Abstract
Purpose
Radiotherapy (RT) after breast-conserving surgery (BCS) is a standard treatment option for the management of ductal carcinoma in situ (DCIS). We sought to determine the survival benefit of RT after BCS on the basis of risk factors for local recurrence.
Patients and Methods
A retrospective longitudinal cohort study was performed to identify patients with DCIS diagnosed between 1988 and 2007 and treated with BCS by using SEER data. Patients were divided into the following two groups: BCS+RT (RT group) and BCS alone (non-RT group). We used a patient prognostic scoring model to stratify patients on the basis of risk of local recurrence. We performed a Cox proportional hazards model with propensity score weighting to evaluate breast cancer mortality between the two groups.
Results
We identified 32,144 eligible patients with DCIS, 20,329 (63%) in the RT group and 11,815 (37%) in the non-RT group. Overall, 304 breast cancer–specific deaths occurred over a median follow-up of 96 months, with a cumulative incidence of breast cancer mortality at 10 years in the weighted cohorts of 1.8% (RT group) and 2.1% (non-RT group; hazard ratio, 0.73; 95% CI, 0.62 to 0.88). Significant improvements in survival in the RT group compared with the non-RT group were only observed in patients with higher nuclear grade, younger age, and larger tumor size. The magnitude of the survival difference with RT was significantly correlated with prognostic score (P < .001).
Conclusion
In this population-based study, the patient prognostic score for DCIS is associated with the magnitude of improvement in survival offered by RT after BCS, suggesting that decisions for RT could be tailored on the basis of patient factors, tumor biology, and the prognostic score.
INTRODUCTION
Ductal carcinoma in situ (DCIS) of the breast is a lesion consisting of an abnormal proliferation of epithelial cells within breast ducts. The incidence of DCIS has increased dramatically since the implementation of breast screening protocols in the 1980s, and it is estimated that approximately 60,000 patients will be diagnosed with DCIS in the United States during 2015.1,2 Although DCIS is not an invasive carcinoma, it displays a broad spectrum of tumor biology and is considered a premalignant lesion.3 A standard surgical option for the local management of both DCIS and invasive disease is breast-conserving surgery (BCS) often followed by postoperative radiation therapy (RT). The benefit of postoperative RT is well established in patients with invasive disease, with large randomized data demonstrating reduced local recurrence rates and improved breast cancer–specific mortality (BCM) for RT-treated patients.4 However, the survival benefit for RT has not yet been clearly established for patients with DCIS.
Given the favorable breast cancer–specific survival of DCIS compared with invasive carcinoma (the 10-year breast cancer–specific survival of DCIS approaches 96% to 98%), prior research efforts have had difficulty achieving adequate sample size and power to examine the survival benefit of RT in the context of in situ disease.5 Whereas prior research for DCIS has shown that mortality risk increases after the development of a second ipsilateral primary invasive breast cancer, prevention of recurrence using RT has not been definitely shown to diminish BCM.6
Many studies have attempted to determine which, if any, patient subgroups may be able to safely avoid RT after BCS.2,7-15 Several clinical factors including age, tumor size, grade, and surgical margin status have been identified as predictive factors that increase the risk for ipsilateral breast tumor recurrence (IBTR) after BCS, suggesting that certain high-risk patients may be most apt to benefit from RT. Prognostic score systems have been developed to help identify this cohort of patients, including the University of Southern California/Van Nuys prognostic index and the patient prognostic score.16-20 We sought to determine the specific survival benefit of RT after BCS among variable risk subpopulations of patients with DCIS. We hypothesized that the addition of RT to BCS may confer a survival benefit to patients with DCIS with clinicopathologic features associated with higher local recurrence risk.
PATIENTS AND METHODS
Study Design and Data Source
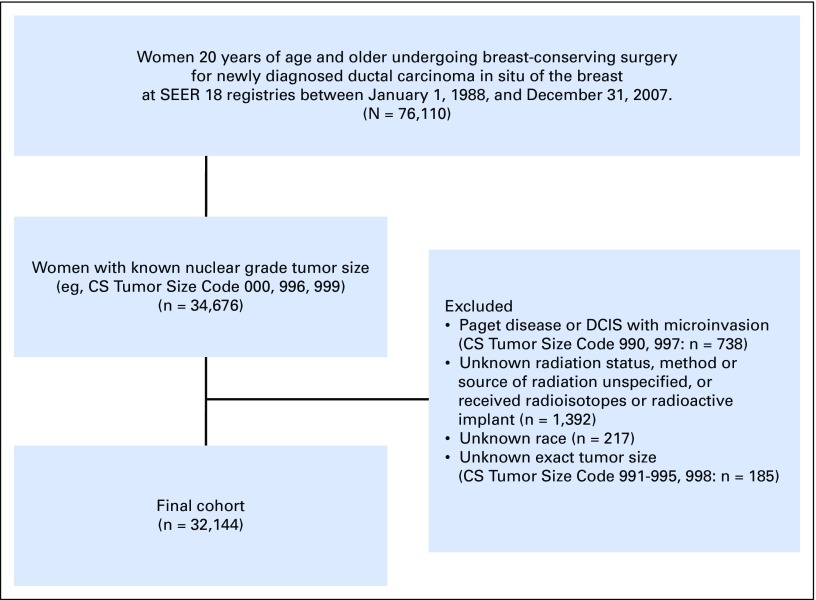
After receiving an exemption from the Partners HealthCare Institutional Review Board, we performed a retrospective longitudinal cohort study using data obtained from the SEER Program of the National Cancer Institute. The SEER database includes incidence and survival data routinely collected from multiple population-based cancer registries.21 For this study, we identified 76,110 women over the age of 20 years who received BCS (site-specific surgery code: 10, 20) after being diagnosed with a first case of DCIS in SEER 17 General Health Service Areas between January 1, 1988, and December 31, 2007 (Appendix Fig A1, online only). Given that DCIS has a good prognosis, we opted for a cohort with a longer median follow-up period and, therefore, excluded patients diagnosed after 2008. Among these patients, 34,676 patients had known nuclear grade and tumor size. Patients with unknown RT status or method or source of RT unspecified and those who received radioisotopes or radioactive implants were excluded. We also excluded patients without information on known prognostic characteristics, including grade, tumor size, and race. Additional exclusion criteria included patients with Paget disease or DCIS with microinvasion, patients registered as multiple cases during the same year, and patients with ipsilateral or contralateral recurrence after breast surgery. The final cohort included 32,144 patients.
