Abstract
Gestational exposure to a fat-rich diet, while elevating maternal circulating fatty acids, increases in the offspring's hypothalamus and amygdala the proliferation and density of neurons that express neuropeptides known to stimulate consummatory behavior. To understand the relationship between these phenomena, this study examined in the brain of postnatal offspring (day 15) the effect of prenatal fat exposure on the transcription factor, peroxisome proliferator-activated receptor (PPAR) β/δ, which is sensitive to fatty acids, and the relationship of PPAR β/δ to the orexigenic neuropeptides, orexin, melanin-concentrating hormone, and enkephalin. Prenatal exposure to a fat-rich diet compared to low-fat chow increased the density of cells immunoreactive for PPAR β/δ in the hypothalamic paraventricular nucleus (PVN), perifornical lateral hypothalamus (PFLH), and central nucleus of the amygdala (CeA), but not the hypothalamic arcuate nucleus or basolateral amygdaloid nucleus. It also increased co-labeling of PPAR β/δ with the cell proliferation marker, BrdU, or neuronal marker, NeuN, and the triple labeling of PPAR β/δ with BrdU plus NeuN, indicating an increase in proliferation and density of new PPAR β/δ neurons. Prenatal fat exposure stimulated the double-labeling of PPAR β/δ with orexin or melanin-concentrating hormone in the PFLH and enkephalin in the PVN and CeA and also triple-labeling of PPAR β/δ with BrdU and these neuropeptides, indicating that dietary fat increases the genesis of PPAR β/δ neurons that produce these peptides. These findings demonstrate a close anatomical relationship between PPAR β/δ and the increased proliferation and density of peptide-expressing neurons in the hypothalamus and amygdala of fat-exposed offspring.
Keywords: prenatal fat, peroxisome proliferator-activated receptor (PPAR) β/δ, orexin, melanin-concentrating hormone, enkephalin, proliferation, hypothalamus, amygdala
1. Introduction
Studies in adult animals (Barson et al., 2011, 2012b) show that consumption of a high-fat diet stimulates the expression of hypothalamic neuropeptides that are known to increase consummatory behavior. These orexigenic peptides include orexin/hypocretin (OX), melanin-concentrating hormone (MCH), and enkephalin (ENK) (Barson et al., 2011, 2012b), which themselves stimulate the intake of a high-fat diet (Barson et al., 2011) as well as drugs of abuse such as alcohol (Barson et al., 2011) and are involved in abuse of nicotine (LeSage et al., 2010, Gold and Lerman, 2012, Plaza-Zabala et al., 2013) and other reinforcing substances (Bodnar, 2014, Khoo and Brown, 2014). Further studies demonstrate that these peptides are also stimulated when dietary fat is introduced early in life, even during gestation (Beck et al., 2006, Chang et al., 2008, Poon et al., 2012), and that their increase in expression, not evident in genetically obese Zucker rats (Cai et al., 2000, Beck et al., 2001) persists possibly with long-term behavioral consequences, including an increase in the intake of and dependence on these substances during adolescence (Chang et al., 2008, Barson et al., 2011, 2012b, Chang et al., 2013). While evidence is limited, there are reports in rat offspring showing gestational exposure to a fat-rich diet to increase OX and MCH expression in the perifornical lateral hypothalamus (PFLH) and ENK expression in the hypothalamic paraventricular nucleus (PVN) (Beck et al., 2006, Chang et al., 2008, Vucetic et al., 2010) and to reduce dopaminergic activity in the mesolimbic system (Vucetic et al., 2010). These prenatal fat-induced neurochemical changes are accompanied by changes in behavior, such as increased fat preference and anxiety (Chang et al., 2008, Sullivan et al., 2010, Vucetic et al., 2010), which may promote further drug use, consistent with a report in mice showing early fat exposure to increase ethanol intake in the adult offspring (Cabanes et al., 2000).
While the physiological and molecular mechanisms that mediate the effects of prenatal fat on these behaviors are not known, one signal possibly serving this function may come from circulating lipids, particularly fatty acids (FAs), which are elevated along with triglycerides (TG) by consumption of a fat-rich diet as well as nicotine or ethanol (Contaldo et al., 1989, Sreekala and Indira, 2008) and are known to be biologically active (Krey et al., 1997). In adult rats, these lipids have been linked to an increase in food intake (Barson et al., 2009, Karatayev et al., 2009), expression of ENK and OX mRNA (Chang et al., 2004, Chang et al., 2008, Vucetic et al., 2010), and release of accumbens DA (Rada et al., 2010, Vucetic et al., 2010). The elevation of FAs in the blood of fat-consuming dams as well as newborn offspring, without changes in glucose and various hormones (Chang et al., 2008), suggests that these lipids may be involved in the stimulatory effect of dietary fat on the genesis and density of the peptide-expressing neurons in the offspring. Whereas evidence is limited, this possibility is supported by studies showing that FAs can stimulate neuronal proliferation and differentiation in vitro (Calderon and Kim, 2004, Kawakita et al., 2006, Chang et al., 2008, Vucetic et al., 2010) and interact directly with several neurochemical signaling pathways, and that a lipid-lowering medication reduces ethanol intake and endogenous expression of OX that stimulates the intake of food and drugs (Clegg et al., 2002, Schneider et al., 2007, Barson et al., 2009, Plaza-Zabala et al., 2010).
The mechanism underlying these FA effects on the brain and behavior is largely unknown, but one likely mediator is the transcription factor, peroxisome proliferator-activated receptor (PPAR), which is highly sensitive to FAs and has an important role in neuronal development as well as in regulating lipid and glucose metabolism (Sarruf et al., 2009, Pyper et al., 2010, Lu et al., 2011). The three isoforms of this receptor are PPAR α that regulates FA catabolism and stimulates neuronal differentiation (Bento-Abreu et al., 2007, Pyper et al., 2010), PPAR β/δ that is less studied but known to regulate cellular processes including neuronal differentiation and maturation (Cimini and Ceru, 2008), and PPAR γ that regulates peripheral adipogenesis, glucose and lipid homeostasis, and inflammatory responses while stimulating neuronal stem cell proliferation and differentiation (Wada et al., 2006, Cimini and Ceru, 2008). The PPAR β/δ isoform has the widest expression, with relatively high levels in the brain, and it contrasts with PPAR γ and PPAR α in remaining elevated postnatally (Braissant and Wahli, 1998, Pistis et al., 2005). In adult rats, PPAR γ in the brain has been suggested to mediate the overeating induced by a high-fat diet (Lu et al., 2011), whereas systemic or oral administration of PPAR γ or PPAR α agonists are found to decrease consumption of nicotine or ethanol (Steensland et al., 2007, Melis et al., 2010, Mascia et al., 2011). In addition to reducing insulin resistance, the PPARs may act through brain neurochemical systems, including hypothalamic neuropeptides, which are believed to mediate feeding and drug-taking behavior (Chikahisa et al., 2008, Sarruf et al., 2009, Schintu et al., 2009). While little is known about the effects of in utero dietary manipulations on brain PPAR, studies in the periphery show that prenatal exposure to dietary fat increases adipose and pancreatic PPAR γ and hepatic PPAR α in adult offspring (Samuelsson et al., 2008, Zhang et al., 2009, Theys et al., 2011) and that systemic administration of PPAR agonists postnatally reverses the adverse effects produced by in utero exposure to alcohol (de la Monte and Wands, 2010, Marche et al., 2011) and nicotine (Rehan et al., 2009).
The experiments described here involving immunofluorescence histochemistry focused on the PPAR β/δ which, in addition to being particularly dense in the brain and to stimulating neuronal proliferation and differentiation, was readily detected in our preliminary tests using a single antibody. Thus, this report examined in the rat the effect of prenatal fat exposure, during the critical time for development of hypothalamic and limbic neuronal systems, on the expression and genesis of PPAR β/δ cells and of peptide neurons that co-express PPAR β/δ. The specific hypothesis to be tested was that the stimulatory effect of prenatal fat exposure on peptide neurogenesis occurs in specific neurons that co-express PPAR β/δ and in specific brain areas where both PPAR β/δ and the peptides are highly expressed and the peptides are known to have a role in promoting consummatory behavior. Positive results supporting this hypothesis would suggest a possible involvement of PPAR β/δ in mediating the stimulatory effects of dietary fat on peptide neurogenesis and behavior.
