Abstract
Purpose
This study investigated the treatment of primary CNS lymphoma with methotrexate, temozolomide (TMZ), and rituximab, followed by hyperfractionated whole-brain radiotherapy (hWBRT) and subsequent TMZ. The primary phase I end point was the maximum tolerated dose of TMZ. The primary phase II end point was the 2-year overall survival (OS) rate. Secondary end points were preirradiation response rates, progression-free survival (PFS), neurologic toxicities, and quality of life.
Patients and Methods
The phase I study increased TMZ doses from 100 to 150 to 200 mg/m2. Patients were treated with rituximab 375 mg/m2 3 days before cycle 1; methotrexate 3.5 g/m2 with leucovorin on weeks 1, 3, 5, 7, and 9; TMZ daily for 5 days on weeks 4 and 8; hWBRT 1.2 Gy twice-daily on weeks 11 to 13 (36 Gy); and TMZ 200 mg/m2 daily for 5 days every 28 days on weeks 14 to 50.
Results
Thirteen patients (one ineligible) were enrolled in phase I of the study. The maximum tolerated dose of TMZ was 100 mg/m2. Dose-limiting toxicities were hepatic and renal. In phase II, 53 patients were treated. Median follow-up for living eligible patients was 3.6 years, and 2-year OS and PFS were 80.8% and 63.6%, respectively. Compared with historical controls from RTOG-9310, 2-year OS and PFS were significantly improved (P = .006 and .030, respectively). In phase II, the objective response rate was 85.7%. Among patients, 66% (35 of 53) had grade 3 and 4 toxicities before hWBRT, and 45% (24 of 53) of patients experienced grade 3 and 4 toxicities attributable to post-hWBRT chemotherapy. Cognitive function and quality of life improved or stabilized after hWBRT.
Conclusion
This regimen is safe, with the best 2-year OS and PFS achieved in any Radiation Therapy Oncology Group primary CNS lymphoma trial. Randomized trials that incorporate this regimen are needed to determine its efficacy compared with other strategies.
INTRODUCTION
The prognosis of primary CNS lymphoma (PCNSL) has improved with the addition of chemotherapeutic regimens that contain high-dose methotrexate (MTX). The use of whole-brain radiotherapy (WBRT) alone is associated with a median overall survival (OS) of approximately 1 year.1,2 The improvement in survival with the addition of pre-WBRT MTX prompted a prospective phase II study (RTOG 9310).3 Patients received preirradiation intravenous (IV) and intrathecal MTX, procarbazine, vincristine, and postirradiation cytarabine. During the study, the radiation dosage for complete responders was reduced from 45 Gy in daily fractions to 36 Gy twice daily, which delayed neurotoxicity without a loss of efficacy. The 30.4-month median OS was determined to be statistically superior to the 11.6-month median OS attained with WBRT alone and was unrelated to selection bias or age.4
The efficacy of MTX is not improved by the addition of agents that are commonly used in the treatment of systemic non-Hodgkin lymphoma,5,6 which provides an opportunity for the identification of newer combinatorial regimens. We hypothesized that including such agents in an MTX-based chemotherapeutic regimen will improve 2-year median OS and progression-free survival (PFS) in patients with PCNSL.
Temozolomide (TMZ), an oral alkylating agent, has reported activity in PCNSL.7-9 In one retrospective series, TMZ monotherapy was explored in elderly patients with PCNSL with severe comorbidities. In 17 patients (age 62 to 90 years), the complete response (CR) rate was 47% and median OS was 21 months. Five patients (29.4%) experienced prolonged responses for ≥ 12 months and survived for > 24 months. Three patients had methylated O6-methylguanine-DNA-methyltransferase (MGMT) promoter, whereas MGMT status was not assessable in two patients. TMZ is known to be active in glioblastoma multiforme when the MGMT promoter is methylated. A proportion of patients with PCNSL also exhibit methylation, which suggests a role for TMZ in this disease.10
Rituximab is a chimeric monoclonal antibody that targets the B-cell surface protein CD20 and is indicated for the treatment of CD20+ non-Hodgkin lymphoma and other B-cell malignancies. Most PCNSLs are CD20+ and potentially responsive to rituximab. Radiographic responses to rituximab have been reported in recurrent PCNSL.11,12
On the basis of this rationale, a phase I and II trial of a MTX-based multibio-chemotherapy regimen in patients with newly diagnosed PCNSL was initiated with the goal of building on the therapeutic gains achieved by RTOG 9310. WBRT is believed to significantly contribute to the cognitive changes in PCNSL. Hyperfractionated WBRT (hWBRT) dosing (120 Gy twice daily for 15 days) that was initiated during RTOG 9310 for the purpose of reducing neurotoxicity risk was maintained in this study.3 This trial was initiated before the results of the study by Thiel et al13 were published, which evaluated the role of radiotherapy in PCNSL. Finally, it was hypothesized that postRT TMZ delivered at a standard dose every 4 weeks for 10 cycles would prolong PFS and OS.
