Abstract
Purpose
Efficacy and toxicity profile of orally administered clofarabine were evaluated in patients with higher-risk myelodysplastic syndrome (MDS).
Patients and Methods
Thirty-two patients were treated, of whom 22 had intermediate-2 or high-risk disease (International Prognostic Scoring System). Median age was 70 years (range, 53 to 86), nine patients had secondary MDS, and 20 patients experienced prior therapy failure with hypomethylating agents. Three doses of clofarabine were evaluated: 40 mg/m2, 30 mg/m2, and 20 mg/m2 daily for 5 days. Courses were repeated every 4 to 8 weeks.
Results
Eight patients (25%) achieved complete remission (CR), three had (9%) hematologic improvement (HI), and three had (9%) clinical benefit (CB; overall response rate, 43%). Responses in patients who experience treatment failure with hypomethylating agents included CR in two (10%), HI in two (10%), and CB in two patients (10%). No patients died within 6 weeks of induction. Renal failure occurred in four patients in the context of myelosuppresssion-associated infectious complications. Common adverse events were gastrointestinal and hepatic. Myelosuppression was common, but prolonged myelosuppression (> 42 days) was rare. The toxicity profile was better with lower doses of clofarabine, whereas response rates did not differ significantly.
Conclusion
Oral clofarabine has achieved a response rate of 43% in patients with higher-risk MDS. The optimal dose and schedule and the appropriate patient population for such therapy remain to be further defined.
INTRODUCTION
Myelodysplastic syndrome (MDS) is a heterogeneous group of clonal hematopoietic stem cell disorders.1 The International Prognostic Scoring System (IPSS) allows prediction of risk of evolution to acute myeloid leukemia (AML) and survival based on percent of marrow blasts, cytogenetic abnormalities, and severity of cytopenias.2,3 The risk of progression to AML varies between 10% and 70% and the median survival times from 3.5 to 5.7 years to 0.4 to 1.2 years for lower and higher-risk patients, respectively.
Therapy remains challenging. Several drugs for MDS therapy are available: lenalidomide for patients with 5q- lower-risk MDS and hypomethylating agents (eg, azacitidine, decitabine) for patients with lower- and higher-risk disease. The complete remission (CR) rates remain modest and in many cases do not significantly outlast treatment duration.4–6 Once patients progress on hypomethylating agents, treatment options are limited to supportive care, intensive chemotherapy, and allogeneic transplantation.
Clofarabine is a second-generation nucleoside analog. It requires intracellular phosphorylation to become biologically active.7 Several mechanisms of action are involved: inhibition of DNA synthesis; disruption of mitochondrial activity resulting in release of proapoptotic proteins; and inhibition of ribonucleotide reductase leading to intracellular depletion of natural nucleosides and enhanced uptake of the analog during DNA synthesis (self-potentiation). Although clofarabine has mainly been used in AML, previous studies have also suggested efficacy in MDS.8–10
The objective of this study was to explore oral clofarabine in the therapy of MDS. In contrast to other nucleoside analogs, clofarabine has enhanced oral bioavailability of around 50% as a result of substitution of a fluorine at the C-2′-position of the arabinofuranosyl moiety, which increases its stability in gastric acid. Oral clofarabine has demonstrated antitumor activity in solid and hematologic tumor xenograft mouse models.11 The higher median age of patients with MDS, the compromised marrow status of these patients, evidence in elderly AML patients that standard doses of clofarabine may be too toxic, desirability of prolonged exposure times, and possibility of outpatient therapy made oral clofarabine an attractive option to investigate.
