In Côte d′Ivoire, human immunodeficiency virus viral load (VL) confirmation of antiretroviral therapy failure would be cost-saving compared with CD4 monitoring. Biannual VL, with reduced frequency for virologically suppressed patients, would be cost-effective. Côte d'Ivoire and similar settings should adopt VL monitoring.
Keywords: HIV, laboratory monitoring, viral load, cost-effectiveness, ART
Abstract
Background. Optimal laboratory monitoring of antiretroviral therapy (ART) for human immunodeficiency virus (HIV) remains controversial. We evaluated current and novel monitoring strategies in Côte d′Ivoire, West Africa.
Methods. We used the Cost-Effectiveness of Preventing AIDS Complications –International model to compare clinical outcomes, cost-effectiveness, and budget impact of 11 ART monitoring strategies varying by type (CD4 and/or viral load [VL]) and frequency. We included “adaptive” strategies (biannual then annual monitoring for patients on ART/suppressed). Mean CD4 count at ART initiation was 154/μL. Laboratory test costs were CD4=$11 and VL=$33. The standard of care (SOC; biannual CD4) was the comparator. We assessed cost-effectiveness relative to Côte d′Ivoire's 2013 per capita GDP ($1500).
Results. Discounted life expectancy was 16.69 years for SOC, 16.97 years with VL confirmation of immunologic failure, and 17.25 years for adaptive VL. Mean time on failed first-line ART was 3.7 years for SOC and <0.9 years for all routine/adaptive VL strategies. VL failure confirmation was cost-saving compared with SOC. Adaptive VL had an incremental cost-effectiveness ratio (ICER) of $4100/year of life saved compared with VL confirmation and increased the 5-year budget by $310/patient compared with SOC. Adaptive VL achieved an ICER <1× GDP if second-line ART and VL costs simultaneously decreased to $156 and $13, respectively.
Conclusions. VL confirmation of immunologic failure is more effective and less costly than CD4 monitoring in Côte d′Ivoire. Adaptive VL monitoring reduces time on failing ART, is cost-effective, and should become standard in Côte d′Ivoire and similar settings.
Access to antiretroviral therapy (ART) for human immunodeficiency virus (HIV)-infected patients in resource-limited settings has improved dramatically over the past 5 years, with 13.5 million patients receiving ART in 2014 in low- and middle-income countries compared with 6.9 million in 2010 [1, 2]. In sub-Saharan Africa, where ART regimens with low barriers to resistance have been widely used, improved access to care has caused growing concerns regarding first-line ART failure and switching to second-line therapies [2]. The best use of second-line ART depends on timely detection of treatment failure, since continued exposure to failing ART leads to increased drug resistance and mortality [3, 4]. Viral load (VL) monitoring promotes earlier switching compared with immunologic monitoring and can reduce exposure time to a virologically failing regimen [3]. The World Health Organization (WHO) currently recommends CD4 monitoring every 6 months and VL monitoring 6 months after ART initiation and every 12 months thereafter [5].
With the expanded availability of second-line ART and increased emphasis on early viral suppression, new questions have arisen on how best to use VL monitoring of ART [5–8]. To address these issues, we compared innovative VL monitoring strategies that reduced monitoring frequency for virally suppressed patients to conventional monitoring strategies. Our objective was to assess the cost-effectiveness and budget impact of current and novel strategies to determine the most efficient use of VL monitoring and to maximize survival benefits in resource-limited settings.
METHODS
Analytic Overview
We used the Cost-Effectiveness of Preventing AIDS Complications (CEPAC)–International model, a microsimulation of HIV disease and treatment, to evaluate the clinical outcomes, costs, cost-effectiveness, and budget impact of alternative types (CD4 count and/or VL) and frequencies of laboratory monitoring [9–11].
We simulated a cohort of HIV-infected patients on or starting ART in Côte d′Ivoire in 2013. We compared projected survival in discounted life years and costs (2013 US dollars [USD]) of various monitoring strategies to biannual CD4 monitoring, which is Côte d′Ivoire's current standard of care (SOC). Outcomes included time spent on failed ART; life expectancy; lifetime cost (in 2013 USD); and incremental cost-effectiveness ratios (ICERs) in dollars per year of life saved ($/YLS). Cost-effectiveness outcomes were discounted at 3% annually [12].
To evaluate budget impact, we simulated a cohort of HIV-infected patients receiving ART over 5 years, from the beginning of 2013 through the end of 2017, including those who had started ART prior to 2013 and new patients initiating ART each year from 2013 to 2017. We used costs from an HIV care center in Abidjan, Côte d′Ivoire [13, 14]. We compared each strategy's 5-year overall and per-person costs to the current SOC.
Strategies
We compared 11 laboratory monitoring strategies that varied by CD4 and VL availability and frequency (Table 1). These included CD4 only, CD4 with VL confirmation of immunologic failure, routine VL with or without CD4, and “adaptive” monitoring, with biannual monitoring for the first year and annual monitoring thereafter for virally suppressed patients. Routine and adaptive monitoring strategies with VL also included a CD4 test for ART initiation and every 2 years (for opportunistic infection [OI] prophylaxis decisions) thereafter. We assumed that second-line ART was available to all patients who failed first-line ART. Cotrimoxazole prophylaxis was used in all strategies [14].
Table 1.