PATIENTS AND METHODS
All patients signed formal consent before enrollment, and the study was approved by the institutional review board of each participating institution. Immunocompetent patients age ≥ 18 years with newly diagnosed PCNSL were eligible for inclusion. All patients were required to have measurable intracranial disease with the diagnosis confirmed by brain biopsy, cerebrospinal fluid cytology, or vitrectomy. Patients with systemic lymphoma, concurrent or past cancers, HIV positivity, or evidence of active hepatitis B were not eligible. A creatinine clearance of ≥ 50 mL/min/1.73 m2 was required.
At the time of diagnosis, all patients underwent brain imaging, preferably with magnetic resonance imaging, a lumbar puncture (unless contraindicated by mass effect), and a slit lamp examination. Repeat imaging was performed at the completion of chemotherapy, after completion of radiation therapy, every 2 months during postradiation TMZ, at completion of TMZ, every 3 months from the end of treatment of 2 years, every 6 months for 3 to 5 years, then annually for 5 years. Radiographic responses were graded as CR, partial response (≥ 50% decrease in enhancing tumor), progressive disease (≥ 25% increase in a lesion; progressive or newly emergent meningeal or ocular disease), or stable disease.
Quality of life (QOL) was assessed by using the Spitzer QOL Index.14 Neurocognitive status was assessed by using the Mini-Mental Status Exam (MMSE).15 QOL and MMSE were performed before treatment, at completion of RT, every 2 months during TMZ, every 3 months from the end of treatment of 2 years, every 6 months for 3 years, annually, and on disease progression.
Treatment
Rituximab 375 mg/m2 was administered 3 days before the initial MTX treatment. MTX 3.5 g/m2 was administered on weeks 1, 3, 5, 7, and 9. Before administration, urine was alkalinized with IV sodium bicarbonate. Each dose was followed 24 hours later by leucovorin 25 mg IV every 6 hours. MTX levels were measured daily. Leucovorin was stopped when MTX level was ≤ 10 µmol/L. TMZ was administered on weeks 4 and 8 at the phase I or II dosages. After completion of chemotherapy, all patients received hWBRT 1.2 Gy twice-daily fractions on weeks 11 to 13 for a total of 36 Gy. TMZ 200 mg/m2 daily for 5 days was administered on weeks 14, 18, 22, 26, 30, 34, 38, 42, 46, and 50 for a total of 10 cycles. An initial dose of 150 mg/m2 for the first cycle was allowed.
In phase I, preirradiation TMZ was initially administered at 100 mg/m2, with dose escalations to 150 and 200 mg/m2. Three patients were planned per cohort, with a dose escalation to the next level if 0 of 3 patients exhibited a dose-limiting toxicity (DLT). If one patient experienced a DLT, an additional 3 patients were enrolled, with escalation to the next level only if 0 of 3 patients experienced a DLT. Otherwise, the previous dose level would be considered the maximum tolerated dose (MTD). In phase II, the MTD was used for the preirradiation TMZ dose.
Statistical Analysis
Statistical analysis was performed on the phase II portion of the study to determine whether this regimen improved OS for patients with PCNSL compared with the prespecified cohort from RTOG 9310, which had observed median and 2-year OS of 37.0 months and 64%, respectively; a 2-year OS of 77% was projected for this study, which corresponded with a 20% relative improvement. Patients who were retrospectively found to be ineligible or who received no protocol drug were excluded from all analyses. Using a one-sample χ2 test with a 0.20 one-sided significance level, 47 evaluable patients would have 87% power to detect the difference between the projected 2-year survival rate and historical control. Assuming a 5% rate for patients who were not evaluable for the primary end point, 52 patients were required for the phase II portion, and patients from the phase I component could be included if they received treatment with the recommended phase II dose of TMZ.
Adverse events were graded by using Common Terminology Criteria for Adverse Events v 2.0 of the National Cancer Institute Common Toxicity Criteria. Late radiation adverse events were graded by using the Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer Late Radiation Morbidity Criteria. The Kaplan-Meier method was used to estimate OS and PFS. An OS event was defined as death as a result of any cause, whereas a PFS event was defined as death as a result of any cause or any progression, whichever comes first. All eligible patients who received any protocol drug were included in the efficacy analysis. Patients alive at the last follow-up were analyzed as censored observations for OS, and patients alive at the last follow-up without disease progression were analyzed as censored observations for PFS. OS and PFS were estimated from the date of registration. Pointwise comparisons of 2-year OS and 2-year PFS rates were based on Kaplan-Meier curves between this study and RTOG 9310. Multivariable analyses on OS and PFS were also performed to calculate the hazard ratios between the two studies, adjusting for patient pretreatment characteristics, such as age, performance status, surgery, etc. Significant MMSE score decline was defined as a decrease of > 3 points14; significant gain was defined as an increase of > 3 points, and no change for any MMSE score change ≤ 3 points. All Spitzer QOL score changes were taken from baseline MMSE/Spitzer QOL score to key evaluation MMSE/Spitzer QOL score.
