Abstract
Streptococcal bone and joint infections are less common than staphylococcal cases. Few studies have reported the cases with well-identified Streptococcus species. Their clinical features and prognosis are not clearly known to date. Moreover, no treatment regimen has yet been clarified. We reviewed the streptococcal bone and joint infection cases managed in our centres from January 2009 to December 2013. We described the epidemiology, clinical and microbiologic characteristics, treatment approach and outcome. Among the 93 cases, 83% of patients were men with a median age of 60 years, and 90% of patients had comorbidities or risk factors. Bacteraemia occurred in 14% of cases. Serious complications occurred in six patients, including severe sepsis (two cases) and infective endocarditis (two cases). Orthopaedic device infections were observed in 35% of cases, including 17 patients with internal osteosynthesis device infection, 14 with prosthetic joint infection and three with vertebral osteosynthesis device infection. The median time between orthopaedic device implantation and onset of infection was 447 days. Fourteen species of Streptococcus were identified, including 97 isolates using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and three isolates using molecular identification. The five most represented species included S. agalactiae (37%), S. dysgalactiae (12%), S. anginosus (11%), S. constellatus (10%) and S. pneumoniae (9%). Streptococci isolates were susceptible to amoxicillin, with the exception of one S. mitis isolate. Remission 1 year after the end of treatment was recorded in 83%. One patient died of infection; eight patients had infections that failed to respond to treatment; and seven patients experienced relapse. Twenty patients (22%) had an unfavourable functional outcome, including 19 amputations and one arthrodesis. Five significant prognostic factors associated with an unfavourable clinical outcome were identified, including peripheral neuropathy (p 0.009), peripheral arterial disease (p 0.019), diabetes mellitus (p 0.031), location in the femur (p 0.0036), location in the foot (p 0.0475), osteitis without an orthopaedic device (p 0.041) and infection caused by S. dysgalactiae (p 0.020). The rate of poor outcomes remains high despite the low number of Streptococcus isolates resistant to antibiotics. Some prognostic factors, such as the presence of S. dysgalactiae, are associated with an unfavourable clinical outcome. Antibiotic regimens of streptococcal bone and joint infections are not standardized and need to be further investigated.
Keywords: Arthritis, bacterial infection, bone and joint infection, human, MALDI-TOF MS, osteitis, osteomyelitis, prosthetic joint infection, streptococci, Streptococcus
Introduction
Although streptococcal bone and joint infections such as arthritis and osteomyelitis are less common than infections due to staphylococci, their role as causative agents of bone and joint infection remains significant [1], [2], [3], [4], [5]. Previous studies of streptococcal bone and joint infection have reviewed the clinical characteristics and the outcome of streptococcal arthritis or joint prosthesis infection by streptococcal groups classified by Lancefield and Sherman [3], [4], [5], [6], [7], [8], [9], [10]. Only a few studies of streptococcal bone and joint infection with well-identified species have been reported [11], [12], [13], [14], [15].
Identification of Streptococcus species is difficult and constantly evolving. The current method used for identification relies on phenotypic conventional techniques (Gram stain, evaluation of hemolysis, Lancefield antigen) that appear to be less sensitive [16]. In addition, new methods have been developed to identify the Streptococcus genus, including PCR amplification of the 16S rRNA gene, which has led to a change in the taxonomy of streptococci [17], [18]. Specific nucleic acid probes for S. pneumoniae and S. agalactiae identification have been developed [19]. Recently rpoB, GroEL, soda and tuf gene sequence–based identification has been used to identify the species of Streptococcus anginosus groups [16], [19], [20], [21].
Matrix-assisted desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) identification has been successfully implemented in routine bacterial identification in clinical laboratories [22]. More recently, MALDI-TOF MS has been used as a reliable routine identification tool for Streptococcus species compared to phenotypic and molecular identification [23], [24], [25].
The objective of this study was to review the clinical characteristics and outcomes of well-defined monomicrobial and polymicrobial bone and joint infections caused by Streptococcus species managed at our referral centre for the treatment of bone and joint infections.
Materials and Methods
Study population
This study was approved by the institutional research ethics board, and written informed consent was provided by each patient. We retrospectively reviewed 93 cases of streptococcal bone and joint infection in 3931 patients (inpatients and outpatients aged >18 years) managed in our referral centre for the treatment of bone or joint infections from January 2009 to December 2013. All cases were managed at four university hospitals with 4000 beds in Marseille, France, including four orthopaedic surgery units, two plastic surgery units and two infectious disease units.
Streptococcal bone and joint infections were diagnosed on the basis of medical history with clinical evidence of infection using biological and/or radiologic compliant data, with at least one positive culture from two or more deep samples based on surgical procedures that excluded the contaminated bacteria, as previously described [26]. Prosthetic joint infection was defined when patients had Infectious Diseases Society of America or Musculoskeletal Infection Society criteria meeting the definition for prosthetic joint infection [27], [28]. Infections involving a prosthetic joint were classified according to the time of onset after implantation: early infection (within 1 month) or chronic infection (after 1 month) [29].
We recorded the patients' medical history including demographic characteristics, comorbidities and risk factors associated with bone and joint infection, clinical presentation, and type and site of infection. We then recorded the medical and/or surgical treatment approach and the duration of relapse-free time after treatment. We evaluated treatment success as the remission rate at 3, 6 and 12 months after the end of antibiotic treatment.
An unfavourable clinical outcome was defined as failure to treat, relapse or death caused by infection. Failure to treat was defined by pain and swelling of the bone or joint, wound drainage, implant site erythema, induration or edema, joint pain, joint effusion, fever, purulent discharge from the wound, sinus tract drainage and persistent positive culture from deep samples based on surgical procedures during the treatment period.
Relapse was defined by pain and swelling of the bone or joint, wound drainage, implant site erythema, induration or edema, joint pain, joint effusion, fever, purulent discharge from the wound, sinus tract drainage and persistent positive culture from deep samples based on surgical procedures after the end of treatment and during follow-up examinations at the clinic. An unfavourable functional outcome was defined as amputation, arthrodesis or severe functional deterioration.
Specimen collection and microbiologic analysis
For all patients, deep samples were obtained by surgical procedures, i.e. joint fluid, crushed tissue or bone biopsy samples inoculated on 5% sheep's blood, chocolate, Mueller-Hinton, trypticase soy and MacConkey agar plates (bioMérieux, Marcy l’Etoile, France) and incubated at 37°C in a 5% CO2 atmosphere and in an anaerobic atmosphere for 10 days. Pure bacterial cultures, obtained by picking isolated colonies, were identified with MALDI-TOF MS and molecular methods, as previously described [22], [30]. The antibiotic susceptibilities of Streptococcus sp. isolates were determined and interpreted according to the recommendations of the French Society for Microbiology and the European Committee on Antimicrobial Susceptibility Testing (CA-SFM/EUCAST, available at http://www.sfm-microbiologie.org/UserFiles/files/casfm/CASFM_EUCAST_V1_0_2014.pdf).
