Abstract
Purpose
Childhood cancer survivors carry a high burden of treatment-related morbidity; however, race/ethnicity–specific risks of adverse outcomes are not well understood.
Methods
Data from the Childhood Cancer Survivor Study, a cohort of survivors of at least 5 years, were used to compare Hispanic (n = 750, 5.4%) and non-Hispanic black (NHB: n = 694, 5.0%) survivors to non-Hispanic white patients (NHW: n = 12,397, 89.6%) for late mortality, subsequent neoplasms, and chronic health conditions.
Results
NHBs and Hispanics reported lower socioeconomic status (SES) and higher prevalence of obesity, and NHBs reported higher prevalence of hypertension. NHBs had higher rate of all-cause mortality (relative rate [RR], 1.4; 95% CI, 1.1 to 1.9; P = .008), which was abrogated (RR, 1.0; 95% CI, 0.8 to 1.4; P = .9) after adjusting for SES. Nonmelanoma skin cancer was not observed among irradiated NHBs, and the risk was lower among Hispanic survivors (RR, 0.3; 95% CI, 0.1 to 0.7) compared with NHWs. Both NHBs and Hispanics demonstrated elevated risks for diabetes; these risks persisted after adjusting for SES and obesity (NHBs: RR, 2.8; 95% CI, 1.1 to 6.7; Hispanics: RR, 3.1; 95% CI, 1.5 to 6.4). NHBs were more likely to report cardiac conditions (RR, 1.8; 95% CI, 1.1 to 2.7), but the risk was attenuated after adjusting for cardiovascular risk factors. Therapeutic exposures did not affect racial/ethnic differences in mortality (all cause or cause specific), chronic health conditions, or subsequent neoplasms.
Conclusion
By and large, NHB and Hispanic childhood cancer survivors experience a comparable burden of morbidity and mortality to their NHW counterparts. The few differences in risk were explained by the racial/ethnic differences in socioeconomic status and/or cardiovascular risk factors.
INTRODUCTION
Therapeutic advances for childhood cancer have resulted in progressive improvements in survival over the last four decades; 5-year survival rates now approach 85%.1 The attendant increase in the number of childhood cancer survivors has demanded (and received) considerable attention related to the long-term effects of cancer and its treatment.2,3 Although previous studies have described the high burden of morbidity and premature mortality borne by childhood cancer survivors, limited attention has been focused on racial/ethnic differences in this burden.4
In the 2010 census, black race made up 12% of the US population, Hispanic origin constituted 16%, and Asian/Pacific Islanders made up 5%. By 2042, the proportion of individuals in the United States belonging to a racial/ethnic background other than non-Hispanic white (NHW) is estimated to exceed 50%.5 Given the significant (and growing) racial/ethnic diversity in the United States, and the paucity of information about race/ethnicity-specific morbidity/mortality in childhood cancer survivors, we describe long-term outcomes experienced by major racial/ethnic groups using the Childhood Cancer Survivor Study (CCSS) cohort, focusing primarily on late mortality, subsequent neoplasms, and chronic health conditions. The primary goal is to determine if there are racial/ethnic differences in these outcomes and to understand the impact of therapeutic exposures, socioeconomic status (SES), and cardiovascular risk factors (CVRFs) on observed differences.
METHODS
Patient Selection
CCSS6 is a retrospective cohort with longitudinal follow-up of childhood cancer survivors diagnosed between 1970 and 1986 in one of 26 institutions and survival of at least 5 years from diagnosis.7,8 Survivors were diagnosed before age 21 years with leukemia, CNS malignancy, Hodgkin lymphoma, non-Hodgkin lymphoma, kidney cancer, neuroblastoma, soft tissue sarcoma, or cancer of the bone. A baseline questionnaire was administered between 1994 and 1999 followed by comprehensive questionnaires in 2000 to 2002, 2002 to 2005, and 2007 to 2009. Information on cancer-related treatment was systematically abstracted for survivors who signed medical record release.
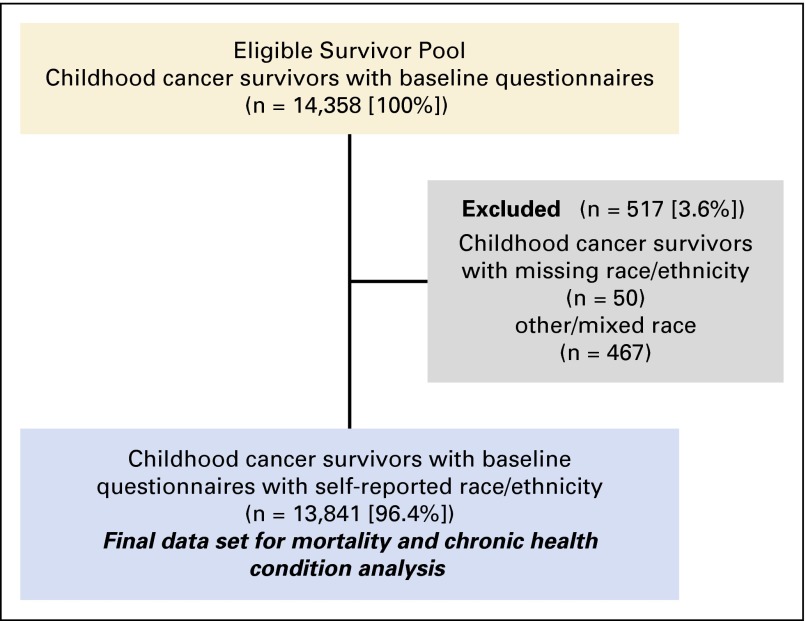
Of the 14,358 childhood cancer survivors who completed the CCSS baseline questionnaire, 13,841 (96.4%) provided information on race/ethnicity and were included in the present analysis (Fig 1). Analyses including treatment variables were restricted to those who provided authorization for medical record release and had treatment exposure data abstracted (n = 12,161, 84.7%).
Fig 1.
CONSORT diagram showing the study participants included in the study.
Outcomes
Four primary outcomes were considered: late mortality, subsequent neoplasms (SNs)/subsequent malignant neoplasms (SMNs), and chronic health conditions.
Late mortality.
Deaths before December 31, 2007 were ascertained using information from the National Death Index. Information on the underlying cause of death was obtained from death certificates for residents in the United States. Cause-specific mortality analyses excluded Canadian residents (n = 971 [NHW, 948; non-Hispanic black (NHB), 15; Hispanic, 8]) because their cause of death could not be systematically determined. In this report, we focus on all-cause mortality and cause-specific mortality due to SNs, SMNs, and cardiac causes.
SNs/SMNs.
SNs were ascertained through self-report or proxy report and/or death certificate, with subsequent confirmation by pathology report review or clinical reports (when pathology reports were unavailable). SNs were placed into the following mutually exclusive categories9,10: SMNs (malignancies with Behavior Code of 3 from the International Classification of Diseases for Oncology), nonmalignant meningioma, nonmelanoma skin cancer (NMSC), and other SNs.
Chronic health conditions.
Common Terminology Criteria for Adverse Events (version 4.03) grading was used11 to classify chronic health conditions.12,13 Grades 3 to 5 chronic health conditions were included, classified as severe or disabling (grade 3), life-threatening (grade 4), or fatal (grade 5).