PATIENTS AND METHODS
Study Group
Patients with a confirmed diagnosis of MDS based on WHO criteria with marrow blasts ≥ 5% or IPSS intermediate or high-risk group, and patients with chronic myelomonocytic leukemia (CMML), were eligible (Table 1).12 Cytogenetic subgroups were defined as established by Greenberg et al2 (good: normal, del(5q) only, del(20q) only, -Y only; intermediate: +8, single miscellaneous, double abnormalities; poor: complex or chromosome 7 abnormalities). All patients provided written informed consent according to institutional guidelines. Prior biologic or targeted therapies (eg, hypomethylating agents) were permitted. Patients receiving prior intensive multiagent chemotherapy were excluded. Patients were required to have Eastern Cooperative Oncology Group performance status ≤ 2, and adequate hepatorenal function (serum creatinine < 2 mg/dL; total bilirubin < 2 mg/dL; ALT < × 3 upper limit of normal). Patients were excluded for active and uncontrolled infections and other uncontrolled and severe intercurrent illness (eg, symptomatic congestive heart failure), and prior treatment with clofarabine. The study was approved by the institutional review board of The University of Texas M. D. Anderson Cancer Center and was conducted in accordance with the basic principles of the Declaration of Helsinki.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | No. | % |
|---|---|---|
| No. | 32 | |
| Median age, years | 70 | |
| Range | 53-86 | |
| ≥ 60 | 27 | 84 |
| Male sex | 23 | 72 |
| Median % of marrow blasts | 11 | |
| Range | 0-28 | |
| Marrow blasts ≥ 20% | 2 | 6 |
| Secondary MDS | 9 | 28 |
| Prior radiation therapy | 3 | 9 |
| Prior chemotherapy | 5 | 16 |
| Prior stem cell transplant | 1 | 3 |
| Median No. of prior MDS therapy | 1 | |
| Range | 1-4 | |
| Previous MDS therapy | 21 | 66 |
| DNMTI (azacitidine, decitabine) | 20 | 63 |
| Other | 6 | 19 |
| WHO classification | ||
| RA | 2 | 6 |
| RAEB-1 | 11 | 34 |
| RAEB-2 | 11 | 34 |
| CMML-1 | 5 | 15 |
| CMML-2 | 1 | 3 |
| AML | 2 | 6 |
| IPSS | ||
| Intermediate-1 | 4 | 12 |
| Intermediate-2 | 15 | 47 |
| High | 5 | 16 |
| Other (CMML, AML) | 8 | 25 |
| IPSS cytogenetic group | ||
| Good | 12 | 38 |
| Intermediate | 9 | 28 |
| Poor | 10 | 31 |
| Insufficient metaphases | 1 | 3 |
| Transfusion dependent | 24 | 75 |
| Red blood cells | 12 | 38 |
| Platelets | 1 | 3 |
| Both | 11 | 34 |
NOTE. Cytogenetic subgroups were defined as established by Greenberg et al2 (good: normal, del(5q) only, del(20q) only, -Y only; intermediate: +8, single miscellaneous, double abnormalities; poor: complex or chromosome 7 abnormalities).
Abbreviations: MDS, myelodysplastic syndrome; DNMTI, DNA methyltransferase inhibitor; RA, refractory anemia; RAEB, refractory anemia with excess blasts; CMML, chronic myelomonocytic leukemia; AML, acute myeloid leukemia; IPSS, International Prognostic Scoring System.
Treatment and Monitoring
The first dose level of clofarabine was 40 mg/m2 orally daily for days 1 through 5. Based on continuous toxicity evaluations, the dose was reduced to 30 mg/m2 and eventually 20 mg/m2 daily for 5 days. Courses were repeated every 4 to 8 weeks. In case of persistent disease, subsequent courses were started regardless of counts. In case of CR, continuation of therapy required recovery of neutrophils to ≥ 0.75 × 109/L and of platelets to ≥ 50 × 109/L. Any drug-related nonhematologic toxicities required recovery to grade ≤ 1 before the next course. The maximum duration of therapy was 12 courses. Dose reductions (from 40 mg/m2 daily for 5 days to 30 mg/m2, 20 mg/m2, 15 mg/m2, and 10 mg/m2) were required for prolonged marrow aplasia (marrow cellularity ≤ 5% without evidence of disease by day ≥ 42), major infections, and any drug-related ≥ grade 3 nonhematologic toxicities. No escalation of clofarabine doses was allowed. Supportive care included anti-infectious prophylaxis (eg, levaquin, valacyclovir, and itraconazole or voriconazole), hematopoietic growth factors, and transfusions as judged indicated by the treating physician. Antifungals were withheld on days when clofarabine was given to avoid exacerbation of liver function abnormalities.
Patients were monitored with CBC and platelet count at least weekly during course 1 and at various frequencies as mandated thereafter. Creatinine, bilirubin, and ALT were monitored weekly during course 1 followed by once every 2 to 4 weeks while on therapy. Marrow aspiration to document remission was required every one to three courses.
