In a multisite, randomized, double-blinded, placebo-controlled pivotal efficacy trial, we documented significant protection against moderate and severe cholera subsequent to experimental challenges with wild-type Vibrio cholerae O1 El Tor at 10 days and 3 months after vaccination.
Keywords: cholera, vaccine, volunteer, challenge, efficacy
Abstract
Background. No licensed cholera vaccine is presently available in the United States. Cholera vaccines available in other countries require 2 spaced doses. A single-dose cholera vaccine that can rapidly protect short-notice travelers to high-risk areas and help control explosive outbreaks where logistics render 2-dose immunization regimens impractical would be a major advance.
PXVX0200, based on live attenuated Vibrio cholerae O1 classical Inaba vaccine strain CVD 103-HgR, elicits seroconversion of vibriocidal antibodies (a correlate of protection) within 10 days of a single oral dose. We investigated the protection conferred by this vaccine in a human cholera challenge model.
Methods. Consenting healthy adult volunteers, 18–45 years old, were randomly allocated 1:1 to receive 1 oral dose of vaccine (approximately 5 × 108 colony-forming units [CFU]) or placebo in double-blind fashion. Volunteers ingested approximately 1 × 105 CFU of wild-type V. cholerae O1 El Tor Inaba strain N16961 10 days or 3 months after vaccination and were observed on an inpatient research ward for stool output measurement and management of hydration.
Results. The vaccine was well tolerated, with no difference in adverse event frequency among 95 vaccinees vs 102 placebo recipients. The primary endpoint, moderate (≥3.0 L) to severe (≥5.0 L) diarrheal purge, occurred in 39 of 66 (59.1%) placebo controls but only 2 of 35 (5.7%) vaccinees at 10 days (vaccine efficacy, 90.3%; P < .0001) and 4 of 33 (12.1%) vaccinees at 3 months (vaccine efficacy, 79.5%; P < .0001).
Conclusions. The significant vaccine efficacy documented 10 days and 3 months after 1 oral dose of PXVX0200 supports further development as a single-dose cholera vaccine.
Clinical Trials Registration. NCT01895855
(See the Editorial Commentary by Harris on pages 1336–7.)
Cholera remains a public health problem among underprivileged populations in many developing countries. Two oral cholera vaccines (OCVs) are licensed in some countries but not the United States, where no cholera vaccine is currently available. Dukoral (Crucell), containing inactivated Vibrio cholerae O1 with recombinant B subunit of cholera toxin (CT), is administered as 2 doses, 1–2 weeks apart; 3 doses are required for children 2–6 years of age. Shanchol (Shantha), containing inactivated V. cholerae O1 and O139, is administered as 2 doses, 2 weeks apart. Dukoral is mainly used to immunize travelers from Europe, whereas Shanchol is intended for control of cholera in developing-country populations.
For travelers on short notice to areas of intense cholera transmission, an OCV that rapidly confers protection after a single dose would be advantageous [1]. Such a vaccine would also be useful for reactive mass vaccination to control cholera in explosive unsettled “virgin soil” epidemics where administering >1 dose is logistically challenging [2].
CVD 103-HgR is a live attenuated V. cholerae serogroup O1, serotype Inaba, classical biotype strain in which the toxigenic A1 (ADP-ribosylating) subunit of CT was deleted and only the nontoxic, immunogenic B (binding) subunit of CT is synthesized [3–5]. The original manufacturer of CVD 103-HgR, the Swiss Serum and Vaccine Institute (SSVI), commercialized the vaccine as Orochol (Switzerland, New Zealand, Australia, and several other countries) and as Mutacol (Canada) to protect travelers, and initiated the licensure process for the US Food and Drug Administration (FDA). FDA licensure was never completed, as SSVI became Berna Biotech in 2000 and manufacture ceased when Crucell acquired Berna Biotech in 2004. In 2009, PaxVax, Inc obtained rights to redevelop CVD 103-HgR. PXVX0200, prepared from new CVD 103-HgR master and working cell banks, demonstrated safety and immunogenicity results similar to the former CVD 103-HgR formulations [6].
The present study, designed with guidance from the FDA, represents the pivotal efficacy trial for FDA licensure of PXVX0200. We used a closely monitored human infection model involving the ingestion of virulent V. cholerae O1 El Tor Inaba strain N16961, 10 days or 3 months after vaccination. The primary endpoint for efficacy was the prevention of moderate (≥3.0 L) to severe (≥5.0 L) cholera diarrhea. Without rehydration therapy, this endpoint would represent potentially life-threatening fluid losses, as the total adult plasma volume approximates 3 L. To identify a possible correlate of protection, we explored the relationship between the vibriocidal antibody responses following vaccination and the clinical outcome of diarrhea following challenge.