Statistical analysis
Data analyses were performed by SPSS 20.0 (IBM SPSS, Chicago, IL, USA). We conducted a descriptive analysis of our population (chi-square test), then analysed the relapse prognostic factors after medicosurgical treatment. Univariate analysis was performed first to identify prognostic variables strongly associated (p <0.2) with risk of relapse (proportional hazard assumptions verified on the representation of Schönefeld residues); then multivariate analysis was performed to assess the predictions specifically for Streptococcus species after adjusting for significant variables in the univariate analysis and/or risk factors such as those reported in the literature including diabetes mellitus, location in the foot and polymicrobial infection [3], [4], [6], [31], [32], [33]. A p value of <0.05 was considered statistically significant. Kaplan-Meier curves were used for graphical illustration. The median follow-up reverse Kaplan-Meier method and the log-rank test were used for comparing curves systematically between variables.
Results
Demographics and clinical characteristics
Of the 93 patients with streptococcal bone and joint infection, 77 patients (83%) were men, yielding a male/female ratio of 4.81; median patient age was 60 years (±17 years; range, 22–92 years). Ninety percent of our patients had comorbidities and/or risk factors. Diabetes mellitus and tobacco use were respectively identified in 34 (37%) and 28 cases (30%). A history of malignancy was observed in 15 patients (16%), including solid cancer in 11 cases (12%) and haematologic malignancy in four cases (4%). Nine patients (10%) were immunocompromised involving corticosteroid treatment in four cases (4%), asplenia in two cases (2%) and HIV infection in two cases (2%). The following risk factors were the most important and frequently observed: chronic wound, peripheral arterial disease, peripheral neuropathy and closed fracture (Table 1).
Table 1.
Clinical relevance of 93 cases of osteoarticular infections due to Streptococcus species
| Characteristic | Total (n = 93), n (%) | Monomicrobial infection (n = 34), n (%) | Polymicrobial infection (n = 59), n (%) |
|---|---|---|---|
| Sex | |||
| Female | 16 (17) | 6 (18) | 10 (17) |
| Male | 77 (83) | 28 (82) | 49 (83) |
| Comorbidities and risk factors | 84 (90) | 25 (74) | 59 (100) |
| Diabetes mellitus | 34 (37) | 10 (29) | 24 (41) |
| Tobacco use | 28 (30) | 10 (29) | 18 (31) |
| Peripheral arterial disease | 27 (29) | 6 (18) | 21 (36) |
| Peripheral neuropathy | 21 (23) | 1 (3) | 20 (34) |
| Malignancy | 15 (16) | 6 (18) | 9 (15) |
| Haematologic malignancy | 4 (4) | 3 (9) | 1 (2) |
| Solid cancer | 11 (12) | 3 (9) | 8 (14) |
| Chronic liver disease | 10 (11) | 1 (3) | 9 (15) |
| Immunodeficiency | 9 (10) | 5 (15) | 4 (7) |
| Corticosteroid treatment | 4 (4) | 1 (3) | 3 (5) |
| Asplenia | 2 (2) | 2 (6) | 0 |
| HIV infection | 2 (2) | 0 | 2 (3) |
| Alcoholism | 7 (8) | 0 | 7 (12) |
| Inflammatory rheumatism | 5 (5) | 2 (6) | 3 (5) |
| Pneumonia | 3 (3) | 1 (3) | 2 (3) |
| Intravenous drug users | 2 (2) | 1 (3) | 1 (2) |
| Chronic wound | 42 (45) | 1 (3) | 41 (69) |
| Closed fracture | 22 (24) | 4 (12) | 18 (31) |
| Open fracture | 8 (9) | 3 (9) | 5 (8) |
| Clinical and biological presentation | |||
| Local inflammation | 64 (69) | 22 (65) | 42 (71) |
| Purulent discharge inside wound | 57 (61) | 9 (26) | 48 (81) |
| Fever | 40 (43) | 20 (59) | 20 (34) |
| Erysipelas | 13 (14) | 2 (6) | 11 (19) |
| Bacteraemia | 13 (14) | 6 (18) | 7 (12) |
| Severe sepsis | 3 (3) | 2 (6) | 1 (2) |
| Endocarditis | 3 (3) | 3 (9) | 0 |
| C-reactive protein rate ≥40 mg/mL | 73 (79) | 28 (82) | 45 (76) |
| Type of infection | |||
| Without orthopaedic devicea | 60 (65) | 17 (50) | 46 (78) |
| Osteitis | 42 (45) | 4 (12) | 38 (64) |
| Arthritis | 12 (13) | 8 (24) | 4 (7) |
| Vertebral osteomyelitis | 9 (10) | 5 (15) | 4 (7) |
| With orthopaedic deviceb | 33 (35) | 17 (50) | 16 (27) |
| Internal osteosynthesis device infection | 17 (18) | 8 (24) | 9 (15) |
| Joint prosthesis infection | 14 (15) | 8 (24) | 6 (10) |
| Vertebral osteosynthesis device infection | 3 (3) | 1 (3) | 2 (3) |
| Infection localization | |||
| Foot | 31 (33) | 2 (6) | 29 (49) |
| Knee | 15 (16) | 9 (26) | 6 (10) |
| Tibia | 14 (15) | 6 (18) | 8 (14) |
| Vertebra | 12 (13) | 8 (24) | 4 (7) |
| Hip | 9 (10) | 5 (15) | 4 (7) |
| Ankle | 8 (9) | 4 (12) | 4 (7) |
| Hand | 4 (4) | 0 | 4 (7) |
| Wrist | 2 (2) | 2 (6) | 0 |
| Sternoclavicular | 1 (1) | 1 (3) | 0 |
| Shoulder | 1 (1) | 1 (3) | 0 |
| Femur | 1 (1) | 0 (0) | 1 (2) |
| Pelvis | 2 (2) | 1 (3) | 1 (2) |
| Multiple localization | 6 (6) | 4 (12) | 2 (3) |
| Orthopaedic device infection delays | |||
| Early infection (month 1) | 4 (12) | 1 (6) | 3 (19) |
| Delayed infection (months 2–6) | 6 (18) | 4 (24) | 2 (13) |
| Late infection (more than month 6) | 23 (70) | 12 (71) | 11 (69) |
Three vertebral infections without osteosynthesis were associated with osteitis and arthritis in one and two, respectively.
One vertebral osteosynthesis device infection was associated with osteitis with internal osteosynthesis device infection.
Local inflammation, which occurred in 64 patients (69%), was the most frequent clinical symptom, followed by purulent discharge inside the wound in 57 patients (61%), fever in 40 (43%) and erysipelas in 13 (14%). Bacteraemia occurred in 13 patients (14%). Serious complications occurred in six patients, including two cases of severe sepsis and two cases of infectious endocarditis. One case of septic shock was recorded (Table 1).
