0.38, P = 0.18). Because myocardial flow reserve can be confounded by resting flow due to dependency on myocardial workload, we further corrected the resting MPRI with RPP and found similar results. Diastolic filling rates were similar for PFR with a trend towards higher tPFR (P = 0.09) on ranolazine vs. placebo (Table 2).
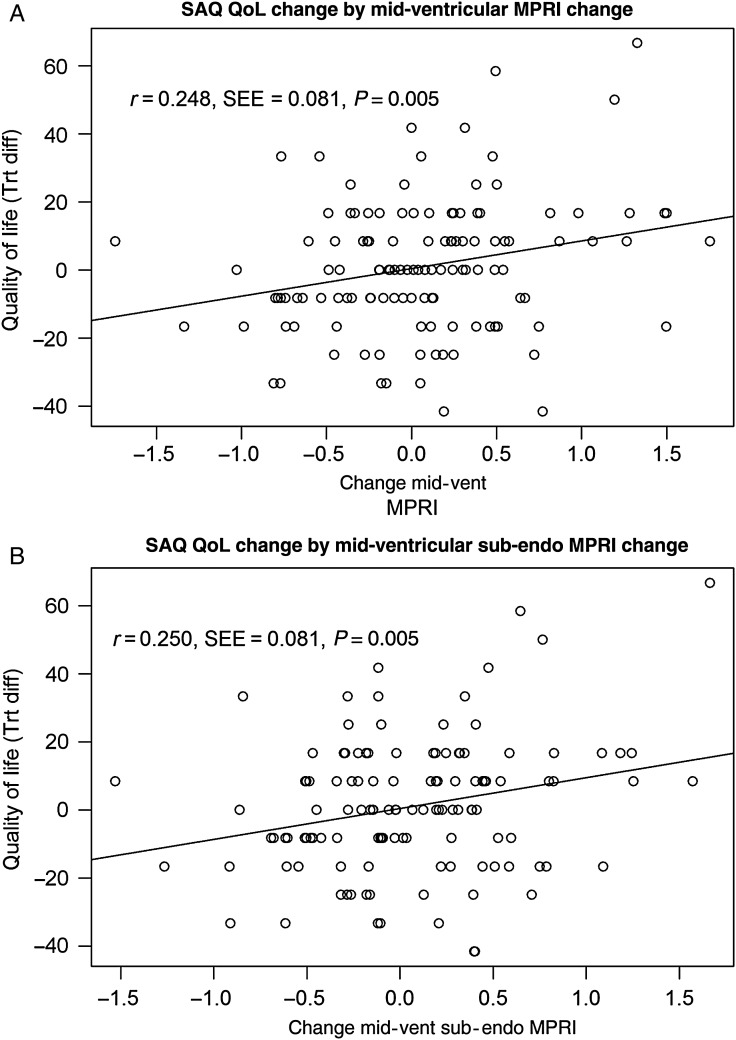
The change in SAQ-7 directly correlated with the change in MPRI (correlation 0.25, P = 0.005). In a multiple linear regression analysis, the MPRI-midventricular change differed by site and prior MI, adjusted for body mass index (BMI), and SAQ QoL change (P = 0.008). All of the angina variables were tried in the model. Two SAQ variables could enter into the models singly, but not at the same time: SAQ QoL and SAQ-7. Each of these had similar associations with the MPRI variables, but QoL has slightly better model fit statistics. The treatment difference in the QoL-depressed question had a significant positive association when added to the model for global mid-ventricular MPRI (P = 0.024), but was not significant for sub-endo or sub-epi mid-ventricular MPRI (P = 0.058 and P = 0.075, respectively). Modelling result demonstrated that as the estimated MPRI mid-ventricular change increased, SAQ QoL change increased, adjusted for BMI, prior MI and site (P = 0.004) (Figure 2A). Similar results were observed with mid-ventricular sub-endocardial MPRI (P = 0.003) (Figure 2B).
