Abstract
Purpose
The clinical efficacy of tamoxifen is suspected to be influenced by the activity of drug-metabolizing enzymes and transporters involved in the formation, metabolism, and elimination of its active forms. We investigated relationships of polymorphisms in transporter genes and CYP2D6 to clinical outcome of patients receiving tamoxifen.
Patients and Methods
We studied 282 patients with hormone receptor–positive, invasive breast cancer receiving tamoxifen monotherapy, including 67 patients who have been previously reported. We investigated the effects of allelic variants of CYP2D6 and haplotype-tagging single nucleotide polymorphisms (tag-SNPs) of ABCB1, ABCC2, and ABCG2 on recurrence-free survival using the Kaplan-Meier method and Cox regression analysis. Plasma concentrations of tamoxifen metabolites were measured in 98 patients receiving tamoxifen 20 mg/d.
Results
CYP2D6 variants were significantly associated with shorter recurrence-free survival (P = .000036; hazard ratio [HR] = 9.52; 95% CI, 2.79 to 32.45 in patients with two variant alleles v patients without variant alleles). Among 51 tag-SNPs in transporter genes, a significant association was found at rs3740065 in ABCC2 (P = .00017; HR = 10.64; 95% CI, 1.44 to 78.88 in patients with AA v GG genotypes). The number of risk alleles of CYP2D6 and ABCC2 showed cumulative effects on recurrence-free survival (P = .000000055). Patients carrying four risk alleles had 45.25-fold higher risk compared with patients with ≤ one risk allele. CYP2D6 variants were associated with lower plasma levels of endoxifen and 4-hydroxytamoxifen (P = .0000043 and .00052), whereas no significant difference was found among ABCC2 genotype groups.
Conclusion
Our results suggest that polymorphisms in CYP2D6 and ABCC2 are important predictors for the prognosis of patients with breast cancer treated with tamoxifen.
INTRODUCTION
Tamoxifen has been widely used for the treatment and prevention of recurrence in patients with estrogen receptor (ER)–positive or progesterone receptor (PR)–positive breast cancers. However, 30% to 50% of patients who receive adjuvant tamoxifen therapy experience relapse and subsequently die of the disease.1,2 Despite decades of research, the mechanisms underlying the ineffectiveness in a subset of the patients are not fully understood.
Tamoxifen is a prodrug that requires metabolic activation to elicit its pharmacologic activity. It is reported that its metabolites, 4-hydroxytamoxifen and 4-hydroxy-N-desmethyltamoxifen (endoxifen), are the active therapeutic moieties.3–5 Compared with the parent drug, these two metabolites have 100-fold greater affinity to ER and 30- to 100-fold greater potency in suppressing estrogen-dependent cell proliferation.3–5 Thus, interindividual differences in the formation and elimination of these active metabolites could be one of the important factors affecting variability in response to tamoxifen. CYP2D6 is a key enzyme for the generation of 4-hydroxytamoxifen and endoxifen.6 Several groups including ours have clarified that loss or decrease of CYP2D6 function by genetic polymorphisms is associated with poorer clinical outcome of tamoxifen treatment in different populations.7–10 Although genetic polymorphisms in CYP2D6 are one of the most important determinants for clinical efficacy of tamoxifen, individual differences in responsiveness to tamoxifen still remain, even if the effects of CYP2D6 polymorphisms are considered, suggesting the existence of another genetic factor(s) contributing to individual differences in the formation and elimination of the active metabolites of tamoxifen. However, no large-scale clinical trial exists that investigates the relationship of polymorphisms in tamoxifen pharmacokinetics (PK) -related genes, including CYP2D6, to clinical efficacy of adjuvant tamoxifen therapy. Tamoxifen is primarily eliminated by hepatic oxidative metabolism; approximately 75% of the dose is excreted into the biliary tract as glucuronides.4 It has been reported that several transporters expressed in cancer cells play important roles in resistance to tamoxifen.11–13 Therefore, adenosine triphosphate–binding cassette (ABC) transporter B1 (ABCB1; also known as multidrug resistance 1 [MDR1]), ABCC2 (also known as multidrug resistance-associated protein 2 [MRP2]), and ABCG2 (also known as breast cancer resistance protein [BCRP]), which have been reported to transport various types of glucuronides or sulfates,14,15 may be involved in the transport of tamoxifen and/or its metabolites, and their genetic variations may influence the individual difference in tamoxifen efficacy.
In this study, we focused on three ABC transporters and comprehensively analyzed their associations, as well as the association of CYP2D6, with clinical outcome after tamoxifen monotherapy, using a larger number of samples. In addition, we investigated the effects of these polymorphisms on the steady-state plasma concentrations of tamoxifen and its metabolites in patients with breast cancer receiving tamoxifen therapy.
PATIENTS AND METHODS
Patients
We had two objectives in this study. The primary objective was to determine the association between genotype of the tamoxifen PK-related genes and recurrence-free survival (genotype-efficacy study). The secondary objective was to determine the association between genotype of the tamoxifen PK-related genes and plasma concentrations of tamoxifen and its metabolites (genotype-PK study) in patients with breast cancer receiving tamoxifen therapy. In the genotype-efficacy study, 282 patients with primary breast cancer (including 67 patients who have been reported previously7) were recruited from September 2007 to April 2009 at Shikoku-*10 collaborative group (Tokushima Breast Care Clinic, Yamakawa Breast Clinic, Shikoku Cancer Center, Kochi University Hospital, and Itoh Surgery and Breast Clinic), Kansai Rosai Hospital, Sapporo Breast Surgical Clinic, and Sapporo Medical University Hospital. All patients were Japanese women pathologically diagnosed with ER- and/or PR-positive, invasive breast cancer without distant spread who received adjuvant tamoxifen monotherapy without any other treatments after surgical treatment between 1986 and 2007. Data on primary breast cancer diagnosis or recurrence were confirmed from the patients' medical records. Patients without recurrence were censored at the date of the last consultation. Recurrence-free survival time was defined as the time from surgical treatment to diagnosis of the recurrence of a breast cancer (locoregional, distant metastasis, and contralateral breast events). Patients received tamoxifen 20 mg/d for 5 years; tamoxifen was stopped at the time a recurrence was identified. ER and PR status was evaluated by enzyme immunoassay or immunohistochemistry. The cutoff for human epidermal growth factor receptor 2 overexpression was defined as 3+ immunohistochemical staining.16 Nodal status was determined according to the International Union Against Cancer TNM classification. For the genotype-PK study, 98 independent patients with breast cancer who had been taking tamoxifen 20 mg daily for at least 4 weeks (patients taking selective serotonin reuptake inhibitors were not included) were recruited at Tokushima Breast Care Clinic (Tokushima, Japan). Blood samples (5 mL) were collected before taking tamoxifen. These studies were approved by the Institutional Review Board of the Institute of Medical Science, The University of Tokyo (Tokyo, Japan), and written informed consent was obtained from all patients.