METHODS
Study Design
This study was approved by institutional review boards at the 3 centers (Baltimore, Maryland; Cincinnati, Ohio; and Burlington, Vermont). Written informed consent was obtained from healthy adults 18–45 years of age screened for eligibility (shown in http://clinicaltrials.gov/show/NCT01895855). Because blood group O individuals are at higher risk for severe cholera (cholera gravis) [7–9], the study population was enriched for blood group O volunteers to assess vaccine efficacy in these high-risk hosts.
Eligible volunteers randomized (1:1) to PXVX0200 vaccine or placebo fasted 60 minutes before and after ingesting the blinded product. Following vaccination, daily oral temperatures and solicited adverse events (AEs) were recorded over 7 days, including diarrhea, abdominal pain, nausea/vomiting, anorexia, headache, and tiredness. The occurrence of unsolicited AEs was recorded through 28 days after vaccination or challenge, whichever was later, and serious AEs through day 180. Blood was collected on days 0, 7, 10, 28, and 180 for participants who were not challenged. Volunteers challenged at day 10 postvaccination had blood collected on days 0, 7, 10 (before challenge), 38 (28 days after challenge), and 180, while volunteers challenged 3 months postvaccination had blood collected on days 0, 7, 10, 28, 90 (before challenge), 118 (28 days after challenge), and 180.
Vaccine
Single-dose PXVX0200 sachets with lyophilized powder containing approximately 5 × 108 colony-forming units (CFU) of CVD 103-HgR were produced according to current good manufacturing practice (cGMP) by PaxVax, Inc. An accompanying buffer powder sachet contained approximately 2.5 g sodium bicarbonate (NaHCO3), approximately 1.6 g ascorbic acid, and approximately 0.2 g lactose. Powders from the vaccine/buffer sachet were suspended in 100 mL of bottled water. Placebo consisted of 100 mL of normal saline.
Challenge
A target of 60% of the total challenge population was to be persons of blood group O. Participants were admitted to an inpatient research ward 1–2 days prior to challenge to complete the screening process. The challenge inoculum was prepared from a frozen cGMP lot vial containing virulent V. cholerae O1 El Tor Inaba strain N16961 [10]. A vial was thawed and appropriately diluted, and the inoculum size was confirmed by quantitative counts. Subjects fasted overnight prior to the ingestion of 120 mL of NaHCO3 solution to neutralize gastric acid followed 1 minute later by ingestion of approximately 1 × 105 CFU of the challenge strain suspended in 30 mL of NaHCO3 solution.
Following challenge, volunteers were closely monitored for illness and every stool was graded: grade 1, firm; grade 2, soft; grade 3, thick liquid; grade 4, opaque watery; and grade 5, rice water. All stools grade 3 or greater were weighed; a gram of loose stool was assumed to be equal to 1 mL volume. Individuals who developed diarrhea were given glucose/electrolytes oral rehydration solution (Jianas Brothers, Kansas City, Missouri) at a volume 1.5 times the diarrheal stool volume. Participants unable to ingest sufficient oral rehydration solution to maintain hydration were given intravenous Lactated Ringer's solution. Ciprofloxacin, 500 mg twice daily for 5 days, was administered when a subject reached 5.0 L of cumulative diarrheal stool output or on day 4 postchallenge, whichever occurred first.
Definition of Diarrhea
For evaluating reactogenicity following vaccination, diarrhea was defined as ≥4 loose stools within a 24-hour period; following challenge, diarrhea, as an efficacy endpoint, was defined as the passage of ≥2 loose stools (grade 3–5) over a 48-hour period ≥200 mL or a single loose stool ≥300 mL. Moderate or severe diarrhea was defined as the passage of at least 3.0 L or 5.0 L of loose stool, respectively.
Bacteriology
Stool specimens were inoculated onto thiosulfate citrate bile salts sucrose (TCBS) agar plates (Eiken, Tokyo, Japan) either directly or after overnight incubation enrichment in alkaline peptone water before plating onto TCBS agar. Up to 2 stools daily were cultured to determine the number of organisms per gram of stool [11]. A rectal swab was obtained if no stool was passed. Suspicious colonies were agglutinated with polyvalent anti-O1 antisera (Remel, Lenexa, Kansas).
Immunology
Serum specimens were tested for classical Inaba vibriocidal antibody [12, 13], with seroconversion defined as ≥4-fold rise in titer over baseline.
Statistical Analysis
Demonstration of efficacy, and their confidence intervals (CIs) [14], against moderate to severe cholera (MSC) at 10 and 90 days postvaccination were the prespecified co–primary aims. Vibriocidal antibody results are summarized by the number and percentage of seroconversions and the geometric mean titer (GMT) with 95% CI at each timepoint. In summarizing safety, categorical endpoints were compared using Fisher exact test, whereas continuous endpoints were compared using Student t test if normally distributed and Wilcoxon rank-sum test if not normally distributed. All P values were derived from 2-sided tests and P ≤ .05 was considered significant. Demographic and safety comparisons were not adjusted for multiplicity.