Race/Ethnicity
Race/ethnicity was ascertained in the baseline questionnaire using the following question, “To which one of the following groups do you belong?”, with the possible options being White, Black, American Indian or Alaskan Native, Asian or Pacific Islander, and Other (specify). Hispanics included those whose self-reported race/ethnicity was White Hispanic, Black Hispanic, Puerto Rican, Hispanic/Mexican, Puerto Rican/Latino (Cuban), Puerto Rican/Latino(Cuban)/White, Not specified/Hispanic, and Other/Hispanic. Survivors were categorized into the following mutually exclusive groups: NHW (n = 12,397), NHB (n = 694), Hispanic/Latino (Hispanic: n = 750), other (American Indian/Alaskan Native/Asian/Pacific Islander: n = 257), mixed race (n = 210), and missing race/ethnicity (n = 50). Survivors in the other/mixed race/missing category were excluded because of numbers in individual groups too small to draw meaningful conclusions (n = 517).
Statistical Analysis
Sociodemographic and treatment variables were compared descriptively across racial/ethnic groups, using χ2 tests for categorical variables and analysis of variance for continuous variables. In multivariable analyses for each outcome, we assessed the effect of race/ethnicity in a base model that adjusted for primary cancer diagnosis, age at diagnosis, attained age (modeled by natural cubic splines), treatment era, and sex. Therapeutic exposures (radiation therapy, cyclophosphamide equivalence dose,14 and anthracycline score were added to the basic model to examine the impact of treatment on the race/ethnicity effect. On the basis of appreciable across-race/ethnicity differences in the distribution of SES (education, annual household income, and health insurance) and/or CVRFs (obesity, dyslipidemia, hypertension, and diabetes), we assessed the impact of SES and CVRFs in racial/ethnic differences in mortality and chronic health outcomes. CVRFs and SES were treated as time-dependent variables.
The proportion of patients who did not consent to release medical record information varied by race/ethnicity (NHWs, 10.5%; NHBs, 33.4%; Hispanics, 19.6%). We corrected for potential bias due to differential nonconsent to release medical record information by using inverse probability weighting technique,15 assuming that nonconsent occurred randomly within each racial/ethnic group.
Mortality.
Follow-up for mortality analysis started at cohort entry (5 years after diagnosis) and ended on the date of death or censoring (US residents: December 31, 2007; Canadian residents: latest questionnaire completion date or December 31, 2007), whichever came earlier. Kaplan-Meier estimates of overall survival were compared across race/ethnicity. Cumulative incidence of cause-specific mortality was estimated by race/ethnicity using Gray’s method.16 Standardized mortality ratio (SMR) represented a ratio of observed and expected mortality rates (expected rates were obtained from the Centers for Disease Control, representing age-, calendar year–, sex-, and race-matched rates in the general population). Piecewise exponential models were used to assess the effect of race/ethnicity on all-cause and cause-specific mortality rates (or SMRs) adjusting for potential confounders (outlined above), using the logarithm of person-years (or expected counts of death for the SMR analysis) as the offset.
SNs/SMNs.
Cumulative incidence of the first SN and SMN after study entry was estimated with death as competing risk. SNs/SMNs diagnosed before study entry but after childhood cancer diagnosis were included as prevalence at study entry. To investigate occurrence of multiple SNs/SMNs by race/ethnicity, a mean cumulative count (MCC)17 was calculated. Cumulative incidence and MCC at 30 years from diagnosis were compared across racial/ethnic groups using permutation test. Relative rates (RRs) of developing any SN, and specific SNs, comparing NHBs and Hispanics to NHWs, were estimated using piecewise exponential models after adjusting for potential confounders listed above.
Chronic health conditions.
Cumulative incidence and MCC of grades 3 to 5 chronic health conditions were evaluated, treating death, SMNs, and late recurrence as competing risks for noncancer chronic conditions and death and late recurrence as competing risks for overall chronic conditions; conditions diagnosed before study entry but after cancer diagnosis were included as prevalence at study entry. Among subjects free of any chronic health condition at study entry, piecewise exponential models were used to compare the rate of developing grades 3 to 5 chronic health conditions across racial/ethnic groups, adjusting for variables listed above. Summary measure of chronic health conditions and specific conditions were examined.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, 1996). Two-sided statistical inferences were used throughout the analyses.
RESULTS
Patient Characteristics
Table 1 shows the characteristics of the 13,841 childhood survivors by race/ethnicity. Compared with NHWs, NHB and Hispanic survivors were younger at cancer diagnosis, more likely to be diagnosed in the recent years, and more likely to have received alkylators/anthracyclines, and at higher doses. Compared with NHWs, NHBs and Hispanics reported lower annual household income and education, were less likely to have health insurance, were more likely to be obese and report diabetes mellitus, but were less likely to report dyslipidemia. Finally, NHBs were more likely and Hispanics less likely to report hypertension.
