Abstract
The contribution of secondary lymphoid tissue homing central memory T cells (TCM) and peripheral tissue homing effector memory T cells (TEM) to allograft rejection is not known. We tested whether TEM is the principal subset responsible for allograft rejection due to the non-lymphoid location of target antigens. Skin allograft rejection was studied after transferring either CD8 TCM or TEM to wild type mice and to mice that lack secondary lymphoid tissues. We found that CD8 TCM and TEM were equally effective at rejecting allografts in wild type hosts. However, CD8 TEM were significantly better than TCM at rejecting allografts in the absence of secondary lymphoid tissues. CD8 TCM were dependent upon secondary lymphoid tissues more than TEM for optimal differentiation into effectors that migrate into the allograft. Recall of either CD8 TCM or TEM led to accumulation of TEM after allograft rejection. These findings indicate that either CD8 TCM or TEM mediate allograft rejection but TEM have an advantage over TCM in immune surveillance of peripheral tissues, including transplanted organs.
Keywords: T cells, memory, transplantation
Introduction
The alloimmune response is a T-cell dependent process that culminates in the rejection of transplanted organs. The immune repertoire of adult humans contains high frequencies of alloreactive memory T cells that play an important role in allograft rejection (1, 2). The presence of donor-specific memory T cells correlates with acute and chronic rejection in humans and hinders tolerance induction in experimental animals (1, 3–7). Immunosuppressive strategies that inhibit naïve T cells are ineffective against memory T cells because of several inherent advantages over their naïve counterparts such as markedly extended lifespan, lower activation threshold, increased proliferative capacity, rapid secretion of multiple effector cytokines, and homing to both lymphoid and non-lymphoid (peripheral) tissues (8–10).
Two subsets of memory T cells have been described in mice and humans based on their homing patterns and effector function: secondary lymphoid tissue-homing CD62Lhi CCR7hi central memory (TCM) T cells with delayed effector function, and peripheral tissue-homing CD62Llo CCR7lo effector memory (TEM) T cells with immediate effector function (11–14). The contribution of CD8 TCM and TEM subsets to protective immunity against bacterial and viral infections is dependent upon the type and location of pathogen. CD8 TCM are superior to TEM at clearing systemic lymphocytic choriomeningitis and vesicular stomatitis virus infections; CD8 TCM are equivalent to TEM at clearing Listeria monocytogenes infection; while CD8 TCM are inferior to TEM at clearing Sendai and vaccinia virus infections, in mice (12, 15–18). However, the relative importance of memory T cell subsets in allograft rejection is less clear (7, 19, 20). Understanding which memory T cell subset is the principal mediator in allograft rejection has biomedical significance because agents that inhibit lymphocyte trafficking are potential candidates for use in clinical transplantation (21).
We hypothesized that peripheral tissue-homing TEM is the principal memory subset responsible for allograft rejection since a transplanted organ represents not only a foreign antigen, but also a complex non-lymphoid peripheral tissue harboring antigen presenting cells (APCs) and endothelial cells that can modulate memory T cell behavior. To test this hypothesis, polyclonal alloreactive memory T cells generated in wild type (wt) hosts were sorted for CD8 TCM or TEM and transferred into wt and splenectomized alymphoplastic (aly/aly) recipients of skin allografts. Splenectomized aly/aly (aly/aly-spleen) mice lack all secondary lymphoid tissues and are therefore suitable for testing the function of transferred CD8 TCM and TEM in non-lymphoid tissues (22). We report that CD8 TCM and TEM were equally effective at mediating allograft rejection in wt hosts that have secondary lymphoid tissues. In contrast, CD8 TEM were significantly more effective than TCM at rejecting skin allografts in aly/aly-spleen hosts that lack all secondary lymphoid tissues. CD8 TEM infiltration into the allograft was independent of secondary lymphoid tissues whereas TCM were dependent upon secondary lymphoid tissues for optimal differentiation into effectors that migrate into the allograft. These results show that either CD8 TCM or TEM reject allografts effectively and CD8 TEM is the principal subpopulation responsible for immune surveillance of transplanted organs.
Materials and Methods
Mice
C57BL/6 (Thy1.2, H-2b; hereafter wt), B6.PL-Thy1a/Cy (Thy1.1, H-2b; hereafter Thy1.1) and BALB/c (H-2d) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Alymphoplasia mice (Map3k14−/−, Thy1.2, H-2b, hereafter aly/aly) were purchased from CLEA (Osaka, Japan) (23). All animals were maintained under SPF conditions and procedures were performed as per IACUC guidelines.
Splenectomy, skin transplantation and co-stimulation blockade
Splenectomy and partial thickness skin transplantation were performed using established techniques (24). Skin grafts were monitored daily and rejection was defined as > 90% graft necrosis. Wt recipients of skin allografts were treated after transplantation with CTLA4-Ig (0.25mg, i.p.) and anti-CD40L (MR1) (0.25mg, i.p.) (Bioexpress Inc, MA) (days 0, 2, 4 and 6). Kaplan-Meier survival analysis was used to assess differences in allograft survival and p value of < 0.05 was considered significant.
Generation, isolation, and adoptive transfer of memory T cells
Thy1.1 mice were immunized with 3 × 107 BALB/c splenocytes (i.p.). 6 – 12 weeks later, spleen and lymph node (LN) cells were enriched for T cells by negative selection via MACS (Miltenyi Biotec, Auburn, CA), and sorted for CD8+CD44highCD62Lhigh (CD8 TCM) and CD8+CD44highCD62Llow (CD8 TEM) populations (> 95% purity) on BD FACS Aria. 1.5 – 2 × 106 CD8 TCM or 4 – 6 × 105 CD8 TEM containing similar numbers of alloreactive IFNγ+ T cells were adoptively transferred in each experiment.
Cell harvest and enumeration after adoptive transfer
Adoptive hosts were sacrificed either at 8 days after transplantation (effector phase) or at 6 – 10 weeks after allograft rejection (secondary memory phase). Cells from spleen, lymph nodes, blood, liver, lungs, bone marrow and skin grafts were harvested as described (24–26). Harvested cells from tissues were counted and analyzed by flow cytometry after gating on CD8+Thy1.1+ population. Antigen-specific cells were analyzed by measuring BALB/c-reactive IFNγ+ T cells within the harvested CD8+Thy1.1+ population by flow cytometry after intracellular cytokine staining (24). Statistical analyses was performed using unpaired Student’s t test and differences with p < 0.05 were considered significant.
Flow cytometry, intracellular cytokine staining and in vivo cytotoxicity
Fluorochrome-tagged antibodies for flow cytometry were purchased from BD Pharmingen (San Diego, CA), eBioscience (San Diego, CA) and R&D systems (Minneapolis, MN). Intracellular IFNγ producing cells were detected after ex-vivo stimulation with BALB/c splenocytes (H-2d) for 6-hrs in the presence of Brefeldin A. In-vivo cytotoxicity of CD8 TCM and TEM was assessed in wt B6 mice pre-treated with anti-NK1.1 (PK136, 300µg) to deplete NK cells. 3-days later, sorted CD8 TCM (1.9 × 106) or TEM (0.5 × 106) containing similar numbers of BALB/c-reactive IFNγ+ T cells were transferred. Equal numbers of CFSE labeled syngeneic H-2b (5 × 106, 2µM, B6) and allogeneic H-2d (5 × 106, 0.2µM, BALB/c) splenocytes were injected. 12-hrs later, in-vivo target cell killing was measured as loss of allogeneic vs syngeneic cells in adoptive hosts of TCM or TEM compared to naïve controls using the formula: 100-[(%H-2d cells in memory T cell recipients/%H-2b cells in memory T cell recipients÷%H-2d cells in naïve mice/%H-2b cells in naïve mice) × 100] (27). Flow acquisition was performed on LSRII analyzers (BD Biosciences, San Diego, CA), and data analyzed using Flowjo software (Treestar, Ashland, OR).