RESULTS
Participants
In total, 197 enrolled volunteers were randomized, 95 to vaccine and 102 to placebo; 63% were male and the mean age was 31 years (range, 18–45 years) (Table 1). Subsequently, 68 subjects were challenged 10 days after vaccination and 66 subjects 3 months after vaccination (consort diagram, Supplementary Figure 1). Subjects selected to be challenged were prioritized based on continued eligibility, availability, and blood type. There was no knowledge of vibriocidal antibody response at the time of challenge.
Table 1.
Subject Demographics
| Characteristic | Challenged |
Not Challenged |
||||
|---|---|---|---|---|---|---|
| Vaccine Day 10 (n = 35) | Placebo Day 10 (n = 33) | Vaccine 3 mo (n = 33) | Placebo 3 mo (n = 33) | Vaccine (n = 27) | Placebo (n = 36) | |
| Age, y | ||||||
| Mean (SD) | 30.5 (6.7) | 31.6 (8.4) | 33.1 (8.2) | 30.3 (7.7) | 30.8 (8.3) | 29.8 (7.5) |
| Median (Min, Max) | 31 (18, 45) | 31 (20, 45) | 32 (18, 45) | 32 (18, 45) | 29 (18, 45) | 28.5 (18, 44) |
| Sex, No. (%) | ||||||
| Female | 10 (28.6) | 15 (45.5) | 6 (18.2) | 13 (39.4) | 11 (40.7) | 18 (50.0) |
| Male | 25 (71.4) | 18 (54.5) | 27 (81.8) | 20 (60.6) | 16 (59.3) | 18 (50.0) |
| Ethnicity, No. (%) | ||||||
| Hispanic/Latino | 2 (5.9) | 1 (3.0) | 1 (3.0) | 1 (3.1) | 2 (7.4) | 2 (5.6) |
| Not Hispanic/Latino | 32 (94.1) | 32 (97.0) | 32 (97.0) | 31 (96.9) | 25 (92.6) | 34 (94.4) |
| Race, No. (%) | ||||||
| Black/African American | 21 (60.0) | 21 (63.6) | 27 (81.8) | 26 (78.8) | 16 (59.3) | 22 (61.1) |
| White | 10 (28.6) | 11 (33.3) | 6 (18.2) | 7 (21.2) | 10 (37.0) | 14 (38.9) |
| Other/unknown | 4 (11.4) | 1 (3.0) | 0 | 0 | 1 (3.7) | 0 |
| Blood group, No. (%) | ||||||
| O | 19 (54.3) | 19 (57.6) | 20 (60.6) | 17 (51.5) | 9 (33.3) | 15 (41.7) |
| Non-O | 16 (45.7) | 14 (42.4) | 13 (39.4) | 16 (48.5) | 18 (66.7) | 21 (58.3) |
Abbreviation: SD, standard deviation.
Vaccine Safety
The vaccine was well tolerated; the frequency of diarrhea during the 7 days after vaccine or placebo was 1.1% and 3.0%, respectively. There were no significant differences in the reports of asthenia, headache, abdominal pain, anorexia, nausea/vomiting, fever, or diarrhea (Supplementary Table 1).
There were 23 and 35 unsolicited AEs reported by 17 vaccinees and 17 placebo recipients, respectively. The single severe AE, back pain, occurred 1 day after receipt of placebo. All serious AEs were self-limited and resolved within several days, and none were deemed to be vaccine-related. Clinical laboratory results did not indicate any safety signals.
Vaccine Immunogenicity
The overall serum Inaba vibriocidal antibody seroconversion rate following vaccination was 90.4% (85 of 94 vaccinees), with 84 of 85 seroconversions evident by day 10 postvaccination, when GMT peaked at 4313 (95% CI, 2873–6476; Table 2). There was no significant difference in vibriocidal antibody seroconversion or GMTs between blood group O or non-O vaccinees. Among placebo recipients, 2 of 102 exhibited vibriocidal seroconversion (Table 2).
Table 2.