Assembly of Key Variables
Using SEER*Stat version 8.2.1, we generated a data table including individual cancer records and patient characteristics and included the following variables: patient identification number, year of diagnosis, age, race, tumor size, nuclear grade, estrogen receptor (ER) status, progesterone receptor (PgR) status, RT, cause-specific death classification, other cause of death classification, survival month, family income, marital status, and SEER registry. All variables were categorized as outlined in Table 1. We used RT codes to classify patients with the code of “beam radiation” into the BCS+RT group (RT group) and those with the code of “none” and “refused” into the BCS alone group (non-RT group).
Table 1.
Patient Characteristics by Receipt of RT
| Characteristic | No. of Patients (%) | P | |
|---|---|---|---|
| Non-RT Group (n = 11,815) | RT Group (n = 20,329) | ||
| Year of diagnosis | < .001 | ||
| 1988-1992 | 151 (1.3) | 128 (0.6) | |
| 1993-1997 | 1,195 (10.1) | 1,299 (6.4) | |
| 1998-2002 | 4,536 (38.4) | 7,140 (35.0) | |
| 2003-2007 | 5,933 (50.2) | 11,762 (57.9) | |
| Age, years | < .001 | ||
| 20-39 | 329 (2.8) | 567 (2.8) | |
| 40-44 | 878 (7.4) | 1,885 (9.3) | |
| 45-49 | 1,403 (11.9) | 2,920 (14.4) | |
| 50-54 | 1,536 (13.0) | 3,186 (15.7) | |
| 55-59 | 1,469 (12.4) | 3,145 (15.5) | |
| 60-64 | 1,304 (11.0) | 2,568 (12.6) | |
| 65-69 | 1,207 (10.2) | 2,336 (11.5) | |
| 70-74 | 1,220 (10.3) | 1,817 (8.9) | |
| 75-79 | 1,126 (9.5) | 1,221 (6.0) | |
| 80+ | 1,343 (11.4) | 684 (3.4) | |
| Race | < .001 | ||
| White | 9,687 (82.0) | 16,261 (80.0) | |
| Black | 951 (8.1) | 1,630 (8.0) | |
| Other | 1,177 (10.0) | 2,438 (12.0) | |
| Marital status | < .001 | ||
| Married | 6,580 (55.7) | 13,015 (64.0) | |
| Single | 4,705 (39.8) | 6,816 (33.5) | |
| Unknown | 530 (4.5) | 498 (2.5) | |
| Tumor size, mm | < .001 | ||
| 1-9 | 7,078 (60.0) | 9,856 (48.5) | |
| 10-19 | 2,844 (24.1) | 6,453 (31.7) | |
| 20-49 | 1,570 (13.3) | 3,582 (17.6) | |
| 50+ | 323 (2.7) | 438 (2.2) | |
| Grade | < .001 | ||
| 1 | 2,626 (22.2) | 2,549 (12.5) | |
| 2 | 5,594 (47.4) | 7,923 (39.0) | |
| 3 | 3,595 (30.4) | 9,857 (48.5) | |
| Estrogen receptor status | < .001 | ||
| Negative | 549 (4.7) | 1,714 (8.4) | |
| Positive | 3,449 (29.1) | 7,947 (39.1) | |
| Unknown | 7,817 (66.2) | 10,668 (52.5) | |
| Progesterone receptor status | < .001 | ||
| Negative | 867 (7.3) | 2,521 (12.4) | |
| Positive | 2,779 (23.5) | 6,522 (32.1) | |
| Unknown | 8,169 (69.1) | 11,286 (55.5) | |
Abbreviation: RT, radiotherapy.