Figure 2.
Mid-ventricular and mid-ventricular sub-endocardial myocardial perfusion reserve index change vs. Seattle Angina Questionnaire quality of life change (ranolazine vs. placebo) model. The observed change in mid-ventricular myocardial perfusion reserve index (A) and mid-ventricular sub-endocardial myocardial perfusion reserve index (B) are plotted against the observed differences in treatment change for Seattle Angina Questionnaire quality of life with a line depicting a simple linear regression. The Pearson correlation and standard error (r, SEE) for this association are shown with the P-value for the test against zero correlation. The multiple regression described in the results showed that as myocardial perfusion reserve index–mid-ventricular change increased, Seattle Angina Questionnaire quality of life change increased, adjusted for body mass index, prior myocardial infarction and site (P = 0.005) with a similar model results for myocardial perfusion reserve index–mid-sub-endocardial change and Seattle Angina Questionnaire quality of life change (P = 0.005). Other variables tested that were not selected by the model include: pharmacological stress heart rate change, age, body mass index, site, history of hypertension, history of diabetes, prior ranolazine use, gender, left ventricular mass, end-diastolic volume, quality of life depressed change, had menopausal symptoms, baseline Seattle Angina Questionnaire angina frequency, Seattle Angina Questionnaire change (each of the five subdomains), adenosine vs. regadenson, and ranolazine dose level.
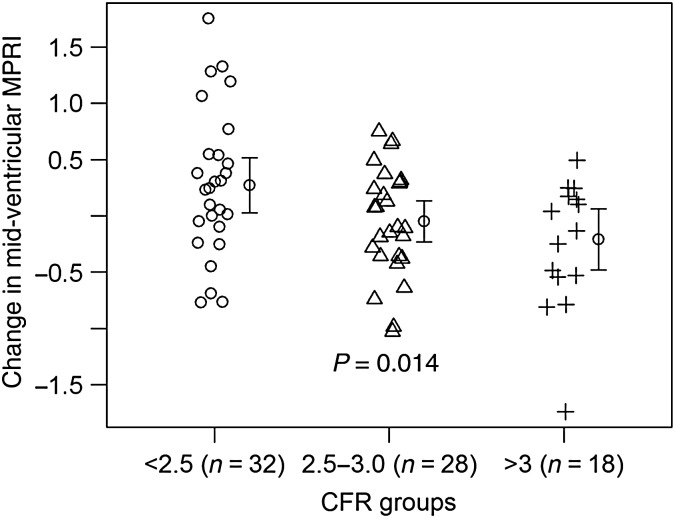
Sub-group CRT analysis of the subjects using a clinically significant threshold of CFR <2.534 had higher global (ANOVA, P = 0.038) and mid-ventricular MPRI improvement during ranolazine compared with CFR 2.5–3 and above 3 (ANOVA, P = 0.014, Figure 3). Among subjects with CFR <2.5, the treatment difference for SAQ angina frequency and SAQ-7 change was higher during ranolazine than placebo (paired t-test 9.43 ± 24.13, P = 0.027 and 6.62 ± 18.46, 0.041, respectively). Sub-group analysis according to typical vs. non-typical angina was negative; however, subjects with prior MI (n = 10) had higher global (0.30, P = 0.047) and sub-epicardial MPRI (0.32, P = 0.027) during ranolazine vs. placebo.
Figure 3.
Myocardial perfusion reserve index change according to qualifying coronary flow reserve in the subset of subjects with invasive coronary reactivity testing. Among subjects with qualifying coronary reactivity testing available coronary flow reserve and both period myocardial perfusion reserve index (n = 78), lower coronary flow reserve had significantly greater mid-ventricular myocardial perfusion reserve index change on ranolazine vs. placebo (P = 0.014). A higher myocardial perfusion reserve index number indicates better myocardial perfusion reserve.