Table 1.
Demographic and Clinical Characteristics Across Racial/Ethnic Groups of Childhood Cancer Survivors
| Characteristic | Non-Hispanic White (n = 12,397) | Non-Hispanic Black (n = 694) | Hispanic (n = 750) | P |
|---|---|---|---|---|
| Age at interview,* years | ||||
| Mean (SD) | 32.9 (9.1) | 28.8 (9.0) | 29.8 (9.0) | < .001 |
| Range | 8.2-58.9 | 9.4-55.0 | 10.8-57.7 | |
| Age at diagnosis, years | ||||
| Mean (SD) | 8.4 (5.9) | 7.4 (5.4) | 7.5 (5.4) | < .001 |
| Range | 0-20.9 | 0-20.8 | 0-20.7 | |
| Interval from diagnosis, years | ||||
| Mean (SD) | 24.5 (6.8) | 21.3 (6.8) | 22.3 (6.9) | < .001 |
| Range | 6.5-39.3 | 8.6-38.9 | 8.8-38.7 | |
| Sex, No. (%) | ||||
| Male | 6,653 (53.7) | 376 (54.2) | 389 (51.9) | .60 |
| Female | 5,744 (46.3) | 318 (45.8) | 361 (48.1) | |
| Age at diagnosis, years, No. (%) | ||||
| 0-4 | 4,895 (39.5) | 315 (45.4) | 322 (42.9) | < .001 |
| 5-9 | 2,703 (21.8) | 152 (21.9) | 204 (27.2) | |
| 10-14 | 2,534 (20.4) | 148 (21.3) | 129 (17.2) | |
| 15-20 | 2,265 (18.3) | 79 (11.4) | 95 (12.7) | |
| Year of diagnosis, No. (%) | ||||
| 1970-1973 | 1,721 (13.9) | 70 (10.1) | 84 (11.2) | < .001 |
| 1974-1977 | 2,596 (20.9) | 108 (15.6) | 142 (18.9) | |
| 1978-1981 | 3,272 (26.4) | 176 (25.4) | 168 (22.4) | |
| 1982-1986 | 4,808 (38.8) | 340 (49.0) | 356 (47.5) | |
| Socioeconomic status,* No. (%) | ||||
| Household income, $ | ||||
| < 20,000 | 1,830 (15.7) | 238 (39.1) | 182 (26.6) | < .001 |
| 20,000-39,999 | 2,711 (23.2) | 158 (26.0) | 192 (28.1) | |
| 40,000-59,999 | 2,328 (19.9) | 88 (14.5) | 124 (18.2) | |
| 60,000+ | 4,814 (41.2) | 124 (20.4) | 185 (27.1) | |
| Education | ||||
| Lower than high school | 1,431 (11.7) | 137 (20.4) | 147 (20.0) | < .001 |
| High school | 2,031 (16.7) | 145 (21.6) | 144 (19.6) | |
| Higher than high school | 8,736 (71.6) | 389 (58.0) | 445 (60.5) | |
| Health insurance | ||||
| Yes | 9,327 (82.0) | 509 (79.5) | 528 (76.4) | < .001 |
| No | 1,362 (12.0) | 121 (18.9) | 158 (22.9) | |
| Canadian resident, No. (%) | 685 (6.0) | 10 (1.6) | 5 (0.7) | |
| Cardiovascular risk factors,* No. (%) | ||||
| Diabetes mellitus | 406 (3.3) | 33 (4.8) | 31 (4.1) | .06 |
| Hypertension | 1,679 (13.5) | 111 (16.0) | 72 (9.6) | .001 |
| Dyslipidemia | 1,029 (8.3) | 28 (4.0) | 43 (5.7) | < .001 |
| Obesity | 2,442 (20.3) | 156 (23.5) | 178 (24.6) | .004 |
| Diagnosis group, No. (%) | ||||
| Acute lymphoblastic leukemia | 3,682 (29.7) | 164 (23.6) | 294 (39.2) | < .001 |
| Acute myeloid leukemia | 299 (2.4) | 20 (2.9) | 18 (2.4) | |
| Other leukemia | 115 (0.9) | 8 (1.2) | 11 (1.5) | |
| CNS tumors | 1672 (13.5) | 74 (10.6) | 77 (10.2) | |
| Hodgkin lymphoma | 1,721 (13.9) | 69 (9.9) | 91 (12.1) | |
| Non-Hodgkin lymphoma | 933 (7.5) | 46 (6.6) | 55 (7.3) | |
| Kidney tumors | 1,046 (8.4) | 120 (17.3) | 60 (8.0) | |
| Neuroblastoma | 829 (6.7) | 52 (7.5) | 37 (4.9) | |
| Soft tissue sarcoma | 1,065 (8.6) | 78 (11.2) | 56 (7.5) | |
| Bone tumors | 1,035 (8.3) | 63 (9.1) | 51 (6.8) | |
| Received radiation therapy, No. (%) | ||||
| Yes | 7,527 (67.5) | 313 (65.8) | 396 (65.7) | .48 |
| No | 3,619 (32.5) | 163 (34.2) | 207 (34.3) | |
| Alkylating agent CED,† mg/m2, No. (%) | ||||
| 0 | 5,394 (53.6) | 204 (47.4) | 240 (45.0) | < .001 |
| 1-3,999 | 1,120 (11.1) | 49 (11.4) | 83 (15.6) | |
| 4,000-7,999 | 1,057 (10.5) | 67 (15.6) | 56 (10.5) | |
| 8,000+ | 2,492 (24.8) | 110 (25.6) | 154 (28.9) | |
| Anthracycline score‡, No. (%) | ||||
| None | 6,797 (63.9) | 231 (52.5) | 334 (59.5) | < .001 |
| 1 | 1,273 (12.0) | 72 (16.4) | 73 (13.0) | |
| 2 | 1,279 (12.0) | 77 (17.5) | 66 (11.8) | |
| 3 | 1,282 (12.1) | 60 (13.6) | 88 (15.7) |
Abbreviation: CED, cyclophosphamide equivalent dose.
For time-dependent variables, information at the last contact is shown.
Green et al.14
Anthracycline scores were no exposure (None) and the tertiles of anthracycline dose, 0.1-204 mg/m2 (1), 205-355 mg/m2 (2), and > 355 mg/m2 (3).
Mortality
Overall mortality.
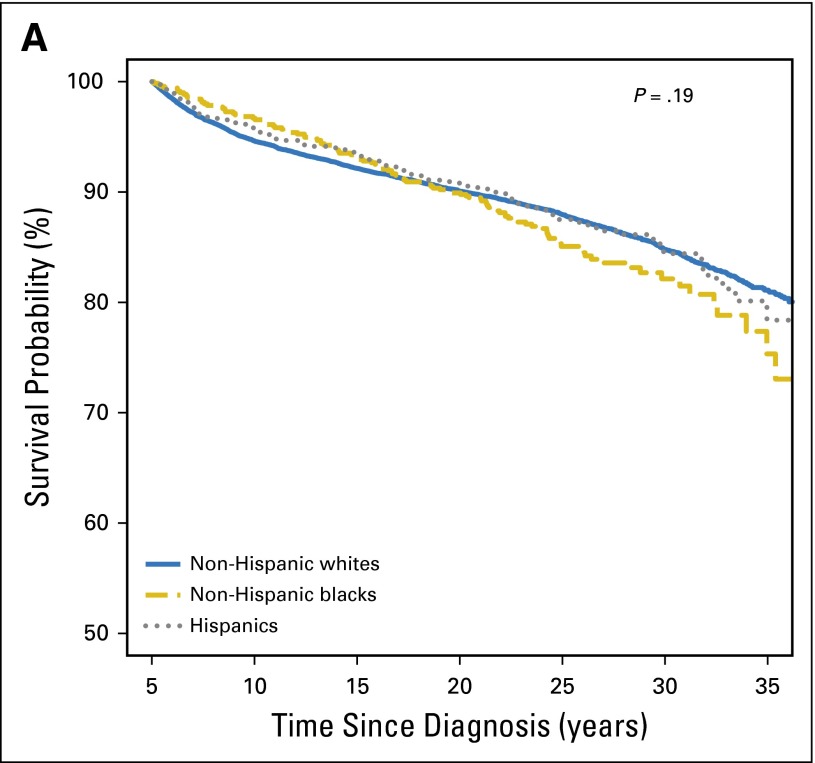
The 30-year overall survival was comparable across racial/ethnic groups (NHWs, 84.8%; 95% CI, 84.1% to 85.5%; NHBs,: 82.1%; 95% CI, 78.4% to 85.3%; Hispanics, 84.4%; 95% CI, 81.0% to 87.3%; P = .19; Fig 2A). The all-cause SMR for NHWs was 6.4 (95% CI, 6.1 to 6.7); the corresponding value for Hispanics (SMR, 5.5; 95% CI, 4.5 to 6.7) did not differ significantly (P = .14) but was significantly lower (SMR, 4.5; 95% CI, 3.7 to 5.4) for NHBs (P < .001). Multivariable analysis (Table 2) revealed that NHBs had higher all-cause mortality rates (RR, 1.5; 95% CI, 1.1 to 2.0; P = .004) when compared with NHWs; addition of treatment exposures to the base model did not modify the racial/ethnic differences in mortality appreciably (RR, 1.4; 95% CI, 1.1 to 1.9; P = .008). However, this difference disappeared after adjustment for SES (RR, 1.0; 95% CI, 0.8 to 1.4; P = .88); addition of CVRFs to the model did not further affect racial/ethnic difference in all-cause mortality. Hispanic survivors were not at increased risk for all-cause mortality. Relative SMRs revealed that NHBs and Hispanics had comparable all-cause SMRs to NHWs; inclusion of therapeutic exposures did not alter this association appreciably (Table 3). Inclusion of SES resulted in a significant reduction in the treatment-adjusted all-cause SMRs for NHBs and Hispanics when compared with NHWs; addition of CVRFs to the model did not alter the association appreciably.