Genotyping
Genomic DNA was extracted from peripheral blood using Qiagen DNA extraction kit (Qiagen, Valencia, CA). Genotyping for key polymorphisms for CYP2D6*4 (1846G>A), CYP2D6*6 (1707delT), CYP2D6*10 (100C>T), CYP2D6*14B (1758G>A), CYP2D6*18 (4125_4133dupGTGCCCACT), CYP2D6*21 (2573_2574insC), CYP2D6*36 (gene conversion to CYP2D7 in exon 9), and CYP2D6*41 (2988G>A) was performed using previously designed primers and probes.17 Determination of allelic ratio and copy number of CYP2D6 gene was performed using ABI PRISM 7900HT (Applied Biosystems, Foster City, CA), as described previously.17,18 To determine the diplotypes, whole-gene deletion (CYP2D6*5) and duplications (CYP2D6*1-*1, CYP2D6*10-*10, CYP2D6*10-36, and CYP2D6*36-*36) were detected following reported protocols.19,20 Multiplication alleles, which consisted of CYP2D6*10 and CYP2D6*36 (ie, CYP2D6*10-*36 and CYP2D6*10-*36-*36), were defined as CYP2D6*10 because the enzyme encoded by CYP2D6*36 has been reported to have negligible activity.21 To evaluate the effects of all CYP2D6 alleles tested in this study, we defined all decreased and null alleles (*4, *5, *10, *10-*10, *14, *21, *36-*36, and *41) as allele V and *1 and *1-*1 alleles as allele wt.
For ABCB1, ABCC2, and ABCG2, we genotyped haplotype-tagging single nucleotide polymorphisms (tag-SNPs) selected with an r2 threshold of 0.8 and minor allele frequency of greater than 10% in the HapMap Japanese population as described in our previous study.22 In addition, we genotyped the following possible functional polymorphisms: rs2032582 (2677G>T/A) and rs3213619 (−129T>C) in ABCB1 and rs2273697 (1249G>A) in ABCC2. A total of 51 SNPs were genotyped using multiplex polymerase chain reaction–based Invader assay (Third Wave Technologies, Madison, WI) as described previously.23
Measurement of Plasma Concentrations of Tamoxifen and Its Metabolites
Plasma concentrations of tamoxifen and its metabolites, N-desmethyltamoxifen, 4-hydroxytamoxifen, and endoxifen, were measured using a high-performance liquid chromatography with time of flight mass spectrometry (HPLC-TOFMS). Tamoxifen (≥ 99%), (Z)-4-hydroxytamoxifen (≥ 98%), and imipramine, an internal standard (IS), were purchased from Sigma-Aldrich (St Louis, MO). N-desmethyltamoxifen (> 98%) was obtained from Toronto Research Chemicals (North York, Ontario, Canada). Endoxifen was used as a mixture of E and Z isomers (25:75) described previously.6
Pretreatment of plasma samples was carried out by protein precipitation and solid phase extraction. Briefly, 100 μL IS solution (imipramine 200 ng/mL in acetonitrile) was added to 100 μL plasma. After mixing and centrifugation (13,000 rpm, 5 minutes, 4°C), 950 μL of 25 mmol/L sodium citrate buffer (pH 5.0) was added to the supernatant. The solutions were then purified by solid phase extraction using BOND ELUTE-C18 cartridges (1 mL, 100 mg; Varian, Lake Forest, CA) following the method described previously.24
HPLC-TOFMS was equipped with a 1200 series HPLC system and a G6210 Time-of-Flight LC/MS (Agilent Technologies, Santa Clara, CA). Chromatographic separations were obtained under gradient conditions using an XBridge C18 column (150 × 3 mm ID, 3.5 μm particle size; Waters, Milford, MA). The mobile phase was consisted of eluent A (10 mmol/L ammonium formate) and eluent B (acetonitrile). The gradient was as follows: 30% B for 2.0 minutes, 0.2 mL/min; 30% B at 2.01 minutes, 0.5 mL/min; 30% B at 3.0 minutes, 0.5 mL/min, 90% B at 20 minutes, 0.5 mL/min; and 98% B at 20.01 minutes, 1.2 mL/min. Injection volume was 20 μL, and all separations were performed at 30°C. The retention times of imipramine (IS), endoxifen, 4-hydroxytamoxifen, N-desmethyltamoxifen, and tamoxifen were 10.68, 12.22, 12.89, 16.29, and 17.39 minutes, respectively. Electrospray ionization-TOFMS was conducted in the positive ion mode. The capillary voltage was set at 4,000 V. A flow rate and a nebulizer pressure of nitrogen gas (heater temperature 360°C) were maintained at 10 L/min and 45 psig, respectively. In TOFMS, the fragmenter voltage was set at 150 V. Two mass-to-charge ratio (m/z) values of imipramine (IS), N-desmethyltamoxifen, tamoxifen, endoxifen, and 4-hydroxytamoxifen were 281.2, 358.2, 372.2, 374.2, and 388.2, respectively.
Standard curves were prepared in the concentration range of 20 to 1,000 ng/mL for tamoxifen and N-desmethyltamoxifen, 3.75 to 187.5 ng/mL for endoxifen, and 1 to 20 ng/mL for 4-hydroxytamoxifen. The interday and intraday variability in precision (expressed as the coefficient of variation) for all compounds ranged from 0.7% to 6.6% and from 5.5% to 9.5%, respectively; the average accuracies were between 94.1% and 102.9%.