Serum Inaba Vibriocidal Antibody Responses to Vaccination, by Day Relative to Vaccination
| Blinded Study Product |
Baseline | Day 7 | Day 10 | Day 28a | Day 90a | Day 180a |
|---|---|---|---|---|---|---|
| No. With Seroconversion (%) | ||||||
| Vaccine | n = 94 | 75 (79.8) | 84 (89.4) | 85 (90.4) | 85 (90.4) | 85 (90.4) |
| Placebo | n = 102 | 2 (2.0) | 2 (2.0) | 2 (2.0) | 2 (2.0) | 2 (2.0) |
| Geometric mean titer (95% CI) | ||||||
| Vaccine | 46.0 (36.5–58.1) | 830.8 (554.5–1245) | 4313 (2873–6476) | 1394 (866.4–2242) | 270.5 (158.3–462.2) | 155.4 (82.2–293.9) |
| Placebo | 63.1 (47.5–83.7) | 65.4 (48.3–88.6) | 64.8 (47.8–87.9) | 50.6 (35.9–71.2) | 48.3 (29.8–78.5) | 62.2 (35.9–107.7) |
Abbreviation: CI, confidence interval.
a Individuals challenged on day 10 were not included in the calculation of 28-, 90-, and 180-day postvaccination immune responses; individuals challenged at 3 months were not included in the calculation of 180-day postvaccination immune responses.
Clinical Response to Challenge at 10 Days and 3 Months Postvaccination
Challenge with virulent V. cholerae O1 elicited MSC diarrhea in 39 of 66 (59.1%) placebo recipients but in only 2 of 35 (5.7%) vaccinees challenged at 10 days (P < .0001; efficacy 90.3%) postvaccination and in only 4 of 33 (12.1%) vaccinees challenged 3 months postvaccination (P < .0001; efficacy 79.5%) (Table 3). The efficacy against MSC among the high-risk blood group O volunteers at 10 days and 3 months was 84.8% (95% CI, 50.4%–100%) and 78.4% (95% CI, 44.2%–100%), respectively. Independent analyses of the 10-day and 3-month data are shown in Supplementary Table 2.
Table 3.
Clinical and Bacteriologic Responses to Challenge
| Vaccine Efficacy (95% CI) orP Value |
||||||
|---|---|---|---|---|---|---|
| Clinical or Bacteriological Endpoint | All Vaccinees | Vaccine Day 10 | Vaccine 3 mo | All Placebos | Day 10 | 3 mo |
| Total challenged | 68 | 35 | 33 | 66 | ||
| No. with severe cholera (%) | 3 (4.4) | 1 (2.9) | 2 (6.1) | 28 (42.4) | 93.3% (56.2%–100%) | 85.7% (46.2%–100%) |
| No. with moderate or severe cholera (%)a | 6 (8.8) | 2 (5.7) | 4 (12.1) | 39 (59.1) | 90.3% (61.7%–100%) | 79.5% (49.1%–100%) |
| No. with mild cholerab or worse (%) | 20 (29.4) | 5 (14.3) | 15 (45.5) | 61 (92.4) | 84.5% (67.0%–100%) | 50.8% (33.6%–66.8%) |
| Median diarrheal stool volume, mL (IQR) | 0 (0–311) | 0 (0–0) | 178 (0–988) | 4377 (1563–8087) | <.0001 | <.0001 |
| Median number of diarrheal stools (IQR) | 0 (0–3.8) | 0 (0–0) | 2.0 (0–5.5) | 22.5 (9.8–39.3) | <.0001 | <.0001 |
| Median peak Vibrio cholerae O1 count, CFU/mL (IQR) | 4.2 × 101 (0–3.5 × 105) | 0 (0–2.7 × 103) | 9.7 × 104 (0–1.7 × 107) | 3.2 × 107 (6.1 × 106–1.2 × 108) | <.0001 | <.0001 |
| No. with fever (%)c | 3 (4.4) | 1 (2.9) | 2 (6.1) | 18 (27.3) | .0025 | .0159 |
| No. with nausea/vomiting (%) | 16 (23.5) | 9 (25.7) | 7 (21.2) | 38 (57.6) | .0032 | .0006 |
| No. with abdominal cramping (%) | 18 (26.5) | 8 (22.9) | 10 (30.3) | 45 (68.2) | <.0001 | .0005 |
| No. with malaise (%) | 16 (23.5) | 8 (22.9) | 8 (24.2) | 41 (62.1) | .0003 | .0006 |
Abbreviations: CFU, colony-forming units; CI, confidence interval; IQR, interquartile range.
a Moderate and severe cholera (study primary endpoint) is defined as ≥3 L and ≥5 L of cumulative diarrheal stool, respectively.
b Mild diarrhea is defined as the passage of ≥2 unformed stools (grade 3–5) over a 48-hour period that equals or exceeds 200 mL or a single unformed stool of ≥300 mL and <3 L total diarrhea.
c Fever is defined as ≥38.0°C (100.4°F).
The median volume and number of diarrheal stools and median peak stool excretion of cholera vibrios were lower among vaccinees challenged at 10 days (P < .0001) and 3 months (P < .0001), compared to controls (Table 3). The incidence of fever, nausea/vomiting, abdominal cramping, and malaise was also lower among vaccinees challenged at 10 days and 3 months (Table 3).