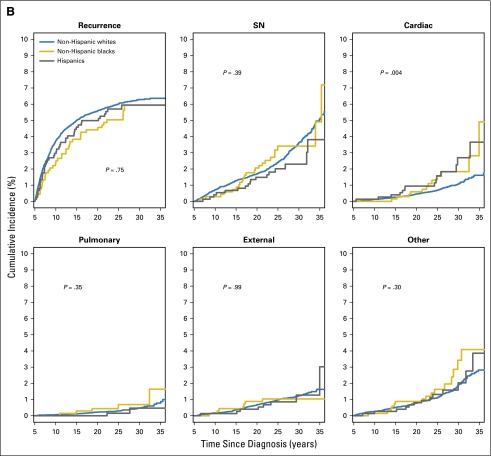
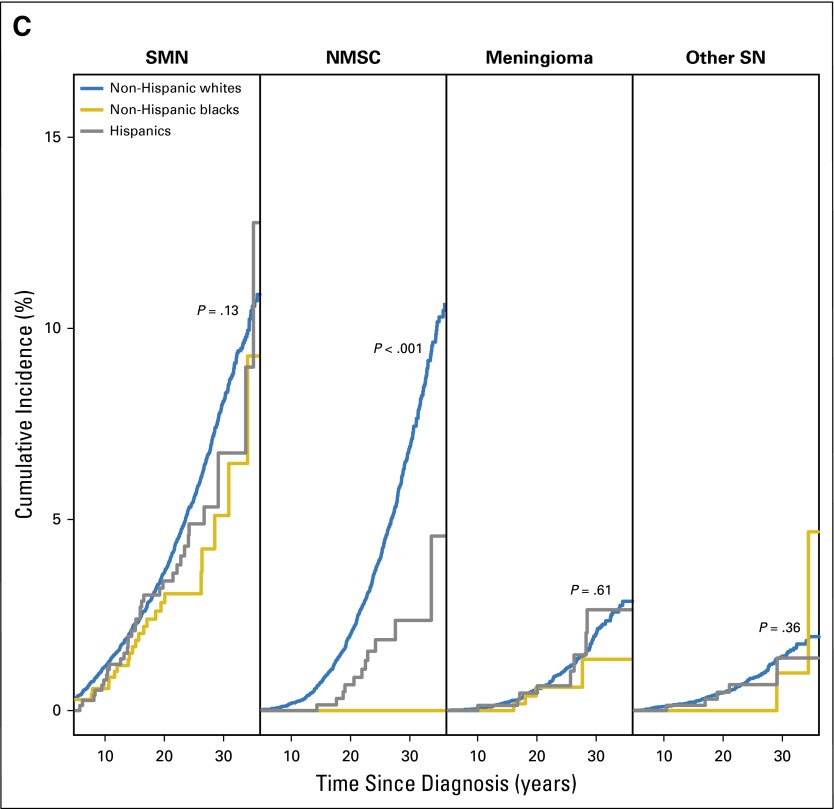
Fig 2.
(A) Survival probability of 5-year survivors of childhood cancer. (B) Cumulative incidence of cause-specific mortality by race/ethnicity in childhood cancer survivors. (C) Cumulative incidence of subsequent neoplasm (SN) categories (subsequent malignant neoplasm [SMN], nonmalignant meningioma, nonmelanoma skin cancer [NMSC], and other SN) by race/ethnicity among childhood cancer survivors.
Table 2.
Excess Risk of All-Cause and Cause-Specific Mortality Across Racial/Ethnic Groups of Childhood Cancer Survivors: Relative Rate
| All-Cause | Subsequent Neoplasm* | Subsequent Malignancy | Cardiac Cause | |||||
|---|---|---|---|---|---|---|---|---|
| RR of Mortality | RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P |
| Adjusted for clinical/demographic variables† | ||||||||
| NHW | Ref | Ref | Ref | Ref | ||||
| NHB | 1.5 (1.1 to 2.0) | .004 | 1.5 (0.9 to 2.4) | .15 | 1.2 (0.7 to 2.1) | .53 | 2.1 (0.7 to 6.3) | .19 |
| Hispanic | 1.1 (0.9 to 1.5) | .35 | 1.0 (0.5 to 1.7) | .92 | 0.9 (0.5 to 1.7) | .77 | 2.0 (0.9 to 4.5) | .09 |
| Adjusted for clinical/demographic variables and treatment‡ | ||||||||
| NHW | Ref | Ref | Ref | Ref | ||||
| NHB | 1.4 (1.1 to 1.9) | .008 | 1.4 (0.9 to 2.4) | .18 | 1.2 (0.7 to 2.1) | .58 | 2.0 (0.7 to 6.2) | .21 |
| Hispanic | 1.1 (0.8 to 1.4) | .53 | 0.9 (0.5 to 1.7) | .82 | 0.9 (0.5 to 1.7) | .68 | 1.8 (0.8 to 4.2) | .15 |
| Adjusted for clinical/demographic variables, treatment, and SES§ | ||||||||
| NHW | Ref | Ref | Ref | Ref | ||||
| NHB | 1.0 (0.8 to 1.4) | .88 | 1.1 (0.6 to 2.0) | .71 | 0.9 (0.5 to 1.8) | .80 | 1.6 (0.6 to 4.8) | .37 |
| Hispanic | 0.9 (0.7 to 1.2) | .46 | 0.8 (0.4 to 1.4) | .37 | 0.7 (0.4 to 1.4) | .38 | 1.5 (0.7 to 3.5) | .34 |
| Adjusted for clinical/demographic variables, treatment, SES, and CVRF|| | ||||||||
| NHW | Ref | Ref | Ref | Ref | ||||
| NHB | 1.0 (0.8 to 1.4) | .89 | 1.1 (0.6 to 2.0) | .65 | 0.9 (0.5 to 1.8) | .87 | 1.2 (0.4 to 3.4) | .70 |
| Hispanic | 0.9 (0.7 to 1.1) | .31 | 0.8 (0.4 to 1.4) | .37 | 0.7 (0.4 to 1.5) | .39 | 1.6 (0.7 to 3.8) | .28 |
Abbreviations: CVRF, cardiovascular risk factors; NHB, non-Hispanic black; NHW, non-Hispanic white; Ref, reference; RR, relative rate; SES, socioeconomic status.
Subsequent neoplasm included both malignancies and benign subsequent neoplasms.
Clinical/demographic variables include sex, age at diagnosis, diagnosis, treatment era, and attained age as natural cubic splines.
Treatment includes radiation therapy, alkylating agent cyclophosphamide equivalent dose, and anthracycline score.
SES includes education, annual household income, and health insurance as time-dependent variables.
||CVRF include obesity, diabetes, hypertension, and dyslipidemia.
Table 3.
