Abstract
Purpose
To describe estradiol (E2), estrone (E1), and estrone sulfate (E1S) levels during the first year of monthly triptorelin plus exemestane or tamoxifen and to assess possible suboptimal suppression while receiving exemestane plus triptorelin.
Patients and Methods
Premenopausal patients with early breast cancer on the Suppression of Ovarian Function Trial who selected triptorelin as the ovarian suppression method and were randomly assigned to exemestane plus triptorelin or tamoxifen plus triptorelin were enrolled until the target population of 120 patients was reached. Blood sampling time points were 0, 3, 6, 12, 18, 24, 36, and 48 months. Serum estrogens were measured with a highly sensitive and specific assay. This preplanned 12-month analysis evaluated E2, E1, E1S, follicle-stimulating hormone, and luteinizing hormone levels in all patients and the proportion of patients with E2 levels greater than 2.72 pg/mL at any time point during treatment with exemestane plus triptorelin.
Results
One hundred sixteen patients (exemestane, n = 86; tamoxifen, n = 30; median age, 44 years; median E2, 51 pg/mL; 55% prior chemotherapy) started triptorelin and had one or more samples drawn. With exemestane plus triptorelin, median reductions from baseline E2, E1, and E1S levels were consistently ≥ 95%, resulting in significantly lower levels than with tamoxifen plus triptorelin at all time points. Among patients on exemestane plus triptorelin, 25%, 24%, and 17% had an E2 level greater than 2.72 pg/mL at 3, 6, and 12 months, respectively. Baseline factors related to on-treatment E2 level greater than 2.72 pg/mL were no prior chemotherapy (P = .06), higher body mass index (P = .05), and lower follicle-stimulating hormone and luteinizing hormone (each P < .01).
Conclusion
During the first year, most patients on exemestane plus triptorelin had E2 levels below the defined threshold of 2.72 pg/mL, consistent with levels reported in postmenopausal patients on aromatase inhibitors, but at each time point, at least 17% of patients had levels greater than the threshold.
INTRODUCTION
Ovarian function suppression (OFS) has been a therapeutic strategy for premenopausal women with endocrine-responsive breast cancer for more than a century.1 In advanced disease, two underpowered phase III trials from the 1990s reported similar efficacy between ovarian ablation (oophorectomy or ovarian radiation) and the use of gonadotropin-releasing hormone agonists (GnRHa).2,3 Studies testing GnRHa alone4-6 or in combination with tamoxifen7-10 or aromatase inhibitors (AIs)6,9-16 have shown clinical activity with effective estrogen suppression for most patients with breast cancer. The combined efficacy analysis of the Suppression of Ovarian Function Trial (SOFT) and Tamoxifen and Exemestane Trial (TEXT) adjuvant trials17 demonstrated a significant benefit in disease-free survival for exemestane plus OFS compared with tamoxifen plus OFS. Furthermore, SOFT reported improved outcomes with the addition of OFS to tamoxifen, and further improvement with OFS plus exemestane, in women who remained premenopausal after prior chemotherapy and in women younger than age 35 years, with striking benefits in this latter group.18 Approximately 95% of patients used GnRHa as an OFS method in these trials. However, studies addressing estrogen levels while receiving GnRHa treatment in benign (endometriosis17-20) or malignant (breast cancer4,5,8,21,22) diseases have shown incomplete OFS for a minority of patients and/or higher mean estrogen levels than those found in postmenopausal women.
Most reports describing endocrine effects of GnRHa, either alone or combined with tamoxifen or AI,6,8,9,11-16,22,23 have short follow-up (3 to 6 months on average), small sample size, or inadequate quantification of estradiol (E2) levels. More informative data regarding GnRHa-related estrogen suppression is needed in the adjuvant setting, particularly for the combination of GnRHa plus AIs, because AIs have a suboptimal effect and may even have stimulatory activity in the presence of residual ovarian function.24,25
The SOFT Estrogen Substudy (SOFT-EST), a prospective substudy of SOFT, aims to describe estrogen levels during the first 4 years of adjuvant treatment in patients receiving the GnRHa triptorelin plus either tamoxifen or the AI exemestane and to determine whether a subgroup experiencing suboptimal estrogen suppression exists among patients on exemestane plus triptorelin. For estrogen measurements, gas chromatography tandem mass spectrometry (GC/MS/MS), a benchmark assay,26,27 was used. We report results of a preplanned analysis during first 12 months of treatment.
PATIENTS AND METHODS
Study Design
The design of the parent SOFT trial has been described elsewhere.17,18 Briefly, 3,066 women remaining premenopausal after (neo)adjuvant chemotherapy or for whom adjuvant tamoxifen alone was considered a suitable treatment were randomly assigned to 5-year treatment with exemestane plus OFS, tamoxifen plus OFS, or tamoxifen alone. OFS was achieved by choice of triptorelin acetate (Decapeptyl Depot, Ipsen, Paris, France; 3.75 mg intramuscularly every 28 ± 3 days), bilateral oophorectomy, or ovarian irradiation. In patients with prior chemotherapy, eligibility was on the basis of local E2 level within premenopausal range, with menses not required. Because chemotherapy can induce transient ovarian suppression, random assignment was permitted up to 8 months after completing chemotherapy, and tamoxifen was allowed until recovery of premenopausal E2 level. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) measurements were not required because tamoxifen may lower gonadotropin levels in some postmenopausal women into premenopausal range. For patients without prior chemotherapy, random assignment was permitted until 12 weeks from breast surgery, and premenopausal status was defined by regular menses (prior 6 months) or local E2 level in the premenopausal range. In the tamoxifen plus OFS group, tamoxifen was started with triptorelin, whereas exemestane was recommended to begin 6 to 8 weeks after triptorelin initiation.
All patients enrolled in SOFT at 24 selected sites who were randomly assigned to tamoxifen plus OFS or exemestane plus OFS and who chose triptorelin as the OFS method were offered participation in the SOFT-EST substudy. An inclusion ratio of 1:3 was planned, to enroll 30 patients receiving tamoxifen plus triptorelin and 90 patients receiving exemestane plus triptorelin. The baseline sample was collected before trial treatment initiation and thereafter at 3, 6, 12, 18, 24, 36, and 48 months, and samples were taken while fasting and before triptorelin injection. Compliance was assessed during visits by patient diaries. The SOLTI Group coordinated sample collection, and the International Breast Cancer Study Group Statistical Center performed the data analysis.
Study Objectives
After study activation, we planned an early analysis providing 12-month results coinciding with first SOFT efficacy results and an analysis providing 4-year results coinciding with a subsequent update of SOFT. The objectives for the 4-year SOFT-EST study are available at ClinicalTrials.gov (Data Supplement). The primary objectives for this 12-month analysis were to describe E2, estrone (E1), and estrone sulfate (E1S) levels at different time points (3, 6, and 12 months) and to assess the proportion of patients receiving exemestane plus triptorelin with E2 levels greater than 2.72 pg/mL (> 10 pmol/L24), a strict threshold to indicate E2 inconsistent with postmenopausal levels on AI, at each postbaseline time point. The secondary objectives were to assess the differential effects of exemestane plus triptorelin versus tamoxifen plus triptorelin on estrogen, FSH, and LH levels; describe estrogen dynamics in exemestane plus triptorelin–treated patients with E2 levels greater than 2.72 pg/mL at any time point; and explore the characteristics of these patients. In patients treated with exemestane plus triptorelin, exploratory thresholds of E2 greater than 10 pg/mL and greater than 20 pg/mL were also summarized, representing less stringent thresholds above which E2 was clearly inconsistent with postmenopausal levels on AI and inconsistent with GnRHa-induced postmenopausal status, respectively.
Sample Management and Hormone Assays
Serum aliquots were stored locally at −20°C until shipment to inVentiv Health Clinical Laboratory (Princeton, NJ) for estrogen analysis and to Hospital Universitari Vall d’Hebron (Barcelona, Spain) for FSH and LH analyses. GC/MS/MS was used to measure E2, E1, and E1S, with a lower limit of quantification (LLQ) of 0.625, 1.56, and 3.13 pg/mL, respectively. Additional data regarding the GC/MS/MS assay and its validation have been described elsewhere.26 No cross-reactivity with exemestane was observed in an ad hoc experiment conducted before testing samples. All samples from the first 12 months of protocol treatment were run consecutively without knowledge of treatment assignment.
LH and FSH levels were determined by electrochemiluminescence with a Cobas 6000 automated analyzer (Roche Diagnostics, Basel, Switzerland). The measurement range was 0.100 to 200 mIU/mL (defined by the lower detection limit and the maximum of the master curve) for both tests.
Statistical Methods
Longitudinal estrogen and FSH/LH levels were summarized descriptively. Values less than the LLQ were imputed at the LLQ. Among patients randomly assigned to exemestane plus triptorelin, the proportions of patients with E2 greater than 2.72 pg/mL at each time point, along with the 95% exact binomial CIs, were reported. Patient characteristics potentially related to estrogen suppression (ie, age, body mass index [BMI], and menstruation status at random assignment; prior chemotherapy and tamoxifen use; smoking history; and baseline estrogen, FSH, and LH levels) were compared between patients who had on-treatment E2 levels greater than 2.72 pg/mL at any time point and patients who did not using t tests, Wilcoxon rank sum tests, or Fisher’s exact tests.
Comparisons of estrogen and gonadotropin levels between treatment groups at each time point were performed using exact Wilcoxon rank sum tests that handled tied data induced by LLQ.28-30 Semiparametric longitudinal modeling of levels over time that adjusted for patient characteristics provided consistent results (Appendix Fig A1, online only).
The protocol estimated, on the basis of a paired Wilcoxon signed rank test (α = .05, two-sided), that the sample size of 120 patients total, and 90 and 30 patients receiving exemestane plus triptorelin and tamoxifen plus triptorelin, respectively, provided 90% power to detect a mean difference between time points of 1.2 pg/mL (and 1.4 and 2.5 pg/mL, respectively). A Wilcoxon rank sum test (α = .05, two-sided) provided 80% power to detect a mean difference between treatment groups of 2.5 pg/mL at any time point.
RESULTS
Study Population
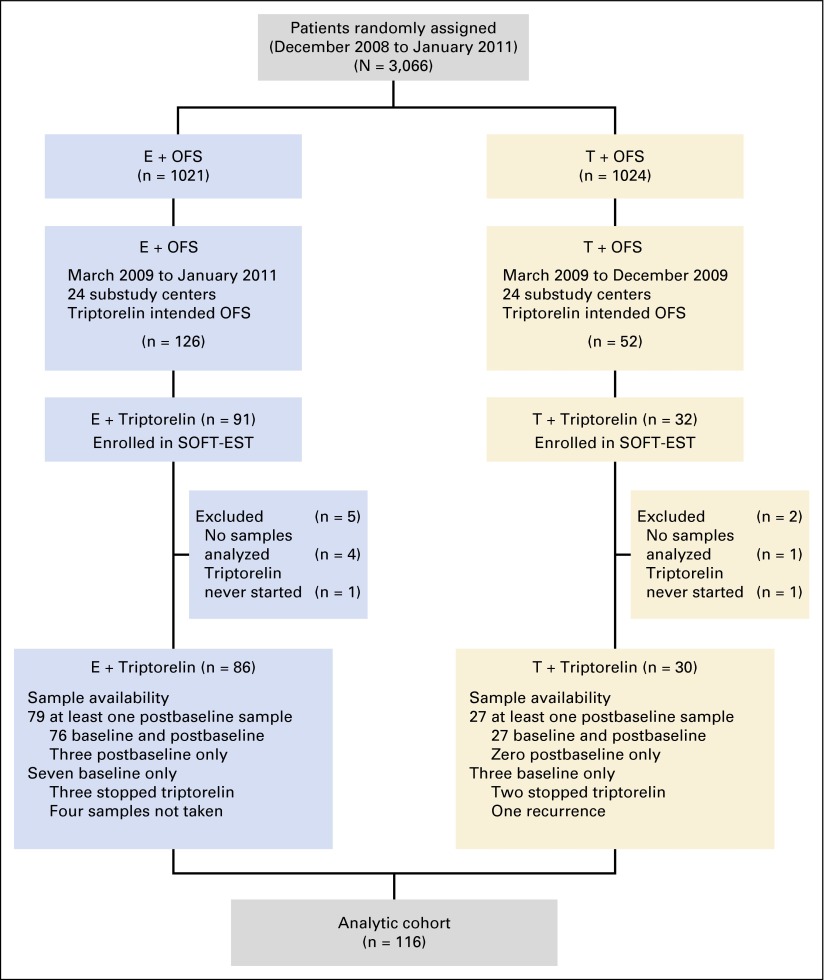
From March 2009 to January 2011, 123 patients were enrolled (tamoxifen plus triptorelin, n = 32; exemestane plus triptorelin, n = 91), of whom 116 patients started triptorelin and had one or more samples analyzed (Fig 1). This group (tamoxifen plus triptorelin, n = 30; exemestane plus triptorelin, n = 86) constituted the analytic cohort (Table 1). Despite meeting the protocol premenopausal definition, 35% of patients in this cohort had baseline E2 levels by GC/MS/MS consistent with postmenopause (≤ 20 pg/mL), which was supported by higher centrally assessed mean FSH and LH values (Appendix Table A1, online only). In the exemestane plus triptorelin group, 56% and 8% of women with or without prior chemotherapy, respectively, had central E2 levels at entry consistent with postmenopausal status (Appendix Table A2, online only).