Streptococcal bone and joint infections without an orthopaedic device were observed in 60 patients (65%), including 42 (45%) with osteitis, 12 (13%) with arthritis and nine (10%) with vertebra osteomyelitis. Of the 42 cases of streptococcal osteitis, 27 were located in the foot, including 17 cases of diabetic foot infection and seven cases of nondiabetic foot infection (four cases of peripheral neuropathy and three cases of peripheral arterial disease). Another 15 cases of streptococcal osteitis were located in the tibia (six cases), knee (two cases), ankle (two cases), femur (two cases), hip (one case), pelvis (one case) and hand (one case). Three cases of vertebra osteomyelitis without an orthopaedic device were associated with osteitis in one case and with arthritis in two cases.
Streptococcal orthopaedic implant infections were observed in 33 patients (35%), including 17 cases (18%) of orthopaedic device infection, 14 cases (15%) of prosthesis joint infection and three cases (3%) of vertebral orthopaedic device infection. One case of vertebral orthopaedic device infection was associated with orthopaedic device infection in the tibia. Most cases of orthopaedic device infection (88%) were chronic infections occurring more than 1 month after implantation (Table 1). The median time delay between orthopaedic device implantation and infection onset was 447 days. In total, the foot represented the main location of osteitis (33%), followed by the knee (15%) and tibia (15%). Six patients (5%) had multiple sites of streptococcal bone and joint infection (Table 1).
Microbiologic characteristics
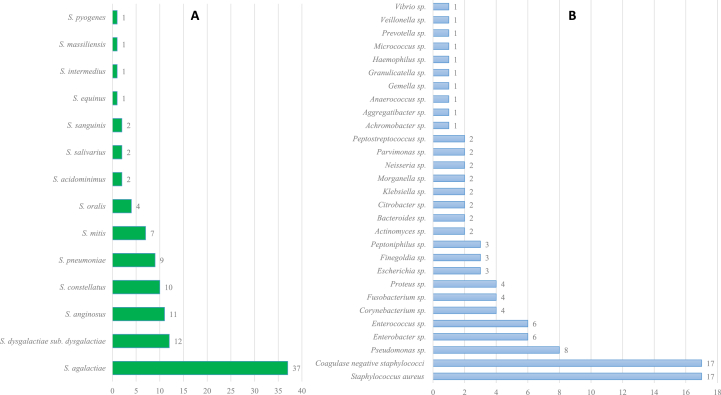
Fourteen species of Streptococcus from 100 streptococcal isolates were identified, of which 97 strains (97%) were identified by MALDI-TOF MS and three (3%) by molecular biology. The five most represented species were S. agalactiae in 37 cases (37%), S. dysgalactiae in 12 cases (12%), S. anginosus in 11 cases (11%), S. constellatus in ten cases (10%) and S. pneumoniae in nine cases (9%) (Fig. 1).
Fig. 1.
Distribution of Streptococcus species (A) and related bacteria (B).
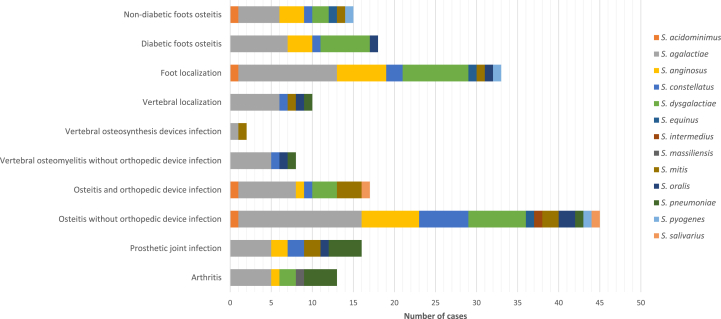
The two most frequent streptococcal species of the 12 cases of streptococcal arthritis were S. agalactiae (42%) and S. pneumoniae (33%). The four main streptococcal species involved in the 42 cases of osteitis without an orthopaedic device were S. agalactiae (36%), S. anginosus (17%), S. dysgalactiae (17%) and S. constellatus (14%). The three main species of the 17 cases of osteitis with internal orthopaedic device infection were S. agalactiae (41%), S. dysgalactiae (18%) and S. mitis (18%). The two most frequent species involved in the 14 cases of joint prosthesis infection were S. agalactiae (36%) and S. pneumoniae (29%). S. agalactiae was the main streptococcal species (47%) involved in the 12 cases of vertebral osteomyelitis with and without an orthopaedic device. The three main streptococcal species involved in the 31 bone and joint infections located in the foot were S. agalactiae (39%), S. dysgalactiae (26%) and S. anginosus (19%); these species were identified in the 18 cases of diabetic foot, at 39%, 33% and 17%, respectively (Fig. 2).
Fig. 2.
Streptococcus species distribution depending on type of infection.
Among the species of streptococci involved in bone and joint infection, S. agalactiae was associated with haematologic malignancy (p 0.023), S. constellatus with solid cancer (p 0.016), S. dysgalactiae with peripheral neuropathy and the foot (p 0.004 and 0.018), S. anginosus with a closed fracture (p 0.019) and S. pneumoniae with arthritis, joint prosthesis infection and in the hip (p 0.015, 0.027 and 0.004).
Thirty-four cases (37%) of streptococcal bone and joint infection were monomicrobial infections; 59 cases (63%) were associated with another bacteria species. The bone and joint infections caused by S. agalactiae and S. pneumoniae were usually monomicrobial infections (p 0.027 and 0.011). Arthritis and vertebral infections without an orthopaedic device were frequently monomicrobial infections (p 0.027 and 0.011). Among the 59 cases of polymicrobial infection, Staphylococcus aureus and coagulase-negative staphylococci were the main bacterial species (Fig. 1). Polymicrobial infections were commonly observed in cases involving peripheral arterial disease, peripheral neuropathy, alcoholism, closed fractures, chronic wounds, location in the foot, and bone and joint infection caused by S. anginosus (p 0.001, 0.001, 0.045, 0.046, 0.0001, 0.001 and 0.006, respectively).
All streptococci isolates were susceptible to amoxicillin, with the exception of one strain of S. mitis that demonstrated reduced susceptibility to amoxicillin. Fifty-six streptococci isolates (48%) demonstrated reduced susceptibility to doxycycline, seven strains (8%) to rifampicin and six strains (9%) to gentamycin.
Medical and surgical treatment
Eighty-two patients (88%) were treated by a combination of surgery with at least one antibiotic that was active against Streptococcus isolates. Eleven patients (12%) received only medical treatment, including five cases (5%) of arthritis, five cases of osteitis without an orthopaedic device, one case of prosthetic joint infection and two cases of vertebral osteomyelitis without an orthopaedic device (Table 2). It should be noted that these two cases were of vertebral osteomyelitis without an orthopaedic device were localized to more than one site, i.e. one case associated with arthritis and one case with osteitis without an orthopaedic device.