Response Criteria
CR required normalization of blood with neutrophils ≥ 1 × 109/L and a platelets ≥ 100 ×109/L with ≤ 5% marrow blasts. Hematologic improvement (HI) was defined as meeting all criteria for CR except for platelet recovery ≥ 100 × 109/L. Clinical benefit was defined according to the following criteria: increase of platelets (untransfused) by 50% and to more than 30 × 109/L; and/or neutrophil increase by 100% and to more than 1 × 109/L; and/or hemoglobin increase by 2 g/dL (untransfused); and/or transfusion independence. Cytogenetic responses were defined as major in case of disappearance of a cytogenetic abnormality and minor in case of a ≥ 50% reduction of abnormal metaphases.13 Response duration was calculated from response date until relapse date or last follow-up. Disease-free survival was calculated from response date until relapse date or last follow-up. Overall survival was calculated from start of treatment date until death or last follow-up date.
Statistical Design
This is a single-arm, open-label, phase II clinical trial to evaluate the efficacy and safety of oral clofarabine in patients with higher-risk MDS. The primary end point for evaluating efficacy was response rate after three courses of therapy. Safety was evaluated by monitoring and recording grade ≥ 3 adverse events and induction mortality (death within 6 weeks from start of therapy). Historical data of azacitidine in patients with MDS showed a remission, toxicity, and mortality rate of around 7%, 20%, and 10%, respectively.5 Using this information, early stopping rules for efficacy, toxicity, and mortality parameters were defined. Continuous variables (eg, age, hematology values) were summarized using the mean or median (range), and frequency tables were used to summarize categoric variables. The distribution of time-to-event time points was estimated using the Kaplan-Meier method.
RESULTS
Patient Characteristics
Patient characteristics are listed in Table 1. Most patients were older than 60 years and had intermediate-2 or high-risk disease (69%) based on the IPSS. Using WHO criteria, two thirds (68%) of the patients were diagnosed with refractory anemia with excess blasts 1 or 2. Two patients with a diagnosis of AML qualified as refractory anemia with excess blasts in transformation by the French-American-British system and were eligible for the study. Patients with AML and CMML were excluded from the IPSS assignment. Nine patients (28%) had secondary MDS based on a history of therapy for a previous malignancy (eg, radiation therapy, chemotherapy, and/or stem-cell transplant). Thirteen patients (41%) had a history of a prior malignancy (lymphoma, 3; CLL, 2; prostate cancer, 2; breast cancer, 1; myeloproliferative disorder, 2; basal cell skin cancer, 5) with overlap of diagnoses in some patients. Cytogenetics were good in 12 patients (38%), intermediate in nine (28%), and poor in 10 (31%).
Twenty-one patients received at least one line of prior MDS therapy not taking into account hematopoietic growth factors. Median number of prior therapies was one (range, one to four). Six patients received prior azacitidine, 12 decitabine, and two both. Other therapies included obatoclax, thalidomide, mercaptopurine, gimatecan, and the proteinase 3–derived PR1 vaccine.
Response
CR was achieved in eight patients (25%), hematologic improvement in three (9%), and clinical benefit in three patients (9%), for an overall response rate of 43% (Table 2). Clinical benefit meant improvement of platelets in one patient, improvement of hemoglobin levels plus neutrophils in the second patient, and neutrophil response in the third patient. Most responses occurred in the 30 mg/m2 group, but patient numbers in the two other groups were small. Response rates of the 24 patients with a diagnosis of MDS based on WHO criteria (excluding CMML and refractory anemia with excess blasts in transition/AML) included CR in five (21%), hematologic improvement in three (13%), and clinical benefit in three patients (13%) for an overall response rate of 47%. Cytogenetic responses occurred in six (40%) of 15 assessable patients and were major in five (33%) and minor in one patient. Three patients had clinical CR and two had clinical benefit.
Table 2.
Response
| Dose (mg/m2) | No. | CR |
HI |
CB |
Total |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| 40 | 6 | — | — | — | — | 1 | 17 | 1 | 17 |
| 30 | 19 | 7 | 37 | 2 | 11 | 2 | 11 | 11 | 59 |
| 20 | 7 | 1 | 14 | 1 | 14 | — | — | 2 | 28 |
| Total | 32 | 8 | 25 | 3 | 9 | 3 | 9 | 14 | 43 |
Abbreviations: CR, complete remission; HI, hematologic improvement; CB, clinical benefit.