Fig 1.
Consort diagram of patient flow from random assignment on the parent Suppression of Ovarian Function Trial (SOFT) to inclusion in the analytic cohort for the SOFT Estrogen substudy (SOFT-EST). E, exemestane; OFS, ovarian function suppression; T, tamoxifen.
Table 1.
Patient and Treatment Characteristics and Baseline (Pretreatment) Hormone Levels in the Analytic Cohort, Overall and According to Treatment Assignment
| Characteristic | No. of Patients (%)* | ||
|---|---|---|---|
| Exemestane Plus Triptorelin (n = 86) | Tamoxifen Plus Triptorelin (n = 30) | All Patients (N = 116) | |
| Age at random assignment, years | |||
| Median (IQR) | 44 (40-48) | 44 (41-48) | 44 (41-48) |
| < 35 | 8 (9.3) | 0 (0.0) | 8 (6.9) |
| 35-39 | 10 (11.6) | 6 (20.0) | 16 (13.8) |
| 40-44 | 27 (31.4) | 9 (30.0) | 36 (31.0) |
| 45-49 | 31 (36.0) | 12 (40.0) | 43 (37.1) |
| Menstruation | |||
| Normal | 39 (45.3) | 18 (60.0) | 57 (49.1) |
| Irregular | 14 (16.3) | 3 (10.0) | 17 (14.7) |
| Amenorrhea | 33 (38.4) | 9 (30.0) | 42 (36.2) |
| Hysterectomy, yes | 2 (2.3) | 0 (0.0) | 2 (1.7) |
| BMI, kg/m2 | |||
| Median (IQR) | 24 (22-28) | 23 (22-26) | 24 (22-28) |
| Normal (< 25) | 45 (52.3) | 19 (63.3) | 64 (55.2) |
| Overweight (25 to < 30) | 26 (30.2) | 5 (16.7) | 31 (26.7) |
| Obese (≥ 30) | 13 (15.1) | 4 (13.3) | 17 (14.7) |
| Unknown | 2 (2.3) | 2 (6.7) | 4 (3.4) |
| Smoking history | |||
| Currently smokes | 25 (29.1) | 3 (10.0) | 28 (24.1) |
| Stopped smoking | 11 (12.8) | 9 (30.0) | 20 (17.2) |
| Never smoked | 48 (55.8) | 18 (60.0) | 66 (56.9) |
| Unknown | 2 (2.3) | 0 (0.0) | 2 (1.7) |
| Prior chemotherapy | |||
| No | 39 (45.3) | 13 (43.3) | 52 (44.8) |
| Yes | 47 (54.7) | 17 (56.7) | 64 (55.2) |
| Chemotherapy regimen | |||
| Anthracycline plus taxane | 34 (72.3) | 10 (58.8) | 44 (68.8) |
| Anthracycline based | 11 (23.4) | 4 (23.5) | 15 (23.4) |
| Taxane based | 2 (4.3) | 3 (17.6) | 5 (7.8) |
| Months from last chemotherapy dose to random assignment, median (IQR) | 4 (2-6) | 4 (2-6) | 4 (2-6) |
| Prior tamoxifen | |||
| No | 58 (67.4) | 22 (73.3) | 80 (69.0) |
| Yes | 28 (32.6) | 8 (26.7) | 36 (31.0) |
| Prior tamoxifen duration, weeks, median (IQR) | 16 (9-20) | 21 (10-23) | 18 (9-21) |
| Hormone levels | |||
| Estradiol, pg/mL | |||
| Median (IQR) | 49.9 (6.8-110.0) | 72.5 (6.2-199.0) | 50.6 (6.5-124.0) |
| No. of missing samples | 3 | 0 | 3 |
| Estrone, pg/mL | |||
| Median (IQR) | 43.6 (24.0-70.0) | 39.2 (24.8-102.2) | 41.8 (24.1-71.3) |
| No. of missing samples | 3 | 0 | 3 |
| Estrone sulfate, pg/mL | |||
| Median (IQR) | 784.0 (315.0-1,320.0) | 1,000.0 (272.2-1,620.0) | 894.0 (307.0-1,380.0) |
| No. of missing samples | 3 | 0 | 3 |
| FSH, IU/L | |||
| Median (IQR) | 19.6 (7.6-47.9) | 13.7 (6.4-41.8) | 15.5 (6.9-46.8) |
| No. of missing samples | 6 | 1 | 7 |
| LH, IU/L | |||
| Median (IQR) | 15.9 (5.9-26.8) | 10.3 (6.9-30.4) | 13.7 (6.0-27.7) |
| No. of missing samples | 5 | 1 | 6 |
Abbreviations: BMI, body mass index; FSH, follicle-stimulating hormone; IQR, interquartile range; LH, luteinizing hormone.
Values are numbers and percentages of patients, unless noted otherwise.
Estrogen Levels Over Time According to Treatment
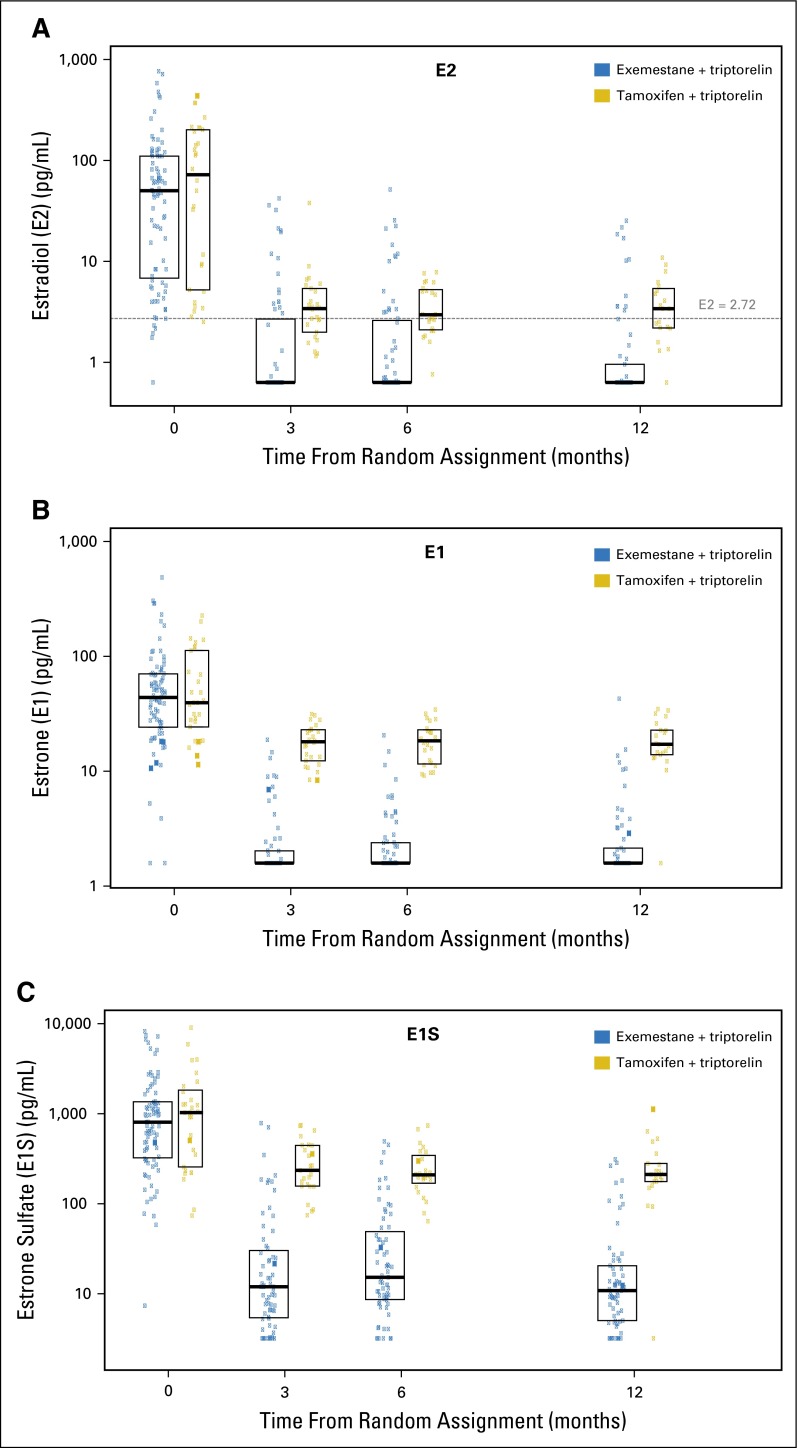
After accounting for missing samples and early discontinuations during the first year, 79 patients treated with exemestane plus triptorelin with at least one postbaseline sample were analyzed. For the three estrogen fractions, a median reduction from baseline of ≥ 95% at all time points was observed in the exemestane plus triptorelin group after treatment initiation (Table 2). Median E2 and E1 levels were 0.625 and 1.56 pg/mL (ie, LLQ), respectively, at all postbaseline time points, whereas median E1S levels were reduced to 11.7, 14.9, and 10.6 pg/mL at 3, 6, and 12 months, respectively (Fig 2 and Appendix Table A3, online only).
Table 2.
Percent Change in Estrogen Levels From Baseline to Each Time Point Among Patients Randomly Assigned to Exemestane Plus Triptorelin
| Estrogen Level | Percentage of Change From Baseline | ||
|---|---|---|---|
| 3 Months | 6 Months | 12 Months | |
| No. of patients* | 64 | 66 | 63 |
| Estradiol, pg/mL | |||
| Mean (SD) | −76 (76) | −82 (62) | −85 (46) |
| Geometric mean | −88 | −90 | −83 |
| Median (IQR) | −96 (−99 to −83) | −96 (−99 to −85) | −97 (−99 to −87) |
| Estrone, pg/mL | |||
| Mean (SD) | −90 (18) | −87 (27) | −82 (61) |
| Geometric mean | −78 | −79 | −91 |
| Median (IQR) | −95 (−98 to −91) | −95 (−98 to −91) | −95 (−98 to −90) |
| Estrone sulfate, pg/mL | |||
| Mean (SD) | −89 (27) | −86 (36) | −93 (13) |
| Geometric mean | −90 | −89 | −92 |
| Median (IQR) | −98 (−99 to −93) | −97 (−99 to −92) | −98 (−99 to −94) |
Abbreviations: IQR, interquartile range; SD, standard deviation;.
Denotes the number of patients with two samples, at baseline and at specified time point, to calculate change.
Fig 2.