2. Experimental procedures
2.1 Animals
Time pregnant, Sprague-Dawley rats (220–240 g) from Charles River Laboratory (Charles River Laboratories International, Inc., Wilmington, MA) were delivered to the animal facility on embryonic day 4 (E4). The dams were individually housed in plastic cages in a fully accredited AAALAC facility (22 °C, with a 12:12 h light–dark cycle, with lights off at noon), according to institutionally approved protocols as specified in the NIH Guide to the Care and Use of Animals and also with approval of the Rockefeller University Animal Care and Use Committee. The rats were maintained ad libitum from E5 until birth on postnatal day 0 (P0) on either a high-fat diet (50% fat) or a standard, low-fat chow diet (13.2% fat), with the high-fat diet dams also having lab chow available for the first 3 days (until E8) as they became fully adapted to the mixed fat-rich diet. The dams’ food intake was measured daily during pregnancy and two times per week during lactation, and body weight of the dams and pups was recorded weekly. On postnatal day 1 (P1), the litters were culled to n = 8, primarily by eliminating the females. Only male offspring were tested, with 1 male pup taken from each litter and the number of rats/group (n=5-8) equal to the number of litters. As in our prior studies of the orexigenic peptides (Chang et al., 2008, Chang et al., 2012, Chang et al., 2013), the offspring were examined at P15, an age immediately before the start of independent feeding which by itself can influence the orexigenic peptides (Leibowitz and Wortley, 2004) and that in fat-exposed offspring exhibits a change in peptide expression which is similar to that revealed at later ages after weaning (Chang et al., 2008).
2.2 Diets
The dams were maintained ad libitum either on the standard rodent chow (13.2% fat, 3.4 kcal/g; PicoLab Rodent Diet 20 5053, Lab Diet, St. Louis, MO) or the high-fat diet (50% fat, 5.2 kcal/g) as described in prior publications (Dourmashkin, et al., 2006, Leibowitz et al., 2004). Specifically, this fat-rich diet consisted of: fat from 75% lard (Armour, Omaha, NE) and 25% vegetable oil (Wesson vegetable oil, Omaha, NE); carbohydrate from 30% dextrin, 30% cornstarch (ICN Pharmaceuticals, Costa Mesa, CA) and 40% sucrose (Domino, Yonkers, NY); and protein from casein (Bioserv, Frenchtown, NJ) with 0.03% L cysteine hydrochloride added (ICN Pharmaceuticals). This diet was supplemented with minerals (USP XIV Salt Mixture Briggs; ICN Pharmaceuticals) and vitamins (Vitamin Diet Fortification Mixture; ICN Pharmaceuticals). The macronutrient composition of this semi-solid fat diet, calculated as percentage of total kilocalories, was 50% fat, 25% carbohydrate, and 25% protein. It was stored at 4 °C until use, and each day, fresh diet was weighed out in metal dishes and placed in the appropriate cages. This high-fat diet is nutritionally complete and found to have no detrimental effects on the health of the animals.
2.3 Brain tissue processing
Serial, 30 μm coronal sections of the hypothalamus and amygdala were cut with a cryostat, and alternate sections were collected for immunofluorescence histochemistry analysis of PPAR β/δ, OX and MCH and for digoxigenin-labeled in situ hybridization histochemistry analysis of ENK. To measure the cell densities of PPAR β/δ and the peptides, 10-12 sections in each brain area of the control and high-fat diet groups were cut at the same anterior-posterior levels relative to Bregma (Paxinos, 2005): PVN, −1.08 to −2.04 mm; PFLH, −2.64 to −3.48 mm; and CeA, −2.04 to −3.12 mm, with the medial-lateral and dorsal-ventral boundaries similar to those previously described (Chang et al., 2004, Chang et al., 2010, Chang et al., 2015). The average cell densities in the high-fat diet and control groups were counted in each area, compared and analyzed statistically.
2.4 5-Bromo-2-deoxyuridine (BrdU) injection
To label proliferating cells in the embryonic hypothalamus and amygdala, the dams that were on high-fat or chow diet were given intraperitoneal (i.p.) injections, every 8 h over 4 days, of BrdU (20 mg/kg, Sigma, St. Louis, MO) in 0.9% NaCl and 0.007 N NaOH, as described in our prior studies (Chang et al., 2008). The animals rapidly became adapted to this injection procedure, showing minimal signs of physical stress and no changes in body weight, and the total amount of BrdU administered is known to be a saturating dose (Desouza et al., 2005, Mandyam et al., 2007). These injections were given from E12-E15, the period of peak cell birth in the hypothalamus and amygdala (Altman and Bayer, 1978, Bayer et al., 1993). The offspring were sacrificed at P15, and their brains were processed using double- and triple-labeling IF or ISH to examine the co-existence of BrdU with PPAR β/δ, NeuN that labels mature neurons, or the neuropeptides, OX, MCH or ENK, as described previously (Chang et al., 2008, Chang et al., 2012, Chang et al., 2013).
2.5 Immunofluorescence histochemistry to measure PPAR β/δ
Immunofluorescence histochemistry was used to characterize the distribution pattern and to quantify the cell density of PPAR β/δ in the hypothalamus and amygdala of postnatal rats. Briefly, and as previously described (Chang et al., 2008, Chang et al., 2013), offspring at P15 (n = 8/group) were decapitated, and their brains were removed, immediately fixed in 4% paraformaldehyde at 4 °C for 48-72 h, and then cryo-protected in 25% sucrose at 4 °C for 60-72 h. Afterwards, brains were frozen and stored at −80 °C. Free-floating cryostat sections (30 μm), incubated with rabbit anti-PPAR β/δ polyclonal antibody and secondary Cy3-conjugated donkey anti-rabbit antibody, were used for PPAR β/δ immunofluorescence histochemistry (See Table 2 for description of working concentrations and vendors for the antibodies). Sections were viewed, and fluorescence images were captured using a Zeiss fluorescence microscope with MetaVue software. The density of immunofluorescent cells, in 8-12 images collected in each area in each animal, was quantified with Image-Pro Plus software (Version 4.5; Media Cybernetics) as described (Chang et al., 2008, Chang et al., 2012, Chang et al., 2013) and is reported here as cells/μm2. In a pilot experiment, immunofluorescence histochemistry of PPARα and γ was conducted with rabbit anti-PPARα (PA1-822A, Pierce Biotechnology, Rockford, IL) and rabbit anti-PPARγ (ab19481, Abcam, MA; 07-466, Millipore, CA), but no PPARα-positive cells and only weakly-stained PPARγ-positive cells were found in the PVN and LH
Table 2.
This table lists the working concentrations of antibodies and vendors for the antibodies used in this report.
| Triple-labeling IF | Combination of primary antibodies | Combination of secondary antibodies |
|---|---|---|
| PPARβ/δ + NeuN + BrdU | Rabbit anti-PPARβ/δ Mouse anti-NeuN Rat anti-BrdU |
Cy3-Donkey anti-Rabbit FITC-Donkey anti-Mouse Cy5-Donkey anti-Rat |
| PPARβ/δ + MCH + BrdU | Rabbit anti-PPARβ/δ Goat anti-MCH Rat anti-BrdU |
Cy3-Donkey anti-Rabbit FITC-Donkey anti-Goat Cy5-Donkey anti-Rat |
| PPARβ/δ + ORX + BrdU | Rabbit anti-PPARβ/δ Goat anti-ORX Rat anti-BrdU |
Cy3-Donkey anti-Rabbit FITC-Donkey anti-Goat Cy5-Donkey anti-Rat |
2.6 Double-labeling and triple-labeling immunofluorescence histochemistry
In P15 offspring (n = 5/group) of dams injected with BrdU (see above), double-labeling IF was used to examine in the PVN, PFLH and CeA the proliferation of PPAR β/δ+ cells using BrdU, the development of mature neurons that co-label PPAR β/δ+ with NeuN, and the co-existence of PPAR β/δ with the PFLH peptides, MCH or OX, using procedures previously described (Chang et al., 2008, Chang et al., 2012, Chang et al., 2013). In addition, triple-labeling IF was used to examine the proliferation and phenotype of PPAR β/δ+ neurons that labeled both BrdU and NeuN in the PVN, PFLH and CeA or both BrdU and the peptides, OX or MCH, in the PFLH (See Table 2 for description of working concentrations and vendors for the antibodies used). To reveal PPAR β/δ+ neurons that were also double-labeled for NeuN+/BrdU+, OX+/BrdU+ and MCH+/BrdU+, triple-labeling IF was performed using three different specific combinations of primary antibodies with corresponding combination of secondary antibodies, respectively, based on previous double-labeling IF procedures (Chang et al., 2008, Chang et al., 2012, Chang et al., 2013). The combinations of primary antibodies and corresponding combinations of secondary antibodies are listed in Table 3. For analysis of double- or triple-labeled PPAR β/δ+ cells with the other markers or peptides, the images were captured with a 20× objective, and the double- and triple-labeled cells were confirmed with a 40× objective and further validated by confocal Z-sectioning with a 40× water-immersion lens on a Zeiss LSM 510 META confocal microscope. The double- and triple-labeled cells were counted and reported as percentage of total single-labeled cells.
Table 3.
