Abstract
Purpose
Tumor lymphocytic infiltration (TLI) has differing prognostic value among various cancers. The objective of this study was to assess the effect of TLI in lung cancer.
Patients and Methods
A discovery set (one trial, n = 824) and a validation set (three trials, n = 984) that evaluated the benefit of platinum-based adjuvant chemotherapy in non–small-cell lung cancer were used as part of the LACE-Bio (Lung Adjuvant Cisplatin Evaluation Biomarker) study. TLI was defined as intense versus nonintense. The main end point was overall survival (OS); secondary end points were disease-free survival (DFS) and specific DFS (SDFS). Hazard ratios (HRs) and 95% CIs associated with TLI were estimated through a multivariable Cox model in both sets. TLI-histology and TLI-treatment interactions were explored in the combined set.
Results
Discovery and validation sets with complete data included 783 (409 deaths) and 763 (344 deaths) patients, respectively. Median follow-up was 4.8 and 6 years, respectively. TLI was intense in 11% of patients in the discovery set compared with 6% in the validation set (P < .001). The prognostic value of TLI in the discovery set (OS: HR, 0.56; 95% CI, 0.38 to 0.81; P = .002; DFS: HR, 0.59; 95% CI, 0.42 to 0.83; P = .002; SDFS: HR, 0.56; 95% CI, 0.38 to 0.82; P = .003) was confirmed in the validation set (OS: HR, 0.45; 95% CI, 0.23 to 0.85; P = .01; DFS: HR, 0.44; 95% CI, 0.24 to 0.78; P = .005; SDFS: HR, 0.42; 95% CI, 0.22 to 0.80; P = .008) with no heterogeneity across trials (P ≥ .38 for all end points). No significant predictive effect was observed for TLI (P ≥ .78 for all end points).
Conclusion
Intense lymphocytic infiltration, found in a minority of tumors, was validated as a favorable prognostic marker for survival in resected non–small-cell lung cancer.
INTRODUCTION
In lung cancer, several attempts have been made to correlate the type and density of immune cells with prognosis. Among T lymphocytes, which comprise 80% of tumor-infiltrating lymphocytes (TILs),1 CD8+ cytotoxic lymphocytes are believed to constitute the effector arm of adaptive immunity against tumor cells that lead to the slowing of growth rates. Studies that examined a correlation between CD8+ TILs and prognosis in non–small-cell lung cancer (NSCLC) are inconsistent.2,3 The largest study2 (1,290 patients with NSCLC) showed an association of CD8+ and prolonged survival but only in squamous cell carcinoma (SCC), which did not confirm the findings of the previous study.3 In some reports, CD3 or concurrent high infiltration of CD4+/CD8+ correlated with longer overall survival (OS).4-6 Similar observations have been made for high CD4/CD8 and CD20 lymphocyte infiltration in stroma.2,3 High FoxP3, Cox2, or density of mature dendritic cells also have been reported to correlate with prognosis of recurrence in NSCLC.7-9 Increased total TILs was associated with longer survival in two studies in a limited series of stage I or large-size (> 5 cm) NSCLC10,11 through univariable analysis. In a more recent tissue microarray series of NSCLC, the degree of lymphocytic infiltration failed to have prognostic value, although CD8 only was associated consistently with better outcome.12 Many reported studies have had limited statistical power, examined multiple factors without correction, included small numbers of patients, and were inhomogeneous with regard to stage and histologic types. These studies did not reach a consensus on how lymphocyte infiltration influences tumor growth and prognosis. In other cancers (breast,13,14 colorectal,15-19 ovarian, cervical,20,21 liver,22 pancreatic,23 esophageal24), CD3, CD4, and CD8 density frequently has been reported to be associated with significantly better OS.
We studied the prognostic value of tumor lymphocytic infiltration (TLI) in a large and relatively homogeneous group of patients with completely resected NSCLC and tested its predictive value for survival benefit in adjuvant cisplatin-based chemotherapy randomized trials. To our knowledge, the study is the first validation of the prognostic role of lymphocytic tumor infiltration in a large series of patients.
PATIENTS AND METHODS
Patients and Pathology Materials
LACE-Bio (Lung Adjuvant Cisplatin Evaluation Biomarker) collaborative group25 patients with a definite diagnosis of NSCLC were included. LACE-Bio pools the results of the following four randomized clinical trials that evaluated the benefit of platinum-based adjuvant chemotherapy compared with observation: International Adjuvant Lung Cancer Trial (IALT)26,27; Adjuvant Navelbine International Trialist Association (ANITA)28; JBR10,29,30 which used cisplatin-based adjuvant chemotherapy (LACE); and Cancer and Leukemia Group B (CALGB) 9633 trial on carboplatin-based adjuvant chemotherapy.31 The accrual period was between 1995 and 2000 for IALT, 1994 and 2000 for ANITA, 1996 and 2003 for CALGB, and 1994 and 2001 for JBR10.
Assay Methods
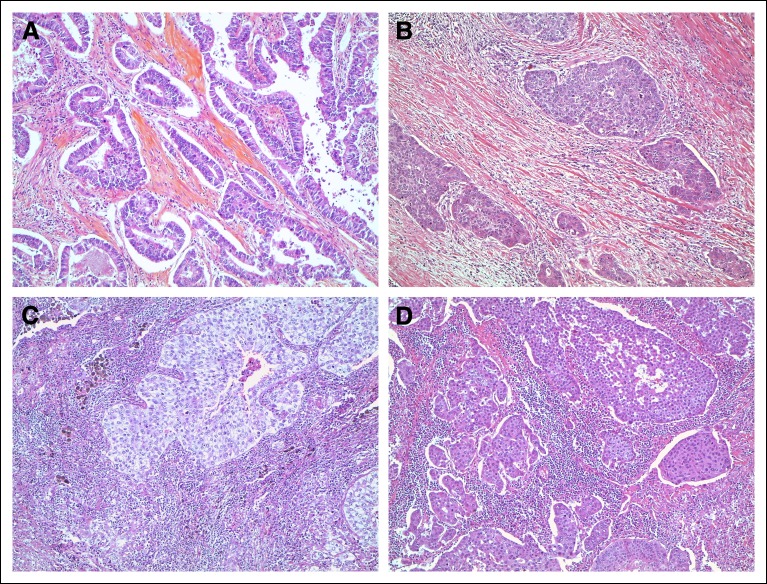
The intensity of TLI was first evaluated by two readers (E.B. and M.S.T.) into four categories (minimal, mild, moderate, and intense) on hematoxylin and eosin–stained representative sections, which were also used to reclassify lung tumor histology according to the new 2015 World Health Organization (WHO) classification for lung tumors.32 A binary scoring system was used to collapse the first three categories into nonintense (Fig. 1). Intense TLI referred to a strong heavy lymphocytic infiltrate (intralobular and/or perilobular) of a density equivalent to that seen in a lymph node with metastasis. We adopted the definition used in breast cancer with predominant lymphocytic infiltration to refer to tumors that show ≥ 50% stromal lymphocytes in the tumor bulk compared with epithelial tumor cells. 33 The evaluation of infiltration and the concordance analysis were conducted in two steps on the pooled analysis in the validation set (ANITA, JBR10, and CALGB) by the two pathologists (E.B. and M.S.T.). In step 1, the first reading was done independently. The agreement analysis showed that the κ varied from 0.42 to 0.79 for four classes (weighted κ) and from 0.44 to 0.85 for two classes (simple κ) across trials. The overall agreement was moderate when consideration was given to infiltration in four classes (κ = 0.59) and good in two classes (κ = 0.72). In step 2, the discordant cases were reviewed to reach the final consensus classification. After the concordance analysis, the lymphocytic infiltration was considered a binary marker for the statistical analysis.
Fig 1.
Histopathologic examples of lymphocytic infiltration. (A) Nonintense lymphocytic infiltration in an adenocarcinoma. (B) Nonintense lymphocytic infiltration in a squamous cell carcinoma. (C) Intense lymphocytic infiltration in an adenocarcinoma. (D) Intense lymphocytic infiltration in a squamous cell carcinoma.