Distributions of (A) estradiol (E2), (B) estrone (E1), and (C) estrone sulfate (E1S) over time according to treatment assignment. Boxes indicate the 25th, 50th, and 75th percentiles. (A) The horizontal dashed line indicates the threshold of 2.72 pg/mL. Levels were significantly different at each postbaseline time point (each P < .001).
The reductions in E2, E1, and E1S were greater in the exemestane plus triptorelin group than in the tamoxifen plus triptorelin group (P < .001 for each postbaseline time point; Fig 2). Estrogen values over time according to treatment group are summarized in Appendix Table A3.
Patients With E2 Greater Than 2.72 pg/mL in Exemestane Plus Triptorelin Group
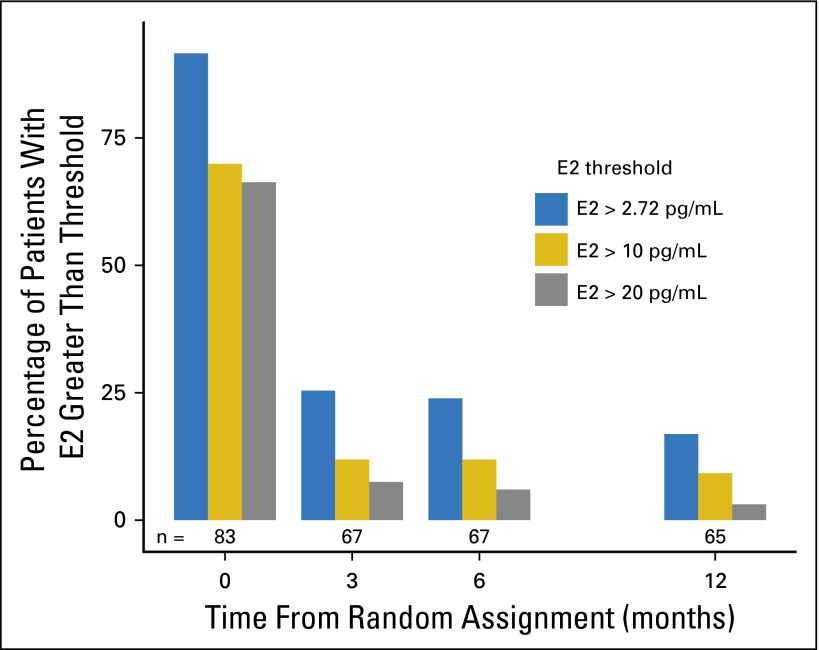
In total, 27 (34.2%; 95% CI, 23.9% to 45.7%) of 79 patients had at least one postbaseline E2 value greater than 2.72 pg/mL. At 3, 6, and 12 months, 25%, 24%, and 17% of patients, respectively, had E2 levels greater than 2.72 pg/mL. These results, and those obtained by exploring additional thresholds of 10 and 20 pg/mL, are summarized in Fig 3 and are further summarized according to prior chemotherapy use in Appendix Table A2. Two patients in the exemestane plus triptorelin group experienced vaginal bleeding more than 3 months after triptorelin initiation, but elevated E2 (41 pg/mL) was centrally demonstrated in only one patient (Appendix Table A4, online only).
Fig 3.
Percentages of patients in the exemestane plus triptorelin group with estradiol (E2) values greater than the predefined threshold (> 2.72 pg/mL, which defines a strict threshold to indicate E2 inconsistent with postmenopausal levels on an aromatase inhibitor) and greater than two additional exploratory thresholds (> 10 and > 20 pg/mL, representing a less strict threshold above which E2 was clearly inconsistent with postmenopausal levels on an aromatase inhibitor and a threshold above which E2 was inconsistent with gonadotropin-releasing hormone agonist–related postmenopausal status, respectively) at each time point. The number of patients tested at each time point is shown at the bottom of the bars.
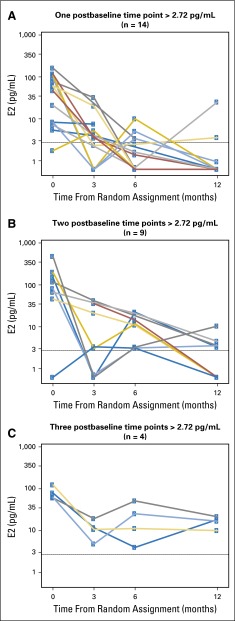
The estrogen levels over time for the 27 patients who had at least one postbaseline E2 level greater than 2.72 pg/mL are displayed in Fig 4. Among them, 14 and nine patients had E2 levels greater than the threshold at one and two postbaseline time points, respectively. Four patients (three of whom were younger than age 35 years and three of whom had not received prior chemotherapy) had E2 levels greater than 2.72 pg/mL at all three postbaseline time points, which corresponds to 8% of the women (four of 48 women) with all three postbaseline samples analyzed (Appendix Table A5, online only).
Fig 4.
Estradiol (E2) levels over time for 27 patients with at least one E2 level greater than 2.72 pg/mL according to the number (one, two, or three) of postbaseline samples with E2 level greater than 2.72 pg/mL. The horizontal dashed line indicates the threshold of 2.72 pg/mL.
These 27 patients had lower baseline FSH (P = .002) and LH (P = .004), had higher BMI (P = .05), and were less likely to have received prior chemotherapy (P = .06) than patients whose E2 levels remained less than 2.72 pg/mL (Table 3).
Table 3.
Characteristics of Patients Randomly Assigned to Exemestane Plus Triptorelin Who Had at Least One Postbaseline Sample Analyzed, According to Occurrence of E2 Level Greater Than 2.72 pg/mL During at Least One Time Point
| Characteristic | No. of Patients (%)* | P | ||
|---|---|---|---|---|
| Postbaseline E2 | All Patients (N = 79) | |||
| All Assay Values < 2.72 pg/mL (n = 52) | At Least One Assay Value > 2.72 pg/mL (n = 27) | |||
| Age at random assignment, years | ||||
| Median (IQR) | 44 (40-48) | 45 (40-48) | 44 (40-48) | .792 |
| < 35 | 4 (7.7) | 4 (14.8) | 8 (10.1) | |
| Menstruation | ||||
| Normal | 12 (23.1) | 2 (7.4) | 14 (17.7) | .162 |
| Irregular | 20 (38.5) | 10 (37.0) | 30 (38.0) | |
| Amenorrhea | 20 (38.5) | 15 (55.6) | 35 (44.3) | |
| BMI, kg/m2, median (IQR) | 24 (22-27) | 27 (23-29) | 24 (22-28) | .054 |
| Smoking history | .926 | |||
| Currently smokes | 15 (28.8) | 9 (33.3) | 24 (30.4) | |
| Stopped smoking | 7 (13.5) | 3 (11.1) | 10 (12.7) | |
| Never smoked | 29 (55.8) | 14 (51.9) | 43 (54.4) | |
| Unknown | 1 (1.9) | 1 (3.7) | 2 (2.5) | |
| Prior chemotherapy | .061 | |||
| No | 19 (36.5) | 16 (59.3) | 35 (44.3) | |
| Yes | 33 (63.5) | 11 (40.7) | 44 (55.7) | |
| Chemotherapy regimen | .788 | |||
| Anthracycline plus taxane | 25 (75.8) | 8 (72.7) | 33 (75.0) | |
| Anthracycline-based | 7 (21.2) | 3 (27.3) | 10 (22.7) | |
| Taxane-based | 1 (3.0) | 0 (0.0) | 1 (2.3) | |
| Prior chemotherapy duration, weeks, median (IQR) | 21 (18-24) | 20 (18-24) | 20 (18-24) | ND |
| Prior tamoxifen | .207 | |||
| No | 32 (61.5) | 21 (77.8) | 53 (67.1) | |
| Yes | 20 (38.5) | 6 (22.2) | 26 (32.9) | |
| Prior tamoxifen duration, weeks, median (IQR) | 18 (12-20) | 12 (10-12) | 16 (10-20) | ND |
| Baseline hormone levels | ||||
| E2, pg/mL, median (IQR) | 41 (5-110) | 65 (27-115) | 50 (6-110) | .183 |
| Estrone, pg/mL, median (IQR) | 41 (24-72) | 47 (22-61) | 42 (24-70) | .780 |
| Estrone sulfate, pg/mL | ||||
| Median (IQR) | 637 (303-1,278) | 854 (490-1,282) | 712 (306-1,288) | .669 |
| FSH, IU/L, median (IQR) | 34 (12-58) | 8 (5-34) | 21 (8-51) | .002 |
| LH, IU/L, median (IQR) | 21 (7-32) | 7 (4-20) | 16 (6-28) | .004 |
Abbreviations: BMI, body mass index; E2, estradiol; FSH, follicle-stimulating hormone; IQR, interquartile range; LH, luteinizing hormone; ND, not done.
Values are numbers and percentages of patients, unless noted otherwise.
FSH and LH Levels Over Time
FSH and LH levels showed a marked reduction after treatment initiation in both groups (Appendix Fig A2, online only). Median FSH values were higher in the exemestane plus triptorelin group compared with the tamoxifen plus triptorelin group (P < .001 at each postbaseline time point). Conversely, LH values were persistently lower (P ≤ .01 at each postbaseline time point) in the exemestane plus triptorelin group compared with the tamoxifen plus triptorelin group.
DISCUSSION
In this study, 66% of premenopausal patients treated with exemestane plus triptorelin showed a profound, persistent reduction in E2 levels during the first 12 months of treatment. This decrease was significantly greater than in the tamoxifen plus triptorelin group at all time points. However, at least 17% of patients had an E2 level greater than 2.72 pg/mL at each time point. Overall, 34% of patients receiving exemestane plus triptorelin had an E2 level greater than the predefined threshold at least once. This finding was more frequent in chemotherapy-naïve patients (46%) and in patients younger than age 35 years (four of eight women; Table 3). We now consider the methodology, prior studies, and the chosen threshold in interpreting the clinical relevance of these results.
The reliable measurement of estrogen levels is challenging given the low levels expected in this study.29 The use of GC/MS/MS, a benchmark assay26,27,31 with high specificity and sensitivity, ensures accuracy. GC/MS/MS has one of the lowest limits of quantification for each estrogen component and has been shown to lack exemestane cross-reactivity,6,32 as we verified. In comparison with other direct and indirect assay results, mean E2 values measured by GC/MS/MS are lower in postmenopausal women (4.0 to 7.3 pg/mL26,27,32) and, importantly, in postmenopausal patients on AI (< 0.65 pg/mL in all samples from letrozole-treated patients26). Furthermore, the complete estrogen profile obtained, which is a unique feature in an international study, provides insight into the estrogen pharmacodynamic effect because E1 is the main product of the aromatase enzyme and E1S is the most abundant estrogen fraction in plasma and, therefore, relevant to the degree of estrogen suppression.
As a result of the high sensitivity of GC/MS/MS and the lower estrogen levels observed, we prospectively selected the E2 threshold of 2.72 pg/mL24 to define suboptimal suppression in patients receiving triptorelin plus exemestane. Using ultrasensitive assays, similar thresholds (2.18 or 2.72 pg/mL) have previously been suggested to determine E2 levels not consistent with postmenopausal status on AIs.33,34 Nevertheless, the clinical implication of these ultra-low E2 thresholds is still uncertain. In the postmenopausal setting, small differences in the degree of aromatase inhibition and estrogen suppression between the third-generation AIs35-38 have not translated into clinically meaningful differences in efficacy in head-to-head comparisons in early or advanced disease.39,40 Therefore, and as in other studies,33,34 we explored two additional less stringent E2 cutoff values, finding that 18% and 13% of patients had E2 levels greater than 10 and greater than 20 pg/mL, respectively, at least once during the 12-month period. However, these were mostly nonpersistent E2 increases, because only six women (8%) and one woman (1%) had E2 values greater than these two thresholds, respectively, at more than one postbaseline time point (Appendix Table A2).
