Abstract
Purpose
Neurofibromatosis type 2 (NF2) is a tumor predisposition syndrome characterized by bilateral vestibular schwannomas (VSs) resulting in deafness and brainstem compression. This study evaluated efficacy and biomarkers of bevacizumab activity for NF2-associated progressive and symptomatic VSs.
Patients and Methods
Bevacizumab 7.5 mg/kg was administered every 3 weeks for 46 weeks, followed by 24 weeks of surveillance after treatment with the drug. The primary end point was hearing response defined by word recognition score (WRS). Secondary end points included toxicity, tolerability, imaging response using volumetric magnetic resonance imaging analysis, durability of response, and imaging and blood biomarkers.
Results
Fourteen patients (estimated to yield > 90% power to detect an alternative response rate of 50% at alpha level of 0.05) with NF2, with a median age of 30 years (range, 14 to 79 years) and progressive hearing loss in the target ear (median baseline WRS, 60%; range 13% to 82%), were enrolled. The primary end point, confirmed hearing response (improvement maintained ≥ 3 months), occurred in five (36%) of 14 patients (95% CI, 13% to 65%; P < .001). Eight (57%) of 14 patients had transient hearing improvement above the 95% CI for WRS. No patients experienced hearing decline. Radiographic response was seen in six (43%) of 14 target VSs. Three grade 3 adverse events, hypertension (n = 2) and immune-mediated thrombocytopenic purpura (n = 1), were possibly related to bevacizumab. Bevacizumab treatment was associated with decreased free vascular endothelial growth factor (not bound to bevacizumab) and increased placental growth factor in plasma. Hearing responses were inversely associated with baseline plasma hepatocyte growth factor (P = .019). Imaging responses were associated with high baseline tumor vessel permeability and elevated blood levels of vascular endothelial growth factor D and stromal cell–derived factor 1α (P = .037 and .025, respectively).
Conclusion
Bevacizumab treatment resulted in durable hearing response in 36% of patients with NF2 and confirmed progressive VS-associated hearing loss. Imaging and plasma biomarkers showed promising associations with response that should be validated in larger studies.
INTRODUCTION
Vestibular schwannomas (VSs) are histologically benign tumors of the eighth nerve that result in hearing loss, imbalance, and brainstem compression. VSs are common, with roughly 3,000 new cases per year in the United States.1 Surgery and radiation therapy (RT) achieve sustained control in more than 95% of sporadic, unilateral VSs.2-4 Germline inactivation of the gene NF2 results in the rare tumor syndrome neurofibromatosis type 2 (NF2), characterized by a bilateral VS and multiple additional schwannomas, meningiomas, and ependymomas.5-7 NF2-associated VSs cause higher morbidity because they are bilateral,8-10 multilobular,11,12 and have poor outcomes with standard therapies.13-16 As a result, most people with NF2 develop significant hearing loss in young adulthood.5,8
Nearly 100% of VSs express vascular endothelial growth factor (VEGF; or VEGF-A).17-19 Pharmacologic inhibition of VEGF in VS murine xenograft models decreases permeability and increases pericyte coverage, consistent with vascular normalization.20-22 Bevacizumab is a humanized immunoglobulin G1 monoclonal blocking antibody specific for VEGF. Anecdotal experience with 31 individuals with NF2-associated VSs treated with bevacizumab showed hearing improvement in 57%, making bevacizumab the first therapy to demonstrate functional and imaging responses in people with NF2.17,23 However, it also requires long-term administration and is associated with chronic toxicity.24
This study was conducted to prospectively confirm the hearing response (HR) rate in a well-defined patient population with hearing loss resulting from NF2-associated VSs, define the duration of benefit during and after treatment with the drug, and identify biomarkers that may predict which individuals are most likely to benefit from bevacizumab.
PATIENTS AND METHODS
This multi-institution, open-label phase II trial enrolled patients with NF2 and documented VS-associated hearing loss. The primary end point was the proportion of patients with confirmed HR in the target ear. Secondary end points included the durability of HR, HR in nontarget evaluable ears, change in VS volumetric magnetic resonance imaging (MRI) measures compared with baseline, safety, and the relationship between imaging and blood biomarkers and HR or radiographic response (RR). The trial was approved by site institutional review boards and the National Cancer Institute Cancer Therapy Evaluation Program. Bevacizumab was supplied by Genentech through a Clinical Research and Development Agreement with the Cancer Therapy Evaluation Program. All patients or their legal guardians provided informed consent.
Patients age 12 years or older meeting National Institutes of Health or Manchester clinical criteria for NF2,25-27 with documented VS-associated hearing loss on serial audiograms over 24 months pre-enrollment and a target ear baseline word recognition score (WRS) lower than 90%, were eligible. Exclusion criteria included prior antiangiogenesis therapy, medical conditions incompatible with bevacizumab, and tumors not amenable to volumetric MRI analysis (Data Supplement).
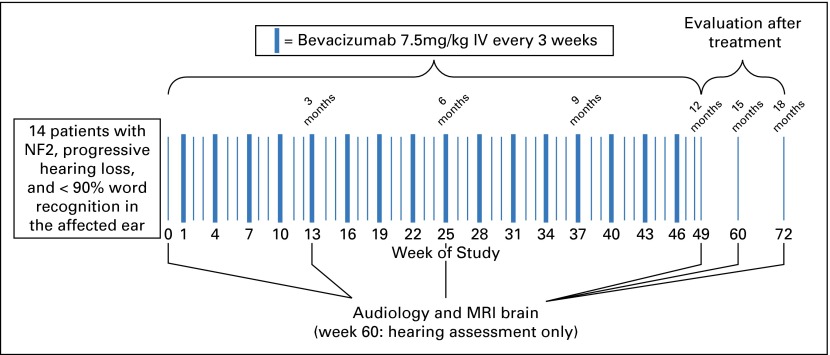
Bevacizumab was administered intravenously at 7.5 mg/kg every 3 weeks for 16 doses. Patients were then assessed for 24 weeks after treatment with the drug (Fig 1).
Fig 1.
Trial schema. IV, intravenous; MRI, magnetic resonance imaging; NF2, neurofibromatosis type 2.
The target tumor was the VS causing documented active, progressive hearing loss. Audiology examinations were performed at baseline, at weeks 13, 25, 49, and 60, and after study treatment. WRS was assessed with a 100-word list of monosyllables delivered via standardized methodology at a sound level determined to yield the optimal score for each participant.28,29 The 95% (P = .05) critical difference table defined statistically significant increased WRS (HR) or decreased WRS (hearing decline; Appendix Table A1, online only).14,28,30 Confirmed HR was defined as an increase in WRS exceeding the 95% critical difference referenced to baseline and maintained across two evaluations over 3 months.
MRI of the brain was performed at baseline, at weeks 13, 25, and 49, and after study treatment. Anatomic and functional imaging protocols were standardized across all sites on a Siemens 3T Verio (Siemens Healthcare, Erlangen, Germany) with published protocols.30,31 Volumetric analysis was performed centrally using the anatomic sequences by independent radiologists blinded to treatment.31 Enhancing tumor volume was outlined on postcontrast images. Median values of each parameter within enhancing tumor were computed. Double baseline MRI was performed to establish the test–retest variability in volumetric analysis of the VS. Change in VS volume compared with baseline was determined for the target and, when feasible, contralateral VSs. RR definitions were as follows: partial response (PR), decrease in tumor volume of 20% or more; minor response (MR), decrease in tumor volume of 5% to 19%; progressive disease (PD), increase in tumor volume of 20% or more; and stable disease (SD) for all others. RR was confirmed at 3 months. Functional MRI sequences, dynamic contrast-enhanced MRI to calculate Ktrans (a measure of vascular permeability), and apparent diffusion coefficient (ADC) were processed using custom-made software in Matlab (MathWorks, Natick, MA), using published approaches.32,33
Adverse events (AEs) were graded and attributed to bevacizumab according the Common Terminology Criteria for Adverse Events (version 4.0) before infusion (every 3 weeks); physical examination was conducted every 6 weeks. Blood pressure was assessed weekly for the first 6 weeks and preinfusion thereafter. For patients younger than 18 years of age, bone toxicity was monitored with laboratory and imaging studies (Data Supplement).
Circulating biomarkers were evaluated in peripheral blood before, during (weeks 25 and 49), and after treatment (week 72). Plasma samples were obtained from fresh blood, aliquots were prepared, frozen, and analyzed for circulating VEGF, placental growth factor (PlGF), VEGF-C, VEGF-D, soluble VEGF receptor 1 (sVEGFR1 or sFLT1), basic fibroblast growth factor, sTie-2, interleukin (IL) -1β, IL-6, IL-8, and tumor necrosis factor-α, using multiplex enzyme-linked immunosorbent assay plates from Meso-Scale Discovery (Gaithersburg, MD). Hepatocyte growth factor (HGF), s-cMET, sVEGFR2, stromal cell–derived factor 1α (SDF1α), angiopoietin (Ang) 1 and Ang2, and carbonic anhydrase IX were measured using single-analyte enzyme-linked immunosorbent assay kits from R&D Systems (Minneapolis, MN). All samples were run in duplicate.