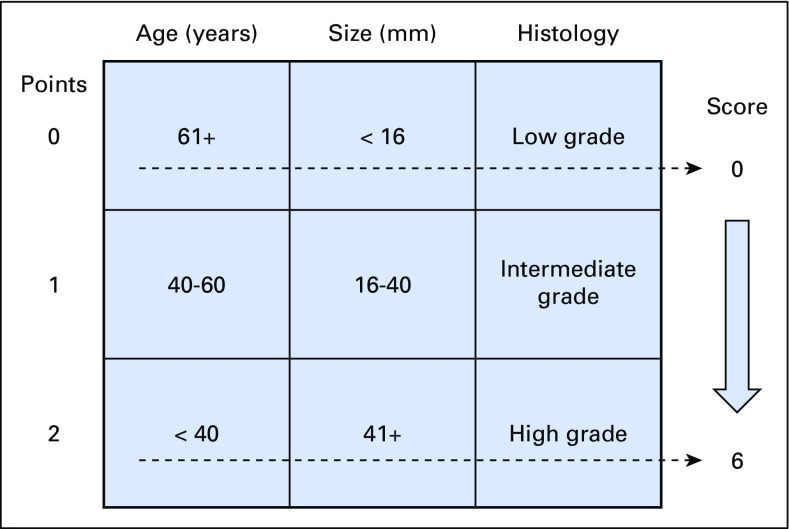
To investigate the benefit of RT on the basis of the risk of IBTR after BCS, we used a patient prognostic score, which was proposed by Smith et al20 (Fig 1), to define an ordinal factor where patients with a score of 0 have the lowest risk and those with a score of 6 have the highest risk of local recurrence.
Fig 1.
Patient prognostic score: risk stratification. Modified from Smith et al.20
Outcome of Interest
The primary outcome of interest was breast cancer–specific death after BCS in patients with DCIS. SEER defines mortality data on the basis of the International Classification of Diseases Revisions 8 to 10. The SEER cause of death recode was used to categorize the cause of death as breast cancer–specific death, other cancer death, death as a result of heart disease, or noncancer cause of death. The time to overall death and breast cancer specific death (overall mortality [OM] and BCM) was calculated as the time period from the date of diagnosis until the last date for which completed vital status data were available (last follow-up date: December 31, 2012). The data regarding deaths were ascertained from death certificates that are coded by state health departments and/or state vital records for each SEER region.22
Statistical Analysis
For this study, we used the same statistical analytic approach as reported in our earlier study that examined the benefit of breast surgery for DCIS.23 In brief, we compared clinicopathologic factors between RT groups and non-RT groups using Pearson or Mantel-Haenszel χ2 tests for categorical and ordinal factors, respectively. For inferring missing values of marital status (n = 1,028; 3.2%), ER status (n = 18,485; 57.5%), and PgR status (n = 19,455; 60.5%), we applied a multiple imputation procedure using IVEware macro version 0.2 (University of Michigan, Ann Arbor, MI) with the following variables: patient age (continuous), race (white, black, or other), nuclear grade, tumor size classification (0.1 to 0.5, 0.6 to 10, 11 to 50, or ≥ 51 mm), receipt of RT, and SEER registry.24,25 To stabilize the results, the procedure was repeated for 10 cycles to produce a single imputed data set (Appendix Table A1, online only). For analysis, the classification of all variables remained consistent in this study except for patient age (5-year age bands) to allow for a nonlinear effect in regression models.
We then used inverse probability propensity score weighting to balance patient characteristics between the RT and non-RT groups.26,27 To calculate propensity scores, baseline characteristics of patient age, year of diagnosis (categorical, 5-year intervals), race, tumor size, nuclear grade, ER status, PgR status, marital status (single or married), and SEER registry were applied to a logistic regression model for receipt of RT.
BCM and OM were compared between RT and non-RT groups using propensity score–weighted log-rank tests and Cox proportional hazards models. Hazard ratios (HRs) of BCM and OM were reported from multivariable models that adjusted for patient age, year of diagnosis, race, tumor size, nuclear grade, and marital status. Because family income was not significant, we did not include it in the models. Interaction tests were performed to explore whether any survival benefit conferred by RT varied across subgroups. Treatment effect modification of RT was evaluated using categorical factors and interaction tests in bivariable weighted Cox models. In addition, we performed a secondary analysis by using a proportional subdistribution hazard model to confirm the HRs of BCM, which was adjusted by competing events such as death from other cancer, death as a result of heart disease, death from other noncancer causes, or death as a result of unknown reasons.28
To assess the consistency of our findings, we conducted four types of sensitivity analyses. First, we repeated analyses after excluding variables for marital, ER, and PgR status, with the missing data exchanged by multiple imputation. Second, we performed the analysis after restriction to patients in the SEER 9 registry, because the RT data in the SEER 9 registry are more accurate than the data in newer SEER registries.29 Third, we repeated analyses after excluding patients without overlapped propensity score between RT group and non-RT group.26,29,30 Last, we performed an additional analysis for the 57,101 patients diagnosed with DCIS during the 20-year period between 1992 and 2011 by using the same multivariable Cox regression hazard model.
We assessed proportional hazard assumption by using a method of Kernel estimation and a time-varying covariate in the Cox regression model. All P values presented are from two-sided tests that use α = .05 to assess the statistical significance of survival benefit by RT. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics in Full SEER Cohort
We identified 32,144 eligible patients with DCIS on the basis of our inclusion and exclusion criteria (Appendix Fig A1). Of this initial cohort, 11,815 patients (36.8%) were stratified into the non-RT group, and 20,329 patients (63.2%) were stratified into the RT group. Patient clinicopathologic factors and SEER cancer registries according to receipt of RT are listed in Table 1 and Appendix Table A2 (online only). Balance in patient characteristics was achieved after multiple imputations and propensity score adjustments for estimating average treatment effect, as shown in Table 2. All clinicopathologic factors were statistically related to the receipt of RT. Patients diagnosed during earlier years, older patients, unmarried patients, patients with low income, patients with small tumor size, and patients with low nuclear grade were less likely to receive RT (P < .001)
Table 2.