Laboratory Monitoring Strategies
| Monitoring Strategy | Laboratory Monitoring Frequency |
|
|---|---|---|
| CD4 Testing Frequency (mo) | Viral Load Testing Frequency (mo) | |
| Biannual CD4 (standard of care) | 6 | … |
| Biannual CD4 + biannual VL | 6 | 6 |
| Biannual CD4 + annual VL | 6 | 12 |
| Annual CD4 + annual VL | 12 | 12 |
| Biannual VL | 24 | 6 |
| Annual VL | 24 | 12 |
| Adaptive: biannual then annual CD4 and VLa | 6 then 12 | 6 then 12 |
| Biannual CD4 + biannual then annual VL (World Health Organization)b | 6 | 6 then 12 |
| Adaptive: biannual then annual VLa | 24 | 6 then 12 |
| Biannual CD4 + VL to confirm failure | 6 | To confirm failure only |
| Annual CD4 + VL to confirm failure | 12 | To confirm failure only |
Abbreviation: VL, viral load.
a In “adaptive” strategies, monitoring frequency decreased from biannual to annual in patients virologically suppressed after 1 year.
b The World Health Organization recommends biannual CD4 monitoring with VL testing 6 months after initiation and every 12 months thereafter.
Main Outcomes
Main outcomes were mean time on virologically failed first-line ART, ICERs for cost-effectiveness, and 5-year budget change for the budget impact analysis. ICERs were calculated by comparing each strategy to the next less costly, nondominated strategy [12]. We labeled strategies “dominated” if they were less effective and more costly than some combination of other strategies [12]. A strategy was defined as “very cost-effective” if its ICER was <1× Cote d′Ivoire's 2013 per capita gross domestic product (GDP; $1500) and “cost-effective” if its ICER was <3× GDP [12, 15]. The 5-year budget change was the 5-year cost difference between each strategy and the SOC (biannual CD4 testing).
CEPAC–International Model
The CEPAC–International model simulates a cohort of patients generated from an initial distribution of age, sex, CD4 count, HIV RNA, and likelihood of treatment adherence [9–11]. Each patient is followed monthly from entry until death. CD4 count determines risk of developing an HIV-related disease or dying from HIV [16]. ART decreases VL, increases CD4 count, and reduces HIV-related mortality [17]. The model tracks “true” CD4 count and VL for each patient monthly; however, ART failure criteria and switching are based on CD4 and VL values “observed” via laboratory testing or development of an OI. Additional model structure details are published and online (http://web2.research.partners.org/cepac) [9–11, 18].
Model Input Parameters
Baseline characteristics, monthly risk of OIs, and mortality were from clinical trials and cohort studies in Côte d'Ivoire and other sub-Saharan African countries (Table 2) [16, 19, 24]. Simulated patients started ART upon model entry. First-line ART was tenofovir and emtricitabine plus efavirenz; second-line was zidovudine and lamivudine plus lopinavir/ritonavir [25]. Overall 6-month viral suppression was 80% for both regimens [19]. Third-line ART was not in the base case, since it is not currently available in Côte d′Ivoire. True ART failure was defined by at least 1 of the following, according to WHO guidelines: immunologic: 50% decrease from peak CD4, return to nadir CD4, or CD4 ≤100/μL; virologic: VL >1000 copies/mL. Clinical failure, defined as WHO stage III/IV disease after at least 6 months on ART, was also included [25]. After observed ART failure, a 6-month adherence reinforcement intervention was implemented with a 30% chance of virologic resuppression [11]. Patients who suppressed after adherence reinforcement were maintained on first-line ART; others were switched to second-line ART.
Table 2.
Monitoring Strategies and Main Model Input Parameters for Analysis Comparing Antiretroviral Therapy Monitoring Strategies in Côte d′Ivoire
| Parameter | Base Case Value | Sensitivity Analyses |
|
|---|---|---|---|
| Range | Reference | ||
| Initial characteristics | |||
| Age, mean (SD), y | 37 (9) | [28–46] | [19] |
| CD4, mean (SD), cells/µL | 154 (102) | [52–256] | [19] |
| Sex, female, % | 75 | … | [19] |
| Characteristics in 2013 | |||
| Age, mean (SD), y | 40 (9) | … | Model derived |
| CD4, mean (SD), cells/µLa | 391 (131) | … | Model derived |
| ART regimen and adherence | |||
| First- and second-line ART | |||
| HIV-1 RNA suppression at 6 mo, % | 80 | [50–90] | [19] |
| Virologic failure after 6 mo, per 100 PY | 15 | [7–22] | [19] |
| Adherence <65%b | 93 | [46–143] | [19] |
| Adherence >95%b | 1.6 | [0.8–2.3] | [19] |
| Monthly CD4 increase, mean (SD), cell/µL | |||
| Between 0 and 2 mo | 76 (19) | [58–97] | [19] |
| ≥2 mo | 4 (1) | … | [19] |
| 6-mo adherence reinforcement | |||
| HIV-1 RNA suppression at 6 mo, % | 30 | [10–60] | Assumption |
| Virologic failure after 6 mo, per 100 PY | 15 | [7–22] | [19] |
| Adherence <65%b | 93 | [46–143] | [19] |
| Adherence >95%b | 1.6 | [0.8–2.3] | |
| Monthly CD4 increase, mean (SD), cell/µL | |||
| Between 0 and 2 mo | 76 (19) | [58–97] | [19] |
| ≥2 mo | 4 (1) | … | [19] |
| Loss to follow-up, per 100 PY | |||
| Adherence <65%b | 13 | [6.5–19.6] | [20] |
| Adherence >95%b | 1.9 | [0.9–2.8] | [20] |
| Costs (2013 US dollars) | |||
| Prophylaxis, annual | 30 | … | [14] |
| Drugs, annual | |||
| First-line ART | 123 | [61–185] | [21] |
| Second-line ART | 391 | [196–587] | [21] |
| 6-mo adherence reinforcementc | 156 | [77–234] | Assumption |
| Laboratory monitoring, per test | |||
| CD4 test | 11 | [5–16] | CeDReS |
| VL test | 33 | [10–50] | CeDReS |
| VL implementation costd | 400 000 | … | [22] |
Confidence interval ranges were derived from input data or estimated by multiplying base case value by .5 (lower bound) and 1.5 (upper bound).
