Abstract
Purpose
Most malignancies are diagnosed in older adults who are potentially susceptible to aging-related health conditions; however, the manifestation of geriatric syndromes during surgical cancer treatment is not well quantified. Accordingly, we sought to assess the prevalence and ramifications of geriatric events during major surgery for cancer.
Patients and Methods
Using Nationwide Inpatient Sample data from 2009 to 2011, we examined hospital admissions for major cancer surgery among elderly patients (ie, age ≥ 65 years) and a referent group age 55 to 64 years. From these observations, we identified geriatric events that included delirium, dehydration, falls and fractures, failure to thrive, and pressure ulcers. We then estimated the collective prevalence of these events according to age, comorbidity, and cancer site and further explored their relationship with other hospital-based outcomes.
Results
Within a weighted sample of 939,150 patients, we identified at least one event in 9.2% of patients. Geriatric events were most common among patients age ≥ 75 years, with a Charlson comorbidity score ≥ 2, and who were undergoing surgery for cancer of the bladder, ovary, colon and/or rectum, pancreas, or stomach (P < .001). Adjusting for patient and hospital characteristics, those patients who experienced a geriatric event had a greater likelihood of concurrent complications (odds ratio [OR], 3.73; 95% CI, 3.55 to 3.92), prolonged hospitalization (OR, 5.47; 95% CI, 5.16 to 5.80), incurring high cost (OR, 4.97; 95% CI, 4.58 to 5.39), inpatient mortality (OR, 3.22; 95% CI, 2.94 to 3.53), and a discharge disposition other than home (OR, 3.64; 95% CI, 3.46 to 3.84).
Conclusion
Many older patients who receive cancer-directed surgery experience a geriatric event, particularly those who undergo major abdominal surgery. These events are linked to operative morbidity, prolonged hospitalization, and more expensive health care. As our population ages, efforts focused on addressing conditions and complications that are more common in older adults will be essential to delivering high-quality cancer care.
INTRODUCTION
The aging population—along with improved health care access and prolonged cancer survivorship—is expected to intensify the cancer burden of our nation during the coming decades.1 On the basis of current projections, the number of older adults age ≥ 65 years will double by midcentury, leading to a nearly 50% increase in the annual cancer incidence.2 By that time, seniors will account for the majority of US patients with cancer.3 Even now, more than one half of new cancer cases in the United States come from this segment of the population.4
Given these epidemiologic trends, cancer will firmly become a disease of the elderly. Whereas cure remains an achievable priority, age-related health concerns can complicate the course of care for older adults. Functional decline, cognitive disorders, frailty, comorbidities, malnutrition, and polypharmacy are common within the elderly population—especially for those with cancer—and have been associated with worsening morbidity, toxicity, and intolerance during cancer treatment.5-9 To better meet these patient needs, multiple organizations have called for greater incorporation of gerontologic principles into cancer care via practice guidelines or interdisciplinary care models.10-12 Recent collaborations between surgical and geriatric societies have also produced guidelines for the preoperative assessment of elderly adults and the management of postoperative delirium.13,14 Such initiatives ask providers and health care systems to devote more time and resources to screening and managing older adults who are potentially at risk for adverse events; however, the extent and magnitude of age-related conditions have not been systematically evaluated on a national level.
In this context, there is an immediate need to better understand the burden of geriatric events among elderly patients who undergo major surgery for cancer. Using a national sample, we examined the presence of dehydration, delirium, falls and fractures, failure to thrive, and pressure ulcers after surgery for the 10 most common solid-organ malignancies in older adults, and explored the potential association with other patient outcomes. In doing so, we identify cancer populations in need of a more integrated, geriatric-based approach to cancer care.
PATIENTS AND METHODS
Data Source
To assess the burden of geriatric events during major cancer surgery, we used the Nationwide Inpatient Sample (NIS) from 2009 to 2011 as provided by the Healthcare Cost and Utilization Project and the Agency for Healthcare Research and Quality (AHRQ). The NIS includes 20% of US inpatient hospitalizations from nonfederal, community hospitals stratified by facility bed size, location, control and/or ownership, teaching status, and region. Abstracted from discharge data, this sample draws from more than 40 states and represents 97% of the US population. The sample also includes established weights to standardize to the general population as well as information on patient demographics, hospital characteristics, discharge diagnoses and procedures, and hospital charges.15 This study was approved by the Institutional Review Board at the University of California, Los Angeles.
Case Selection, Patient Demographics, and Hospital Characteristics
To focus on the older population, we created a sample consisting of adults age ≥ 65 along with a referent group of adults age 55 to 64 years. We then selected surgical admissions for the 10 most common solid-organ malignancies in the United States—on the basis of age-specific cancer incidence rates4—by identifying cases with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) code for cancer of the bladder, breast, colon and/or rectum, endometrium, kidney, lung, ovary, pancreas, prostate, or stomach, along with a corresponding procedure code for surgical resection (Appendix Table A1, online only). In total, we identified an unweighted cohort of 190,014 patients who underwent major surgery for treatment of cancer.
On the basis of the information provided in NIS, we identified patient age, gender, and race/ethnicity for each observation. We assessed pre-existing comorbidity by using a modification of the Charlson comorbidity index, socioeconomic position on the basis of median ZIP code income, and admission acuity (elective v urgent or emergent hospitalization).16,17
In addition, we used established methodology to identify minimally invasive surgery for patients undergoing nephrectomy, cystectomy, colorectal surgery, gastrectomy, lung surgery, prostatectomy, oophorectomy, or hysterectomy.18,19 We also distinguished organ-sparing techniques for patients undergoing surgery for cancer of the kidney, bladder, lung, pancreas, ovary, or stomach. To control for hospital characteristics that can influence outcomes,20,21 we used hospital-level measures related to bed size, location, control and/or ownership, teaching status, and region. Finally, we determined the annual procedure-specific volume for each hospital, stratified into four equally sized quartiles.