Patient Characteristics Weighted by Propensity Score
| Characteristic | No. of Patients (%) | |
|---|---|---|
| Non-RT Group (n = 11,815) | RT Group (n = 20,329) | |
| Year of diagnosis | ||
| 1988-1992 | 105 (0.9) | 178 (0.9) |
| 1993-1997 | 922 (7.8) | 1,569 (7.7) |
| 1998-2002 | 4,298 (36.2) | 7,376 (36.1) |
| 2003-2007 | 6,561 (55.2) | 11,320 (55.4) |
| Age, years | ||
| 20-39 | 345 (2.9) | 578 (2.8) |
| 40-44 | 1,010 (8.5) | 1,744 (8.5) |
| 45-49 | 1,582 (13.3) | 2,740 (13.4) |
| 50-54 | 1,730 (14.6) | 2,997 (14.7) |
| 55-59 | 1,711 (14.4) | 2,924 (14.3) |
| 60-64 | 1,433 (12.1) | 2,445 (12.0) |
| 65-69 | 1,306 (11.0) | 2,242 (11.0) |
| 70-74 | 1,146 (9.6) | 1,936 (9.5) |
| 75-79 | 863 (7.3) | 1,488 (7.3) |
| 80+ | 760 (6.4) | 1,349 (6.6) |
| Race | ||
| White | 9,597 (80.7) | 16,532 (80.9) |
| Black | 967 (8.1) | 1,641 (8.0) |
| Others | 1,322 (11.1) | 2,270 (11.1) |
| Marital status | ||
| Married | 7,440 (62.6) | 12,820 (62.7) |
| Single | 4,446 (37.4) | 7,623 (37.3) |
| Tumor size, mm | ||
| 1-9 | 6,200 (52.2) | 10,760 (52.6) |
| 10-19 | 3,442 (29.0) | 5,892 (28.8) |
| 20-49 | 1,933 (16.3) | 3,267 (16.0) |
| 50+ | 311 (2.6) | 524 (2.6) |
| Grade | ||
| 1 | 1,906 (16.0) | 3,285 (16.1) |
| 2 | 4,901 (41.2) | 8,564 (41.9) |
| 3 | 5,079 (42.7) | 8,594 (42.0) |
| Estrogen receptor status | ||
| Negative | 2,082 (17.5) | 3,469 (17.0) |
| Positive | 9,804 (82.5) | 16,974 (83.0) |
| Progesterone receptor status | ||
| Negative | 3,158 (26.6) | 5,302 (25.9) |
| Positive | 8,728 (73.4) | 15,141 (74.1) |
Abbreviation: RT, radiotherapy.
Survival Benefit of RT
With a median follow-up time of 96 months from diagnosis (interquartile range, 69 to 127 months), there were 304 breast cancer–specific deaths (0.9%), 827 deaths from other cancer causes (2.6%), 837 deaths from heart disease (2.6%), 1,575 deaths from other noncancer causes (4.9%), and 97 deaths from unknown causes (0.3%; Table 3 lists data for cohort weighted by propensity score; Appendix Table A3, online only, lists data for original patient cohort). The 10-year BCM rate weighted by inverse propensity score was 1.8% in the RT group and 2.1% in the non-RT group (absolute difference, 0.3%; log-rank test, P = .003; HR, 0.73; 95% CI, 0.62 to 0.88). There was no statistically significant departure from the proportional hazard assumption in the Cox regression hazard model (P = .59). After adjusting for other clinical factors, age (P = .004), nuclear grade (P = .007), and tumor size (P = 0.02) were each identified as statistically significant effect modifiers of RT for BCM.
Table 3.
Cause of Death With Patient Cohort Weighted by Propensity Score
| Cause of Death | No. of Patients (%) | Total No. of Patients (%) | |
|---|---|---|---|
| Non-RT Group | RT Group | ||
| Alive | 10,278 (87) | 18,222 (89.6) | 28,500 (88.7) |
| Cause of death | |||
| Breast cancer | 153 (1.3) | 164 (0.8) | 317 (1.0) |
| Other cancer | 328 (2.8) | 512 (2.6) | 840 (2.6) |
| Heart disease | 356 (3.0) | 470 (2.3) | 826 (2.6) |
| Other noncancer | 654 (5.5) | 912 (4.5) | 1,566 (4.9) |
| Unknown reason | 46 (0.4) | 49 (0.2) | 95 (0.3) |
| Total | 11,815 | 20,329 | 32,144 |
Abbreviation: RT, radiotherapy.