Study Design
Samples were reviewed independently by E.B. (IALT, ANITA, JBR10, CALGB) and M.S.T. (ANITA, JBR10, CALGB), and TLI intensity was assigned. IALT was used as the discovery set and ANITA, JBR10, and CALGB as the validation set. The primary end point was OS, defined as the time from random assignment to the date of death, whatever the cause. Secondary end points were disease-free survival (DFS), defined as the time from random assignment to the time of the first event (progression, death), and specific DFS (SDFS), defined as the time from random assignment to a cancer-related event (ie, noncancer deaths were censored at the date of last follow-up [eg, death due to toxicity]). Patients with no events were censored at the date of their last follow-up.
Statistical Analysis
Patient characteristics (demographic clinic, tumor, and outcomes) of the discovery set are described. Median follow-up was estimated with the reverse Kaplan-Meier method.34 Correlation between TLI and the baseline patient and tumor characteristics were assessed by using a logistic regression.
The prognostic effect of TLI was first evaluated on the discovery set. Survival rates of TLI (intense, nonintense) were estimated by Kaplan-Meier method with Rothman CIs and survival curves compared with the log-rank test. The hazard ratio (HR) and 95% CI associated with TLI were estimated through a multivariable Cox regression model that controlled for sex, age (< 55, 55 to 64, ≥ 65 years), tumor stage (I, II, and III), type of surgery (pneumonectomy, lobectomy/other), WHO performance status (0, ≥ 1), and histology (SCC, adenocarcinoma [ADC], and others). The prognostic value of TLI was estimated in both arms but restricted to the control arm if an interaction existed between TLI and treatment. The assessment of the proportional hazard hypothesis was tested by using martingale residuals.35,36 When this hypothesis was rejected for some covariates, the covariates were then used for stratification in the Cox model. In the validation set, patient and tumor characteristics of individuals with and without TLI results were compared by using a logistic regression stratified by trial. Patient characteristics with TLI results were also compared with those in the discovery set. We repeated the same discovery set inferential analyses with the addition of stratification by trial. We also investigated the heterogeneity of the prognostic effect of TLI among trials. Two interaction terms (TLI × histology and TLI × treatment) were tested on the combined set (discovery + validation) as an exploratory analysis. For the former, we extended the analysis by differentiating ADC according to the main pattern predominant variant (lepidic [LEP], acinar/papillary, micropapillary/solid).
Analysis was performed on all patients analyzable on the basis of the initial treatment assignment. Significance levels were set to 1% for discovery set analyses, 5% for validation set analyses, and 1% for discovery plus validation set analyses (combination of the four trials). An α-level of 1% was used in the discovery set analysis to account for the multiple markers tested (Appendix Table A1, online only) and in the exploratory combined analysis. Statistical analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC). Results reporting follows REMARK (Reporting Recommendations for Tumour Marker Prognostic Studies) guidelines.37
RESULTS
Patient Cohorts
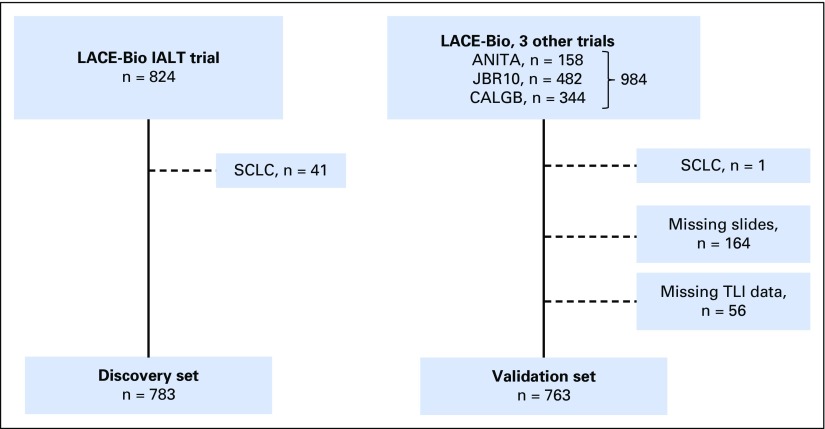
The TLI results were available in 783 of 824 discovery set patients (95%) and in 763 of 984 (ANITA, n = 158; JBR10, n = 482; CALGB, n = 344) validation set patients (77.5%; Appendix Fig A1, online only). The median follow-up was 4.8 years (range, 0.7 to 7.4 years) and 6.0 years (range, 0.1 to 11.3 years) for the discovery and validation sets, respectively. In the validation set, comparison between patients with TLI scores (n = 763) and without TLI scores (164 with no tissue and 56 with missing TLI results) showed a significant difference in terms of T of TNM (P = .01) and histology (P < .001) (Appendix Table A2, online only). Heterogeneity was observed between the discovery and validation sets (Table 1), with 35% stage I in discovery versus 59% in validation, 33% ADC in discovery versus 49% in validation, and 11% intense TLI in discovery versus 6% (4% to 7% across trials) in validation. This TLI imbalance between the two data sets persisted after adjustment for sex, age, stage, type of surgery, WHO performance status, and histology (P < .001). Although TLI was significantly correlated to stage (P = .01), N (P = .01), and T (P < .001) in the discovery set (Appendix Table A3, online only), it was correlated to histology (P = .001) in the validation set, with a higher proportion of intense TLI for patients with SCC (11%) compared with those with ADC (4%) or other histology (3%) (Appendix Table A4, online only). We observed 409 (52%) and 344 (45%) deaths in the discovery and validation sets, respectively.
Table 1.
Patient Characteristics of Discovery, Validation, and Combined Sets
| Characteristic | Discovery Set (n = 783), No. (%) | Validation Set* (n = 763), No. (%) | P† | Combined sets (n = 1,546), No. (%) |
|---|---|---|---|---|
| Sex | ||||
| Male | 638 (81) | 533 (70) | < .001 | 1,171 (76) |
| Female | 145 (19) | 230 (30) | 375 (24) | |
| Mean age (years) | 58.4 | 60.1 | < .001 | 59.3 |
| < 55 | 237 (30) | 209 (27) | < .001 | 446 (29) |
| 55-64 | 343 (44) | 289 (38) | 632 (41) | |
| > 64 | 203 (26) | 265 (35) | 468 (30) | |
| Treatment | ||||
| No chemotherapy | 382 (49) | 385 (50) | .51 | 767 (50) |
| Chemotherapy | 401 (51) | 378 (50) | 779 (50) | |
| Stage | ||||
| I | 271 (35) | 451 (59) | < .001 | 722 (47) |
| II | 273 (35) | 264 (35) | 537 (35) | |
| III | 239 (31) | 44 (6) | 283 (18) | |
| Unknown | 0 | 4 | 4 | |
| N of TNM | ||||
| N0 | 367 (47) | 459 (61) | < .001 | 826 (54) |
| N1 | 223 (28) | 260 (34) | 483 (31) | |
| N2, 3, 4 | 193 (25) | 38 (5) | 231 (15) | |
| Unknown | 0 | 6 | 6 | |
| T of TNM | ||||
| T1 | 119 (15) | 61 (8) | < .001 | 180 (12) |
| T2 | 466 (60) | 677 (89) | 1,143 (74) | |
| T3, 4 | 198 (25) | 21 (3) | 219 (14) | |
| Unknown | 0 | 4 | 4 | |
| Type of surgery | < .001 | |||
| Lobectomy/other | 469 (60) | 590 (78) | 485 (31) | |
| Pneumonectomy | 314 (40) | 171 (22) | 1,059 (69) | |
| Unknown | 0 | 2 | 2 | |
| WHO PS | ||||
| 0 | 440 (56) | 385 (51) | .034 | 825 (54) |
| ≥ 1 | 343 (44) | 373 (49) | 716 (46) | |
| Unknown | 0 | 5 | 5 | |
| Histology | ||||
| Squamous cell carcinoma | 422 (54) | 285 (37) | < .001 | 707 (46) |
| Adenocarcinoma | 261 (33) | 373 (49) | 634 (41) | |
| Other NSCLC‡ | 100 (13) | 105 (14) | 205 (13) | |
| TLI§ | ||||
| Nonintense | 697 (89) | 714 (94) | < .001 | 1,411 (91) |
| Intense | 86 (11) | 49 (6) | 135 (9) | |
| No. of deaths | 409 (52) | 344 (45) | 753 (49) | |
| No. of events‖ | 456 (58) | 396 (52) | 852 (55) | |
| No. of specific events | 398 (51) | 345 (45) | 743 (48) | |
| Median (range) follow-up (years)¶ | 4.8 (0.7-7.4) | 6.0 (0.1-11.3) | 5.4 (0.1-11.3) |
Abbreviations: NSCLC, non–small-cell lung cancer; PS, performance status; TLI, tumor lymphocytic infiltration.