Table 2.
Treatment of 93 cases of osteoarticular infections caused by Streptococcus species
| Characteristic | n (%) |
|---|---|
| Antibiotic | |
| Amoxicillin | 55 (59) |
| Rifampicin | 34 (37) |
| Clindamycin | 23 (25) |
| Cotrimoxazole | 21 (23) |
| Fluoroquinolone | 20 (22) |
| Ceftriaxone | 20 (22) |
| Vancomycin | 11 (12) |
| Ticarcillin-clavulanate | 11 (12) |
| Aminoglycoside | 9 (10) |
| Piperacillin-tazobactam | 5 (5) |
| Imipenem-cilastatin | 4 (4) |
| Teicoplanin | 5 (5) |
| Doxycycline | 2 (2) |
| Hyperbaric oxygen therapy | 16 (17) |
| Osteoarticular infection without orthopaedic device | 60 (65) |
| Vertebral osteomyelitis | 9 (10) |
| Medical treatment only | 2 (2) |
| Surgical treatment | 7 (8) |
| Surgical debridement | 3 (3) |
| Surgical debridement and establishment of internal osteosynthesis device | 4 (4) |
| Arthritis | 12 (13) |
| Medical treatment only | 5 (5) |
| Surgical treatment | 7 (8) |
| Surgical debridement | 7 (8) |
| Surgical debridement and establishment of internal osteosynthesis device | 0 |
| Amputation | 0 |
| Osteitis | 42 (45) |
| Medical treatment only | 5 (5) |
| Surgical treatment | 37 (40) |
| Surgical debridement | 21 (23) |
| Surgical debridement and establishment of internal osteosynthesis device | 1 (1) |
| Amputation | 18 (19) |
| Osteoarticular infection with orthopaedic device | 33 (35) |
| Vertebral osteomyelitis | 3 (3) |
| Medical treatment only | 0 |
| Surgical treatment | 3 (3) |
| Surgical debridement without removal | 0 |
| Osteosynthesis removal and implantation of new device | 3 (3) |
| Prosthetic joint | 14 (15) |
| Medical treatment only | 1 (1) |
| Surgical treatment | 13 (14) |
| Surgical debridement without removal | 6 (6) |
| One-stage exchange strategy | 0 |
| Two-stage exchange strategy | 7 (8) |
| Amputation | 0 |
| Internal osteosynthesis device infection | 17 (18) |
| Medical treatment only | 0 |
| Surgical treatment | 17 (18) |
| Surgical debridement without removal | 1 (1) |
| Osteosynthesis removal | 15 (16) |
| Osteosynthesis removal and implantation of new device | 0 |
| Amputation | 1 (1) |
Amoxicillin therapy was provided in 55 cases (59%), rifampicin in 34 cases (37%), clindamycin in 23 cases (25%), quinolones in 20 cases (22%), cotrimoxazole in 21 cases (23%) and ceftriaxone in 20 cases (21%). Twenty-one cases (23%) were treated by only one antibiotic; the main antibiotics used were amoxicillin and ceftriaxone in 12 cases and three cases, respectively. The median dose of amoxicillin used was 9 g/day (range, 6–12 g/day).
Antibiotic combinations were recorded in 72 cases (77%). The most frequently used associated antibiotic was amoxicillin–rifampicin in 24 cases (26%), followed by clindamycin–cotrimoxazole in ten cases (11%) (Table 2). Short courses of initial treatment with intravenous antibiotics were provided in 49 cases (53%). The mean time of antibiotic duration was 124 days (±68 days; range, 16–350 days).
Forty-four patients (47%) were treated directly by oral antibiotics. Forty-nine patients (53%) were treated by intravenous antibiotics with a mean time of intravenous antibiotic treatment of 62 days (median, 34 ± 63 days; range, 2–238 days). Thirty-three patients (35%) were changed secondarily to oral antibiotics at a mean time of 28 days (median, 18 ± 27 days; range, 2–103 days); 16 patients were treated by intravenous antibiotics until the end of treatment.
Surgical treatment was performed in 50 cases (83%) of bone and joint infection without an orthopaedic device and in 32 cases (97%) of orthopaedic device–related infection. Ten cases (83%) of streptococcal vertebral osteomyelitis were treated with surgical debridement, including establishment of vertebral osteosynthesis in our cases. Seven cases (58%) of streptococcal arthritis without orthopaedic device infection were treated with surgical debridement and five cases (42%) with antibiotic treatment without surgical debridement. Thirty-seven cases (88%) of streptococcal osteitis without orthopaedic device infection were treated by surgery, including 21 patients with surgical debridement and 18 patients with amputation. Thirteen cases (93%) of streptococcal prosthetic joint infection were treated by surgery, including six patients with surgical debridement with retention and seven patients with prosthesis replacement by a two-stage exchange strategy. All the cases of internal osteosynthesis device infection were treated by surgery, including 15 cases of osteosynthesis removal, one case of surgical debridement without removal and one case of amputation (Table 2).
Follow-up and clinical outcomes
A total of 93 patients were evaluated during an average follow-up time of 22 months (±15 months; range, 1–58 months). Remission at 1 year after the end of treatment was recorded in 76 patients (82%). Sixteen patients (17%) had an unfavourable clinical outcome related to infection, including one death, eight cases (9%) of failure to treat and seven cases (8%) of relapse. The median time to failure to treat was 141 days after starting treatment, and the median time to relapse was 218 days after the end of treatment. The clinical outcome of failure and relapsed cases is detailed in Table 3.
Table 3.