RESULTS
Twelve patients (six male and six female; median age 60 years) were treated in the phase I portion. An additional patient (enrolled in the 150 mg/m2 arm) was ineligible because of carmustine wafer implantation at the time of surgery. At TMZ 100 mg/m2, there was one DLT (grade 3 renal). At TMZ 150 mg/m2, there were three DLTs: grade 3 and 4 hepatic (2 patients) and grade 3 renal (1 patient). All toxicities were reversible. The MTD of TMZ in combination with MTX in patients with PCNSL who were treated with this regimen was 100 mg/m2. This preirradiation TMZ dose was used in the phase II portion of this trial.
Fifty-three patients were enrolled in the phase II portion, including those who were treated in the phase I at the phase II dose. Clinical characteristics are detailed in Table 1.
Table 1.
Phase II Clinical Characteristics (N = 53)
| Characteristic | No. of Patients (%) |
|---|---|
| Male | 25 (47) |
| Female | 28 (53) |
| Age, years | |
| Median (range) | 57 (24-73) |
| < 60 | 33 (62) |
| ≥ 60 | 20 (38) |
| Diagnostic procedure | |
| Gross total resection | 9 (17) |
| Subtotal resection | 7 (13) |
| Stereotactic/open biopsy | 37 (70) |
| Histologic diagnosis | |
| Diffuse large cell | 48 (90) |
| Nodular | 1 (2) |
| Unknown | 4 (8) |
| Performance status (Zubrod) | |
| 0 | 12 (23) |
| 1 | 30 (56) |
| 2 | 11 (21) |
Response
Among patients, 45 (85%) of 53 completed initial chemotherapy as per protocol, and 42 patients (79%) received hWBRT (Table 2). For those patients who did not receive hWBRT, reasons include patient withdrawal or refusal (n = 3), toxicity (n = 1), tumor progression (n = 2), and unknown (n = 5). On completion of preirradiation chemotherapy, 35 patients were assessable for radiographic response. CRs were seen in 18 patients (51%), partial responses in 12 patients (34%), and progressive disease in two patients (6%); 3 patients (9%) had no measurable disease at the initiation of treatment and on completion. Incomplete data were received on 18 patients.
Table 2.
Treatment Completion (N = 53)
| Treatment | No. of Patients (%) |
|---|---|
| Completed chemotherapy | |
| Per protocol | 45 (85.0) |
| Variation, acceptable | 0 (0.0) |
| Deviation, unacceptable | 2 (3.8) |
| Incomplete chemotherapy | 4 (7.5) |
| Not evaluable | 2 (3.8) |
| Completed WBRT | 42 (79) |
Abbreviation: WBRT, whole-brain radiation therapy.