Primary Outcome Measures
To reflect the scope of health concerns pertinent to the geriatric population, we developed a composite measure for geriatric events on the basis of ICD-9 codes that indicate dehydration (ie, 276.5x), delirium (ie, 290.11, 290.3, 290.41, 291.0, 292.81, 293.0, 293.1, 348.31, 349.82), falls and fractures (ie, 800–829, E880–E888), failure to thrive (ie, 783.2x, 783.7, 260–263), and pressure ulcers (ie, 707.0x, 707.2x, 707.8, 707.9).22-24 Our claims-based definition focuses on events that tend to be acute, incorporates the AHRQ Patient Safety Indicators for pressure ulcer and hip fractures, and consists of codes used previously to examine geriatric conditions in administrative data.22-27
Secondary Outcome Measures
To assess the potential impact of geriatric events, we examined several additional outcomes: inpatient complications; length of stay; hospitalization costs; and disposition status. As described previously,28 we drew from the Complication Screening Program and AHRQ Patient Safety Indicators and identified inpatient complications by using specific ICD-9 codes for accidental puncture or laceration, acute renal failure, cardiac complications, gastrointestinal complications, genitourinary complications, neurologic events, postoperative hemorrhage, postoperative infection (eg, pneumonia and Clostridium difficile), pulmonary failure, sepsis, venous thromboembolism, wound complications, and miscellaneous complications.23,24,29-31 Because length of stay and expenditures vary by disease, we created indicator variables for hospitalizations in the top deciles of admission length and cost, the latter of which was estimated from total charges, cost-to-charge ratios, and adjustment factors as provided by Healthcare Cost and Utilization Project.32,33 Finally, disposition status was assessed in two ways. First, we monitored the occurrence of inpatient mortality during the hospital admission. Second, we determined if a patient expired or transferred to another facility versus discharged to home.
Statistical Analysis
Applying the appropriate sampling frame and weights, we obtained national estimates for our composite geriatric measure. Next, we compared patient demographics and hospital characteristics according to the occurrence of a geriatric event by using χ2 testing. We then examined the proportion of cases with a geriatric event for each cancer type with respect to age and age-adjusted comorbidity.
To further investigate the relationship between age and geriatric events, we fitted multivariable logistic regression models that adjusted for patient demographics (ie, gender, race/ethnicity, socioeconomic status, and admission acuity), cancer type, and hospital characteristics (ie, bed size, location, control and/or ownership, teaching status, region, and procedure volume). Holding these variables constant, we calculated the predicted probability of a geriatric event for patients age 55 to 64, 65 to 74, and ≥ 75. As some of our geriatric events could reflect pre-existing conditions, we repeated our analyses for patients who underwent elective surgery and those without baseline comorbidity. In addition, because such events may be related to disease severity, we estimated the probability of geriatric events for patients who underwent minimally invasive and/or organ-sparing surgery, which tend to be performed for less aggressive malignancies.
Next, as an exploratory analysis, we examined the relationship between geriatric events and our secondary outcome measures. For each outcome, we fit multivariable logistic regression models that adjusted for patient characteristic, cancer type, and hospital factors. We then determined the model-adjusted probability for each outcome according to exposure to a geriatric event.
Finally, to gauge the robustness of our findings, we performed several additional sensitivity analyses. First, we refitted our primary models for each specific cancer to gauge the consistency of our findings across cancer type. Second, we examined each component of our primary outcome measure separately to determine whether the relationships with age and our secondary outcomes persisted. Third, we fitted multilevel, mixed-effect models in the unweighted sample given the hierarchical nature of the dataset. Fourth, given measurable differences between patients with and without a geriatric event, we reassessed the relationship between geriatric events and our secondary outcomes using propensity score weighting. To do so, we weighted each observation by the inverse probability of a geriatric event, which was calculated by using logistic regression adjusted for our described covariates.
All statistical testing was two sided and completed using STATA software (STATA, College Station, TX; Computing Resource Center, Santa Monica, CA), and carried out at the 5% significance level.
RESULTS
From 2009 to 2011, we identified a weighted sample of 939,150 patients age ≥ 55 admitted to the hospital for major cancer surgery. Overall, 9.2% (95% CI, 8.8% to 9.7%) experienced at least one geriatric event during the inpatient hospitalization. Among those who experienced a geriatric event, specific conditions occurred as follows: nutrition-related events (failure to thrive and dehydration), 81.3% (95% CI, 80.2% to 82.3%); delirium, 17.1% (95% CI, 16.1% to 18.0%); and mobility-related events (pressure ulcers, falls, and fractures), 9.6% (95% CI, 9.0% to 10.2%). As detailed in Table 1, these events occurred more often among the very old, adults with greater comorbidity, and patients who underwent nonelective procedures (P < .001). Our aging-related conditions also seemed to be more common in nonteaching institutions (P < .001) and lower-volume centers (P < .001).
Table 1.