Similar results have been reported in the adjuvant Hormonal Bone Effects (HOBOE) trial,9 in which patients were randomly assigned to receive triptorelin plus either letrozole or tamoxifen, and hormone levels were evaluated at baseline and after 6 months. Consistent with our findings, median E2 levels were lower in the AI group than in the tamoxifen group. The median on-treatment FSH and LH levels showed a decline in both treatment groups, with LH levels significantly lower and FSH significantly higher in the AI group compared with the tamoxifen group. These complex gonadotropin dynamics probably result from the direct suppressive effect of the GnRHa, together with the decrease in E2 that removes E2 physiologic feedback on gonadotropins (FSH is more sensitive to this than LH) and the direct effect of tamoxifen on the pituitary.
Aside from the AI used, the HOBOE trial differs from SOFT-EST in several respects. The HOBOE trial was a single-institution study in which premenopausal status was determined before chemotherapy, and only one postbaseline time point was assessed for 81 patients (letrozole plus triptorelin, n = 51; tamoxifen plus triptorelin, n = 30). The main objective was to compare endocrine effects between treatments, including adrenal function, but not to explore suboptimal estrogen suppression in the letrozole plus triptorelin group. A much less sensitive electrochemiluminescence immunoassay (LLQ, 5 pg/mL) was used for E2 measurements, and E1 and E1S were not studied.9
Other studies have addressed estrogen suppression with GnRHa plus AI in the neoadjuvant and metastatic settings. Many are flawed by the use of low-sensitivity assays,4,8,9,13,15,16 short follow-up duration,6,9,11,12,14,15,22,23 and small numbers of patients.6,11,12,22,23 In addition to the accurate estrogen measurements, the SOFT-EST substudy constitutes, to our knowledge, the largest series addressing estrogen levels in premenopausal women on GnRHa plus AI, with the longest sampling duration (until 48 months), and uniquely conducted in the context of an international phase III trial.
Our study is limited by early discontinuations, missing samples, and the uncertain clinical value associated with isolated E2 increases. Overall, 48 patients in the exemestane plus triptorelin group had all three postbaseline samples analyzed within the first year of treatment. However, 8% of patients (four of 48 patients) had E2 levels greater than 2.72 pg/mL at all three time points, which would most likely have an unfavorable impact on the prognosis of these patients.
A high proportion of E2 and E1 values less than the LLQ were observed (> 81% and > 83% at any time point, respectively; Appendix Table A3), demonstrating that a profound estrogen reduction is possible in premenopausal women receiving exemestane plus triptorelin. Conversely, to understand why a minority of women and samples showed E2 values greater than 2.72 pg/mL is complex, and the SOFT-EST study was not designed to assess all possible reasons. Compliance is particularly relevant for a compound with a 24-hour half-life such as exemestane, although because of the irreversible nature of its aromatase inhibition, estrogen levels remain suppressed for 4 days after a 25-mg single-dose administration.41 Other variables that can influence pharmacodynamic effects of AIs, such as polymorphisms in the CYP19 aromatase gene,42,43 were not studied. In addition, compliance with the every-28-day triptorelin injections is relevant, but there was no evidence of missed injections or overt delays between injections (Appendix Tables A6-A8, online only). Variability of the interval between the blood draw and the last triptorelin injection could lead to variability in E2 levels, but no pattern was evident (Appendix Fig A3, online only).
The analysis of potential predictive factors for suboptimal suppression, albeit exploratory, reinforces the additional role of FSH and LH levels to better define a truly premenopausal status, particularly after chemotherapy, which is superior to that provided by locally assessed E2 levels that defined eligibility for the SOFT trial. Even with serial estrogen and gonadotropin assessments, establishing a definitive menopausal status remains elusive, and the possibility of a later recovery still exists, as illustrated by E2 levels greater than 2.72 pg/mL observed during triptorelin plus exemestane among women with baseline postmenopausal levels (Figs 4A and 4B). Of note, higher BMI was marginally associated with increases in E2 greater than 2.72 pg/mL. The relationship of obesity with higher E2 and E1S has been recently reported in postmenopausal patients with advanced breast cancer on nonsteroidal AIs.44 However, its impact on clinical resistance to AIs in premenopausal plus ovarian suppression and postmenopausal populations is not clear.44-47
The findings of this substudy should be viewed in light of the results of SOFT and TEXT trials. The SOFT and TEXT combined analysis17 showed improved disease-free survival with exemestane plus OFS compared with tamoxifen plus OFS, whereas the SOFT trial showed benefits in freedom from breast cancer with tamoxifen plus OFS compared with tamoxifen alone, which were further improved with exemestane plus OFS in patient who received prior chemotherapy and in the youngest patients. Therefore, exemestane plus OFS has emerged as a new option in adjuvant endocrine therapy for premenopausal women. Considering that treatment with a GnRHa plus AI will be increasingly adopted, knowing whether a patient has suboptimal estrogen suppression in real time will become clinically important. Of note, although age was not related to isolated suboptimal suppression in our substudy, the population younger than age 35 years was small (eight women, all in the exemestane plus triptorelin group), and we observed that sustained suboptimal suppression was mainly seen in these youngest women (three patients, two of whom were chemotherapy naïve). In contrast to SOFT results (greatest benefit from OFS in population younger than age 35 years), this finding might be explained by the lower proportion of patients younger than age 35 in SOFT-EST who received prior chemotherapy (50% v 94% in SOFT). Additionally, our substudy revealed that 56% of women who received prior chemotherapy may actually have been postmenopausal at random assignment, which raises the possibility of a diluted effect of OFS in SOFT.
In conclusion, in our study, the majority of premenopausal patients with breast cancer treated with exemestane plus triptorelin had a profound reduction in estrogen levels during the first 12 months of treatment, which was similar to that reported in postmenopausal patients on AI. One-third of patients had an E2 level inconsistent with that expected for a postmenopausal level on AI (< 2.72 pg/mL) at least once. Further analysis of the 4-year data will better establish the dynamics of estrogen levels over time at the individual patient level.
Supplementary Material
Acknowledgment
We thank the patients, physicians, nurses, trial coordinators, and pathologists who participated in the Suppression of Ovarian Function Trial Estrogen Substudy, and the SOLTI Group, the International Breast Cancer Study Group, and Pfizer for study support. We acknowledge Cecilia Guzman, Medical Head Oncology Spain and Portugal Cluster, for her contribution in launching this substudy, and Raquel Espallargas, Senior Study Coordinator at Vall d’Hebron Institute of Research Hospital, for her contribution in the substudy development and sample management.
Appendix
Suppression of Ovarian Function Trial (SOFT)-EST Investigators and the SOLTI and International Breast Cancer Study Group (IBCSG) Participating Centers
SOFT/TEXT Steering Committee: P.A. Francis (Chair, SOFT Co-Chair), G.F. Fleming (SOFT Co-Chair), M.M. Regan (Trial Statistician), R. Torrisi, L. Blacher, H. Bonnefoi, E. Ciruelos, A.S. Coates, M. Colleoni, N. Dif, R.D. Gelber, A. Goldhirsch, T. Goulioti, T. Heckman-Scolese, A. Hiltbrunner, R. Kammler, R. Maibach, O. Ortmann, O. Pagani, E.A. Perez, K.N. Price, M. Rabaglio, B. Ruepp, K. Tryfonidis, K. Scott, H. Shaw, G. Viale, G. von Minckwitz, B.A. Walley, D. Zardavas, L. Cisar (Pfizer), and E. Chetaille (Ipsen).
IBCSG Coordinating Center, Bern, Switzerland: A. Hiltbrunner (Director), R. Kammler, R. Maibach, M. Rabaglio, S. Roux, B. Ruepp, and P. Sicher.
IBCSG Statistical Center, Dana-Farber Cancer Institute, Boston, MA: R.D. Gelber (Director), M.M. Regan (Group Statistician), J. Aldridge, M. Bonetti, Y. Feng, A. Giobbie-Hurder, K.P. Gray, H. Huang, W. Luo, K.N. Price, and L. Zickl.
IBCSG Data Management Center, Frontier Science & Technology Research Foundation, Amherst, NY: L. Blacher (Director), K. Scott (Data Management Section Head), M. Blackwell, A. Cesario, A. Dickinson, K. Donahue, M. Greco, P. Gonzalez, T. Heckman-Scolese, R. Hecker, R. Hinkle, M. Kalera, K. Lupejkis, A. Mora de Karausch, V. Palermo, H. Shaw, R. Starkweather, and J. Swick-Jemison.
Participating Centers and Principal Investigators
Breast International Group (BIG)
SOLTI, SPAIN
Hospital Universitario 12 de Octubre, Madrid; E. Ciruelos
Hospital Son Llatzer, Palma de Mallorca; J.G. Catalán
Hospital Clinic i Provincial de Barcelona, Barcelona; M. Muñoz
Hospital Universitari Vall D' Hebron and Vall d’Hebron Institute of Oncology, Universitat Autònoma de Barcelona, Barcelona; M. Bellet
Instituto Valenciano de Oncologia, Valencia; M.A. Climent
Hospital Son Dureta (Palma de Mallorca), Palma de Mallorca; A. Avella
H.U. Arnau de Vilanova, Lleida; A. Llombart
Hospital Clinico Universitario de Valencia, Valencia; A. Lluch
Hospital Ramon Y Cajal, Madrid; N. Martinez Jañez
Hospital Sant Joan de Reus, Reus; M. Melé
Hospital Dr Negrin, Las Palmas de Gran Canari; U. Bohn
Centro Oncologico MD Anderson, Madrid; A. González Martín
Hospital Sant Pau i Santa Tecla, Tecla; C. Pérez Segura
IBCSG, HUNGARY
National Institute of Oncology, Budapest; I. Láng
IBCSG, ITALY
Fondazione Salvatore Maugeri, Pavia; L. Pavesi
Dipartimento di Oncologia, Azienda Ospedaliero-Universitaria di Udine, Udine; F. Puglisi
IBCSG, PERU
Instituto de Enfermedades Neoplásicas, Lima; H.L. Gomez
IBCSG, SWEDEN
Sahlgrenska University Hospital, Gothenburg; P. Karlsson
SWISS GROUP FOR CLINICAL CANCER RESEARCH (SAKK), SWITZERLAND
Centre Hospitalier Universitaire Vaudois, Lausanne; K. Zaman
Onkologiezentrum Thun-Berner Oberland, Thun; D. Rauch
Brust-Zentrum Zurich, Zurich; C. Rageth
Kantonsspital Graubünden; R. von Moos
EUROPEAN ORGANIZATION FOR RESEARCH AND TREATMENT OF CANCER, FRANCE
Centre Rene Huguenin, Saint-Cloud; E. Brain
EUROPEAN ORGANIZATION FOR RESEARCH AND TREATMENT OF CANCER, PORTUGUAL
Centro de Lisboa, Lisboa; A. Moreira
Fig A1.
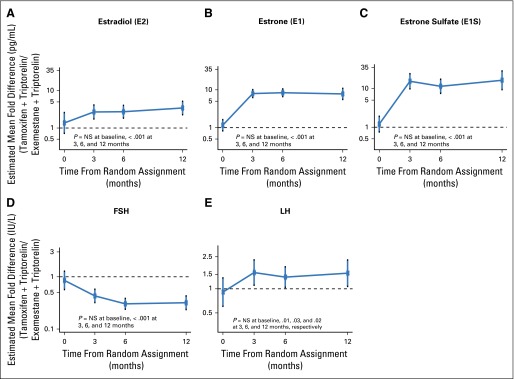
Estimated mean fold-difference between treatment groups (tamoxifen plus triptorelin relative to exemestane plus triptorelin) for each hormone level over time. Levels (log10-transformed) were modeled using generalized estimating equation (GEE) as a function of time point, treatment assignment, the treatment-by-time interaction, and patient characteristics, accounting for correlation of longitudinal values. Mean fold-differences are plotted with 95% CIs. SE used robust (sandwich) variance calculation. The horizontal dashed line at 1 indicates no difference. NS=not statistically significant.
Fig A2.