Discussion
In this short-term mechanistic trial, an anti-anginal agent effective for effort angina in patients with obstructive CAD, late Na current blockade with ranolazine, did not generally improve symptoms, myocardial perfusion reserve index, or diastolic filling in symptomatic subjects with no obstructive CAD but evidence of CMD. These findings suggest that short-term late sodium current inhibition with ranolazine is not generally effective for symptoms in this novel population. However, changes in angina and myocardial perfusion reserve index were directly related, indicating that symptoms are related to ischaemia in this population, supporting the case that therapies with the potential to improve ischaemia should be tested. Myocardial perfusion reserve index improved in sub-group of subjects with lower baseline CFR, consistent with prior study findings.42,43
Invasively determined CRT is perhaps a more rigorous determination of CFR and comparable with PET.20,38,39 Among the entire cohort, slightly more than half underwent a clinically indicated invasive CRT, and 38% had low CFR <2.5. Our result suggests that late sodium current inhibition with ranolazine may beneficially improve angina and myocardial perfusion reserve index in such a rigorously defined CMD population with more severe CMD. This result suggests that subjects with more severe CMD may benefit and should be included in future trials testing strategies to improve ischaemic morbidity and mortality.
Interestingly, stress HR and RPP on ranolazine was ∼4 b.p.m. lower vs. peak stress HR during placebo. Stress HR is an important determinant of myocardial oxygen demand, while pharmacological stress predominantly alters myocardial oxygen supply via arteriolar dilation (both coronary and systemic), the HR increase is mediated by reflex increases in beta-adrenegic activity. Furthermore, we observed a lower RPP during pharmacological stress on ranolazine vs. placebo supporting the suggestion that myocardial oxygen demand was lower during stress on ranolazine. To our knowledge, this is the first description of such an effect of ranolazine in humans. The pharmacological basis for our finding is suggested from experimental studies which have shown attenuated ranolazine dose-dependent isoproterenol increases in HR (beta1 receptor) and decreases in blood pressure (beta2 receptor) in rat and conscious dog models40,41 and also supported by beta-adrenergic receptor binding data.41 The results indicate that ranolazine has a weak effect to antagonize beta-adrenergic receptors during stress. A minority of our subjects were on beta-blockers or non-dihydropyridine calcium current blockers, although these and the other anti-anginal medications were withdrawn for the stress testing. This HR effect also contributed to a small reduction in RPP supporting the suggestion that myocardial oxygen demand during stress was somewhat lower at the same MPRI. We may have observed different myocardial perfusion reserve and symptom responses if had we used exercise testing, as in previous ranolazine trials.8–14
Our current results differ from the pilot study22 used to design this CMD mechanistic trial. The current subjects had a much lower prevalence of typical or ‘effort angina’ (31 vs. 95%, respectively), which may explain the lack of anti-anginal benefit measured by the SAQ in the current results. Current subjects were also more obese (BMI 29.3 ± 7.5 vs. 25.6 ± 3.8, respectively), which may have impacted drug distribution and effect. While baseline SAQ scores were comparable, our pre-specified analysis plan compared baseline-treatment SAQ scores (a more conservative measure), vs. direct comparison of treatment periods in prior studies.22,42 Qualifying myocardial perfusion reserve was higher (better) in the current cohort vs. our pilot (overall CMRI MPRI 1.8 ± 0.5 vs. 1.40 ± 0.43, respectively). While we did not have pilot measures of diastolic filling, LV end-diastolic pressure in CRT was comparable in the current and pilot (14.8 ± 5.1 vs. 14.71 ± 5 mmHg, respectively).