This table lists the combinations of primary antibodies and corresponding combinations of secondary antibodies used in immunofluorescence histochemistry.
| Antibody | Working concentration | Catalog #, Vendor |
|---|---|---|
| Rabbit ant-PPARβ/δ | 1:200 | Polyclonal, PA1-823A, Pierce Biotechnology, IL |
| Rat anti-BrdU | 1:100 | ab6326, Abcam, MA |
| Mouse anti-NeuN | 1:50 | MAB377, Millipore-Chemicon, CA |
| Goat anti-Orexin-A(C-19) | 1:100 | Polyclonal, sc-8070, Santa Cruz Biotechnology, CA |
| Goat Anti-Orexin B(C-19) | 1:100 | Polyclonal, sc-8071, Santa Cruz Biotechnology, CA |
| Goat anti-pro-MCH (C-20) | 1:100 | Polyclonal, sc-14509, Santa Cruz Biotechnology, CA |
| Cy3-Donkey anti-Rabbit | 1:100 | 711-165-152, JacksonImmunoResearch Lab. Inc. PA |
| FITC-Donkey anti-Mouse | 1:50 | 715-095-150, JacksonImmunoResearch Lab. Inc. PA |
| FITC-Donkey anti-Goat | 1:50 | 705-095-147, JacksonImmunoResearch, Lab. Inc. PA |
| Cy5-Donkey anti-Rat | 1:100 | 712-175-150, JacksonImmunoResearch Lab. Inc. PA |
| FITC-Donkey anti-Rat | 1:100 | 712-095-150, JacksonImmunoResearch, Lab. Inc. PA |
2.7 Digoxigenin-labeled in situ hybridization histochemistry with double-labeling immunofluorescence histochemistry
Double-labeling IF of PPAR β/δ with BrdU combined with digoxigenin-labeled ISH for ENK was performed to determine whether the PPAR β/δ+/BrdU+ double-labeled cells in the PVN and CeA also expressed ENK, using procedures described in our previous reports (Chang et al., 2008, Chang et al., 2012, Chang et al., 2013). Briefly, the brains of P15 offspring of dams injected with BrdU were cut with a cryostat, and 30 μm free-floating coronal sections were processed first for ISH of ENK. After the ENK signal was visualized in NBT/BCIP, the sections were briefly washed in 0.1 M Tris-HCl containing 0.1 M NaCl and 50 mM MgCl2 (pH 9.5), then in PBS, and were then treated in 0.2 N HCl for 60 min at 37 °C. After a 45 min wash in Borate buffer (0.1 M, pH 8.5) and 30 min wash in PB (0.1 M, pH 7.4), the sections were blocked in 0.5% TritonX-100, 5% normal donkey serum 0.01 M PBS for 2 h, and were then incubated in the mixture of rabbit anti-PPAR β/δ and rat anti-BrdU at 4 °C for 72 h. After washing in PBS for 10 min × 4, sections were incubated in the mixture of Cy3-donkey anti-rabbit and FITC-donkey anti-rat at room temperature for 2 h. After wash in PBS for 10 min × 3, the sections were mounted and cover-slipped with VECTASHIELD mounting medium (H-1000, Vector, Burlingame, CA). Triple-labeled cells were analyzed on a Zeiss microscope with MetaVue software. The ENK-expressing neurons with dark blue digoxigenin-labeled signal were viewed and captured first with the differential interference contrast (DIC) filter, then the red rhodamine/Cy3 fluorescence filter, and green FITC fluorescence filter were consecutively applied to reveal the PPAR β/δ + signal (red) and BrdU+ signal (green) separately in the same field. The images were merged, and the double- and triple-labeled cells were counted and reported as a percentage of total single-labeled cells.
2.8 Data Analysis
Differences in the effects of prenatal diet on single-labeled PPAR β/δ+ cell or PPAR β/δ+ cells co-labeled with different markers or peptides within each diet group were tested with a repeated-measures ANOVA followed up by pairwise comparisons using Tukey's HSD. PPAR β/δ+ cells triple-labeled with different markers and/or peptide were analyzed separately using unpaired Student's t-tests. Data were determined to be distributed normally using the Shapiro-Wilk test. Significance was determined at p < 0.05, and data are reported as mean ± standard error of the mean (S.E.M.).
3. Results
3.1 Prenatal fat-rich diet and PPAR β/δ levels in the hypothalamus and amygdala of postnatal offspring
Our first goal was to characterize using immunofluorescence (IF) the distribution pattern of cells that label PPAR β/δ in the hypothalamus and amygdala of postnatal rats (P15) and then compare this pattern in offspring exposed in utero to a fat-rich diet to that in control offspring exposed to a low-fat, chow diet (n=8/group). In the chow offspring, a moderate density of PPAR β/δ+ cells, exhibiting staining mostly in the nucleus, was detected in three areas, the PVN, PFLH and central nucleus of the amygdala (CeA), with very few or no PPAR β/δ+ cells seen in the hypothalamic arcuate nucleus (ARC) or basolateral amygdaloid nucleus (BLA), as shown in Figs. 1A and 1B, consistent with other studies (Moreno et al., 2004). Analysis of the effect of prenatal diet on PPAR β/δ+ cells in these five brain areas revealed an overall significant main effect of fat compared to chow (F(1,13) = 20.20, p < 0.01). This main effect reflected a significant, fat-induced increase (+17%, p < 0.01) in PPAR β/δ+ cells in the PVN, PFLH, and CeA, with no change detected in the ARC (ns) or BLA (ns) (Fig. 1A), as illustrated in the photomicrographs of the PVN, PFLH and CeA (Fig. 1B). The prenatal high-fat diet compared to control increased, by 25-39%, the density of total PPAR β/δ+ cells in the PVN, PFLH and CeA and also increased the percentage of PPAR β/δ+ cells that were neurons, from 24% to 56% in the PVN, from 8% to 28% in the PFLH, and from 20% to 53% in the CeA. These results reveal the existence of PPAR β/δ+ cells in specific but not all areas of the hypothalamus and amygdala and show that prenatal exposure to fat leads to a site-specific elevation of PPAR β/δ-immunoreactive neurons in the offspring brain.
Fig. 1.
Prenatal fat increases the density of total PPAR β/δ+ cells relative to chow control diet (n = 8/group), as shown in P15 offspring and assessed by immunofluorescence histochemistry. A: Density of PPAR β/δ+ cells (all cell types) indicated by cells/um2 × 10−4. B: Photomicrographs illustrating this effect of fat vs. chow on the density of PPAR β/δ+ cells. Data are mean ± S.E.M., *p < 0.05 vs. control. Abbreviations: F: fornix; V: ventricle; PVN: hypothalamic paraventricular nucleus; PFLH: perifornical lateral hypothalamus; CeA: central nucleus of amygdala; ARC: arcuate nucleus; BLA: basolateral amygdaloid nucleus.
3.2 Prenatal fat-rich diet and genesis of PPAR β/δ-expressing neurons in the offspring
This experiment examined whether prenatal fat exposure compared to low-fat chow affects the proliferation and density of neurons that express PPAR β/δ in the hypothalamus and amygdala. The dams were injected during pregnancy with the cell proliferation marker, 5-Bromo-2-deoxyuridine (BrdU; see Methods), and the P15 offspring were examined using single-, double- and triple-labeling IF, with antibodies against PPAR β/δ, BrdU and Neuronal Nuclei (NeuN) (a marker of mature neurons) in the PVN, PFLH and CeA. We used single-labeling IF to measure the density of PPAR β/δ+, BrdU+ and NeuN+ cells, double-labeling IF to measure the density of PPAR β/δ+/BrdU+ or PPAR β/δ+/NeuN+ cells, and triple-labeling IF to measure the density of PPAR β/δ+/BrdU+/NeuN+ cells. Analysis of the data revealed that there was an overall main effect of prenatal diet on the density of PPAR+ cells (F(2,8) = 40.97, p < 0.01) across the three brain areas, which reflected a significant fat-induced increase (+14%, p < 0.01) in PPAR β/δ+ cells compared to chow control (averaging 4.76 × 10 −4/um2 across the three areas), confirming the results in Experiment 1. Further, as previously described (Chang et al., 2008), examination of BrdU+ cells revealed an overall significant main effect of the prenatal diet (F(1,8) = 58.02, p < 0.01), reflecting a 30% increase (p < 0.01) in the density of BrdU+ cells in the PVN, PFLH and CeA in the fat compared to chow group (averaging 4.72 × 10−4 cells/um2 across the three areas). A similar analysis of NeuN+ cells showed a main effect of prenatal fat (F(1,8) = 51.92, p < 0.01), reflecting a significant increase in the PVN (p < 0.01), PFLH (p < 0.01) and CeA (p < 0.01) compared to chow (averaging 6.27 × 10−4 cells/um2). Double-labeling IF revealed an overall significant main effect of diet on the density of PPAR β/δ+/BrdU+ (F(1,8) = 50.22, p < 0.01) and PPAR β/δ+/NeuN+ (F(1,8) = 38.51, p < 0.01) cells across the three brain areas, with fat compared to chow showing a significantly greater percentage of these double-labeled cells relative to total number of PPAR β/δ+ cells (Fig. 2) and a significant increase in their density specifically in the PVN (p < 0.01), PFLH (p < 0.01) and CeA (p < 0.01). Analysis of the PPAR β/δ+ cells that were positive for both BrdU and NeuN revealed an overall main effect of diet on the density of PPAR β/δ+/BrdU+/NeuN+ (F(1,8) = 30.00, p < 0.01) across the three brain areas, as indicated by a significantly greater percentage in the fat compared to chow offspring of these triple-labeled cells relative to total PPAR β/δ+ cells (Fig. 3A) and a significant increase in their density in the PVN (p < 0.01), PFLH (p < 0.01) and CeA (p < 0.01), as illustrated by typical examples in the photomicrographs (Fig. 3B). Examination of the ARC and BLA in the chow and fat-exposed offspring revealed no PPAR β/δ+ cells that co-labeled BrdU or NeuN. Together, these data demonstrate that PPAR β/δ exists in neurons and that prenatal fat exposure has a significant stimulatory effect on the proliferation and density of PPAR β/δ-ir neurons in specific areas of the hypothalamus and amygdala.