Patient and Hospital Characteristics Among Older Adults With and Without an Acute Geriatric Event in the Weighted Sample
| Characteristic | No Event, % (95% CI) (n = 852,473) | Any Event, % (95% CI) (n = 86,677)* | P |
|---|---|---|---|
| Age, years | |||
| 55-64 | 38.1 (37.4 to 38.8) | 22.1 (21.3 to 23.0) | < .001 |
| 65-74 | 37.0 (36.6 to 37.5) | 32.3 (31.5 to 33.1) | |
| ≥ 75 | 24.8 (24.1 to 25.5) | 45.6 (44.5 to 46.8) | |
| Race/ethnicity | |||
| White | 69.9 (67.3 to 72.4) | 69.0 (66.1 to 71.7) | .113 |
| Black | 8.5 (7.7 to 9.3) | 9.7 (8.8 to 10.8) | |
| Hispanic/Latino | 5.3 (4.5 to 6.2) | 5.0 (4.3 to 5.9) | |
| Asian | 2.1 (1.8 to 2.5) | 2.1 (1.7 to 2.5) | |
| Other/unknown | 14.2 (11.7 to 17.0) | 14.2 (11.4 to 17.5) | |
| Female sex | 49.1 (47.7 to 50.4) | 50.5 (49.4 to 51.7) | .044 |
| Income by zip code, quartile | |||
| Bottom | 22.9 (21.4 to 24.4) | 26.4 (24.9 to 28.1) | < .001 |
| 2nd | 24.9 (23.6 to 26.2) | 25.4 (24.0 to 26.8) | |
| 3rd | 25.6 (24.6 to 26.6) | 25.4 (24.3 to 26.6) | |
| Top | 26.7 (24.3 to 29.1) | 22.7 (20.6 to 25.0) | |
| Charlson comorbidity score | |||
| 0 | 46.1 (45.3 to 46.9) | 24.0 (23.1 to 24.9) | < .001 |
| 1 | 22.2 (21.8 to 22.6) | 19.2 (18.6 to 19.9) | |
| ≥ 2 | 31.7 (30.9 to 32.5) | 56.8 (55.8 to 57.8) | |
| Year | |||
| 2009 | 33.7 (30.1 to 37.4) | 30.9 (27.7 to 34.3) | .013 |
| 2010 | 32.5 (28.9 to 36.4) | 32.7 (29.6 to 35.9) | |
| 2011 | 33.8 (30.0 to 37.8) | 36.4 (32.9 to 40.2) | |
| Elective admission | 86.7 (85.4 to 87.8) | 58.6 (56.8 to 60.3) | < .001 |
| Hospital bed size | |||
| Small | 10.9 (8.8 to 13.5) | 9.4 (8.1 to 11.0) | .107 |
| Medium | 20.9 (18.7 to 23.3) | 22.1 (19.9 to 24.4) | |
| Large | 68.2 (65.1 to 71.1) | 68.5 (65.8 to 71.1) | |
| Hospital region | |||
| Northeast | 21.1 (18.0 to 24.5) | 18.0 (15.5 to 20.7) | .007 |
| Midwest | 23.6 (21.1 to 26.4) | 26.5 (23.5 to 29.6) | |
| South | 35.7 (32.7 to 38.8) | 36.4 (33.7 to 39.3) | |
| West | 19.6 (17.2 to 22.3) | 19.1 (17.0 to 21.4) | |
| Hospital control | |||
| Government | 10.4 (8.1 to 13.2) | 9.8 (7.7 to 12.5) | .121 |
| Private, nonprofit | 79.7 (76.4 to 82.7) | 79.0 (75.9 to 81.8) | |
| Private, invest | 9.9 (8.1 to 12.0) | 11.1 (9.4 to 13.2) | |
| Rural hospital | 7.4 (6.4 to 8.5) | 8.3 (7.2 to 9.5) | .038 |
| Nonteaching hospital | 40.2 (37.2 to 43.3) | 44.6 (41.6 to 47.6) | < .001 |
| Hospital patient volume, quartile | |||
| Bottom | 24.5 (22.7 to 26.5) | 27.4 (25.4 to 29.5) | < .001 |
| 2nd | 25.2 (23.3 to 27.2) | 27.1 (25.0 to 29.4) | |
| 3rd | 25.2 (23.2 to 27.3) | 25.0 (22.8 to 27.3) | |
| Top | 25.1 (21.3 to 29.3) | 20.5 (17.1 to 24.3) |
NOTE. Comparisons were performed by using χ2 testing carried out at the 5% significance level.
Geriatric event identified on the basis of International Classification of Diseases, Ninth Revision, Clinical Modification codes that indicate dehydration (276.5x), delirium (290.11, 290.3, 290.41, 291.0, 292.81, 293.0, 293.1, 348.31, 349.82), falls and fractures (800-829, E880-E888), failure to thrive (783.2x, 783.7, 260-263), and pressure ulcers (707.0x, 707.2x, 707.8, 707.9).
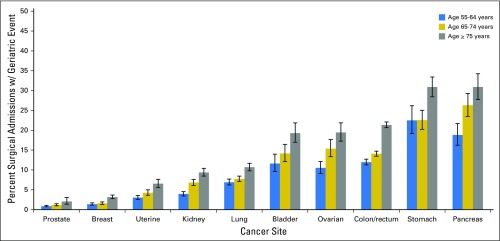
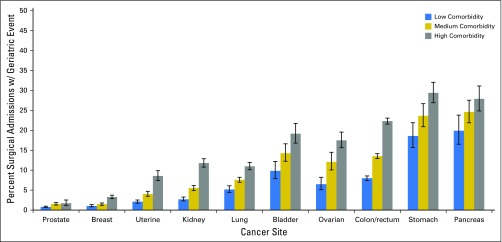
As highlighted in Figures 1 and 2, the occurrence of events varied significantly with cancer site, age, and comorbidity. Patients who underwent operations for prostate cancer (1.0%; 95% CI, 0.9% to 1.2%), breast cancer (2.0%; 95% CI, 1.8% to 2.2%), or endometrium cancer (4.2%; 95% CI, 3.7% to 4.7%) had the lowest proportion of patients with a geriatric event. Patients treated surgically for kidney cancer (6.2%; 95% CI, 5.7% to 6.7%) or lung cancer (8.4%; 95% CI, 7.8% to 9.0%) had moderate rates of geriatric events. Patients who underwent cancer-directed surgery for the following cancers had the highest proportion of geriatric events: ovary, 14.4% (95% CI, 12.9% to 15.9%); bladder, 15.3% (95% CI, 13.5% to 17.3%); colon and/or rectum, 16.6% (95% CI, 16.1% to 17.2%); pancreas, 25.2% (95% CI, 22.9% to 27.7%); and stomach, 25.5% (95% CI, 23.5% to 27.5%). Across all cancer sites, patients age ≥ 75 years and those with high comorbidity were significantly more likely to experience a geriatric event compared with their younger or healthier counterparts (P < .05). The prevalence of geriatric events by type, cancer site, and age is reported in Appendix Table A2 (online only).