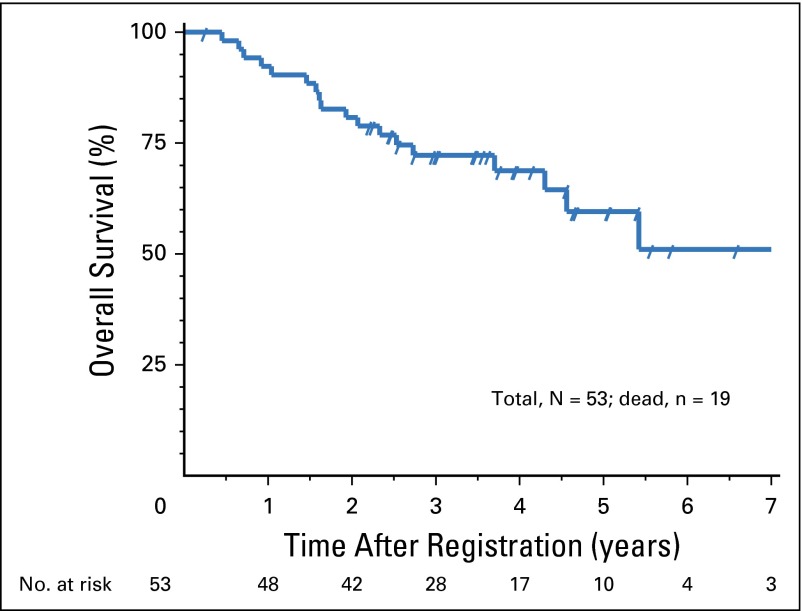
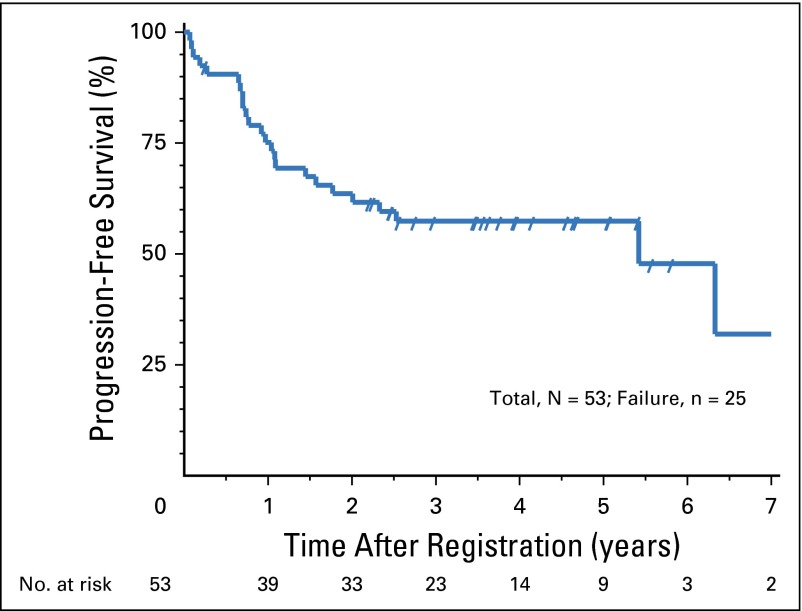
The median follow-up time for eligible living patients was 3.6 years. The 2-year OS for the entire cohort was 80.8% (P = .006). The estimated median OS was 7.5 years, with a 95% CI of 4.3 years to an upper limit not reached (Fig 1). The 2-year PFS was 63.6% (P = .03; compared with 50% in RTOG 9310). The estimated median PFS was 5.4 years with a 95% CI of 1.8 to 7.3 years (Fig 2). Pointwise comparisons of 2-year OS and 2-year PFS on the basis of Kaplan-Meier curves between this study and RTOG 9310 essentially showed no difference from the data presented above. On the basis of multivariable analyses on OS and PFS, results were favorable for this study compared with RTOG 9310. Specifically, the hazard ratio for OS was 0.44, with a 95% CI of 0.25 to 0.80 (P = .007); the hazard ratio for PFS was 0.52, with a 95% CI of 0.30 to 0.89 (P = .018).
Fig 1.
Overall survival.
Fig 2.
Progression-free survival.
Toxicity
Grade 3 and 4 adverse events definitely, probably, or possibly related to phase II treatment are reported in Tables 3 and 4 and Appendix Table A1 (online only). Most toxicities were grade 3, occurring before radiation therapy. Toxicities that were directly attributable to radiation and postradiation toxicities occurred less often. Hematologic toxicities that occurred during hWBRT were attributed to prior chemotherapy.
Table 3.
Grade 3 and 4 Toxicity: Chemotherapy Toxicity Before Start of WBRT (N = 53)
| Toxicity | Grade 3 | Grade 4 |
|---|---|---|
| Auditory/hearing | 0 | 0 |
| Cardiovascular | 2 | 0 |
| Constitutional | 3 | 1 |
| Febrile neutropenia | 1 | 0 |
| GI | 1 | 1 |
| Hematologic | 9 | 2 |
| Hepatic | 6 | 0 |
| Metabolic | 6 | 0 |
| Neurologic | 5 | 0 |
| Ocular | 1 | 0 |
| Pain | 2 | 1 |
| Renal/GU | 3 | 0 |
Abbreviations: GU, genitourinary; WBRT, whole-brain radiation therapy.
Table 4.
Grade 3 and 4 Toxicity: Chemotherapy and Acute Radiotherapy Toxicity After Start of WBRT (n = 42)
| Toxicity | Grade 3 | Grade 4 |
|---|---|---|
| Auditory/hearing | 1 | 0 |
| Cardiovascular | 1 | 0 |
| Coagulation | 1 | 0 |
| Constitutional | 1 | 0 |
| Dermatological | 1 | 0 |
| GI | 2 | 0 |
| Hematologic | 1 | 5 |
| Metabolic | 1 | 0 |
| Musculoskeletal | 0 | 0 |
| Ocular | 1 | 0 |
Abbreviation: WBRT, whole-brain radiation therapy.