Fig. 2.
Prenatal fat stimulates PPAR β/δ+ cell proliferation compared to control chow diet as shown in P15 offspring (n = 5/group) and assessed using double-labeling immunofluorescence histochemistry. A: Prenatal fat increases the percentage of PPAR β/δ+/BrdU+ cells relative to the total number of PPAR β/δ+ cells. B: Prenatal fat vs. chow increases the percentage of PPAR β/δ+/NeuN+ cells relative to the total number of PPAR β/δ+ cells. Data are mean ± S.E.M., *p < 0.05 vs. chow control.
Fig. 3.
Prenatal fat stimulates the proliferation of PPAR β/δ+ neurons compared to chow control diet as shown in P15 offspring (n = 5/group) and assessed by triple-labeling immunofluorescence histochemistry. A: Prenatal fat increases the percentage of PPAR β/δ+/NeuN+/BrdU+ cells relative to the total number of PPAR β/δ+ cells. Data are mean ± S.E.M., *p < 0.05 vs. chow control. B: Photomicrographs illustrate this effect of fat vs. chow control. In the PVN and PFLH, single-labeled BrdU+ (blue), PPAR β/δ+ (red), and NeuN+ (green) cells; double-labeled PPAR+/NeuN+ (yellow), PPAR+/BrdU+ (purple), Brdu+/NeuN+ (green/aqua) cells; and tripled-labeled PPAR+/NeuN+/BrdU+ (white) cells, as indicated by arrowheads for the double-labeled and triple-labeled cells. Images on the far right are higher magnifications of images identified with a white square. See legend to Fig.1 for abbreviations. Scale bar = 100 μm.
3.3 Prenatal fat-rich diet and genesis of peptide neurons in the PFLH that co-express PPAR β/δ
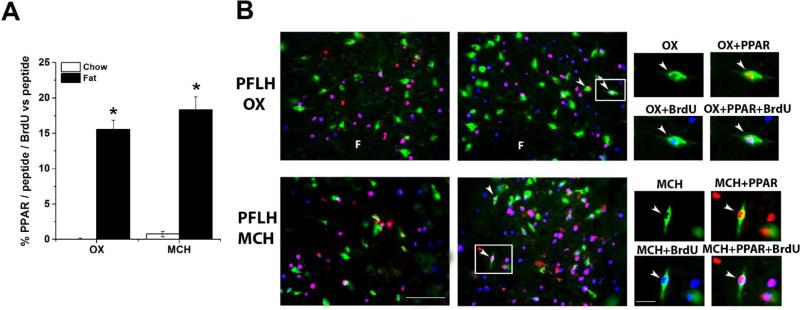
The goal of this experiment was to determine whether PPAR β/δ exists specifically in neurons that express the orexigenic peptides, OX or MCH in the PFLH, in the P15 offspring and whether prenatal fat exposure stimulates the genesis and density of these peptide neurons that contain PPAR β/δ. As previously shown (Chang et al., 2008), analysis of the effect of prenatal diet exposure revealed an overall main effect of fat on the density of OX+ (t(8) = 9.93, p < 0.01) and MCH+ (t(8) = 9.51, p < 0.01) neurons, with the fat diet significantly increasing the density OX+ (+23%, p < 0.01) and MCH+ (+26%, p < 0.01) neurons compared to the chow control (averaging 2.63 vs 2.15 × 10−5 cells/um2 and 3.08 vs 2.41 × 10−5 cells/um2, respectively, across the three areas). Analysis of the PPAR β/δ+ neurons that express OX or MCH in the PFLH revealed a significant stimulatory effect of prenatal fat vs chow diet on the density of PPAR β/δ+/OX+ (t(8) = 7.51, p < 0.01) and PPAR β/δ+/MCH+ (t(8) = 3.56, p < 0.01), as indicated by a greater percentage of these double-labeled neurons relative to the total number of peptide neurons (Table 1). Further, while in the chow offspring there were no peptide+/BrdU+ neurons that contained PPAR β/δ, the prenatal fat-exposed offspring had a significantly greater density of PPAR β/δ+/OX+/BrdU+(t(8) = 12.01, p < 0.01) and PPAR β/δ+/MCH+/BrdU+ (t(8) = 7.80, p < 0.01) neurons, as indicated by a greater percentage of the triple-labeled neurons relative to the peptide+ neurons (Fig. 4A) and illustrated in the photomicrographs (Fig. 4B). Further analysis of the fat-exposed offspring showed that 82% of the OX+/BrdU+ neurons and 78% of the MCH+/BrdU+ neurons also co-expressed PPAR β/δ, with the anatomical localization of these double- and triple-labeled PPAR β/δ+/peptide+/BrdU+ neurons in the PFLH similar in the high-fat diet and control groups. This indicates that prenatal fat exposure stimulates the proliferation of specific peptide neurons in the PFLH that co-express PPAR β/δ.
Table 1.
Prenatal fat increases the percentage of OX+/ PPAR β/δ+ and MCH+/PPAR β/δ+ neurons relative to the total number of peptide+ neurons in the PFLH and of ENK+/ PPAR β/δ+ neurons relative to the total number of ENK+ neurons in the PVN and CeA.
| Percentage of Peptide+/PPAR β/δ+ Neurons vs Peptide Neurons | ||||
|---|---|---|---|---|
| PFLH | PVN | CeA | ||
| OX+/PPAR β/δ+ vs OX+ | MCH+/PPAR β/δ+ vs MCH+ | ENK+/PPAR β/δ+ vs ENK+ | ENK+/PPAR β/δ+ vs ENK+ | |
| Chow | 4.53 ± 0.01 | 1.11 ± 0.02 | 4.78 ± 0.73 | 11.80 ± 1.10 |
| Fat | 20.72 ± 1.32* | 25.76 ± 1.66* | 16.03 ± 1.12* | 28.04 ± 3.21* |
Data are mean ± S.E.M.
p < 0.05 vs. control.
Fig. 4.
Prenatal fat stimulates the proliferation of PFLH OX+/PPAR β/δ+ and MCH+/PPAR β/δ+ peptide neurons compared to chow control diet as shown in P15 offspring (n = 5/group) and assessed by triple-labeling immunofluorescence histochemistry. A: Prenatal fat increases the percentage of peptide+/PPAR β/δ+/BrdU+ relative to the total number of peptide+ neurons. Data are mean ± S.E.M., *p < 0.05 vs. chow control. B: Photomicrographs illustrate this effect of fat vs. chow control group. In PFLH, (top): single-labeled OX+ (green), PPAR β/δ+ (red) and BrdU (blue); double-labeled OX+/PPAR β/δ+ (red nucleus in yellow-green soma) and OX+/BrdU+ (blue nucleus in green/aqua soma); and tripled-labeled OX+/ PPAR β/δ+/BrdU+ (purple nucleus in white-grey green soma) cells. In the PFLH (bottom): single-labeled MCH (green), PPAR β/δ (red) and BrdU (blue); double-labeled MCH+/PPAR β/δ+ (red nucleus in yellow and green soma), MCH+/BrdU+ (blue nucleus in green/aqua and green soma); and tripled-labeled MCH+/PPAR β/δ+/BrdU+ (purple nucleus in white and green soma). Double and triple-labeled cells are indicated by arrowheads. Images on the far right are higher magnifications of images identified with a white square. See legend to Fig.1 for abbreviations. Scale bar = 100 μm.