Clinical outcome of 93 cases of bone and joint infection due to Streptococcus species
| Characteristic | Value |
|---|---|
| Remission at 1 year after end of treatment | 76 (82) |
| Death | 7 (8) |
| During treatment period | 4 (4) |
| After end of treatment | 3 (3) |
| Causes of death | |
| Death by infection | 1 (1) |
| Death by cancer | 3 (3) |
| Death by acute respiratory distress syndrome caused by severe pneumonia | 2 (2) |
| Death by suicide | 1 (1) |
| Unfavourable clinical outcomes | 16 (17) |
| Death by infection | 1 (1) |
| Failure during treatment | 8 (9) |
| Median time to failure | 141 days |
| Evolution | |
| Remission after further ATB | 1 (1) |
| Failure after further treatment | 4 (4) |
| Wound care only | 2 (2) |
| Wound care with suppressive antibiotic therapy | 2 (2) |
| Remission after osteosynthesis removal and antibiotic therapy | 1 (1) |
| Amputation | 2 (2) |
| Relapse | 7 (8) |
| Median time to relapse (after end of treatment) | 218 days |
| Evolution (after further treatment) | |
| Remission | 2 (2) |
| Amputation | 5 (5) |
| Amputation | 19 (20) |
| Median time to amputation (from date of infection) | 447 days |
| Type of infection | |
| Diabetic foot | 11 (12) |
| Ischemic foot | 1 (1) |
| Chronic wound of foot | 1 (1) |
| Chronic osteitis | 5 (5) |
| Joint prosthesis infection | 1 (1) |
| Delay of amputation | |
| Before starting antibiotic treatmenta | 3 (3) |
| Diabetic foot | 3 (3) |
| During antibiotic treatment | 10 (11) |
| Neoplastic transformation | 1 (1) |
| Ischemic foot | 1 (1) |
| Diabetic foot | 5 (5) |
| Chronic osteitis | 2 (2) |
| Joint prosthesis infection | 1 (1) |
| Treatment failure | 0 |
| After end of antibiotic treatmenta | 7 (8) |
| Neoplastic transformation | 0 |
| Ischemic foot | 0 |
| Diabetic foot | 4 (4) |
| Chronic osteitis | 3 (3) |
| Joint prosthesis infection | 0 |
| Failure to treatment | 2 (2) |
| Relapse | 5 (5) |
| Reason for amputation | |
| Treatment failure | 2 (2) |
| Relapse | 6 (6) |
| Other | 11 (12) |
| Neoplastic transformation of foot osteitis | 1 (1) |
| Ischemic foot | 1 (1) |
| Diabetic foot | 6 (6) |
| Chronic osteitis | 2 (2) |
| Joint prosthesis infection | 1 (1) |
Data are presented as n (%) unless otherwise indicated.
Unfavourable clinical outcome was included in cases of treatment failure and relapse.
Twenty patients (22%) had an unfavourable functional outcome, including 19 amputations and one arthrodesis. Amputation was performed in 19 patients (20%), with a median time to amputation from date of infection of 447 days. Diabetic foot was the main type of streptococcal bone and joint infection, leading to amputation in 11 cases, followed by chronic osteitis in five cases, ischemic foot in one case, a chronic foot wound in one case and joint prosthesis infection in one case.
Three amputations were performed before initiating antibiotic treatment; all were diabetic foot infections. Ten amputations were performed during antibiotic treatment involving diabetic foot infection in five cases, chronic osteitis in two cases, neoplastic transformation in one case, ischemic foot in one case and prosthetic joint infection in one case. Seven amputations were performed after the end of antibiotic treatment involving relapses in six cases, diabetic foot infection in four cases, chronic osteitis in three cases and failure to treat in two cases. One patient had two amputations: one before treatment and one after the end of antibiotic treatment for relapse (Table 3).
Seven patients (8%) died, including four during the treatment period and three after the end of treatment. One patient died of infection; two died of acute respiratory distress syndrome related to severe pneumonia; three deaths were related to cancer; and there was one case of suicide.
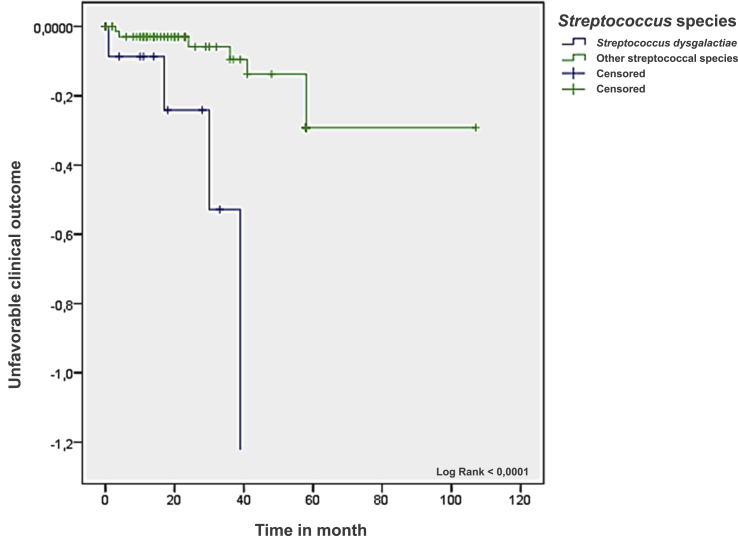
In the univariate analysis (Table 4), five significant prognostic factors associated with an unfavourable clinical outcome were identified, including peripheral neuropathy (odds ratio (95% confidence interval): 5.25 (1.56–17.71) p 0.009), peripheral arterial disease (4.55 (1.39–14.93) p 0.019), diabetes mellitus (4.07 (1.23–13.49) p 0.031), location in the femur (relative risk 26 (2.91–232.62) p 0.0036), location in the foot (relative risk 2.10 (1.01–4.37) p 0.0475), osteitis without an orthopaedic device (3.75 (1.08–13.06) p 0.041) and infection caused by S. dysgalactiae (5.40 (1.41–20.65) p 0.020) (Table 4). We did not identify any specific factors in the multivariate analysis adjusted for diabetes mellitus, location in the foot and polymicrobial bone and joint infection. The Kaplan-Meier curve showed that bone and joint infections caused by S. dysgalactiae have worse clinical outcomes than other streptococcal species (Fig. 3).
Table 4.