Neurocognitive and QOL Evaluations
Neurocognitive and QOL evaluations were performed for patients in the phase II portion of the trial. The median baseline MMSE score was 28 and at each of the follow-up points, 29. The mean improvement in MMSE score from baseline to each time point was 2.1 after WBRT, 2.0 at 6 months after diagnosis, and 1.4 at year 3 (Appendix Table A2, online only).
A significant decline in MMSE score from baseline, defined by a decrease of > 3 points, was seen in a minority of patients. Significant declines in MMSE score were seen in one (3%) of 33 assessable patients post-RT and in one (2.6%) of 38 assessable patients (for whom information was received) 6 months post-RT. An increase in MMSE score at 3 years was more pronounced in patients age ≥ 60 years.
QOL was assessed by Spitzer QOL scores. These were measured at baseline, after completion of WBRT, and at 6 months and 3 years after diagnosis. Median Spitzer QOL scores increased in assessable patients from a baseline of 6 to 7 after radiation therapy, 8 at 6 months, and 10 at 3 years. The mean score change was 0.7 after radiation therapy, 1.2 at 6 months, and 2.3 at 3 years (Appendix Tables A3 and A4, online only).
DISCUSSION
The use of MTX-based chemotherapeutic regimens before WBRT in patients with PCNSL is associated with improvement in median OS and PFS. Other agents that are commonly used in the treatment of systemic lymphoma do not impact survival. Efforts to improve outcome have focused on intensifying treatment by increasing the dose of MTX to 8 g/m2, adding high-dose Ara-C either concurrently or sequentially to MTX, and evaluating marrow-ablative chemotherapy with hematopoietic salvage in first remission.16,17 These dose-intensive regimens are often not feasible for patients age > 70 years or those with impaired renal function. In addition, no clear evidence exists that dose intensification improves outcomes compared with MTX alone. It is therefore a key therapeutic goal to identify other active agents to be used in conjunction with MTX.
On the basis of pilot clinical data that suggest the activity of TMZ and rituximab in PCNSL, we hypothesized that these agents, when combined with standard high-dose MTX, could improve outcomes in this disease. Building on the experience with preirradiation, MTX-based chemotherapy in RTOG 9310, we designed an approach that included WBRT to consolidate the estimated 30% of patients who could be expected to achieve less than a CR. Given the neurocognitive toxicity of WBRT, we chose to use a hyperfractionated dose that was piloted in a subset of patients who received treatment in RTOG 9310.4 Finally, we hypothesized that TMZ was well suited as a maintenance agent, an approach not widely explored in the treatment of patients with PCNSL.
To our knowledge, this is the first prospective cooperative group study to report the use of rituximab and TMZ in conjunction with MTX for the primary treatment of this disease, followed by hWBRT. Rubenstein et al18 reported the results of a phase II trial (CALGB 50202) in which patients received MTX, TMZ, and rituximab, followed by dose-intensive consolidation with cytarabine and etoposide; WBRT was not used. The 2-year PFS was 57% and the estimated 2-year OS was 70%. Our current study and the study by Rubenstein et al18 clearly support the notion that the combination of rituximab, TMZ, and high-dose MTX is active in PCNSL. There are, however, few other similarities between this study and CALGB 50202. Whereas both used these agents, the number of cycles of rituximab and TMZ and the dose of MTX were different. Most notably, the study by Rubenstein et al18 did not include treatment with WBRT; an objective was to eliminate the need for WBRT to decrease the risk of neurocognitive toxicity. Instead, chemotherapy was intensified with the addition of etoposide and cytarabine for patients who achieved a CR after six cycles of MTX, rituximab, and TMZ. Among patients, 29 (66%) achieved a CR—implying that 34% of patients had radiographically visible residual disease after six cycles—and only 27 patients (52%) completed the entire regimen, which indicated a high rate of progression, toxicity, and/or intolerance. In contrast, in NRG Oncology RTOG 0227, 85% of patients completed the protocol treatment, demonstrating a high rate of compliance.
Compared with the prespecified end points of RTOG 9310, which confirmed pre-WBRT high-dose MTX, vincristine, procarbazine, WBRT, and post-RT Ara-C as a widely adopted treatment for patients with PCNSL, the 2-year PFS and OS rates achieved in NRG Oncology RTOG 0227 were encouraging. Both clinical trials used MTX for five cycles before radiation therapy, though at a higher dose in this study (2.5 v 3.5 g/m2). The oral agents also differed (procarbazine v TMZ); no intraventricular MTX or vincristine were used in NRG Oncology RTOG 0227; and rituximab was not used in RTOG 9310. A major difference was the duration of postradiation treatment. RTOG 9310 used cytarabine 3 g/m2/d for 2 days over two cycles 4 weeks apart. The current study used TMZ continuously for 10 cycles after the completion of hWBRT. Finally, the WBRT dose used in RTOG 9310 was higher at 45 Gy for all but a subset of patients who achieved a CR who were treated with a twice-daily regimen of 36 Gy as in the current study.