Nine patients had secondary MDS: seven (78%) responded including four patients achieving CR (44%), two (22%) clinical benefit, and one (11%) hematologic improvement. Twenty patients received prior hypomethylating agents. Two patients (10%) achieved CR, two (10%) hematologic improvement, and two (10%) clinical benefit for an overall response rate of 30%. There was one response (hematologic improvement) among six patients after treatment with azacitidine and five responses in 12 patients after treatment with decitabine (differences not significant).
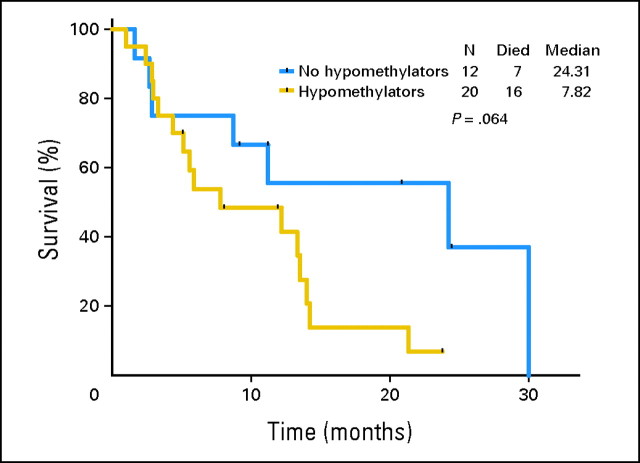
Median response duration was 5.1 months (range, 0.5 to 20 months). Median disease-free survival (CR patients only) was 7.8 months (range, 3.2 to 20 months). Median overall survival of all patients was 9.2 months (range, 1.6 to 30.1 months) with nine patients still alive off-study. Median survival of responding patients was 13.8 months (range, 1.6 to 24.5+ months) and median survival of CR patients was 20.9 months (range, 8.8 to 24.5+ months) with five of eight patients still alive. There was a trend for better survival of patients who did not receive prior therapy with hypomethylating agents (Fig 1).
Fig 1.
Overall survival of patients treated with oral clofarabine who have or have not received prior therapy with hypomethylating agents.
Time to Response, Hospitalizations and Infections, Myelosuppression
Eleven patients required one course and three patients required two courses to respond. The median time to response was 34 days (range, 25 to 113 days) and for CR patients 29 days (range, 25 to 84 days). Among nonresponders, the median number of courses received was one (range, one to three). Twenty-eight patients (88%) received the induction course in an ambulatory care setting. Only four patients were hospitalized for therapy including one patient in a laminar air flow room. Fifty-three percent of the patients received hematopoietic growth factor support at some point during induction therapy, mainly erythropoietin (53%) and/or filgrastim (41%). Almost all patients (94%) received anti-infectious prophylaxis, such as levafloxacin (80%), valacyclovir (70%), and fluconazole (47%). Sixteen patients (50%) developed at least one infectious episode during induction and infectious complications were the major reason for hospital admissions. Most commonly observed were pneumonia in 11 patients (34%), fever of unknown origin in six (19%), and bacterial sepsis in three patients (9%). Eight patients experienced more than one infection. The median number of hospitalizations during the first course was two (range, 0 to three) and the median number of days spent hospitalized was 14 (range, 0 to 69). The number of patients with infections based on dose reveals the following: three (50%) of six patients at 40 mg/m2, nine (47%) of 19 at 30 mg/m2, and one (14%) of seven patients at 20mg/m2.
Consolidation Therapy
Ten patients (31%) received consolidation therapy (one patient in the 40 mg/m2 group, seven at 30 mg/m2, and two at 20 mg/m2). The median number of consolidation courses was one (range, one to eight). All consolidation courses were administered on an outpatient basis. The median total number of courses delivered (including induction) was two (range, one to nine). Fourteen (74%) of 19 patients who required dose reductions on subsequent courses. Dose reductions occurred in three of four patients receiving a starting dose of 40 mg/m2 (subsequent doses delivered at 20 mg/m2), in 10 of 11 patients who started at 30 mg/m2 (dose reductions to 7.5 mg/m2 in two patients with ≥ 2 courses), and in one of four patients who started at 20 mg/m2 (second course delivered at 10 mg/m2 and the third at 7.5 mg/m2). The most common reasons for dose reductions were infectious complications and prolonged thrombocytopenia or neutropenia followed by grade ≥ 3 skin rashes, transaminase elevations, and fatigue.