The primary end point was HR, defined as increased WRS above the 95% critical threshold and maintained across at least two time points compared with baseline WRS. Using a one-stage design based on a null hypothesis of response rate at 5%, a total of 14 patients with confirmed progressive hearing loss were estimated to yield more than 90% power to detect an alternative response rate of 50% at alpha level of 0.05. The trial required four or more responders of 14 to reject the null hypothesis. Baseline patient and disease characteristics are presented with standard descriptive summaries. Proportion of HR was estimated using binomial distribution along with 95% CIs. The binomial exact test was used for testing proportions. Pearson correlation coefficient was used to estimate a correlation between continuous variables. All P values are reported as two sided. All analyses were conducted using SAS software (version 9.2; SAS Institute, Cary, NC).
The percentage of changes in the blood and imaging biomarkers from before, during, and after treatment were summarized using descriptive statistics. Blood biomarker analysis is reported per patient and imaging biomarker analysis per tumor. The differences before and during treatment in blood and imaging biomarkers were assessed with paired statistics. Signed rank test was used to assess the significance of the change over time, and Wilcoxon signed rank test was used to test the difference between HR and RR groups. Tumor reduction was calculated on the basis of the percentage of change in volume from baseline to week 25 for all tumors. Correlation between RR for target VS and median ADC and Ktrans at baseline was determined using the Spearman correlation test.
RESULTS
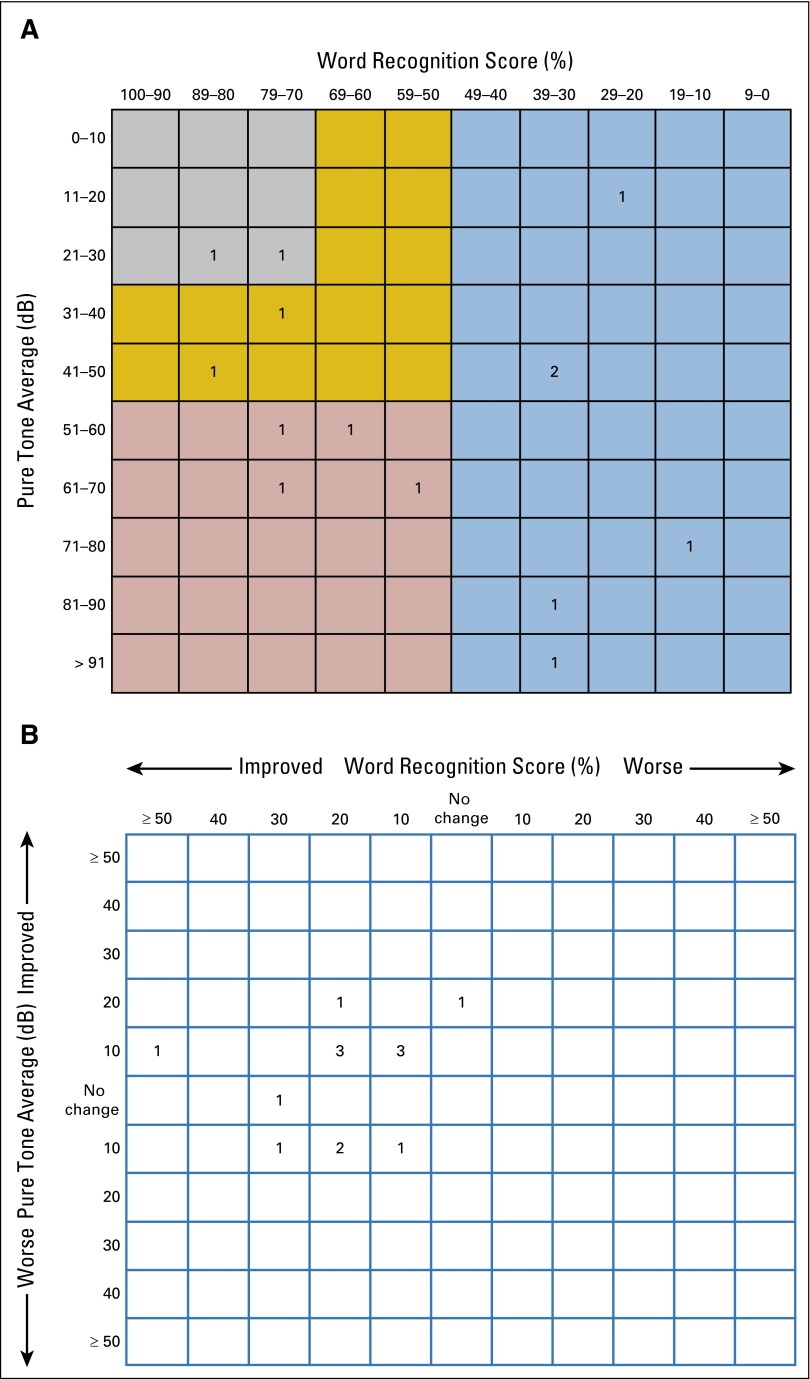
Fourteen patients (10 female), with a median age of 30 years (range, 14 to 79 years), were enrolled between November 2010 and August 2011 (Table 1). Eight participants had undergone prior surgery: six on the nontarget ear and two bilaterally. Three participants had received prior RT (one to target and two to nontarget VS) 15 to 120 months before receiving bevacizumab (Appendix Table A2, online only). All patients were evaluable for response and toxicity. Median baseline target ear WRS was 60% (range, 13% to 82%). Only four (28%) of 14 target ears had serviceable hearing (ie, class A or B) per the American Academy of Otolaryngology-Head and Neck Society Hearing Committee guidelines (Appendix Fig A1, online only).34 Nine (64%) of 14 patients were anacusic in the nontarget ear.
Table 1.
Baseline Patient Demographics and Clinical Characteristics
| Characteristic | No. (%) | P | ||
|---|---|---|---|---|
| Total (N = 14) | Confirmed Hearing Response (n = 5) | No Confirmed Hearing Response (n = 9) | ||
| Age, years | .2 | |||
| Median | 30.5 | 26.0 | 32.0 | |
| Range | 14-79 | 14-33 | 14-79 | |
| Sex | .6 | |||
| Male | 4 (29) | 1 (20) | 3 (33) | |
| Female | 10 (71) | 4 (80) | 6 (67) | |
| Race | ||||
| White | 12 (87) | 3 (60) | 9 (100) | .1 |
| Karnofsky PS, % | .7 | |||
| 90 | 7 (50) | 3 (60) | 4 (44) | |
| 70-80 | 7 (50) | 2 (40) | 5 (56) | |
| WRS in target ear, % | .7 | |||
| Median | 60.5 | 72.0 | 56.0 | |
| Range | 13-82 | 20-78 | 13-82 | |
| Tumor volume in target ear, mL | .9 | |||
| Median | 3.0 | 2.3 | 3.4 | |
| Range | 0.7-23 | 1.2-22 | 0.7-23 | |
Abbreviations: PS, performance status; WRS, word recognition score.
Five of (36%) 14 patients (95% CI, 13% to 65%; P < .001) achieved the primary end point of confirmed HR in the target ear. This was achieved by week 13 in four of five patients and maintained continuously throughout treatment. No patient experienced hearing decline while receiving bevacizumab, despite progressive hearing loss being required for enrollment. Of the five patients evaluable for HR in the nontarget ear, four had confirmed HR (80%; 95% CI, 28% to 99%; Table 2). In total, nine (47%) of 19 evaluable ears (95% CI, 24% to 71%) achieved confirmed HR (Table 2). Pre- and post-treatment hearing scattergrams are presented in Appendix Figure A1.
Table 2.
Hearing and Imaging Response Data During 12 Months of Bevacizumab Treatment
| Response | Overall Response | Confirmed Response* | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Hearing | ||||
| Target ear | 8 of 14 | 57 | 5 of 14 | 36 |
| Contralateral ear | 4 of 5 | 80 | 4 of 5 | 80 |
| All ears | 12 of 19 | 63 | 9 of 19 | 47 |
| Imaging | ||||
| Target VS | ||||
| PR (≥ 20% decrease) | 6 of 14 | 43 | 2 of 14 | 14 |
| MR (5%–19% decrease) | 4 of 14 | 29 | 7 of 14 | 50 |
| SD | 4 of 14 | 29 | 5 of 14 | 36 |
| PD (≥ 20% increase) | 0 of 14 | 0 | 0 of 14 | 0 |
| Contralateral VS | ||||
| PR (≥ 20% decrease) | 6 of 14 | 43 | 3 of 14 | 21 |
| MR (5%–19% decrease) | 6 of 14 | 43 | 6 of 14 | 43 |
| SD | 2 of 14 | 14 | 5 of 14 | 36 |
| PD (≥ 20% increase) | 0 of 14 | 0 | 0 of 14 | 14 |
Abbreviations: MR, minor response; PD, progressive disease; PR, partial response; SD, stable disease; VS, vestibular schwannoma.