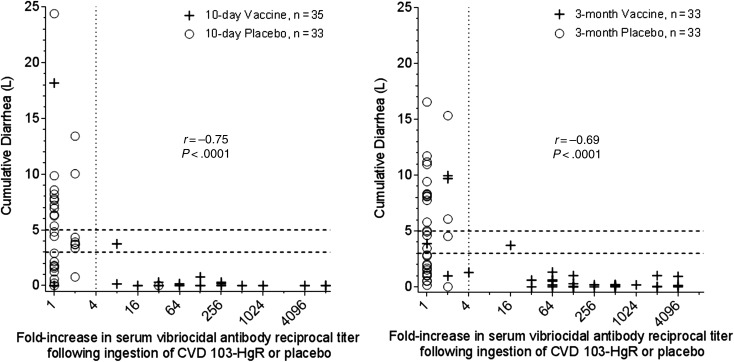
Correlation of Vibriocidal Antibody Response to Clinical Outcome
There was a strong correlation between serum vibriocidal antibody seroconversion and protection against MSC. Only 2 of the 62 (3.2%) vaccinees who manifested ≥4-fold titer rise had MSC vs 4 of the 6 (75%) vaccinees who failed to seroconvert (P = .00026; Figure 1). The 2 vaccinees who developed MSC despite seroconversion exhibited only modest reciprocal titers (80 and 160) postvaccination. Indeed, no vaccinee who seroconverted and achieved a day 10 titer ≥320 experienced MSC (Supplementary Figure 2).
Figure 1.
Correlation of serum vibriocidal titer fold-increase in response to vaccination and the cumulative diarrheal purge volume in response to cholera challenge.
DISCUSSION
Cholera causes fear for its ability to rapidly dehydrate (leading to hypovolemic shock and death unless rehydration is promptly instituted), propensity for explosive outbreaks, and pandemic behavior. With careful organization, 2-dose OCVs can be delivered to at-risk populations preemptively. By contrast, a single-dose vaccine is more practical for travelers and for reactive vaccination to control explosive epidemics, particularly in unsettled situations [2].
This study documents protection of US adults against potent experimental cholera challenge that caused MSC diarrhea (≥3.0 L) in 59% of control subjects overall and in 69% of high-risk blood group O controls, reproducing results observed with the previous commercial formulation of CVD 103-HgR in North American adults [1, 3, 15, 16]. Because travelers to high-risk cholera areas often must leave on short notice, some subjects were challenged a mere 10 days after vaccination and a high level of protection (90% vaccine efficacy) was shown. This corroborates past studies that showed efficacy within 8–10 days postvaccination [15]. Collectively, results of these multiple challenges with 2 distinct commercial formulations of CVD 103-HgR (Orochol/Mutacol and PXVX0200) constitute convincing evidence of the vaccine's ability to elicit protection rapidly.
The vibriocidal antibodies manifested by immunologically naive hosts such as North Americans following vaccination or cholera illness are mostly immunoglobulin M [17], and decline rapidly to approach baseline within 1–6 months [13]. Because initial experimental cholera illness protects for ≥3 years against rechallenge [18], the persistence of high vibriocidal antibody titers per se is not a prerequisite for protection. This study provides evidence that in immunologically naive North Americans serum vibriocidal antibody seroconversion is a reliable correlate of protection and may constitute a surrogate for an as yet uncharacterized mechanistic intestinal protective response [19].
Enterotoxigenic V. cholerae O1 resides in brackish water environmental reservoirs where horizontal gene transfer can ensue. N16961, a V. cholerae O1 El Tor Inaba strain from Bangladesh, has been the benchmark challenge strain since the National Institute of Allergy and Infectious Diseases prepared a cGMP frozen lot to allow multisite challenges with a standardized inoculum, thereby permitting insights to be drawn on the relative efficacy of different vaccines and formulations by either concurrent or historical comparisons [10]. N16961, which expresses El Tor CT and toxin coregulated pili (TCP), differs from many currently circulating El Tor “hybrid strains” that express classical biotype cholera enterotoxin B subunit and sometimes classical TCP [20–24]. Some hybrid strains are hypertoxigenic in vitro [24] and may be clinically more virulent [25], thereby resembling classical biotype strains [26]. A hybrid El Tor strain cGMP inoculum is not available for challenge studies. However, the genetic changes in the hybrid strains should not diminish the ability of CVD 103-HgR to protect, as it is classical biotype and encodes both classical CT B subunit and TCP. In past challenges, a single oral dose of either CVD 103-HgR or its progenitor CVD 103 (before a gene encoding resistance to Hg++ was inserted into the hlyA locus) conferred significant protection against challenge with classical biotype strains that produce classical CT, including the highly toxigenic Ogawa 395 and Inaba 569B strains [1, 3, 15].