Statistical Analysis
Recurrence-free survival curves were estimated using the Kaplan-Meier method. Statistical significance of a relationship between clinical outcome and genetic polymorphism was assessed using the trend log-rank test. Cox proportional hazards analysis was used to identify significant prognostic clinical factors and to test for an independent contribution of genetic factors to recurrence-free survival. To examine potential confounding, age was treated as a continuous variable, tumor size was treated as an ordinal variable, and the other covariates were treated as categoric variables. Genotypes were analyzed by assigning an ordinal score to each genotype (1 for homozygous nonrisk allele, 2 for heterozygous risk allele, and 3 for homozygous risk allele). The significant subsets of variables in the univariate analysis were used in multivariate analysis. All polymorphisms evaluated in this study were tested for deviation from Hardy-Weinberg equilibrium with the use of a χ2 test. Risk alleles were defined as the alleles associated with shorter recurrence-free survival. Combination effects were investigated by adding up the number of risk alleles of CYP2D6 and ABCC2 genes. The differences in plasma concentrations of tamoxifen and its metabolites among genotypes were evaluated by a Kruskal-Wallis test. Statistical tests provided two-sided P values, and a significance level of P < .05 was used. In the screening of transporter genes, we used a significance level of P < .00098 to adjust multiple testing by the strict Bonferroni's correction. Statistical analyses were carried out using SPSS (version 17.0; SPSS, Chicago, IL) and the R statistic program (http://www.r-project.org/).
RESULTS
Patient Characteristics
To evaluate the effect of tamoxifen on recurrence-free survival, we recruited 282 Japanese patients receiving adjuvant tamoxifen monotherapy without any other anticancer treatments in this study. Table 1 lists the characteristics of these 282 patients who were pathologically diagnosed to have an ER- and/or PR-positive, invasive breast cancer. Their median age at the time of surgery was 51 years old (range, 31 to 83 years), and the median follow-up period was 7.1 years (range, 0.8 to 23.5 years). Among the characteristics listed in Table 1, tumor size and nodal status showed significant associations with recurrence-free survival (P = .037 and .049, respectively) in the Cox proportional hazards analysis.
Table 1.
Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | No. of Patients (N = 282) | % |
|---|---|---|
| Age at surgery, years | ||
| Median | 51 | |
| Range | 31-83 | |
| Menopausal status | ||
| Premenopausal | 123 | 43.6 |
| Postmenopausal | 149 | 52.8 |
| Unknown | 10 | 3.6 |
| Tumor size, cm | ||
| ≤ 2 | 159 | 56.4 |
| 2.1-5 | 106 | 37.6 |
| > 5 | 2 | 0.7 |
| Unknown | 15 | 5.3 |
| Nodal status | ||
| Negative | 230 | 81.6 |
| Positive | 48 | 17.0 |
| Unknown | 4 | 1.4 |
| ER status | ||
| Positive | 208 | 73.8 |
| Negative | 25 | 8.9 |
| Unknown | 49 | 17.3 |
| PR status | ||
| Positive | 195 | 69.1 |
| Negative | 36 | 12.8 |
| Unknown | 51 | 18.1 |
| HER2 | ||
| Positive* | 5 | 1.8 |
| Negative | 97 | 34.4 |
| Unknown | 180 | 63.8 |
| Events | ||
| No event | 241 | 85.5 |
| Locoregional events | 9 | 3.2 |
| Distant metastasis events | 22 | 7.8 |
| Contralateral breast events | 10 | 3.5 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Score of 3+ in immunohistochemistry.
Associations Between Genotypes and Clinical Outcome
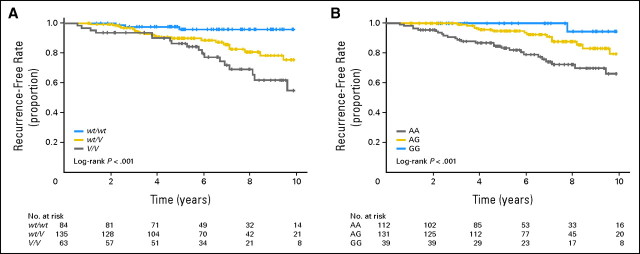
We determined CYP2D6 genotypes of these 282 patients (Table 2). The allele frequency of CYP2D6*10, which is considered to have decreased enzymatic activity and is known to be present at a relatively high frequency in Asian populations, was 37.2%. The frequencies of the alleles observed in this study were comparable to those reported previously.17,20 To evaluate the effects of the CYP2D6 alleles, we examined all of the decreased and null alleles (*4, *5, *10, *10-*10, *14, *21, *36-*36, and *41) as allele V and *1 and *1-*1 alleles as allele wt. Kaplan-Meier estimates indicated that the patients carrying one or two variant alleles (wt/V or V/V) had significantly shorter recurrence-free survival compared with patients with homozygous wild-type alleles (wt/wt; log-rank P = .0002; Fig 1A). In Cox proportional hazards analysis, the CYP2D6 genotype was an independent indicator of recurrence-free survival after adjustment of tumor size and nodal status (trend P = .000036, Table 3). The adjusted hazard ratios (HRs) of patients with wt/V and V/V genotypes, relative to patients with wt/wt, were 4.44 (95% CI, 1.31 to 15.00) and 9.52 (95% CI, 2.79 to 32.45), respectively.
Table 2.
Genotype Frequency of CYP2D6
| Combined CYP2D6 genotype† | No. of Patients | % |
|---|---|---|
| wt/wt | ||
| *1/*1 | 83 | 29.4 |
| wt/V | ||
| *1/*4 | 1 | 0.4 |
| *1/*5 | 17 | 6.0 |
| *1/*10 | 105 | 37.2 |
| *1/*10-*10 | 3 | 1.1 |
| *1/*14 | 1 | 0.4 |
| *1/*21 | 2 | 0.7 |
| *1/*36-*36 | 1 | 0.4 |
| *1/*41 | 5 | 1.8 |
| *1-*1/*10 | 1 | 0.4 |
| V/V | ||
| *5/*5 | 1 | 0.4 |
| *5/*10 | 9 | 3.2 |
| *5/*21 | 1 | 0.4 |
| *5/*41 | 2 | 0.7 |
| *10/*10 | 45 | 16.0 |
| *10/*10-*10 | 1 | 0.4 |
| *10/*21 | 1 | 0.4 |
| *10/*36-*36 | 2 | 0.7 |
| *10/*41 | 1 | 0.4 |
All decreased or null alleles (*4, *5, *10, *10-*10, *14, *21, *36-*36, and *41) were defined as an allele V, and *1 and *1-*1 alleles were defined as wt.
Fig 1.
Kaplan-Meier estimates of recurrence-free survival for CYP2D6 and ABCC2 rs3740065 genotypes. (A) Comparison of 282 patients with wt/wt, wt/V, and V/V of CYP2D6. (B) Comparison of 282 patients with AA, AG, and GG genotypes at rs3740065 in ABCC2.
Table 3.