Confirmed response was defined as maintained across two evaluations at least 3 months apart.
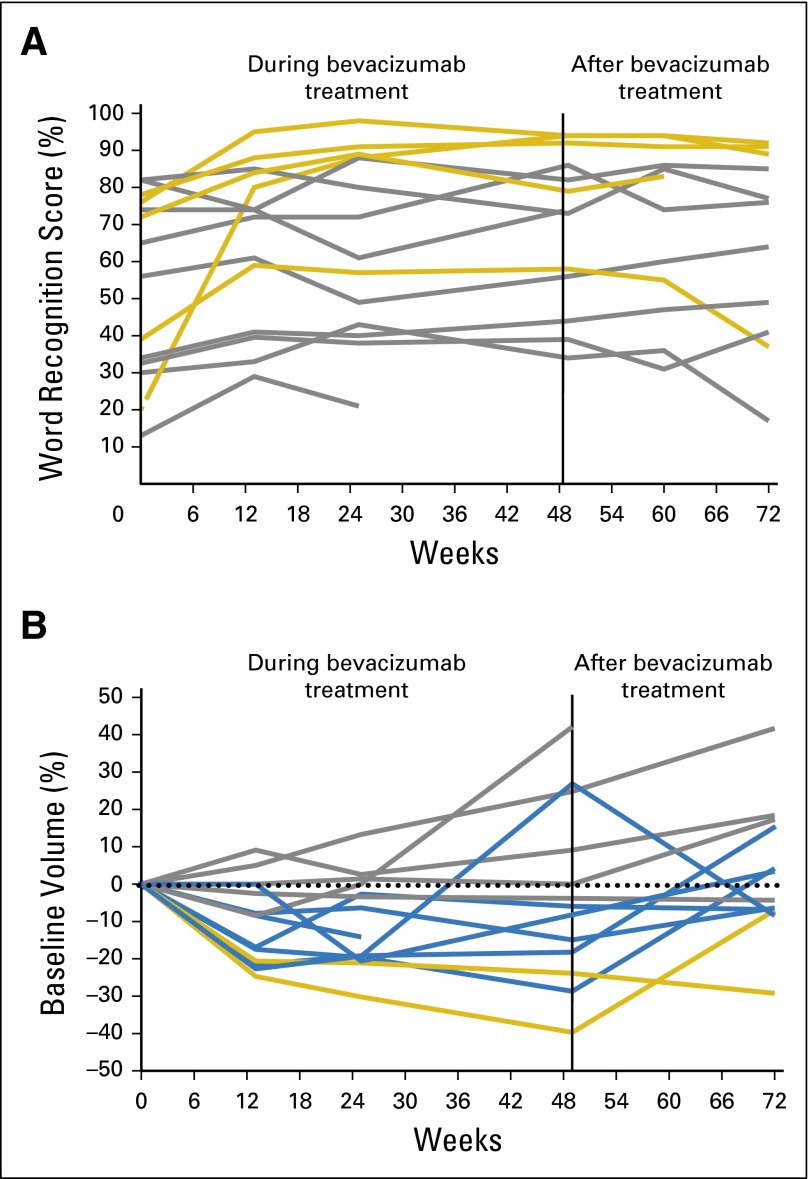
Bevacizumab was stopped after 12 months to assess durability of response. Three (60%) of five patients with confirmed HR in the target ear maintained HR 6 months after treatment with study drug (Fig 2A). Similarly, two of four patients with confirmed HR in the nontarget ear maintained HR 6 months after treatment with study drug. In total, five of nine (target and nontarget) ears with confirmed HR maintained this for 6 months after treatment with study drug.
Fig 2.
Changes in (A) word recognition score and (B) tumor volume during (through week 49) and after treatment (weeks 60 and 72) for target ears by best confirmed response: (A) yellow line, hearing response; grey line, stable disease; (B) grey line, stable disease; blue line, minor response; yellow line, partial response.
The median baseline target VS volume was 3.0 cc (range, 0.7 to 23 cc). The mean difference in volume across the two baseline assessments was 0.02 mL (P = .83), confirming the reproducibility of volumetric measurements. A total of 28 VSs (14 target and 14 contralateral) were evaluable for RR. PR at any time point was achieved in six (43%) of 14 target and six (43%) of 14 nontarget ears (Table 2). Confirmed PR (imaging response maintained across two evaluation time points) was seen in two (14%) of 14 target VSs (95% CI, 2% to 43%). Maximal reduction was 39.7% at week 49. Confirmed MR occurred in seven (50%) of 14 target VSs (95% CI, 23% to 77%). One person with confirmed MR had received RT to the target ear 10 years earlier, and theoretically, late recovery from RT could have influenced RR (Appendix Table A2). No VS meeting criteria for MR or PR at any time point developed PD while receiving treatment, but VSs with best response of SD developed PD at week 49 (Fig 2B). Regarding nontarget VSs, three (21%) of 14 tumors (95% CI, 5% to 51%) had confirmed PR, and six (43%) of 14 (95% CI, 18% to 71%) had confirmed MR. Of note, two patients in whom PR was achieved in nontarget VSs had received prior RT 15 and 48 months before enrollment, respectively, which could potentially have influenced RR. In total, five (18%) of 28 VSs (95% CI, 6% to 37%) achieved confirmed PR, and 13 (46%) of 28 (95% CI, 28% to 66%) VSs had confirmed MR. Of the 18 of 28 VSs with confirmed PR or MR, nine (50%) maintained durability of RR 6 months off of drug (Fig 2B).
There was no significant correlation between HR and RR when analyzed by patient or by target VS (r = 0.34; 95% CI, −0.14 to 0.82; P = .23). There was also no significant correlation between WRS and tumor volume over time (r = 0.287; 95% CI, −0.29 to 0.71; P = .32). Finally, there was no significant relationship between HR and baseline factors including age, sex, or baseline WRS (Table 1).
There were 124 AEs possibly related to bevacizumab. Of these, 121 were classified as grade 1 to 2 (Table 3). The three grade 3 AEs were two episodes of hypertension that responded to monotherapy and one episode of idiopathic thrombocytopenia purpura that required treatment termination at week 16 but resolved after 6 months without the drug. A second patient discontinued treatment after 13 of 16 planned doses because of surgery required for another tumor. No bone toxicity occurred in the two patients age younger than 18 years. Three of seven female patients with normal menstruation at baseline developed grade 1 to 2 irregular menstruation that resolved after stopping treatment. There were 11 additional episodes of grade 1 to 2 bleeding (Table 3).
Table 3.
Total AEs Possibly, Probably, or Definitively Related to Bevacizumab in Patients With NF2 and Progressive VSs (N = 14)
| AE | No. (%) | ||
|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | |
| Abdominal pain | 2 (14) | ||
| ALT increased | 5 (36) | 3 (21) | |
| Allergic rhinitis | 1 (7) | ||
| Anemia | 1 (7) | ||
| Anorexia | 1 (7) | 1 (7) | |
| AST increased | 8 (57) | ||
| Bruising | 2 (14) | ||
| CPK increased | 1 (7) | ||
| Diarrhea | 2 (14) | 1 (7) | |
| Dizziness | 1 (7) | ||
| Dry skin | 2 (14) | ||
| Dyspepsia | 2 (14) | ||
| Dyspnea | 2 (14) | ||
| Electrocardiogram QT corrected interval prolonged | 1 (7) | ||
| Epistaxis | 8 (57) | 2 (14) | |
| Fatigue | 9 (64) | 4 (29) | |
| Headache | 2 (14) | ||
| Hemoglobinuria | 1 (7) | ||
| Hemolysis | 1 (7) | ||
| Hemorrhoidal hemorrhage | 2 (14) | ||
| Hoarseness | 1 (7) | ||
| Hyperglycemia | 4 (29) | ||
| Hypermagnesemia | 2 (14) | ||
| Hypertension | 2 (14) | ||
| Hypomagnesemia | 1 (7) | ||
| Increased blood bicarbonate | 1 (7) | ||
| Irregular menstruation* | 5 (71) | 1 (14) | |
| Menorrhagia* | 2 (29) | 1 (14) | |
| Mucositis oral | 1 (7) | ||
| Nausea | 5 (36) | 2 (14) | |
| Oral hemorrhage | 2 (14) | ||
| Oral pain | 1 (7) | ||
| Palpitations | 2 (14) | ||
| Peripheral sensory neuropathy | 1 (7) | ||
| Platelet count decreased | 2 (14) | ||
| Proteinuria | 7 (50) | 3 (21) | |
| Rectal hemorrhage | 1 (7) | ||
| Respiratory, thoracic, and mediastinal disorders | 1 (7) | ||
| Sore throat | 4 (29) | ||
| Thrombocytopenia purpura | 1 (7) | ||
| Vertigo | 1 (7) | ||
| Voice alteration | 1 (7) | ||
| Vomiting | 1 (7) | ||
| Weight gain | 1 (7) | ||
| Weight loss | 1 (7) | ||
| Wound complication | 1 (7) | ||
Abbreviations: AE, adverse event; CPK, creatine phosphokinase; VS, vestibular schwannoma.