Excess Risk of All-Cause and Cause-Specific Mortality Across Racial/Ethnic Groups of Childhood Cancer Survivors: Relative Standardized Mortality Rate
| All-Cause | Subsequent Neoplasm* | Subsequent Malignancy | Cardiac Cause | |||||
|---|---|---|---|---|---|---|---|---|
| Relative SMR (95% CI) | P | Relative SMR (95% CI) | P | Relative SMR (95% CI) | P | Relative SMR (95% CI) | P | |
| Adjusted for clinical/demographic variables† | ||||||||
| NHW | Ref | Ref | Ref | Ref | ||||
| NHB | 0.8 (0.6 to 1.1) | .15 | 1.2 (0.7 to 1.9) | .56 | 0.9 (0.5 to 1.7) | .85 | 0.8 (0.3 to 2.4) | .70 |
| Hispanic | 1.0 (0.7 to 1.2) | .71 | 0.9 (0.5 to 1.6) | .66 | 0.8 (0.4 to 1.6) | .52 | 2.0 (0.9 to 4.6) | .09 |
| Adjusted for clinical/demographic variables and treatment‡ | ||||||||
| NHW | Ref | Ref | Ref | Ref | ||||
| NHB | 0.8 (0.6 to 1.0) | .09 | 1.1 (0.7 to 1.9) | .62 | 0.9 (0.5 to 1.7) | .80 | 0.8 (0.3 to 2.4) | .66 |
| Hispanic | 0.9 (0.7 to 1.2) | .51 | 0.8 (0.5 to 1.5) | .58 | 0.8 (0.4 to 1.5) | .45 | 1.9 (0.8 to 4.3) | .15 |
| Adjusted for clinical/demographic variables, treatment, and SES§ | ||||||||
| NHW | Ref | Ref | Ref | Ref | ||||
| NHB | 0.6 (0.4 to 0.8) | < .001 | 0.9 (0.5 to 1.6) | .71 | 0.7 (0.4 to 1.4) | .35 | 0.6 (0.2 to 1.9) | .42 |
| Hispanic | 0.8 (0.6 to 1.0) | .05 | 0.7 (0.4 to 1.3) | .22 | 0.7 (0.3 to 1.3) | .23 | 1.6 (0.7 to 3.7) | .30 |
| Adjusted for clinical/demographic variables, treatment, SES, and CVRF|| | ||||||||
| NHW | Ref | Ref | Ref | Ref | ||||
| NHB | 0.6 (0.4 to 0.8) | < .001 | 0.9 (0.5 to 1.6) | .76 | 0.7 (0.4 to 1.4) | .39 | 0.5 (0.2 to 1.3) | .15 |
| Hispanic | 0.7 (0.6 to 1.0) | 0.03 | 0.7 (0.4 to 1.2) | .21 | 0.7 (0.3 to 1.3) | .23 | 1.7 (0.7 to 3.9) | .25 |
NOTE. Rates for Hispanic are available from the Centers for Disease Control and Prevention only for years 1999 and after. We used models to estimate the Hispanic rates before 1999, with the assumption that Hispanics are very similar to whites in terms of how the mortality rates have changed.
Abbreviations: CVRF, cardiovascular risk factors; NHB, non-Hispanic black; NHW, non-Hispanic white; Ref, reference; SES, socioeconomic status; SMR, standardized mortality ratio.
Subsequent neoplasm included both malignancies and benign subsequent neoplasms.
Clinical/demographic variables include sex, age at diagnosis, diagnosis, treatment era, and attained age as natural cubic splines.
Treatment includes radiation therapy, alkylating agent cyclophosphamide equivalent dose, and anthracycline score.
SES includes education, annual household income, and health insurance as time-dependent variables.
||CVRF include obesity, diabetes, hypertension, and dyslipidemia.
Cause-specific mortality.
Across all racial/ethnic groups, recurrence of primary disease was the leading cause of death, followed by SNs and cardiovascular causes. The cumulative incidence of cause-specific mortality was comparable across racial/ethnic groups for all causes, with the exception of cardiovascular mortality (P = .004), where the 30-year cumulative incidence was 2.7% for Hispanics, 1.8% for NHBs, and 1.1% for NHWs (Fig 2B). For cardiovascular deaths, SMRs were similar for NHWs (SMR, 5.3; 95% CI, 4.4 to 6.4) and NHBs (SMR, 5.5; 95% CI, 2.8 to 9.6) but were higher for Hispanics (SMR, 12.5; 95% CI, 6.8 to 21.0). There were no racial/ethnic difference in the SMRs for SN-related deaths (NHWs, SMR, 11.5; 95% CI, 10.4 to 12.8; Hispanics, SMR, 8.5; 95% CI, 5.0 to 13.6; NHBs, SMR, 11.9; 95% CI, 7.5 to 17.8). Multivariable analyses revealed no racial/ethnic differences either in cause-specific RR of mortality or in relative SMRs (Tables 2 and 3).
SNs/SMNs
SNs.
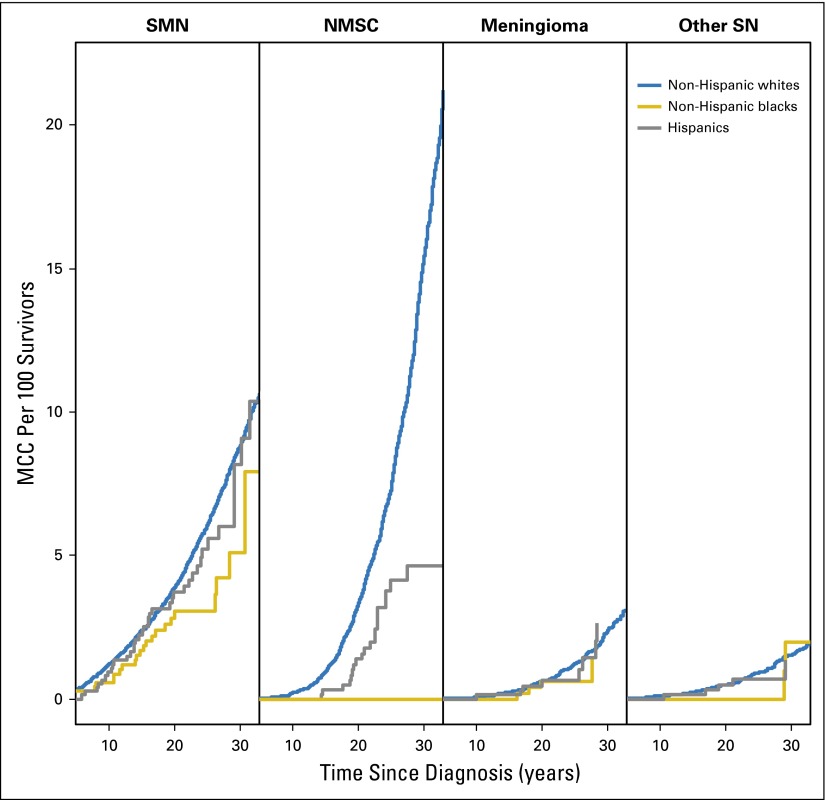
A total of 1,459 (11.8%) NHWs, 28 NHBs (4%), and 52 (6.9%) Hispanics developed SNs. The 30-year cumulative incidence was higher among NHWs (16.1%; 95% CI, 15.2% to 16.9%) as compared with NHBs (7.4%; 95% CI, 3.8% to 11.0%) or Hispanics (10.6%; 95% CI, 7.2% to 14.0%; P < .001). These differences were attributable to the significantly higher 30-year cumulative incidence of NMSC (P < .001) in NHWs (6.9%) compared with 0% and 2.4% in NHBs and Hispanics, respectively (Fig 2C). The average number of SNs (MCC) experienced per 100 survivors by 30 years from primary cancer diagnosis was 27.6 for NHWs, 8.4 for NHBs, and 16.8 for Hispanics (P = .004). The MCC per 100 survivors for SMN, nonmalignant meningioma, NMSC, and other SNs by race/ethnicity is shown in Appendix Figure A1 (online only). Multivariable analysis, adjusting for clinical variables, demographic variables, treatment, SES, and CVRFs revealed that NHBs were significantly less likely to be diagnosed with SNs (RR, 0.6; 95% CI, 0.4 to 0.9; P = .02) when compared with NHWs (Table 4); Hispanics did not differ significantly from NHWs (RR, 0.8; 95% CI, 0.6 to 1.1; P = .3).