Results
Characterization of polyclonal alloreactive CD8 memory T cell subsets
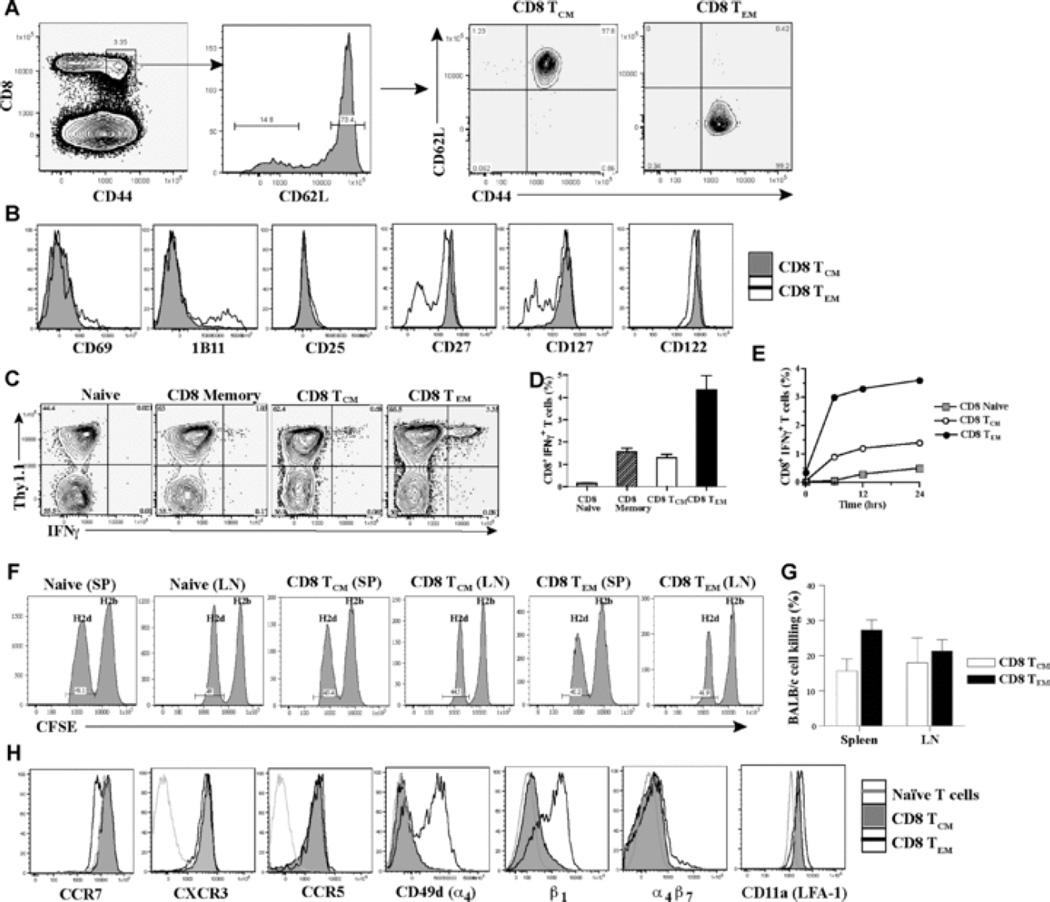
Alloreactive memory T cells were generated in wt hosts to study polyclonal CD8 TCM and TEM subsets. Briefly, B6 Thy1.1 mice were immunized with BALB/c splenocytes. 6 – 12 weeks later, spleen and LN cells were harvested and sorted for CD8 memory T cell subsets by gating on CD8+CD44highCD62Lhigh cells (CD8 TCM) and CD8+CD44highCD62Llow cells (CD8 TEM) (Fig. 1A). Phenotypic analysis showed that CD8 TEM contained a sub-population that is CD69high, 1B11high, CD27low and CD127low, suggesting a more activated phenotype and less dependence on co-stimulation and/or cytokines than TCM (Fig. 1B) (17, 28, 29). Ex-vivo alloreactive IFNγ production was used to detect antigen-specific cells within the polyclonal CD8 TCM and TEM subsets since antigen-specific MHC-tetramer+ cells in either subset were IFNγ+ upon ex-vivo recall in viral studies (12). BALB/c-reactive IFNγ+ T cells constituted 1.6 ± 0.3% of unfractionated CD8 memory T cells, 1.3 ± 0.3% of CD8 TCM, and 4.4 ± 1.3% of CD8 TEM subset (Figs. 1C–1D). Intracellular TNFα correlated with IFNγ but Granzyme B and IL-2 were not detected above background (data not shown) (12). BALB/c-reactive, IFNγ+ cells within CD8 TCM and TEM increased over time upon ex-vivo re-stimulation and were approximately 3 – 4 fold more in TEM than TCM subset (Fig. 1E). These findings suggest that CD8 TEM contained 3 – 4 fold more alloreactive memory T cells than TCM.
Fig. 1. Characterization of polyclonal alloreactive CD8 memory T cell subsets.
Thy1.1 mice were immunized with BALB/c splenocytes (3 × 107, i.p.) and spleen and LN cells were harvested 6 – 12 weeks later to sort for CD8 TCM and TEM. (A) Sorting of CD8 memory T cell subsets. CD8 memory T cell subsets were sorted after gating on CD8+CD44highCD62Lhigh cells (CD8 TCM) and CD8+CD44highCD62Llow cells (CD8 TEM). Sorted cells were tested for purity prior to adoptive transfer. (B) Phenotype of CD8 TCM and TEM. Expression of CD69, 1B11, CD25, CD27, CD127 and CD122 on CD8 TCM and TEM is shown (representative FACS plots of 5 – 6 experiments). (C) Ex-vivo IFNγ production by CD8 TCM and TEM. Sorted CD8 TCM and TEM were assessed for IFNγ production by intracellular cytokine staining after 5-hr ex-vivo stimulation with BALB/c splenocytes. IFNγ production within the Thy1.1+ population is shown after gating on CD8+ T cells. Naïve T cells from unimmunized Thy1.1 mice (Naïve) and unfractionated sorted CD8+ CD44high memory T cells (CD8 memory) from immunized Thy1.1 mice that contained both CD62Lhigh and CD62Llow cells were used as controls. Representative FACS plots of 4 – 5 experiments are shown. (D) Quantitation of alloreactive IFNγ+ T cells within CD8 TCM and TEM subsets after 5-hr ex-vivo stimulation with BALB/c splenocytes. Percent of IFNγ+ T cells within naïve, unfractionated CD8 memory (memory) and sorted CD8 TCM and TEM populations. Mean ± SD of 4 – 5 experiments. (E) Alloreactive IFNγ+ T cells within CD8 TCM and TEM subsets upon ex-vivo stimulation with BALB/c splenocytes over time. Percent of IFNγ+ T cells within naïve, sorted CD8 TCM and TEM populations after ex-vivo stimulation with BALB/c splenocytes at 6hrs, 12hrs and 24hrs. (F) In-vivo cytotoxicity of alloreactive CD8 TCM and TEM subsets in wt adoptive hosts. Wt mice were treated with anti-NK1.1 (PK136, 300µg, i.p.) to deplete NK cells. 3-days later, sorted CD8 TCM (1.9 × 106) or CD8 TEM (5 × 105) containing similar numbers of BALB/c-reactive IFNγ+ T cells were transferred to wt adoptive hosts followed by injection of equal numbers of CFSE labeled H-2b (5 × 106, 2µM B6, syngeneic) and H-2d (5 × 106, 0.2µM, BALB/c, allogeneic) splenocytes on the same day. 12-hrs later, in vivo killing of target cells was measured in harvested spleen and LN cells as loss of H-2d target cells compared to loss of H-2b syngeneic cells in adoptive hosts of memory T cells (TCM or TEM) versus naïve B6 control mice (as described in Materials and Methods) (representative FACS plots of n = 3 mice/grp are shown). (G) Quantitation of in-vivo specific lysis of allogeneic cells by alloreactive CD8 TCM and TEM subsets in wt adoptive hosts. Percent of BALB/c-cell lysis in harvested spleen and LNs from wt adoptive hosts of CD8 TCM or TEM. Mean ± SD of n = 3 mice/grp. (H) Expression of chemokine and adhesion receptors on CD8 TCM and TEM. Expression of CCR7, CXCR3, CCR5, CD49d (α4-integrin), β1-integrin, α4β7 and CD11a (LFA-1) on CD8 TCM and TEM subsets shown in comparison to CD8+ CD44low naïve T cells (representative FACS plots of 3 – 4 experiments).