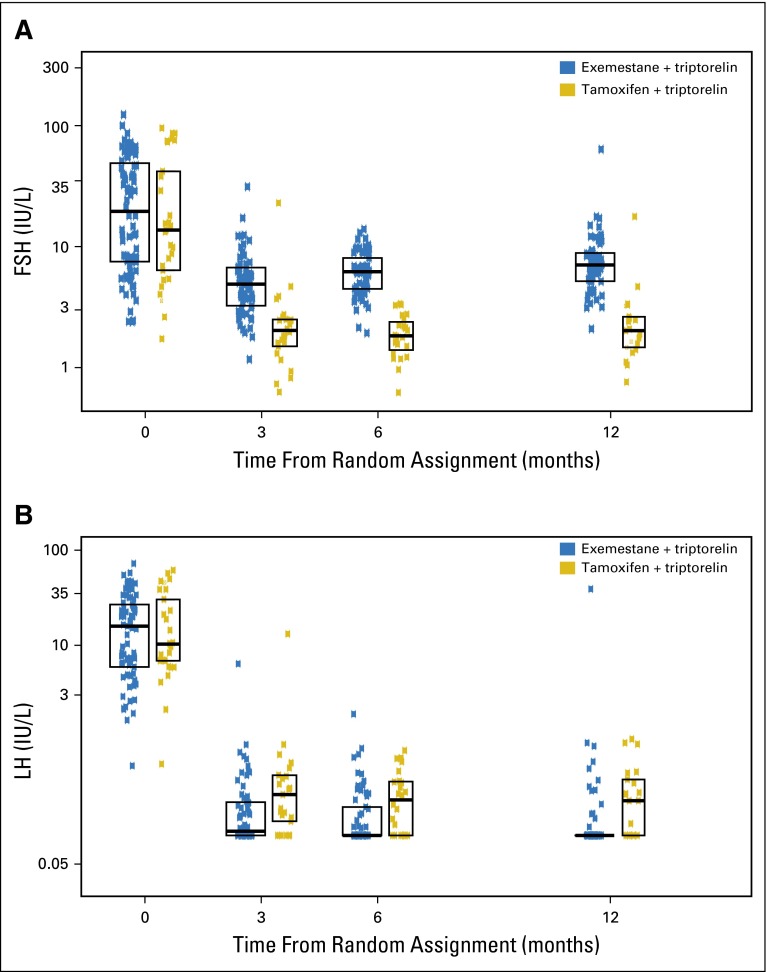
Distribution of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels over time according to treatment assignment. Boxes indicate the 25th, 50th, and 75th percentiles.
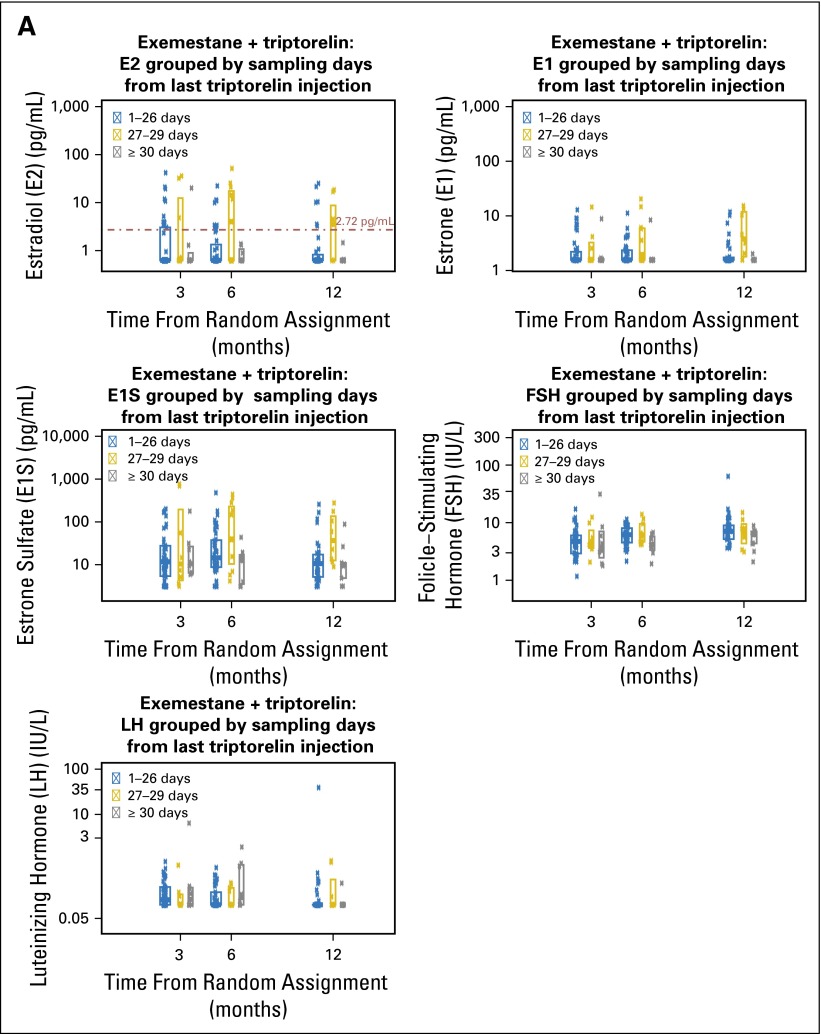
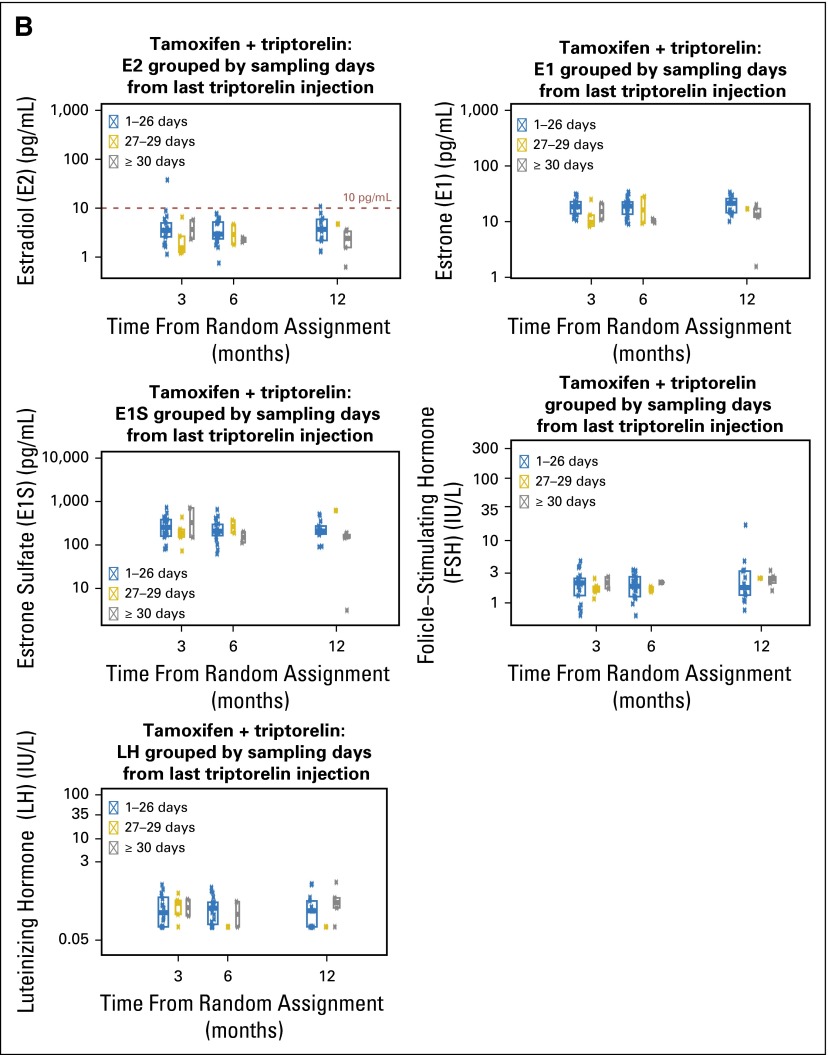
Fig A3.
Distribution of estrogen and gonadotropin levels at each scheduled sampling time grouped by sampling days from last triptorelin injection.
Treatment adherence and timing of sampling relative to triptorelin injections
Among all 106 patients with at least one postbaseline sample in this 1-year analysis, the median duration receiving triptorelin injections was 12 months. Triptorelin injections were stopped early by eight patients before 12 months; these patients were included in the analysis until the time of early discontinuation and were adherent with injections before early discontinuation. For all other patients, there was no indication of missed injections during the 12-month period. The same eight patients also stopped the assigned oral endocrine therapy early. Seven additional patients stopped exemestane but remained on triptorelin; early discontinuation of exemestane may have led to some values of estradiol (E2) greater than 2.72 pg/mL after stopping, but these few values were unlikely to substantially affect the results. For all other patients, there was no indication of nonadherence with assigned oral endocrine therapy. Postbaseline E2 values for 15 patients who stopped protocol treatment early are listed in Tables A6 and A7 according to treatment assignment.
The interval of time between the blood draw sample and the last triptorelin injection could potentially account for variability in E2 levels. For approximately 75% of patients we had complete, accurate data in the database for dates of triptorelin injections and blood samples. We calculated the duration of the interval (in days) for each blood draw. Table A8 shows the number of patients with complete date information for dates of triptorelin injections and blood samples, grouped by the interval between blood sampling and the last injection of triptorelin, at each scheduled blood draw time point. Approximately 15% of patients had samples 30 or more days since their last injection, which also indicates that the triptorelin injections were not always given within the 28 ± 3–day window. Figures A3A and A3B plot the estrogen and follicle-stimulating hormone and luteinizing hormone levels according to the interval between blood sampling and the last triptorelin injection, by treatment assignment. There is no clear pattern in the relationship of the sampling interval with E2 level; there is variability in E2 levels even within the 1- to 26-day interval from the last injection and remarkably small variability when the sample was obtained 30 or more days from last injection.
Table A1.
Patient Characteristics According to Groups Defined by Baseline E2 20 pg/mL or Less Versus E2 Greater Than 20 pg/mL
| Baseline E2 ≤ 20 pg/mL (n = 39) | Baseline E2 > 20 pg/mL (n = 74) | Total (N = 113)* | |
|---|---|---|---|
| Treatment assignment | |||
| Exemestane plus triptorelin | 28 (71.8) | 55 (74.3) | 83 (73.5) |
| Tamoxifen plus triptorelin | 11 (28.2) | 19 (25.7) | 30 (26.5) |
| Age at random assignment, years | |||
| Median (IQR) | 45 (42, 48) | 44 (39, 48) | 44 (40, 48) |
| < 35 | 2 (5.1) | 6 (8.1) | 8 (7.1) |
| ≥ 35 | 37 (94.9) | 68 (91.9) | 105 (92.9) |
| Menstruation before random assignment | |||
| Normal | 8 (20.5) | 49 (66.2) | 57 (50.4) |
| Irregular | 5 (12.8) | 11 (14.9) | 16 (14.2) |
| Persistent amenorrhea | 26 (66.7) | 14 (18.9) | 40 (35.4) |
| BMI, kg/m2 | |||
| Median (IQR) | 26 (24, 28) | 23 (22, 27) | 24 (22, 28) |
| Smoking history | |||
| Currently smokes | 8 (20.5) | 19 (25.7) | 27 (23.9) |
| Stopped smoking | 10 (25.6) | 10 (13.5) | 20 (17.7) |
| Never smoked | 21 (53.8) | 43 (58.1) | 64 (56.6) |
| Unknown | 0 (0.0) | 2 (2.7) | 2 (1.8) |
| Prior chemotherapy | |||
| No | 4 (10.3) | 47 (63.5) | 51 (45.1) |
| Yes | 35 (89.7) | 27 (36.5) | 62 (54.9) |
| Chemotherapy regimen | |||
| Anthracycline-based | 11 (31.4) | 3 (11.1) | 14 (22.6) |
| Anthracycline + taxane | 21 (60.0) | 22 (81.5) | 43 (69.4) |
| Taxane-based | 3 (8.6) | 2 (7.4) | 5 (8.1) |
| Prior chemotherapy duration, weeks | |||
| Median (IQR) | 18 (18, 24) | 24 (18, 24) | 20 (18, 24) |
| Prior tamoxifen | |||
| No | 26 (66.7) | 52 (70.3) | 78 (69.0) |
| Yes | 13 (33.3) | 22 (29.7) | 35 (31.0) |
| Prior tamoxifen duration, weeks | |||
| Median (IQR) | 18 (14, 20) | 16 (8, 22) | 18 (8, 21) |
| Baseline hormone levels | |||
| E2, pg/mL | |||
| Median (IQR) | 4 (3, 7) | 110 (54, 170) | 51 (6,124) |
| E1, pg/mL | |||
| Median (IQR) | 19 (15, 28) | 60 (41, 106) | 42 (24, 71) |
| E1S, pg/mL | |||
| Median (IQR) | 265 (146, 396) | 1205 (890, 2,342) | 894 (307, 1,380) |
| FSH (IU/L) | |||
| Median (IQR) | 60 (30, 73) | 8 (6, 20) | 15 (7, 47) |
| LH (IU/L) | |||
| Median (IQR) | 25 (17, 44) | 8 (5, 21) | 14 (6, 28) |
Abbreviations: BMI, body mass index; E1, estrone; E1S, estrone sulfate; E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; IQR, interquartile range.