3.4 Prenatal fat-rich diet and genesis of ENK neurons in the PVN and CeA that co-express PPAR β/δ
This experiment examined whether the PPAR β/δ-ir cells stimulated by prenatal fat exposure are also those that express another orexigenic peptide, ENK, in the PVN and CeA, where a fat-rich diet is known to stimulate this peptide. A combination of in situ hybridization (ISH) and IF was used to measure, respectively, the density of neurons that express ENK and are immunoreactive for PPAR β/δ and BrdU. Consistent with previously published studies, prenatal fat exposure produced a significant increase (+25%) in the density of ENK neurons in the PVN (t(8) = 11.77, p < 0.01) and CeA (t(8) = 10.46, p < 0.01) compared to chow offspring (5.08 × 10−4 cells/ um2 and 6.09 × 10−4 cells/ um2, respectively). Although the chow rats had only a few ENK neurons that contained PPAR β/δ in the hypothalamus (Table 1), prenatal fat exposure significantly increased the density of PPAR β/δ+/ENK+ neurons in the PVN (t(8) = 8.26, p < 0.001) and CeA (t(8) = 8.84, p < 0.001), as indicated by a greater percentage of ENK-expressing neurons that exhibited double labeling. Similarly, while in the chow offspring there were no PPAR β/δ+/ENK+/BrdU+ neurons, the fat-exposed offspring had a significantly greater density of these PPAR β/δ+/ENK+/BrdU+ neurons in the PVN (t(8) = 13.98, p < 0.01) and CeA (t(8) = 7.35, p < 0.01), as indicated by a greater percentage of triple-labeled neurons relative to ENK+ neurons (Fig. 5A) and illustrated in the photomicrographs (Fig. 5B). Further analysis in the fat-exposed offspring showed that 78% of the ENK+/BrdU+ neurons in the PVN and 55% of the ENK+/BrdU+ neurons in the CeA also co-expressed PPAR β/δ, with these double- and triple-labeled PPAR β/δ+/ENK+/BrdU+ neurons similarly located in these nuclei of the high-fat diet and control groups. Examination of the ARC and BLA in the chow and fat-exposed offspring yielded very different results, with no ENK+ neurons in either group found to be immunoreactive for PPAR β/δ or BrdU. These findings indicate that PPAR β/δ exists in ENK-expressing neurons and that prenatal exposure to fat stimulates, specifically in the PVN and CeA, the proliferation of these ENK neurons that contain PPAR β/δ.
Fig. 5.
Prenatal fat stimulates the proliferation of ENK+/PPAR β/δ+ peptide neurons compared to chow control diet in the PVN and the CeA as shown in P15 offspring (n = 5/group) and assessed by triple-labeling of digoxigenin-labeled in situ hybridization of ENK combined with double-labeling immunofluorescence histochemistry of PPAR β/δ and BrdU. A: Prenatal fat increases the percentage of ENK+/PPAR β/δ+/BrdU+ relative to the total number of ENK+ cells. Data are mean ± S.E.M., *p < 0.05 vs. chow control. B: Photomicrographs illustrate this effect of fat vs. chow control group. In PVN (top) and CeA (bottom): single-labeled ENK+ (black), PPAR+ β/δ (red) and BrdU (green); double-labeled ENK+/PPAR β/δ+ (red nucleus with black perikaryon), ENK+/BrdU+ (green nucleus with black perikaryon); and tripled-labeled ENK+/ PPAR β/δ +/BrdU+ (yellowish nucleus with black perikaryon) cells as indicated by arrowheads for the double-labeled and triple-labeled cells. Images on the far right are higher magnifications of images identified with a white square. See legend to Fig.1 for abbreviations. Scale bar = 100 μm.
4. Discussion
Studies have demonstrated that prenatal exposure to dietary fat increases the expression and genesis of orexigenic peptide-expressing neurons in the offspring, in association with an increase in circulating FAs in the pregnant dams and postnatal offspring (Beck et al., 2006, Chang et al., 2008, Poon et al., 2012). With evidence showing FAs to be involved in neuronal proliferation and differentiation (Calderon and Kim, 2004, Kawakita et al., 2006), the goal of this study was to determine whether PPAR β/δ, a receptor activated by binding FA ligands, co-exists with the orexigenic peptide neurons and is in fact stimulated by prenatal fat exposure together with the peptides and increased neurogenesis. In postnatal offspring of pregnant rats maintained for 10 days on a diet high (50%) versus low (13%) in fat content, we demonstrate here that this early exposure to fat increases the proliferation of PPAR+ cells and the density of PPAR+ neurons in specific, but not all, areas of the hypothalamus and amygdala. In addition, we show that prenatal fat exposure stimulates the genesis and density of particular peptide neurons, OX and MCH in the PFLH or ENK in the PVN and CeA, which co-express PPAR β/δ.
With most studies to date examining PPAR β/δ in peripheral organs, there is limited information on its anatomical distribution in the brain. In adult animals, PPAR β/δ is shown to be widely and densely expressed throughout multiple brain areas, including the hypothalamus and limbic system (Woods et al., 2003, Moreno et al., 2004, Gofflot et al., 2007). The present study in postnatal animals is consistent with these findings, demonstrating a moderately dense population of PPAR β/δ-ir cells in specific areas of the hypothalamus, PVN and PFLH, and also the amygdala, CeA. It further demonstrates, in addition, a degree of anatomical specificity within these two structures, with very low levels of PPAR β/δ-ir cells detected in the ARC and BLA. The evidence that PPAR γ is expressed within the ARC (Sarruf et al., 2009) where PPAR β/δ is absent provides further support for the idea that PPAR β/δ is functionally different from the other two PPAR isoforms (Wada et al., 2006, Bento-Abreu et al., 2007, Cimini and Ceru, 2008, Pyper et al., 2010). Our study also reveals a close, anatomical relationship between PPAR β/δ and the orexigenic peptides, as indicated by the existence of PPAR β/δ+/OX+ and PPAR β/δ+/MCH+ co-labeled neurons in the PFLH and of PPAR β/δ+/ENK+ co-labeled neurons in the PVN and CeA of the chow offspring. The only other evidence anatomically relating this transcription factor to orexigenic peptides is from a study of PPAR γ, showing this isoform to colocalize with the orexigenic peptide, agouti-related protein, in the ARC (Sarruf et al., 2009).
There is little information on the effect of dietary fat on PPAR β/δ in the periphery or brain, with most studies focusing on the other PPAR isoforms, PPAR γ and PPAR α. In adult animals, measurements in peripheral organs have yielded mixed results, with consumption of a fat-rich diet found to either stimulate or have no effect on the expression of PPAR γ and PPAR α (Kannisto et al., 2006, McIlroy et al., 2013, Penna-de-Carvalho et al., 2014, Shamsi et al., 2014, Shen et al., 2014). The only available evidence in the brain shows an increase in mRNA levels of PPAR γ in the hypothalamus of diet-induced obese mice, with no change in expression of PPAR α or PPAR β/δ (Diano et al., 2011). The few reports examining the effect of prenatal exposure to fat on the offspring have again yielded mixed results, with fat exposure causing an increase in or having no effect on the expression of PPAR γ and PPAR α in the periphery (Zhang et al., 2005, Zhang et al., 2009, Theys et al., 2011, Magliano et al., 2013, Zheng et al., 2014). The present study provides the first evidence for a significant, prenatal fat-induced change in PPAR β/δ in the offspring brain. The stimulatory effect of fat exposure on the density of PPAR β/δ+ cells was evident in specific regions of the hypothalamus, the PVN and PFLH, and medial amygdala, the CeA, but not the ARC in the basal hypothalamus or the BLA in the lateral amygdala. This distribution pattern indicates that prenatal fat exposure alters the early development of PPAR β/δ+ cell in an anatomically-specific manner.
Previous reports have shown that PPAR β/δ is expressed in neurons in various brain areas, including the hypothalamus, caudate putamen and thalamus of adult rodents (Kremarik-Bouillaud et al., 2000, Woods et al., 2003, Moreno et al., 2004) and the neocortex of the rodent embryo (Cimini et al., 2005). In the present study, approximately 7% of the PPAR β/δ+ cells in control rats were found to be neurons, as indicated by the presence of PPAR β/δ+/NeuN+ double-labeled cells. Prenatal exposure to fat had a profound stimulatory effect on these PPARβ/δ+ neurons, increasing their density to approximately 44% in the PVN, PFLH and CeA. Further analysis revealed that prenatal fat also increased the density of PPAR β/δ+/peptide+ neurons in these specific areas, as indicated by an increased percentage of PPAR β/δ+/OX+ and PPAR β/δ+/MCH+ in the PFLH and of PPAR+/ENK+ neurons in the PVN and CEA relative to their respective peptides. Together, this evidence indicates that prenatal fat exposure affects the development not only of the PPAR β/δ+ cells but also of the peptide neurons that co-label PPAR β/δ+.
Most notable are our findings that prenatal fat exposure affects the proliferation and genesis of PPAR β/δ+ neurons and specifically of orexigenic peptide neurons that co-express PPAR β/δ+. This is demonstrated by a significant increase in the density of PPAR β/δ neurons in the PVN, PFLH and CeA that co-label BrdU, a marker of cell proliferation, and also of OX and MCH neurons in the PFLH and ENK neurons in the PVN and CeA that co-express PPAR β/δ together with BrdU. While not directly tested in this study, it is possible that PPAR β/δ has a functional role in mediating the stimulatory effect of prenatal fat on the genesis of PPAR β/δ+ neurons and of PPAR β/δ+/peptide+ co-labeled neurons in the hypothalamus and amygdala. This idea is supported by other studies in the periphery and brain, showing PPAR β/δ to stimulate the proliferation and differentiation of neurons (Di Loreto et al., 2007, D'Angelo et al., 2011, Yu et al., 2012, Benedetti et al., 2014). These effects of PPAR β/δ may involve the growth factor BDNF and the ERK pathway, which are modulated by PPAR β/δ (D'Angelo et al., 2011) and activated in offspring of dams consuming a fat-rich diet during pregnancy (Peleg-Raibstein et al., 2012, Tajima et al., 2012) and which can themselves promote neurogenesis (Hetman and Xia, 2000, Islam et al., 2009). They may also involve FAs, which are elevated on a fat-rich diet and can stimulate the orexigenic peptide neurons as well as the expression or protein levels of PPAR β/δ (Poon et al., 2015). Together, this evidence indicates that prenatal fat exposure has a profound and specific effect on the proliferation of PPAR β/δ neurons that co-express orexigenic peptides.
