Introduction
Leprosy, or Hansen's disease, is caused by the acid-fast bacillus Mycobacterium leprae. Leprosy is transmitted by human-to-human contact, although zoonotic transmission has been described, and contact with the nine-banded armadillo (Dasypus novemcinctus) is a risk factor for development of leprosy.1, 2, 3, 4 Cases 1 and 2 in this case series show zoonotic transmission from armadillos. An additional source of M leprae infection may be soil or land contaminated by leprosy-infected armadillos.3, 5, 6 Cases 3 and 4 support this potential mode of transmission.
Florida recently experienced an increased incidence of leprosy, including 72 confirmed cases since 2010. We report 4 patients seen over 2 years in Central Florida, an area in which leprosy is not endemic.
Case reports
Case 1
An otherwise healthy 55-year-old white man from Central Florida presented to clinic in January 2014 with a 2-year history of enlarging, pink, anesthetic skin lesions on the left ankle and thigh. The patient denied a history of travel to areas where leprosy is endemic, and the patient had not previously come in contact with individuals with leprosy. He reported multiple exposures to armadillos over the last decade; the most significant exposure was when his vehicle struck an armadillo. Armadillo carcass transferred to the patient's shoes and skin on his left arm and ankle. He recalled smelling his left arm before wiping off the remains.
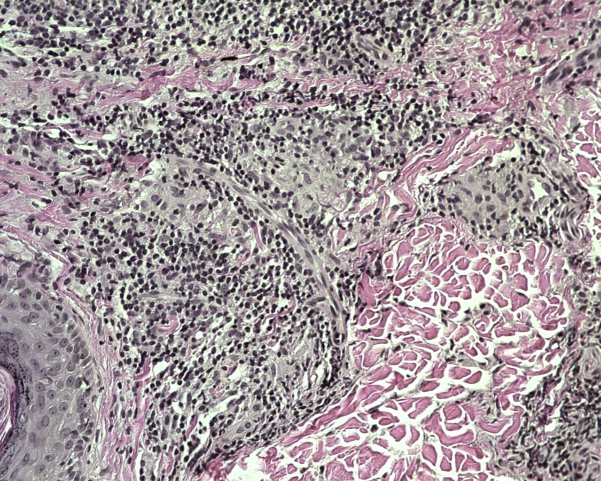
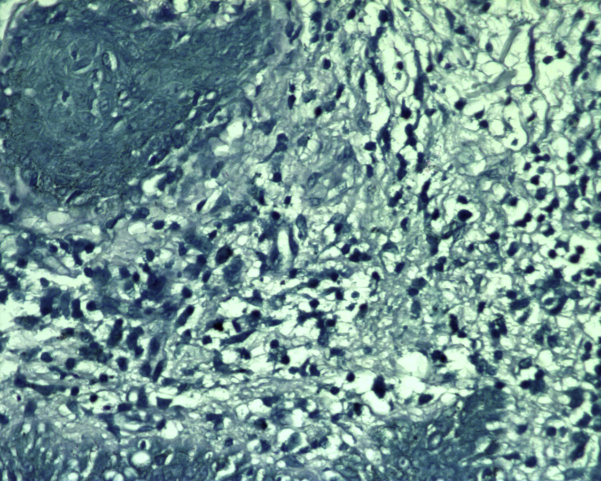
On physical examination, 8- to 15-cm hypopigmented, annular plaques with erythematous borders and central anesthesia to touch and pinprick were located on the left lower extremity (Fig 1). Two- to 5-cm annular, erythematous plaques were found on the scalp, face, trunk, and left upper extremity. Punch biopsies from ankle and leg lesions found mycobacterial infection with granulomatous inflammatory response suggestive of borderline tuberculoid leprosy (Fig 2). A punch biopsy of the upper midback was performed, and acid-fast bacilli were seen after application of a Fite stain (Fig 3). Biopsy specimens from the left lower extremity, which were sent to the National Hansen's Disease Program (NHDP), showed granulomatous infiltrates, and given the clinical findings, the patient had borderline tuberculoid leprosy diagnosed.
Fig 1.