Unfavourable clinical outcome and prognostic factors of 93 cases of streptococcal osteoarticular infections
| Patient characteristic | Odds ratio | 95% confidence interval |
p | |
|---|---|---|---|---|
| Upper | Lower | |||
| Male sex | 3.25 | — | — | 0.450 |
| Comorbidities and risk factors | ||||
| Tobacco use | 0.62 | — | — | 0.750 |
| Alcoholism | 2.96 | — | — | 0.238 |
| Chronic liver disease | 2.65 | — | — | 0.189 |
| Peripheral neuropathy | 5.25 | 1.56 | 17.71 | 0.009 |
| Peripheral arterial disease | 4.55 | 1.39 | 14.93 | 0.019 |
| Diabetes mellitus | 4.07 | 1.23 | 13.49 | 0.031 |
| Malignancy | 0.40 | — | — | 0.683 |
| Immunodeficiency | 0.64 | — | — | 1.000 |
| Inflammatory rheumatism | 1.36 | — | — | 0.584 |
| Pneumonia | 5.69 | — | — | 0.291 |
| Chronic wound | 2.55 | — | — | 0.147 |
| Open fracture | 0.75 | — | — | 1.000 |
| Closed fracture | 1.36 | — | — | 0.733 |
| Orthopaedic device | 1.01 | — | — | 1.000 |
| Prosthetic joint | — | — | — | 0.114 |
| Type of infection | ||||
| Arthritis | — | — | — | 0.201 |
| Osteitis without orthopaedic device | 3.75 | 1.08 | 13.06 | 0.041 |
| Vertebral osteomyelitis | 0.88 | — | — | 1.000 |
| Prosthetic joint infection | — | — | — | 0.114 |
| Osteosynthesis device infection | 1.19 | — | — | 0.726 |
| Vertebral osteosynthesis device infection | — | — | — | 1.000 |
| Localization of infection | ||||
| Sternoclavicular | — | — | — | 1.000 |
| Shoulder | — | — | — | 1.000 |
| Wrist | — | — | — | 1.000 |
| Hand | — | — | — | 1.000 |
| Vertebra | 0.56 | — | — | 1.000 |
| Pelvis | — | — | — | 1.000 |
| Hip | 0.64 | — | — | 1.000 |
| Femur | — | — | — | 0.157 |
| Knee | — | — | — | 0.115 |
| Tibia | 2.60 | — | — | 0.223 |
| Ankle | 0.75 | — | — | 1.000 |
| Foot | 1.70 | — | — | 0.371 |
| Multiple localization | — | — | — | 0.584 |
| Streptococcus species | ||||
| acidominimus | — | 1.000 | ||
| agalactiae | 1.12 | — | — | 1.000 |
| anginosus | — | — | — | 0.344 |
| constellatus | 1.62 | — | — | 0.628 |
| dysgalactiae | 5.40 | 1.41 | 20.65 | 0.020 |
| equinus | — | 1.000 | ||
| intermedius | — | 1.000 | ||
| massiliensis | — | 1.000 | ||
| mitis | 0.88 | — | — | 1.000 |
| oralis | 1.85 | — | — | 0.502 |
| pneumoniae | — | — | — | 0.344 |
| pyogenes | — | — | — | 1.000 |
| salivarius | — | — | — | 1.000 |
| sanguinis | — | — | — | 1.000 |
| Polymicrobial infection | 2.44 | — | — | 0.238 |
| Antibiotic | ||||
| Amoxicillin | 0.84 | — | — | 0.774 |
| Third-generation cephalosporin | 1.01 | — | — | 1.000 |
| Ticarcillin–clavulanate | 0.50 | — | — | 1.000 |
| Piperacillin–tazobactam | 0.73 | — | — | 1.000 |
| Imipenem–cilastatin | — | — | — | 1.000 |
| Rifampicin | 0.99 | — | — | 1.000 |
| Doxycycline | 5.69 | — | — | 0.291 |
| Aminoglycoside | 0.64 | — | — | 1.000 |
| Clindamycin | 2.76 | — | — | 0.101 |
| Fluoroquinolones | 2.05 | 0.305 | ||
| Cotrimoxazole | 2.42 | 0.167 | ||
| Vancomycin and teicoplanin | — | — | — | 0.115 |
| Hyperbaric oxygen therapy | 0.73 | — | — | 1.000 |
| Surgical treatment | — | — | — | 0.201 |
Fig. 3.
Unfavourable clinical outcome according to bone and joint infection caused by Streptococcus dysgalactiae vs. other streptococcal species by Kaplan-Meier test.
Discussion
Here we report a large series of streptococcal bone and joint infections identified at the species levels, representing 2.4% of the bone and joint infections followed up in our reference centres over the past 5 years. Streptococcal bone and joint infections, which are less common than staphylococci, were reported to be approximately 2% to 15.4% in the literature [1], [2], [34], [35], [36]. As for the cases of staphylococci, strategy and treatment approaches have improved the management over the last 20 years. There are now specific treatment protocols dedicated to specific staphylococcal species [37]. However, no similar approaches exist for streptococcal bone and joint infections.
Streptococcal bone and joint infections have been reported to be frequent in women (56–62%) [6], [15], which contrasts with our finding that only 17% of the cases in our study involved women. Men were affected more often than women, and therefore the demographic characteristics of streptococcal bone and joint infection in our patients were similar to those found in bone and joint infection caused by staphylococci or anaerobic bacterial bone and joint infection [26], [38].
Numerous risk factors have previously been associated with bone and joint infections, including malignancy, immunodeficiency, diabetes mellitus, age over 65 years, chronic alcoholism, inflammatory rheumatism [3], [4], [6], [31], diabetic foot and decubitus ulcers, especially for osteomyelitis [31]. Most cases of streptococcal bone and joint infection in our study presented a comorbidity or a risk factor at a rate higher than in previous studies (36–38%) [6], [15]. This finding could explain the significant rate of amputation (20%), death (8%) and unfavourable clinical outcomes (17%). Beyond the well-known comorbidity and risk factors for streptococcal bone and joint infection, we observed a significant association between haematologic malignancy and infection with S. agalactiae (p 0.023) and solid cancer and infection with S. constellatus (p 0.016). This observation has been poorly reported in previous studies. We have also identified three main comorbidities that were significantly associated with unfavourable clinical outcomes: peripheral neuropathy (p 0.009), peripheral arterial disease (p 0.019) and diabetes mellitus (p 0.031).
Most of our cases of streptococcal orthopaedic device infection (88% of the 33 cases) were chronic infections occurring after 1 month of implantation, as reported in previous studies [8], [15]. The identification of streptococcal isolates from blood cultures in our series (14% of cases) was less frequent than that in the literature (27–72%).
We identified 14 species of 100 streptococcal isolates involved in bone and joint infection almost exclusively by MALDI-TOF MS. Only three isolates (S. agalactiae, S. pneumoniae and S. anginosus) required molecular identification. S. agalactiae was the most frequent species (37% of cases) in our study; it was observed in 12% to 75% of cases in previous studies [6], [10], [12], [15], [32], [33], [39]. S. pneumoniae is known as a pathogen of arthritis without an orthopaedic device [6] and vertebral osteomyelitis [12]. Nevertheless, we have identified four cases of pneumococcal prosthetic joint infection and one case of orthopaedic device infection; these types of infection are poorly reported in the literature.
Among the 14 Streptococcus species involved in bone and joint infection in our study, S. dysgalactiae was the second streptococcal species (12%). Only a few cases of bone and joint infection caused by S. dysgalactiae (formally group C and/or group G streptococci) have been reported [6], [10], [12], [39], [40], [41].
The cases of streptococcal osteitis are located mainly in the foot [31]; 64% of our streptococcal osteitis cases (42 cases) were located in the foot, including 17 diabetes mellitus patients (63%). Diabetic foot osteomyelitis infections are polymicrobial in more than 50% of cases [32], [33], and these infections frequently occur with Streptococcus species more than with S. aureus [31], [33], although coinfection with S. aureus or Pseudomonas aeruginosa has been reported [32], [33].