Nonhematologic Toxicities
Most of the nonhematologic adverse effects did not exceed grade 2, were reversible, and were predominantly related to gastrointestinal disturbances (eg, nausea, emesis, transaminase and bilirubin elevations), skin rashes (including palmoplantar dysesthesias), and constitutional complaints such as fatigue and anorexia (Table 3). Four patients developed acute renal failure (ARF) requiring renal replacement therapy (Table 4). In all, ARF occurred in the context of infectious complications and sepsis. One patient had concomitant grade 3 tumor lysis syndrome and two patients had prolonged neutropenia. All patients died at a median of 27 days after the onset of ARF (ie, 18, 20, 34, and 61 days). Two of the patients were off study at the time of death. Three patients (9%) died while on study on days 19 and 30 of course 2, respectively, and on day 51 of course 1. Two deaths occurred at the 30 mg/m2 dose and one at 40 mg/m2. The deaths were due to prolonged neutropenia and infectious complications aggravated by ARF in two patients.
Table 3.
Nonhematologic Toxicities (n = 32)
| Toxicity | Grade |
|||
|---|---|---|---|---|
| ≤ 2 |
> 2 |
|||
| No. | % | No. | % | |
| Nausea | 27 | 84 | 0 | 0 |
| Skin rash | 16 | 50 | 2 | 6 |
| Emesis | 16 | 50 | 0 | 0 |
| AST elevations | 15 | 47 | 7 | 22 |
| Fatigue | 13 | 41 | 2 | 6 |
| ALT elevations | 11 | 34 | 5 | 16 |
| Headache | 11 | 34 | 0 | 0 |
| Hyperbilirrubinemia | 10 | 31 | 3 | 9 |
| Pruritus | 9 | 28 | 0 | 0 |
| Increase of creatinine | 7 | 22 | 1 | 3 |
| Palmoplantar dysesthesia | 7 | 22 | 0 | 0 |
| Anorexia | 7 | 22 | 0 | 0 |
| Diarrhea | 6 | 19 | 3 | 9 |
| Mucositis | 6 | 19 | 0 | 0 |
| Elevations of alkaline phosphatase | 6 | 19 | 0 | 0 |
| Myalgia | 6 | 19 | 0 | 0 |
| Constipation | 6 | 19 | 0 | 0 |
| Dizziness | 5 | 16 | 0 | 0 |
| Acute renal failure | 0 | 0 | 4 | 13 |
| Edema | 4 | 13 | 0 | 0 |
| Gastrointestinal bleeding | 0 | 0 | 3 | 9 |
| Elevations of serum lipase | 0 | 0 | 1 | 3 |
| Pleural effusion | 0 | 0 | 1 | 3 |
| Supraventricular tachycardia | 0 | 0 | 1 | 3 |
| Tumor lysis syndrome | 0 | 0 | 1 | 3 |
NOTE. Toxicities during first course only.
Table 4.
Summary of Patients With Acute Renal Failure
| Age (years) | Sex | Baseline Creatinine (mg/dL) | Onset | Dose (mg/m2) | Associated Event | Other Possible Nephrotoxics |
|---|---|---|---|---|---|---|
| 58 | Male | 1.7 | Course 2 day 6 | 20* | Fungal pneumonia, sepsis (E. faecium) | Liposomal amphotericin B |
| 70 | Male | 1.8 | Course 2 day 8 | 20† | Sepsis (K. pneumoniae), pneumonia | Levofloxacin |
| 72 | Male | 1.8 | Course 1 day 31 | 30 | Pneumonia, sepsis, tumor lysis syndrome | Moxifloxacin |
| 54 | Female | 0.8 | Course 1 day 10 | 30 | Pneumonia, sepsis (E. faecalis), supraventricular arrhythmia | Vancomycin, amikacin |
NOTE. All four patients required renal replacement therapy.
Reduced from 40 mg/m2 starting dose.
Reduced from 30 mg/m2 starting dose.