Validation set included ANITA (Adjuvant Navelbine International Trialist Association), CALGB (Cancer and Leukemia Group B), and JBR10 trials.
P value from χ2 and t test for categorical and continuous covariates, respectively.
Other NSCLC included large-cell, adenosquamous, sarcomatoid, basaloid, and unclassifiable NSCLC.
This significant difference between the two data sets persists after adjustment for covariates (sex, age, stage, type of surgery, WHO PS, and histology).
Events that defined the disease-free survival included progression or death.
Median follow-up was estimated by using the reverse Kaplan-Meier (Schemper method).
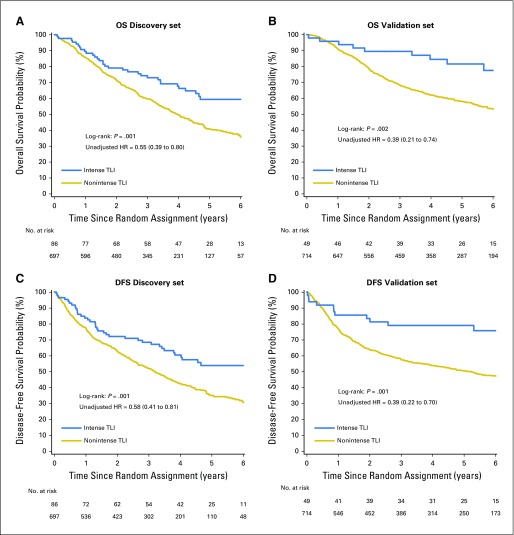
Prognostic Value of TLI (Discovery Set)
The unadjusted survival curves for the discovery set (Figs 2A and 2C) show that the intense TLI group had longer OS than the nonintense TLI group (log-rank P = .001), with 59% 5-year OS (95% CI, 47% to 70%) in the intense TLI group compared with 40% (95% CI, 36% to 45%) in the nonintense TLI. Similar results were observed for DFS (log-rank P = .001), with 54% 5-year DFS (95% CI, 42% to 64%) compared with 35% (95% CI, 31% to 39%; Figs 2A and 2C) for SDFS (Appendix Fig A2, online only). The difference on outcomes according to TLI remained statistically significant after controlling for covariates (OS: HR, 0.56; 95% CI, 0.39 to 0.81; P = .002; DFS: HR, 0.59; 95% CI, 0.42 to 0.83; P = .002; SDFS: HR, 0.56; 95% CI, 0.38 to 0.82; P = .003; Table 2; Appendix Tables A5 and A6, online only). Note that the prognostic effect was estimated in all patients because no interaction between treatment and TLI was observed (P = .92 and .95 for OS and DFS, respectively). The hypothesis of proportionality was violated for some covariates, but the results did not substantially change when stratified in the Cox model (data not shown).
Fig 2.
Survival curves for tumor lymphocytic infiltration (TLI; intense and nonintense) for overall survival (OS; A and B) and disease-free survival (DFS; C and D) in the discovery (A and C) and validation (B and D) sets. The P value of the log-rank test and the unadjusted hazard ratios (HRs) and 95% CIs are reported.
Table 2.
Prognostic Value of TLI for OS and DFS Estimated From Unadjusted and Adjusted Cox Models on Discovery (n = 783) and Validation set (n = 753)
| Discovery Set | Validation Set | |||||||
|---|---|---|---|---|---|---|---|---|
| TLI | Event/No. of Patients | Unadjusted HR (95% CI) | Adjusted* HR (95% CI) | Adjusted P | Event/No. of Patients | Unadjusted HR (95% CI) | Adjusted* HR (95% CI) | Adjusted P |
| OS | ||||||||
| Nonintense | 377/697 | 1.00 | 1.00 | .002 | 329/704 | 1.00 | 1.00 | .01 |
| Intense | 32/86 | 0.55 (0.39 to 0.80) | 0.56 (0.39 to 0.81) | 10/49 | 0.39 (0.21 to 0.74) | 0.45 (0.23 to 0.85) | ||
| DFS | ||||||||
| Nonintense | 419/697 | 1.00 | 1.00 | .002 | 378/704 | 1.00 | 1.00 | .005 |
| Intense | 37/86 | 0.58 (0.41 to 0.81) | 0.59 (0.42 to 0.83) | 12/49 | 0.39 (0.22 to 0.70) | 0.44 (0.24 to 0.78) | ||
| SDFS | ||||||||
| Nonintense | 368/697 | 1.00 | 1.00 | .003 | 331/704 | 1.00 | 1.00 | .008 |
| Intense | 30/86 | 0.54 (0.37 to 0.79) | 0.56 (0.38 to 0.82) | 10/49 | 0.38 (0.20 to 0.71) | 0.42 (0.22 to 0.80) | ||
Abbreviations: DFS, disease-free survival; HR, hazard ratio; OS, overall survival; SDFS, specific disease-free survival; TLI, tumor lymphocytic infiltration.
Ten patients with missing type of surgery (n = 2), stage (n = 4), or performance status (n = 5) were excluded from the multivariable analyses.
Validation of the Prognostic Value of TLI
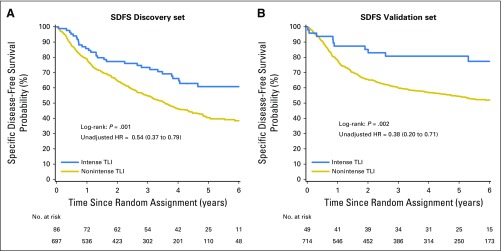
OS was longer in the intense TLI group than in the nonintense TLI group (P = .002). This was similar for DFS (P = .001; Figs 2B and 2D) and SDFS (Appendix Fig A2). The 5-year OS and DFS were estimated at 85% (95% CI, 70% to 92%) and 79% (95% CI, 65% to 88%) in patients with intense TLI compared with 58% (95% CI, 54% to 62%) and 50% (95% CI, 47% to 54%) in patients with nonintense TLI. Because no significant interaction existed between treatment covariate and TLI (P = .99 and .88 for OS and DFS, respectively), the adjusted HRs were estimated on both arms. We confirmed the prognostic effect of TLI (HR, 0.45; 95% CI, 0.23 to 0.85; P = .01) in favor of longer OS for patients with intense TLI (Table 2). Similar results were observed for DFS (HR, 0.44; 95% CI, 0.24 to 0.78; P = .005; Appendix Table A7, online only) and SDFS (HR, 0.42; 95% CI, 0.22 to 0.80; P = .008; Appendix Table A8, online only). These effects were homogeneous among trials (P = .76 and .38 for OS and DFS, respectively). The reduction of risk of an event was slightly higher in the validation set than in the discovery set. However, we observed a violation of the proportional hazard for some covariates, but the results did not change after stratification on these covariates (data not shown).