Over the past decade, the RTOG 9310 regimen has been modified by others in the hope of maintaining high tumor control rates and lowering the incidence of late neurocognitive toxicity. Morris et al19 reported high response rates, long-term disease control, and minimal neurotoxicity. In their approach, rituximab was added to the pre-RT regimen. Of note is the reduction in the WBRT dose from 45 to 23.40 Gy in daily 1.8 Gy fractions. Among patients 52 received treatment in this fashion had median PFS and OS of 3.3 and 6.6 years, respectively. Median PFS was 7.7 years for patients age < 60 years and 1.7 years for patients age ≥ 60 years. Median OS has not been reached for younger patients and is 5.5 years for older patients. Neurotoxicity in long-term survivors is reported as minimal, and correlative magnetic resonance imaging demonstrated moderate white matter changes.
The ongoing randomized phase II RTOG 1114 study is further testing this approach by using a pre-RT rituximab, MTX, procarbazine, and vincristine regimen with a reduced dose of WBRT (23.40 Gy) or no WBRT, followed by cytarabine.20 This approach challenges the notion that the WBRT dose of 45 Gy in the Primary CNS Lymphoma Study Group (G-PCNSL-SG-1) trial13 is the optimal dose. Although WBRT omission did not result in inferior OS, PFS was reduced, in particular in patients who did not achieve a CR to chemotherapy. The question remains as to whether elimination or deferral of WBRT is superior to reduced-dose WBRT approaches that maintain enhanced PFS without late neurocognitive toxicity.
Treatment was well tolerated. There were no unexpected or grade 5 toxicities, and with median follow-up of 43 months on surviving patients, the late post-WBRT toxicity rate remains low (Appendix Table A1). The most consistent cognitive finding was an early and sustained improvement in MMSE scores from baseline, consistent with tumor response. For patients alive at 3 years, none experienced a protocol-defined drop in MMSE score. Sustained improvement in QOL is consistent with this observation and, at least in part, can be attributed to successful tumor treatment. In addition, the plateau of PFS and OS beyond 4 years suggests that patients without progression are not dying of late toxicity as has been reported in other series.21
Given the superiority of the 2-year PFS and OS in the current study compared with that of RTOG 9310 as well as the low incidence of late neurotoxicity, cognitive decline, decreased QOL, and leukoencephalopathy, this regimen is a potential standard of care for patients with PCSNL. A randomized trial comparing this regimen with a dose-reduced or non-WBRT–containing regimen as in RTOG 1114 is justified. Such a trial would further define whether lower-dose WBRT with maintenance chemotherapy as in NRG Oncology RTOG 0227 is superior to treatment regimens that use further dose-reduced or no WBRT.
Appendix
Table A1.
Grade 3 and 4 Late Radiotherapy (n = 42)
| Toxicity | Grade 3 | Grade 4 |
|---|---|---|
| Brain | 1 | 0 |
| Encephalopathy | 0 | 0 |
| Eye | 0 | 0 |
| Skin (within RT field) | 0 | 0 |
| Subcutaneous tissue | 0 | 0 |
| Hearing loss | 1 | 0 |
| Leukoencephalopathy | 1 | 0 |
| Thrombocytopenia | 0 | 1 |
Abbreviation: RT, radiotherapy.
Table A2.
MMSE Scores and Change From Baseline
| MMSE Score | Baseline | Post-RT | Month 6 | Week 50 | Year 3 |
|---|---|---|---|---|---|
| Total score | |||||
| No. of patients assessed | 50 | 34 | 40 | 26 | 17 |
| Median | 28 | 29 | 29 | 29 | 29 |
| Minimum-maximum | 6-30 | 19-30 | 15-30 | 21-30 | 26-30 |
| Q1-Q3 | 23-29 | 26-30 | 26.5-30 | 27-30 | 28-30 |
| Change in score, No. (%) | |||||
| No. of patients assessed | 33 | 38 | 25 | 17 | |
| Decrease > 3 | 1 (3.0) | 1 (2.6) | 1 (4.0) | 0 (0.0) | |
| Stable MMSE | 24 (72.7) | 29 (76.3) | 19 (76.0) | 15 (88.2) | |
| Increase > 3 | 8 (24.2) | 8 (21.1) | 5 (20.0) | 2 (11.8) | |
| Median change by age (No.) | |||||
| No. of patients assessed | 33 | 38 | 25 | 17 | |
| < 60 | 1 (23) | 1.5 (26) | 1(15) | 0.5 (10) | |
| ≥ 60 | 1.5 (10) | 1 (12) | 1(10) | 2.0 (7) |
Abbreviations: MMSE, Mini-Mental Status Exam; Q1, first quartile; Q3, third quartile; RT, radiotherapy.