Excludes three patients without baseline samples.
Table A2.
Proportions of Patients Treated With Exemestane Plus Triptorelin With an Estradiol Level Above the Predefined Threshold (2.72 pg/mL) and With Respect to Two Additional Thresholds According to Use of Prior Chemotherapy
| E2 > 2.72 (pg/mL) | E2 > 10 (pg/mL) | E2 > 20 (pg/mL) | E2 ≤ 20 (pg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Point, Months | No. of Patients | Total | No Prior Chemotherapy | Prior Chemotherapy | Total | No Prior Chemotherapy | Prior Chemotherapy | Total | No Prior Chemotherapy | Prior Chemotherapy | Total | No Prior Chemotherapy | Prior Chemotherpy |
| 0 (Baseline) | 83 | 76 (92) | 37 (97) | 39 (87) | 58 (70) | 36 (95) | 22 (49) | 55 (66) | 35 (92) | 20 (44) | 28 (34) | 3 (8) | 25 (56) |
| 3 | 67 | 17 (25) | 10 (37) | 7 (18) | 8 (12) | 7 (26) | 1 (2) | 5 (7) | 4 (15) | 1 (2) | 62 (93) | 23 (85) | 39 (98) |
| 6 | 67 | 16 (24) | 9 (29) | 7 (19) | 8 (12) | 4 (13) | 4 (11) | 4 (6) | 1 (3) | 3 (8) | 63 (94) | 30 (97) | 33 (92) |
| 12 | 65 | 11 (17) | 8 (27) | 3 (9) | 6 (9) | 5 (17) | 1 (3) | 2 (3) | 2 (7) | 0 (0) | 63 (97) | 28 (93) | 35 (100) |
| 3, 6, or 12 | |||||||||||||
| ≥ 1 with E2 > cutoffs | 79 | 27 (34.2) | 16 (45.7) | 11 (25) | 14 (17.7) | 10 (28.6) | 4 (9.1) | 10 (12.7) | 6 (17.1) | 4 (9.1) | 69 (87.3) | 29 (82.9) | 40 (90.9) |
| ≥ 2 with E2 > cutoffs | 13 (16.5) | 6 (7.6) | 1 (1.3) | ||||||||||
NOTE. Data are presented as No. (%). Estradiol (E2) greater than 2.72 pg/mL defines a strict threshold to indicate E2 inconsistent with postmenopausal levels on an aromatase inhibitor. E2 greater than 10 pg/mL defines a less-strict threshold, and thus above which E2 was clearly inconsistent with postmenopausal levels on an aromatase inhibitor. E2 greater than 20 pg/mL defines a threshold above which E2 was inconsistent with gonadotropin-releasing hormone agonist–related postmenopausal status.
Table A3.
Estrogen Levels at Each Time Point According to Treatment Assignment
| Treatment Assignment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Exemestane Plus Triptorelin (n = 86) | Tamoxifen Plus Triptorelin (n = 30) | |||||||
| Estrogen Levels | Baseline | 3 Months | 6 Months | 12 Months | Baseline | 3 Months | 6 Months | 12 Months |
| Samples expected, No. | 86 | 83 | 80 | 78 | 30 | 28 | 27 | 26 |
| E2, pg/mL | ||||||||
| No. of samples analyzed | 83 | 67 | 67 | 65 | 30 | 26 | 24 | 20 |
| Geometric mean | 32.7 | 1.3 | 1.3 | 1 | 40.4 | 3.4 | 3.2 | 3.2 |
| Median (IQR)* | 49.9 (6.8, 110) | 0.6 (0.6, 2.7) | 0.6 (0.6, 2.6) | 0.6 (0.6, 0.9) | 72.5 (6.2, 199) | 3.4 (2.1, 5.3) | 2.9 (2.1, 5.1) | 3.4 (2.2, 5.2) |
| Mean (SD) | 95.4 (149.91) | 4 (8.44) | 3.6 (7.95) | 2.5 (5.07) | 114.5 (124.99) | 4.9 (6.98) | 3.8 (2.02) | 4 (2.77) |
| Range | (0.6, 766) | (0.6, 41.9) | (0.6, 51.4) | (0.6, 25.1) | (2.5, 436) | (1.1, 37.7) | (0.8, 7.8) | (0.6, 10.8) |
| No. (%) < LLQ | 4 (5) | 61 (91) | 54 (81) | 57 (88) | 0 (0) | 4 (15) | 5 (21) | 6 (30) |
| E1, pg/mL | ||||||||
| No. of samples analyzed | 83 | 67 | 67 | 65 | 30 | 26 | 24 | 21 |
| Geometric mean | 41 | 2.2 | 2.2 | 2.2 | 45.1 | 16.9 | 17.2 | 16.6 |
| Median (IQR) | 43.6 (24, 70) | 1.6 (1.6, 2) | 1.6 (1.6, 2.3) | 1.6 (1.6, 2.1) | 39.2 (24.8, 102.2) | 17.9 (12.4, 22.7) | 18.2 (11.6, 22.5) | 17 (13.8, 22.6) |
| Mean (SD) | 65.3 (77.24) | 2.9 (3.33) | 2.8 (3.17) | 3.4 (5.75) | 64.4 (57.84) | 18.2 (6.88) | 18.7 (7.54) | 19 (8.4) |
| Range | (1.6, 486) | (1.6, 18.6) | (1.6, 20.4) | (1.6, 42.5) | (11.3, 226) | (8.3, 31.2) | (9.1, 34.2) | (1.6, 34.5) |
| No. (%) < LLQ | 5 (6) | 64 (96) | 57 (85) | 54 (83) | 0 (0) | 3 (12) | 4 (17) | 5 (24) |
| E1S, pg/mL | ||||||||
| No. of samples analyzed | 83 | 67 | 67 | 65 | 30 | 26 | 24 | 21 |
| Geometric mean | 710.3 | 15.4 | 20.6 | 13.1 | 787.5 | 239.9 | 216.8 | 193.6 |
| Median (IQR) | 784 (315, 1,320) | 11.7 (5.3, 29.6) | 14.9 (8.4, 48.1) | 10.6 (4.9, 20) | 1,000 (272, 1,620) | 229 (154.8, 420.8) | 204 (173.8, 321.2) | 206 (172, 273) |
| Mean (SD) | 1,377.9 (1,752.51) | 56 (133.71) | 54.5 (96.93) | 34.2 (65.11) | 1,501.5 (1,897.57) | 295 (192.65) | 259 (167.23) | 278.5 (236.38) |
| Range | (7.2, 8,000) | (3.1, 766) | (3.1, 480) | (3.1, 303) | (72.2, 8,770) | (72.8, 725) | (62.5, 718) | (3.1, 1,090) |
| No. (%) < LLQ | 5 (6) | 25 (37) | 22 (33) | 19 (29) | 0 (0) | 3 (12) | 4 (17) | 5 (24) |
Abbreviations: E1, estrone; E1S, estrone sulfate; E2, estradiol; IQR, interquartile range, SD, standard deviation; LLQ, lower limit of quantification.
Values are rounded to one decimal, including values at LLQ for each estrogen fraction (eg, E2 = 0.6 was rounded to one decimal from 0.625).
Table A4.
Characteristics of the Two Patients in the Exemestane Plus Triptorelin Group Who Experienced Vaginal Bleeding
| Triptorelin Injection Dates | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID | Time Point, Months | Blood Draw Date | E2 (pg/mL) | E1 (pg/mL) | E1S (pg/mL) | FSH (IU/L) | LH (IU/L) | Age | BMI | Menstruation | Prior Chemotherapy | Prior Tamoxifen | First | Second | Third | Fourth |
| A* | 0 | August 3, 2001 | 117 | 49 | 192 | 5.5 | 2.9 | 47 | Overweight | Normal | No | No | ||||
| 3 | November 2, 2001 | 41.9 | 9.1 | 27.8 | 11.2 | 0.2 | August 3, 2001 | August 31, 2001 | September 29, 2001 | October 27, 2001 | ||||||
| 6 | — | November 23, 2001 | December 21, 2001 | January 18, 2002 | ||||||||||||
| 9 | February 16, 2002 | March 16, 2002 | April 13, 2002 | |||||||||||||
| 12 | August 2, 2002 | 3.5 | 10.4 | 22.8 | 6 | 0.9 | May 11, 2002 | June 8, 2002 | July 5, 2002 | August 2, 2002 | ||||||
| B† | 0 | March 9, 2001 | 120.4 | 5.2 | 71.2 | NA | NA | 40 | Normal | Irregular | Yes | Yes | ||||
| 3 | June 11, 2001 | 0.6 | 1.6 | 3.9 | 5.0 | 0.6 | March 9, 2001 | April 6, 2001 | May 4, 2001 | |||||||
| 6 | August 31, 2001 | 1.3 | 8.4 | 43.5 | 4.1 | 1.9 | June 1, 2001 | June 29, 2001 | July 27, 2001 | |||||||
| 9 | ||||||||||||||||
| Postoophorectomy | December 22, 2001 | 0.6 | 1.6 | 3.13 | 146.5 | 54.1 | ||||||||||
NOTE. Visit time points were 0 (baseline), 3, 6, 9, and 12 months. Sampling time points were 0, 3, 6, and 12 months. In blood draw and injection dates, the years were changed for the purpose of anonymity.
Abbreviations: BMI, body mass index; E1, estrone; E1S, estrone sulfate; E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Patient A was administered exemestane from August 2001 to December 2001, then started tamoxifen; a 6-month sample was not obtained. The change to tamoxifen was made because the patient continued to have regular menses.
Patient B had vaginal bleeding 6 months after triptorelin initiation and proceeded to oophorectomy on the basis of premenopausal local E2 levels taken on August 18, 2001; a postoophorectomy sample was obtained 3 months later.
Table A5.