TLI and Histology or Treatment Interaction
Exploratory analysis showed that the histology-TLI interaction was marginally significant for OS (P = .06) with an HR of 0.62 (95% CI, 0.42 to 0.93), 0.69 (95% CI, 0.40 to 1.19), and 0.12 (95% CI, 0.03 to 0.47) for SCC (n = 81 intense TLI + 624 nonintense TLI), ADC (n = 38 + 590), and other NSCLC (n = 16 + 187), respectively. The results in the other subgroup should be interpreted with caution because of the small sample size. When we excluded that category, the interaction term was no longer significant (P = .73), which shows that the effect of TLI was homogeneous within SCC and ADC. After regrouping these two histologies, the interaction term was significant (P = .02). We repeated the histology-TLI interaction analysis to include ADC according to its subtypes (LEP was excluded because no patient with LEP had intense TLI) and found no significant effect of TLI in the acinar/papillary and micropapillary/solid subtypes (data not shown). Similar results were observed for DFS and SDFS (data not shown).
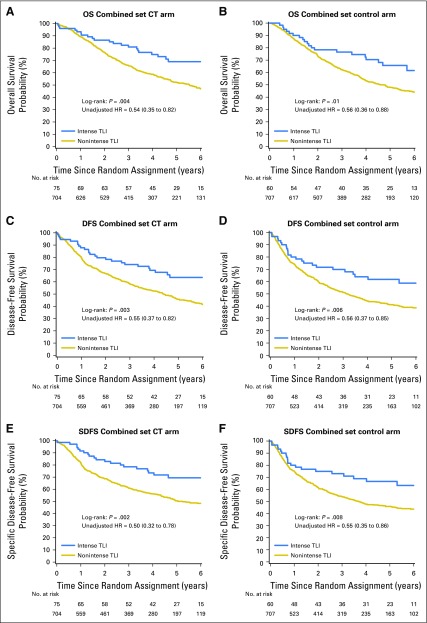
As previously shown in the discovery and validation sets, the treatment-TLI interaction, estimated on the combined set, was not significant for OS, DFS, and SDFS (P = .96, .99, and .78, respectively; Table 3; Appendix Table A9, online only, for SDFS). The corresponding Kaplan-Meier curves that compared intense versus nonintense TLI by treatment group are reported in Appendix Fig A3, online only.
Table 3.
Treatment Interaction With Tumor Lymphocytic Infiltration for Overall Survival and Disease-Free Survival Estimated From a Multivariable Cox Model on the Combined Data Set (n = 1,536*)
| Overall Survival | Disease-Free Survival | |||||
|---|---|---|---|---|---|---|
| Tumor Lymphocytic Infiltration | CT Deaths/No. of Patients | Observation Deaths/No. of Patients | CT v No CT, HR (95% CI) | CT Events/No. of Patients | Observation Events/No. of Patients | CT v No CT, HR (95% CI) |
| Nonintense | 341/699 | 365/702 | 0.88 (0.76 to 1.02) | 382/699 | 415/702 | 0.84 (0.73 to 0.97) |
| Intense | 22/75 | 20/60 | 0.90 (0.49 to 1.64) | 26/75 | 23/60 | 0.84 (0.48 to 1.48) |
| Interaction test: P = .96 | Interaction test: P = .99 | |||||
Abbreviations: CT, chemotherapy; HR, hazard ratio.
Ten patients were excluded from the analysis due to missing covariates.
DISCUSSION
We have demonstrated the independent prognostic value of TLI on survival by using two different data sets, with intense lymphocytic infiltration (that mimics lymph node involvement) predicting longer survival (OS, DFS, and SDFS) in patients with NSCLC. As expected, SCC histology was more frequent in the IALT than in the JBR10, CALGB, ANITA patients. Nonetheless, given the size of the data sets and the set of prognostic factors considered for adjustment, the current study provided reliable evidence of the prognostic role of TLI. We note, however, a different risk reduction in the discovery set compared with the validation set, regardless of the end points, that may be explained by the different trial populations. For example, the HRs of OS were around 0.56 and 0.45 in the discovery and validation sets, respectively. We updated the prognostic effect of TLI by pooling the discovery and validation sets, which was possible because no heterogeneity across trials was highlighted (P = .77, .40, .64 for OS, DFS, and SDFS, respectively). This gives a more accurate estimation of the prognostic effect of TLI with an HR of 0.53 (95% CI, 0.39 to 0.73; P < .001), 0.55 (95% CI, 0.41 to 0.73; P < .001), and 0.52 (95% CI, 0.38 to 0.72; P < .001) for OS, DFS, and SDFS, respectively.
Whether the association of intense lymphocytic infiltration with survival is direct or indirect or whether this survival advantage is attributable to one or a set of phenotypic subtypes of immune cells (CD4+, CD8+, CD20, regulatory T cells, macrophages 1 and 2, and dendritic cells) was not investigated in the current study. When prognostic immune markers were investigated in another NSCLC study,38 the prognostic effect of immune infiltrates was mediated by both protumor and antitumor immune populations but without evidence that this balance could be measured (with validated cutoffs) to predict outcomes in individual patients.
The prognostic value of epithelial versus stromal lymphocytic infiltration has been reported in NSCLC,39 with only high-density CD4+/CD8+ stromal lymphocyte infiltration being an independent positive prognostic indicator for patients with resected NSCLC. This suggests that these cells mediated a strong antitumor immune response, but this study used tissue microassays for the analyses.6 We believe that discrimination between stromal and epithelial infiltration may add more confusion than precision due to lack of interobserver reproducibility, and from a pathology viewpoint, it adds little because by definition, the tumor cell environment includes stroma, which includes penetrating blood vessels and inflammatory or immune cells.
The prognostic effect was not statistically different in ADC versus SCC. We noted a highly significant effect in the other NSCLC category. The risk of death in patients with intense infiltration decreased by 88% (HR, 0.12; 95% CI, 0.03 to 0.47) in the other compared with 38% (HR, 0.62; 95% CI, 0.45 to 0.85) in the SCC plus ADC group (P for interaction = .02). Although heterogeneous, the other category includes patients at high risk for early death, such as basaloid; sarcomatoid; and large-cell carcinoma, which is known to have the worst prognosis.40 This is reminiscent of Epstein-Barr virus–dependent large-cell carcinoma with high lymphocytic infiltration (so-called lymphoepithelial-like carcinoma32) where pathologic classification and prognosis are strongly correlated with the presence of heavy lymphocytic infiltration, irrespective of composition (CD8+ supposed).
The association of tumor cells with lymphocytes has led to the postulate that adaptive immunity maintains occult cancer in an equilibrium state. This concept, inferred from a mouse model,41 illustrates how immunity is able to control and shape cancer and delay malignant tumor progression.42 However, by forcing the selective evolution of malignant cells, tumors ultimately escape their attack through the immune system, a phenomenon called immune editing.43 Therefore, TLI may identify a sensitive therapeutic window before immune editing and tumor escape occur, where immunotherapy or immunomodulation may take place. Immune editing may take several forms, each common in lung cancer, such as mutations (epidermal growth factor receptor, P53); escape from CD8 cytolytic apoptosis (mitochondrial apoptosis dismissal, complement systems defects, death receptor ligands [fas/tumor necrosis factor–related apoptosis-inducing ligand inactivation]); raise of ligand-receptor checkpoints (CTLA4, PD1-PDL1); loss of HLA class 1 or 2 (major histocompatibility complex antigens); or ultimately, illegitimate expression of germ cell antigens (testis- and placenta-restricted antigens), in a context of immune escape.44 PDL1 mRNA expression has been shown to associate with increased TILs and better outcome in breast carcinoma.13
In contrast to previous breast cancer studies, the current international study performed to date in the largest NSCLC population (n = 1,546) randomly assigned to chemotherapy versus surgery alone (LACE), we did not observe any predictive effect of lymphocytic infiltration (no interaction between TLI and treatment). In a phase III adjuvant breast cancer trial in patients with node-positive disease randomly assigned on either anthracycline and anthracycline plus taxane arms, an interaction of lymphocytic infiltration and treatment with survival was found only in the subgroup of 297 patients positive for HER2 (P = .06), with a survival benefit for lymphocytic infiltration found only among the patients receiving single-agent anthracycline.14 In a neoadjuvant chemotherapy breast cancer cohort, high numbers of infiltrating CD4+ T cells after palliative chemotherapy correlated with clinical response.45
In summary, the current study shows that intense lymphocytic infiltration is an independent prognostic factor in patients with completely resected NSCLC but is not predictive of a differential survival benefit from adjuvant chemotherapy. The results raise the question about whether lymphocytic infiltration should be considered a stratification factor in trials that test immunotherapy or immunomodulation. Therefore, as suggested recently for CD8 density level in NSCLC, which predicted survival independently of all other variables and within each pathologic stage, intense lymphocytic infiltration could be a good candidate marker for establishing a TNM immunoscore.46
Acknowledgment
We thank Nicolas Lemaitre and Célia Barrachina for technical assistance.