Recurrences and Hazard Ratios for Recurrence-Free Survival in Patients With Breast Cancer Treated With Tamoxifen
| Variable | No. of Patients | No. of Recurrences | Adjusted Hazard Ratio* | 95% CI | P (trend test) |
|---|---|---|---|---|---|
| CYP2D6 genotype | .000036 | ||||
| wt/wt | 84 | 3 | 1.00 (reference) | ||
| wt/V | 135 | 20 | 4.44 | 1.31 to 15.00 | |
| V/V | 63 | 18 | 9.52 | 2.79 to 32.45 | |
| ABCC2 rs3740065 | .00017 | ||||
| GG | 39 | 1 | 1.00 (reference) | ||
| AG | 131 | 14 | 3.52 | 0.46 to 26.79 | |
| AA | 112 | 26 | 10.64 | 1.44 to 78.88 | |
| No. of risk alleles of CYP2D6 and ABCC2 rs3740065 | .000000055 | ||||
| 0 | 13 | 0 | |||
| 1 | 52 | 1 | 1.00 (reference)† | ||
| 2 | 109 | 8 | 4.93 | 0.61 to 39.63 | |
| 3 | 86 | 23 | 19.98 | 2.69 to 148.65 | |
| 4 | 22 | 9 | 45.25 | 5.58 to 366.81 |
Adjusted for tumor size and nodal status.
The reference category included ≤ one risk allele.
We also genotyped tag-SNPs in ABC transporter genes, ABCB1, ABCC2, and ABCG2, which are suspected to be involved in the biliary excretion of tamoxifen or its metabolites (Appendix Table A1, online only).15 Among 51 SNPs tested, five SNPs in ABCC2 showed P < .05 by log-rank test for recurrence-free survival. The lowest P value was observed at rs3740065 in ABCC2 (log-rank P = .0002, Fig 1B), which was significant after the adjustment for multiple testing by the strict Bonferroni's correction. rs11190303 in ABCC2 also showed a significant association (log-rank P = .00048), which is in strong linkage disequilibrium (LD) with rs3740065 (r2 of LD = 0.79). No significant associations between genotypes and recurrence-free survival were observed for the remaining SNPs. Cox proportional hazards analysis revealed that rs3740065 in ABCC2 was an independent indicator of recurrence-free survival (trend P = .00017, Table 3) and that the adjusted HR for patients carrying rs3740065 AA, compared with patients carrying GG genotype as a reference, was 10.64 (95% CI, 1.44 to 78.88; Table 3), indicating rs3740065 A to be a risk allele of recurrence.
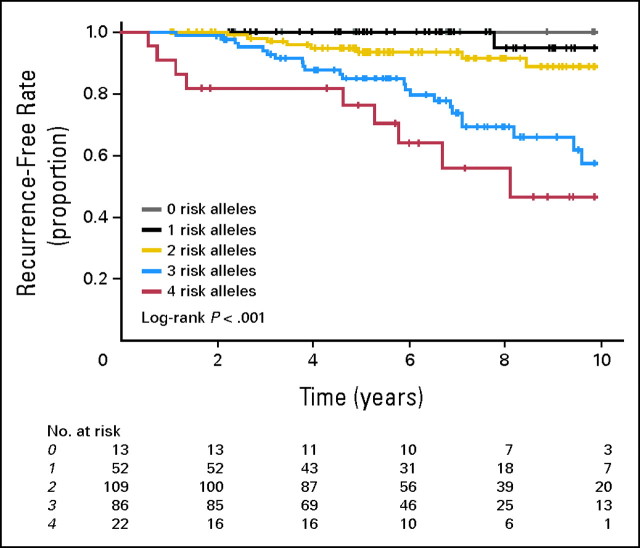
We then investigated a combined effect of CYP2D6 and ABCC2 genotypes on the recurrence-free survival by classifying the patients into five groups (zero, one, two, three, and four risk allele groups) according to the number of risk alleles of CYP2D6 and ABCC2 genes. Kaplan-Meier analysis revealed the number of risk alleles of these two genes to have cumulative effects on recurrence-free survival (log-rank P = .000000083, Fig 2). Adjusted HRs for risk of recurrence computed for patients carrying two or more risk alleles increased from 4.93-fold (two risk alleles) to 45.25-fold (four risk alleles; trend P = .000000055, Table 3).
Fig 2.
Kaplan-Meier estimates of recurrence-free survival for combination effects of CYP2D6 and ABCC2 genotypes. The patients were classified into five groups (zero, one, two, three, or four alleles) based on the number of risk alleles of these two genes.
Associations Between Genotypes and Plasma Concentrations
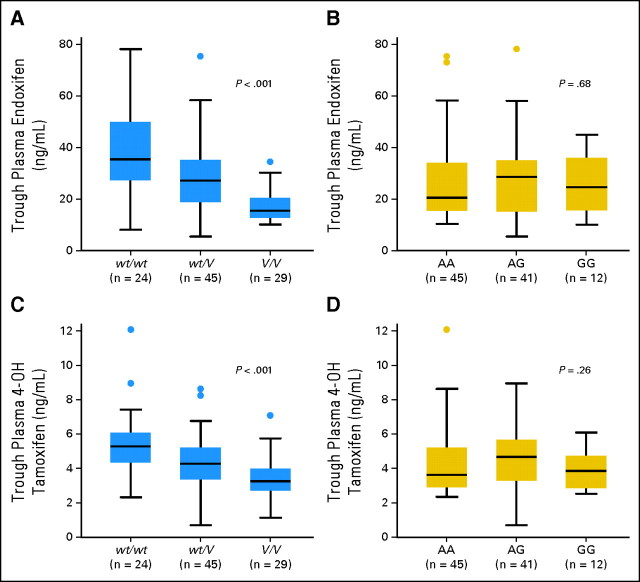
To analyze the effects of CYP2D6 and ABCC2 genotypes on the plasma concentrations of tamoxifen and its metabolites, we recruited 98 independent patients with breast cancer taking tamoxifen 20 mg/d as an adjuvant therapy. The steady-state plasma concentrations of endoxifen among the three genotypic groups of wt/wt, wt/V, and V/V were significantly different (P = .0000043, Fig 3A). Median plasma concentrations of endoxifen in patients with V/V and wt/V were 15.5 and 27.2 ng/mL, respectively, which were 43.8% and 76.8% of the concentration in patients with wt/wt (35.4 ng/mL). The differences in plasma 4-hydroxytamoxifen concentrations among CYP2D6 genotype groups were also statistically significant (P = .00052, Fig 3C). When compared among three genotype groups for CYP2D6*10, similar results were observed (Appendix Fig A1, online only). However, there was no significant association between genotypes of ABCC2 rs3740065 and plasma concentrations of endoxifen and 4-hydroxytamoxifen (Figs 3B and 3D). Plasma tamoxifen and N-desmethyltamoxifen levels were also not significantly different among the three genotype groups (data not shown).