In-vivo cytotoxicity of CD8 TCM and TEM was assessed after transfer to wt adoptive hosts pre-treated with anti-NK1.1 (PK136) antibody to inhibit NK cell mediated killing of allogeneic cells (30). Since CD8 TEM contained 3 – 4 fold more alloreactive IFNγ+ cells than TCM (Fig. 1D), 3 – 4 fold more total TCM (1.9 × 106) than TEM (0.5 × 106) were transferred to wt hosts to compare similar numbers of antigen-specific cells. Spleen and LN from adoptive hosts were analyzed to assess killing since splenocyte target cells were found mainly in these tissues and not in peripheral tissues (data not shown). BALB/c-cell killing by CD8 TEM was significantly better than TCM in the spleen (27 ± 3% vs 15 ± 3%, p = 0.04, n = 3, Figs. 1F–1G), and comparable to TCM in the LN (21 ± 3% vs 18 ± 7%, p = 0.1, NS, n = 3, Figs. 1F–1G). Therefore, both CD8 TCM and TEM killed allogeneic cells in-vivo but TEM were better cytotoxic cells than TCM.
Alloreactive CD8 TCM and TEM were assessed for the expression of chemokine and adhesion receptors that guide their migration and recruitment into inflammatory sites. As reported, CCR7 expression was higher on CD8 TCM than TEM cells (Fig. 1H) (12, 31). CXCR3, CCR5 and LFA-1 were upregulated on both subsets whereas VLA-4 (α4β1) expression was restricted to CD8 TEM (Fig. 1H). These findings suggest that both subsets can migrate in response to chemokines such as MIG, IP-10, I-TAC, MIP-1α, MIP-1β, and RANTES produced at inflammatory sites, including transplanted organs (32, 33). However, following migration, CD8 TEM that express VLA-4 (α4β1) can extravasate readily into allografts (34–38) and hence, might have an advantage over TCM in causing allograft rejection.
Allograft rejection in adoptive hosts of CD8 TCM and TEM
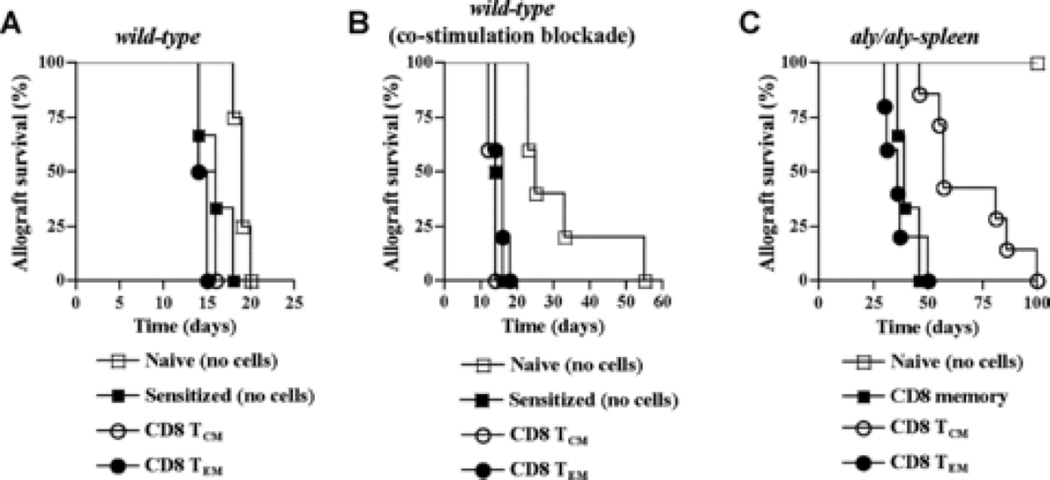
To test which alloreactive CD8 memory T cell subset is more effective at allograft rejection, skin allograft survival was compared in adoptive hosts of CD8 TCM and TEM. Sorted CD8 TCM and TEM (Fig. 1A) were transferred into wt recipients of BALB/c-skin allografts. Since CD8 TEM contained 3 – 4 fold more alloreactive IFNγ+ T cells than TCM (Fig. 1D), 3 – 4 fold more total TCM (1.5 × 106 – 2 × 106) than TEM (0.5 × 106 – 0.6 × 106) were transferred in each experiment to compare similar numbers of antigen-specific cells. Wt hosts of CD8 TCM or TEM rejected skin allografts comparable to sensitized recipients (MST = 15, 15, and 16 days, respectively, n = 3 – 4/grp; p = 0.4, NS), and not significantly different from naïve mice (MST = 18 days, n = 4; p = 0.06 and 0.4, respectively, NS) (Fig. 2A). Since endogenous naïve T cells also contributed to observed skin allograft rejection in wt hosts, recipients were treated with CTLA4-Ig and anti-CD40L to inhibit naïve T cell activation to better assess rejection mediated by transferred CD8 TCM and TEM (5, 6, 39). Recall of either CD8 TCM or TEM subset in wt recipients treated with co-stimulation blockade led to skin allograft rejection that was comparable to sensitized recipients (MST = 14, 16 and 15 days, respectively, n = 4 – 6/grp; p = 0.06 and 0.5, respectively, NS) and significantly accelerated than naïve recipients (MST = 25 days, n = 4; p = 0.02) (Fig. 2B). Therefore, in wt hosts, both CD8 TCM and TEM subsets were equally effective in mediating allograft rejection.
Fig. 2. Allograft rejection in adoptive hosts of CD8 TCM and TEM.