Survival Benefit of RT According to Patient Prognostic Score
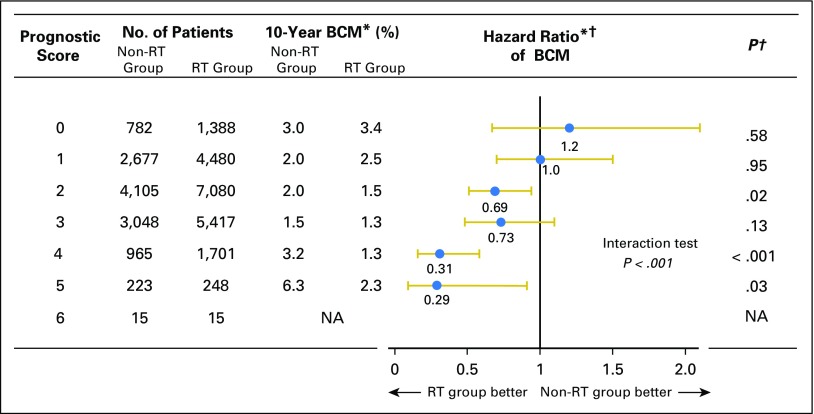
When examining the benefit of RT stratified by factors associated with a risk of local recurrence used in the patient prognostic score, we found that the survival for the RT group was significantly better than that observed in the non-RT group for patients with higher nuclear grade, younger age, and larger tumor size. A statistically significant reduction in BCM with RT was not observed among patients without these prognostic factors (Appendix Tables A4 and A5, online only). Moreover, the magnitude of improved survival among patients treated with RT was significantly correlated with the patient prognostic score (P < .001), whereby patients with low scores demonstrated no significant difference in BCM (score 0: absolute difference, −0.4%; HR, 1.2; 95% CI, 0.67 to 2.06; P = .58; score 1: absolute difference, −0.5%; HR, 1.0; 95% CI, 0.70 to 1.47; P = .95, respectively) compared with patients with higher scores of 4 or 5, who saw a near 70% reduction in BCM (score 4: absolute difference, 1.9%; HR, 0.31; 95% CI, 0.16 to 0.58; P < .001; score 5: absolute difference, 4.0%; HR, 0.29; 95% CI, 0.09 to 0.91; P = .03; Figs 2 and 3). These findings were comparable with those in the secondary analysis through the proportional subdistribution hazards model (Appendix Table A6, online only).
Fig 2.
Hazard ratio comparing breast cancer mortality (BCM) between radiotherapy (RT) group and non-RT group according to prognostic score. (*) Weighted by inverse propensity score. (†) Multivariate analysis adjusted by age of patients, year of diagnosis, race, tumor size, nuclear grade, and marital status. NA, not applicable.
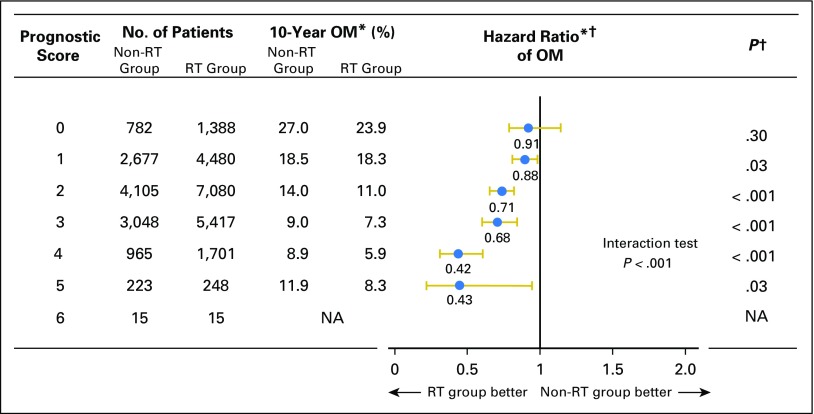
Fig 3.
Hazard ratio comparing overall mortality (OM) between radiotherapy (RT) group and non-RT group according to prognostic score. (*) Weighted by inverse propensity score. (†) Multivariate analysis adjusted by age of patients, year of diagnosis, race, tumor size, nuclear grade, and marital status. NA, not applicable.
In sensitivity analyses performed after the exclusion of variables for hormone receptor status and marital status, after restriction of patients within SEER 9, and after exclusion of patients without overlapped propensity score, we observed similar findings. In an additional analysis of the patients diagnosed between 1992 and 2011, the results were consistent with the primary analysis.
DISCUSSION
In our large population-based cohort study, we observed low BCM in women with DCIS, a reassuring finding that is consistent with prior reports.5,6 However, our findings suggest a possible heterogeneous treatment effect of RT that may be most important when certain risk factors (ie, large tumor size, young age, and high nuclear grade) are present. These clinicopathologic factors have been used together to produce a recurrence risk scoring system called the patient prognostic score.16-19,31 Our findings suggest that patients with low prognostic scores experienced a small difference in breast cancer–specific survival outcome when RT was combined with BCS, whereas patients with high prognostic scores treated with RT and BCS demonstrated a statistically significant difference in outcome compared with those in whom only BCS was used. In addition, overall BCM was only approximately 1%, whereas mortality from other causes was approximately 10% in our study. These results, when taken together with our earlier findings on DCIS, suggest that further research investigating the overdiagnosis and overtreatment of breast cancer is warranted and that a less invasive and more individualized local treatment strategy on the basis of one’s probability of local recurrence should be considered.23,32-35
Local recurrence after BCS for DCIS is significant, because nearly half of patients with IBTR are diagnosed with invasive ductal carcinoma, which is associated with the potential for distant recurrence and an increased risk of death.2,6 In observational studies and in a randomized clinical trial, several investigators have attempted to identify a cohort of patients with DCIS with a low probability of local recurrence for whom RT could be safely avoided after BCS.11,16-20 Smith et al20 proposed the patient prognostic score, which was designed to predict one’s risk of IBTR using well-known predictive factors including patient age, tumor size, and grade. They investigated 14,202 patients with DCIS in the SEER database and found that the likelihood of IBTR increases by 22% with every 1-point increase in the prognostic score.