Events that could only have occurred among seven female patients. Percentage represents No. of events occurring among seven female patients with baseline normal menstruation.
We explored potential associations between baseline functional imaging markers, ADC and Ktrans, as well as changes in ADC and Ktrans during treatment, with HR and RR. ADC and Ktrans values were evaluable for 12 target VSs and nine contralateral VSs. Baseline ADC values were not associated with HR or RR in target ears. However, dynamic changes in ADC from baseline to week 25 were associated with HR in target ears (P = .019), with a median decrease in ADC of 9% in patients with HR. Ktrans was not significantly associated with HR, but baseline Ktrans values were associated with RR at week 25 across all evaluable tumors (n = 21; P = .037), and patients achieving RR in a target VS had higher baseline Ktrans than nonresponders (0.30 v 0.07, respectively; P = .051).
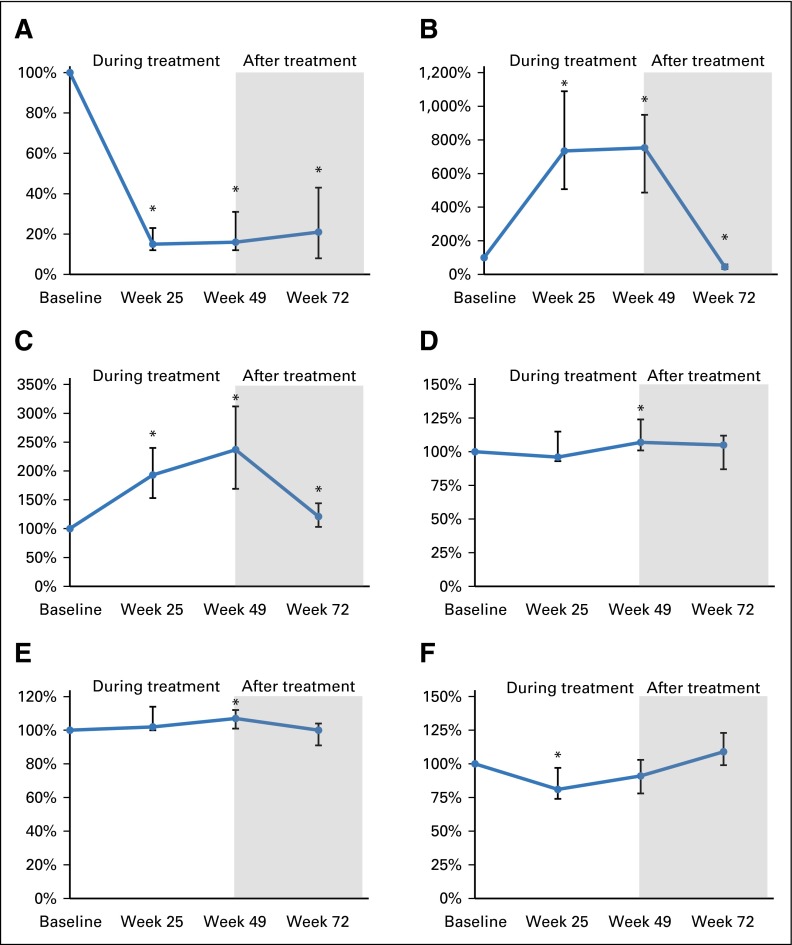
Bevacizumab was associated with decreased plasma levels of free VEGF across all time points in all patients. At weeks 25 and 49, this was accompanied by increased levels of total VEGF, which significantly dropped after treatment (Fig 3; Appendix Table A3, online only). Bevacizumab was also associated with increased plasma levels of PlGF at all time points and of VEGF-D and SDF1α at week 49 (Fig 3; Appendix Table A3). Finally, bevacizumab was associated with a transient decrease in Ang2 levels at week 25 (Fig 3; Appendix Table A3).
Fig 3.
Line graphs showing changes over time in plasma (A) free vascular endothelial growth factor (VEGF), (B) total VEGF (free plus bound), (C) placental growth factor (PlGF), (D) VEGF-D, (E) stromal cell–derived factor 1α (SDF1α), and (F) angiopoietin 2 for all participants (N = 14). Anti-VEGF therapy with bevacizumab decreased the plasma levels of free VEGF and increased the levels of antibody-bound VEGF, PlGF, and SDF1α in patients with neurofibromatosis type 2. Vertical bars indicate interquartile range; (*) indicates P < .05.
HR was associated with lower baseline HGF (P = .019), decreased plasma carbonic anhydrase IX at weeks 25 and 49 (P = .010 and .035, respectively), and increased plasma sVEGFR2 at week 25 (P = .004; Appendix Table A4; Appendix Fig A2, online only). RR was associated with higher baseline levels of VEGF-D and SDF1α (P = .037 and .025, respectively), decreased s-cKIT at week 25 (P = .023), and decreased sTie2 at week 49 (P = .034; Appendix Table A5, online only).
DISCUSSION
The most common and universally life-altering consequence of NF2 is hearing loss, with the majority of affected individuals experiencing progression to deafness in their third decade.5,6,8,10 Bevacizumab administered on a compassionate-use basis to people with NF2 resulted in HR in 57% of evaluable patients.17,23 This outcome was unprecedented, heralding the possibility of effective therapy for these tumors. However, much uncertainty remains regarding the optimal patient population, dosing strategy, and long-term durability. The results of this prospective efficacy study have confirmed the proportion of patients with NF2 and symptomatic VS who achieved durable HR with bevacizumab (36%; 95% CI, 13% to 65%; P < .001) as well as the durability of response during and after treatment and presented several candidate biomarkers that may ultimately allow rational selection of patients for therapy.
HR was selected as the primary end point because it is clinically meaningful and provides evidence of drug activity, given that durable hearing improvement with NF2-associated VSs is improbable either spontaneously or with RT or resection.8,15,16 HR assessed by WRS is quantifiable, reliable, and feasible for measuring hearing function over time.28,29 We required that statistically significant HR be maintained for at least 3 months to overcome concerns about spurious HR and with the awareness that NF2-associated VSs are chronic tumors for which short-term efficacy would have little value. Natural history data show that only 16% of people with NF2 have spontaneous HR if baseline WRS is lower than 90%.8 The finding of a 36% (95% CI, 13% to 65%) confirmed HR in people with NF2, documented progressive hearing loss, and a median baseline WRS of 60% represents noteworthy therapeutic benefit. Moreover, the unconfirmed HR rate of 57% in this prospective study is identical to the results of large retrospective series17,23 and far superior to spontaneous HR in natural history studies.8
Although bevacizumab was well tolerated in this study, there were three serious AEs, and three of seven female patients who had normal menstruation at baseline developed menstrual irregularities. However, all women recovered baseline menstrual function after stopping treatment with bevacizumab. This experience echoes recent reports of ovarian failure in women with breast cancer treated with bevacizumab. Given the age of people with NF2 considered for treatment, this AE should be expressly discussed with women considering bevacizumab therapy and monitored during treatment.
An important finding is that 55% of patients who achieved HR in any ear maintained this response for up to 6 months after treatment with study drug. Similar durability was seen with RR. These results suggest that after HR is achieved, multiweek dosing intervals or drug holidays capitalizing on the long half-life of bevacizumab may be feasible. Interestingly, analysis of antiangiogenic therapies across a variety of cancers also suggested alternative dosing strategies may be more efficacious based on markers of vessel normalization and oxygenation.35 Analysis of blood markers in our study was also consistent with this hypothesis. Specifically, we saw unexpectedly high baseline VEGF levels, comparable to those in brain cancer.32,33 Second, there was a sustained decrease in the circulating levels of free VEGF (with a corresponding increase in total VEGF) and a transient decrease in Ang2 during bevacizumab therapy, a pattern reminiscent of biologic response to anti-VEGF therapy in cancer35 but not previously recognized in nonmalignant tumor syndromes like NF2. Third, bevacizumab treatment was associated with increased plasma levels of PlGF, VEGF-D, and SDF1α over time. These have been proposed as markers of resistance to anti-VEGF therapy in brain cancer20,35 and may hold similar value as potential biomarkers for antiangiogenic therapy for benign nerve sheath tumors. Together these data indicate that circulating markers of vessel normalization and oxygenation may support alternative dosing strategies in both cancers and benign tumor syndromes.