Abbreviations: ART, antiretroviral therapy; CeDReS, Centre de diagnostic et de Recherche sur le SIDA et les Affections Opportunistes at Treichville University Hospital, Abidjan, Côte d′Ivoire; HIV, human immunodeficiency virus; PY, person-years; SD, standard deviation; VL, viral load.
a CD4 for patients on ART beginning at various points from 2008 to 2012.
b Values from 65% to 95% were linearly interpolated. For more information, see [23].
c The adherence reinforcement involved 6 adherence training sessions (1/month) and weekly short message service reminders, estimated as the cost of 6 routine care visits.
d We assumed that 5 VL machines in the 5 areas of highest HIV prevalence in Côte d′Ivoire would cover all patients on ART. Implementation cost/machine would be $80 000 (see “Methods” section).
Estimates of direct costs were from the payer perspective. These included costs to the Côte d′Ivoire Ministry of Health, The United States President's Emergency Plan for AIDS Relief (PEPFAR), and patients. Drug costs were from Médecins Sans Frontières and Clinton Health Access Initiative databases [21]. CD4 and VL test costs ($11 and $33) were from a reference laboratory, Centre de Diagnostic et de Recherche sur le SIDA et les Affections Opportunistes, at Treichville University Hospital, Abidjan, Côte d′Ivoire. Indirect costs were not included. We assumed that 5 laboratories in regions of high HIV prevalence would be sufficient to monitor VL for those on ART in Côte d′Ivoire [26]. Implementation would cost approximately $80 000 per laboratory, including cost of a VL machine (COBAS AmpliPREP and TAQMAN 48) and training [22]. Thus, we estimated the initial overhead cost for implementing routine VL testing in Côte d′Ivoire at $400 000. This value was included in both our cost-effectiveness and budget impact analyses (Supplementary Material 2).
Cohort Description
Patients on ART at the Beginning of 2013 in Côte d′Ivoire
By UNAIDS estimates, approximately 110 000 adults were on ART at the beginning of 2013 and 27 000 new patients started ART during 2013 in Côte d′Ivoire [27]. To model patients on ART in 2013, we simulated the following 5 cohorts for each monitoring strategy: patients suppressed on first-line ART; patients on failed first-line ART; patients suppressed on second-line ART; patients on failed second-line ART; and patients initiating ART in 2013 (hereafter the "new ART" cohort). Patients in the first 4 cohorts (hereafter the “prior ART” cohorts) initiated ART prior to 2013 and were on first- or second-line ART for various durations. Characteristics of the prior ART cohorts in 2013 were from an initial simulation of patients starting ART each year between 2008 and 2012 to obtain an epidemic steady state (Table 2, Supplementary Table 2).
Patients on ART Between 2013 and the End of 2017
We simulated 5 additional cohorts for the budget impact analysis, 1 initiating ART each year between 2013 and 2017. We assumed that 110 000 patients were on ART at the beginning of 2013 and an additional 27 000 patients initiated ART each year from 2013 to 2017, for a total of 245 000 patients [27].
Analyses
Base Case and Sensitivity Analyses
For the cost-effectiveness analysis, the prior ART and new ART cohorts were simulated as the base case for each strategy. For the budget impact analysis, we scaled the results to the total of 245 000 patients.
In 1-way sensitivity analyses, we varied main parameters, including ART suppression, mean initial CD4, monitoring costs, and ART costs, to evaluate the sensitivity of our model output to variation in the input variables. One-way sensitivity analysis ranges were 95% confidence intervals from the literature, 50% ranges (0.5× and 1.5× the base case), or extreme values. In a 3-way sensitivity analysis, we simultaneously varied 3 of the most influential parameters from 1-way sensitivity analyses: VL test cost, second-line ART cost, and probability of resuppression.
RESULTS
Base Case Analysis
Mean Time on Failed First-Line ART
Mean time on failed first-line ART was 3.65 years for SOC monitoring. Biannual or annual CD4 monitoring with VL confirmation of failure increased this to 4.32 years and 5.18 years. Routine or adaptive VL monitoring, alone or with CD4 monitoring, decreased mean time on failed first-line ART to 0.90 years for annual VL and 0.70 years for adaptive VL. Time on failed first-line ART was lowest (0.54 years) for the following 2 strategies: biannual VL monitoring alone and biannual CD4 and VL.