DISCUSSION
Despite a variety of therapies in MDS (eg, hematopoietic growth factor support, immune modulation, lenalidomide, hypomethylating agents, histone deacetylase inhibitors), treatment of patients with higher-risk MDS remains challenging and long-term prognosis is often poor.4–6,14 Hypomethylating agents achieve response rates of up to 60%, and have demonstrated improved survival (in the case of azacitidine) in higher-risk MDS.6,14 However, not all patients are susceptible to the effects of these drugs and even those who derive some benefit will ultimately lose their response and progress. Clofarabine is a second-generation deoxyadenosine analog with extensive experience in adult AML and hints of activity in MDS.7,9,15 The development of oral clofarabine seemed ideal for patients with MDS. Oral clofarabine in a daily-for-5-days schedule has been studied in solid and hematologic tumor xenograft mouse models, demonstrating bioavailability of around 50% and excellent antitumor activity.11,16,17 The starting dose of 40 mg/m2 was chosen conservatively given the older age and limited marrow function of patients with MDS. This dose corresponded to half of the 40 mg/m2 intravenous (IV) dose, which was found to be excessive in elderly patients with AML. After six patients had been enrolled, it became apparent that the starting dose was not as well-tolerated as expected, making it necessary to reduce the dose and to assess both toxicity and response profile at lower doses. The reason to de-escalate even further to 20 mg/m2/dose was partly influenced by a similar de-escalation in studies of IV clofarabine in MDS where doses as low as 10 mg IV are being evaluated in ongoing studies.
We report a response rate of 43% (25% CR). Responses in patients who had previously been treated with hypomethylating agents were 30% (10% CR). Responses were not censored for use of hematopoietic growth factors as it is unlikely that responses were influenced by their use. Only seven (50%) of the responders received growth factors during induction, and in at least five of those patients the respective growth factor did not contribute to the clinical response. Considering patients with WHO-defined MDS only, the response rates remain comparable so that the response rate is characteristic for MDS and is not biased by an admixture of patients with AML. Most patients received two courses of therapy including induction. Reasons for not continuing therapy were lack of response or progression. Few patients discontinued therapy because of constitutional symptoms, infectious complications, or myelosuppression. Prolonged myelosuppression (defined as persistent treatment-related cytopenias lasting for ≥ 6 weeks) was infrequent. However, poor baseline marrow function of most patients, aggravated by chemotherapy, contributed to infectious events in half of the patients.
The toxicity profile of clofarabine centered on gastrointestinal and hepatic adverse events. Most toxicities did not exceed grade 2 and were reversible. Acute renal failure occurred in four patients in the context of septic complications and decreased renal baseline renal function in three of those patients. All patients required renal replacement therapy and died. When using clofarabine, caution should be applied in observing renal function and every attempt should be made to avoid concomitant administration of nephrotoxic drugs.
The question arises whether clofarabine at 20 mg/m2/dose is safer, and at least as effective as clofarabine at the higher dose levels. Although only seven patients were accrued at the lowest dose, this group had the lowest rate of infections (14% compared with 47% at 30 mg/m2 and 50% at 40 mg/m2) and required the least number of dose reductions in subsequent courses (one of four patients compared with 10 of 11 and three of four patients, respectively). In contrast, two of seven patients responded indicating at least as much activity as at 40 mg/m2 where only one of six patients responded. A lower dose may therefore not jeopardize activity and be delivered more safely over a more protracted course of time. To validate this observation, further plans include expansion at doses of 20 mg/m2 and lower.
In terms of dose and schedule, preclinical studies of a lung and colon tumor murine model are of interest, where markedly greater regression of tumor was observed when clofarabine was given daily for up to 30 days (in case of the colon tumor murine model) at a lower total dose per course of treatment.18,19 Although based on solid tumor models, the lessons learned could as well be applicable to MDS.