Finally, the frequently observed absence of a significant correlation between hearing and tumor size in NF2-associated VSs was borne out in this study. However, there were interesting associations between HR and dynamic changes in ADC from baseline to week 25 as well as lower absolute levels of HGF at baseline in patients achieving HR. These findings suggest that HR may be related to reduced tumor-associated edema and improved oxygenation rather than direct impact on tumor volume.22 RR was associated with baseline Ktrans and degree of reduction in plasma free VEGF, VEGF-D, and sTie-2, suggesting a pharmacodynamic relationship between targeting circulating VEGF and reducing hyperpermeable blood vessels.20,36 Lastly, the preliminary findings of Ang2 and sTie-2 changing in response to bevacizumab in people with NF2 is notable because, first, similar patterns are observed with antiangiogenesis therapy in brain cancer; second, Ang2 and Tie-2 are important factors in proangiogenic pathways in general; and third, both proteins have been implicated in schwannomas.20,32,37
In conclusion, this prospective study confirms the efficacy and safety of bevacizumab in the subset of people with NF2 and progressive, symptomatic VSs. The data, although from a small, single-arm study, expand the understanding of required dosing intervals to maintain HR, potentially allowing lower doses over time, and identify several potential blood and imaging biomarkers that, if validated, will allow targeting therapy to the people with the highest likelihood of benefit. The ongoing subsequent study of bevacizumab for children and young adults with hearing loss resulting from an NF2-associated VS (ClinicalTrials.gov identifier NCT01767792) will further investigate the findings from this study.
Supplementary Material
Acknowledgment
We thank Helen Chen, MD, for her support and oversight of this study via the Cancer Therapy Evaluation Program collaboration; Shannon Langmead, Andrea Baldwin, and Krista Follmer for their excellent care of patients enrolled in this study; Robert Evers, Hugh Wall, Trinity Urban, and Dominique Jennings for their assistance with the imaging assessments; Rhonda Jackson for her administrative support with the manuscript; Anna Khachatryan for technical support for the biomarker studies; and the Galloway Family Foundation for their support of this study.
Appendix
Table A1.
Clinical Criteria for Definition of Hearing Response on the Basis of 100-Word Hearing Test
| Baseline Word Recognition Score (%) | 95% Critical Difference (%) | Word Recognition Score During Study (%) | |
|---|---|---|---|
| Definition of Hearing Improvement | Definition of Hearing Decline | ||
| 0 | 0-3 | ≥ 4 | NA |
| 1 | 0-6 | ≥ 7 | NA |
| 2 | 0-8 | ≥ 9 | NA |
| 3 | 0-9 | ≥ 10 | NA |
| 4 | 1-11 | ≥ 12 | 0 |
| 5 | 1-12 | ≥ 13 | 0 |
| 6 | 1-14 | ≥ 15 | 0 |
| 7 | 2-15 | ≥ 16 | ≤ 1 |
| 8 | 2-17 | ≥ 18 | ≤ 1 |
| 9 | 3-18 | ≥ 19 | ≤ 2 |
| 10 | 4-19 | ≥ 20 | ≤ 3 |
| 11 | 4-21 | ≥ 22 | ≤ 3 |
| 12 | 5-22 | ≥ 23 | ≤ 4 |
| 13 | 6-23 | ≥ 24 | ≤ 5 |
| 14 | 6-25 | ≥ 26 | ≤ 5 |
| 15 | 7-26 | ≥ 27 | ≤ 6 |
| 16 | 8-27 | ≥ 28 | ≤ 7 |
| 17 | 8-28 | ≥ 29 | ≤ 7 |
| 18 | 9-29 | ≥ 30 | ≤ 8 |
| 19 | 10-31 | ≥ 32 | ≤ 9 |
| 20 | 11-32 | ≥ 33 | ≤ 10 |
| 21 | 11-33 | ≥ 34 | ≤ 10 |
| 22 | 12-34 | ≥ 35 | ≤ 11 |
| 23 | 13-35 | ≥ 36 | ≤ 12 |
| 24 | 14-36 | ≥ 37 | ≤ 13 |
| 25 | 14-37 | ≥ 38 | ≤ 13 |
| 26 | 15-39 | ≥ 40 | ≤ 14 |
| 27 | 16-40 | ≥ 41 | ≤ 15 |
| 28 | 17-41 | ≥ 42 | ≤ 16 |
| 29 | 18-42 | ≥ 43 | ≤ 17 |
| 30 | 19-43 | ≥ 44 | ≤ 18 |
| 31 | 19-44 | ≥ 45 | ≤ 18 |
| 32 | 20-45 | ≥ 46 | ≤ 19 |
| 33 | 21-46 | ≥ 47 | ≤ 20 |
| 34 | 22-47 | ≥ 48 | ≤ 21 |
| 35 | 23-48 | ≥ 49 | ≤ 22 |
| 36 | 24-49 | ≥ 50 | ≤ 23 |
| 37 | 25-50 | ≥ 51 | ≤ 24 |
| 38 | 26-51 | ≥ 52 | ≤ 25 |
| 39 | 26-52 | ≥ 53 | ≤ 25 |
| 40 | 27-53 | ≥ 54 | ≤ 26 |
| 41 | 28-54 | ≥ 55 | ≤ 27 |
| 42 | 29-55 | ≥ 56 | ≤ 28 |
| 43 | 30-56 | ≥ 57 | ≤ 29 |
| 44 | 31-57 | ≥ 58 | ≤ 30 |
| 45 | 32-58 | ≥ 59 | ≤ 31 |
| 46 | 33-59 | ≥ 60 | ≤ 32 |
| 47 | 34-60 | ≥ 61 | ≤ 33 |
| 48 | 35-61 | ≥ 62 | ≤ 34 |
| 49 | 36-62 | ≥ 63 | ≤ 35 |
| 50 | 37-63 | ≥ 64 | ≤ 36 |
| 51 | 38-64 | ≥ 65 | ≤ 37 |
| 52 | 39-65 | ≥ 66 | ≤ 38 |
| 53 | 40-66 | ≥ 67 | ≤ 39 |
| 54 | 41-67 | ≥ 68 | ≤ 40 |
| 55 | 42-68 | ≥ 69 | ≤ 41 |
| 56 | 43-69 | ≥ 70 | ≤ 42 |
| 57 | 44-70 | ≥ 71 | ≤ 43 |
| 58 | 45-71 | ≥ 72 | ≤ 44 |
| 59 | 46-72 | ≥ 73 | ≤ 45 |
| 60 | 47-73 | ≥ 74 | ≤ 46 |
| 61 | 48-74 | ≥ 75 | ≤ 47 |
| 62 | 49-74 | ≥ 75 | ≤ 48 |
| 63 | 50-75 | ≥ 76 | ≤ 49 |
| 64 | 51-76 | ≥ 77 | ≤ 50 |
| 65 | 52-77 | ≥ 78 | ≤ 51 |
| 66 | 53-78 | ≥ 79 | ≤ 52 |
| 67 | 54-79 | ≥ 80 | ≤ 53 |
| 68 | 55-80 | ≥ 81 | ≤ 54 |
| 69 | 56-81 | ≥ 82 | ≤ 55 |
| 70 | 57-81 | ≥ 82 | ≤ 56 |
| 71 | 58-82 | ≥ 83 | ≤ 57 |
| 72 | 59-83 | ≥ 84 | ≤ 58 |
| 73 | 60-84 | ≥ 85 | ≤ 59 |
| 74 | 61-85 | ≥ 86 | ≤ 60 |
| 75 | 63-86 | ≥ 87 | ≤ 62 |
| 76 | 64-86 | ≥ 87 | ≤ 63 |
| 77 | 65-87 | ≥ 88 | ≤ 64 |
| 78 | 66-88 | ≥ 89 | ≤ 65 |
| 79 | 67-89 | ≥ 90 | ≤ 66 |
| 80 | 68-89 | ≥ 90 | ≤ 67 |
| 81 | 69-90 | ≥ 91 | ≤ 68 |
| 82 | 71-91 | ≥ 92 | ≤ 70 |
| 83 | 72-92 | ≥ 93 | ≤ 71 |
| 84 | 73-92 | ≥ 93 | ≤ 72 |
| 85 | 74-93 | ≥ 94 | ≤ 73 |
| 86 | 75-94 | ≥ 95 | ≤ 74 |
| 87 | 77-94 | ≥ 95 | ≤ 76 |
| 88 | 78-95 | ≥ 96 | ≤ 77 |
| 89 | 79-96 | ≥ 97 | ≤ 78 |
| 90 | 81-96 | ≥ 97 | ≤ 80 |
| 91 | 82-97 | ≥ 98 | ≤ 81 |
| 92 | 83-98 | ≥ 99 | ≤ 82 |
| 93 | 85-98 | ≥ 99 | ≤ 84 |
| 94 | 86-99 | 100 | ≤ 85 |
| 95 | 88-99 | 100 | ≤ 87 |
| 96 | 89-99 | 100 | ≤ 88 |
| 97 | 91-100 | NA | ≤ 90 |
| 98 | 92-100 | NA | ≤ 91 |
| 99 | 94-100 | NA | ≤ 93 |
| 100 | 97-100 | NA | ≤ 96 |
NOTE. Upper and lower limits for the 95% critical differences for percentage scores are adapted from Thornton and Raffin.27 Patients with baseline word recognition scores greater than 90% or lower than 6% (bold font) were ineligible for the study because of ceiling and floor effects, respectively.