Summary of Four Patients Assigned Exemestane Plus Triptorelin With Persistently Suboptimal E2 Less Than 2.72 pg/mL Throughout Follow-Up
| Triptorelin Injection Dates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID | Time Point, Months | Blood Draw Date | E2 (pg/mL) | E1 (pg/mL) | E1S (pg/mL) | FSH (IU/L) | LH (IU/L) | First | Second | Third | Fourth |
| C1 | 0 | August 5, 2001 | 79.1 | 58.8 | 625 | 2.4 | 1.6 | ||||
| 3 | October 22, 2001 | 11.8 | 8.9 | 181 | 3.7 | 0.1 | August 5, 2001 | September 1, 2001 | September 29, 2001 | October 20, 2001 | |
| 6 | January 19, 2002 | 4 | 2 | 39.3 | 6.4 | 0.1 | November 24, 2001 | December 22, 2001 | January 19, 2002 | ||
| 9 | April 13, 2002 | 1.8 | 33.9 | 29.8 | 5.4 | 0.1 | February 16, 2002 | March 16, 2002 | April 13, 2002 | ||
| 12 | July 7, 2002 | 18.5 | 13.5 | 176 | 5.6 | 0.1 | May 11, 2002 | June 8, 2002 | July 7, 2002 | August 5, 2002 | |
| C2 | 0 | November 10, 2001 | 60.6 | 47.5 | 582 | 5.7 | 2.6 | ||||
| 3 | January 27, 2002 | 19.4 | 6.8 | 167 | 6.8 | 0.1 | November 10, 2001 | December 8, 2001 | January 5, 2002 | February 2, 2002 | |
| 6 | April 27, 2002 | 51.4 | 20.4 | 439 | 4.8 | 0.1 | March 2, 2002 | March 30, 2002 | April 27, 2002 | ||
| 9 | May 25, 2002 | June 22, 2002 | July 20, 2002 | ||||||||
| 12 | November 10, 2002 | 21.6 | 10.2 | 257 | 7.5 | 0.1 | August 17, 2002 | September 14, 2002 | October 12, 2002 | November 9, 2002 | |
| C3 | 0 | December 4, 2001 | 62.8 | 30.6 | 923 | 8.4 | 4.8 | ||||
| 3 | March 4, 2002 | 4.8 | 4.2 | 55.1 | 5.5 | 0.2 | December 4, 2001 | January 4, 2002 | February 4, 2002 | March 4, 2002 | |
| 6 | June 3, 2002 | 25.4 | 14.7 | 363 | 4.2 | 0.3 | April 8, 2002 | May 6, 2002 | June 3, 2002 | ||
| 9 | July 1, 2002 | August 26, 2002 | |||||||||
| 12 | November 18, 2002 | 16.9 | 4.5 | 105 | 8 | 0.1 | September 23, 2002 | October 21, 2002 | November 18, 2002 | ||
| C4 | 0 | November 18, 2001 | 124 | 61.3 | 934 | 4 | 4.9 | ||||
| 3 | February 21, 2002 | 10.7 | 5.5 | 76.8 | 7.2 | 0.1 | November 18, 2001 | December 17, 2001 | January 14, 2002 | February 11, 2002 | |
| 6 | May 23, 2002 | 11.3 | 4.4 | 84.2 | 6.5 | 0.1 | March 11, 2002 | April 8, 2002 | May 6, 2002 | ||
| 9 | June 4, 2002 | July 1, 2002 | July 29, 2002 | August 27, 2002 | |||||||
| 12 | November 22, 2002 | 10.1 | 3.2 | 96.6 | 5.4 | 0.1 | September 23, 2002 | October 21, 2002 | November 20, 2002 | ||
NOTE. For all patients, case report forms indicate exemestane taken per protocol throughout period. For the blood draw and injection dates, the years were changed arbitrarily to 2001-2002 for the purpose of anonymity.
Abbreviations: E1, estrone; E1S, estrone sulfate; E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Table A6.
Exemestane Plus Triptorelin: Patients With Treatment Nonadherence (n = 10) and Sampling Time From Last Triptorelin Injection
| Patient ID | Visit Time Point, Months | Blood Draw, Months | Blood Sample Since Last Injection, Days | E2 (pg/mL) | Duration of Exemestane, Months | Duration of Triptorelin, Months |
|---|---|---|---|---|---|---|
| A1 | 3 | 3 | 6 | 41.9 | 4.4 | 12 |
| 6 | 6 | 4.4 | 12 | |||
| 9 | 4.4 | 12 | ||||
| 12 | 12* | 28 | 3.55* | 4.4 | 12 | |
| A2 | 3 | 3 | 28 | 0.625 | 4.2 | 12 |
| 6 | 6 | 4.2 | 12 | |||
| 9 | 4.2 | 12 | ||||
| 12 | 4.2 | 12 | ||||
| A3 | 3 | 3 | 28 | 32.1 | 8.5 | 9.2 |
| 6 | 6 | 28 | 0.625 | 8.5 | 9.2 | |
| 9 | 9 | 7 | 0.625 | 8.5 | 9.2 | |
| A4 | 3 | 3 | 11.8 | 12 | ||
| 6 | 6 | 4 | 1.03 | 11.8 | 12 | |
| 9 | 11.8 | 12 | ||||
| 12 | 12 | 16 | 0.715 | 11.8 | 12 | |
| A5 | 3 | 3 | 38 | 0.625 | 9 | 12 |
| 6 | 6 | 25 | 0.625 | 9 | 12 | |
| 9 | 9 | 12 | ||||
| A6 | 3 | 3* | 23 | 2.33* | 1.2 | 12 |
| 6 | 6* | 19 | 2.47* | 1.2 | 12 | |
| 9 | 1.2 | 12 | ||||
| 12 | 12* | 28 | 3.57* | 1.2 | 12 | |
| A7 | 3 | 3 | 7.48 | 0.3 | 4.3 | |
| 6 | 6 | 0.3 | 4.3 | |||
| 12 | 12 | 7.38 | 0.3 | 4.3 | ||
| A8 | 3 | 3 | 31 | 0.625 | 7.8 | 12 |
| 6 | 6 | 29 | 0.625 | 7.8 | 12 | |
| 9 | 7.8 | 12 | ||||
| 12 | 7.8 | 12 | ||||
| A9 | 3 | 3 | 6 | 0.625 | 3.5 | 3.7 |
| 6 | 3.5 | 3.7 | ||||
| A10 | 3 | 3 | 21 | 3.95 | 12 | 12 |
| 6 | 6 | 14 | 1.6 | 12 | 12 | |
| 9 | 12 | 12 | ||||
| 12 | 12 | 28 | 0.625 | 12 | 12 |
Abbreviations: E2, estradiol.
The patient was on tamoxifen at this blood draw.
Table A7.
Tamoxifen Plus Triptorelin: Patients With Treatment Nonadherence (n = 5) and Sampling Time From Last Triptorelin Injection
| Patient ID | Visit Time Point, Months | Blood Draw, Months | Blood Sample Since Last Injection, Days | E2 (pg/mL) | Duration of Tamoxifen, Months | Duration of Triptorelin, Months |
|---|---|---|---|---|---|---|
| B1 | 3 | 3 | 26 | 37.7 | 11.4 | 2.8 |
| B2 | 3 | 3 | 29 | 1.21 | 10.6 | 11.5 |
| 6 | 6 | 35 | 2.03 | 10.6 | 11.5 | |
| 9 | 10.6 | 11.5 | ||||
| 12 | 12 | 47 | 1.57 | 10.6 | 11.5 | |
| B3 | 3 | 3 | 10 | 6.78 | 10.1 | 11.2 |
| 6 | 6 | 19 | 6.33 | 10.1 | 11.2 | |
| 9 | 10.1 | 11.2 | ||||
| 12 | 12 | 10.1 | 11.2 | |||
| B4 | 3 | 3 | 6 | 2.68 | 4 | 12 |
| 6 | 6 | 13 | 2.92 | 4 | 12 | |
| 9 | 4 | 12 | ||||
| 12 | 12* | 108* | 0.625* | 4 | 12 | |
| B5 | 3 | 3 (ED) | 5.35 | 3 | 1 |
Abbreviations: E2, estradiol; ED, early discontinuation
The patient was on aromatase inhibitor plus triptorelin at this blood draw; there is uncertainty about the value of 108 days since last injection.
Table A8.
Number of Patients Grouped by Sampling Days from Last Triptorelin Injection at Each Scheduled Blood Draw Time Point
| Time Point, Months | Exemestane Plus Triptorelin | Tamoxifen Plus Triptorelin | ||||
|---|---|---|---|---|---|---|
| 1-26 Days | 27-29 Days | 30 Days or More | 1-26 Days | 27-29 Days | 30 Days or More | |
| 3 | 42 | 8 | 9 | 17 | 5 | 2 |
| 6 | 43 | 11 | 8 | 19 | 2 | 2 |
| 12 | 37 | 8 | 11 | 13 | 1 | 5 |
NOTE. Data are restricted to those patients for whom we have complete date information. Data are presented as the No. of patients having a blood sample drawn within the indicated number of days from their last triptorelin injection.
Footnotes
Written on behalf of the Suppression of Ovarian Function Trial Estrogen Substudy (SOFT-EST) Investigators, the SOLTI Group and the International Breast Cancer Study Group. The investigators in SOFT-EST and the central coordination leadership and staff are listed in the Appendix (online only).
Processed as a Rapid Communication manuscript.
SOFT-EST was supported by Pfizer International Oncology, Spain and Portugal Cluster, and the International Breast Cancer Study Group (IBCSG). IBCSG was supported by the Frontier Science and Technology Research Foundation, the Swiss Group for Clinical Cancer Research, National Cancer Institute (Grant No. CA75362), and the Swiss Cancer Research/Oncosuisse.
Pfizer had no role in the reporting or interpretation of the substudy, other than a minority representation on the SOFT Steering Committee.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00975676.