In our study, polymicrobial foot osteomyelitis was identified in 29 of the 59 cases of polymicrobial bone and infections with streptococcal involved. The polymicrobial nature of foot osteomyelitis in diabetic foot infection and the role of streptococci have been clearly demonstrated in a study of the microbiome of diabetic foot osteomyelitis using conventional culture techniques and 16S rRNA sequencing [42]. Besides diabetic foot osteomyelitis, the polymicrobial nature of nondiabetic foot osteomyelitis including foot osteomyelitis in patients with peripheral arterial disease, peripheral neuropathy and chronic wound were poorly reported. We have identified that, except for diabetic foot osteomyelitis with streptococcal involved (seven cases) that were mixed infection, 85% of 13 nondiabetic foot osteomyelitis with streptococcal involved were mixed infection. To confirm these results, further study is required to elucidate the role and significance of streptococci in polymicrobial foot osteomyelitis with streptococcal involvement.
In our study, foot osteitis was associated with polymicrobial infection in 93% of cases and coinfection with S. aureus in 38% of cases. Polymicrobial infections represent a significant risk factor for limb loss [32], especially when associated with S. aureus strains resistant to antibiotics [32]. These infections were identified as a prognostic factor associated with relapse in our study. The rate of unfavourable clinical outcome was high (17%) despite the very low rate of streptococcus isolate–reduced susceptibility to amoxicillin (1%) in our study. We think that antibiotic treatment regimens need to be further investigated in future studies, particularly for each well-defined Streptococcus species.
Conclusion
Streptococcal bone and joint infections are rare and are usually polymicrobial. Most streptococcal orthopaedic device infections were chronic infections. Surprisingly, the proportion of unfavourable clinical outcomes and amputations related to infection was considerable despite of low rate of antimicrobial resistance. Therefore, therapeutic failure compared to staphylococci infection is not due to multidrug-resistant microbial strains or to the selection of resistant strains during therapy; rather, it seems to depend on the Streptococcus species involved. Our study clearly determined that some prognostic factors, such as S. dysgalactiae, were associated with unfavourable clinical outcomes.
Acknowledgements
The authors thank C. Leautier, Y. Russo and C. Peruffo for their help on clinical data with this study. The results of this study were presented at the Joint 55th Interscience Conference on Antimicrobial Agents and Chemotherapy and 28th International Congress of Chemotherapy meeting in San Diego, CA, USA.
Conflict of Interest
None declared.
References
- 1.Lipsky B.A., Weigelt J.A., Gupta V., Killian A., Peng M.M. Skin, soft tissue, bone, and joint infections in hospitalized patients: epidemiology and microbiological, clinical, and economic outcomes. Infect Control Hosp Epidemiol. 2007;28:1290–1298. doi: 10.1086/520743. [DOI] [PubMed] [Google Scholar]
- 2.Phillips J.E., Crane T.P., Noy M., Elliott T.S.J., Grimer R.J. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943–948. doi: 10.1302/0301-620X.88B7.17150. [DOI] [PubMed] [Google Scholar]
- 3.Ryan M.J., Kavanagh R., Wall P.G., Hazleman B.L. Bacterial joint infections in England and Wales: analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36:370–373. doi: 10.1093/rheumatology/36.3.370. [DOI] [PubMed] [Google Scholar]
- 4.Kaandorp C.J., Dinant H.J., van de Laar M.A., Moens H.J., Prins A.P., Dijkmans B.A. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis. 1997;56:470–475. doi: 10.1136/ard.56.8.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan D.S., Fisher D., Merianos A., Currie B.J. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect. 1996;117:423–428. doi: 10.1017/s0950268800059070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubost J.J., Soubrier M., De Champs C., Ristori J.M., Sauvezie B. Streptococcal septic arthritis in adults. A study of 55 cases with a literature review. Jt Bone Spine Rev Rhum. 2004;71:303–311. doi: 10.1016/S1297-319X(03)00122-2. [DOI] [PubMed] [Google Scholar]
- 7.Dubost J.J., Couderc M., Tatar Z., Tournadre A., Lopez J., Mathieu S. Three-decade trends in the distribution of organisms causing septic arthritis in native joints: single-center study of 374 cases. Joint Bone Spine. 2014;81:438–440. doi: 10.1016/j.jbspin.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Everts R.J., Chambers S.T., Murdoch D.R., Rothwell A.G., McKie J. Successful antimicrobial therapy and implant retention for streptococcal infection of prosthetic joints. ANZ J Surg. 2004;74:210–214. doi: 10.1111/j.1445-2197.2004.02942.x. [DOI] [PubMed] [Google Scholar]
- 9.Gavet F., Tournadre A., Soubrier M., Ristori J.M., Dubost J.J. Septic arthritis in patients aged 80 and older: a comparison with younger adults. J Am Geriatr Soc. 2005;53:1210–1213. doi: 10.1111/j.1532-5415.2005.53373.x. [DOI] [PubMed] [Google Scholar]
- 10.Meehan A.M., Osmon D.R., Duffy M.C.T., Hanssen A.D., Keating M.R. Outcome of penicillin-susceptible streptococcal prosthetic joint infection treated with debridement and retention of the prosthesis. Clin Infect Dis. 2003;36:845–849. doi: 10.1086/368182. [DOI] [PubMed] [Google Scholar]
- 11.Corvec S., Illiaquer M., Touchais S., Boutoille D., van der Mee-Marquet N., Quentin R. Clinical features of group B streptococcus prosthetic joint infections and molecular characterization of isolates. J Clin Microbiol. 2011;49:380–382. doi: 10.1128/JCM.00581-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murillo O., Roset A., Sobrino B., Lora-Tamayo J., Verdaguer R., Jiménez-Mejias E. Streptococcal vertebral osteomyelitis: multiple faces of the same disease. Clin Microbiol. 2014;20:O33–O38. doi: 10.1111/1469-0691.12302. [DOI] [PubMed] [Google Scholar]