PXVX0200 is well tolerated and highly immunogenic in stimulating vibriocidal antibody, corroborating the previous safety and immunogenicity record of CVD 103-HgR. A phase 3 trial of 3 different production lots of PXVX0200 (NCT02094586) and a phase 3 trial in elderly adults (NCT02100631) are being conducted that will provide additional data assessing the clinical tolerability and immunogenicity of PXVX0200 as a cholera vaccine for travelers from the United States. Importantly for future clinical trials in other venues and populations, serum vibriocidal seroconversion, an immunologic correlate that significantly predicted protection, has been accepted by the FDA as the regulatory criterion for immunologic bridging. Only a volunteer challenge study that measures baseline and postvaccination sera from all challenged vaccinees and records all cases following a “point-source exposure” has the unique ability to identify seroconversion as a correlate of protection [27]. Because the low incidence of cholera infection in travelers makes field efficacy trials in travelers infeasible, a placebo-controlled cholera challenge study in healthy volunteers was the only practical way of demonstrating clinical efficacy of the vaccine in the relevant population. If PXVX0200 is approved by the FDA, this would be the first vaccine where demonstration of clinical efficacy was based on a challenge study in healthy volunteers, although other national regulatory authorities (eg, of Canada, Switzerland, Australia) licensed the previous commercial formulation of CVD 103-HgR on efficacy data from challenge studies. This could be a model for other travel vaccines where disease incidence precludes a field efficacy trial and no relevant animal model exists.
Previously, a formulation (Orochol E) containing 1-log higher dosage (109 CFU) was needed to achieve adequate immunogenicity in persons living in underprivileged conditions in developing countries [28–30]. We are undertaking clinical studies with a PXVX0200 formulation containing 1-log higher CFU for developing-country populations to assess its future utility for reactive vaccination campaigns, preemptive curtailment of seasonal epidemics, and protecting populations in refugee camps and similar high-risk venues.
Two previous studies addressed the ability of Orochol E (109 CFU) to prevent cholera in developing-country subjects. A large-scale, double-blind randomized (at the level of the individual), placebo-controlled field trial was conducted in a densely populated cholera-hyperendemic area in North Jakarta, Indonesia, where 33 696 participants received vaccine and 33 812 received placebo [31]. Communities with the highest incidence of cholera in the previous 4 years within the endemic subdistricts were targeted for inclusion. The overall point estimate of vaccine efficacy was only 14%, over 4 years of follow-up (1993–1997). However, following the vaccination phase of this field trial, there was a significant fall in the number of cholera cases in the population compared to the 4 years before the field trial. Whereas several hundred cholera cases were expected to occur among placebo controls during the 4 years of follow-up, only 50 cases were detected despite intensive surveillance. This suggested that administration of OCV to a notable proportion of the high-risk target population had diminished the overall risk of cholera in these areas. At the time of that trial there was no explanation for this, as the currently appreciated powerful effect of indirect protection with OCV had not yet been reported [32, 33]. A reanalysis of the mid-1980s field trial of inactivated OCVs in Bangladesh first elucidated the potent indirect protection that OCVs can achieve [32].
Thus, the striking reduction of cholera cases in the North Jakarta field trial may be explained by the expected powerful additional effect of indirect protection. This concept of indirect protection is now so widely accepted that for more than a decade, large-scale randomized controlled field trials of cholera [34] and typhoid vaccines [35, 36] have used cluster randomized study designs to preclude what transpired in the North Jakarta trial [31].
A second opportunity to evaluate the ability of CVD 103-HgR to prevent cholera occurred during a cholera epidemic on the island of Pohnpei in Micronesia [2]. Subsequent to a reactive mass immunization campaign using a single oral dose of Orochol E conducted by the World Health Organization (WHO), the incidence of suspected cholera cases was 5 times lower among vaccinated compared with unvaccinated persons. WHO epidemiologists estimated that 45% of the island's population was vaccinated and calculated 79% vaccine effectiveness (95% CI, 72%–85%) in preventing cholera under field conditions. Based on these data, there is optimism that the higher-dosage PXVX0200 formulation may be an effective, logistically practical means for cholera control in developing-country populations.