With an earlier report from this lab showing maternal consumption of the fat-rich diet to increase circulating FA levels in the offspring at birth and P15 as well as in the dams (Chang et al., 2008), it is possible that these elevated FAs, common ligands for PPARs (Krey et al., 1997), are involved in increasing the density and promoting the development of orexigenic peptides that co-express PPAR β/δ. This possibility is supported by studies showing that FAs can activate PPAR-expressing neurons and microglia in vitro (Bento-Abreu et al., 2007, Antonietta Ajmone-Cat et al., 2012) and stimulate expression of the different PPAR isoforms in the periphery (Melis et al., 2010). They are also found to regulate the differentiation and migration of neurons in the brain (Tabernero et al., 2001, Polo-Hernandez et al., 2014) and stimulate neuronal proliferation and differentiation in vitro (Calderon and Kim, 2004, Kawakita et al., 2006). There is evidence that FAs can interact directly with several neurochemical signaling pathways (Yamamoto et al., 2005, Parab et al., 2007) and stimulate ENK in PC12 cells (Mally et al., 2004, Parab et al., 2007). Also, a PPAR α agonist via a reduction in circulating levels of FAs has been shown to reduce expression of OX in the PFLH (Barson et al., 2009). In addition to directly stimulating neurogenesis, PPAR β/δ is believed to have a neuroprotective role during development by opposing neuronal inflammation and oxidative stress (Schnegg and Robbins, 2011). This PPAR isoform may be acting similarly in the hypothalamus and amygdala, protecting the peptide neurons in the PVN, PFLH and CeA against the negative effects of FAs and maternal fat exposure and then consequently increasing the density of those neurons that contain PPAR β/δ.
In conclusion, this report presents novel findings indicating that prenatal exposure to a fat-rich diet has a strong stimulatory effect on the genesis of PPAR β/δ neurons and of particular peptide neurons that co-express PPAR β/δ and are highly responsive to dietary fat in adults. These effects are found to be anatomically specific, occurring in the PFLH where OX and MCH are expressed and in the PVN and CeA where ENK is expressed, but not in the BLA or ARC where PPAR β/δ-ir cells are sparse and another orexigenic peptide, neuropeptide Y, is negatively affected by a fat-rich diet (Leibowitz et al., 2007, Barson et al., 2012a). With the evidence that prenatal fat exposure increases the offspring's propensity to overconsume food and drugs of abuse (Cabanes et al., 2000, Chang et al., 2008, Bocarsly et al., 2012, Morganstern et al., 2013), these behavioral effects may be mediated in part by these peptides that are known to stimulate consummatory behavior (Barson et al., 2011, 2012b). While not investigated here, the possibility that PPAR β/δ contributes to these behaviors through its actions on the peptide neurons is consistent with the evidence that this PPAR isoform colocalizes with the orexigenic peptides, binds fatty acids, and is highly responsive during gestation to a fat-rich diet and, thus, is well positioned to regulate the genesis and differentiation of these peptide-expressing neurons in the hypothalamus and amygdala that promote consummatory behavior. With this report examining the effect of prenatal fat only in male offspring and during the postnatal period, future studies of this idea should also examine female offspring, in light of evidence that the hypothalamic response to fat-rich diet is sexually dimorphic (Morselli et al., 2015), and both sexes at different ages, during adolescence as well as adulthood.
Highlights.
Prenatal fat exposure stimulates PPAR β/δ immunoreactive cells in offspring brain
Prenatal fat increases the proliferation and density of PPAR β/δ neurons
Prenatal fat stimulates co-existence of PPAR β/δ with orexigenic peptides
Prenatal fat stimulates genesis and differentiation of PPAR β/δ and peptide neurons
Increased of peptides neurons may stimulate consummatory behavior in offspring
Acknowledgements
The authors would like to thank Dr. Jessica R. Barson and Dr. Kinning Poon for their guidance with manuscript preparation. This research was supported National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number 1R21 AA020593. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We extend gratitude to The Rockefeller University's Bio-Imaging Resource Center for use of their equipment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Bayer SA. Development of the diencephalon in the rat. I. Autoradiographic study of the time of origin and settling patterns of neurons of the hypothalamus. J Comp Neurol. 1978;182:945–971. doi: 10.1002/cne.901820511. [DOI] [PubMed] [Google Scholar]
- Antonietta Ajmone-Cat M, Lavinia Salvatori M, De Simone R, Mancini M, Biagioni S, Bernardo A, Cacci E, Minghetti L. Docosahexaenoic acid modulates inflammatory and antineurogenic functions of activated microglial cells. J Neurosci Res. 2012;90:575–587. doi: 10.1002/jnr.22783. [DOI] [PubMed] [Google Scholar]
- Barson JR, Carr AJ, Soun JE, Sobhani NC, Leibowitz SF, Hoebel BG. Opioids in the nucleus accumbens stimulate ethanol intake. Physiol Behav. 2009;98:453–459. doi: 10.1016/j.physbeh.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Karatayev O, Gaysinskaya V, Chang GQ, Leibowitz SF. Effect of dietary fatty acid composition on food intake, triglycerides, and hypothalamic peptides. Regul Pept. 2012a;173:13–20. doi: 10.1016/j.regpep.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Morganstern I, Leibowitz SF. Similarities in hypothalamic and mesocorticolimbic circuits regulating the overconsumption of food and alcohol. Physiol Behav. 2011;104:128–137. doi: 10.1016/j.physbeh.2011.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Morganstern I, Leibowitz SF. Neurobiology of consummatory behavior: mechanisms underlying overeating and drug use. ILAR J. 2012b;53:35–58. doi: 10.1093/ilar.53.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Beck B, Kozak R, Moar KM, Mercer JG. Hypothalamic orexigenic peptides are overexpressed in young Long-Evans rats after early life exposure to fat-rich diets. Biochem Biophys Res Commun. 2006;342:452–458. doi: 10.1016/j.bbrc.2006.01.158. [DOI] [PubMed] [Google Scholar]
- Beck B, Richy S, Dimitrov T, Stricker-Krongrad A. Opposite regulation of hypothalamic orexin and neuropeptide Y receptors and peptide expressions in obese Zucker rats. Biochem Biophys Res Commun. 2001;286:518–523. doi: 10.1006/bbrc.2001.5420. [DOI] [PubMed] [Google Scholar]
- Benedetti E, D'Angelo B, Cristiano L, Di Giacomo E, Fanelli F, Moreno S, Cecconi F, Fidoamore A, Antonosante A, Falcone R, Ippoliti R, Giordano A, Cimini A. Involvement of peroxisome proliferator-activated receptor beta/delta (PPAR beta/delta) in BDNF signaling during aging and in Alzheimer disease: possible role of 4-hydroxynonenal (4-HNE). Cell Cycle. 2014;13:1335–1344. doi: 10.4161/cc.28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento-Abreu A, Tabernero A, Medina JM. Peroxisome proliferator-activated receptor-alpha is required for the neurotrophic effect of oleic acid in neurons. J Neurochem. 2007;103:871–881. doi: 10.1111/j.1471-4159.2007.04807.x. [DOI] [PubMed] [Google Scholar]
- Bocarsly ME, Barson JR, Hauca JM, Hoebel BG, Leibowitz SF, Avena NM. Effects of perinatal exposure to palatable diets on body weight and sensitivity to drugs of abuse in rats. Physiol Behav. 2012;107:568–575. doi: 10.1016/j.physbeh.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opiates and behavior: 2013. Peptides. 2014;62:67–136. doi: 10.1016/j.peptides.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Braissant O, Wahli W. Differential expression of peroxisome proliferator-activated receptor-alpha, -beta, and -gamma during rat embryonic development. Endocrinology. 1998;139:2748–2754. doi: 10.1210/endo.139.6.6049. [DOI] [PubMed] [Google Scholar]
- Cabanes A, de Assis S, Gustafsson JA, Hilakivi-Clarke L. Maternal high n-6 polyunsaturated fatty acid intake during pregnancy increases voluntary alcohol intake and hypothalamic estrogen receptor alpha and beta levels among female offspring. Dev Neurosci. 2000;22:488–493. doi: 10.1159/000017480. [DOI] [PubMed] [Google Scholar]
- Cai XJ, Lister CA, Buckingham RE, Pickavance L, Wilding J, Arch JR, Wilson S, Williams G. Down- regulation of orexin gene expression by severe obesity in the rats: studies in Zucker fatty and zucker diabetic fatty rats and effects of rosiglitazone. Brain research Molecular brain research. 2000;77:131–137. doi: 10.1016/s0169-328x(00)00041-3. [DOI] [PubMed] [Google Scholar]
- Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Barson JR, Karatayev O, Chang SY, Chen YW, Leibowitz SF. Effect of chronic ethanol on enkephalin in the hypothalamus and extra-hypothalamic areas. Alcohol Clin Exp Res. 2010;34:761–770. doi: 10.1111/j.1530-0277.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Davydova Z, Leibowitz SF. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2004;145:3904–3912. doi: 10.1210/en.2003-1582. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Leibowitz SF. Prenatal exposure to nicotine stimulates neurogenesis of orexigenic peptide-expressing neurons in hypothalamus and amygdala. J Neurosci. 2013;33:13600–13611. doi: 10.1523/JNEUROSCI.5835-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Leibowitz SF. Prenatal exposure to ethanol stimulates hypothalamic CCR2 chemokine receptor system: Possible relation to increased density of orexigenic peptide neurons and ethanol drinking in adolescent offspring. Neuroscience. 2015;310:163–175. doi: 10.1016/j.neuroscience.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Liang SC, Barson JR, Leibowitz SF. Prenatal ethanol exposure stimulates neurogenesis in hypothalamic and limbic peptide systems: possible mechanism for offspring ethanol overconsumption. Neuroscience. 2012;222:417–428. doi: 10.1016/j.neuroscience.2012.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikahisa S, Tominaga K, Kawai T, Kitaoka K, Oishi K, Ishida N, Rokutan K, Sei H. Bezafibrate, a peroxisome proliferator-activated receptors agonist, decreases body temperature and enhances electroencephalogram delta-oscillation during sleep in mice. Endocrinology. 2008;149:5262–5271. doi: 10.1210/en.2008-0285. [DOI] [PubMed] [Google Scholar]
- Cimini A, Benedetti E, Cristiano L, Sebastiani P, D'Amico MA, D'Angelo B, Di Loreto S. Expression of peroxisome proliferator-activated receptors (PPARs) and retinoic acid receptors (RXRs) in rat cortical neurons. Neuroscience. 2005;130:325–337. doi: 10.1016/j.neuroscience.2004.09.043. [DOI] [PubMed] [Google Scholar]
- Cimini A, Ceru MP. Emerging roles of peroxisome proliferator-activated receptors (PPARs) in the regulation of neural stem cells proliferation and differentiation. Stem Cell Rev. 2008;4:293–303. doi: 10.1007/s12015-008-9024-2. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Woods SC, Seeley RJ. Eating elicited by orexin-a, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143:2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- Contaldo F, D'Arrigo E, Carandente V, Cortese C, Coltorti A, Mancini M, Taskinen MR, Nikkila EA. Short-term effects of moderate alcohol consumption on lipid metabolism and energy balance in normal men. Metabolism. 1989;38:166–171. doi: 10.1016/0026-0495(89)90257-6. [DOI] [PubMed] [Google Scholar]
- D'Angelo B, Benedetti E, Di Loreto S, Cristiano L, Laurenti G, Ceru MP, Cimini A. Signal transduction pathways involved in PPARbeta/delta-induced neuronal differentiation. J Cell Physiol. 2011;226:2170–2180. doi: 10.1002/jcp.22552. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Role of central nervous system insulin resistance in fetal alcohol spectrum disorders. J Popul Ther Clin Pharmacol. 2010;17:e390–404. [PMC free article] [PubMed] [Google Scholar]
- Desouza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci. 2005;29:414–426. doi: 10.1016/j.mcn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Di Loreto S, D'Angelo B, D'Amico MA, Benedetti E, Cristiano L, Cinque B, Cifone MG, Ceru MP, Festuccia C, Cimini A. PPARbeta agonists trigger neuronal differentiation in the human neuroblastoma cell line SH-SY5Y. J Cell Physiol. 2007;211:837–847. doi: 10.1002/jcp.20996. [DOI] [PubMed] [Google Scholar]
- Diano S, Liu ZW, Jeong JK, Dietrich MO, Ruan HB, Kim E, Suyama S, Kelly K, Gyengesi E, Arbiser JL, Belsham DD, Sarruf DA, Schwartz MW, Bennett AM, Shanabrough M, Mobbs CV, Yang X, Gao XB, Horvath TL. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat Med. 2011;17:1121–1127. doi: 10.1038/nm.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gofflot F, Chartoire N, Vasseur L, Heikkinen S, Dembele D, Le Merrer J, Auwerx J. Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell. 2007;131:405–418. doi: 10.1016/j.cell.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Gold AB, Lerman C. Pharmacogenetics of smoking cessation: role of nicotine target and metabolism genes. Hum Genet. 2012 doi: 10.1007/s00439-012-1143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetman M, Xia Z. Signaling pathways mediating anti-apoptotic action of neurotrophins. Acta Neurobiol Exp (Wars) 2000;60:531–545. doi: 10.55782/ane-2000-1374. [DOI] [PubMed] [Google Scholar]
- Islam O, Loo TX, Heese K. Brain-derived neurotrophic factor (BDNF) has proliferative effects on neural stem cells through the truncated TRK-B receptor, MAP kinase, AKT, and STAT-3 signaling pathways. Curr Neurovasc Res. 2009;6:42–53. doi: 10.2174/156720209787466028. [DOI] [PubMed] [Google Scholar]
- Kannisto K, Chibalin A, Glinghammar B, Zierath JR, Hamsten A, Ehrenborg E. Differential expression of peroxisomal proliferator activated receptors alpha and delta in skeletal muscle in response to changes in diet and exercise. Int J Mol Med. 2006;17:45–52. [PubMed] [Google Scholar]
- Karatayev O, Gaysinskaya V, Chang GQ, Leibowitz SF. Circulating triglycerides after a high-fat meal: predictor of increased caloric intake, orexigenic peptide expression, and dietary obesity. Brain Res. 2009;1298:111–122. doi: 10.1016/j.brainres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139:991–997. doi: 10.1016/j.neuroscience.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Khoo SY, Brown RM. Orexin/hypocretin based pharmacotherapies for the treatment of addiction: DORA or SORA? CNS Drugs. 2014;28:713–730. doi: 10.1007/s40263-014-0179-x. [DOI] [PubMed] [Google Scholar]
- Kremarik-Bouillaud P, Schohn H, Dauca M. Regional distribution of PPARbeta in the cerebellum of the rat. J Chem Neuroanat. 2000;19:225–232. doi: 10.1016/s0891-0618(00)00065-x. [DOI] [PubMed] [Google Scholar]
- Krey G, Braissant O, L'Horset F, Kalkhoven E, Perroud M, Parker MG, Wahli W. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- Leibowitz KL, Chang GQ, Pamy PS, Hill JO, Gayles EC, Leibowitz SF. Weight gain model in prepubertal rats: prediction and phenotyping of obesity-prone animals at normal body weight. Int J Obes (Lond) 2007;31:1210–1221. doi: 10.1038/sj.ijo.0803634. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Wortley KE. Hypothalamic control of energy balance: different peptides, different functions. Peptides. 2004;25:473–504. doi: 10.1016/j.peptides.2004.02.006. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA. Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology (Berl) 2010;209:203–212. doi: 10.1007/s00213-010-1792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, Bandyopadhyay G, Nalbandian S, Fan W, Gayen JR, Mahata SK, Webster NJ, Schwartz MW, Olefsky JM. Brain PPAR-gamma promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med. 2011;17:618–622. doi: 10.1038/nm.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliano DC, Bargut TC, de Carvalho SN, Aguila MB, Mandarim-de-Lacerda CA, Souza-Mello V. Peroxisome proliferator-activated receptors-alpha and gamma are targets to treat offspring from maternal diet-induced obesity in mice. PLoS One. 2013;8:e64258. doi: 10.1371/journal.pone.0064258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mally P, Mishra R, Gandhi S, Decastro MH, Nankova BB, Lagamma EF. Stereospecific regulation of tyrosine hydroxylase and proenkephalin genes by short-chain fatty acids in rat PC12 cells. Pediatr Res. 2004;55:847–854. doi: 10.1203/01.PDR.0000119365.21770.45. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Harburg GC, Eisch AJ. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience. 2007;146:108–122. doi: 10.1016/j.neuroscience.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marche K, Danel T, Bordet R. Fetal alcohol-induced hyperactivity is reversed by treatment with the PPARalpha agonist fenofibrate in a rat model. Psychopharmacology (Berl) 2011;214:285–296. doi: 10.1007/s00213-010-1960-2. [DOI] [PubMed] [Google Scholar]
- Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, Scherma M, Fratta W, Fadda P, Barnes C, Redhi GH, Yasar S, Le Foll B, Tanda G, Piomelli D, Goldberg SR. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry. 2011;69:633–641. doi: 10.1016/j.biopsych.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy GD, Delibegovic M, Owen C, Stoney PN, Shearer KD, McCaffery PJ, Mody N. Fenretinide treatment prevents diet-induced obesity in association with major alterations in retinoid homeostatic gene expression in adipose, liver, and hypothalamus. Diabetes. 2013;62:825–836. doi: 10.2337/db12-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Carta S, Fattore L, Tolu S, Yasar S, Goldberg SR, Fratta W, Maskos U, Pistis M. Peroxisome proliferator-activated receptors-alpha modulate dopamine cell activity through nicotinic receptors. Biol Psychiatry. 2010;68:256–264. doi: 10.1016/j.biopsych.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Morganstern I, Lukatskaya O, Moon SH, Guo WR, Shaji J, Karatayev O, Leibowitz SF. Stimulation of nicotine reward and central cholinergic activity in Sprague-Dawley rats exposed perinatally to a fat-rich diet. Psychopharmacology (Berl) 2013;230:509–524. doi: 10.1007/s00213-013-3178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli E, Frank AP, Palmer BF, Rodriguez-Navas C, Criollo A, Clegg DJ. A sexually dimorphic hypothalamic response to chronic high-fat diet consumption. Int J Obes (Lond) 2015 doi: 10.1038/ijo.2015.114. [DOI] [PubMed] [Google Scholar]
- Parab S, Nankova BB, La Gamma EF. Differential regulation of the tyrosine hydroxylase and enkephalin neuropeptide transmitter genes in rat PC12 cells by short chain fatty acids: concentration-dependent effects on transcription and RNA stability. Brain Res. 2007;1132:42–50. doi: 10.1016/j.brainres.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain, in Sterotaxic Coordinates. Academic Press, Inc.; San Diego, CA: 2005. [Google Scholar]
- Peleg-Raibstein D, Luca E, Wolfrum C. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav Brain Res. 2012;233:398–404. doi: 10.1016/j.bbr.2012.05.027. [DOI] [PubMed] [Google Scholar]
- Penna-de-Carvalho A, Graus-Nunes F, Rabelo-Andrade J, Mandarim-de-Lacerda CA, Souza-Mello V. Enhanced pan-peroxisome proliferator-activated receptor gene and protein expression in adipose tissue of diet-induced obese mice treated with telmisartan. Exp Physiol. 2014;99:1663–1678. doi: 10.1113/expphysiol.2014.081596. [DOI] [PubMed] [Google Scholar]
- Pistis M, Muntoni AL, Pillolla G, Perra S, Cignarella G, Melis M, Gessa GL. Gamma-hydroxybutyric acid (GHB) and the mesoaccumbens reward circuit: evidence for GABA(B) receptor-mediated effects. Neuroscience. 2005;131:465–474. doi: 10.1016/j.neuroscience.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Flores A, Martin-Garcia E, Saravia R, Maldonado R, Berrendero F. A role for hypocretin/orexin receptor-1 in cue-induced reinstatement of nicotine-seeking behavior. Neuropsychopharmacology. 2013;38:1724–1736. doi: 10.1038/npp.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci. 2010;30:2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo-Hernandez E, Tello V, Arroyo AA, Dominguez-Prieto M, de Castro F, Tabernero A, Medina JM. Oleic acid synthesized by stearoyl-CoA desaturase (SCD-1) in the lateral periventricular zone of the developing rat brain mediates neuronal growth, migration and the arrangement of prospective synapses. Brain Res. 2014;1570:13–25. doi: 10.1016/j.brainres.2014.04.038. [DOI] [PubMed] [Google Scholar]
- Poon K, Alam M, Karatayev O, Barson JR, Leibowitz SF. Regulation of the orexigenic neuropeptide, enkephalin, by PPARdelta and fatty acids in neurons of the hypothalamus and forebrain. J Neurochem. 2015 doi: 10.1111/jnc.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K, Barson JR, Fagan SE, Leibowitz SF. Developmental changes in embryonic hypothalamic neurons during prenatal fat exposure. Am J Physiol Endocrinol Metab. 2012;303:E432–441. doi: 10.1152/ajpendo.00238.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyper SR, Viswakarma N, Yu S, Reddy JK. PPARalpha: energy combustion, hypolipidemia, inflammation and cancer. Nucl Recept Signal. 2010;8:e002. doi: 10.1621/nrs.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Barson JR, Leibowitz SF, Hoebel BG. Opioids in the hypothalamus control dopamine and acetylcholine levels in the nucleus accumbens. Brain Res. 2010;1312:1–9. doi: 10.1016/j.brainres.2009.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehan VK, Asotra K, Torday JS. The effects of smoking on the developing lung: insights from a biologic model for lung development, homeostasis, and repair. Lung. 2009;187:281–289. doi: 10.1007/s00408-009-9158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD, Schwartz MW. Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology. 2009;150:707–712. doi: 10.1210/en.2008-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, Carta AR. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. Eur J Neurosci. 2009;29:954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- Schnegg CI, Robbins ME. Neuroprotective Mechanisms of PPARdelta: Modulation of Oxidative Stress and Inflammatory Processes. PPAR Res. 2011;2011:373560. doi: 10.1155/2011/373560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Shamsi BH, Ma C, Naqvi S, Xiao Y. Effects of pioglitazone mediated activation of PPAR-gamma on CIDEC and obesity related changes in mice. PLoS One. 2014;9:e106992. doi: 10.1371/journal.pone.0106992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Song SJ, Keum N, Park T. Olive leaf extract attenuates obesity in high-fat diet-fed mice by modulating the expression of molecules involved in adipogenesis and thermogenesis. Evid Based Complement Alternat Med. 2014;2014:971890. doi: 10.1155/2014/971890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekala S, Indira M. Effect of exogenous selenium on nicotine induced hyperlipidemia in rats. Indian J Physiol Pharmacol. 2008;52:132–140. [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, Smith MS, Coleman K, Grove KL. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. 2010;30:3826–3830. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabernero A, Lavado EM, Granda B, Velasco A, Medina JM. Neuronal differentiation is triggered by oleic acid synthesized and released by astrocytes. J Neurochem. 2001;79:606–616. doi: 10.1046/j.1471-4159.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- Tajima M, Ikarashi N, Imahori Y, Okaniwa T, Saruta K, Ishii M, Kusunoki Y, Kon R, Toda T, Ochiai W, Yamada H, Sugiyama K. Consumption of a high-fat diet during pregnancy decreases the activity of cytochrome P450 3a in the livers of offspring. Eur J Pharm Sci. 2012;47:108–116. doi: 10.1016/j.ejps.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Theys N, Ahn MT, Bouckenooghe T, Reusens B, Remacle C. Maternal malnutrition programs pancreatic islet mitochondrial dysfunction in the adult offspring. J Nutr Biochem. 2011;22:985–994. doi: 10.1016/j.jnutbio.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Nakajima A, Katayama K, Kudo C, Shibuya A, Kubota N, Terauchi Y, Tachibana M, Miyoshi H, Kamisaki Y, Mayumi T, Kadowaki T, Blumberg RS. Peroxisome proliferator-activated receptor gamma-mediated regulation of neural stem cell proliferation and differentiation. J Biol Chem. 2006;281:12673–12681. doi: 10.1074/jbc.M513786200. [DOI] [PubMed] [Google Scholar]
- Woods JW, Tanen M, Figueroa DJ, Biswas C, Zycband E, Moller DE, Austin CP, Berger JP. Localization of PPARdelta in murine central nervous system: expression in oligodendrocytes and neurons. Brain Res. 2003;975:10–21. doi: 10.1016/s0006-8993(03)02515-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Kanno T, Nagata T, Yaguchi T, Tanaka A, Nishizaki T. The linoleic acid derivative FR236924 facilitates hippocampal synaptic transmission by enhancing activity of presynaptic alpha7 acetylcholine receptors on the glutamatergic terminals. Neuroscience. 2005;130:207–213. doi: 10.1016/j.neuroscience.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Yu S, Levi L, Siegel R, Noy N. Retinoic acid induces neurogenesis by activating both retinoic acid receptors (RARs) and peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta). J Biol Chem. 2012;287:42195–42205. doi: 10.1074/jbc.M112.410381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang C, Terroni PL, Cagampang FR, Hanson M, Byrne CD. High-unsaturated-fat, high-protein, and low-carbohydrate diet during pregnancy and lactation modulates hepatic lipid metabolism in female adult offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R112–118. doi: 10.1152/ajpregu.00351.2004. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang F, Didelot X, Bruce KD, Cagampang FR, Vatish M, Hanson M, Lehnert H, Ceriello A, Byrne CD. Maternal high fat diet during pregnancy and lactation alters hepatic expression of insulin like growth factor-2 and key microRNAs in the adult offspring. BMC Genomics. 2009;10:478. doi: 10.1186/1471-2164-10-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Xiao X, Zhang Q, Yu M, Xu J, Wang Z. Maternal high-fat diet modulates hepatic glucose, lipid homeostasis and gene expression in the PPAR pathway in the early life of offspring. Int J Mol Sci. 2014;15:14967–14983. doi: 10.3390/ijms150914967. [DOI] [PMC free article] [PubMed] [Google Scholar]