Abbreviation: NA, not applicable.
Table A2.
Timing and Form of Prior Therapy for Participants Undergoing Surgery or RT for Target or Nontarget VS Before Initiating Bevacizumab (n = 8 of 14)
| Target Ear Surgery (year) | Time From Surgery to Bevacizumab (months) | RT to Target Ear (year) | Time From RT to Bevacizumab (months) | Nontarget Ear Surgery (year) | Time From Surgery to Bevacizumab (months) | RT to Nontarget Ear (year) | Time to Bevacizumab From RT (months) |
|---|---|---|---|---|---|---|---|
| 1992 | 216 | ||||||
| 1972 | 468 | ||||||
| 2006 | 60 | 1996 | 180 | ||||
| 2010 | 5 | 2007 (24 Gy) | 48 | ||||
| 2006 | 58 | ||||||
| 2010 (13 Gy) | 15 | ||||||
| 2008 | 36 | ||||||
| 1996 | 180 | 2001 (40 Gy) | 120 | 1996 | 180 | ||
| 2009 | 22 |
Abbreviations: RT, radiation therapy; VS, vestibular schwannoma.
Table A3.
Blood Circulating Biomarker Levels at Baseline and Changes During Treatment in Patients With NF2-Associated VSs
| Biomarker | Median (IQR) | |||
|---|---|---|---|---|
| Pretreatment | Percent Change | |||
| During Treatment | Post-Treatment | |||
| Baseline (pg/ml; n = 13) | Week 25 (n = 13) | Week 49 (n = 12) | Week 72 (n = 11) | |
| Free VEGF | 182 (107-237) | 0.15 (0.12-0.23) | 0.16 (0.12-0.31) | 0.21 (0.80-0.43) |
| P | NA | < .001 | < .001 | < .001 |
| Total VEGF (free plus bound) | 131 (68-197) | 7.34 (5.07-10.89) | 7.53 (4.87-9.49) | 0.45 (0.32-0.60) |
| P | NA | < .001 | < .001 | < .001 |
| PlGF | 35 (33-41) | 1.93 (1.53-2.40) | 2.37 (1.69-3.12) | 1.21 (1.03-1.44) |
| P | NA | .0034 | < .001 | .014 |
| VEGF-C | 234 (190-316) | 0.88 (0.71-1.35) | 1.20 (0.62-1.65) | 0.70 (0.41-0.81) |
| P | NA | .95 | .68 | .054 |
| VEGF-D | 1,019 (879-1,366) | 0.96 (0.93-1.15) | 1.07 (1.01-1.24) | 1.05 (0.87-1.12) |
| P | NA | .68 | .034 | .58 |
| sVEGFR1 | 79 (64-94) | 0.89 (0.80-1.12) | 0.92 (0.79-1.31) | 0.86 (0.82-0.95) |
| P | NA | .68 | .97 | .068 |
| sVEGFR2 | 11,108 (9,296-12,065) | 0.95 (0.93-1.05) | 0.92 (0.88-1.05) | 0.96 (0.86-1.13) |
| P | NA | .50 | .38 | .64 |
| bFGF | 79 (43-93) | 1.02 (0.48-1.18) | 1.28 (0.69-1.57) | 0.50 (0.24-0.74) |
| P | NA | .38 | .27 | .054 |
| Ang1 | 5,274 (3,996-6,953) | 1.02 (0.48-1.18) | 1.28 (0.73-2.26) | 0.58 (0.29-0.95) |
| P | NA | 1.00 | .23 | .067 |
| Ang2 | 1,654 (1,422-1,938) | 0.81 (0.74-0.97) | 0.91 (0.78-1.03) | 1.09 (0.99-1.23) |
| P | NA | .0093 | .23 | .10 |
| sTie2 | 6,937 (5,773-8,271) | 0.97 (0.87-1.03) | 0.89 (0.85-1.03) | 1.00 (0.90-1.21) |
| P | NA | .19 | .34 | .83 |
| HGF | 848 (772-1,016) | 1.01 (0.90-1.20) | 1.06 (1.01-1.31) | 1.09 (0.97-1.17) |
| P | NA | .45 | .052 | .24 |
| s-cMet | 1,349 (1,186-1,478) | 0.97 (0.92-1.06) | 0.99 (0.93-1.04) | 1.00 (0.96-1.03) |
| P | NA | .38 | .678 | .90 |
| CAIX | 47 (32-52) | 1.84 (0.75-2.16) | 1.52 (0.64-2.11) | 1.16 (0.64-1.71) |
| P | NA | .094 | .052 | .24 |
| IL-1α | 1.4 (1.0-2.4) | 0.97 (0.84-1.07) | 1.15 (0.72-3.02) | 1.03 (0.70-1.39) |
| P | NA | .64 | .34 | .70 |
| IL-6 | 2.4 (1.9-3.1) | 1.23 (0.97-1.63) | 1.01 (0.84-1.30) | 1.07 (0.91-1.45) |
| P | NA | .094 | .68 | .21 |
| IL-8 | 4.8 (3.3-5.8) | 1.08 (0.88-1.23) | 1.07 (0.81-1.41) | 0.98 (0.67-1.24) |
| P | NA | .59 | .52 | .64 |
| TNF-α | 7.8 (7.5-1.0) | 1.00 (0.91-1.06) | 0.99 (0.85-1.23) | 0.88 (0.71-1.30) |
| P | NA | .89 | .68 | 1.00 |
| SDF1α | 1,972 (1,789-2,107) | 1.02 (1.00-1.14) | 1.07 (1.01-1.12) | 1.00 (0.91-1.04) |
| P | NA | .38 | .0093 | .52 |
NOTE. Bold font indicates statistical significance. P values determined from Wilcoxon sign rank test for percentage of change after treatment.
Abbreviations: Ang, angiopoietin; bFGF, basic fibroblast growth factor; CAIX, carbonic anhydrase IX; HGF, hepatocyte growth factor; IL, interleukin; IQR, interquartile range; NA, not applicable; NF2, neurofibromatosis type 2; PlGF, placental growth factor; SDF1α, stromal cell–derived factor 1α; sVEGFR, soluble vascular endothelial growth factor receptor; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor; VS, vestibular schwannoma.
Table A4.
Association Between Blood Circulating Biomarker Levels in Hearing Responders (n = 5) Versus Nonresponders (n = 8) Treated With Bevacizumab
| Biomarker | Median (IQR) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (pg/ml), n=13 | Percent Change | |||||||
| Week 25 (n = 13) | Week 49 (n = 12) | Week 72 (n = 11) | ||||||
| Responders (n = 5) | Nonresponders (n = 8) | Responders (n = 5) | Nonresponders (n = 8) | Responders (n = 5) | Nonresponders (n = 7) | Responders (n = 4) | Nonresponders (n = 7) | |
| sVEGFR2 | 10,691 (8,417-11,108) | 11,739 (9,911-13,842) | 1.06 (1.05-1.21) | 0.93 (0.87-0.93) | 1.03 (0.93-1.07) | 0.88 (0.85-1.01) | 1.13 (1.01-1.18) | 0.87 (0.84-0.90) |
| P | .19 | .0043 | .074 | .014 | ||||
| HGF | 745 (602-821) | 957 (841-1,054) | 1.11 (1.03-1.20) | 0.92 (0.86-1.21) | 1.23 (1.05-1.38) | 1.03 (0.99-1.22) | 1.03 (1.03-1.19) | 1.12 (0.85-1.17) |
| P | .019 | .092 | .42 | .65 | ||||
| CAIX | 50 (47-71) | 32 (20-51) | 0.72 (0.54-0.75) | 2.04 (1.40-2.32) | 0.59 (0.51-0.69) | 1.81 (1.49-2.63) | 0.95 (0.64-1.16) | 1.67 (1.11-2.01) |
| P | .14 | .010 | .035 | .12 | ||||
NOTE. Bold font indicates statistical significance.
Abbreviations: CAIX, carbonic anhydrase IX; HGF, hepatocyte growth factor; IQR, interquartile range; sVEGFR, soluble vascular endothelial growth factor receptor.
Table A5.