Table A3.
Spitzer QOL Scores at Each Time Point
| Spitzer QOL score | Baseline | Post-RT | Month 6 | Week 50 | Year 3 |
|---|---|---|---|---|---|
| No. of patients assessed | 52 | 34 | 40 | 27 | 16 |
| Median | 6 | 7 | 8 | 9 | 10 |
| Minimum-maximum | 0-10 | 0-10 | 0-10 | 0-10 | 3-10 |
| Q1-Q3 | 4-8 | 5-10 | 5.5-10 | 6-10 | 9-10 |
Abbreviations: Q1, first quartile; Q3, third quartile; QOL, quality of life; RT, radiotherapy.
Table A4.
Spitzer Score Changes From Baseline to Each Time Point
| Spitzer score | Post-RT (n = 34) | Month 6 (n = 40) | Week 50 (n = 27) | Year 3 (n = 16) |
|---|---|---|---|---|
| No. of patients assessed for change | 33 | 39 | 26 | 16 |
| Mean | 0.7 | 1.2 | 2.3 | 2.3 |
| Standard deviation | 3.9 | 3.2 | 3.0 | 2.9 |
| Median | 1 | 1 | 2.5 | 2 |
| Minimum-maximum | −7 to 10 | −6 to 10 | −4 to 10 | −2 to 10 |
| Q1-Q3 | −1 to 2 | −1 to 3 | 1 to 4 | 0 to 4 |
Abbreviations: Q1, first quartile; Q3, third quartile; RT, radiotherapy.
Footnotes
Supported by Grants No. U10CA21661, U10CA180868, and U10CA180822 from the National Institutes of Health, National Cancer Institute, and by Merck.
Presented in part at the 11th Annual Meeting of the Society for Neuro-Oncology, Orlando, FL, November 16-19, 2006, and at the 2013 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Jon Glass, Christopher J. Schultz, Minesh P. Mehta
Provision of study materials or patients: Christopher J. Schultz, Maria Werner-Wasik, Marcia K. Liepman
Collection and assembly of data: Jon Glass, Christopher J. Schultz, Daniel Brat, Nancy Bartlett, John H. Suh, Maria Werner-Wasik, Marcia K. Liepman, Mark Augspurger, Felix Bokstein, Minesh P. Mehta
Data analysis and interpretation: Jon Glass, Minhee Won, Christopher J. Schultz, Nancy Bartlett, Barbara Jean Fisher, Joseph A. Bovi, Matthew C. Solhjem, Minesh P. Mehta
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase I and II Study of Induction Chemotherapy With Methotrexate, Rituximab, and Temozolomide, Followed By Whole-Brain Radiotherapy and Postirradiation Temozolomide for Primary CNS Lymphoma: NRG Oncology RTOG 0227
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Jon Glass
Consulting or Advisory Role: Novocure
Minhee Won
No relationship to disclose
Christopher J. Schultz
Research Funding: Elekta AB (Inst)
Daniel Brat
No relationship to disclose
Nancy Bartlett
No relationship to disclose
John H. Suh
Research Funding: Varian Medical Systems
Other Relationship: Elekta AB
Maria Werner-Wasik
Stock or Other Ownership: Medidata Solutions
Research Funding: Elekta AB
Barbara Jean Fisher
No relationship to disclose
Marcia K. Liepman
Consulting or Advisory Role: Pfizer
Mark Augspurger
No relationship to disclose
Felix Bokstein
No relationship to disclose
Joseph A. Bovi
No relationship to disclose
Matthew C. Solhjem
No relationship to disclose
Minesh P. Mehta
Leadership: Pharmacyclics
Stock or Other Ownership: Pharmacyclics
Consulting or Advisory Role: Cavion, Novartis, Novocure
Research Funding: Novocure (Inst), Celldex (Inst)
Other Relationship: Monteris
REFERENCES
- 1.Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin’s lymphoma of the brain: Can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23:9–17. doi: 10.1016/0360-3016(92)90538-s. [DOI] [PubMed] [Google Scholar]
- 2.Nelson DF. Radiotherapy in the treatment of primary central nervous system lymphoma (PCNSL). J Neurooncol. 1999;43:241–247. doi: 10.1023/a:1006206602918. [DOI] [PubMed] [Google Scholar]
- 3.DeAngelis LM, Seiferheld W, Schold SC, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20:4643–4648. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Seiferheld W, Schultz C, et al. Secondary analysis of Radiation Therapy Oncology Group study (RTOG) 9310: An intergroup phase II combined modality treatment of primary central nervous system lymphoma. J Neurooncol. 2005;74:201–205. doi: 10.1007/s11060-004-6596-9. [DOI] [PubMed] [Google Scholar]