Appendix
Fig A1.
Flowchart. Patients with missing slides correspond to patients whose blocks were not sent for histologic review. Patients with missing TLI correspond to patients with blocks of insufficient performance for quantitative histologic review and evaluation. ANITA, Adjuvant Navelbine International Trialist Association; CALGB, Cancer and Leukemia Group B; IALT, International Adjuvant Lung Cancer Trial; LACE-Bio, Lung Adjuvant Cisplatin Evaluation-Biomarker; SCLC, small-cell lung cancer; TLI, tumor lymphocytic infiltration.
Fig A2.
Survival curves for tumor lymphocytic infiltration (TLI; intense and nonintense) for specific disease-free survival (SDFS) on discovery (A) and validation (B) sets. The P value of the log-rank test and the unadjusted hazard ratios (HRs) and 95% CIs are reported.
Fig A3.
Survival curves for tumor lymphocytic infiltration (TLI; intense and nonintense) for overall survival (OS; A and B), disease-free survival (DFS; C and D,) and specific disease-free survival (SDFS; E and F) on the combined set (discovery + validation) by treatment arm. The P value of the log-rank test and the unadjusted hazard ratios (HRs) and 95% CIs are reported. CT, chemotherapy.
Table A1.
Markers of Which the Prognostic and Predictive Values Have Been Evaluated on the IALT
| Family | Markers | Reference |
|---|---|---|
| Drug transporters | MRP1 MRP2 | Filipits M, et al: Clin Cancer Res 13:3892-3898, 2007 |
| Apoptosis I | P53 Bax Bcl2 | Brambilla E, et al: Lung Cancer 49:S10-S11, 2005 (suppl 2) |
| Apoptosis II | Fas FasL | Brambilla E, et al: J Thorac Oncol 2:S444-S445, 2007 (suppl 4) |
| Cell cycle regulators | Cyclin D1 Cyclin D3 Cyclin E P16 P27 Ki67 | Filipits M, et al: J Clin Oncol 25:2735-2740, 2007 |
| Telomerase/telomere-related proteins and DNA repair enzymes | ERCC1 | Olaussen KA, et al: N Engl J Med 355:983-991, 2006 Besse B, et al: Ann Oncol 22:575-581, 2011 |
| Mutation | TP53 KRAS | Ma X, et al: Ann Oncol 19:viii61-viii62, 2008 (suppl 8) |
| Tissue microarrays | MSH2 PARP1 | Olaussen KA, et al: Lung Cancer 80:216-222, 2013 |
Abbreviation: IALT, International Adjuvant Lung Cancer Trial.
Table A2.
Characteristics Comparison of Patients in the Validation Set (n = 763) and Without Slides or With Missing Information on TLI (n = 220*)
| Characteristic | No. of Patients in Validation Set (%) | No. of Patients Without Slides or With Missing TLI (%) | P† |
|---|---|---|---|
| Sex | .70 | ||
| Male | 533 (70) | 144 (65) | |
| Female | 230 (30) | 76 (35) | |
| Age, years | .62 | ||
| < 55 | 209 (27) | 65 (30) | |
| 55-64 | 289 (38) | 80 (36) | |
| > 64 | 265 (35) | 75 (34) | |
| Treatment | .33 | ||
| No chemotherapy | 385 (50) | 103 (47) | |
| Chemotherapy | 378 (50) | 117 (53) | |
| Stage | .57 | ||
| I | 451 (59) | 162 (75) | |
| II | 264 (35) | 47 (22) | |
| III | 44 (6) | 8 (4) | |
| Unknown | 4 | 3 | |
| N of TNM | .63 | ||
| N0 | 459 (61) | 164 (76) | |
| N1 | 260 (34) | 47 (22) | |
| N2 | 38 (5) | 5 (2) | |
| Unknown | 6 | 4 | |
| T of TNM | .01 | ||
| T1 | 61 (8) | 23 (11) | |
| T2 | 677 (89) | 192 (88) | |
| T3, 4 | 21 (3) | 2 (1) | |
| Unknown | 4 | 3 | |
| Type of surgery | .98 | ||
| Lobectomy/other | 590 (78) | 179 (83) | |
| Pneumonectomy | 171 (22) | 37 (17) | |
| Unknown | 2 | 4 | |
| WHO PS | .31 | ||
| 0 | 385 (51) | 109 (50) | |
| 1, 2 | 373 (49) | 108 (50) | |
| Unknown | 5 | 3 | |
| Histology | < .001 | ||
| Squamous cell carcinoma | 285 (37) | 70 (33) | |
| Adenocarcinoma | 373 (49) | 91 (42) | |
| Other NSCLC‡ | 105 (14) | 54 (25) | |
| Unknown | 0 | 5 |
Abbreviations: NSCLC, non–small-cell lung cancer; PS, performance status; TLI, tumor lymphocytic infiltration.
The validation set included 763 patients from ANITA (Adjuvant Navelbine International Trialist Association), CALGB (Cancer and Leukemia Group B), and JBR10 with a nonmissing TLI, and 220 patients were excluded due to missing slides (n = 164) or missing TLI (n = 56).
Statistical test was calculated from a logistic regression model stratified by trial that excluded patients with missing values in the corresponding analysis.
Other NSCLC included large-cell, adenosquamous, sarcomatoid, basaloid, and unclassifiable NSCLC.
Table A3.
Association Between Tumor Lymphocytic Infiltration and Covariates on the Discovery Set (n = 783)
| Tumor Lymphocytic Infiltration | |||
|---|---|---|---|
| Characteristic | Nonintense (n = 697), No. (%) | Intense (n = 86), No. (%) | P* |
| Sex | |||
| Male | 570 (89) | 68 (11) | |
| Female | 127 (88) | 18 (12) | |
| Age, years | |||
| < 55 | 216 (91) | 21 (9) | |
| 55-64 | 299 (87) | 44 (13) | |
| > 64 | 182 (90) | 21 (10) | |
| Stage | .014 | ||
| I | 238 (88) | 33 (12) | |
| II | 234 (86) | 39 (14) | |
| III | 225 (94) | 14 (6) | |
| N of TNM | |||
| N0 | 327 (89) | 40 (11) | |
| N1 | 188 (84) | 35 (16) | |
| N2 | 182 (94) | 11 (6) | |
| T of TNM | |||
| T1 | 94 (79) | 25 (21) | |
| T2 | 416 (89) | 50 (11) | |
| T3 | 187 (94) | 11 (6) | |
| Type of surgery | .177 | ||
| Lobectomy/other | 409 (87) | 60 (13) | |
| Pneumonectomy | 288 (92) | 26 (8) | |
| WHO PS | |||
| 0 | 394 (90) | 46 (10) | |
| ≥ 1 | 303 (88) | 40 (12) | |
| Histology | |||
| Squamous cell carcinoma | 371 (88) | 51 (12) | |
| Adenocarcinoma | 239 (92) | 22 (8) | |
| Other NSCLC† | 87 (87) | 13 (13) | |
Abbreviations: NSCLC, non–small-cell lung cancer; PS, performance status; TLI, tumor lymphocytic infiltration.
Adjusted P value was computed by using a likelihood ratio test from a multivariable logistic regression model. Patients with missing covariate data were excluded. The selection variable process was a univariable analysis with P < .20. N and T of TNM stage, correlated with stage, were not included in the selection process.