AUTHOR CONTRIBUTIONS
Conception and design: Meritxell Bellet, Prudence A. Francis, Josefa Morales, Josep Vázquez, Karen N. Price, Meredith M. Regan
Administrative support: Josefa Morales, Josep Vázquez
Provision of study materials or patients: Josefa Morales, Josep Vázquez
Collection and assembly of data: Meritxell Bellet, Prudence A. Francis, István Láng, Eva Ciruelos, Ana Lluch, Miguel Angel Climent, Gustavo Catalán, Antoni Avella, Uriel Bohn, Antonio González-Martin, Roberto Catalán, Analía Azaro, Agnita Rajasekaran, Josefa Morales, Meredith M. Regan
Data analysis and interpretation: Meritxell Bellet, Kathryn P. Gray, Prudence A. Francis, Roser Ferrer, Gini F. Fleming, Karen N. Price, Meredith M. Regan
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Twelve-Month Estrogen Levels in Premenopausal Women With Hormone Receptor–Positive Breast Cancer Receiving Adjuvant Triptorelin Plus Exemestane or Tamoxifen in the Suppression of Ovarian Function Trial (SOFT): The SOFT-EST Substudy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Meritxell Bellet
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca
Kathryn P. Gray
No relationship to disclose
Prudence A. Francis
Honoraria: Pierre Fabre
Travel, Accommodations, Expenses: Amgen, Roche
István Láng
No relationship to disclose
Eva Ciruelos
No relationship to disclose
Ana Lluch
Consulting or Advisory Role: Novartis, Roche, Pfizer
Miguel Angel Climent
Honoraria: Pfizer
Consulting or Advisory Role: Pfizer
Speakers' Bureau: Pfizer
Travel, Accommodations, Expenses: Pfizer
Gustavo Catalán
No relationship to disclose
Antoni Avella
No relationship to disclose
Uriel Bohn
No relationship to disclose
Antonio González-Martin
No relationship to disclose
Roser Ferrer
No relationship to disclose
Roberto Catalán
No relationship to disclose
Analía Azaro
No relationship to disclose
Agnita Rajasekaran
No relationship to disclose
Josefa Morales
No relationship to disclose
Josep Vázquez
Research Funding: Pfizer, Novartis, Roche, Eisai, Sanofi, Bayer, Puma Biotechnology, Pierre Fabre Medicament, Genentech, AstraZeneca
Gini F. Fleming
Research Funding: Corcept
Other Relationship: Aetna Zentaris
Karen N. Price
No relationship to disclose
Meredith M. Regan
Research Funding: Veridex, OncoGenex, Pfizer, Ipsen, Novartis, Merck, Ferring, Celgene, AstraZeneca
REFERENCES
- 1.Beatson GW. 1896. On treatment of inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment, with illustrative cases. Lancet 2:104-107, 162-165,
- 2.Boccardo F, Rubagotti A, Perrotta A, et al. Ovarian ablation versus goserelin with or without tamoxifen in pre-perimenopausal patients with advanced breast cancer: Results of a multicentric Italian study. Ann Oncol. 1994;5:337–342. doi: 10.1093/oxfordjournals.annonc.a058837. [DOI] [PubMed] [Google Scholar]
- 3.Taylor CW, Green S, Dalton WS, et al. Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: An intergroup study. J Clin Oncol. 1998;16:994–999. doi: 10.1200/JCO.1998.16.3.994. [DOI] [PubMed] [Google Scholar]
- 4.Blamey RW, Jonat W, Kaufmann M, et al. Goserelin depot in the treatment of premenopausal advanced breast cancer. Eur J Cancer. 1992;28A:810–814. doi: 10.1016/0959-8049(92)90120-q. [DOI] [PubMed] [Google Scholar]
- 5.Schmid P, Untch M, Kossé V, et al. Leuprorelin acetate every-3-months depot versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant treatment in premenopausal patients with node-positive breast cancer: The TABLE study. J Clin Oncol. 2007;25:2509–2515. doi: 10.1200/JCO.2006.08.8534. [DOI] [PubMed] [Google Scholar]
- 6.Jannuzzo MG, Di Salle E, Spinelli R, et al. Estrogen suppression in premenopausal women following 8 weeks of treatment with exemestane and triptorelin versus triptorelin alone. Breast Cancer Res Treat. 2009;113:491–499. doi: 10.1007/s10549-008-9949-9. [DOI] [PubMed] [Google Scholar]
- 7.Walker KJ, Walker RF, Turkes A, et al. Endocrine effects of combination antioestrogen and LH-RH agonist therapy in premenopausal patients with advanced breast cancer. Eur J Cancer Clin Oncol. 1989;25:651–654. doi: 10.1016/0277-5379(89)90200-9. [DOI] [PubMed] [Google Scholar]
- 8.Klijn JG, Beex LV, Mauriac L, et al. Combined treatment with buserelin and tamoxifen in premenopausal metastatic breast cancer: A randomized study. J Natl Cancer Inst. 2000;92:903–911. doi: 10.1093/jnci/92.11.903. [DOI] [PubMed] [Google Scholar]
- 9.Rossi E, Morabito A, De Maio E, et al. Endocrine effects of adjuvant letrozole + triptorelin compared with tamoxifen + triptorelin in premenopausal patients with early breast cancer. J Clin Oncol. 2008;26:264–270. doi: 10.1200/JCO.2007.13.5319. [DOI] [PubMed] [Google Scholar]
- 10.Masuda N, Sagara Y, Kinoshita T, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): A double-blind, randomised phase 3 trial. Lancet Oncol. 2012;13:345–352. doi: 10.1016/S1470-2045(11)70373-4. [DOI] [PubMed] [Google Scholar]
- 11.Celio L, Martinetti A, Ferrari L, et al. Premenopausal breast cancer patients treated with a gonadotropin-releasing hormone analog alone or in combination with an aromatase inhibitor: A comparative endocrine study. Anticancer Res. 1999;19:2261–2268. [PubMed] [Google Scholar]
- 12.Forward DP, Cheung KL, Jackson L, et al. Clinical and endocrine data for goserelin plus anastrozole as second-line endocrine therapy for premenopausal advanced breast cancer. Br J Cancer. 2004;90:590–594. doi: 10.1038/sj.bjc.6601557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torrisi R, Bagnardi V, Pruneri G, et al. Antitumour and biological effects of letrozole and GnRH analogue as primary therapy in premenopausal women with ER and PgR positive locally advanced operable breast cancer. Br J Cancer. 2007;97:802–808. doi: 10.1038/sj.bjc.6603947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung KL, Agrawal A, Folkerd E, et al. Suppression of ovarian function in combination with an aromatase inhibitor as treatment for advanced breast cancer in pre-menopausal women. Eur J Cancer. 2010;46:2936–2942. doi: 10.1016/j.ejca.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Carlson RW, Schurman CM, et al. Phase II trial of anastrozole plus goserelin in the treatment of hormone receptor-positive, metastatic carcinoma of the breast in premenopausal women. J Clin Oncol. 2010;28:3917–3921. doi: 10.1200/JCO.2009.24.9565. [DOI] [PubMed] [Google Scholar]
- 16.Park IH, Ro J, Lee KS, et al. Phase II parallel group study showing comparable efficacy between premenopausal metastatic breast cancer patients treated with letrozole plus goserelin and postmenopausal patients treated with letrozole alone as first-line hormone therapy. J Clin Oncol. 2010;28:2705–2711. doi: 10.1200/JCO.2009.26.5884. [DOI] [PubMed] [Google Scholar]
- 17.Pagani O, Regan MM, Walley BA, et al. TEXT and SOFT Investigators. International Breast Cancer Study Group Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 372:1673, 2015. [DOI] [PubMed]
- 19.Soysal S, Soysal ME, Ozer S, et al. The effects of post-surgical administration of goserelin plus anastrozole compared to goserelin alone in patients with severe endometriosis: A prospective randomized trial. Hum Reprod. 2004;19:160–167. doi: 10.1093/humrep/deh035. [DOI] [PubMed] [Google Scholar]
- 20.Reichel RP, Schweppe KW, Zoladex Endometriosis Study Group Goserelin (Zoladex) depot in the treatment of endometriosis. Fertil Steril. 1992;57:1197–1202. [PubMed] [Google Scholar]
- 21.Filicori M, Flamigni C, Cognigni G, et al. Comparison of the suppressive capacity of different depot gonadotropin-releasing hormone analogs in women. J Clin Endocrinol Metab. 1993;77:130–133. doi: 10.1210/jcem.77.1.8325935. [DOI] [PubMed] [Google Scholar]
- 22.Dowsett M, Stein RC, Coombes RC. Aromatization inhibition alone or in combination with GnRH agonists for the treatment of premenopausal breast cancer patients. J Steroid Biochem Mol Biol. 1992;43:155–159. doi: 10.1016/0960-0760(92)90201-s. [DOI] [PubMed] [Google Scholar]
- 23.Dowsett M, Doody D, Miall S, et al. Vorozole results in greater oestrogen suppression than formestane in postmenopausal women and when added to goserelin in premenopausal women with advanced breast cancer. Breast Cancer Res Treat. 1999;56:25–34. doi: 10.1023/a:1006289811540. [DOI] [PubMed] [Google Scholar]
- 24.Smith IE, Dowsett M, Yap YS, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: Caution and suggested guidelines. J Clin Oncol. 2006;24:2444–2447. doi: 10.1200/JCO.2005.05.3694. [DOI] [PubMed] [Google Scholar]
- 25.Burstein HJ, Mayer E, Patridge AH, et al. Inadvertent use of aromatase inhibitors in patients with breast cancer with residual ovarian function: Cases and lessons. Clin Breast Cancer. 2006;7:158–161. doi: 10.3816/cbc.2006.n.026. [DOI] [PubMed] [Google Scholar]
- 26.Santen RJ, Demers L, Ohorodnik S, et al. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72:666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Lee JS, Ettinger B, Stanczyk FZ, et al. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab. 2006;91:3791–3797. doi: 10.1210/jc.2005-2378. [DOI] [PubMed] [Google Scholar]
- 28.Hothorn T, Hornik K, van de Wiel MA, et al. A lego system for conditional inference. Am Stat. 2006;60:257–263. [Google Scholar]
- 29.Hothorn T, Hornik K, van de Wiel MA, et al. Implementing a class of permutation tests: The coin package. J Stat Softw. 2008;28:1–23. [Google Scholar]
- 30.Hajek J, Sidak Z, Sen PK. Theory of Rank Tests. San Diego, CA, : Academic Press; 1999. [Google Scholar]
- 31.Folkerd EJ, Lønning PE, Dowsett M. Interpreting plasma estrogen levels in breast cancer: Caution needed. J Clin Oncol. 2014;32:1396–1400. doi: 10.1200/JCO.2013.53.9411. [DOI] [PubMed] [Google Scholar]
- 32.Lønning PE, Geisler J, Krag LE, et al. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol. 2005;23:5126–5137. doi: 10.1200/JCO.2005.07.097. [DOI] [PubMed] [Google Scholar]
- 33.Guerrero A, Gavilá J, Folkerd E, et al. Incidence and predictors of ovarian function recovery (OFR) in breast cancer (BC) patients with chemotherapy-induced amenorrhea (CIA) who switched from tamoxifen to exemestane. Ann Oncol. 2013;24:674–679. doi: 10.1093/annonc/mds464. [DOI] [PubMed] [Google Scholar]
- 34.Henry NL, Xia R, Banerjee M, et al. Predictors of recovery of ovarian function during aromatase inhibitor therapy. Ann Oncol. 2013;24:2011–2016. doi: 10.1093/annonc/mdt149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geisler J, Haynes B, Anker G, et al. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002;20:751–757. doi: 10.1200/JCO.2002.20.3.751. [DOI] [PubMed] [Google Scholar]
- 36.Dixon JM, Renshaw L, Young O, et al. Letrozole suppresses plasma estradiol and estrone sulphate more completely than anastrozole in postmenopausal women with breast cancer. J Clin Oncol. 2008;26:1671–1676. doi: 10.1200/JCO.2007.13.9279. [DOI] [PubMed] [Google Scholar]
- 37.Geisler J, Helle H, Ekse D, et al. Letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels. Clin Cancer Res. 2008;14:6330–6335. doi: 10.1158/1078-0432.CCR-07-5221. [DOI] [PubMed] [Google Scholar]
- 38.Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J Clin Oncol 29:2342-2349, 2011. [DOI] [PMC free article] [PubMed]
- 39.Rose C, Vtoraya O, Pluzanska A, et al. An open randomised trial of second-line endocrine therapy in advanced breast cancer: Comparison of the aromatase inhibitors letrozole and anastrozole. Eur J Cancer. 2003;39:2318–2327. doi: 10.1016/s0959-8049(03)00630-0. [DOI] [PubMed] [Google Scholar]
- 40.Goss PE, Ingle JN, Pritchard KI, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27—A randomized controlled phase III trial. J Clin Oncol. 2013;31:1398–1404. doi: 10.1200/JCO.2012.44.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spinelli R, Januzzo MG, Pogessi I, et al. Pharmacokinetics (PK) of aromasin (exemestane, EXE) after single and repeated doses in healthy postmenopausal volunteers (HPV) Eur J Cancer. 1999;35(suppl 4):S295. [Google Scholar]
- 42.Wang L, Ellsworth KA, Moon I, et al. Functional genetic polymorphisms in the aromatase gene CYP19 vary the response of breast cancer patients to neoadjuvant therapy with aromatase inhibitors. Cancer Res. 2010;70:319–328. doi: 10.1158/0008-5472.CAN-09-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lunardi G, Piccioli P, Bruzzi P, et al. Plasma estrone sulfate concentrations and genetic variation at the CYP19A1 locus in postmenopausal women with early breast cancer treated with letrozole. Breast Cancer Res Treat. 2013;137:167–174. doi: 10.1007/s10549-012-2306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Folkerd EJ, Dixon JM, Renshaw L, et al. Suppression of plasma estrogen levels by letrozole and anastrozole is related to body mass index in patients with breast cancer. J Clin Oncol. 2012;30:2977–2980. doi: 10.1200/JCO.2012.42.0273. [DOI] [PubMed] [Google Scholar]
- 45.Sestak I, Distler W, Forbes JF, et al. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411–3415. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]
- 46.Ewertz M, Gray KP, Regan MM, et al. Obesity and risk of recurrence or death after adjuvant endocrine therapy with letrozole or tamoxifen in the Breast International Group 1-98 trial. J Clin Oncol. 2012;30:3967–3975. doi: 10.1200/JCO.2011.40.8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfeiler G, Königsberg R, Fesl C, et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: An analysis of the prospective ABCSG-12 trial. J Clin Oncol. 2011;29:2653–2659. doi: 10.1200/JCO.2010.33.2585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.