Fig 1.
Proportion of patients with a geriatric event according to cancer site and age. Age stratified into three groups: age 55 to 64, age 65 to 74, and age ≥ 75 years. Proportions are derived from the number of patients with at least one geriatric event divided by the number of patients treated surgically. The association between geriatric events and age was assessed by using χ2 testing and found to be significant for all sites (P < .001).
Fig 2.
Proportion of patients with a geriatric event according to cancer site and age-adjusted Charlson score. Age-adjusted Charlson score stratified into low, medium, and high comorbidity terciles. Proportions are derived from the number of patients with at least one geriatric event divided by the number of patients treated surgically. The association between geriatric events and age-adjusted comorbidity was assessed by using χ2 testing. Significance was noted for all sites (P < .001).
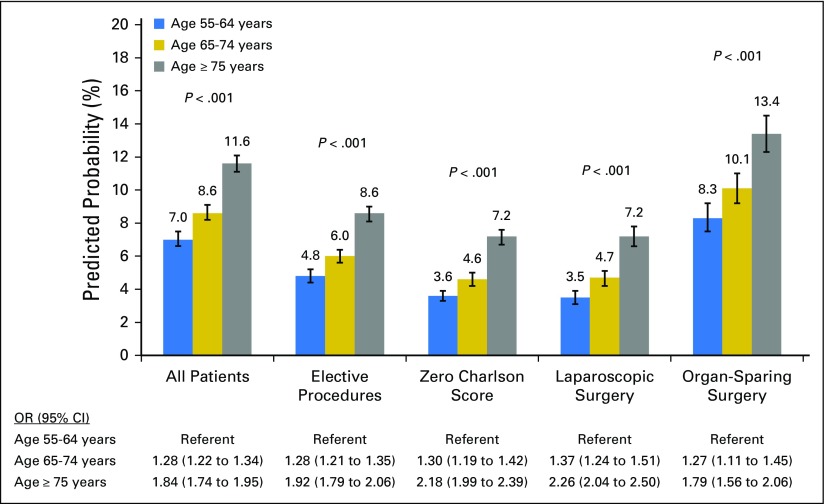
Adjusting for patient and hospital characteristics, the predicted probability of a geriatric event increased substantially with age strata; elderly patients (ie, age 65 to 74 years) and very elderly patients (ie, age ≥ 75 years) encountered a 22.9% and 65.7% higher probability of a geriatric event, respectively, compared with patients age 55 to 64 years. Whereas the likelihood of experiencing an event was lower among elective cases, laparoscopic surgeries, and patients without comorbidity, the relative probability of a geriatric event remained substantially higher for patients ages 65 to 74 and ≥ 75 years (Fig 3).
Fig 3.
Model-adjusted probability of a geriatric event according to age and patient population. Probabilities presented for the entire cohort, elective cases, patients with a Charlson score of 0, laparoscopic surgery, and organ-sparing surgery. Predicted probabilities are derived from multivariable logistic regression models that adjusted for patient demographics (ie, gender, race/ethnicity, socioeconomic status, and admission acuity), cancer type, and hospital characteristics (ie, bed size, location, control/ownership, teaching status, region, and procedure volume). OR, odds ratio.
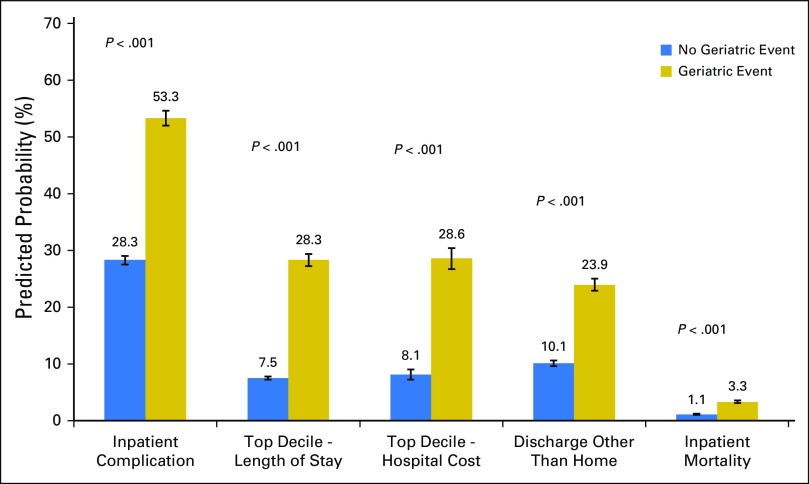
Finally, these events seem to be associated with other adverse outcomes. As depicted in Figure 4, patients with a geriatric event had a nearly two-fold higher probability of a concurrent inpatient complication compared with those without a geriatric event (odds ratio [OR], 3.73; 95% CI, 3.55 to 3.92). These events also seemed to be linked to prolonged hospitalization (OR, 5.47; 95% CI, 5.16 to 5.80) and high cost (OR, 4.97; 95% CI, 4.58 to 5.39). Furthermore, patients who experienced a geriatric event were less likely to be discharged to home (OR, 0.27; 95% CI, 0.26 to 0.29) and were more likely to die during the index hospitalization (OR, 3.22; 95% CI, 2.94 to 3.53) compared with patients who did not experience such events. These relationships remained significant across cancer type, for each specific event, and through our exploratory mixed-effect and propensity-weighted models.
Fig 4.
Model-adjusted probability of associated outcomes by the presence of a geriatric event. Reported outcomes include inpatient complications, prolonged hospitalization (top decile by cancer site), high cost (top decile by cancer site), inpatient mortality, and discharge disposition. Predicted probabilities are derived from multivariable, logistic regression models that adjusted for patient demographics (ie, gender, race/ethnicity, socioeconomic status, and admission acuity), cancer type, and hospital characteristics (ie, bed size, location, control/ownership, teaching status, region, and procedure volume).