Our current results are also not consistent with two recent, but smaller studies in subjects with no obstructive CAD and CFR assessed by transthoracic Doppler. One studied 58 patients (only 19 women), with angina and abnormal myocardial perfusion, in a double-blind, placebo-controlled parallel trial of ranolazine 500 mg twice daily or placebo for 8-weeks. Ranolazine increased CFR and most SAQ domains vs. placebo.42 Another studied 46 patients with effort angina, abnormal exercise tests, CFR <2.5 and persistent symptoms on anti-anginal agents, randomized to ranolazine (n = 15: 12 women, 375 mg twice daily), ivabradine or placebo (n = 15: 12 women) for 4-weeks. Ranolazine improved the SAQ vs. placebo and achieved better results vs. ivabradine: exercise time to 1-mm ST-segment depression and duration.43 More men, more typical effort angina, use of exercise testing, direct treatment period SAQ comparison, use of directly-measured CFR, and more severe CMD likely contributed to these different results.
Women comprised 96% of our population, precluding conclusions regarding response in men. Pivotal chronic angina trials with ranolazine were comprised mostly of men (∼75%).8–10 One was designed to determine dose–response and efficacy and found improvement in exercise time with doses used in the current trial, without suggestion of differential effects among men vs. women.8 Another found that ranolazine had similar angina and nitroglycerine use in women as in men, although less improvement on exercise testing-related angina.9 Importantly, subjects in these prior ranolazine trials had effort angina due to obstructive CAD.
Study limitations
Although the largest study to date in subjects with no obstructive CAD but CMD, our study by design was a mechanistic trial. Its strengths include a rigorous crossover design, use of validated measures and core laboratories, and CMRI and CRT evaluation. Limitations include a short-term 2-week exposure to ranolazine, although exposure duration was the same as a prior trial.10 Also, by design, only a subset qualified by clinically ordered CRT, and these subjects may have had lower CFR than those who qualified by CMRI, raising the possibility that more severe CMD explained the beneficial ranolazine response observed in this sub-group. The CFR analysis suggesting this concept is a sub-group, and therefore underpowered with a wide standard deviation, although significant and consistent with prior CFR studies. Use of angina diaries for angina is often limited by inappropriate input of multiple entries in a single sitting (so-called hoarding) and low compliance (as low as 11%).44 While we used objectively assessed MPRI, a validated quantitative myocardial perfusion reserve method, MPRI is based on the relative upslopes of arrival of myocardial contrast at stress and rest, which correlates with, but is not a direct measure of, CFR. Our future CMRI work will use our newly developed radial imaging and absolute CFR computation.45 The use of diastolic filling as a measure of diastolic function is a limitation as new approaches including tissue tagging acquisition,21 and post-processing diastolic function software46 appear to be improved measures; prior work has shown that ranolazine improves LV diastolic function.47,48 The SAQ, used and validated among predominantly elderly men from a Veterans Administration hospital with effort angina due to obstructive CAD, may not measure the atypical ‘angina-equivalents’ such as shortness of breath, fatigue, indigestion, and weakness in this predominantly female population. Indeed, other symptoms may have completely different causes, including dyspnoea can be explained by obesity and palpitations are a frequent component of the post-menopausal syndrome. Furthermore, we modified the SAQ to a 2-week period to fit the protocol. Finally, we used selected SF-36, MOS-166, and HIS-GWB QoL variables, which may be less reliable than the full measures; however, the practice of using selected general QoL measures as part of lengthy batteries of questionnaires assessing disease-specific QoL is common.29
Conclusions
In this mechanistic trial, short-term late Na current blockade (ranolazine) effective for effort angina in patients with obstructive CAD did not significantly improve symptoms or myocardial perfusion reserve index in subjects with no obstructive CAD but evidence of CMD. This suggests that short-term late sodium current inhibition is not generally effective for SAQ angina in this novel population. Changes in the SAQ and myocardial perfusion reserve index were directly related, indicating that symptoms are related to myocardial perfusion reserve, supporting the notion that strategies to improve ischaemia should be tested in these subjects.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
C.N.B.M., C.J.P., E.H., C.L.S., P.K.M., L.T., D.B. and L.S. performed statistical analysis. C.N.B.M., C.J.P., C.S. and E.H. handled funding and supervision. C.N.B.M., C.J.P., E.H., C.L.S., P.K.M., M.M., J.W., L.T., D.B., L.S., J.P., G.H.B., D.A., J.J.S., G.C.-W. and A.R. acquired the data. C.N.B.M., C.J.P., E.H., C.L.S., P.K.M., L.T., D.B. and L.S. conceived and designed the research. C.N.B.M., C.J.P., E.H., C.L.S., P.K.M., M.M., J.W., L.T., D.B., L.S., J.P., G.H.B., D.A., J.J.S., G.C.-W. and A.R. drafted the manuscript. C.N. B.M., C.J.P., E.H., C.L.S., P.K.M., M.M., J.W., L.T., D.B., L.S., J.P., G.H.B., D.A., J.J.S., G.C.-W. and A.R. made critical revision of the manuscript for key intellectual content.