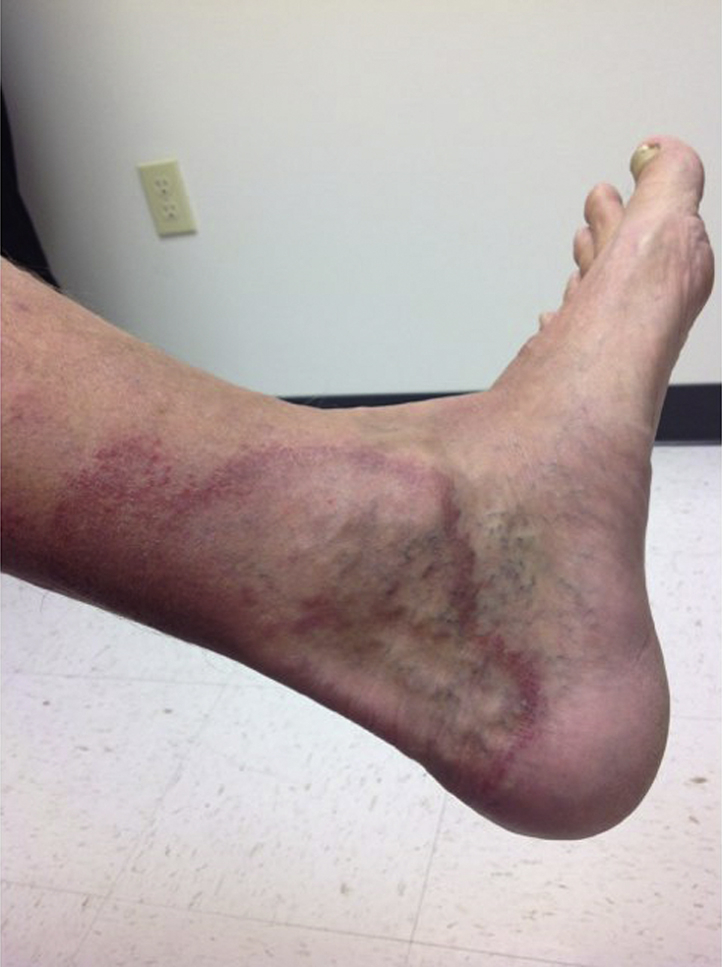
Borderline tuberculoid leprosy. Case 1: Annular, hypopigmented, anesthetic dermal plaques over the medial left ankle.
Fig 2.
Borderline tuberculoid leprosy. Case 1: Granulomatous infiltrate. (Hematoxylin-eosin stain; original magnification: ×40.)
Fig 3.
Borderline tuberculoid leprosy. Case 1: Punch biopsy from the upper midback shows acid-fast bacilli. (Fite stain; original magnification: ×100.)
The patient received a combined daily regimen of dapsone (100 mg), minocycline (100 mg), and rifampin (600 mg) for 24 months. He responded well with no adverse events or immunologically mediated reactions. The smaller lesions disappeared within a week of treatment, with the larger lesions remaining as hyperpigmented anesthetic areas. There is no sign of relapse 12 months after completing treatment.
Case 2
An otherwise healthy 75-year-old white man from Central Florida presented to the clinic in November 2015 with a 5-year history of annular pink lesions on the trunk and thighs and a 3-year history of worsening paresthesias of the feet and distal fingers. He was being treated with gabapentin for peripheral neuropathy of unknown origin for more than 1 year. The patient denied a history of travel to areas where leprosy is endemic, and the patient had not come in contact with individuals with leprosy. He is a hunter and reported direct contact with armadillos and their carcasses over the last decade.
On physical examination, multiple asymmetric, erythematous, and annular plaques with central clearing were distributed on the trunk, thighs, and upper arms. The lesions, feet, and distal fingers were anesthetic to touch and pinprick. A punch biopsy found granulomatous dermatitis and epithelioid histiocytes surrounded by lymphocytes with perineural affinity. Histopathologic examination, after application of Fite stain, found the presence of multiple bacilli. The biopsy specimen was sent to the NHDP, and histopathology findings were diagnostic for leprosy. The patient has not begun treatment.
Case 3
A healthy 70-year-old white man from Central Florida presented to clinic in January 2014 with concern for increasing number of lesions previously diagnosed as cutaneous sarcoidosis in 2012. The patient denied a history of travel to areas where leprosy is endemic, and the patient had not previously come in contact with individuals with leprosy. The patient denied direct contact with armadillos, but reported clearing an acre of overgrown wild land behind his house, which was known to be inhabited by armadillos for more than 20 years.
On physical examination, 8 annular, erythematous plaques, some with central clearing, were located on the trunk, back, and extremities. The plaques were anesthetic to touch and pinprick. Two punch biopsies from truncal lesions found granulomatous inflammatory infiltrate with scattered foci of necrosis (this histologic pattern is not typically seen with leprosy). Fite stain found rare acid-fast bacilli. The NHDP confirmed the presence of acid-fast bacilli and positive polymerase chain reaction for M leprae DNA. Tuberculoid Hansen's disease was diagnosed.