DISCUSSION
The US population continues to grow older, bringing major change to the cancer landscape.2,10 In addition to increasing the number of patients with cancer, the aging population will push to the forefront a distinctive set of health concerns—those related to frailty, function, cognitive decline, malnutrition, and other syndromes. Even now, as defined in this study, these affect approximately one in 10 patients older than age 54 who undergo cancer surgery in the United States. With even higher rates observed among the very old (ie, patients age ≥ 75 years), the fastest growing segment of the US population,2 geriatric events during cancer-directed surgery are likely to become even more prevalent.
In addition to patient age, the risk for geriatric events seems to be driven by cancer site. Geriatric events occurred in 1.0% to 25.5% of surgical cases according to cancer location, with the highest frequency noted for cancers requiring major abdominal surgery. For these procedures, our estimates seem to be consistent with the published literature. Our observed rate of falls and fractures and pressure ulcers are in line with previous population-based estimates.34 Though failure to thrive and dehydration are reported infrequently, failure to thrive seems to be a leading reason for readmission to the hospital.35,36 With respect to delirium, published rates vary widely, ranging from 5% to 50%, depending on a multitude of parameters, including patient population, surgical indication, and performed procedure.14 The relative contribution from delirium noted here stands slightly below this range, likely as a result of under-reporting of delirium when using administrative data.25,37 Even so, the estimated prevalence of delirium is consistent with previous claims-based assessments and seems higher than that reported for orthopedic surgery.26 Taken together, geriatric events seem to be a major category of postoperative morbidity, especially for those who undergo abdominal cancer surgery.
Irrespective of cancer type, these age-related events seem to indicate worse surgical outcomes. Patients with dehydration, delirium, falls and fractures, failure to thrive, or pressure ulcers have a nearly two-fold greater probability of a concurrent medical or surgical complication. As observed with other types of complications,38,39 these events may also escalate resource use (ie, hospital and postacute care days) and hospitalization cost. Although we cannot infer causality (eg, geriatric events could occur as both an inciting or ensuing incident), specific interventions aimed at addressing geriatric needs during surgery have demonstrated reductions in delirium, medical complications, hospitalization length, and cost.40-44 Given their associated ramifications, geriatric events during cancer surgery need to be reduced, especially as health care systems aim to improve patient outcomes and curtail the rising cost of cancer care in the United States.10
These results should be considered in the context of several limitations. First, the use of administrative data to identify these events relies largely on coding accuracy. To the extent possible, we used either validated measures or diagnoses codes used previously in population-based assessments.22-26 These metrics have demonstrated higher levels of accuracy within surgical cohorts and when applying more current coding algorithms.27,29,31 Second, although our selected events are typically acute, they may, in some cases, represent pre-existing conditions rather than postsurgical events.27,45 However, our sensitivity analyses found similar results for our healthiest patients and elective cases where these selected conditions are unlikely to exist at baseline. Even in the event of misclassification, geriatric conditions present on admission likely carry similar ramifications for hospital care. Third, given the cross-sectional nature of our dataset, we are unable to assess longitudinal outcomes pertinent to the geriatric population, such as rehospitalization and functional recovery. It is worth noting, though, that skilled nursing facility use, which was greater among patients with a geriatric event, has been linked to an increased number of 30-day readmissions for patients who underwent major surgery involving the gastrointestinal tract.35,46 Fourth, and most important, as with any observational study, our findings remain subject to potential bias. In particular, the absence of cancer staging may lead to residual confounding. To offset this limitation, we examined the likelihood of geriatric events among patients who underwent laparoscopic and/or organ-sparing surgery—techniques typically reserved for less aggressive disease. Even within these subgroups, the likelihood of a geriatric event increased significantly with age, confirming the robustness of our analyses. As mentioned in the preceding paragraph, the relationship between geriatric events and our secondary outcomes may be bidirectional. Though our findings did not differ when using mixed-effect and propensity-weighted models, future studies will be needed to clarify the causal relationship between geriatric events and other clinical outcomes.
These limitations notwithstanding, our findings have important implications for surgical cancer care in the context of an aging population. As highlighted through recent guidelines and proposed care models,10-14 integrating geriatric care—comprehensive geriatric assessments, polypharmacy management, physical conditioning, social support—within oncologic practice may enhance quality by addressing health concerns that are frequently encountered by older adults. Whereas geriatric oncology programs or coordinated care teams with geriatricians may represent the most straightforward approach, access to such services may be limited, especially when considering the limited geriatrics-trained workforce.10,47 As such, it will be critical to develop core competency in geriatric medicine for surgical and medical oncologists and other members of the cancer care team. The Geriatrics-for-Specialists Initiative of the American Geriatric Society is one ongoing effort that aims to enhance the uptake and application of geriatric medicine by surgical and medical specialists.48 Among several milestones, this initiative has piloted geriatric education programs in select specialty residency programs.49 Though advanced training in medical oncology typically offers exposure to similar curricula, embedding geriatric education in oncology fellowship programs in gynecology, surgery, and urology may prove especially prudent. Along with other modalities (eg, practice guidelines and core competency tested on board certification),50 specialty cancer providers may be more empowered to care for these high-risk, elderly adults.
Although greater knowledge and comfort with geriatric concerns remain essential, certain patients with cancer who are at high risk may benefit from a more integrated, team-based approach. As highlighted in Figures 1 and 2, as many as one third of very old patients who undergo major abdominal surgery for treatment of cancer experience a geriatric event. For these patients, comanagement models that actively involve geriatric-trained providers may increase adherence to evidence-based care and reduce overall morbidity. Used previously for patients undergoing orthopedic procedures, these models span the entire treatment episode, from planning through recovery, and incorporate thorough geriatric assessment and tailored perioperative care from a comanaging geriatrician or internist.42-44 Previous studies suggest that such approaches can reduce acute geriatric events as well as other complications and total hospitalization length.40-44 Future studies in oncology will be critical to understand the role and effectiveness of such care processes in reducing geriatric events. Until then, building the working knowledge in geriatrics and selectively applying geriatric-based team care to high-risk patients may provide the most practical means for delivering optimal care to older patients who require cancer surgery.