Although the National Comprehensive Cancer Network clinical guidelines do not mandate RT for low-risk DCIS,36 RT after BCS is widely recognized as an acceptable treatment option and has become a standard approach for DCIS management in the United States.37-39 Recent studies have sought to determine which subgroups of patients may be able to avoid RT, using IBTR as the primary end point to assess whether RT should be used. Young patient age, large tumor size, and high nuclear grade have been reported as predictive factors of IBTR after BCS,7-12,14-20 yet data have been lacking on whether RT portends an improved survival in the treatment of DCIS. One randomized clinical trial investigated the efficacy of RT for DCIS with low recurrence risk features. The rate of local failure of patients with BCS alone was 6.7% during 7 years of follow-up and was significantly reduced by RT, whereas distant recurrence–free survival and overall survival remained identical between RT and non-RT groups.11 Our results suggest that the omission of RT for patients with low prognostic scores is safe, given that it does not seem to improve survival compare with BCS alone.
Several researchers have investigated whether a gene profiling tool, the DCIS Score Assay, can predict local recurrence risk after BCS for DCIS.40,41 In a cohort of patients from the Eastern Cooperative Oncology Group 5194 study, nuclear grade, margin width, and Van Nuys prognostic index were not significant predictive factors of an ipsilateral breast event.41 However, the number of patients for this analysis was small (n = 327), possibly precluding the detection of small differences in recurrence rates. In addition, it is possible that the DCIS score maintains a collinear relationship with nuclear grade, such that the score inappropriately reduces the explanatory power of nuclear grade in the Cox regression model. The patient prognostic score uses classic clinical factors that have been established for predicting IBTR, whereas the DCIS score needs further validation to confirm how much additional prognostic information could be derived from its use.
There are several limitations in our study. Because unmeasured confounders such as surgical margin status, endocrine therapy, patient comorbidities, and reasons for treatment selection were not available in the SEER database and may have influenced overall results, our results should be interpreted with some caution. If surgical margins were positive, a physician would be more likely to recommend RT and the benefit would be underestimated in this study. In contrast, if RT was selectively avoided in patients with medical comorbidities, the survival benefit associated with its use would be overestimated. In view of this limitation, it is reassuring to note that one previous National Comprehensive Cancer Network study demonstrated no significant relationship between the presence of patient comorbidities and receipt of RT after BCS in the setting of DCIS.39 Regarding the agreement of RT implementation between Medicare and the SEER database, substantial agreement was reported in one study (κ = 0.77), and almost perfect agreement was observed in another study (κ = 0.87).42,43 Finally, we recognize that it may be more straightforward to use the prognostic score to select high-risk patients in whom the RT benefit is clear, as opposed to the more challenging scenario of selecting lower risk patients, in whom RT provides negligible absolute benefit despite the significant reduction in local recurrence. Therefore, thorough counseling on the risk-benefit profile should be required for informed decision making.
The strength of our study is that it is the first to investigate the survival benefit of RT after BCS for DCIS according to individualized patient risk factors. By using a large population-based registry, it was possible to detect the absolute difference of survival rates between the RT and non-RT groups. Furthermore, our results provide information to guide individual treatment options according to prognostic scores that will predict the survival benefit of RT.
In conclusion, our study validates the prognostic score of DCIS, which can be used to predict not only local recurrence but also the magnitude of survival benefit offered by RT after BCS. As an oncology community, we must be cognizant of overtreatment for this disease process that has low BCM. Further prospective studies will be needed to confirm our findings and tailor RT for DCIS.
Acknowledgment
We thank Koji Takebe, Takebe Clinic, and Hajime Uno, Division of Population Sciences and Biostatistics and Computational Biology, Dana-Farber Cancer Institute, for giving us the idea for this study.
Appendix
Fig A1.
Flow diagram of patient population. CS, collaborative stage; DCIS, ductal carcinoma in situ.
Table A1.
Patient Characteristics After Multiple Imputation for Unknown Data
| Characteristic | No. of Patients (%) | P | |
|---|---|---|---|
| Non-RT Group (n = 11,815) | RT Group (n = 20,329) | ||
| Marital status | < .001 | ||
| Married | 6,940 (58.4) | 13,427 (65.7) | |
| Single | 4,946 (41.6) | 7,016 (34.3) | |
| Estrogen receptor status | < .001 | ||
| Negative | 1,684 (14.2) | 3,794 (18.6) | |
| Positive | 10,202 (85.8) | 16,649 (81.4) | |
| Progesterone receptor status | < .001 | ||
| Negative | 2,639 (22.2) | 5,721 (28.0) | |
| Positive | 9,247 (77.8) | 14,722 (72.0) | |
Abbreviation: RT, radiotherapy.
Table A2.
Original Registries Data
| Registry | No. of Patients (%) | P | |
|---|---|---|---|
| Non-RT Group (n = 11,886) | RT Group (n = 20,443) | ||
| Alaska Natives, 1992+ | 25 (0.2) | 16 (0.1) | < .001 |
| Atlanta (metropolitan), 1975+ | 383 (3.2) | 971 (4.8) | |
| California excluding SF/SJM/LA | 2,347 (19.8) | 3,637 (17.8) | |
| Connecticut, 1973+ | 904 (7.6) | 1,528 (7.5) | |
| Detroit (metropolitan), 1973+ | 797 (6.7) | 2,041 (10.0) | |
| Hawaii, 1973+ | 200 (1.7) | 963 (4.7) | |
| Iowa, 1973+ | 267 (2.3) | 837 (4.1) | |
| Kentucky, 2000+ | 249 (2.1) | 574 (2.8) | |
| Los Angeles, 1992+ | 2,371 (20.0) | 2,299 (11.3) | |
| Louisiana, 2000+ | 203 (1.7) | 450 (2.2) | |
| New Jersey, 2000+ | 981 (8.3) | 1,572 (7.7) | |
| New Mexico, 1973+ | 272 (2.3) | 379 (1.9) | |
| Rural Georgia, 1992+ | 18 (0.2) | 19 (0.1) | |
| San Francisco-Oakland SMSA, 1973+ | 1,425 (12.0) | 1,705 (8.3) | |
| San Jose-Monterey, 1992+ | 343 (2.9) | 884 (4.3) | |
| Seattle (Puget Sound), 1974+ | 811 (6.8) | 2,156 (10.6) | |
| Utah, 1973+ | 290 (2.4) | 412 (2.0) | |
Abbreviations: LA, Los Angeles; RT, radiotherapy; SF, San Francisco; SJM, San Jose-Monterey; SMSA, standard metropolitan statistical area.