Association Between Blood Circulating Biomarker Levels in Radiographic Responders (n = 9) Versus Nonresponders (n = 4) Treated With Bevacizumab
| Biomarker | Median (IQR) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (pg/mL; n = 13) | Percent Change | |||||||||
| Week 25 (n = 13) | Week 49 (n = 12) | Week 72 (n = 11) | ||||||||
| Responders (n = 9) | Nonresponders (n = 4) | Responders (n = 9) | Nonresponders (n = 4) | Responders (n = 8) | Nonresponders (n = 4) | Responders (n = 8) | Nonresponders (n = 3) | |||
| Free VEGF | 233 (172-281) | 107 (93-149) | 0.12 (0.11-0.15) | 0.24 (0.22-0.37) | 0.14 (0.09-0.21) | 0.31 (0.22-0.40) | 0.21 (0.09-0.44) | 0.21 (0.06-0.43) | ||
| P | .19 | .025 | .075 | .76 | ||||||
| Total VEGF (free plus bound) | 134 (106-197) | 68 (66-139) | 4.98 (1.63-6.95) | 12.23 (8.93-15.50) | 6.53 (3.80-7.97) | 6.91 (3.87-13.63) | 0.44 (0.34-0.56) | 0.53 (0.17-0.60) | ||
| P | .25 | .034 | .67 | 1.0 | ||||||
| VEGF-D | 1,243 (1,016-1,412) | 813 (627-949) | 0.96 (0.90-1.04) | 1.17 (1.05-1.23) | 1.07 (1.0-1.33) | 1.13 (1.01-1.24) | 1.08 (0.96-1.13) | 0.98 (0.83-1.05) | ||
| P | .037 | .054 | .93 | .13 | ||||||
| SDF1α | 2,040 (1,906-2,185) | 1,762 (1,707-1,875) | 1.09 (1.00-1.14) | 0.92 (0.81-1.07) | 1.09 (1.05-1.13) | 1.01 (0.99-1.05) | 1.0 (0.88-1.03) | 0.97 (0.92-1.11) | ||
| P | .025 | .11 | .15 | .76 | ||||||
| sTie2 | 8,055 (6,000-8,362) | 6,355 (5,416-7,509) | 0.89 (0.82-1.01) | 1.03 (0.98-1.05) | 0.87 (0.82-0.93) | 1.12 (0.95-1.47) | 1.01 (0.85-1.14) | 1.00 (0.91-1.22) | ||
| P | .40 | .14 | .034 | .76 | ||||||
| s-cKIT | 1,237 (914-1,243) | 1,017 (822-1,427) | 0.94 (0.87-1.00) | 1.16 (1.15-1.18) | 1.05 (0.98-1.53) | 1.09 (1.02-1.24) | 1.05 (0.87-1.12) | 1.01 (0.91-1.14) | ||
| P | .70 | .023 | .87 | .64 | ||||||
NOTE. Bold font indicates statistical significance.
Abbreviations: IQR, interquartile range; SDF1α, stromal cell–derived factor 1α; VEGF, vascular endothelial growth factor.
Fig A1.
Scattergrams of (A) baseline hearing function for all target ears, as recommended by the Hearing Committee of the American Academy of Otolaryngology-Head and Neck Society, and (B) best change in hearing for all target ears after treatment with bevacizumab. Color code: gray, class A; gold, class B; red, class C; blue, class D. Classes A and B are considered serviceable, and classes C and D are considered unserviceable.34
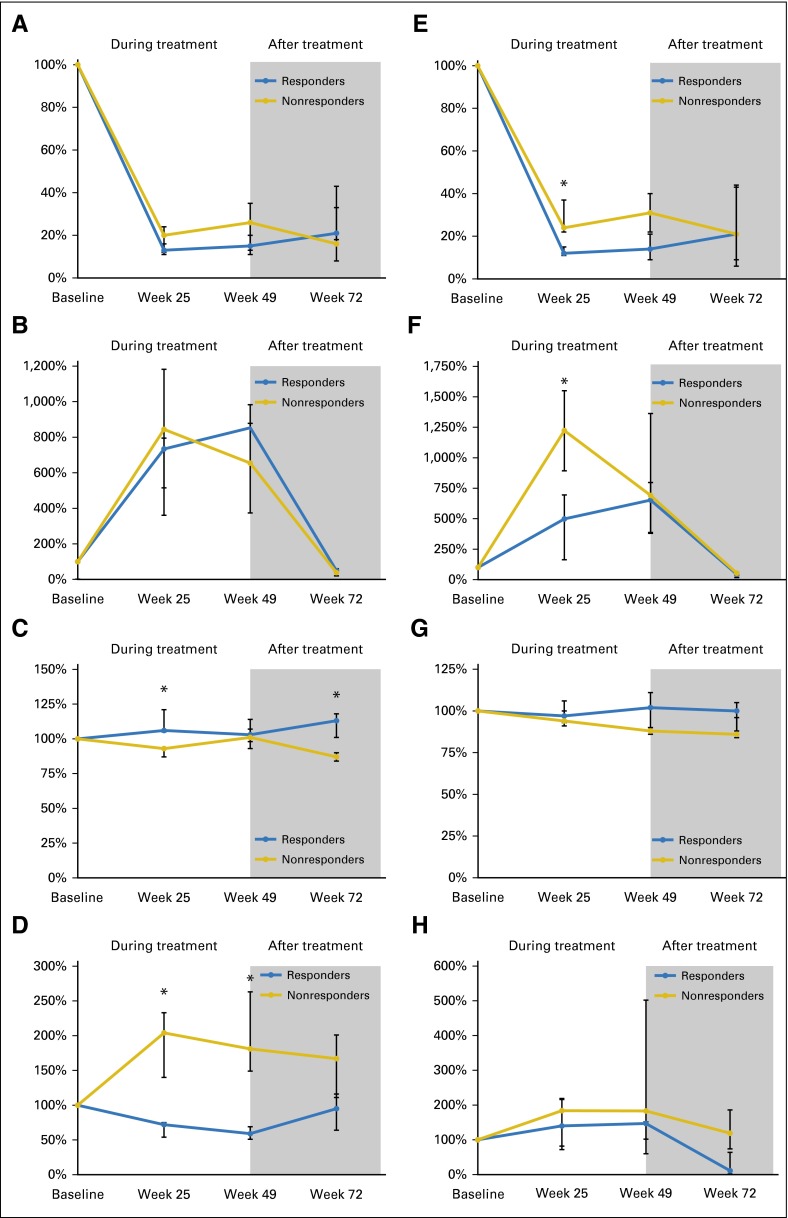
Fig A2.
Line graphs for (A to D) patients with confirmed hearing response (HR; n = 5) versus nonresponders (n = 9) and (E to H) confirmed radiographic responders (n = 9) versus nonresponders (n = 5) showing changes over time in (A, E) relative free vascular endothelial growth factor (VEGF), (B, F) total VEGF (free plus bound), (C, G) soluble VEGF receptor 2, and (D, H) carbonic anhydrase IX. Data are presented as median values with interquartile ranges; (*) indicates a significant difference between relative biomarker concentrations in responders and nonresponders (P < .05).
Footnotes
Supported by the Cancer Therapy Evaluation Program, Galloway Family Foundation, and National Cancer Institute Center for Cancer Research Intramural Research Program and National Institute on Deafness and Other Communication Disorders Intramural Research Program, National Institutes of Health (Bethesda, MD).