Sorted CD8 TCM (1.5 × 106 – 2 × 106) or TEM (0.4 × 106 – 0.6 × 106) containing similar numbers of BALB/c-reactive IFNγ+ T cells were transferred into wt and aly/aly-spleen adoptive hosts followed by BALB/c-skin transplantation 2-days later. (A) Skin allograft rejection mediated by CD8 TCM and TEM in wt hosts. BALB/c-skin allograft survival was tested in wt recipients of CD8 TCM or TEM and compared to unimmunized naïve mice (Naïve, no cells) and sensitized mice (Sensitized, no cells) harboring endogenous memory T cells that had rejected BALB/c-skin allografts 8 – 12 weeks earlier (n = 3 – 4 mice/grp). (B) Skin allograft rejection mediated by CD8 TCM and TEM in wt hosts treated with co-stimulation blockade. Wt recipients of CD8 TCM or CD8 TEM, unimmunized naïve mice (Naïve, no cells) and sensitized mice (Sensitized, no cells) were treated with CTLA4-Ig and anti-CD40L, 0.25mg each (i.p.), on day 0, 2, 4 and 6 after BALB/c-skin transplantation and allograft survival was compared (n = 4 – 6 mice/grp). (C) Skin allograft rejection mediated by CD8 TCM and TEM in aly/aly-spleen hosts. Aly/aly-spleen recipients of CD8 TCM or CD8 TEM or unfractionated CD8 memory T cells (1 × 106) (CD8 memory) and aly/aly-spleen naïve mice without memory T cell transfer (Naïve, no cells) underwent BALB/c-skin transplantation and allograft survival was compared (n = 4 – 7 mice/grp).
CD8 TCM have a resting phenotype and do not express VLA-4, suggesting that further differentiation is required for their extravasation and function in non-lymphoid tissues (Figs. 1B and 1H). It is not clear if the preferential location of CD8 TCM within secondary lymphoid tissues suggests dependence on these tissues for differentiation into effectors analogous to naïve T cells (22). To address this question, sorted CD8 TCM (1.5 × 106 – 2 × 106) or TEM (0.4 × 106 – 0.6 × 106) containing similar numbers of alloreactive IFNγ+ T cells were transferred to aly/aly-spleen mice and BALB/c-skin allograft rejection was tested. Skin allograft rejection in aly/aly-spleen recipients of CD8 TCM was significantly delayed than in recipients of TEM (MST = 57 days, n = 7 vs MST = 36 days, n = 5, respectively; p = 0.008, Fig. 2C). Allografts in aly/aly-spleen mice that did not receive memory T cells survived > 100 days compared to recipients of memory T cells (n = 4; p = 0.0002, Fig. 2C). These data show that alloreactive CD8 TEM were significantly more effective than TCM in causing allograft rejection in the absence of secondary lymphoid tissues. However, in adoptive hosts of CD8 TEM, skin allograft rejection was accelerated in wt than in aly/aly-spleen recipients (MST 16 and 36 days, respectively, n = 5 – 6/grp; p = 0.003) (Figs. 2B–2C) suggesting that secondary lymphoid organs support recall of both subsets.
Recall and differentiation of CD8 TCM and TEM in adoptive hosts
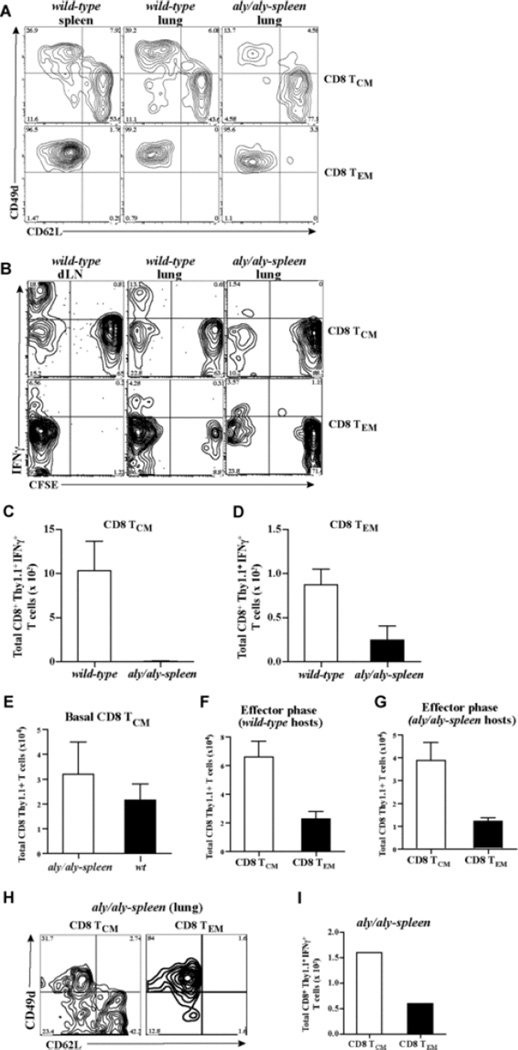
To understand why allograft rejection was delayed in aly/aly-spleen hosts of CD8 TCM, differentiation of CD8 TCM and TEM into effectors and migration into the allograft was compared in adoptive hosts. Sorted CD8 TCM (2 × 106) or TEM (0.5 × 106) containing similar numbers of alloreactive IFNγ+ T cells were transferred into wt and aly/aly-spleen hosts that underwent BALB/c-skin transplantation. 8 days later, cells were harvested from lymphoid and non-lymphoid tissues in adoptive hosts, and analyzed after gating on CD8+Thy1.1+ T cells. A large population of CD8 TCM had acquired a CD49dhigh CD62Llow effector phenotype in wt hosts (50 ± 10%, n = 3) compared to aly/aly-spleen hosts (15 ± 4%, n = 4) suggesting that differentiation of CD8 TCM into effectors was impaired in the absence of secondary lymphoid tissues (Fig. 3A). CD8 TEM retained a CD49dhigh CD62Llow effector phenotype in harvested tissues of both adoptive hosts (Fig. 3A). BALB/c-reactive IFNγ+ T cells derived from CD8 TCM and TEM were found mainly in the CD49dhigh population (data not shown). At 8 days after skin transplantation, BALB/c-reactive IFNγ+ T cells present in the harvested CD8+Thy1.1+ T cells were found exclusively within the proliferated CFSElow population in adoptive hosts of CD8 TCM and TEM suggesting differentiation into alloreactive effectors (Fig. 3B). Transferred CD8 TCM and TEM contained CD44hi memory phenotype T cells of irrelevant specificities that possibly underwent bystander proliferation in adoptive hosts contributing to CFSElow cells within the CD8+Thy1.1+ population that were not IFNγ+ (Fig. 3B) (40–42). CFSElow IFNγ+ effector T cells from CD8 TCM in wt constituted a larger population of harvested CD8+Thy1.1+ cells than in aly/aly-spleen hosts (15 ± 5% vs 1.5 ± 0.5%, n = 3, respectively) compared to CFSElow IFNγ+ effectors from TEM (6 ± 3% vs 3 ± 2%, n = 3, respectively) (Fig. 3B). The greater proliferative advantage of alloreactive CD8 TCM in wt hosts contributed to 100-fold more alloreactive IFNγ+ effectors from TCM compared to a 4-fold increase in alloreactive effectors from TEM in wt than in aly/aly-spleen hosts (p = 0.008 and p = 0.06, NS, respectively, n = 3 – 4, Figs. 3C–3D). Alloreactive effector T cells from CD8 TCM compared to TEM were 13-fold more in wt hosts (p = 0.03, n = 3 – 4) and 3-fold less in aly/aly-spleen hosts (p = 0.07, NS, n = 3 – 4) at 8 days (Figs. 3C–3D). Fewer alloreactive IFNγ+ effector T cells from CD8 TCM in aly/aly-spleen hosts was not due to impaired survival since TCM were recovered from naïve adoptive hosts in comparable numbers (Fig. 3E) (24), and ratio of recovered TCM to TEM (3.5:1) in allograft recipients was similar to cells transferred (Figs. 3F–3G). At 30-days after transplantation, more CD8 TCM in aly/aly-spleen hosts had acquired a CD49dhigh CD62Llow effector phenotype and differentiated into 3-fold more alloreactive IFNγ+ effector T cells than TEM (Figs. 3H–3I). These findings suggested that differentiation of CD8 TCM into effectors was significantly delayed in the absence of secondary lymphoid tissues.