Table A3.
Cause of Death: Original Patient Cohort
| Cause of Death | No. of Patients (%) | Total No. of Patients (%) | |
|---|---|---|---|
| Non-RT Group | RT Group | ||
| Alive | 9,850 (83.4) | 18,657 (91.8) | 28,507 (88.7) |
| Cause of death | |||
| Breast cancer–specific death | 151 (1.3) | 153 (0.8) | 304 (0.9) |
| Other cancer cause of death | 378 (3.2) | 449 (2.2) | 827 (2.6) |
| Death from heart disease | 506 (4.3) | 328 (1.6) | 837 (2.6) |
| Other noncancer cause of death | 876 (7.4) | 699 (3.4) | 1,575 (4.9) |
| Death from unknown reason | 54 (0.5) | 43 (0.2) | 97 (0.3) |
| Total | 11,815 | 20,329 | 32,144 |
Abbreviation: RT, radiotherapy.
Table A4.
HRs Comparing BCM Between Non-RT Group and RT Group According to Clinicopathologic Factors
| Subgroup | No. of Patients | Weighted 10-Year BCM Rate (%) | Weighted Multivariable* HR | 95% CI* | P* | ||
|---|---|---|---|---|---|---|---|
| Non-RT Group | RT Group | Non-RT Group | RT Group | ||||
| Age, years | |||||||
| < 40 | 329 | 567 | 2.8 | 1.2 | 0.34 | 0.11 to 1.06 | .06 |
| 40-60 | 5,545 | 11,697 | 1.4 | 0.9 | 0.51 | 0.38 to 0.69 | < .001 |
| > 60 | 5,941 | 8,065 | 3.0 | 3.0 | 0.94 | 0.75 to 1.2 | .59 |
| Tumor size, mm | |||||||
| < 16 | 9,493 | 15,255 | 1.9 | 1.7 | 0.81 | 0.65 to 1.00 | .05 |
| 16-40 | 1,919 | 4,484 | 2.7 | 2.1 | 0.64 | 0.45 to 0.92 | .02 |
| > 40 | 403 | 590 | 3.4 | 1.5 | 0.43 | 0.17 to 1.1 | .08 |
| Grade | |||||||
| 1 | 2,626 | 2,549 | 1.5 | 1.9 | 0.91 | 0.58 to 1.43 | .67 |
| 2 | 5,594 | 7,923 | 1.9 | 2.0 | 0.87 | 0.66 to 1.16 | .35 |
| 3 | 3,595 | 9,857 | 2.6 | 1.6 | 0.52 | 0.39 to 0.68 | < .001 |
| Total patients | 11,815 | 20,329 | 2.1 | 1.8 | 0.74 | 0.62 to 0.88 | < .001 |
Abbreviations: BCM, breast cancer mortality; HR, hazard ratio; RT, radiotherapy.
Multivariable analysis adjusted by age of patients, year of diagnosis, race, tumor size, nuclear grade, and marital status.
Table A5.
HRs Comparing OM Between Non-RT Group and RT Group According to Clinicopathologic Factors
| Subgroup | No. of Patients | Weighted 10-Year OM Rate (%) | Weighted Multivariable* HR | 95% CI* | P* | ||
|---|---|---|---|---|---|---|---|
| Non-RT Group | RT Group | Non-RT Group | RT Group | ||||
| Age, years | |||||||
| < 40 | 329 | 567 | 4.5 | 1.8 | 0.30 | 0.12 to 0.74 | .009 |
| 40-60 | 5,545 | 11,697 | 4.6 | 3.7 | 0.68 | 0.58 to 0.80 | < .001 |
| > 60 | 5,941 | 8,065 | 26.6 | 23.1 | 0.78 | 0.73 to 0.84 | < .001 |
| Tumor size, mm | |||||||
| < 16 | 9,493 | 15,255 | 13.5 | 12.0 | 0.81 | 0.74 to 0.87 | .05 |
| 16-40 | 1,919 | 4,484 | 16.4 | 12.1 | 0.64 | 0.56 to 0.74 | < .001 |
| > 40 | 403 | 590 | 18.2 | 18.0 | 0.58 | 0.41 to 0.82 | .003 |
| Grade | |||||||
| 1 | 2,626 | 2,549 | 14.4 | 13.8 | 1.01 | 0.93 to 1.27 | .29 |
| 2 | 5,594 | 7,923 | 14.4 | 12.6 | 0.79 | 0.71 to 0.88 | < .001 |
| 3 | 3,595 | 9,857 | 14.0 | 11.1 | 0.66 | 0.59 to 0.73 | < .001 |
| Total patients | 11,815 | 20,329 | 14.2 | 12.2 | 0.76 | 0.71 to 0.81 | < .001 |
Abbreviations: HR, hazard ratio; OM, overall mortality; RT, radiotherapy.