Funding
This work was supported by an unrestricted research grant from Gilead and by contracts from the National Heart, Lung and Blood Institutes, nos N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, a GCRC grant MO1-RR00425 from the National Center for Research Resources, the National Center for Research Resources, grant UL1RR033176, the NIH/National Center for Advancing Translational Sciences (NCATS), UCLA CTSI grant UL1TR000124 and UF CTSI grant UL1TR001427, grant R01 HL089765, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Denville, New Jersey, the Women's Guild of Cedars-Sinai Medical Center, Los Angeles, California, the Edythe L. Broad Women's Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, California, the Constance Austin Women's Heart Research Fellowship, the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, and the Erika Glazer Women's Heart Health Project, Cedars-Sinai Medical Center, Los Angeles. Funding to pay the Open Access publication charges for this article was provided by the Barbra Streisand Women's Heart Center, Los Angeles CA, USA.
Conflict of interest: C.N.B.M. reports receiving consulting monies from Amgen, Medscape, Pfizer, Research Triangle Institute, and research grants from the NIH. C.N.B.M. also reports receiving research support from the Flight Attendant Medical Research Institute unrelated to this work, and payment for lectures from AACE, ACC, Florida Hospital, Mayo Scottsdale, Mayo Cancun, NAMS, Practice Point Communications, Pri-Med, Scripps Clinic, Vox Media, VBWG, UCLA, University of Chicago, Northwestern, Radcliffe Institute, UCSF and served on the grant review committee for Gilead. E.M.H. reports receiving research grants from Gilead, the NIH/NHLBI, Fujisawa Healthcare, Amarin, Amgen, Astra Zeneca, Baxter, Boehringer Ingleheim, Catadasis, Cytori, Daiichi-Sankyo, Esperion, Genentech, Gilead, ISIS pharmaceuticals, Mesoblast, Neostem, sanofi aventis, United Therapeutics. C.L.S. reports receiving research grants from Gilead. P.K.M. reports receiving research grants from General Electric and Gilead, and payment for lectures from Little Company of Mary, Dignity Health John F. Kennedy Hospital, Kaiser Permanente, San Diego Institute of Cardiology, and Emory. M.B.M. reports research a research grant from Gilead Sciences, serves on the board for the UCLA School of Nursing and consulting monies from Sanofi- Regeneron. J.W. reports receiving payment for lectures from Practice Point Communications. L.E.J.T. reports receiving research grants from Gilead Science and the NIH, NHLBI. J.W.P. reports receiving research grants from the NIH. R.D.A. reports receiving consulting monies from BioSense Webster. J.J.S. reports receiving research grants from the NIH, consulting and honorarium from the CTRC. A.R. reports receiving research grants from the NIH. C.J.P. reports receiving research grants from Gilead, the NIH/NHLBI, NIH/NCATS, Fujisawa, serves on the board for Lilly/Cleveland Clnic, Mesoblast, NHLBI, Amarin, AstraZeneca, received consulting monies from Amarin, AstraZeneca, Abbott Labs, Bayer Healthcare, Gilead, Janssen, Lilly/Cleveland Clinic, DSMB, Merck, Mesoblast DSMB, NHLBI DSMB, NHLBI Progenitor Cell Consortium, SLACK, Inc., and unrestricted education grants from AHA, Amorcyte/Neostem, Athersys, Baxter Healthcare, Capricor, Inc., Cytori, Fujisawa HealthCare, Inc., Gilead Sciences, Inc., InfraReDx, inVentive Health Clinical LLC, Pfizer, and sanofi-aventis.