In conclusion, geriatric events arise regularly after cancer surgery, especially for the very old and sick and those who require major abdominal surgery. These events may add to operative morbidity and resource use, straining both patients and the cancer care delivery system. Efforts aimed at addressing age-related health concerns and reducing associated morbidity will be essential as the number of older adults with cancer continues to grow.
Appendix
Data provided by the Nationwide Inpatient Sample, Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality in collaboration with Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Florida, Georgia, Hawaii, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming.
Table A1.
Case Identification for Study Cohort
| Cancer Site | ICD-9 Diagnosis Code | ICD-9 Procedure Code |
|---|---|---|
| Bladder | 188, 188.0-9 | 57.6, 57.7x, 68.8 |
| Breast | 174.x | 85.21, 85.22, 85.23, 85.4x |
| Colon/rectum | 153.x, 154.x | 45.7x, 45.8x, 48.4x, 48.5x, 48.6x, 17.3x |
| Endometrium | 182.x | 68.3x, 68.4x, 68.5x, 68.6x, 68.7x, 68.8, 68.9 |
| Kidney | 189, 189.0, 189.8,189.9 | 55.4, 55.4, 55.51, 55.52, 55.54 |
| Lung | 162.x | 32.20, 32.29, 32.3x, 32.4x, 32.5x, 32.6, 32.9 |
| Ovary | 183.x | 65.3x, 65.4x, 65.5x, 65.6x |
| Prostate | 185 | 60.4, 60.5, 60.62 |
| Pancreas | 157.x | 52.5x, 52.6, 52.7 |
| Stomach | 151.x | 43.5, 43.6, 43.7, 43.8x, 43.9x |
NOTE. Observations required concurrent codes for both diagnosis and procedure for study inclusion.
Abbreviation: ICD-9, International Classification of Disease, Ninth Revision, Clinical Modification.
Table A2.
Prevalence of Geriatric Events by Type, Cancer Site, and Age
| Cancer Site | Nutrition-Related Events* | Delirium | Mobility-Related Events† | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | P | Age | P | Age | P | |||||||
| 55-64 | 65-74 | ≥ 75 | 55-64 | 65-74 | ≥ 75 | 55-64 | 65-74 | ≥ 75 | ||||
| Prostate | 0.7 (0.6-0.9) | 1.0 (0.8-1.3) | 1.7 (1.0-2.6) | < .001 | 0.1 (0.0-0.2) | 0.2 (0.1-0.3) | 0.4 (0.2-0.9) | .093 | < 0.1 (0.0-0.1) | < 0.1 (0.0-0.1) | 0.2 (0.1-0.7) | .015 |
| Breast | 0.9 (0.7-1.2) | 0.9 (0.7-1.1) | 2.0 (1.7-2.4) | < .001 | 0.1 (0.0-0.2) | 0.3 (0.2-0.5) | 0.5 (0.4-0.7) | < .001 | 0.4 (0.2-0.5) | 0.5 (0.3-0.7) | 0.9 (0.7-1.2) | < .001 |
| Uterine | 2.5 (2.1-3.0) | 3.4 (2.8-4.1) | 4.8 (3.0-5.8) | < .001 | 0.2 (0.1-0.4) | 0.7 (0.5-0.9) | 1.2 (0.8-1.7) | < .001 | 0.4 (0.3-0.6) | 0.5 (0.3-0.7) | 0.8 (0.6-1.2) | .019 |
| Kidney | 3.0 (2.6-3.5) | 4.8 (4.2-5.5) | 6.5 (5.8-7.4) | < .001 | 0.7 (0.5-1.0) | 1.9 (1.6-2.3) | 2.7 (2.2-3.3) | < .001 | 0.4 (0.3-0.6) | 0.5 (0.3-0.7) | 0.9 (0.6-1.3) | .004 |
| Lung | 5.3 (4.7-6.0) | 5.2 (4.7-5.8) | 6.8 (6.0-7.6) | < .001 | 1.5 (1.2-1.8) | 2.4 (2.0-2.7) | 3.7 (3.2-4.2) | < .001 | 0.6 (0.4-0.8) | 0.8 (0.7-1.0) | 1.3 (1.0-1.6) | < .001 |
| Ovary | 9.7 (8.3-11.3) | 13.7 (11.7-16.0) | 16.7 (14.6-19.0) | < .001 | 0.8 (0.5-1.2) | 1.7 (1.2-2.3) | 2.7 (2.0-3.7) | < .001 | 0.5 (0.3-0.8) | 1.0 (0.6-1.5) | 1.6 (1.1-2.5) | .001 |
| Bladder | 9.8 (7.8-12.2) | 11.0 (9.2-13.0) | 13.9 (11.8-16.4) | < .001 | 1.7 (1.2-2.5) | 3.3 (2.5-4.3) | 5.2 (4.2-6.3) | < .001 | 0.7 (0.4-1.3) | 1.1 (0.8-1.6) | 1.4 (1.0-2.1) | .116 |
| Colon/rectum | 10.8 (10.1-11.5) | 12.1 (11.5-12.7) | 17.6 (16.9-18.3) | < .001 | 1.1 (1.0-1.3) | 1.9 (1.7-2.2) | 3.5 (3.2-3.8) | < .001 | 0.8 (0.7-1.0) | 1.1 (1.0-1.3) | 2.5 (2.3-2.8) | < .001 |
| Pancreas | 17.5 (14.9-20.4) | 22.4 (19.6-25.4) | 26.3 (23.2-29.6) | < .001 | 1.4 (0.8-2.4) | 3.9 (2.9-5.3) | 5.5 (4.1-7.4) | < .001 | 0.8 (0.4-1.5) | 1.7 (1.2-2.4) | 2.2 (1.5-3.2) | .013 |
| Stomach | 20.2 (17.1-23.7) | 19.6 (17.3-22.1) | 26.2 (23.7-28.9) | < .001 | 2.3 (1.5-3.3) | 4.0 (3.2-5.1) | 4.5 (3.5-5.8) | .006 | 1.3 (0.8-2.2) | 1.4 (0.9-2.1) | 2.5 (1.8-3.4) | .023 |
NOTE. Comparisons were performed by using χ2 testing carried out at the 5% significance level. Prevalence rates are reported along with 95% confidence intervals.