Fig 3.
Steady-state plasma concentrations of (A, B) endoxifen and (C, D) 4-hydroxytamoxifen (4-OH tamoxifen) according to genotypes. (A, C) Comparison among wt/wt, wt/V, and V/V of CYP2D6. (B, D) Comparison among AA, AG, and GG genotypes at rs3740065 in ABCC2. The horizontal line indicates the median concentration, the box covers the 25th to 75th percentiles, and the maximum length of each whisker is 1.5 × the interquartile range; dots outside the whiskers are outliers.
DISCUSSION
Adjuvant tamoxifen treatment substantially improves the 10-year survival of patients with ER-positive breast cancer, with a significant reduction in breast cancer recurrence and mortality.2 Although aromatase inhibitors have demonstrated superiority to tamoxifen as adjuvant therapy for early breast cancer in postmenopausal women, there are some reports indicating that aromatase inhibitors are associated with a higher risk of osteoporosis than tamoxifen.25,26 Therefore, tamoxifen still plays a major therapeutic role in ER-positive breast cancer. We previously reported that CYP2D6*10 was significantly associated with clinical outcome in 67 patients with breast cancer receiving adjuvant tamoxifen monotherapy.7 One group replicated this association; they also found a significant association between CYP2D6*10 and disease-free survival in 152 patients.27 Lim et al28 reported a significant association between CYP2D6*10 and time to progression for patients with metastatic breast cancer receiving tamoxifen, which supports our results. We studied additional patients with breast cancer who received tamoxifen monotherapy and confirmed the significant associations of CYP2D6*10 with recurrence-free survival in the additionally collected sample set (n = 175; log-rank P = .0048). When sample sets of the previous and current studies were combined, the patients with CYP2D6*1/*10 and CYP2D6*10/*10 showed significantly shorter recurrence-free survival compared with patients with CYP2D6*1/*1 (log-rank P = .00027, Appendix Fig A1). We also clarified that CYP2D6*10 was associated with lower plasma levels of endoxifen using 98 patients with breast cancer who were receiving tamoxifen therapy (Appendix Fig A1). These results suggest that a lower clinical efficacy in the tamoxifen-treated patients with CYP2D6*10/*10 may be caused by lower systemic exposure to endoxifen.
We found that two SNPs in ABCC2 were significantly associated with recurrence-free survival of the patients receiving tamoxifen monotherapy. ABCC2 is a member of the ABC transporter, which is involved in the transport of many kinds of drugs and glucuronide conjugates. An in vitro study reporting that ABCC2 was expressed at higher levels in tamoxifen-resistant breast cancer cells suggested that active metabolites of tamoxifen were transported by ABCC2 from breast cancer cells.29 Although we measured steady-state plasma concentrations of tamoxifen and its metabolites, we could not find significant differences among the ABCC2 rs3740065 genotype groups (Fig 3). From these lines of evidence, we suspect that ABCC2 might regulate local exposure of endoxifen to breast cancer cells. The SNP most significantly associated with recurrence-free survival (rs3740065) in this study was one of the tag-SNPs located in intron 29 of the ABCC2 gene. Although we also genotyped one nonsynonymous SNP rs2273697 (I417V),30 we could not find any association of this SNP with recurrence-free survival (log-rank P = .88, r2 of LD with rs3740065 = 0.05). Therefore, we speculate that rs3740065 SNP or some other genetic variations linked to rs3740065 in ABCC2 may be associated with increased expression levels or transport activity of ABCC2 in breast cancer tissue, causing the lower exposure of breast cancer cells to endoxifen. However, further functional analysis will be needed to clarify the biologic mechanisms associated with clinical outcome of tamoxifen therapy.
In summary, our results indicate that genetic polymorphisms in CYP2D6 and ABCC2 may be important predictors for the clinical outcome of tamoxifen treatment for patients with breast cancer. These findings have the potential to improve the ability of physicians to select optimal hormonal therapy for the treatment of hormone receptor–positive breast cancer.
Acknowledgment
We express our heartfelt gratitude to all the study participants. We thank Yuka Kikuchi and Shoko Higuchi for technical assistance. We thank all other members and staff for their contribution to the sample collection and the completion of our study. We thank Dr Eun S. Lee (National Cancer Center, Korea) for her helpful discussion.
Appendix
Fig A1.
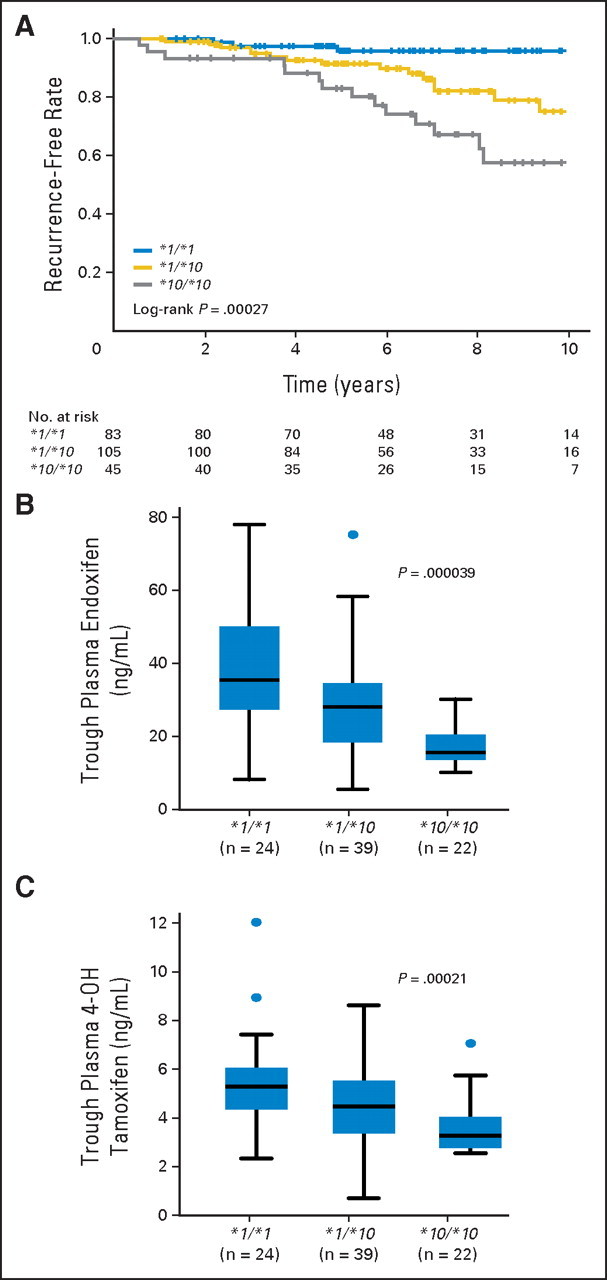
Association of CYP2D6*10 with recurrence-free survival and plasma concentrations of tamoxifen metabolites in patients with breast cancer. (A) Kaplan-Meier estimates of recurrence-free survival for patients with CYP2D6*1/*1, *1/*10, and *10/*10 genotypes. Steady-state plasma concentrations of (B) endoxifen and (C) 4-hydroxytamoxifen (4-OH tamoxifen) according to CYP2D6*10 genotypes. The horizontal line indicates the median concentration, the box covers the 25th to 75th percentiles, and the maximum length of each whisker is 1.5 × the interquartile range; dots outside the whiskers are outliers.