Presented in part at the Children’s Tumor Foundation Neurofibromatosis Conference, Monterey, CA, June 8-11, 2013, and European Association of Neurosurgical Societies, Tel Aviv, Israel, November 11-14, 2013.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Jaishri O. Blakeley, Xiaobu Ye, Chris F. Halpin, Brigitte C. Widemann, Scott R. Plotkin
Financial support: Jaishri O. Blakeley
Administrative support: Jaishri O. Blakeley
Provision of study materials or patients: Jaishri O. Blakeley, Brigitte Widemann, Scott R. Plotkin
Collection and assembly of data: Jaishri O. Blakeley, Dan. G. Duda, Chris F. Halpin, Amanda L. Bergner, Laura M. Fayad, Shivani Ahlawat, Michael A. Jacobs, Christopher Zalewski, Eva Dombi, Brigitte Widemann, Scott R. Plotkin
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Efficacy and Biomarker Study of Bevacizumab for Hearing Loss Resulting From Neurofibromatosis Type 2–Associated Vestibular Schwannomas
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Jaishri O. Blakeley
Honoraria: Mayo Clinic Cancer Center, New York University Clinical Cancer Center, AbbVie
Research Funding: GlaxoSmithKline (Inst), Eli Lilly (Inst), Sanofi (Inst)
Xiaobu Ye
No relationship to disclose
Dan G. Duda
Consulting or Advisory Role: Hexal
Research Funding: Merrimack (Inst), Health Care Pharma (Inst)
Chris F. Halpin
Honoraria: Auris Medical AG
Amanda L. Bergner
Employment: Ambry Genetics
Honoraria: UpToDate
Alona Muzikansky
No relationship to disclose
Vanessa L. Merker
No relationship to disclose
Elizabeth R. Gerstner
No relationship to disclose
Laura M. Fayad
Research Funding: Association of University Radiologists: General Electric Radiology Research Academic Fellowship, Siemens
Shivani Ahlawat
No relationship to disclose
Michael A. Jacobs
Other Relationship: Siemens Medical
Rakesh K. Jain
Honoraria: Roche Norway
Consulting or Advisory Role: Ophthotech, SynDevRx, SPARC, XTuit Pharmaceuticals, Sun Pharma Advanced Research
Research Funding: Roche (Inst), MedImmune (Inst)
Travel, Accommodations, Expenses: Roche
Other Relationship: Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla World Healthcare Fund, Tekla Healthcare Opportunities Fund
Christopher Zalewski
No relationship to disclose
Eva Dombi
No relationship to disclose
Brigitte C. Widemann
No relationship to disclose
Scott R. Plotkin
Research Funding: Genentech (Inst)
REFERENCES
- 1.Stangerup SE, Tos M, Thomsen J, et al. True incidence of vestibular schwannoma? Neurosurgery. 2010;67:1335–1340. doi: 10.1227/NEU.0b013e3181f22660. discussion 1340. [DOI] [PubMed] [Google Scholar]
- 2.Carlson ML, Link MJ, Wanna GB, et al. Management of sporadic vestibular schwannoma. Otolaryngol Clin North Am. 2015;48:407–422. doi: 10.1016/j.otc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Flickinger JC, Kondziolka D, Niranjan A, et al. Results of acoustic neuroma radiosurgery: An analysis of 5 years’ experience using current methods. J Neurosurg. 2013;119(suppl):1–6. doi: 10.3171/jns.2001.94.1.0001. [DOI] [PubMed] [Google Scholar]
- 4.Lunsford LD, Niranjan A, Flickinger JC, et al. Radiosurgery of vestibular schwannomas: Summary of experience in 829 cases. J Neurosurg. 2013;119(suppl):195–199. [PubMed] [Google Scholar]
- 5.Asthagiri AR, Parry DM, Butman JA, et al. Neurofibromatosis type 2. Lancet. 2009;373:1974–1986. doi: 10.1016/S0140-6736(09)60259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans DG. Neurofibromatosis type 2 (NF2): a clinical and molecular review. Orphanet J Rare Dis. 2009;4:16. doi: 10.1186/1750-1172-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: A twenty first century perspective. Lancet Neurol. 2007;6:340–351. doi: 10.1016/S1474-4422(07)70075-3. [DOI] [PubMed] [Google Scholar]
- 8.Plotkin SR, Merker VL, Muzikansky A, et al. Natural history of vestibular schwannoma growth and hearing decline in newly diagnosed neurofibromatosis type 2 patients. Otol Neurotol. 2014;35:e50–e56. doi: 10.1097/MAO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 9.Blakeley JO, Evans DG, Adler J, et al. Consensus recommendations for current treatments and accelerating clinical trials for patients with neurofibromatosis type 2. Am J Med Genet A. 2012;158A:24–41. doi: 10.1002/ajmg.a.34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher LM, Doherty JK, Lev MH, et al. Concordance of bilateral vestibular schwannoma growth and hearing changes in neurofibromatosis 2: Neurofibromatosis 2 natural history consortium. Otol Neurotol. 2009;30:835–841. doi: 10.1097/MAO.0b013e3181b2364c. [DOI] [PubMed] [Google Scholar]
- 11.Dewan R, Pemov A, Kim HJ, et al. Evidence of polyclonality in neurofibromatosis type 2-associated multilobulated vestibular schwannomas. Neuro-oncol. 2015;17:566–573. doi: 10.1093/neuonc/nou317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stivaros SM, Stemmer-Rachamimov AO, Alston R, et al. Multiple synchronous sites of origin of vestibular schwannomas in neurofibromatosis type 2. J Med Genet. 2015;52:557–562. doi: 10.1136/jmedgenet-2015-103050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun S, Liu A. Long-term follow-up studies of Gamma Knife surgery for patients with neurofibromatosis type 2. J Neurosurg. 2014;121(suppl):143–149. doi: 10.3171/2014.8.GKS141503. [DOI] [PubMed] [Google Scholar]
- 14.Plotkin SR, Ardern-Holmes SL, Barker FG, II, et al. Hearing and facial function outcomes for neurofibromatosis 2 clinical trials. Neurology. 2013;81(suppl 1):S25–S32. doi: 10.1212/01.wnl.0000435746.02780.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe JG, Radatz MW, Walton L, et al. Clinical experience with gamma knife stereotactic radiosurgery in the management of vestibular schwannomas secondary to type 2 neurofibromatosis. J Neurol Neurosurg Psychiatry. 2003;74:1288–1293. doi: 10.1136/jnnp.74.9.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samii M, Matthies C, Tatagiba M. Management of vestibular schwannomas (acoustic neuromas): Auditory and facial nerve function after resection of 120 vestibular schwannomas in patients with neurofibromatosis 2. Neurosurgery. 1997;40:696–705. doi: 10.1097/00006123-199704000-00007. discussion 705-706. [DOI] [PubMed] [Google Scholar]
- 17.Plotkin SR, Stemmer-Rachamimov AO, Barker FG, II, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361:358–367. doi: 10.1056/NEJMoa0902579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalgorf DM, Rowsell C, Bilbao JM, et al. Immunohistochemical investigation of hormone receptors and vascular endothelial growth factor concentration in vestibular schwannoma. Skull Base. 2008;18:377–384. doi: 10.1055/s-0028-1096198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutsimpelas D, Stripf T, Heinrich UR, et al. Expression of vascular endothelial growth factor and basic fibroblast growth factor in sporadic vestibular schwannomas correlates to growth characteristics. Otol Neurotol. 2007;28:1094–1099. doi: 10.1097/MAO.0b013e31814b2787. [DOI] [PubMed] [Google Scholar]
- 20.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong HK, Lahdenranta J, Kamoun WS, et al. Anti-vascular endothelial growth factor therapies as a novel therapeutic approach to treating neurofibromatosis-related tumors. Cancer Res. 2010;70:3483–3493. doi: 10.1158/0008-5472.CAN-09-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 23.Plotkin SR, Merker VL, Halpin C, et al. Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: A retrospective review of 31 patients. Otol Neurotol. 2012;33:1046–1052. doi: 10.1097/MAO.0b013e31825e73f5. [DOI] [PubMed] [Google Scholar]
- 24.Slusarz KM, Merker VL, Muzikansky A, et al. Long-term toxicity of bevacizumab therapy in neurofibromatosis 2 patients. Cancer Chemother Pharmacol. 2014;73:1197–1204. doi: 10.1007/s00280-014-2456-2. [DOI] [PubMed] [Google Scholar]
- 25.The Consensus Development Panel National Institutes of Health Consensus Development Conference statement on acoustic neuroma, December 11-13, 1991. Arch Neurol. 1994;51:201–207. doi: 10.1001/archneur.1994.00540140115021. [DOI] [PubMed] [Google Scholar]
- 26.Evans DG, Huson SM, Donnai D, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84:603–618. [PubMed] [Google Scholar]
- 27.Baser ME, Friedman JM, Wallace AJ, et al. Evaluation of clinical diagnostic criteria for neurofibromatosis 2. Neurology. 2002;59:1759–1765. doi: 10.1212/01.wnl.0000035638.74084.f4. [DOI] [PubMed] [Google Scholar]
- 28.Halpin C, Rauch SD. Using audiometric thresholds and word recognition in a treatment study. Otol Neurotol. 2006;27:110–116. doi: 10.1097/00129492-200601000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Thornton AR, Raffin MJ. Speech-discrimination scores modeled as a binomial variable. J Speech Hear Res. 1978;21:507–518. doi: 10.1044/jshr.2103.507. [DOI] [PubMed] [Google Scholar]
- 30.Plotkin SR, Halpin C, Blakeley JO, et al. Suggested response criteria for phase II antitumor drug studies for neurofibromatosis type 2 related vestibular schwannoma. J Neurooncol. 2009;93:61–77. doi: 10.1007/s11060-009-9867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris GJ, Plotkin SR, Maccollin M, et al. Three-dimensional volumetrics for tracking vestibular schwannoma growth in neurofibromatosis type II. Neurosurgery. 2008;62:1314–1319. doi: 10.1227/01.neu.0000333303.79931.83. discussion 1319-1320. [DOI] [PubMed] [Google Scholar]
- 32.Batchelor TT, Gerstner ER, Emblem KE, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci USA. 2013;110:19059–19064. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28:2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurgel RK, Jackler RK, Dobie RA, et al. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg. 2012;147:803–807. doi: 10.1177/0194599812458401. [DOI] [PubMed] [Google Scholar]
- 35.Jain RK, Duda DG, Willett CG, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6:327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li KL, Djoukhadar I, Zhu X, et al. Vascular biomarkers derived from dynamic contrast-enhanced MRI predict response of vestibular schwannoma to antiangiogenic therapy in type 2 neurofibromatosis. Neuro Oncol. 2016;18:275–282. doi: 10.1093/neuonc/nov168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boin A, Couvelard A, Couderc C, et al. Proteomic screening identifies a YAP-driven signaling network linked to tumor cell proliferation in human schwannomas. Neuro-oncol. 2014;16:1196–1209. doi: 10.1093/neuonc/nou020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.