Cost-Effectiveness Analysis
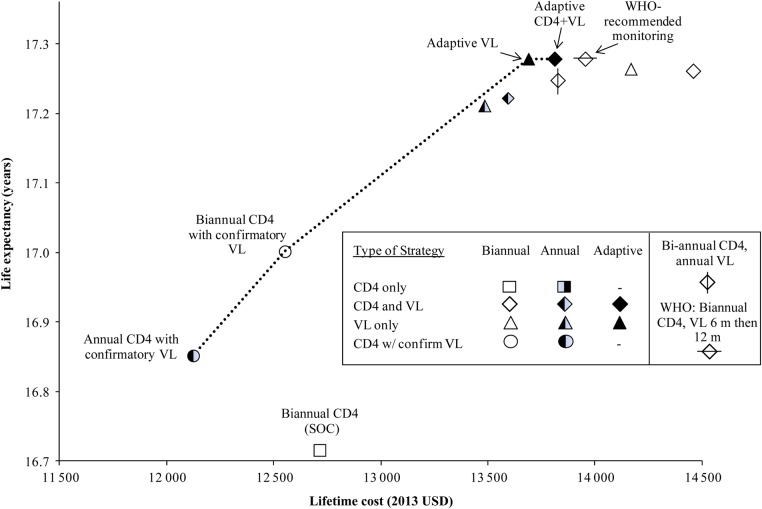
Discounted life expectancy ranged from 16.69 years for the SOC to 17.25 years for adaptive VL monitoring with or without CD4 and WHO-recommended monitoring (biannual CD4 and VL at 6 months followed by every 12 months; Table 3). Strategies with confirmatory VL increased life expectancy to 16.82–16.97 years, while strategies using routine or adaptive VL monitoring further increased life expectancy to 17.18–17.25 years. Lifetime per-person costs ranged from $12 130 for annual CD4 with confirmatory VL to $14 460 for biannual CD4 and VL monitoring. Of the strategies using routine or adaptive VL, annual VL was least expensive ($13 490), followed by annual CD4 and VL ($13 600), then adaptive VL ($13 700). The following 4 strategies were nondominated and on the efficiency frontier, denoting the maximum life expectancy gain that can be achieved for a given financial outlay (Figure 1): annual CD4 with confirmatory VL, biannual CD4 with confirmatory VL, adaptive VL, and adaptive CD4 and VL. All strategies with routine CD4 and/or routine VL, including the SOC and WHO-recommended strategies, were dominated (Table 3). Adaptive VL had an ICER of $4100/YLS compared with biannual CD4 with confirmatory VL. Adaptive CD4 and VL slightly increased life expectancy compared with adaptive VL alone but also increased costs (ICER: $1 993 500/YLS). WHO-recommended monitoring was more expensive ($13 960 compared with $13 820) and yielded lower life expectancy than adaptive CD4 and VL and was thus dominated (Table 3).
Table 3.
Clinical and Economic Outcomes of Implementing Monitoring Strategies for Human Immunodeficiency Virus–Infected Patients on Antiretroviral Therapy in Côte d′Ivoire
| Monitoring Strategy | Mean Time on Failed First-Line Antiretroviral Therapy (y) | Discounted Life Expectancy (y) | Discounted Lifetime Cost (USD) | Incremental Cost-Effectiveness Ratio (USD/YLS) |
|---|---|---|---|---|
| Annual CD4 + VL to confirm failure | 5.18 | 16.82 | 12 130 | … |
| Biannual CD4 + VL to confirm failure | 4.32 | 16.97 | 12 560 | 2900 |
| Biannual CD4 (standard of care) | 3.65 | 16.69 | 12 720 | Dominated |
| Annual VL | 0.90 | 17.18 | 13 490 | Dominated |
| Annual CD4 + annual VL | 0.90 | 17.19 | 13 600 | Dominated |
| Adaptive: biannual then annual VLa | 0.70 | 17.25 | 13 700 | 4100 |
| Adaptive: biannual then annual CD4 and VLa | 0.70 | 17.25 | 13 820 | 1 993 500 |
| Biannual CD4 + annual VL | 0.85 | 17.22 | 13 830 | Dominated |
| Biannual CD4 + biannual then annual VLb (World Health Organization) | 0.73 | 17.25 | 13 960 | Dominated |
| Biannual VL | 0.54 | 17.23 | 14 170 | Dominated |
| Biannual CD4 + biannual VL | 0.54 | 17.23 | 14 460 | Dominated |
All costs and life years discounted at 3% per year (see “Methods” section). Costs are in 2013 US dollars. Numbers were rounded for clarity; recalculating incremental cost-effectiveness ratio (ICER) using these rounded numbers would not be accurate. In the cost-effectiveness analysis, life expectancy was projected for 110 000 patients initiating antiretroviral therapy at the beginning of 2013. Dominated: More costly and lower life expectancy or higher ICER than another, more effective strategy. Bolded strategies are non-dominated.
Abbreviations: USD, 2013 US dollars; VL, viral load; YLS, years of life saved.
a Adaptive strategies included biannual testing and then annual testing if a patient was suppressed after 12 months.
b The World Health Organization recommends biannual CD4 monitoring with VL testing 6 months after initiation, then every 12 months thereafter, not contingent on virologic suppression.