- 13.Raad J., Peacock J.E., Jr. Septic arthritis in the adult caused by Streptococcus pneumoniae: a report of 4 cases and review of the literature. Semin Arthritis Rheum. 2004;34:559–569. doi: 10.1016/j.semarthrit.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Sendi P., Christensson B., Uçkay I., Trampuz A., Achermann Y., Boggian K. Group B streptococcus in prosthetic hip and knee joint–associated infections. J Hosp Infect. 2011;79:64–69. doi: 10.1016/j.jhin.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Zeller V., Lavigne M., Biau D., Leclerc P., Ziza J.M., Mamoudy P. Outcome of group B streptococcal prosthetic hip infections compared to that of other bacterial infections. Jt Bone Spine Rev Rhum. 2009;76:491–496. doi: 10.1016/j.jbspin.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Summanen P.H., Rowlinson M.C., Wooton J., Finegold S.M. Evaluation of genotypic and phenotypic methods for differentiation of the members of the Anginosus group streptococci. Eur J Clin Microbiol Infect Dis. 2009;28:1123–1128. doi: 10.1007/s10096-009-0758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Facklam R. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev. 2002;15:613–630. doi: 10.1128/CMR.15.4.613-630.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drancourt M., Berger P., Raoult D. Systematic 16S rRNA gene sequencing of atypical clinical isolates identified 27 new bacterial species associated with humans. J Clin Microbiol. 2004;42:2197–2202. doi: 10.1128/JCM.42.5.2197-2202.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drancourt M., Roux V., Fournier P.E., Raoult D. rpoB gene sequence-based identification of aerobic Gram-positive cocci of the genera Streptococcus, Enterococcus, Gemella, Abiotrophia, and Granulicatella. J Clin Microbiol. 2004;42:497–504. doi: 10.1128/JCM.42.2.497-504.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glazunova O.O., Raoult D., Roux V. Partial sequence comparison of the rpoB, sodA, groEL and gyrB genes within the genus Streptococcus. Int J Syst Evol Microbiol. 2009;59:2317–2322. doi: 10.1099/ijs.0.005488-0. [DOI] [PubMed] [Google Scholar]
- 21.Hoshino T., Fujiwara T., Kilian M. Use of phylogenetic and phenotypic analyses to identify nonhemolytic streptococci isolated from bacteremic patients. J Clin Microbiol. 2005;43:6073–6085. doi: 10.1128/JCM.43.12.6073-6085.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seng P., Abat C., Rolain J.M., Colson P., Lagier J.C., Gouriet F. Identification of rare pathogenic bacteria in a clinical microbiology laboratory: impact of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2013;51:2182–2194. doi: 10.1128/JCM.00492-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arinto-Garcia R., Pinho M.D., Carriço J.A., Melo-Cristino J., Ramirez M. Comparing matrix-assisted laser desorption ionization–time of flight mass spectrometry and phenotypic and molecular methods for identification of species within the Streptococcus anginosus group. J Clin Microbiol. 2015;53:3580–3588. doi: 10.1128/JCM.01892-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen C.S., Dam-Nielsen C., Arpi M. Matrix-assisted laser desorption/ionization-time of flight mass spectrometry identification of large colony beta-hemolytic streptococci containing Lancefield groups A, C, and G. Infect Dis. 2015;47:575–579. doi: 10.3109/23744235.2015.1043940. [DOI] [PubMed] [Google Scholar]
- 25.Chen J.H.K., She K.K.K., Wong O.Y., Teng J.L.L., Yam W.C., Lau S.K.P. Use of MALDI Biotyper plus ClinProTools mass spectra analysis for correct identification of Streptococcus pneumoniae and Streptococcus mitis/oralis. J Clin Pathol. 2015;68:652–656. doi: 10.1136/jclinpath-2014-202818. [DOI] [PubMed] [Google Scholar]
- 26.Seng P., Barbe M., Pinelli P.O., Gouriet F., Drancourt M., Minebois A. Staphylococcus caprae bone and joint infections: a re-emerging infection? Clin Microbiol Infect. 2014;20:O1042–O1048. doi: 10.1111/1469-0691.12743. [DOI] [PubMed] [Google Scholar]
- 27.Parvizi J., Zmistowski B., Berbari E.F., Bauer T.W., Springer B.D., Della Valle C.J. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osmon D.R., Berbari E.F., Berendt A.R., Lew D., Zimmerli W., Steckelberg J.M. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerli W. Clinical presentation and treatment of orthopaedic implant–associated infection. J Intern Med. 2014;276:111–119. doi: 10.1111/joim.12233. [DOI] [PubMed] [Google Scholar]
- 30.Drancourt M., Raoult D. rpoB gene sequence-based identification of Staphylococcus species. J Clin Microbiol. 2002;40:1333–1338. doi: 10.1128/JCM.40.4.1333-1338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lew D.P., Waldvogel F.A. Osteomyelitis. Lancet. 2004;364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 32.Aziz Z., Lin W.K., Nather A., Huak C.Y. Predictive factors for lower extremity amputations in diabetic foot infections. Diabet Foot Ankle. 2011;2 doi: 10.3402/dfa.v2i0.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavery L.A., Sariaya M., Ashry H., Harkless L.B. Microbiology of osteomyelitis in diabetic foot infections. J Foot Ankle Surg. 1995;34:61–64. doi: 10.1016/S1067-2516(09)80103-8. [DOI] [PubMed] [Google Scholar]
- 34.Titécat M., Senneville E., Wallet F., Dezèque H., Migaud H., Courcol R.J. Bacterial epidemiology of osteoarticular infections in a referent center: 10-year study. Orthop Traumatol Surg Res. 2013;99:653–658. doi: 10.1016/j.otsr.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Grammatico-Guillon L., Baron S., Gettner S. Hospital surveillance of bone and joint infections in France: analysis of the National Hospital Discharge Database 2008 (PMSI) Bull Epidemiol Hebdomadaire. 2013 [Google Scholar]
- 36.Arciola C.R., An Y.H., Campoccia D., Donati M.E., Montanaro L. Etiology of implant orthopedic infections: a survey on 1027 clinical isolates. Int J Artif Organs. 2005;28:1091–1100. doi: 10.1177/039139880502801106. [DOI] [PubMed] [Google Scholar]
- 37.Legout L., Senneville E. Periprosthetic joint infections: clinical and bench research. ScientificWorldJournal. 2013;2013:549091. doi: 10.1155/2013/549091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter G., Vernier M., Pinelli P.O., Million M., Coulange M., Seng P. Bone and joint infections due to anaerobic bacteria: an analysis of 61 cases and review of the literature. Eur J Clin Microbiol Infect Dis. 2014;33:1355–1364. doi: 10.1007/s10096-014-2073-3. [DOI] [PubMed] [Google Scholar]
- 39.Trampuz A., Zimmerli W. Prosthetic joint infections: update in diagnosis and treatment. Swiss Med Wkly. 2005;135:243–251. doi: 10.4414/smw.2005.10934. [DOI] [PubMed] [Google Scholar]
- 40.Park M.J., Eun I.S., Jung C.Y., Ko Y.C., Kim Y.J., Kim C.K. Streptococcus dysgalactiae subspecies dysgalactiae infection after total knee arthroplasty: a case report. Knee Surg Relat Res. 2012;24:120–123. doi: 10.5792/ksrr.2012.24.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernández-Martínez A.I., Pascual M.R., Cimas D., Esteban J. [Septic arthritis due to Streptococcus dysgalactiae ssp. equisimilis] Enferm Infecc Microbiol Clin. 2008;26:670–671. doi: 10.1016/s0213-005x(08)75285-6. [DOI] [PubMed] [Google Scholar]
- 42.van Asten S.A., La Fontaine J., Peters E.J., Bhavan K., Kim P.J., Lavery L.A. The microbiome of diabetic foot osteomyelitis. Eur J Clin Microbiol Infect Dis. 2016;35:293–298. doi: 10.1007/s10096-015-2544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]