Fig. 3. Differentiation of CD8 TCM and TEM in adoptive hosts.
Sorted CD8 TCM (2 × 106) or TEM (0.5 × 106) containing similar numbers of BALB/c-reactive IFNγ+ T cells were transferred to wt and aly/aly-spleen mice followed by BALB/c-skin transplantation 2-days later. CD8 TCM and TEM were labeled with 2µM CFSE prior to adoptive transfer to assess proliferation in adoptive hosts at 8 days after BALB/c-skin transplantation. Differentiation and proliferation of CD8 TCM and TEM was assessed after skin transplantation. Cells were harvested from liver (LV), lungs (LG), bone marrow (BM), blood and skin allograft (SK) in both wt and aly/aly-spleen hosts. Spleen (SP), draining lymph node (dLN) and non-draining LN cells were also harvested in wt hosts. (A) Phenotype of activated CD8 TCM and TEM in wt and aly/aly-spleen hosts at 8-days. Expression of CD49d and CD62L is shown after gating on CD8+Thy1.1+ population within the harvested cells from spleen and lungs in wt mice, and lungs in aly/aly-spleen mice. Harvested cells from LN, BM and liver tissues were similar in phenotype (data not shown). Representative FACS plots of 3 – 4 experiments are shown. (B) Proliferation of alloreactive CD8 TCM and TEM after recall in adoptive hosts at 8-days. BALB/c-reactive IFNγ+ T cells within the harvested cells from all tissues were assessed by intracellular cytokine staining and analyzed after gating on CD8+Thy1.1+ population. CFSE dilution and IFNγ are shown after gating on CD8+Thy1.1+ T cells. Representative FACS plots of 3 experiments are shown. (C – D) Quantitation of BALB/c-reactive IFNγ+ T cells derived from CD8 TCM (C) and CD8 TEM (D) in wt and aly/aly-spleen adoptive hosts at 8-days after BALB/c-skin transplantation. BALB/c-reactive IFNγ+ T cells within the harvested cells from all tissues were assessed by intracellular cytokine staining and enumerated after gating on CD8+Thy1.1+ population. Mean ± SD of 3 – 4 mice/grp. (E – G) Quantitation of CD8+Thy1.1+ cells from harvested tissues in adoptive hosts of CD8 TCM at 8 days after transfer (E) or at 8 days after transplantation in adoptive hosts of CD8 TCM and TEM (F – G) (Mean ± SD of 3 – 4 mice/grp). (H) Phenotype of activated CD8 TCM and TEM in aly/aly-spleen hosts at 30-days after BALB/c-skin transplantation (similar to 3A). (I) Quantitation of BALB/c-reactive IFNγ+ T cells derived from CD8 TCM and TEM in aly/aly-spleen adoptive hosts at 30-days after BALB/c-skin transplantation (similar to 3C-D).
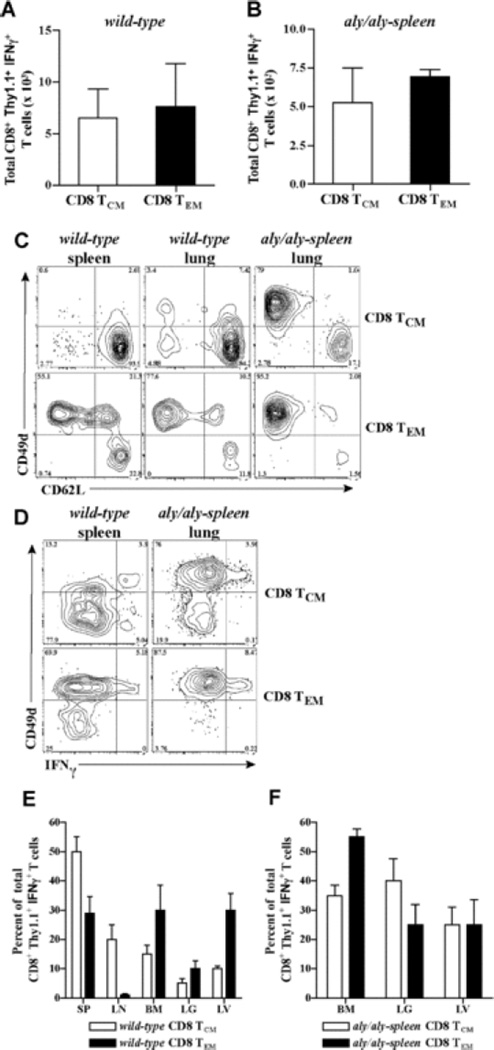
Migration of alloreactive effectors derived from CD8 TCM and TEM and their infiltration into skin allografts was compared in adoptive hosts. Alloreactive IFNγ+ effector T cells from either CD8 TCM or TEM migrated broadly to non-lymphoid tissues and infiltrated into skin allografts to a comparable extent in wt hosts (Fig. 4A; p = 0.08, NS, n = 3 – 4, Fig. 4C). Despite greater numbers of total alloreactive effectors from CD8 TCM than TEM in wt hosts (Figs. 3B – 3D), alloreactive effectors from TEM were comparable to TCM in skin allografts suggesting that early migration and accumulation of TEM in the allograft contributed to equally effective allograft rejection as TCM (Fig. 2B). In aly/aly-spleen allograft recipients, alloreactive IFNγ+ effector T cells from CD8 TCM were found mainly in lungs and liver but not in skin allograft, whereas alloreactive effectors derived from TEM were found in lungs, bone marrow and skin allograft at 8 days (Fig. 4B; p = 0.01, n = 3 – 4, Fig. 4D). Alloreactive IFNγ+ effector T cells from both subsets were found in blood, lung, liver, skin allograft (Fig. 4E) and more alloreactive effectors from TEM than TCM had infiltrated skin allografts (Fig. 4E–4F) despite fewer total alloreactive IFNγ+ effectors derived from TEM (Fig. 3I) in aly/aly-spleen recipients at 30-days. Thus, CD8 TEM infiltrated skin allografts early after transplantation whereas differentiation of TCM into alloreactive effectors that infiltrate the allograft was delayed in the absence of secondary lymphoid tissues contributing to significantly delayed allograft rejection.
Fig. 4. Migration of alloreactive effectors in adoptive hosts of CD8 TCM and TEM.