Other NSCLC included large-cell, adenosquamous, sarcomatoid, basaloid, and unclassifiable NSCLC.
Table A4.
Association Between Tumor Lymphocytic Infiltration and Covariates on the Validation Set (n = 763)
| Tumor Lymphocytic Infiltration | |||
|---|---|---|---|
| Characteristic | Nonintense (n = 714), No. (%) | Intense (n = 49), No. (%) | P* |
| Sex | |||
| Male | 501 (94) | 32 (6) | |
| Female | 213 (93) | 17 (7) | |
| Age, years | |||
| < 55 | 197 (94) | 12 (6) | |
| 55-64 | 269 (93) | 20 (7) | |
| > 64 | 248 (94) | 17 (6) | |
| Stage | |||
| I | 421 (93) | 30 (7) | |
| II | 246 (93) | 18 (7) | |
| III | 43 (98) | 1 (2) | |
| Unknown | 4 | 0 | |
| N of TNM | |||
| N0 | 429 (93) | 30 (7) | |
| N1 | 241 (93) | 19 (7) | |
| N2 | 38 (100) | 0 (0) | |
| Unknown | 6 | 0 | |
| T of TNM | |||
| T1 | 57 (93) | 4 (7) | |
| T2 | 633 (94) | 44 (7) | |
| T3 | 20 (95) | 1 (5) | |
| Unknown | 4 | 0 | |
| Type of surgery | |||
| Lobectomy/other | 550 (93) | 40 (7) | |
| Pneumonectomy | 162 (95) | 9 (5) | |
| Unknown | 2 | 0 | |
| WHO PS | |||
| 0 | 362 (94) | 23 (6) | |
| ≥ 1 | 347 (93) | 26 (7) | |
| Unknown | 5 | 0 | |
| Histology | < .001 | ||
| Squamous cell carcinoma | 255 (89) | 30 (11) | |
| Adenocarcinoma | 357 (96) | 16 (4) | |
| Other NSCLC† | 102 (97) | 3 (3) | |
Abbreviations: NSCLC, non–small-cell lung cancer; PS, performance status.
Adjusted P value was computed by using a likelihood ratio test from a multivariable logistic regression model stratified by trial. Patients with missing covariate data were excluded. The selection variable process was a univariable analysis with P < .20. N and T of TNM stage, correlated with stage, were not included in the selection process.
Other NSCLC included large-cell, adenosquamous, sarcomatoid, basaloid, and unclassifiable NSCLC.
Table A5.
Prognostic Value of Tumor Lymphocytic Infiltration for Overall Survival and Disease-Free Survival Estimated From a Multivariable Cox Model on the Discovery Set (n = 783)
| Overall Survival | Disease-Free Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Deaths/No. of Patients | HR | 95% CI | P | Events/No. of Patients | HR | 95% CI | P |
| Age, years | .29 | .51 | ||||||
| < 55 | 112/237 | 1.00 | 0.93 to 1.49 | 130/237 | 1.00 | 0.84 to 1.32 | ||
| 55-64 | 182/343 | 1.18 | 0.93 to 1.59 | 197/343 | 1.06 | 0.90 to 1.48 | ||
| ≥ 65 | 115/203 | 1.22 | 129/203 | 1.16 | ||||
| Sex | .007 | .03 | ||||||
| Male | 350/638 | 1.00 | 0.51 to 0.90 | 382/638 | 1.00 | 0.57 to 0.97 | ||
| Female | 59/145 | 0.67 | 74/145 | 0.75 | ||||
| WHO PS | .22 | .04 | ||||||
| 0 | 219/440 | 1.00 | 0.93 to 1.39 | 241/440 | 1.00 | 1.01 to 1.48 | ||
| ≥ 1 | 190/343 | 1.14 | 215/343 | 1.22 | ||||
| Tumor stage | < .001 | < .001 | ||||||
| I | 94/271 | 1.00 | 1.46 to 2.50 | 111/271 | 1.00 | 1.48 to 2.46 | ||
| II | 150/273 | 1.91 | 2.42 to 4.19 | 169/273 | 1.91 | 2.29 to 3.86 | ||
| III | 165/239 | 3.18 | 176/239 | 2.97 | ||||
| Type of surgery | .96 | 0.79 to 1.21 | .85 | |||||
| Pneumonectomy | 184/314 | 1.00 | 0.80 to 1.24 | 202/314 | 1.00 | |||
| Other | 225/469 | 0.99 | 254/469 | 0.98 | ||||
| Treatment arm | 0.77 to 1.14 | .51 | 0.75 to 1.08 | .24 | ||||
| No chemotherapy | 200/382 | 1.00 | 227/382 | 1.00 | ||||
| Chemotherapy | 209/401 | 0.94 | 229/401 | 0.90 | ||||
| Histology | .02 | .002 | ||||||
| Squamous cell carcinoma | 221/422 | 1.00 | 0.93 to 1.51 | 238/422 | 1.00 | 1.12 to 1.76 | ||
| Adenocarcinoma | 129/261 | 1.19 | 1.11 to 2.00 | 155/261 | 1.41 | 1.14 to 2.02 | ||
| Other NSCLC* | 59/100 | 1.49 | 63/100 | 1.52 | ||||
| Tumor lymphocytic infiltration | 0.39 to 0.81 | .002 | 0.42 to 0.83 | .002 | ||||
| Nonintense/no infiltration | 377/697 | 1.00 | 419/697 | 1.00 | ||||
| Intense infiltration | 32/86 | 0.56 | 37/86 | 0.59 | ||||
Abbreviations: HR, hazard ratio; NSCLC, non–small-cell lung cancer; PS, performance status.
Other NSCLC included large-cell, adenosquamous, sarcomatoid, basaloid, and unclassifiable NSCLC.
Table A6.
Prognostic Value of Tumor Lymphocytic Infiltration for Specific Disease-Free Survival Estimated From a Multivariable Cox Model on the Discovery Set (n = 783)
| Specific Disease-Free Survival | ||||
|---|---|---|---|---|
| Variable | Events/No. of Patients | HR | 95% CI | P |
| Age, years | .95 | |||
| < 55 | 123/237 | 1.00 | 0.77 to 1.23 | |
| 55-64 | 169/343 | 0.97 | 0.77 to 1.31 | |
| ≥ 65 | 106/203 | 1.01 | ||
| Sex | .03 | |||
| Male | 331/638 | 1.00 | 0.56 to 0.98 | |
| Female | 67/145 | 0.74 | ||
| WHO PS | 0.97 to 1.46 | .09 | ||
| 0 | 215/440 | 1.00 | ||
| ≥ 1 | 183/343 | 1.19 | ||
| Tumor stage | < .001 | |||
| I | 97/271 | 1.00 | 1.38 to 2.39 | |
| II | 137/273 | 1.82 | 2.49 to 4.31 | |
| III | 164/239 | 3.27 | ||
| Type of surgery | 0.85 to 1.33 | .60 | ||
| Pneumonectomy | 169/314 | 1.00 | ||
| Other | 229/469 | 1.06 | ||
| Treatment arm | 0.73 to 1.08 | .22 | ||
| No chemotherapy | 201/382 | 1.00 | ||
| Chemotherapy | 197/401 | 0.89 | ||
| Histology | < .001 | |||
| Squamous cell carcinoma | 199/422 | 1.00 | 1.17 to 1.89 | |
| Adenocarcinoma | 142/261 | 1.48 | 1.12 to 2.21 | |
| Other NSCLC* | 57/100 | 1.63 | ||
| Tumor lymphocytic infiltration | 0.38 to 0.82 | .003 | ||
| Nonintense/no infiltration | 368/697 | 1.00 | ||
| Intense infiltration | 30/86 | 0.56 | ||
NOTE: Similar results were observed with a Cox model stratified on covariates that violate the hypothesis of proportional hazards.
Abbreviations: HR, hazard ratio; NSCLC, non–small-cell lung cancer; PS, performance status.