Figure 1.
Cost-effectiveness efficiency frontier. The increase in life expectancy and cost as strategies become more effective and more costly. Adding viral load (VL) improves survival for all strategies; more frequent VL further improves survival. All adaptive strategies are above and to the left of the corresponding nonadaptive strategies, highlighting the benefit of adaptive monitoring. Neither the standard of care (SOC) nor the World Health Organization (WHO) –recommended strategy (biannual CD4 and VL monitoring at 6 months, followed by every 12 months thereafter) is on the efficiency frontier. Abbreviation: USD, 2013 US dollars.
Budget Impact Analysis
For patients already on ART or starting ART in 2013, SOC monitoring had a projected 5-year budget impact of $642.2 million ($3670/person; Table 4). Biannual CD4 monitoring with VL confirmation of failure decreased the 5-year outlay to $640.7 million ($3660/person). Adaptive VL monitoring increased this outlay to $699.2 million ($3980/person), an increase of 8.9% from the SOC. WHO monitoring would require a 10.9% increase in the 5-year budget.
Table 4.
Budget Impact Analysis Over 5 Years of Laboratory Monitoring Strategies for Human Immunodeficiency Virus–Infected Patients on Antiretroviral Therapy in Côte d′Ivoire
| Monitoring Strategy | Monitoring |
Antiretroviral Therapy Regimen |
Overall |
||||
|---|---|---|---|---|---|---|---|
| Cost (1000 USD) | % of Overall Cost | Cost (1000 USD) | % of Overall Cost | Cost (1000 USD)a | 5-Year Cost per Person (USD)b | % Budget Increasec | |
| Annual CD4 testing + VL to confirm failure | 15 000 | 2.4 | 118 000 | 18.9 | 623 700 | 3570 | −2.9 |
| Biannual CD4 testing + VL to confirm failure | 26 800 | 4.2 | 122 400 | 19.1 | 640 700 | 3660 | −0.2 |
| Biannual CD4 testing (standard of care) | 21 500 | 3.4 | 128 900 | 20.1 | 642 200 | 3670 | … |
| Annual VL testing | 40 700 | 6.1 | 139 100 | 20.7 | 671 800 | 3830 | 4.6 |
| Annual CD4 + annual VL testing | 45 500 | 6.7 | 139 100 | 20.6 | 676 500 | 3860 | 5.3 |
| Biannual CD4 + annual VL testing | 57 300 | 8.3 | 142 100 | 20.5 | 691 900 | 3940 | 7.8 |
| Adaptive: biannual then annual VLd | 53 500 | 7.6 | 152 100 | 21.8 | 699 200 | 3980 | 8.9 |
| Adaptive: biannual then annual CD4 and VLd | 62 200 | 8.8 | 152 000 | 21.5 | 707 800 | 4030 | 10.2 |
| Biannual CD4 + biannual then annual VLe (World Health Organization) | 66 400 | 9.3 | 152 100 | 21.4 | 712 100 | 4070 | 10.9 |
| Biannual VL | 71 200 | 9.9 | 154 100 | 21.4 | 718 900 | 4090 | 11.9 |
| Biannual CD4 + biannual VL | 86 100 | 11.7 | 154 100 | 21.0 | 733 900 | 4170 | 14.3 |
All costs are undiscounted. Bolded strategies are non-dominated.
Abbreviations: USD, 2013 US dollars; VL, viral load.
a In the budget impact analysis, 5-year outcomes were projected for 110 000 patients on antiretroviral therapy (ART) at the beginning of 2013 and 27 000 additional patients starting ART each year from 2013 to 2017 (See “Methods” section for details).
b Per person cost was calculated by dividing the total cost over 5 years by the total number of patients ever in care (prior ART cohorts and new ART cohorts [patients entering care each year]), weighted by total amount of time spent in care per patient and adjusted for mortality (see Supplementary Material 1).
c Budget increase is compared with the standard of care.
d Adaptive strategies included biannual testing and then annual testing if a patient was suppressed after 12 months.
e The World Health Organization recommends biannual CD4 monitoring with VL testing 6 months after ART initiation, then every 12 months thereafter, not contingent on virologic suppression.