Finally, it remains unclear what kind of patient with MDS is most likely to respond to clofarabine. This study focused on patients with higher-risk MDS, most of whom have failed to respond to hypomethylating agents. In our study, median survival of these patients after clofarabine has been 7.8 months. This compares to a median survival of 4.3 months as reported for patients with MDS who experience treatment failure with decitabine therapy.20 As hypomethylating agents are widely used and appropriate first-line drugs in most patients with MDS, clofarabine might be reasonable salvage therapy in this group. Identification of a “responder profile” would be an important next step. Alternatively, a combination of clofarabine with hypomethylating agents in sequence or concurrently could be devised upfront in higher-risk MDS.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00299156.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Stefan Faderl, Genzyme Oncology (C); Farhad Ravandi, Genzyme Oncology (C); Varsha Gandhi, Genzyme (U); Hagop M. Kantarjian, Genzyme (U) Stock Ownership: None Honoraria: None Research Funding: Stefan Faderl, Genzyme Oncology; Jorge E. Cortes, Genzyme; Varsha Gandhi, Genzyme; Hagop M. Kantarjian, Genzyme Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Stefan Faderl, Varsha Gandhi, William Plunkett, Hagop M. Kantarjian
Administrative support: Anna Byrd, Monica Kwari
Provision of study materials or patients: Stefan Faderl, Guillermo Garcia-Manero, Zeev Estrov, Farhad Ravandi, Gautam Borthakur, Jorge E. Cortes, Susan O'Brien, Hagop M. Kantarjian
Collection and assembly of data: Stefan Faderl, Anna Byrd, Monica Kwari
Data analysis and interpretation: Stefan Faderl, Hagop M. Kantarjian
Manuscript writing: Stefan Faderl
Final approval of manuscript: Stefan Faderl, Guillermo Garcia-Manero, Zeev Estrov, Farhad Ravandi, Gautam Borthakur, Jorge E. Cortes, Susan O'Brien, Varsha Gandhi, William Plunkett, Anna Byrd, Monica Kwari, Hagop M. Kantarjian
REFERENCES
- 1.Catenacci DVT, Schiller GJ. Myelodysplastic syndromes: A comprehensive review. Blood Rev. 2005;19:301–319. doi: 10.1016/j.blre.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg P, Cox C, LeBeau MM, et al. International Scoring System for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 3.Malcovati L, Della Porta MG, Cazzola M. Predicting survival and leukemic evolution in patients with myelodysplastic syndrome. Haematologica. 2006;91:1588–1590. [PubMed] [Google Scholar]
- 4.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 5.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with myelodysplastic syndrome: A study of the Cancer and Leukemia Group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Issa J-P, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian HM, Jeha S, Gandhi V, et al. Clofarabine: Past, present, and future. Leuk Lymphoma. 2007;48:1922–1930. doi: 10.1080/10428190701545644. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian HM, Gandhi V, Kozuch P, et al. Phase I clinical and pharmacology study of clofarabine in patients with solid and hematologic cancers. J Clin Oncol. 2003;21:1167–1173. doi: 10.1200/JCO.2003.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian H, Gandhi V, Cortes J, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–2386. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 10.Faderl S, Gandhi V, O'Brien S, et al. Results of a phase 1-2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory leukemias. Blood. 2005;105:940–947. doi: 10.1182/blood-2004-05-1933. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi T, Kanaqawa J, Akinaga S, et al. Antitumor activity of 2-chloro-9-(2-deoxy-fluoro-beta-D-arabinofuranosyl)adenine, a novel deoxyadenosine analog, against human colon tumor xenografts by oral administration. Cancer Chemother Pharmacol. 1999;43:233–240. doi: 10.1007/s002800050889. [DOI] [PubMed] [Google Scholar]
- 12.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 14.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantarjian HM, Erba HP, Claxton D, et al. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 2009;27:3659–3663. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- 16.Bonate PL, Arthaud L, Cantrell WR, et al. Discovery and development of clofarabine: A nucleoside analogue for treating cancer. Nat Rev Drug Discov. 2006;5:855–863. doi: 10.1038/nrd2055. [DOI] [PubMed] [Google Scholar]
- 17.Carson DA, Wasson DB, Esparza LM, et al. Oral antilymphocyte activity and induction of apoptosis by 2-chloro-2′arabino-fluoro-2′- deoxyadenosine. Proc Natl Acad Sci U S A. 1992;89:2970–2974. doi: 10.1073/pnas.89.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waud W. Birmingham, AL: Southern Research Institute; 2002. Response of subcutaneous NCI-H460 lung tumor to treatment with clofarabine (report ILEX-16) [Google Scholar]
- 19.San Antonio, TX: ILEX Products Inc; 2004. Clofarabine (PO), oxaliplatin, 5-FU, CPT-11 and fludarabine vs. HT-29 human colon tumor xenograft model (final report CLO. 032204-03) [Google Scholar]
- 20.Jabbour E, Garcia-Manero G, Shan J, et al. Outcome of patients (pts) with myelodysplastic syndrome (MDS) and chronic myelomonocytic leukemia (CMML) post decitabine failure. Blood. 2008;112:585. abstr 1659. [Google Scholar]