The patient received a combined daily regimen of rifampin (600 mg) and minocycline (100 mg) for 12 months. Prednisone was prescribed at the onset of treatment to suppress a Jarisch-Herxheimer reaction. The lesions resolved to the patient's native skin color. There is no sign of relapse 7 months after completing treatment.
Case 4
An otherwise healthy 73-year-old white woman from Central Florida presented to the clinic in November 2015 with a 4-month history of one nonenlarging, erythematous solitary lesion on the lateral upper portion of the left arm. The patient had not previously come in contact with individuals with leprosy but traveled to Botswana, Zimbabwe, and South Africa (World Health Organization level 1 classification for low endemicity) 2 years before presentation for a 2-week stay. The patient denied direct contact with armadillos but reported that she frequently gardens in her backyard, which is inhabited by several armadillos.
On physical examination, a single nontender, mildly erythematous lesion was located on the left posterior part of the upper arm. The lesion was anesthetic to touch and pinprick. A punch biopsy found superficial and deep perivascular infiltrates composed of lymphocytes and multinucleated histiocytes with noncaseating granulomas; Fite stain showed rare acid-fast bacilli. Biopsy was sent to the NHDP and polymerase chain reaction results were positive. The patient has not begun treatment.
Discussion
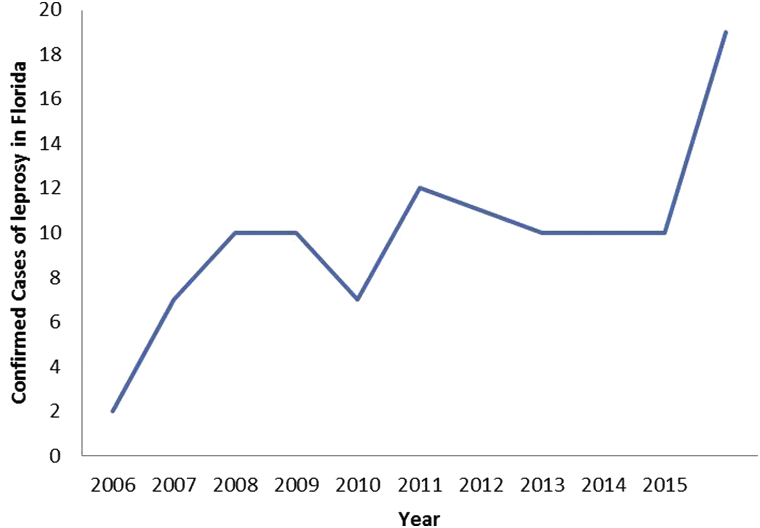
The incidence of leprosy in Florida has recently increased, particularly within Central Florida (Fig 4).
Fig 4.
Increasing incidence of leprosy in Florida. Confirmed cases of leprosy as reported to the Florida Department of Health and including the 4 cases presented in this report.
In the United States, there are approximately 150 new cases of leprosy each year.7 Since 2010, 72 cases of documented leprosy were reported to the Florida Department of Health.8 An outbreak of 19 confirmed cases of leprosy occurred in 2015, with additional cases pending confirmation. In speaking with other Central Florida dermatologists, we suspect an underrepresentation of the total number of cases because of underreporting by private dermatology practices. The increased incidence also leads to question whether improved diagnostic suspicion plays a significant role.
Leprosy in patients from nonendemic areas, such as Central Florida, necessitates an effort to describe the transmission of the disease. Historical evidence suggests that M leprae spreads between humans by nasal droplets via long-term contact with an infected host.9 However, one-third of leprosy patients deny contact with an infected human and have not traveled to a region where leprosy is endemic. In such circumstances, human leprosy is linked to exposure to nine-banded armadillos, the only known nonhuman reservoirs for M leprae.5, 10, 11, 12 Direct and indirect armadillo exposures are significant risk factors for contracting leprosy in nonendemic regions within the United States.10, 13, 14 Cases 1 and 2 exemplify leprosy resulting from direct exposure to armadillos via skin contact and respiratory transmission. Additionally, environmental sources of leprosy have been proposed, including the possible roles of soil, water, and plants from armadillo-inhabited lands.3 Although viable M leprae has been isolated from soil of endemic regions, the exact role of the environment in the transmission of leprosy has yet to be determined.6 Cases 3 and 4 present the possibility of leprosy transmission from indirect exposure to M leprae within armadillo-inhabited land.