Nutrition-related events encompass dehydration and failure to thrive.
Mobility-related events encompass pressure ulcers, falls, and fractures.
Footnotes
Supported by the Veterans Affairs Office of Academic Affiliations through the Robert Wood Johnson Foundation Clinical Scholars Program (H.-J.T.), the American Cancer Society Postdoctoral Fellowship Program (Grant No. 126217-PF-14-028-01-CPHPS to H.-J.T.), the National Institute on Alcohol Abuse and Alcoholism (Grant No. K24AA15957 to A.A.M.), and the National Institute on Aging (Grants No. P30AG021684 and P30AG028748 to A.A.M.).
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Hung-Jui Tan, Debra Saliba, Alison A. Moore, Mark S. Litwin
Collection and assembly of data: Hung-Jui Tan, Lorna Kwan
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Burden of Geriatric Events Among Older Adults Undergoing Major Cancer Surgery
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or www.jco.ascopubs.org/site/ifc.
Hung-Jui Tan
No relationship to disclose
Debra Saliba
No relationship to disclose
Lorna Kwan
No relationship to disclose
Alison A. Moore
No relationship to disclose
Mark S. Litwin
No relationship to disclose
REFERENCES
- 1.American Society of Clinical Oncology The state of cancer care in America, 2015: A report by the American Society of Clinical Oncology. J Oncol Pract. 2015;11:79–113. doi: 10.1200/JOP.2015.003772. [DOI] [PubMed] [Google Scholar]
- 2. United States Census: U.S. Census Bureau projections show a slower growing, older, more diverse nation a half century from now. http://www.census.gov/newsroom/releases/archives/population/cb12-243.html.
- 3.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2010. Bethesda, MD: National Cancer Institute; 2013. [Google Scholar]
- 5.Spyropoulou D, Pallis AG, Leotsinidis M, et al. Completion of radiotherapy is associated with the Vulnerable Elders Survey-13 score in elderly patients with cancer. J Geriatr Oncol. 2014;5:20–25. doi: 10.1016/j.jgo.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Audisio RA, Pope D, Ramesh HS, et al. PACE Participants Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol. 2008;65:156–163. doi: 10.1016/j.critrevonc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Mohile SG, Fan L, Reeve E, et al. Association of cancer with geriatric syndromes in older Medicare beneficiaries. J Clin Oncol. 2011;29:1458–1464. doi: 10.1200/JCO.2010.31.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puts MT, Hardt J, Monette J, et al. Use of geriatric assessment for older adults in the oncology setting: A systematic review. J Natl Cancer Inst. 2012;104:1133–1163. doi: 10.1093/jnci/djs285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute of Medicine . Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 11. National Comprehensive Cancer Network: Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Senior Adult Oncology V.2.2014. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 12.Extermann M, Aapro M, Bernabei R, et al. Task Force on CGA of the International Society of Geriatric Oncology Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–252. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Chow WB, Rosenthal RA, Merkow RP, et al. American College of Surgeons National Surgical Quality Improvement Program. American Geriatrics Society Optimal preoperative assessment of the geriatric surgical patient: A best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg. 2012;215:453–466. doi: 10.1016/j.jamcollsurg.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 14.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults Postoperative delirium in older adults: Best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220:136.e1–148.e1. doi: 10.1016/j.jamcollsurg.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 15. Healthcare Cost and Utilization Project: Overview of the National (Nationwide) Inpatient Sample (NIS). http://www.hcup-us.ahrq.gov/nisoverview.jsp. [Google Scholar]
- 16.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 18.Miller DC, Taub DA, Dunn RL, et al. Laparoscopy for renal cell carcinoma: Diffusion versus regionalization? J Urol. 2006;176:1102–1106, discussion 1106-1107. doi: 10.1016/j.juro.2006.04.101. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JE, Chang DC, Parsons JK, et al. The first national examination of outcomes and trends in robotic surgery in the United States. J Am Coll Surg. 2012;215:107–114, discussion 114-106. doi: 10.1016/j.jamcollsurg.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Silber JH, Williams SV, Krakauer H, et al. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care. 1992;30:615–629. doi: 10.1097/00005650-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Ghaferi AA, Osborne NH, Birkmeyer JD, et al. Hospital characteristics associated with failure to rescue from complications after pancreatectomy. J Am Coll Surg. 2010;211:325–330. doi: 10.1016/j.jamcollsurg.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 22.D’Arcy LP, Stearns SC, Domino ME, et al. Is geriatric care associated with less emergency department use? J Am Geriatr Soc. 2013;61:4–11. doi: 10.1111/jgs.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agency for Healthcare Research and Quality: AHRQ Quality Indicators – Guide to the Patient Safety Indicators. http://www.qualityindicators.ahrq.gov/Modules/PSI_TechSpec.aspx.