Table 4.
Relative Rate of Endocrine and Cardiac Complications and Subsequent Neoplasms Across Racial/Ethnic Groups of Childhood Cancer Survivors
| Subsequent Neoplasms* | Subsequent Malignancies | Cardiovascular | Endocrine | |||||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P | |
| Adjusted for clinical/demographic variables† | ||||||||
| NHW | Ref | Ref | Ref | Ref | ||||
| NHB | 0.6 (0.4 to 0.9) | .009 | 0.8 (0.5 to 1.4) | .50 | 1.9 (1.2 to 2.9) | .005 | 0.8 (0.5 to 1.4) | .48 |
| Hispanic | 0.8 (0.6 to 1.1) | .21 | 1.1 (0.7 to 1.6) | .62 | 1.2 (0.8 to 1.8) | .41 | 1.5 (1.1 to 2.1) | .02 |
| Adjusted for clinical/demographic variables and treatment‡ | ||||||||
| NHW | Ref | Ref | Ref | Ref | ||||
| NHB | 0.5 (0.3 to 0.8) | .005 | 0.8 (0.5 to 1.3) | .37 | 1.8 (1.1 to 2.7) | .01 | 0.8 (0.5 to 1.3) | .39 |
| Hispanic | 0.8 (0.6 to 1.1) | .25 | 1.1 (0.7 to 1.6) | .66 | 1.2 (0.8 to 1.8) | .46 | 1.5 (1.1 to 2.2) | .01 |
| Adjusted for clinical/demographic variables, treatment, and SES§ | ||||||||
| NHW | Ref | Ref | Ref | Ref | ||||
| NHB | 0.6 (0.4 to 0.9) | .01 | 0.8 (0.5 to 1.4) | .45 | 1.6 (1.0 to 2.4) | .04 | 0.9 (0.5 to 1.5) | .63 |
| Hispanic | 0.8 (0.6 to 1.2) | .29 | 1.1 (0.7 to 1.6) | .77 | 1.1 (0.7 to 1.6) | .77 | 1.6 (1.2 to 2.3) | .005 |
| Adjusted for clinical/demographic variables, treatment, SES, and CVRF|| | ||||||||
| NHW | Ref | Ref | Ref | Ref | ||||
| NHB | 0.6 (0.4 to 0.9) | .02 | 0.9 (0.5 to1.4) | .59 | 1.5 (1.0 to 2.3) | .08 | 0.9 (0.5 to 1.4) | .59 |
| Hispanic | 0.8 (0.6 to 1.1) | .25 | 1.0 (0.7 to 1.6) | .91 | 1.0 (0.6 to 1.5) | .86 | 1.6 (1.2 to 2.3) | .005 |
Abbreviations: CVRF, cardiovascular risk factors; NHB, non-Hispanic black; NHW, non-Hispanic white; Ref, reference; RR, relative rate; SES, socioeconomic status.
Subsequent neoplasm included both malignancies and benign subsequent neoplasms.
Clinical/demographic variables include sex, age at diagnosis, diagnosis, treatment era, and attained age as natural cubic splines.
Treatment includes radiation therapy, alkylating agent cyclophosphamide equivalent dose, and anthracycline score.
SES includes education, annual household income, and health insurance as time-dependent variables.
||CVRF include obesity, diabetes, hypertension, and dyslipidemia.
SMNs.
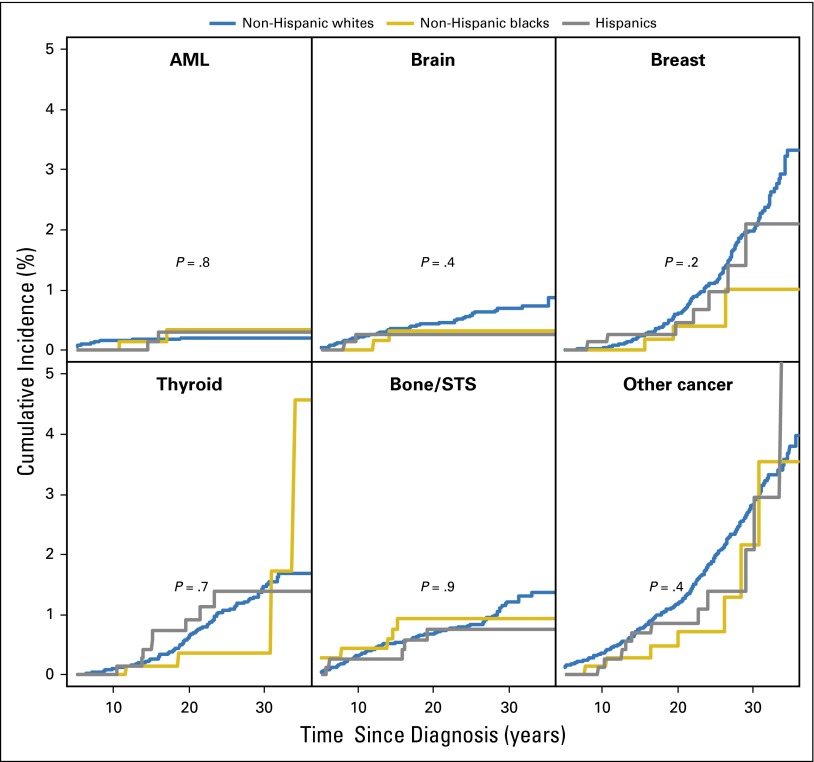
The 30-year cumulative incidence of any SMNs was higher in NHWs (8.1%) versus NHBs (5.1%) or Hispanics (6.7%; P = .07); however, the incidence of specific types of SMNs was comparable across racial/ethnic groups (Appendix Fig A2, online only). The average number of SMNs per 100 survivors at 30 years from diagnosis was higher in NHWs (8.7) versus Hispanics (8.1) and NHBs (5.1; P = .02). Multivariable analysis (Table 4) revealed that the risk of developing SMNs was comparable across all racial/ethnic groups. However, NHBs (RR, 0.0; 95% CI, 0.0 to 0.2; P < .001) and Hispanics (RR, 0.3; 95% CI, 0.1 to 0.7; P = .005) were significantly less likely to develop NMSC compared with NHWs; restricting the analysis to those exposed to radiation revealed similar associations (NHBs: RR, 0.0; 95% CI, 0.0 to 0.2; P < .001; Hispanics: RR, 0.3; 95% CI, 0.1 to 0.7; P = .005).
Chronic Health Conditions
All grades 3 to 5 chronic health conditions.