Other NSCLC included large-cell, adenosquamous, sarcomatoid, basaloid, and unclassifiable NSCLC.
Table A7.
Prognostic Value of Tumor Lymphocytic Infiltration for Overall Survival and Disease-Free Survival Estimated From a Multivariable Cox Model on the Validation Set (n = 753*)
| Overall Survival | Disease-Free Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| center | Deaths/No. of Patients | HR | 95% CI | P | Events/No. of Patients | HR | 95% CI | P |
| Age, years | .14 | .51 | ||||||
| < 55 | 85/208 | 1.00 | 0.84 to 1.47 | 101/208 | 1.00 | 0.84 to 1.41 | ||
| 55-64 | 129/288 | 1.11 | 0.99 to 1.76 | 151/288 | 1.09 | 0.90 to 1.53 | ||
| ≥ 65 | 125/257 | 1.32 | 138/257 | 1.17 | ||||
| Sex | 0.48 to 0.82 | < .001 | 0.55 to 0.89 | .004 | ||||
| Male | 262/524 | 1.00 | 296/524 | 1.00 | ||||
| Female | 77/229 | 0.62 | 94/229 | 0.70 | ||||
| WHO PS | .16 | 0.94 to 1.41 | .16 | |||||
| 0 | 158/381 | 1.00 | 1.00 | 183/381 | 1.00 | |||
| ≥ 1 | 181/372 | 1.17 | 0.94 to 1.45 | 207/372 | 1.15 | |||
| Tumor stage | < .001 | < .001 | ||||||
| I | 180/446 | 1.00 | 1.16 to 2.02 | 205/446 | 1.00 | 1.17 to 1.94 | ||
| II | 126/263 | 1.53 | 1.47 to 3.95 | 147/263 | 1.51 | 1.31 to 3.21 | ||
| III | 33/44 | 2.41 | 38/44 | 2.05 | ||||
| Type of surgery | 0.57 to 0.99 | .05 | 0.59 to 0.99 | .04 | ||||
| Pneumonectomy | 91/170 | 1.00 | 104/170 | 1.00 | ||||
| Other | 248/583 | 0.76 | 286/583 | 0.76 | ||||
| Treatment arm | 0.67 to 1.03 | .09 | 0.65 to 0.97 | .02 | ||||
| No chemotherapy | 185/380 | 1.00 | 211/380 | 1.00 | ||||
| Chemotherapy | 154/373 | 0.83 | 179/373 | 0.79 | ||||
| Histology | .005 | .007 | ||||||
| Squamous cell carcinoma | 119/283 | 1.00 | 1.12 to 1.91 | 139/283 | 1.00 | 1.14 to 1.87 | ||
| Adenocarcinoma | 168/367 | 1.46 | 1.15 to 2.27 | 195/367 | 1.47 | 1.03 to 1.96 | ||
| Other NSCLC† | 52/103 | 1.62 | 56/103 | 1.42 | ||||
| Tumor lymphocytic infiltration | 0.23 to 0.85 | .014 | 0.24 to 0.78 | .005 | ||||
| Nonintense/no infiltration | 329/704 | 1.00 | 378/704 | 1.00 | ||||
| Intense infiltration | 10/49 | 0.45 | 12/49 | 0.44 | ||||
Abbreviations: HR, hazard ratio; NSCLC, non–small-cell lung cancer; PS, performance status.
Ten patients with missing type of surgery (n = 2), stage (n = 4), or PS (n = 5) were excluded from the multivariable analyses.
Other NSCLC included large-cell, adenosquamous, sarcomatoid, basaloid, and unclassifiable NSCLC.
Table A8.
Prognostic Value of Tumor Lymphocytic Infiltration for Specific Disease-Free Survival Estimated From a Multivariable Cox Model on Validation set (n = 753*)
| Specific Disease-Free Survival | ||||
|---|---|---|---|---|
| Variable | Events/No. of Patients | HR | 95% CI | P |
| Age, years | .70 | |||
| < 55 | 91/208 | 1.00 | 0.86 to 1.47 | |
| 55-64 | 139/288 | 1.12 | 0.80 to 1.43 | |
| ≥ 65 | 111/257 | 1.07 | ||
| Sex | 0.58 to 0.98 | .03 | ||
| Male | 253/524 | 1.00 | ||
| Female | 88/229 | 0.75 | ||
| WHO PS | 0.91 to 1.40 | .27 | ||
| 0 | 162/381 | 1.00 | ||
| ≥ 1 | 179/372 | 1.13 | ||
| Tumor stage | < .001 | |||
| I | 173/446 | 1.00 | 1.28 to 2.19 | |
| II | 135/263 | 1.67 | 1.23 to 3.21 | |
| III | 33/44 | 1.90 | ||
| Type of surgery | 0.56 to 0.96 | .27 | ||
| Pneumonectomy | 92/170 | 1.00 | ||
| Other | 249/583 | 0.73 | ||
| Treatment arm | 0.60 to 0.92 | .007 | ||
| No chemotherapy | 190/380 | 1.00 | ||
| Chemotherapy | 151/373 | 0.74 | ||
| Histology | .001 | |||
| Squamous cell carcinoma | 112/283 | 1.00 | 1.24 to 2.11 | |
| Adenocarcinoma | 177/367 | 1.62 | 1.14 to 2.24 | |
| Other NSCLC† | 52/103 | 1.59 | ||
| Tumor lymphocytic infiltration | 0.22 to 0.80 | .008 | ||
| Nonintense/no infiltration | 331/704 | 1.00 | ||
| Intense infiltration | 10/49 | 0.42 | ||
NOTE: The P value of heterogeneity across trials was .59. Similar results were observed with a Cox model stratified on covariates that violate the hypothesis of proportional hazards.
Abbreviations: HR, hazard ratio; NSCLC, non–small-cell lung cancer; PS, performance status.
Ten patients with missing type of surgery (n = 2), stage (n = 4), or PS (n = 5) were excluded from the multivariable analyses.
Other NSCLC included large-cell, adenosquamous, sarcomatoid, basaloid, and unclassifiable NSCLC.
Table A9.
Treatment Interaction With Tumor Lymphocytic Infiltration for Specific Disease-Free Survival Estimated From a Multivariable Cox Model on the Combined Data Set (n = 1,536*)
| Specific Disease-Free Survival | |||
|---|---|---|---|
| Tumor lymphocytic infiltration | CT Events/No. of Patients | Observation Events/No. of Patients | CT v No CT HR (95% CI) |
| Nonintense | 328/699 | 371/702 | 0.82 (0.71 to 0.95) |
| Intense | 20/75 | 20/60 | 0.75 (0.40 to 1.39) |
| Interaction test: P = .78 | |||
Abbreviations: CT, chemotherapy; HR, hazard ratio.
Ten patients were excluded from the analysis due to missing covariates.
Footnotes
Written on behalf of the LACE-Bio (Lung Adjuvant Cisplatin Evaluation Biomarker) Collaborative Group.