- 5.Schultz C, Scott C, Sherman W, et al. Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas: Initial report of Radiation Therapy Oncology Group protocol 88-06. J Clin Oncol. 1996;14:556–564. doi: 10.1200/JCO.1996.14.2.556. [DOI] [PubMed] [Google Scholar]
- 6.Glass J, Shustik C, Hochberg FH, et al. Therapy of primary central nervous system lymphoma with pre-irradiation methotrexate, cyclophosphamide, doxorubicin, vincristine, and dexamethasone (MCHOD). J Neurooncol. 1996;30:257–265. doi: 10.1007/BF00177277. [DOI] [PubMed] [Google Scholar]
- 7.Herrlinger U, Küker W, Platten M, et al. First-line therapy with temozolomide induces regression of primary CNS lymphoma. Neurology. 2002;58:1573–1574. doi: 10.1212/wnl.58.10.1573. [DOI] [PubMed] [Google Scholar]
- 8.Reni M, Ferreri AJ, Landoni C, et al. Salvage therapy with temozolomide in an immunocompetent patient with primary brain lymphoma. J Natl Cancer Inst. 2000;92:575–576. doi: 10.1093/jnci/92.7.575. [DOI] [PubMed] [Google Scholar]
- 9.Reni M, Ferreri AJ. Therapeutic management of refractory or relapsed primary central nervous system lymphomas. Ann Hematol. 2001;80(suppl 3):B113–B117. doi: 10.1007/pl00022772. [DOI] [PubMed] [Google Scholar]
- 10.Kurzwelly D, Glas M, Roth P, et al. Primary CNS lymphoma in the elderly: Temozolomide therapy and MGMT status. J Neurooncol. 2010;97:389–392. doi: 10.1007/s11060-009-0032-0. [DOI] [PubMed] [Google Scholar]
- 11.Raizer J, DeAngelis L, Zelenetz A, et al. Activity of rituximab in primary central nervous system lymphoma PCNSL. J Clin Oncol. 2000;19(abstr 642) [Google Scholar]
- 12.Ruhstaller TW, Amsler U, Cerny T. Rituximab: Active treatment of central nervous system involvement by non-Hodgkin’s lymphoma? Ann Oncol. 2000;11:374–375. doi: 10.1023/a:1008371602708. [DOI] [PubMed] [Google Scholar]
- 13.Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11:1036–1047. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- 14.Spitzer WO, Dobson AJ, Hall J, et al. Measuring the quality of life of cancer patients: A concise QL-index for use by physicians. J Chronic Dis. 1981;34:585–597. doi: 10.1016/0021-9681(81)90058-8. [DOI] [PubMed] [Google Scholar]
- 15.Tangalos EG, Smith GE, Ivnik RJ, et al. The Mini-Mental State Examination in general medical practice: Clinical utility and acceptance. Mayo Clin Proc. 1996;71:829–837. doi: 10.4065/71.9.829. [DOI] [PubMed] [Google Scholar]
- 16.Illerhaus G, Marks R, Ihorst G, et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24:3865–3870. doi: 10.1200/JCO.2006.06.2117. [DOI] [PubMed] [Google Scholar]
- 17.Kiefer T, Hirt C, Späth C, et al. Long-term follow-up of high-dose chemotherapy with autologous stem-cell transplantation and response-adapted whole-brain radiotherapy for newly diagnosed primary CNS lymphoma: Results of the multicenter Ostdeutsche Studiengruppe Hamatologie und Onkologie OSHO-53 phase II study. Ann Oncol. 2012;23:1809–1812. doi: 10.1093/annonc/mdr553. [DOI] [PubMed] [Google Scholar]
- 18.Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31:3061–3068. doi: 10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: Final results and long-term outcome. J Clin Oncol. 2013;31:3971–3979. doi: 10.1200/JCO.2013.50.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cancer Institute Basic trial information: Rituximab, methotrexate, procarbazine hydrochloride, vincristine sulfate, and cytarabine with or without radiation therapy in treating patients with primary central nervous system lymphoma. http://www.cancer.gov/clinicaltrials/search/view?cdrid=703682&version=HealthProfessional&protocolsearchid=13523935.
- 21.Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: The next step. J Clin Oncol. 2000;18:3144–3150. doi: 10.1200/JCO.2000.18.17.3144. [DOI] [PubMed] [Google Scholar]