No significant difference was observed in the 30-year cumulative incidence of grade 3 to 5 chronic health conditions by race/ethnicity (NHBs, 44.4%; Hispanics, 41.9%; NHWs, 42.9%; P = .84). The average number of grades 3 to 5 chronic conditions experienced per 100 survivors by 30 years after diagnosis was also comparable (NHBs, 69.9; Hispanics, 71.0; NHWs, 71.6; P = .57). An examination of specific chronic health conditions revealed racial/ethnic differences in cardiovascular and endocrine outcomes.
Cardiovascular outcomes.
Multivariable analysis adjusting for clinical and demographic variables revealed that NHBs were more likely to report grades 3 to 5 cardiovascular conditions (RR, 1.9; 95% CI, 1.2 to 2.9; P = .005; Table 4). Adjustment for therapeutic variables did not alter the association appreciably (RR, 1.8; 95% CI, 1.1 to 2.7; P = .01). Inclusion of SES and CVRFs in the model resulted in attenuation of the association (RR, 1.5; 95% CI, 1.0 to 2.3; P = .08). Hispanic survivors did not differ from NHWs in the risk of cardiovascular disease (Table 4). Similar trends were observed for the individual cardiovascular outcomes (stroke, congestive heart failure, and coronary artery disease; Appendix Table A1, online only).
Endocrine outcomes.
Multivariable analysis adjusting for clinical and demographic variables revealed that although NHBs were not at increased risk for endocrine conditions when compared with NHWs, Hispanics were at a significantly increased risk (RR, 1.5; 95% CI, 1.1 to 2.1; P = .02; Table 4). This risk did not change appreciably on adjustment for treatment exposures (RR, 1.5; 95% CI, 1.1 to 2.2; P = .01) or SES and CVRFs (RR, 1.6; 95% CI, 1.2 to 2.3; P = .003). Among the endocrine conditions evaluated, both NHBs and Hispanics were more likely to report diabetes when compared with NHWs (Appendix Table A1). These differences persisted after adjusting for clinical, demographic, and therapeutic variables; SES; and CVRFs (excluding diabetes; NHBs, RR, 2.8; 95% CI, 1.1 to 6.7; P = .03; Hispanics, RR, 3.1; 95% CI, 1.5 to 6.4; P = .003).
DISCUSSION
Childhood cancer survivors carry a significant burden of morbidity and are at risk for premature mortality.9,13,18-20 In 2008, approximately 33% of the US population identified themselves as belonging to a racial or ethnic minority population; by 2050, this number is expected to increase to 50%.21 Whether the burden of morbidity borne by the growing population of childhood cancer survivors differs by race and ethnicity has not been assessed comprehensively. CCSS offers a unique opportunity to address this issue, because the cohort includes a large number of childhood cancer survivors from self-reported racial/ethnic minority backgrounds followed for two or more decades. This study finds that therapeutic exposures did not have a race/ethnicity-specific impact on morbidity or mortality. However, NHBs had a higher all-cause mortality than NHWs, but this difference was explained by SES. Both NHBs and Hispanics had a higher risk of diabetes that persisted after adjustment for clinical characteristics, therapeutic exposures, SES, and CVRFs. Finally, irradiated NHBs and Hispanics had a significantly lower risk of NMSCs when compared with NHWs.
Racial and ethnic minorities in the United States are in poorer health and experience more significant problems accessing care.22 Health insurance coverage is an important determinant of access to health care, in particular, preventive care, and has been dependent on income and education. The difference in mortality rates among NHBs and NHWs in the general population is reduced after taking education and income into account.23 We found an overrepresentation of NHB and Hispanic childhood cancer survivors with low income and education and without health insurance when compared with NHW survivors. As seen in the general population, the low SES among the NHB survivors explained the higher risk of all-cause mortality. When compared with the age-, sex-, and race-specific general population, NHB and Hispanic survivors did not reveal elevated all-cause mortality rates relative to NHWs. However, adjustment for SES resulted in a significant reduction in the relative excess in all-cause mortality among the Hispanic and NHB survivors, suggesting that low SES likely contributes to excess mortality among the minority general populations.
In the general population, the higher risk of cardiovascular disease among NHBs and Hispanics is ascribed (in part) to the higher prevalence of CVRFs.24,25 We find a higher prevalence of hypertension among NHB survivors and a higher prevalence of obesity among NHB and Hispanic survivors. The higher risk of cardiovascular disease overall as well as stroke, heart attack, and heart failure reported by the NHB childhood cancer survivors was explained by the higher prevalence of CVRFs. NHB and Hispanic survivors were more likely to report diabetes, and the higher prevalence of obesity among NHBs and Hispanics did not explain this excess risk, identifying an area that needs further investigation.
The risk of developing invasive SMNs was comparable across all racial/ethnic groups of survivors. However, similar to the extremely low risk of ultraviolet radiation–related NMSC observed among blacks and Hispanics in the general population,26,27 we found that NHB and Hispanic survivors were significantly less likely to develop radiation-related NMSC. People with dark skin have more melanin, and melanin helps protect against ultraviolet radiation. However, despite the fact that therapeutic radiation affords a higher dose (and intensity) of radiation than ultraviolet radiation, the NHB survivors did not report any NMSC, and the Hispanic survivors reported a much reduced risk. These observations suggest that either the protection afforded by the melanin is very effective or there is an alternative (genetic) explanation for the protection from radiation-related NMSC afforded to the NHB and Hispanic survivors.
Findings from this study need to be placed within the context of its limitations. Despite the large size and ethnic/racial diversity of this cohort, the number of minority survivors is not large enough to examine rare outcomes. The outcomes were obtained by self-report, although SNs/SMNs were verified by pathology reports, and vital status and cause of death were obtained from the National Death Index. These limitations notwithstanding, findings from this study indicate that, by and large, NHB and Hispanic childhood cancer survivors experience a comparable burden of morbidity and mortality to their NHW counterparts. Most importantly, therapeutic exposures did not have a differential impact on adverse outcomes, and the few differences in risk were explained by the racial/ethnic differences in SES and/or CVRFs. Specific morbidities (in particular diabetes) were more prevalent among Hispanics and NHBs, were only partially explained by SES and/or CVRFs, and need to be studied in greater detail.
Appendix
Fig A1.
Mean cumulative count (MCC) per 100 survivors of subsequent neoplasm (SN) categories (subsequent malignant neoplasm [SMNs], nonmalignant meningioma, nonmelanoma skin cancer [NMSC], and other SN) by race/ethnicity.
Fig A2.
Cumulative incidence (%) of specific types of subsequent malignant neoplasm by race/ethnicity. AML, acute lymphoblastic leukemia; STS, soft tissue sarcoma.
Table A1.