Supplementary Material
References
- 1. Pepine CJ, Ferdinand KC, Shaw L, Light-McGroary KA, Shah R, Gulati M, Duvernoy C, Walsh MN, Bairey Merz CN. Emergence of non-obstructive coronary artery disease: a woman's problem and need for change in definition on angiography. J Am Coll Cardiol 2015;66:1918–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jespersen L, Abildstrom SZ, Hvelplund A, Galatius S, Madsen JK, Pedersen F, Hojberg S, Prescott E. Symptoms of angina pectoris increase the probability of disability pension and premature exit from the workforce even in the absence of obstructive coronary artery disease. Eur Heart J 2013;34:3294–3303. [DOI] [PubMed] [Google Scholar]
- 3. Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Sopko G, Women's Ischemia Syndrome Evaluation I. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health—National Heart, Lung, and Blood Institute—sponsored Women's Ischemia Syndrome Evaluation. Circulation 2006;114:894–904. [DOI] [PubMed] [Google Scholar]
- 4. Johnson BD, Shaw LJ, Buchthal SD, Bairey Merz CN, Kim HW, Scott KN, Doyle M, Olson MB, Pepine CJ, den Hollander J, Sharaf B, Rogers WJ, Mankad S, Forder JR, Kelsey SF, Pohost GM. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:2993–2999. [DOI] [PubMed] [Google Scholar]
- 5. Pepine CJ. Ischemic heart disease in women. J Am Coll Cardiol 2006;47(3 Suppl):S1–S3. [DOI] [PubMed] [Google Scholar]
- 6. von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:722–725. [DOI] [PubMed] [Google Scholar]
- 7. Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chaitman BR. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation 2006;113:2462–2472. [DOI] [PubMed] [Google Scholar]
- 9. Chaitman BR, Pepine CJ, Parker JO, Skopal J, Chumakova G, Kuch J, Wang W, Skettino SL, Wolff AA. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA 2004;291:309–316. [DOI] [PubMed] [Google Scholar]
- 10. Chaitman BR, Skettino SL, Parker JO, Hanley P, Meluzin J, Kuch J, Pepine CJ, Wang W, Nelson JJ, Hebert DA, Wolff AA. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol 2004;43:1375–1382. [DOI] [PubMed] [Google Scholar]
- 11. Timmis AD, Chaitman BR, Crager M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J 2006;27:42–48. [DOI] [PubMed] [Google Scholar]
- 12. Wenger NK, Chaitman B, Vetrovec GW. Gender comparison of efficacy and safety of ranolazine for chronic angina pectoris in four randomized clinical trials. Am J Cardiol 2007;99:11–18. [DOI] [PubMed] [Google Scholar]
- 13. Rousseau MF, Pouleur H, Cocco G, Wolff AA. Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. Am J Cardiol 2005;95:311–316. [DOI] [PubMed] [Google Scholar]
- 14. Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol 2006;48:566–575. [DOI] [PubMed] [Google Scholar]
- 15. Kosiborod M, Arnold SV, Spertus JA, McGuire DK, Li Y, Yue P, Ben-Yehuda O, Katz A, Jones PG, Olmsted A, Belardinelli L, Chaitman BR. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina. Results from the TERISA randomized clinical trial (type 2 diabetes evaluation of ranolazine in subjects with chronic stable angina). J Am Coll Cardiol 2013;61:2038–2045. [DOI] [PubMed] [Google Scholar]
- 16. Task Force M, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Guidelines ESCCfP, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 17. Pepine CJ. Angina pectoris in a contemporary population: characteristics and therapeutic implications. TIDES Investigators. Cardiovasc Drugs Ther 1998;12(Suppl 3):211–216. [DOI] [PubMed] [Google Scholar]