Table A1.
Association of 51 SNPs in ABC Transporter Genes With Recurrence-Free Survival
| Gene and SNP ID | Chromosomal Location | Position in Gene | Allele* |
No. of Patients With Genotype |
Minor Allele Frequency |
Hardy-Weinberg Equilibrium P | Log-Rank P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event |
No Event |
|||||||||||||
| 1 | 2 | 11 | 12 | 22 | 11 | 12 | 22 | Event | No Event | |||||
| ABCB1 | ||||||||||||||
| rs10278483 | 87169901 | Intron 1 | T | C | 34 | 7 | 0 | 192 | 44 | 4 | 0.09 | 0.11 | .57 | .47 |
| rs2157929 | 87140076 | Intron 1 | T | C | 31 | 8 | 2 | 170 | 62 | 7 | 0.15 | 0.16 | .35 | .47 |
| rs10233247 | 87109774 | Intron 1 | A | G | 27 | 8 | 0 | 189 | 50 | 2 | 0.11 | 0.11 | .37 | .84 |
| rs1978095 | 87089577 | Intron 1 | T | C | 23 | 13 | 5 | 134 | 94 | 12 | 0.28 | 0.25 | .83 | .77 |
| rs13233308 | 87082896 | Intron 1 | C | T | 15 | 16 | 10 | 75 | 117 | 48 | 0.44 | 0.44 | .49 | .69 |
| rs4148732 | 87071985 | Intron 1 | A | G | 32 | 9 | 0 | 178 | 58 | 4 | 0.11 | 0.14 | .60 | .32 |
| rs2188524 | 87068371 | Intron 1 | A | G | 34 | 7 | 0 | 192 | 48 | 0 | 0.09 | 0.10 | .069 | .43 |
| rs3213619 | 87068129 | Exon 2 (5′ UTR) | T | C | 36 | 5 | 0 | 211 | 29 | 0 | 0.06 | 0.06 | .28 | .58 |
| rs3789243 | 87058822 | Intron 4 | C | T | 15 | 18 | 8 | 93 | 107 | 39 | 0.41 | 0.39 | .29 | .93 |
| rs1202184 | 87051837 | Intron 5 | A | G | 20 | 16 | 5 | 102 | 119 | 19 | 0.32 | 0.33 | .12 | .62 |
| rs17327442 | 87050926 | Intron 5 | T | A | 31 | 10 | 0 | 184 | 52 | 4 | 0.12 | 0.13 | .84 | .65 |
| rs1202172 | 87048910 | Intron 5 | T | G | 33 | 8 | 0 | 204 | 35 | 0 | 0.10 | 0.07 | .16 | .77 |
| rs10256836 | 87038709 | Intron 5 | G | C | 29 | 12 | 0 | 166 | 68 | 6 | 0.15 | 0.17 | .50 | .23 |
| rs2235018 | 87037301 | Intron 6 | A | G | 24 | 15 | 2 | 153 | 82 | 5 | 0.23 | 0.19 | .14 | .69 |
| rs4148734 | 87031533 | Intron 8 | C | T | 28 | 13 | 0 | 162 | 72 | 6 | 0.16 | 0.18 | .32 | .32 |
| rs868755 | 87027866 | Intron 9 | A | C | 14 | 20 | 6 | 68 | 126 | 44 | 0.40 | 0.45 | .28 | .98 |
| rs2235046 | 87012002 | Intron 17 | A | G | 17 | 19 | 5 | 88 | 126 | 26 | 0.35 | 0.37 | .068 | .45 |
| rs1922242 | 87011603 | Intron 17 | A | T | 19 | 18 | 4 | 101 | 114 | 25 | 0.32 | 0.34 | .41 | .40 |
| rs3789246 | 87005963 | Intron 20 | G | A | 30 | 9 | 2 | 166 | 63 | 9 | 0.16 | 0.17 | .19 | .70 |
| rs2032582 | 86998554 | Exon 22 (Ala893Thr/Ser) | G | A/T | 13 | 22 | 6 | 82 | 124 | 35 | 0.41 | 0.40 | .21 | .53 |
| rs7787082 | 86994987 | Intron 22 | G | A | 13 | 21 | 6 | 92 | 105 | 40 | 0.41 | 0.39 | .43 | .91 |
| rs2373588 | 86991096 | Intron 22 | C | T | 14 | 20 | 6 | 76 | 100 | 61 | 0.40 | 0.47 | .034 | .38 |
| rs2235067 | 86987858 | Intron 23 | G | A | 38 | 3 | 0 | 226 | 14 | 0 | 0.04 | 0.03 | .60 | .96 |
| rs1002205 | 86979110 | Intron 26 | C | G | 14 | 21 | 6 | 102 | 105 | 33 | 0.40 | 0.36 | .61 | .61 |
| rs1045642 | 86976581 | Exon 27 (Ile1145Ile) | C | T | 11 | 24 | 6 | 77 | 116 | 47 | 0.44 | 0.44 | .84 | .50 |