Relative Rate of Specific Cardiovascular, Endocrine, and Neoplastic Complications Across Racial/Ethnic Groups of Childhood Cancer Survivors
| Complication | Not Adjusted for SES/CV Risk Factors | Adjusted for SES Risk Factors | Adjusted for CV Risk Factors | Adjusted for CV Risk Factors and SES | ||||
|---|---|---|---|---|---|---|---|---|
| NHB | Hispanic | NHB | Hispanic | NHB | Hispanic | NHB | Hispanic | |
| Diabetes*† | 2.8 (1.2 to 6.7) | 3.1 (1.5 to 6.3) | 3.0 (1.2 to 7.3) | 3.2 (1.5 to 6.7) | 2.5 (1.1 to 6.0) | 2.9 (1.4 to 6.0) | 2.8 (1.1 to 6.7) | 3.1 (1.5 to 6.4) |
| P | .02 | .002 | .01 | .002 | .04 | .003 | .03 | .003 |
| Stroke‡ | 2.3 (1.2 to 4.5) | 1.2 (0.6 to 2.4) | 1.8 (0.9 to 3.6) | 1.0 (0.5 to 2.1) | 2.3 (1.1 to 4.6) | 1.2 (0.6 to 2.6) | 1.7 (0.8 to 3.5) | 1.1 (0.5 to 2.3) |
| P | .01 | .69 | .07 | .98 | .02 | .55 | .14 | .78 |
| CHF‡ | 1.8 (1.1 to 2.9) | 0.9 (0.5 to 1.7) | 1.6 (1.0 to 2.6) | 0.9 (0.5 to 1.6) | 1.4 (0.9 to 2.3) | 1.0 (0.5 to 1.8) | 1.3 (0.8 to 2.1) | 0.9 (0.5 to 1.6) |
| P | .02 | .85 | .06 | .69 | .15 | .92 | .31 | .65 |
| CAD‡ | 2.0 (0.9 to 4.5) | 1.6 (0.8 to 3.1) | 2.0 (0.9 to 4.3) | 1.3 (0.6 to 2.6) | 2.0 (0.9 to 4.3) | 1.3 (0.6 to 2.7) | 1.5 (0.7 to 3.3) | 1.1 (0.5 to 2.2) |
| P | .08 | .22 | .08 | .46 | .09 | .46 | .27 | .87 |
| NMSC | 0.0 (0.0 to 0.2) | 0.3 (0.1 to 0.7) | 0.0 (0.0 to 0.1) | 0.3 (0.1 to 0.8) | 0.0 (0.0 to 0.1) | 0.3 (0.1 to 0.7) | 0.0 (0.0 to 0.2) | 0.3 (0.1 to 0.8) |
| P | < .001 | .005 | < .001 | .01 | < .001 | 0005 | < .001 | .01 |
| NMSC§ | 0.0 (0.0 to 0.2) | 0.3 (0.1 to 0.7) | 0.0 (0.0 to 0.2) | 0.3 (0.1 to 0.7) | 0.0 (0.0 to 0.2) | 0.3 (0.1 to 0.7) | 0.0 (0.0 to 0.2) | 0.3 (0.1 to 0.7) |
| P | < .001 | .005 | < .001 | .01 | < .001 | .006 | < .001 | .01 |
NOTE. Data presented as RR (95% CI). RR uses non-Hispanic whites as reference group. Unless stated otherwise, all models adjusted for sex, age at diagnosis, diagnosis, treatment era, attained age as natural cubic splines, and treatment including any radiation, surgery (splenectomy when the outcome is SN), alkylating agent cyclophosphamide equivalent dose, and anthracycline score.
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; CV, cardiovascular; NHB, non-Hispanic blace; NMSC, nonmelanoma skin cancer; RR, relative rate; SES, socioeconomic status.
The only CV risk factor included in the model was obesity.
Without adjustment for age as cubic splines to make the model converge.
The model does not converge for the reduced number of people that have treatment data. Used all survivors and did not adjust for treatment (analyses of all other outcomes suggested that treatment adjustment does not change the race/ethnicity effect) to evaluate the effect of SES/CV.
Analysis restricted to survivors exposed to radiation therapy.
Footnotes
Supported by the National Cancer Institute Grant No. CA55727 (G.T.A.), Cancer Center Support (CORE) Grant No. CA21765 (R.G.), and the American Lebanese-Syrian Associated Charities.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Wendy M. Leisenring, Kirsten K. Ness, Leslie L. Robison, Gregory T. Armstrong, Yutaka Yasui, Smita Bhatia
Financial support: Leslie L. Robison, Gregory T. Armstrong
Administrative support: Leslie L. Robison, Gregory T. Armstrong
Provision of study materials or patients: Leslie L. Robison, Gregory T. Armstrong
Collection and assembly of data: Leslie L. Robison, Gregory T. Armstrong
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Racial/Ethnic Differences in Adverse Outcomes Among Childhood Cancer Survivors: The Childhood Cancer Survivor Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Qi Liu
No relationship to disclose
Wendy M. Leisenring
Research Funding: Merck (Inst)
Kirsten K. Ness
No relationship to disclose
Leslie L. Robison
No relationship to disclose
Gregory T. Armstrong
No relationship to disclose
Yutaka Yasui
No relationship to disclose
Smita Bhatia
No relationship to disclose
REFERENCES
- 1.Howlader NN, Krapcho M, Garshell J, et al. SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2012/
- 2.Armenian SH, Robison LL. Childhood cancer survivorship: An update on evolving paradigms for understanding pathogenesis and screening for therapy-related late effects. Curr Opin Pediatr. 2013;25:16–22. doi: 10.1097/MOP.0b013e32835b0b6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia S, Armenian SH, Armstrong GT, et al. Collaborative research in childhood cancer survivorship: The current landscape. J Clin Oncol. 2015;33:3055–3064. doi: 10.1200/JCO.2014.59.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellino SM, Casillas J, Hudson MM, et al. Minority adult survivors of childhood cancer: A comparison of long-term outcomes, health care utilization, and health-related behaviors from the childhood cancer survivor study. J Clin Oncol. 2005;23:6499–6507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]
- 5.Colby SL. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau; 2015. https://www.census.gov/library/publications/2015/demo/p25-1143.html. [Google Scholar]
- 6.St Jude Children’s Research Hospital The Childhood Cancer Survivor Study. http://ccss.stjude.org/
- 7.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 9.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Therapy Evaluation Program . Common terminology criteria for adverse events. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 12.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 14.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Little RJA, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: J Wiley & Sons; 2002. doi: 10.1002/9781119013563. [Google Scholar]
- 16.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 17.Dong H, Robison LL, Leisenring WM, et al. Estimating the burden of recurrent events in the presence of competing risks: The method of mean cumulative count. Am J Epidemiol. 2015;181:532–540. doi: 10.1093/aje/kwu289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304:172–179. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 20.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matise TC, Ambite JL, Buyske S, et al. The next PAGE in understanding complex traits: Design for the analysis of Population Architecture Using Genetics and Epidemiology (PAGE) study. Am J Epidemiol. 2011;174:849–859. doi: 10.1093/aje/kwr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agency for Healthcare Research and Quality: National Healthcare Disparities Report: 2003–2006; Institute of Medicine, Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC, National Academy of Sciences, 2003. [Google Scholar]
- 23.Guralnik JM, Land KC, Blazer D, et al. Educational status and active life expectancy among older blacks and whites. N Engl J Med. 1993;329:110–116. doi: 10.1056/NEJM199307083290208. [DOI] [PubMed] [Google Scholar]
- 24.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: A report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [Erratum: Circulation 127:3841, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolly S, Vittinghoff E, Chattopadhyay A, et al. Higher cardiovascular disease prevalence and mortality among younger blacks compared to whites. Am J Med. 2010;123:811–818. doi: 10.1016/j.amjmed.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Little EG, Eide MJ. Update on the current state of melanoma incidence. Dermatol Clin. 2012;30:355–361. doi: 10.1016/j.det.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 27.American Cancer Society . Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]