Sorted CD8 TCM (2 × 106) or TEM (0.5 × 106) containing similar numbers of BALB/c-reactive IFNγ+ T cells were transferred to wt and aly/aly-spleen mice followed by BALB/c-skin transplantation 2-days later. Alloreactive effectors from CD8 TCM and TEM were assessed after BALB/c-skin transplantation. Cells were harvested from liver (LV), lungs (LG), bone marrow (BM), blood and skin allograft (SK) in both wt and aly/aly-spleen hosts. Spleen (SP), draining lymph node (dLN) and non-draining LN cells were also harvested in wt hosts. BALB/c-reactive IFNγ+ T cells within the harvested cells from all tissues were assessed by intracellular cytokine staining after gating on CD8+Thy1.1+ population to identify alloreactive effectors derived from CD8 TCM and TEM. (A – B) Tissue distribution of alloreactive effector T cells generated from transferred CD8 TCM and TEM in wt (A) and aly/aly-spleen (B) hosts. CD8+Thy1.1+ IFNγ+ T cells harvested from each tissue is shown as % of total CD8+Thy1.1+ IFNγ+ T cells harvested from all tissues in that recipient (Mean ± SD of 3 – 4 mice/grp). CD8+ Thy1.1+ IFNγ+ T cells harvested from blood and non-draining LN were < 1% of total and are not shown. (C – D) Quantitation of BALB/c-reactive IFNγ+ T cells within the harvested CD8+ Thy1.1+ population from skin allografts in either wt (C) or aly/aly-spleen (D) adoptive hosts of CD8 TCM and TEM (Mean ± SD of 3 – 4 mice/grp). (E) Tissue distribution of alloreactive effector T cells generated from transferred CD8 TCM and TEM in aly/aly-spleen hosts at 30-days after BALB/c-skin transplantation (similar to 4B). (F) Quantitation of BALB/c-reactive IFNγ+ T cells within the harvested CD8+ Thy1.1+ population from skin allografts in aly/aly-spleen adoptive hosts of CD8 TCM and TEM at 30-days after BALB/c-skin transplantation.
Persistence of CD8 TCM and TEM in adoptive hosts after allograft rejection
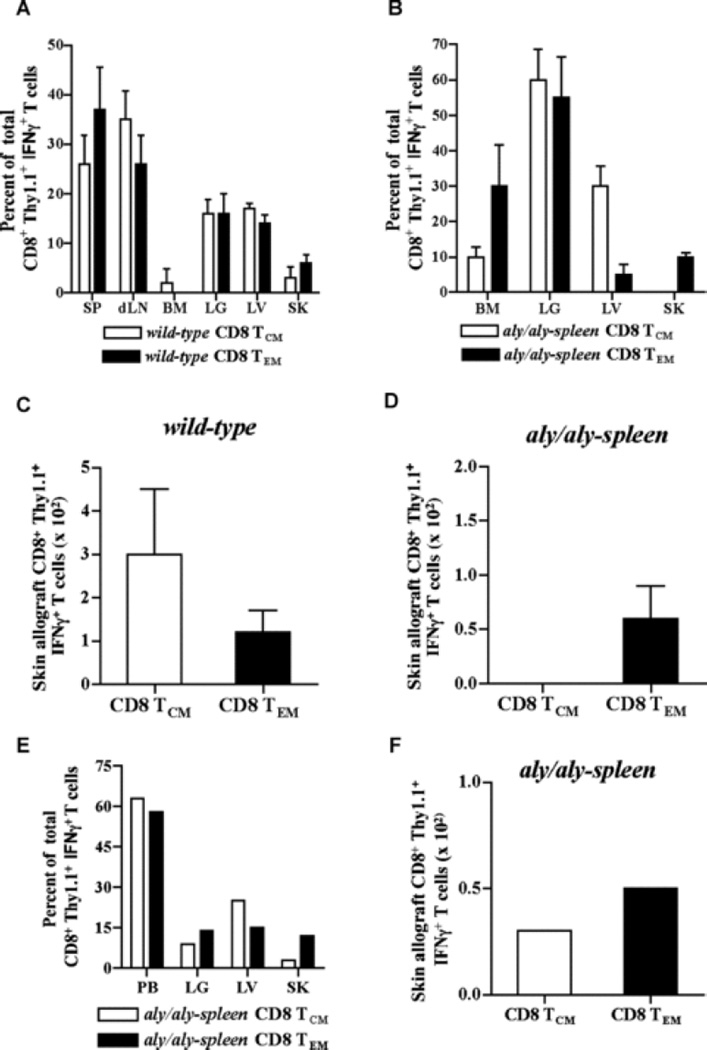
Hallmark of memory T cell recall is the generation of secondary memory T cells that mediate better protective immunity (43, 44). To test which alloreactive CD8 memory subset persists as secondary memory T cells after allograft rejection, sorted CD8 TCM (2 × 106) or TEM (0.5 × 106) containing similar numbers of alloreactive memory T cells were transferred to wt and aly/aly-spleen skin allograft recipients. 6 – 10 weeks after rejection, cells were harvested from lymphoid and non-lymphoid tissues and analyzed after gating on CD8+Thy1.1+ T cells. BALB/c-reactive IFNγ+ T cells within the harvested CD8+Thy1.1+ population were measured to assess antigen-specific secondary memory T cells derived from TCM and TEM. Alloreactive IFNγ+ T cells derived from CD8 TCM and TEM were comparable in harvested tissues of adoptive hosts (n = 4 – 5/grp) (Figs. 5A and 5B) suggesting that both subsets persist as secondary memory T cells in either wt or aly/aly-spleen adoptive hosts after allograft rejection. Recall of CD8 TCM in wt hosts led to both CD62Lhigh CD49dlow TCM and CD62Llow CD49dhigh TEM that contained alloreactive IFNγ+ T cells compared to CD62Llow CD49dhigh alloreactive TEM alone in aly/aly-spleen hosts (Figs. 5C and 5D). CD8 TEM persisted as CD62Llow CD49dhigh, alloreactive TEM in either wt or aly/aly-spleen hosts (Figs. 5C and 5D). Thus, CD8 TCM alone differentiated into alloreactive TCM secondary memory after allograft rejection in wt and not in aly/aly-spleen hosts suggesting that generation of TCM secondary memory requires secondary lymphoid tissues and/or that non-lymphoid tissues promote differentiation of TCM to TEM (29). TCM vs TEM phenotype of alloreactive secondary memory T cells was confirmed by assessing their tissue distribution in adoptive hosts. In wt hosts, alloreactive IFNγ+ T cells from CD8 TCM were found mainly in spleen and LNs than non-lymphoid tissues while those from TEM were found in spleen, bone marrow and liver (n = 4) (Fig. 5E). Alloreactive IFNγ+ T cells from either CD8 TCM or TEM were comparable in bone marrow, lung and liver tissues in aly/aly-spleen hosts (n = 5) (Fig. 5F). Thus, either CD8 TCM or TEM persisted as alloreactive secondary memory in lymphoid and non-lymphoid tissues and both subsets led to TEM after allograft rejection that can function independently of secondary lymphoid tissues.
Fig. 5. Persistence of CD8 TCM and TEM in adoptive hosts after allograft rejection.
Sorted CD8 TCM (2 × 106) or TEM (0.5 × 106) containing similar numbers of BALB/c-reactive IFNγ+ T cells were transferred to wt and aly/aly-spleen mice followed by BALB/c-skin transplantation 2-days later. BALB/c-reactive IFNγ+ T cells derived from transferred CD8 TCM or CD8 TEM were assessed in adoptive hosts 6 – 10 weeks after allograft rejection. Cells were harvested from liver (LV), lungs (LG), bone marrow (BM) and blood in both wt and aly/aly-spleen hosts. Spleen (SP) and LN cells were also harvested in wt hosts. (A – B) Quantitation of alloreactive secondary memory T cells generated from CD8 TCM and TEM in wt (A) and aly/aly-spleen (B) adoptive hosts. BALB/c-reactive IFNγ+ T cells within the harvested cells from all tissues were assessed by intracellular cytokine staining and enumerated after gating on CD8+Thy1.1+ population (Mean ± SD of 4 – 5 mice/grp). (C – D) Phenotype of secondary memory T cells derived from transferred CD8 TCM or TEM in wt and aly/aly-spleen hosts. Expression of CD49d and CD62L is shown after gating on CD8+Thy1.1+ population within the harvested cells from spleen and lungs in wt mice, and lungs in aly/aly-spleen mice (C). Expression of CD49d and IFNγ is shown after gating on CD8+Thy1.1+ population within the harvested cells from spleen in wt mice and lungs in aly/aly-spleen mice (D). Harvested cells from LNs, BM and liver tissues were comparable in phenotype (data not shown). Representative FACS plots of 4 – 5 experiments are shown. (E – F) Tissue distribution of BALB/c-reactive IFNγ+ T cells derived from transferred CD8 TCM or TEM in wt (E) and aly/aly-spleen (F) hosts. CD8+Thy1.1+ IFNγ+ T cells harvested from each tissue is shown as % of total CD8+Thy1.1+ IFNγ+ T cells harvested from all tissues in that recipient (Mean ± SD of 4 – 5 mice/grp). CD8+Thy1.1+ IFNγ+ T cells harvested from blood were < 1% of total and are not shown.