Sensitivity Analysis
Cost-Effectiveness Analysis
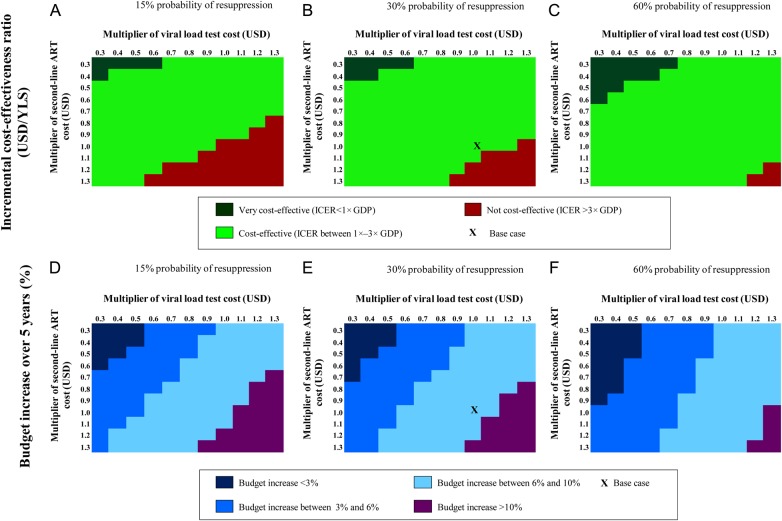
In 1-way sensitivity analysis, the cost-effectiveness of adaptive VL compared with biannual CD4 monitoring with VL confirmation was most sensitive to initial mean CD4 count, second-line ART cost, VL test cost, and probability of resuppression. Decreasing initial mean CD4 from 152/µL to 52/µL decreased the ICER from $4100 to $3200; increasing initial mean CD4 to 256/µL increased the ICER to $6900 (Supplementary Figure 2). A 50% decrease in second-line ART cost decreased the ICER of the adaptive VL strategy from $4100 to $2500/YLS, while a 50% decrease in VL test costs decreased the ICER of adaptive VL to $3000/YLS. Simultaneously varying second-line ART cost, VL test cost, and probability of resuppression in a 3-way sensitivity analysis substantially changed the cost-effectiveness and percentage budget increase of the adaptive monitoring strategies (Figure 2B). The ICER decreased to <1× Côte d′Ivoire's GDP when we simultaneously reduced annual second-line ART cost and VL test cost to 40% of base case (to $156 and $13, respectively), with a probability of resuppression of 30%. This strategy also had ICER <1× GDP when we simultaneously reduced annual second-line ART cost to 30% of base case cost (from $391 to $117) and VL test cost to 60% of base case cost (from $33 to $20). If probability of resuppression was 60% (base case: 30%), then smaller reductions in VL test cost and second-line ART cost made the adaptive strategy ICER <1× GDP (Figure 2C).
Figure 2.
Three-way sensitivity analysis. A–C, Variation in the incremental cost-effectiveness ratio (ICER) of the adaptive strategy when second-line antiretroviral therapy (ART) cost, viral load (VL) test cost, and probability of resuppression with the adherence intervention are varied. D–F, Variation in 5-year percent budget increase under the same variations. In the adaptive strategy, testing frequency is decreased from biannual to annual for patients suppressed after 1 year. The comparator strategy for adaptive VL monitoring changes throughout this figure. Abbreviations: GDP, gross domestic product; USD, US dollars; YLS, years of life saved.
Budget Impact Analysis
In 1-way sensitivity analysis, the 5-year budget impact was most sensitive to VL test cost, second-line ART cost, and first-line ART cost (Supplementary Figure 3). In 3-way sensitivity analysis, when probability of resuppression was 30%, total budget increase was <3% if second-line ART cost was 50% of base case ($196) and VL test cost was 40% of base case ($13; Figure 2E). Other parameters varied in sensitivity analysis, including first- and second-line ART efficacy, loss to follow-up, and mean age at ART initiation, had little effect on the budget impact results.
DISCUSSION
The WHO recommends VL monitoring of first-line ART if possible; however, there are many countries in sub-Saharan Africa where this remains largely unavailable, including Côte d′Ivoire, Malawi, Rwanda, and Zambia [28, 29]. Our goal was to determine the clinical outcomes, cost-effectiveness, and budget impact of various monitoring strategies using current data on ART availability and efficacy. “Adaptive” VL monitoring, which included biannual monitoring followed by annual monitoring after 1 year of viral suppression, substantially decreased time spent on failed first-line ART, which would markedly decrease time for HIV transmission [30]. Additionally, biannual CD4 monitoring with VL failure confirmation improved survival and was cost-saving compared with the current Côte d′Ivoire SOC (biannual CD4 monitoring). Therefore, the SOC, which reflects current clinical care in Côte d′Ivoire, cannot be justified on cost-effectiveness grounds. Adaptive VL monitoring further increased survival and was cost-effective compared with biannual CD4 with confirmatory VL (ICER: $4100/YLS).
In sensitivity analyses, we found that the cost-effectiveness of the adaptive strategy depended mostly on the initial CD4 count of the cohort, second-line ART cost, and VL test cost. Since the clinical benefit of VL monitoring is from switching patients to second-line ART before immunologic decline or an OI, VL monitoring will be even more cost-effective as second-line ART costs decrease further. This would have a bigger impact on cost-effectiveness than VL test cost itself.
We found a similar benefit as in previous studies to routine VL compared with biannual CD4 monitoring, with a life expectancy increase of 1.1 undiscounted life years for routine biannual VL compared with biannual CD4. Prior modeling analyses have reported life expectancy increases of 0.7–1.8 undiscounted life years [31–35]. Phillips et al found an undiscounted life expectancy increase of 0.7 years with VL monitoring compared with CD4, while Braithwaite et al compared biannual VL to confirmatory VL and found a difference of 0.5 discounted life years [33, 36]. Our analysis differs from all of these in our evaluation of adaptive VL monitoring strategies, which have not previously been examined.