- 18. Goykhman P, Mehta PK, Agarwal M, Shufelt C, Slomka PJ, Yang Y, Xu Y, Shaw LJ, Berman DS, Merz NB, Thomson LE. Reproducibility of myocardial perfusion reserve—variations in measurements from post processing using commercially available software. Cardiovasc Diagn Ther 2012;2:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishimori ML, Martin R, Berman DS, Goykhman P, Shaw LJ, Shufelt C, Slomka PJ, Thomson LE, Schapira J, Yang Y, Wallace DJ, Weisman MH, Bairey Merz CN. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc Imaging 2011;4:27–33. [DOI] [PubMed] [Google Scholar]
- 20. Thomson LE, Wei J, Agarwal M, Haft-Baradaran A, Shufelt C, Mehta PK, Gill EB, Johnson BD, Kenkre T, Handberg EM, Li D, Sharif B, Berman DS, Petersen JW, Pepine CJ, Bairey Merz CN. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging 2015;3:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nelson MD, Szczepaniak LS, Wei J, Haftabaradaren A, Bharadwaj M, Sharif B, Mehta P, Zhang X, Thomson LE, Berman DS, Li D, Bairey Merz CN. Diastolic dysfunction in women with signs and symptoms of ischemia in the absence of obstructive coronary artery disease: a hypothesis-generating study. Circ Cardiovasc Imaging 2014;7:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehta PK, Goykhman P, Thomson LE, Shufelt C, Wei J, Yang Y, Gill E, Minissian M, Shaw LJ, Slomka PJ, Slivka M, Berman DS, Bairey Merz CN. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging 2011;4:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 24. Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circ Cardiovasc Qual Outcome 2014;7:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 1989;64:651–654. [DOI] [PubMed] [Google Scholar]
- 26. Anderson C, Laubscher S, Burns R. Validation of the Short Form 36 (SF-36) health survey questionnaire among stroke patients. Stroke 1996;27:1812–1816. [DOI] [PubMed] [Google Scholar]
- 27. Hays R, Sherbourne C, Mazel R. User's Manual for the Medical Outcomes Study (MOS) Core: Measures of Health-related Quality of Life. MR-162- RC. Santa Monica: RAND; 1994. p. 172. [Google Scholar]
- 28. Ware J, Johnston S, Davies A, Brook R. Conceptualization and Measurement of Health for Adults in the Health Insurance Study. Santa Monica: RAND; 1987. p. 180. [Google Scholar]
- 29. Handberg EM, Eastwood JA, Eteiba W, Johnson BD, Krantz DS, Thompson DV, Vaccarino V, Bittner V, Sopko G, Pepine CJ, Merz NB, Rutledge TR. Clinical implications of the Women's Ischemia Syndrome Evaluation: inter-relationships between symptoms, psychosocial factors and cardiovascular outcomes. Women's Health 2013;9:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation 1988;77:43–52. [DOI] [PubMed] [Google Scholar]
- 31. Chih S, Macdonald PS, Feneley MP, Law M, Graham RM, McCrohon JA. Reproducibility of adenosine stress cardiovascular magnetic resonance in multi-vessel symptomatic coronary artery disease. J Cardiovasc Magn Reson 2010;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elkington AG, Gatehouse PD, Ablitt NA, Yang GZ, Firmin DN, Pennell DJ. Interstudy reproducibility of quantitative perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2005;7:815–822. [DOI] [PubMed] [Google Scholar]