- 24.Iezzoni LI, Daley J, Heeren T, et al. Identifying complications of care using administrative data. Med Care. 1994;32:700–715. doi: 10.1097/00005650-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Heisler CA, Melton LJ, III, Weaver AL, et al. Determining perioperative complications associated with vaginal hysterectomy: Code classification versus chart review. J Am Coll Surg. 2009;209:119–122. doi: 10.1016/j.jamcollsurg.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin RY, Heacock LC, Fogel JF. Drug-induced, dementia-associated and non-dementia, non-drug delirium hospitalizations in the United States, 1998-2005: An analysis of the national inpatient sample. Drugs Aging. 2010;27:51–61. doi: 10.2165/11531060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Zrelak PA, Utter GH, Tancredi DJ, et al. How accurate is the AHRQ Patient Safety Indicator for hospital-acquired pressure ulcer in a national sample of records? J Healthc Qual. 2015;37:287–297. doi: 10.1111/jhq.12052. [DOI] [PubMed] [Google Scholar]
- 28.Tan HJ, Wolf JS, Jr, Ye Z, et al. Complications and failure to rescue after laparoscopic versus open radical nephrectomy. J Urol. 2011;186:1254–1260. doi: 10.1016/j.juro.2011.05.074. [DOI] [PubMed] [Google Scholar]
- 29.Weingart SN, Iezzoni LI, Davis RB, et al. Use of administrative data to find substandard care: Validation of the complications screening program. Med Care. 2000;38:796–806. doi: 10.1097/00005650-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17:1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 31.Lawthers AG, McCarthy EP, Davis RB, et al. Identification of in-hospital complications from claims data. Is it valid? Med Care. 2000;38:785–795. doi: 10.1097/00005650-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 32. Healthcare Cost and Utilization Project: Cost-to-Charge Ratio Files. http://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp.
- 33. Sun Y, Friedman B; Healthcare Cost and Utilization Project: Tools for More Accurate Inpatient Cost Estimates with HCUP Databases, 2009. http://www.hcup-us.ahrq.gov/reports/methods/2011_04.pdf.
- 34.Sukumar S, Roghmann F, Trinh VQ, et al. National trends in hospital-acquired preventable adverse events after major cancer surgery in the USA. BMJ Open. 2013;3:e002843. doi: 10.1136/bmjopen-2013-002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu M, Jacobs BL, Montgomery JS, et al. Sharpening the focus on causes and timing of readmission after radical cystectomy for bladder cancer. Cancer. 2014;120:1409–1416. doi: 10.1002/cncr.28586. [DOI] [PubMed] [Google Scholar]
- 36.Kassin MT, Owen RM, Perez SD, et al. Risk factors for 30-day hospital readmission among general surgery patients. J Am Coll Surg. 2012;215:322–330. doi: 10.1016/j.jamcollsurg.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katznelson R, Djaiani G, Tait G, et al. Hospital administrative database underestimates delirium rate after cardiac surgery. Can J Anaesth. 2010;57:898–902. doi: 10.1007/s12630-010-9355-8. [DOI] [PubMed] [Google Scholar]
- 38.Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: A report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531–537. doi: 10.1016/j.jamcollsurg.2004.05.276. [DOI] [PubMed] [Google Scholar]
- 39.Dimick JB, Pronovost PJ, Cowan JA, et al. Complications and costs after high-risk surgery: Where should we focus quality improvement initiatives? J Am Coll Surg. 2003;196:671–678. doi: 10.1016/S1072-7515(03)00122-4. [DOI] [PubMed] [Google Scholar]
- 40.Miura LN, DiPiero AR, Homer LD. Effects of a geriatrician-led hip fracture program: Improvements in clinical and economic outcomes. J Am Geriatr Soc. 2009;57:159–167. doi: 10.1111/j.1532-5415.2008.02069.x. [DOI] [PubMed] [Google Scholar]
- 41.Gustafson Y, Brännström B, Berggren D, et al. A geriatric-anesthesiologic program to reduce acute confusional states in elderly patients treated for femoral neck fractures. J Am Geriatr Soc. 1991;39:655–662. doi: 10.1111/j.1532-5415.1991.tb03618.x. [DOI] [PubMed] [Google Scholar]
- 42.González-Montalvo JI, Alarcón T, Mauleón JL, et al. The orthogeriatric unit for acute patients: A new model of care that improves efficiency in the management of patients with hip fracture. Hip Int. 2010;20:229–235. doi: 10.1177/112070001002000214. [DOI] [PubMed] [Google Scholar]
- 43.Harari D, Hopper A, Dhesi J, et al. Proactive care of older people undergoing surgery (‘POPS’): Designing, embedding, evaluating and funding a comprehensive geriatric assessment service for older elective surgical patients. Age Ageing. 2007;36:190–196. doi: 10.1093/ageing/afl163. [DOI] [PubMed] [Google Scholar]
- 44.Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: A randomized trial. J Am Geriatr Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 45.Rosen AK, Itani KM, Cevasco M, et al. Validating the patient safety indicators in the Veterans Health Administration: Do they accurately identify true safety events? Med Care. 2012;50:74–85. doi: 10.1097/MLR.0b013e3182293edf. [DOI] [PubMed] [Google Scholar]
- 46.Kelly KN, Iannuzzi JC, Rickles AS, et al. Risk factors associated with 30-day postoperative readmissions in major gastrointestinal resections. J Gastrointest Surg. 2014;18:35–43, discussion 43-34. doi: 10.1007/s11605-013-2354-7. [DOI] [PubMed] [Google Scholar]
- 47.Institute of Medicine: Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- 48.Solomon DH, Burton JR, Lundebjerg NE, et al. The new frontier: Increasing geriatrics expertise in surgical and medical specialties. J Am Geriatr Soc. 2000;48:702–704. doi: 10.1111/j.1532-5415.2000.tb04734.x. [DOI] [PubMed] [Google Scholar]
- 49.Potter JF, Burton JR, Drach GW, et al. Geriatrics for residents in the surgical and medical specialties: Implementation of curricula and training experiences. J Am Geriatr Soc. 2005;53:511–515. doi: 10.1111/j.1532-5415.2005.53173.x. [DOI] [PubMed] [Google Scholar]
- 50.Bell RH, Jr, Drach GW, Rosenthal RA. Proposed competencies in geriatric patient care for use in assessment for initial and continued board certification of surgical specialists. J Am Coll Surg. 2011;213:683–690. doi: 10.1016/j.jamcollsurg.2011.08.004. [DOI] [PubMed] [Google Scholar]