| rs2235047 | 86976468 | Intron 27 | T | G | 14 | 22 | 5 | 94 | 109 | 36 | 0.39 | 0.38 | .90 | .97 |
| rs6979885 | 86975397 | Intron 27 | G | A | 29 | 12 | 0 | 173 | 62 | 5 | 0.15 | 0.15 | .55 | .41 |
| rs1882478 | 86974954 | Intron 27 | C | T | 11 | 22 | 8 | 64 | 127 | 49 | 0.46 | 0.47 | .28 | .48 |
| ABCC2 | ||||||||||||||
| rs12268782 | 101523996 | 5′ upstream region | G | A | 24 | 15 | 2 | 174 | 61 | 6 | 0.23 | 0.15 | .83 | .082 |
| rs2804398 | 101548624 | Intron 7 | T | A | 30 | 8 | 3 | 175 | 58 | 8 | 0.17 | 0.15 | .062 | .79 |
| rs2756109 | 101548736 | Intron 7 | G | T | 10 | 22 | 9 | 110 | 102 | 28 | 0.49 | 0.33 | .58 | .0031 |
| rs2273697 | 101553805 | Exon 10 (Ile417Val) | G | A | 32 | 7 | 1 | 196 | 43 | 2 | 0.11 | 0.10 | .89 | .66 |
| rs11190291 | 101556000 | Intron 11 | C | T | 32 | 7 | 1 | 196 | 43 | 2 | 0.11 | 0.10 | .89 | .66 |
| rs2002042 | 101577921 | Intron 19 | C | T | 19 | 18 | 4 | 114 | 101 | 26 | 0.32 | 0.32 | .66 | .83 |
| rs3740065 | 101595683 | Intron 29 | A | G | 26 | 14 | 1 | 86 | 117 | 38 | 0.20 | 0.40 | .94 | .00020† |
| rs12762549 | 101610761 | 3′ downstream region | G | C | 12 | 20 | 8 | 100 | 110 | 30 | 0.45 | 0.35 | .98 | .074 |
| rs2862691 | 101612513 | 3′ downstream region | C | T | 17 | 19 | 5 | 138 | 86 | 17 | 0.35 | 0.25 | .48 | .042 |
| rs11598781 | 101623010 | 3′ downstream region | C | T | 17 | 19 | 5 | 136 | 90 | 15 | 0.35 | 0.25 | .92 | .036 |
| rs11190303 | 101625199 | 3′ downstream region | C | T | 27 | 13 | 0 | 103 | 103 | 34 | 0.16 | 0.36 | .31 | .00048† |
| ABCG2 | ||||||||||||||
| rs2622604 | 89297948 | Intron 1 | C | T | 31 | 9 | 1 | 200 | 41 | 0 | 0.13 | 0.09 | .32 | .45 |
| rs6532049 | 89286550 | Intron 1 | C | T | 31 | 8 | 1 | 167 | 67 | 7 | 0.13 | 0.17 | .78 | .36 |
| rs2622624 | 89288430 | Intron 1 | G | A | 22 | 16 | 2 | 137 | 88 | 16 | 0.25 | 0.25 | .86 | .85 |
| rs1564481 | 89280289 | Intron 1 | C | T | 19 | 18 | 3 | 92 | 117 | 32 | 0.30 | 0.38 | .54 | .083 |
| rs2231137 | 89280138 | Exon 2 (Val12Met) | G | A | 24 | 15 | 2 | 164 | 69 | 8 | 0.23 | 0.18 | .87 | .30 |
| rs2231142 | 89271347 | Exon 5 (Gln141Lys) | C | A | 15 | 23 | 3 | 121 | 97 | 22 | 0.35 | 0.29 | .84 | .17 |
| rs11931123 | 89269132 | Intron 5 | T | G | 31 | 9 | 0 | 172 | 63 | 6 | 0.11 | 0.16 | .90 | .42 |
| rs1871744 | 89258653 | Intron 6 | T | C | 22 | 18 | 1 | 137 | 88 | 16 | 0.24 | 0.25 | .90 | 1.00 |
| rs2622621 | 89249944 | Intron 9 | G | C | 18 | 18 | 4 | 105 | 102 | 34 | 0.33 | 0.35 | .32 | .74 |
| rs7681519 | 89246864 | Intron 10 | G | C | 27 | 9 | 4 | 175 | 63 | 3 | 0.21 | 0.14 | .85 | .51 |
| rs2622611 | 89244050 | Intron 10 | G | T | 32 | 4 | 5 | 163 | 68 | 10 | 0.17 | 0.18 | .020 | .92 |
| rs2231164 | 89234881 | Intron 14 | A | G | 12 | 21 | 8 | 68 | 121 | 52 | 0.45 | 0.47 | .84 | .71 |
Abbreviations: SNP, single nucleotide polymorphism; ID, identification; UTR, untranslated region.
Major allele in no event group was defined as allele 1.
Statistically significant after Bonferroni's correction based on 51 independent effective tests (P < .00098).