VL monitoring, even when adaptive strategies are used, will likely increase total HIV care costs, primarily due to increased survival and concomitant ART costs. Thus, while the adaptive strategy has an ICER <3× Côte d′Ivoire's per capita GDP, it would increase the total 5-year budget by 8.9%. Though allocating additional resources to HIV monitoring would be challenging, initiatives are ongoing to develop low-cost VL assays, including point-of-care tests [22, 31, 37]. Additionally, Roche introduced a ceiling price of $9.40 in 2014 for VL testing, indicating that VL tests may soon achieve prices similar to CD4 tests [38]. Governments and organizations such as the Clinton Health Access Initiative and Médecins Sans Frontières will likely continue to drive down the cost of second-line ART; since 2010, that cost has decreased from $550/year to $390/year [21, 39]. Over the next several years, further decreases in second-line ART costs and VL test costs, as well as reduced clinic visit frequencies for virally suppressed patients, would lessen the impact of adaptive monitoring on constrained national budgets.
The WHO recommends VL monitoring 6 months after ART initiation and every 12 months thereafter, as well as biannual CD4 monitoring [25]. Our analysis is the first to fully evaluate this recommendation, and we found that including biannual CD4 with VL monitoring increased costs, but not survival, compared with VL monitoring with CD4 only every 2 years. CD4 monitoring, with its inherent variability, can lead to ART switching without virologic failure; thus, adding CD4 monitoring to routine or adaptive VL increases costs but does not improve outcomes [4, 40].
This analysis has several limitations. Models are simplifications of complex clinical and healthcare systems, and all needed data are not always available. There are scant data on the efficacy of adherence interventions, so we assumed a low efficacy (30%), though recent evidence suggests this could be much higher [11, 41]. Second, in our budget impact analysis, we assumed that a constant number of patients would initiate ART each year from 2013 to 2017. New data on the benefits of earlier ART initiation, coupled with recent WHO recommendations, will likely increase the number of patients starting ART annually, which would affect our total budget estimates [5, 7, 8]. Third, we did not directly evaluate the impact of different monitoring strategies on HIV transmission. By reducing time on failing first-line ART before switching and increasing the overall percentage of patients virologically suppressed, adaptive VL monitoring would lower HIV transmission rates, making it even more cost-effective [6].
Resource constraints in Côte d′Ivoire and other sub-Saharan African nations have limited access to VL monitoring. Achieving the best outcomes for HIV-infected patients, however, requires timely switching to second-line ART after virologic failure. Using VL to confirm failure diagnosis would be cost-saving and would improve life expectancy in settings where CD4 monitoring alone is the SOC. Incorporating routine or adaptive VL monitoring will improve switch timing, reduce time on failed ART, and further improve clinical outcomes. Adaptive VL monitoring is cost-effective at current costs and will become increasingly affordable with reductions in VL and second-line ART costs. VL monitoring should be implemented in Côte d′Ivoire and similar resource-limited settings.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Disclaimer. The manuscript's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was funded by the National Institute of Allergy and Infectious Diseases (R01 AI058736) and the Agence Nationale de Recherches sur le SIDA et les hépatites virales in Paris, France.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Actual and projected numbers of people receiving antiretroviral therapy in low- and middle-income countries by WHO region and in high-income countries across WHO regions, 2003–2015. Available at: http://www.who.int/hiv/data/art_2003_2015.png?ua=1 Accessed 11 November 2015.
- 2.UNAIDS. How AIDS changed everything. Available at: http://www.unaids.org/sites/default/files/media_asset/MDG6Report_en.pdf Accessed 11 November 2015.
- 3.Petersen ML, Tran L, Geng EH et al. Delayed switch of antiretroviral therapy after virologic failure associated with elevated mortality among HIV-infected adults in Africa. AIDS 2014; 28:2097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schooley RT. Viral load testing in resource-limited settings. Clin Infect Dis 2007; 44:139–40. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Guidelines on when to start antiretroviral therapy and pre-exposure prophylaxis for HIV. Available at: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1 Accessed 11 November 2015. [PubMed]
- 6.Cohen MS, Chen YQ, McCauley M et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Temprano ANRS Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–22. [DOI] [PubMed] [Google Scholar]
- 8.Insight START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anglaret X, Scott CA, Walensky RP et al. Could early antiretroviral therapy entail more risks than benefits in sub-Saharan African HIV-infected adults? A model-based analysis. Antivir Ther 2013; 18:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedberg KA, Losina E, Weinstein MC et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med 2001; 344:824–31. [DOI] [PubMed] [Google Scholar]
- 11.Ouattara EN, Ross EL, Yazdanpanah Y et al. Clinical impact and cost-effectiveness of making third-line antiretroviral therapy available in sub-Saharan Africa: a model-based analysis in Côte d'Ivoire. J Acquir Immune Defic Syndr 2014; 66:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA 1996; 276:1253–8. [PubMed] [Google Scholar]
- 13.Anglaret X, Chêne G, Attia A et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d'Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet 1999; 353:1463–8. [DOI] [PubMed] [Google Scholar]
- 14.Yazdanpanah Y, Losina E, Anglaret X et al. Clinical impact and cost-effectiveness of co-trimoxazole prophylaxis in patients with HIV/AIDS in Côte d'Ivoire: a trial-based analysis. AIDS 2005; 19:1299–308. [DOI] [PubMed] [Google Scholar]
- 15.The World Bank. GDP per capita (current US$). Available at: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD Accessed 10 February 2014.