- 33. Agarwal M, Shufelt C, Mehta PK, Gill E, Berman DS, Li D, Sharif B, Li N, Bairey Merz CN, Thomson LE. Cardiac risk factors and myocardial perfusion reserve in women with microvascular coronary dysfunction. Cardiovasc Diagn Ther 2013;3:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schrimpf D, Pilz LR. Adaptive randomization procedures for the web-based randomization system RANDI2. Int J Clin Pharmacol Ther 2012;50:85–86. [DOI] [PubMed] [Google Scholar]
- 36. Holm S. A simple sequentially rejective multiple test procedure. Scand J Statist 1979;6:65–70. [Google Scholar]
- 37. Pocock S. Clinical Trials—a Practical Approach. Chichester—New York—Brisbane—Toronto—Singapore: John Wiley & Sons; 1983, p265 S. [Google Scholar]
- 38. De Bruyne B, Baudhuin T, Melin JA, Pijls NH, Sys SU, Bol A, Paulus WJ, Heyndrickx GR, Wijns W. Coronary flow reserve calculated from pressure measurements in humans. Validation with positron emission tomography. Circulation 1994;89:1013–1022. [DOI] [PubMed] [Google Scholar]
- 39. Hozumi T, Yoshida K, Akasaka T, Asami Y, Ogata Y, Takagi T, Kaji S, Kawamoto T, Ueda Y, Morioka S. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol 1998;32:1251–1259. [DOI] [PubMed] [Google Scholar]
- 40. Letienne R, Vie B, Puech A, Vieu S, Le Grand B, John GW. Evidence that ranolazine behaves as a weak beta1- and beta2-adrenoceptor antagonist in the rat [correction of cat] cardiovascular system. Naunyn Schmiedebergs Arch Pharmacol 2001;363:464–471. [DOI] [PubMed] [Google Scholar]
- 41. Zhao G, Walsh E, Shryock JC, Messina E, Wu Y, Zeng D, Xu X, Ochoa M, Baker SP, Hintze TH, Belardinelli L. Antiadrenergic and hemodynamic effects of ranolazine in conscious dogs. J Cardiovasc Pharmacol 2011;57:639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tagliamonte E, Rigo F, Cirillo T, Astarita C, Quaranta G, Marinelli U, Caruso A, Romano C, Capuano N. Effects of ranolazine on noninvasive coronary flow reserve in patients with myocardial ischemia but without obstructive coronary artery disease. Echocardiography 2015;32:516–521. [DOI] [PubMed] [Google Scholar]
- 43. Villano A, Di Franco A, Nerla R, Sestito A, Tarzia P, Lamendola P, Di Monaco A, Sarullo FM, Lanza GA, Crea F. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol 2013;112:8–13. [DOI] [PubMed] [Google Scholar]
- 44. Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient non-compliance with paper diaries. Bmj 2002;324:1193–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou JZ, Bi X, Yang H, Dharmakumar R, Arsanjani R, Bairey Merz C, Berman DS, Li D, Sharif B. First-pass perfusion CMR with reduced dark-rim artifact and instantaneous image reconstruction using optimized cartesian sampling and apodization. J Cardiovasc Magn Reson 2015;17(Supple 1):P001.(in press). [Google Scholar]
- 46. Nelson MD, Shaw J, Wei J, Mehta P, Motwani M, Thomson L, Berman DS, Thompson R, Li D, Bairey Merz CN, Sharif B. Subclinical left ventricular systolic and diastolic dysfunction in women with signs and symptoms of ischemia but no obstructive coronary artery disease: Novel insight using ‘CINE’ tracking in the NHLBI WISE study. Soc Cardiovasc Magn Reson; (under review). [Google Scholar]
- 47. Cocco G, Rousseau MF, Bouvy T, Cheron P, Williams G, Detry JM, Pouleur H. Effects of a new metabolic modulator, ranolazine, on exercise tolerance in angina pectoris patients treated with beta-blocker or diltiazem. J Cardiovasc Pharmacol 1992;20:131–138. [PubMed] [Google Scholar]
- 48. Hayashida W, van Eyll C, Rousseau MF, Pouleur H. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther 1994;8:741–747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.