Footnotes
See accompanying editorial on page 1273
Supported in part by Grants-in-Aid from the Kobayashi Institute for Innovative Cancer Chemotherapy (K.K.), the Japan Research Foundation for Clinical Pharmacology (K.K.), and Leading Project of Ministry of Education, Culture, Sports, Science and Technology of Japan (Y.N.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Kazuma Kiyotani, Taisei Mushiroda, Yusuke Nakamura, Hitoshi Zembutsu
Financial support: Yusuke Nakamura
Administrative support: Yusuke Nakamura
Provision of study materials or patients: Fuminori Aki, Koichi Hirata, Yuichi Takatsuka, Minoru Okazaki, Shozo Ohsumi, Takashi Yamakawa, Mitsunori Sasa
Collection and assembly of data: Kazuma Kiyotani, Taisei Mushiroda, Chiyo K. Imamura, Naoya Hosono, Michiaki Kubo, Yusuke Tanigawara, David A. Flockhart, Zeruesenay Desta, Todd C. Skaar, Hitoshi Zembutsu
Data analysis and interpretation: Kazuma Kiyotani, Taisei Mushiroda, Tatsuhiko Tsunoda, Mitsunori Sasa, Hitoshi Zembutsu
Manuscript writing: Kazuma Kiyotani, Taisei Mushiroda, Michiaki Kubo, Yusuke Nakamura, Hitoshi Zembutsu
Final approval of manuscript: Kazuma Kiyotani, Taisei Mushiroda, Chiyo K. Imamura, Naoya Hosono, Tatsuhiko Tsunoda, Michiaki Kubo, Yusuke Tanigawara, David A. Flockhart, Zeruesenay Desta, Todd C. Skaar, Fuminori Aki, Koichi Hirata, Yuichi Takatsuka, Minoru Okazaki, Shozo Ohsumi, Takashi Yamakawa, Mitsunori Sasa, Yusuke Nakamura, Hitoshi Zembutsu
REFERENCES
- 1.Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 3.Borgna JL, Rochefort H. Hydroxylated metabolites of tamoxifen are formed in vivo and bound to estrogen receptor in target tissues. J Biol Chem. 1981;256:859–868. [PubMed] [Google Scholar]
- 4.Lien EA, Solheim E, Lea OA, et al. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res. 1989;49:2175–2183. [PubMed] [Google Scholar]
- 5.Johnson MD, Zuo H, Lee KH, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 6.Desta Z, Ward BA, Soukhova NV, et al. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: Prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310:1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- 7.Kiyotani K, Mushiroda T, Sasa M, et al. Impact of CYP2D6*10 on recurrence-free survival in breast cancer patients receiving adjuvant tamoxifen therapy. Cancer Sci. 2008;99:995–999. doi: 10.1111/j.1349-7006.2008.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borges S, Desta Z, Li L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: Implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80:61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23:9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 10.Goetz MP, Knox SK, Suman VJ, et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101:113–121. doi: 10.1007/s10549-006-9428-0. [DOI] [PubMed] [Google Scholar]
- 11.Sugimoto Y, Tsukahara S, Ishikawa E, et al. Breast cancer resistance protein: Molecular target for anticancer drug resistance and pharmacokinetics/pharmacodynamics. Cancer Sci. 2005;96:457–465. doi: 10.1111/j.1349-7006.2005.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.König J, Nies AT, Cui Y, et al. Conjugate export pumps of the multidrug resistance protein (MRP) family: Localization, substrate specificity, and MRP2-mediated drug resistance. Biochim Biophys Acta. 1999;1461:377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 13.Callaghan R, Higgins CF. Interaction of tamoxifen with the multidrug resistance P-glycoprotein. Br J Cancer. 1995;71:294–299. doi: 10.1038/bjc.1995.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin SC, Choi JS, Li X. Enhanced bioavailability of tamoxifen after oral administration of tamoxifen with quercetin in rats. Int J Pharm. 2006;313:144–149. doi: 10.1016/j.ijpharm.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Moeder CB, Giltnane JM, Harigopal M, et al. Quantitative justification of the change from 10% to 30% for human epidermal growth factor receptor 2 scoring in the American Society of Clinical Oncology/College of American Pathologists guidelines: Tumor heterogeneity in breast cancer and its implications for tissue microarray based assessment of outcome. J Clin Oncol. 2007;25:5418–5425. doi: 10.1200/JCO.2007.12.8033. [DOI] [PubMed] [Google Scholar]
- 17.Hosono N, Kato M, Kiyotani K, et al. CYP2D6 genotyping for functional-gene dosage analysis by allele copy number detection. Clin Chem. 2009;55:1546–1554. doi: 10.1373/clinchem.2009.123620. [DOI] [PubMed] [Google Scholar]
- 18.Hosono N, Kubo M, Tsuchiya Y, et al. Multiplex PCR-based real-time invader assay (mPCR-RETINA): A novel SNP-based method for detecting allelic asymmetries within copy number variation regions. Hum Mutat. 2008;29:182–189. doi: 10.1002/humu.20609. [DOI] [PubMed] [Google Scholar]
- 19.Johansson I, Lundqvist E, Dahl ML, et al. PCR-based genotyping for duplicated and deleted CYP2D6 genes. Pharmacogenetics. 1996;6:351–355. doi: 10.1097/00008571-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Soyama A, Saito Y, Kubo T, et al. Sequence-based analysis of the CYP2D6*36-CYP2D6*10 tandem-type arrangement, a major CYP2D6*10 haplotype in the Japanese population. Drug Metab Pharmacokinet. 2006;21:208–216. doi: 10.2133/dmpk.21.208. [DOI] [PubMed] [Google Scholar]
- 21.Johansson I, Oscarson M, Yue QY, et al. Genetic analysis of the Chinese cytochrome P4502D locus: Characterization of variant CYP2D6 genes present in subjects with diminished capacity for debrisoquine hydroxylation. Mol Pharmacol. 1994;46:452–459. [PubMed] [Google Scholar]
- 22.Kiyotani K, Mushiroda T, Kubo M, et al. Association of genetic polymorphisms in SLCO1B3 and ABCC2 with docetaxel-induced leukopenia. Cancer Sci. 2008;99:967–972. doi: 10.1111/j.1349-7006.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohnishi Y, Tanaka T, Ozaki K, et al. A high-throughput SNP typing system for genome-wide association studies. J Hum Genet. 2001;46:471–477. doi: 10.1007/s100380170047. [DOI] [PubMed] [Google Scholar]
- 24.Williams LD, Twaddle NC, Churchwell MI, et al. Quantification of tamoxifen and metabolites and soy isoflavones in human plasma using liquid chromatography with electrospray ionization tandem mass spectrometry. J AOAC Int. 2006;89:1168–1173. [PubMed] [Google Scholar]
- 25.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 26.Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: Update of study BIG 1-98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Sun Y, Yao L, et al. Association between CYP2D6*10 genotype and survival of breast cancer patients receiving tamoxifen treatment. Ann Oncol. 2008;19:1423–1429. doi: 10.1093/annonc/mdn155. [DOI] [PubMed] [Google Scholar]
- 28.Lim HS, Ju Lee H, Seok Lee K, et al. Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol. 2007;25:3837–3845. doi: 10.1200/JCO.2007.11.4850. [DOI] [PubMed] [Google Scholar]
- 29.Choi HK, Yang JW, Roh SH, et al. Induction of multidrug resistance associated protein 2 in tamoxifen-resistant breast cancer cells. Endocr Relat Cancer. 2007;14:293–303. doi: 10.1677/ERC-06-0016. [DOI] [PubMed] [Google Scholar]
- 30.Haenisch S, May K, Wegner D, et al. Influence of genetic polymorphisms on intestinal expression and rifampicin-type induction of ABCC2 and on bioavailability of talinolol. Pharmacogenet Genomics. 2008;18:357–365. doi: 10.1097/FPC.0b013e3282f974b7. [DOI] [PubMed] [Google Scholar]