Discussion
We addressed the roles of alloreactive CD8 TCM and TEM subsets in allograft rejection using an in vivo polyclonal system that is analogous to physiologic immune responses in the transplant setting. Alloreactive memory T cells were generated in wt hosts, sorted into CD8 TCM and TEM subsets and transferred to wt or aly/aly-spleen hosts that lack secondary lymphoid tissues to compare skin allograft rejection. We find that CD8 TCM and TEM were equally effective at mediating skin allograft rejection in wt hosts. However, in hosts that lack secondary lymphoid tissues, CD8 TEM were significantly more effective than TCM in mediating skin allograft rejection. Optimal differentiation of CD8 TCM into effectors that migrate into the allograft was impaired in the absence of secondary lymphoid tissues leading to delayed allograft rejection. Following allograft rejection, either CD8 TCM or TEM can persist as alloreactive TEM secondary memory that function independently of secondary lymphoid tissues.
Allograft rejection by CD8 TCM and TEM was addressed in an in-vivo polyclonal system since (a) alloimmune responses target a diverse array of antigenic epitopes and are polyclonal (45); (b) function of a monoclonal population of cells such as TCR-tg T cells may not be representative of physiologic immune responses; and (c) TCR-tg T cells result in artificially high antigen specific precursor frequencies that skew the generation of TCM vs TEM and alter the outcome of immune responses (46–48). Antigen-specific cells present within the polyclonal CD8 TCM and TEM subsets were detected by identifying alloreactive IFNγ+ T cells. CD8 TCM or TEM containing similar numbers of alloreactive IFNγ+ cells were transferred to accurately compare function of CD8 TCM vs TEM that led to transfer of 3 – 4 fold more total CD8 TCM than TEM in each experiment. Using this approach might have lead to transfer of more alloreactive CD8 TCM than TEM and possibly underestimated the functional advantages of TEM over TCM in vivo. Inspite of transferring more total CD8 TCM, TEM were equally effective to TCM in wt hosts and better than TCM in aly/aly-spleen hosts in rejecting allografts. When total number of transferred CD8 TCM was matched to TEM (0.5 × 106 each), skin allograft survival in aly/aly-spleen hosts was prolonged >100 days (data not shown).
Secondary lymphoid organs supported differentiation of both CD8 TCM and TEM resulting in increased alloreactive effectors and accelerated skin allograft rejection in wt compared to aly/aly-spleen hosts. It is possible that residual activation of endogenous T and B cells despite co-stimulation blockade also contributed to allograft rejection in wt recipients. CD8 TCM and TEM mediated equally effective skin allograft rejection in wt hosts whereas TEM functioned significantly better than TCM in aly/aly-spleen hosts. Differentiation of CD8 TCM into effectors that upregulated VLA-4 and migrated into the allograft was significantly delayed in the absence of secondary lymphoid tissues. Alloreactive CD8 TEM expressed VLA-4 constitutively that could have contributed to early infiltration into the allograft independent of differentiation in secondary lymphoid tissues. CD8 TEM were also better than TCM in lysis of allogeneic target cells in-vivo. These subtle advantages of CD8 TEM over TCM led to more rapid allograft rejection by TEM in hosts that lacked secondary lymphoid tissues but were of lesser significance in wt hosts that supported differentiation of both subsets. It is possible that optimal activation of CD8 TCM requires interaction with antigen-bearing professional APCs that is promoted in secondary lymphoid organs (49). Since skin allograft rejection is a lymph-node dependent response (22, 50) and more likely to be dependent on CD8 TCM than TEM, it remains to be determined whether CD8 TCM and TEM respond similarly to other organ allografts (such as heart and kidney) that are less dependent on LN and more dependent on spleen (51–53).
Migration to sites where antigen is located and extravasation into inflamed tissues is essential for immune surveillance (54, 55). Although either CD8 TCM or TEM subset can cause allograft rejection, ability of CD8 TEM to extravasate directly into allografts is an advantage over TCM in promoting allograft rejection when activation requirements are constrained. The findings reported here implicate alloreactive CD8 TEM as the principal subset responsible for immune surveillance of allografts. Similarly, TEM control viruses residing in peripheral tissues and limit metastatic invasion in colorectal cancer (55–57). Our findings along with these reports emphasize the role of TEM in immune surveillance of non-lymphoid tissues.
Acknowledgements
We are indebted to Dr. Fadi Lakkis for helpful discussions and greatly appreciate critical review of the manuscript by Drs. Robert Hendricks, Jennifer Woodward and Rosemary Hoffman. We thank Lonnette Diggs (Yale University) and Autumn Marlowe (University of Pittsburgh) for technical assistance.
Abbreviations used in this paper
- aly
alymphoplasia
- TCM
central memory
- TEM
effector memory
Footnotes
This work is supported by NIH grant AI059137 (GC) and John Merrill Transplant Research Scholar Award (GC) from the American Society of Nephrology and the American Society of Transplantation. MHO is a recipient of the American Society of Transplantation International Fellowship Award.