Supported by research grants from Ligue Nationale Contre le Cancer (France); le Programme National d’Excellence Specialisé cancer du poumon de l’Institut National du Cancer (France); National Cancer Institute (United States); Canadian Cancer Society Research Institute (Canada); an unrestricted grant from Sanofi; personal funding from the investigators; the Gustave Roussy Foundation; the Princess Margaret Cancer Foundation; and European contract EU-FP7 Curelung, Plateforme Detection Moléculaire In Situ of Centre Hospitalier Universitaire Grenoble, Délégation à la Recherche Clinique et à l’innovation.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Elisabeth Brambilla, Gwénaël Le Teuff, Ariane Dunant, Robert Pirker, Jean-Yves Douillard, Martin Filipits, Rafael Rosell, Robert Kratzke, Jean-Charles Soria, Frances A. Shepherd, Lesley Seymour, Ming Sound Tsao
Administrative support: Lesley Seymour
Provision of study materials or patients: Jean-Yves Douillard, Robert Kratzke, Jean-Charles Soria, Frances A. Shepherd, Lesley Seymour, Ming Sound Tsao
Collection and assembly of data: Elisabeth Brambilla, Gwénaël Le Teuff, Sylvie Lantuejoul, Ariane Dunant, Stephen Graziano, Robert Kratzke, Helmut Popper, Lesley Seymour, Ming Sound Tsao
Data analysis and interpretation: Elisabeth Brambilla, Gwénaël Le Teuff, Sophie Marguet, Ariane Dunant, Thierry Le Chevalier, Martin Filipits, Jean-Charles Soria, Frances A. Shepherd, Lesley Seymour, Ming Sound Tsao
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non–Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Elisabeth Brambilla
No relationship to disclose
Gwénaël Le Teuff
No relationship to disclose
Sophie Marguet
No relationship to disclose
Sylvie Lantuejoul
Consulting or Advisory Role: Pfizer, Novartis, Roche, Bristol-Myers Squibb, MSD, Boehringer Ingelheim
Research Funding: Pfizer
Ariane Dunant
No relationship to disclose
Stephen Graziano
Consulting or Advisory Role: Helsinn
Robert Pirker
Consulting or Advisory Role: Boehringer Ingelheim, Synta, Merck, Bristol-Myers Squibb, AstraZeneca, Pfizer
Speakers’ Bureau: Pierre Fabre, Eli Lilly, Boehringer Ingelheim, AstraZeneca
Jean-Yves Douillard
No relationship to disclose
Thierry Le Chevalier
No relationship to disclose
Martin Filipits
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme
Speakers’ Bureau: AstraZeneca, Novartis, Pfizer, Roche, Merck Sharp & Dohme
Travel, Accommodations, Expenses: Novartis, Roche
Rafael Rosell
No relationship to disclose
Robert Kratzke
No relationship to disclose
Helmut Popper
Consulting or Advisory Role: Novartis, AstraZeneca, Boehringer Ingelheim, Pfizer, Hoffmann-La Roche, Bristol-Myers Squibb
Research Funding: Unrestricted research grants from Roche, Boehringer Ingelheim, AstraZeneca, Eli Lilly
Jean-Charles Soria
No relationship to disclose
Frances A. Shepherd
No relationship to disclose
Lesley Seymour
Stock or Other Ownership: AstraZeneca
Honoraria: Innate Pharma
Consulting or Advisory Role: Boehringer Ingelheim
Research Funding: Pfizer (Inst), AstraZeneca (Inst), Astex Pharmaceuticals (Inst), Innate Pharma (Inst)
Ming Sound Tsao
Consulting or Advisory Role: AstraZeneca/MedImmune, Roche/Genentech, Merck, Pfizer
Research Funding: Pfizer (Inst)
REFERENCES
- 1.Kataki A, Scheid P, Piet M, et al. Tumor infiltrating lymphocytes and macrophages have a potential dual role in lung cancer by supporting both host-defense and tumor progression. J Lab Clin Med. 2002;140:320–328. doi: 10.1067/mlc.2002.128317. [DOI] [PubMed] [Google Scholar]
- 2.Ruffini E, Asioli S, Filosso PL, et al. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009;87:365–371. doi: 10.1016/j.athoracsur.2008.10.067. discussion 371-372. [DOI] [PubMed] [Google Scholar]
- 3.Wakabayashi O, Yamazaki K, Oizumi S, et al. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94:1003–1009. doi: 10.1111/j.1349-7006.2003.tb01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson SK, Kerr KM, Chapman AD, et al. Immune cell infiltrates and prognosis in primary carcinoma of the lung. Lung Cancer. 2000;27:27–35. doi: 10.1016/s0169-5002(99)00095-1. [DOI] [PubMed] [Google Scholar]
- 5.Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gooden MJM, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieu-Nosjean M-C, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu K, Nakata M, Hirami Y, et al. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol. 2010;5:585–590. doi: 10.1097/JTO.0b013e3181d60fd7. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 10.Horne ZD, Jack R, Gray ZT, et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. 2011;171:1–5. doi: 10.1016/j.jss.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 11.Kilic A, Landreneau RJ, Luketich JD, et al. Density of tumor-infiltrating lymphocytes correlates with disease recurrence and survival in patients with large non-small-cell lung cancer tumors. J Surg Res. 2011;167:207–210. doi: 10.1016/j.jss.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Schalper KA, Brown J, Carvajal-Hausdorf D, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst. 2015;107:dju435. doi: 10.1093/jnci/dju435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schalper KA, Velcheti V, Carvajal D, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20:2773–2782. doi: 10.1158/1078-0432.CCR-13-2702. [DOI] [PubMed] [Google Scholar]
- 14.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 15.Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 16.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann Oncol. 2014;25:1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 18.Di Caro G, Bergomas F, Grizzi F, et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res. 2014;20:2147–2158. doi: 10.1158/1078-0432.CCR-13-2590. [DOI] [PubMed] [Google Scholar]
- 19.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 20.Nedergaard BS, Ladekarl M, Nyengaard JR, et al. A comparative study of the cellular immune response in patients with stage IB cervical squamous cell carcinoma. Low numbers of several immune cell subtypes are strongly associated with relapse of disease within 5 years. Gynecol Oncol. 2008;108:106–111. doi: 10.1016/j.ygyno.2007.08.089. [DOI] [PubMed] [Google Scholar]
- 21.Piersma SJ, Jordanova ES, van Poelgeest MIE, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 22.Shi J-Y, Gao Q, Wang Z-C, et al. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res. 2013;19:5994–6005. doi: 10.1158/1078-0432.CCR-12-3497. [DOI] [PubMed] [Google Scholar]
- 23.Fukunaga A, Miyamoto M, Cho Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Schumacher K, Haensch W, Röefzaad C, et al. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 25.Pignon J-P, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 26.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 27.Arriagada R, Dunant A, Pignon J-P, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28:35–42. doi: 10.1200/JCO.2009.23.2272. [DOI] [PubMed] [Google Scholar]
- 28.Douillard J-Y, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 29.Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: Updated survival analysis of JBR-10. J Clin Oncol. 2010;28:29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 31.Strauss GM, Herndon JE, II, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Travis WD, Brambilla E, Burke A, et al. WHO Classification of the Tumours of the Lung, Pleura, Thymus and Heart. IARC Press; 2015. (ed 4). Lyon, France, IARC Press. [DOI] [PubMed] [Google Scholar]
- 33.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 35.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 36.Collins GS, de Groot JA, Dutton S, et al. External validation of multivariable prediction models: A systematic review of methodological conduct and reporting. BMC Med Res Methodol. 2014;14:40. doi: 10.1186/1471-2288-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK) Br J Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki K, Kachala SS, Kadota K, et al. Prognostic immune markers in non-small cell lung cancer. Clin Cancer Res. 2011;17:5247–5256. doi: 10.1158/1078-0432.CCR-10-2805. [DOI] [PubMed] [Google Scholar]
- 39.Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 40.Howlader N, Noone A, Krapcho M, et al. (eds): SEER Cancer Statistics Review, 1975-2010. Bethesda, MD, National Cancer Institute, 2013. http://seer.cancer.gov/csr/1975_2010.
- 41.Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 42.DuPage M, Cheung AF, Mazumdar C, et al. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell. 2011;19:72–85. doi: 10.1016/j.ccr.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 44.Rousseaux S, Debernardi A, Jacquiau B, et al. Ectopic activation of germline and placental genes identifies aggressive metastasis-prone lung cancers. Sci Transl Med. 2013;5:186ra66. doi: 10.1126/scitranslmed.3005723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Péguillet I, Milder M, Louis D, et al. High numbers of differentiated effector CD4 T cells are found in patients with cancer and correlate with clinical response after neoadjuvant therapy of breast cancer. Cancer Res. 2014;74:2204–2216. doi: 10.1158/0008-5472.CAN-13-2269. [DOI] [PubMed] [Google Scholar]
- 46.Donnem T, Hald SM, Paulsen EE, et al. Stromal CD8+ T-cell density: A promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res 21:2635-2643, 2015. [DOI] [PubMed]