- 16.Anglaret X, Minga A, Gabillard D et al. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Côte d'Ivoire. Clin Infect Dis 2012; 54:714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Losina E, Yazdanpanah Y, Deuffic-Burban S et al. The independent effect of highly active antiretroviral therapy on severe opportunistic disease incidence and mortality in HIV-infected adults in Côte d'Ivoire. Antivir Ther 2007; 12:543–51. [PMC free article] [PubMed] [Google Scholar]
- 18.Walensky RP, Ross EL, Kumarasamy N et al. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med 2013; 369:1715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messou E, Chaix ML, Gabillard D et al. Association between medication possession ratio, virologic failure and drug resistance in HIV-1-infected adults on antiretroviral therapy in Côte d'Ivoire. J Acquir Immune Defic Syndr 2011; 56:356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox MP, Ive P, Long L, Maskew M, Sanne I. High rates of survival, immune reconstitution, and virologic suppression on second-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr 2010; 53:500–6. [DOI] [PubMed] [Google Scholar]
- 21.Clinton Health Access Initiative (CHAI). Antiretroviral (ARV) ceiling price list. Available at: http://45.55.138.94/content/uploads/2015/05/CHAI-ARV-Ceiling-Price-2014-Final_English.pdf Accessed 11 November 2015.
- 22.UNITAID. HIV/AIDS Diagnostic Technology Landscape, 2nd Edition Available at: http://www.unitaid.eu/images/marketdynamics/publications/UNITAID-HIV_Diagnostics_Landscape-2nd_edition.pdf Accessed 11 November 2015.
- 23.Ross E, Tanser F, Pei P et al. The impact of the 2013 WHO antiretroviral therapy guidelines on the feasibility of HIV population prevention trials. HIV Clin Trials 2014; 15:185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danel C, Moh R, Minga A et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in West Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet 2006; 367:1981–9. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Available at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf Accessed 11 November 2015.
- 26.Rapport annuel des indicateurs VIH du secteur santé en Côte d'Ivoire, 2012: direction de l'information, de la planification et de l'evaluation (DIPE) (not published). Accessed 11 November 2015.
- 27.UNAIDS. AidsInfo: Côte d'Ivoire data. Available at: http://www.unaids.org/en/dataanalysis/datatools/aidsinfo/ Accessed 11 November 2015.
- 28.UNAIDS. Report on the global AIDS epidemic. Available at: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf Accessed 11 November 2015.
- 29.Tagar E, Sundaram M, Condliffe K et al. Multi-country analysis of treatment costs for HIV/AIDS (MATCH): facility-level ART unit cost analysis in Ethiopia, Malawi, Rwanda, South Africa and Zambia. PLoS One 2014; 9:e108304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–404. [DOI] [PubMed] [Google Scholar]
- 31.Estill J, Egger M, Johnson LF et al. Monitoring of antiretroviral therapy and mortality in HIV programmes in Malawi, South Africa and Zambia: mathematical modelling study. PLoS One 2013; 8:e57611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimmel AD, Weinstein MC, Anglaret X et al. Laboratory monitoring to guide switching antiretroviral therapy in resource-limited settings: clinical benefits and cost-effectiveness. J Acquir Immune Defic Syndr 2010; 54:258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips AN, Pillay D, Miners AH, Bennett DE, Gilks CF, Lundgren JD. Outcomes from monitoring of patients on antiretroviral therapy in resource-limited settings with viral load, CD4 cell count, or clinical observation alone: a computer simulation model. Lancet 2008; 371:1443–51. [DOI] [PubMed] [Google Scholar]
- 34.Braithwaite RS, Nucifora KA, Yiannoutsos CT et al. Alternative antiretroviral monitoring strategies for HIV-infected patients in east Africa: opportunities to save more lives? J Int AIDS Soc 2011; 14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keebler D, Revill P, Braithwaite S et al. Cost-effectiveness of different strategies to monitor adults on antiretroviral treatment: a combined analysis of three mathematical models. Lancet Glob Health 2014; 2:e35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braithwaite SR, Nucifora KA, Toohey C et al. How do different eligibility guidelines for antiretroviral therapy affect the cost-effectiveness of routine viral load testing in sub-Saharan Africa? AIDS 2014; 28(suppl 1):S73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Working Group on Modelling of Antiretroviral Therapy Monitoring Strategies in Sub-Saharan A Phillips A, Shroufi A et al. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature 2015; 528:S68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.UNAIDS. Landmark HIV diagnostic access program will save $150m and help achieve new global goals on HIV. Available at: http://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2014/september/20140925prviralload Accessed 10 February 2016.
- 39.Médecins Sans Frontières. Untangling the web of antiretroviral price reductions. 15th Edition Available at: http://d2pd3b5abq75bb.cloudfront.net/2013/09/11/10/25/44/896/MSF_Access_UTW_16th_Edition_2013.pdf Accessed May 20 2015.
- 40.De Beaudrap P, Thiam M, Diouf A et al. Risk of virological failure and drug resistance during first- and second-line antiretroviral therapy in a 10-year cohort in Senegal: results from the ANRS 1215 cohort. J Acquir Immune Defic Syndr 2013; 62:381–7. [DOI] [PubMed] [Google Scholar]
- 41.Jobanputra K, Parker LA, Azih C et al. Factors associated with virological failure and suppression after enhanced adherence counselling, in children, adolescents and adults on antiretroviral therapy for HIV in Swaziland. PLoS One 2015; 10:e0116144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.