References
- 1.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-γ-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 2.Akbar AN, Amlot PL, Timms A, Lombardi G, Lechler R, Janossy G. The development of primed/memory CD8+ lymphocytes in vitro and in rejecting kidneys after transplantation. Clin Exp Immunol. 1990;81:225–231. doi: 10.1111/j.1365-2249.1990.tb03322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Najafian N, Salama A, Fedoseyeva E, Benichou G, Sayegh M. Enzyme-linked immunosorbent spot assay analysis of peripheral blood lymphocyte reactivity to donor HLA-DR peptides: potential novel assay for prediction of outcomes for renal transplant recipients. J Am Soc Nephrol. 2002;13:252–259. doi: 10.1681/ASN.V131252. [DOI] [PubMed] [Google Scholar]
- 4.Pearl JP, Parris J, Hale DA, Hoffman SC, Bernstein WB, McCoy KL, et al. Immunocompetent T cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 5.Valujskikh A, Pantenburg B, Heeger P. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhai Y, Meng L, Gao F, Busuttil R, Kupiec-Weglinski J. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169:4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 7.Adams A, Williams A, Jones T, Shirasugi N, Durham M, Kaech S, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kedl RM, Mescher MF. Qualitative differences between naive and memory T cells make a major contribution to the more rapid and efficient memory CD8+ T cell response. J Immunol. 1998;161:674–683. [PubMed] [Google Scholar]
- 9.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 10.Gourley TS, Wherry EJ, Masopust D, Ahmed R. Generation and maintenance of immunological memory. Semin Immunol. 2004;16:323–333. doi: 10.1016/j.smim.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 12.Wherry E, Teichgraber V, Becker T, Masopust D, Kaech S, Antia R, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 13.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8+ T cells. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klonowski KD, Marzo AL, Williams KJ, Lee S-J, Pham Q-M, Lefrancois L. CD8 T cell recall responses are regulated by the tissue tropism of the memory cell and pathogen. J Immunol. 2006;177:6738–6746. doi: 10.4049/jimmunol.177.10.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts A, Woodland D. Cutting edge: Effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J Immunol. 2004;172:6533–6537. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- 17.Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 18.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Chen Y, Fairchild RL, Heeger PS, Valujskikh A. Lymphoid sequestration of alloreactive memory CD4 T cells promotes cardiac allograft survival. J Immunol. 2006;176:770–777. doi: 10.4049/jimmunol.176.2.770. [DOI] [PubMed] [Google Scholar]
- 20.Tang AL, Bingaman AW, Kadavil EA, Leeser DB, Farber DL. Generation and functional capacity of polyclonal alloantigen-specific memory CD4 T cells. Am J Transplant. 2006;6:1275–1284. doi: 10.1111/j.1600-6143.2006.01317.x. [DOI] [PubMed] [Google Scholar]
- 21.Yabu JM, Vincenti F. Novel immunosuppression: small molecules and biologics. Semin Nephrol. 2007;27:479–486. doi: 10.1016/j.semnephrol.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic 'ignorance' of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6:686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 23.Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S, et al. A new mutation aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol. 1994;24:429–434. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 24.Obhrai J, Oberbarnscheidt M, Hand T, Diggs L, Chalasani G, Lakkis F. Effector T cell differentiation and memory T cell maintenance outside secondary lymphoid organs. J Immunol. 2006;176:4051–4058. doi: 10.4049/jimmunol.176.7.4051. [DOI] [PubMed] [Google Scholar]
- 25.Chervenick PA, Boggs DR, Marsh JC, Cartwright GE, Wintrobe MM. Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol. 1968;215:353–360. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- 26.Nasr IW, Reel M, Oberbarnscheidt MH, Mounzer RH, Baddoura FK, Ruddle NH, et al. Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection. Am J Transplant. 2007;7:1071–1079. doi: 10.1111/j.1600-6143.2007.01756.x. [DOI] [PubMed] [Google Scholar]
- 27.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 28.Baars PA, Sierro S, Arens R, Tesselaar K, Hooibrink B, Klenerman P, et al. Properties of murine (CD8+)CD27- T cells. Eur J Immunol. 2005;35:3131–3141. doi: 10.1002/eji.200425770. [DOI] [PubMed] [Google Scholar]
- 29.Marzo AL, Yagita H, Lefrancois L. Cutting edge: migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J Immunol. 2007;179:36–40. doi: 10.4049/jimmunol.179.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brehm MA, Daniels KA, Ortaldo JR, Welsh RM. Rapid conversion of effector mechanisms from NK to T cells during virus-induced lysis of allogeneic implants in vivo. J Immunol. 2005;174:6663–6671. doi: 10.4049/jimmunol.174.11.6663. [DOI] [PubMed] [Google Scholar]
- 31.Unsoeld H, Krautwald S, Voehringer D, Kunzendorf U, Pircher H. CCR7+ and CCR7− memory T cells do not differ in immediate effector cell function. J Immunol. 2002;169:638–641. doi: 10.4049/jimmunol.169.2.638. [DOI] [PubMed] [Google Scholar]
- 32.El-Sawy T, Fahmy N, Fairchild R. Chemokines: directing leukocyte infiltration into allografts. Curr Opin Immunol. 2002;14:562–568. doi: 10.1016/s0952-7915(02)00382-5. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho-Gasper M, Stephen Billing J, Spriewald BM, Wood KJ. Chemokine gene expression during allograft rejection: Comparison of two quantitative PCR techniques. J Immunol Methods. 2005;301:41–52. doi: 10.1016/j.jim.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Bevilacqua MP. Endothelial-Leukocyte Adhesion Molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 35.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 36.Mobley JL, Dailey MO. Regulation of adhesion molecule expression by CD8 T cells in vivo. I. Differential regulation of gp90MEL-14 (LECAM-1), Pgp-1, LFA-1, and VLA-4 alpha during the differentiation of cytotoxic T lymphocytes induced by allografts. J Immunol. 1992;148:2348–2356. [PubMed] [Google Scholar]
- 37.Stegall MD, Dean PG, Ninova D, Cohen AJ, Shepard GM, Gup C, et al. α4 integrin in iselt allograft rejection. Transplantation. 2001;71:1549–1555. doi: 10.1097/00007890-200106150-00011. [DOI] [PubMed] [Google Scholar]
- 38.Christensen JP, Andersson EC, Scheynius A, Marker O, Thomsen A. Alpha 4 integrin directs virus-activated CD8+ T cells to sites of infection. J Immunol. 1995;154:5293–5301. [PubMed] [Google Scholar]
- 39.Larsen C, Elwood E, Alexander D, Ritchie S, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 40.Judge A, Zhang X, Fujii H, Surh C, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tough DF, Zhang X, Sprent J. An IFN-gamma-dependent pathway controls stimulation of memory phenotype CD8+ T cell turnover in vivo by IL-12, IL-18, and IFN-gamma. J Immunol. 2001;166:6007–6011. doi: 10.4049/jimmunol.166.10.6007. [DOI] [PubMed] [Google Scholar]
- 42.Eberl G, Brawand P, Macdonald HR. Selective bystander proliferation of memory CD4+ and CD8+ T cells upon NK T or T cell activation. J Immunol. 2000;165:4305–4311. doi: 10.4049/jimmunol.165.8.4305. [DOI] [PubMed] [Google Scholar]
- 43.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 44.Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherman LA, Chattopadhyay S. The molecular basis of allorecognition. Annu Rev Immunol. 1993;11:385–402. doi: 10.1146/annurev.iy.11.040193.002125. [DOI] [PubMed] [Google Scholar]
- 46.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 47.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204:299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barker CF, Billingham RE. The role of afferent lymphatics in the rejection of skin homografts. J Exp Med. 1968;128:197–221. doi: 10.1084/jem.128.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medawar PB. The homograft reaction. Proc Roy Soc (London), Ser B. 1957;149:145–148. doi: 10.1098/rspb.1958.0058. [DOI] [PubMed] [Google Scholar]
- 52.Vetto RM, Lawson RK. The role of vascular endothelium in the afferent pathway as suggested by the alymphatic renal homotransplant. Transplantation. 1967;5:1537–1539. [Google Scholar]
- 53.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. J Exp Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stock AT, Jones CM, Heath WR, Carbone FR. Cutting edge: central memory T cells do not show accelerated proliferation or tissue infiltration in response to localized herpes simplex virus-1 infection. J Immunol. 2006;177:1411–1415. doi: 10.4049/jimmunol.177.3.1411. [DOI] [PubMed] [Google Scholar]
- 55.Bachmann MF, Wolint P, Schwarz K, Oxenius A. Recall proliferation potential of memory CD8+ T cells and antiviral protection. J Immunol. 2005;175:4677–4685. doi: 10.4049/jimmunol.175.7.4677. [DOI] [PubMed] [Google Scholar]
- 56.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 57.Wüthrich C, Kesari S, Kim W-K, Williams K, Gelman R, Elmeric D, et al. Characterization of lymphocytic infiltrates in progressive multifocal leukoencephalopathy: Co-localization of CD8 T cells with JCV-infected glial cells. Neurology. 2006;68:985–990. doi: 10.1080/13550280600716604. [DOI] [PubMed] [Google Scholar]