The estimated range of infected armadillos includes Florida.4 Early studies document armadillos infected with M leprae at multiple locations in Texas and Louisiana. Later studies show populations of infected armadillos east of the Mississippi River including regions of Mississippi and Alabama.7 This case series provides evidence that zoonotic leprosy exists in Central Florida. However, the extent to which leprosy is spreading to previously uninfected populations of Florida remains unknown. Genotyping armadillos inhabiting Central Florida is crucial to explain leprosy transmission and geographical spread.
Historically, leprosy is one of the most socially stigmatizing diseases, although early diagnosis and treatment often prevents complications. Health care providers must be aware of the increasing incidence of cases of leprosy in Florida and have a high degree of suspicion for leprosy. The public must take precautions against direct contact with armadillos and indirect agricultural contact in armadillo-inhabited areas until the possible role of the environment in the transmission of leprosy is better understood.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.van Beers S.M., Hatta M., Klatser P.R. Patient contact is the major determinant in incident leprosy: implications for future control. Int J Lepr Other Mycobact Dis. 1999;67(2):119–128. [PubMed] [Google Scholar]
- 2.Moet F., Meima A., Oskam L., Richardus J. Risk factors for the development of clinical leprosy among contacts, and their relevance for targeted interventions. Lepr Rev. 2004;75(4):310–326. [PubMed] [Google Scholar]
- 3.Truman R., Fine P. ‘Environmental’ sources of Mycobacterium leprae: issues and evidence. Lepr Rev. 2010;81:89–95. [PubMed] [Google Scholar]
- 4.Truman R.W., Singh P., Sharma R. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364(17):1626–1633. doi: 10.1056/NEJMoa1010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lane J.E., Walsh D.S., Meyers W.M., Klassen-Fischer M.K., Kent D.E., Cohen D.J. Borderline tuberculoid leprosy in a woman from the state of Georgia with armadillo exposure. J Am Acad Dermatol. 2006;55(4):714–716. doi: 10.1016/j.jaad.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 6.Lavania M., Katoch K., Katoch V.M. Detection of viable Mycobacterium leprae in soil samples: insights into possible sources of transmission of leprosy. Infect Genet Evol. 2008;8(5):627–631. doi: 10.1016/j.meegid.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Loughry W.J., Truman R.W., McDonough M., Tilak M., Garnier S., Delsuc F. Is Leprory spreading amoug nine-banded armadillos in the southeastern United States? J Wildl Dis. 2009;45(1):144–152. doi: 10.7589/0090-3558-45.1.144. [DOI] [PubMed] [Google Scholar]
- 8.Health FDo. Florida charts: Hansen's Disease (Leprosy). 2015; http://www.floridacharts.com/charts/OtherIndicators/NonVitalIndNoGrpCountsDataViewer.aspx?cid=0174
- 9.Scollard D.M., Adams L.B., Gillis T.P., Krahenbuhl J.L., Truman R.W., Williams D.L. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19(2):338–381. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce S., Schroeder T.L., Ellner K., Rubin H., Williams T., Wolf J.E., Jr. Armadillo exposure and Hansen's disease: an epidemiologic survey in southern Texas. J Am Acad Dermatol. 2000;43(2 Pt 1):223–228. doi: 10.1067/mjd.2000.106368. [DOI] [PubMed] [Google Scholar]
- 11.Lumpkin L.R., Cox G.F., Wolf J.E. Leprosy in five armadillo handlers. J Am Acad Dermatol. 1983;9(6):899–903. doi: 10.1016/s0190-9622(83)70206-9. [DOI] [PubMed] [Google Scholar]
- 12.Abide J.M., Webb R.M., Jones H.L., Young L. Three indigenous cases of leprosy in Mississippi delta. South Med J. 2008;101(6):635–638. doi: 10.1097/SMJ.0b013e31816f8610. [DOI] [PubMed] [Google Scholar]
- 13.Clark B., Murray C., Horvath L., Deye G., Rasnake M., Longfield R. Case-control study of armadillo contact and Hansen's Disease. Am J Trop Med Hyg. 2008;78(6):962–967. [PubMed] [Google Scholar]
- 14.Deps P.D., Alves B.L., Gripp C.G. Contact with armadillos increases the risk of leprosy in Brazil: A case control study. Indian J Dermatol Venereol Leprol. 2008;74(4):338. doi: 10.4103/0378-6323.42897. [DOI] [PubMed] [Google Scholar]