Our first priority for PXVX0200 is to achieve FDA licensure so that travelers from the United States can have access to a cholera vaccine. Although the incidence of cholera among US travelers is not typically high, during certain periods, as when cholera returned to South America in 1991 after a century of absence [37] and during the postearthquake epidemic in Haiti [38], travel-associated US cases markedly increased. Looking to the future, if licensed, we would encourage that PXVX0200 be particularly targeted for at-risk travelers to endemic or epidemic regions (especially those far from healthcare or visiting friends or relatives [39, 40]), hosts especially vulnerable to severe cholera such as individuals of blood group O or with hypochlorhydria (eg, from medications that suppress or neutralize gastric acid), and persons at risk for complications, such as individuals with cardiac or renal impairment.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We are grateful for the participation of subjects and care provided by staff at each of the clinical sites. We thank the clinical and laboratory personnel who supported this study at University of Maryland, Baltimore: Melissa Billington, Lisa Chrisley, Sofie Livio, Mardi Reymann; Cincinnati Children's Hospital Medical Center: Amy Hoeper, Michelle Dickey, Susan Parker; University of Vermont: Cathy Larsson, Libby Lucas, Marya Carmolli; members of the Safety Monitoring Committee (Drs David Sack, Herbert DuPont, and Jason Harris); staff at Theorem Clinical Research for help in data management (Rebecca Schwarz, Mat Davis, and Lisa Moore); and the staff at Focus Diagnostics for the vibriocidal antibody assays (Jason Fernandez, Kenneth Van Horn, Keith Gottlieb, and Sarah Daijogo). Maggie Sisti at PaxVax was instrumental in coordinating this multisite study.
Disclaimer. The views expressed are solely those of the authors and does not represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Financial support. This work was fully funded by PaxVax, Inc.
Potential conflicts of interest. M. G., M. L., and J. K. S. were employed by PaxVax, Inc. W. H. C., M. B. C., B. D. K., R. C. B., D. G., R. A. K., C. E. L., F. S., and M. M. L. received research funding from PaxVax, Inc. M. M. L. is the patent holder of CVD 103-HgR. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Levine MM, Kaper JB. Live oral cholera vaccine: from principle to product. Bull Inst Pasteur 1995; 93:243–53. [Google Scholar]
- 2.Calain P, Chaine JP, Johnson E et al. Can oral cholera vaccination play a role in controlling a cholera outbreak? Vaccine 2004; 22:2444–51. [DOI] [PubMed] [Google Scholar]
- 3.Levine MM, Kaper JB, Herrington D et al. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet 1988; 2:467–70. [DOI] [PubMed] [Google Scholar]
- 4.Ketley JM, Michalski J, Galen J, Levine MM, Kaper JB. Construction of genetically marked Vibrio cholerae O1 vaccine strains. FEMS Microbiol Lett 1993; 111:15–21. [DOI] [PubMed] [Google Scholar]
- 5.Kaper JB, Levine MM. Recombinant attenuated Vibrio cholerae strains used as live oral vaccines. Res Microbiol 1990; 141:901–6. [DOI] [PubMed] [Google Scholar]
- 6.Chen WH, Greenberg RN, Pasetti MF et al. Safety and immunogenicity of single-dose live oral cholera vaccine strain CVD 103-HgR, prepared from new master and working cell banks. Clin Vaccine Immunol 2014; 21:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhuri A, De S. Cholera and blood-groups. Lancet 1977; 2:404. [DOI] [PubMed] [Google Scholar]
- 8.Barua D, Paguio AS. ABO blood groups and cholera. Ann Hum Biol 1977; 4:489–92. [DOI] [PubMed] [Google Scholar]
- 9.Levine MM, Nalin DR, Rennels MB et al. Genetic susceptibility to cholera. Ann Hum Biol 1979; 6:369–74. [DOI] [PubMed] [Google Scholar]
- 10.Sack DA, Tacket CO, Cohen MB et al. Validation of a volunteer model of cholera with frozen bacteria as the challenge. Infect Immun 1998; 66:1968–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rennels MB, Levine MM, Daya V, Angle P, Young C. Selective vs. nonselective media and direct plating vs. enrichment technique in isolation of Vibrio cholerae: recommendations for clinical laboratories. J Infect Dis 1980; 142:328–31. [DOI] [PubMed] [Google Scholar]
- 12.Benenson AS, Saad A, Mosley WH. Serological studies in cholera. 2. The vibriocidal antibody response of cholera patients determined by a microtechnique. Bull World Health Organ 1968; 38:277–85. [PMC free article] [PubMed] [Google Scholar]
- 13.Clements ML, Levine MM, Young CR et al. Magnitude, kinetics, and duration of vibriocidal antibody responses in North Americans after ingestion of Vibrio cholerae. J Infect Dis 1982; 145:465–73. [DOI] [PubMed] [Google Scholar]
- 14.Farrington CP, Gay NJ. Interval-censored survival data with informative examination times: parametric models and approximate inference. Stat Med 1999; 18:1235–48. [DOI] [PubMed] [Google Scholar]
- 15.Tacket CO, Losonsky G, Nataro JP et al. Onset and duration of protective immunity in challenged volunteers after vaccination with live oral cholera vaccine CVD 103-HgR. J Infect Dis 1992; 166:837–41. [DOI] [PubMed] [Google Scholar]
- 16.Tacket CO, Cohen MB, Wasserman SS et al. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El Tor Inaba three months after vaccination. Infect Immun 1999; 67:6341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Losonsky GA, Yunyongying J, Lim V et al. Factors influencing secondary vibriocidal immune responses: relevance for understanding immunity to cholera. Infect Immun 1996; 64:10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine MM, Black RE, Clements ML, Cisneros L, Nalin DR, Young CR. Duration of infection-derived immunity to cholera. J Infect Dis 1981; 143:818–20. [DOI] [PubMed] [Google Scholar]
- 19.Saha D, LaRocque RC, Khan AI et al. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J Infect Dis 2004; 189:2318–22. [DOI] [PubMed] [Google Scholar]
- 20.Mukhopadhyay AK, Takeda Y, Balakrish Nair G. Cholera outbreaks in the El Tor biotype era and the impact of the new El Tor variants. Curr Top Microbiol Immunol 2014; 379:17–47. [DOI] [PubMed] [Google Scholar]
- 21.Mutreja A, Kim DW, Thomson NR et al. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature 2011; 477:462–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair GB, Qadri F, Holmgren J et al. Cholera due to altered El Tor strains of Vibrio cholerae O1 in Bangladesh. J Clin Microbiol 2006; 44:4211–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morita M, Ohnishi M, Arakawa E et al. Emergence and genetic diversity of El Tor Vibrio cholerae O1 that possess classical biotype ctxB among travel-associated cases of cholera in Japan. J Med Microbiol 2010; 59:708–12. [DOI] [PubMed] [Google Scholar]
- 24.Son MS, Megli CJ, Kovacikova G, Qadri F, Taylor RK. Characterization of Vibrio cholerae O1 El Tor biotype variant clinical isolates from Bangladesh and Haiti, including a molecular genetic analysis of virulence genes. J Clin Microbiol 2011; 49:3739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddique AK, Nair GB, Alam M et al. El Tor cholera with severe disease: a new threat to Asia and beyond. Epidemiol Infect 2010; 138:347–52. [DOI] [PubMed] [Google Scholar]
- 26.Bart KJ, Huq Z, Khan M, Mosley WH. Seroepidemiologic studies during a simultaneous epidemic of infection with El Tor Ogawa and classical Inaba Vibrio cholerae. J Infect Dis 1970; 121(suppl 121):17+. [DOI] [PubMed] [Google Scholar]
- 27.Pasetti MF, Levine MM. Insights from natural infection-derived immunity to cholera instruct vaccine efforts. Clin Vaccine Immunol 2012; 19:1707–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotuzzo E, Butron B, Seas C et al. Safety, immunogenicity, and excretion pattern of single-dose live oral cholera vaccine CVD 103-HgR in Peruvian adults of high and low socioeconomic levels. Infect Immun 1993; 61:3994–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol 2010; 8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suharyono, Simanjuntak C, Witham N et al. Safety and immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR in 5–9-year-old Indonesian children. Lancet 1992; 340:689–94. [DOI] [PubMed] [Google Scholar]
- 31.Richie EE, Punjabi NH, Sidharta YY et al. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine 2000; 18:2399–410. [DOI] [PubMed] [Google Scholar]
- 32.Ali M, Emch M, von Seidlein L et al. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet 2005; 366:44–9. [DOI] [PubMed] [Google Scholar]
- 33.Longini IM Jr., Nizam A, Ali M, Yunus M, Shenvi N, Clemens JD. Controlling endemic cholera with oral vaccines. PLoS Med 2007; 4:e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sur D, Lopez AL, Kanungo S et al. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet 2009; 374:1694–702. [DOI] [PubMed] [Google Scholar]
- 35.Sur D, Ochiai RL, Bhattacharya SK et al. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Engl J Med 2009; 361:335–44. [DOI] [PubMed] [Google Scholar]
- 36.Khan MI, Soofi SB, Ochiai RL et al. Effectiveness of Vi capsular polysaccharide typhoid vaccine among children: a cluster randomized trial in Karachi, Pakistan. Vaccine 2012; 30:5389–95. [DOI] [PubMed] [Google Scholar]
- 37.Cholera situation in the Americas. An update. Epidemiol Bull 1991; 12:1–4. [PubMed] [Google Scholar]
- 38.Newton AE, Heiman KE, Schmitz A et al. Cholera in United States associated with epidemic in Hispaniola. Emerg Infect Dis 2011; 17:2166–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahon BE, Mintz ED, Greene KD, Wells JG, Tauxe RV. Reported cholera in the United States, 1992–1994: a reflection of global changes in cholera epidemiology. JAMA 1996; 276:307–12. [PubMed] [Google Scholar]
- 40.Steinberg EB, Greene KD, Bopp CA, Cameron DN, Wells JG, Mintz ED. Cholera in the United States, 1995–2000: trends at the end of the twentieth century. J Infect Dis 2001; 184:799–802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.