Multivariable analysis adjusted by age of patients, year of diagnosis, race, tumor size, nuclear grade, and marital status.
Table A6.
Patient Prognostic Score and HR Comparing BCM Between RT Group and Non-RT Group: HR From Cox Regression Model and Proportional Subdistribution Hazards Model
| Patient Prognostic Score | Weighted Multivariable* HR of BCM (95%CI) | |
|---|---|---|
| Cox Regression Model | Proportional Subdistribution Hazards Model | |
| 0 | 1.2 (0.67 to 2.1) | 0.76 (0.33 to 1.8) |
| 1 | 1.0 (0.70 to 1.5) | 0.93 (0.57 to 1.5) |
| 2 | 0.69 (0.51 to 0.94) | 0.66 (0.46 to 0.96) |
| 3 | 0.73 (0.48 to 1.1) | 0.70 (0.43 to 1.1) |
| 4 | 0.31 (0.16 to 0.58) | 0.22 (0.10 to 0.49) |
| 5 | 0.29 (0.09 to 0.91) | 0.29 (0.09 to 0.91) |
| 6 | NA | NA |
Abbreviations: BCM, breast cancer mortality; HR, hazard ratio; NA, not applicable; RT, radiotherapy.
Multivariable analysis adjusted by age of patients, year of diagnosis, race, tumor size, nuclear grade, and marital status.
Footnotes
See accompanying editorial on page 1172
Supported by the National Institutes of Health Grant No. R25CA089017.
Y.S. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Yasuaki Sagara, Rachel A. Freedman, Ines Vaz-Luis, Melissa Anne Mallory, Stephanie M. Wong, Fatih Aydogan, William T. Barry, Mehra Golshan
Administrative support: Stephen DeSantis, Mehra Golshan
Collection and assembly of data: Yasuaki Sagara, Stephen DeSantis, Mehra Golshan
Data analysis and interpretation: Yasuaki Sagara, Rachel A. Freedman, Ines Vaz-Luis, Melissa Anne Mallory, Stephanie M. Wong, William T. Barry, Mehra Golshan
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Patient Prognostic Score and Associations With Survival Improvement Offered by Radiotherapy After Breast-Conserving Surgery for Ductal Carcinoma In Situ: A Population-Based Longitudinal Cohort Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Yasuaki Sagara
No relationship to disclose
Rachel A. Freedman
Research Funding: Genentech, Puma, Eisai
Ines Vaz-Luis
No relationship to disclose
Melissa Anne Mallory
No relationship to disclose
Stephanie M. Wong
No relationship to disclose
Fatih Aydogan
No relationship to disclose
Stephen DeSantis
No relationship to disclose
William T. Barry
No relationship to disclose
Mehra Golshan
Consulting or Advisory Role: Abbvie
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Virnig BA, Tuttle TM, Shamliyan T, et al. Ductal carcinoma in situ of the breast: A systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 3.Allred DC, Mohsin SK, Fuqua SA. Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer. 2001;8:47–61. doi: 10.1677/erc.0.0080047. [DOI] [PubMed] [Google Scholar]
- 4.Darby S, McGale P, Correa C, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correa C, McGale P, Taylor C, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narod SA, Iqbal J, Giannakeas V, et al. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1:888–896. doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
- 7.Arvold ND, Punglia RS, Hughes ME, et al. Pathologic characteristics of second breast cancers after breast conservation for ductal carcinoma in situ. Cancer. 2012;118:6022–6030. doi: 10.1002/cncr.27691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31:2382–2387. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solin LJ, Gray R, Hughes LL, et al. Surgical excision without radiation for ductal carcinoma in situ of the breast: 12-year results from the ECOG-ACRIN E5194 study. J Clin Oncol. 2015;33:3938–3944. doi: 10.1200/JCO.2015.60.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerlikowske K, Molinaro A, Cha I, et al. Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. J Natl Cancer Inst. 2003;95:1692–1702. doi: 10.1093/jnci/djg097. [DOI] [PubMed] [Google Scholar]
- 11.McCormick B, Winter K, Hudis C, et al. RTOG 9804: A prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol. 2015;33:709–715. doi: 10.1200/JCO.2014.57.9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vicini FA, Recht A. Age at diagnosis and outcome for women with ductal carcinoma-in-situ of the breast: A critical review of the literature. J Clin Oncol. 2002;20:2736–2744. doi: 10.1200/JCO.2002.07.137. [DOI] [PubMed] [Google Scholar]
- 13.Wang SY, Chu H, Shamliyan T, et al. Network meta-analysis of margin threshold for women with ductal carcinoma in situ. J Natl Cancer Inst. 2012;104:507–516. doi: 10.1093/jnci/djs142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong JS, Chen YH, Gadd MA, et al. Eight-year update of a prospective study of wide excision alone for small low- or intermediate-grade ductal carcinoma in situ (DCIS) Breast Cancer Res Treat. 2014;143:343–350. doi: 10.1007/s10549-013-2813-6. [DOI] [PubMed] [Google Scholar]
- 15.Wong JS, Kaelin CM, Troyan SL, et al. Prospective study of wide excision alone for ductal carcinoma in situ of the breast. J Clin Oncol. 2006;24:1031–1036. doi: 10.1200/JCO.2005.02.9975. [DOI] [PubMed] [Google Scholar]
- 16.Silverstein MJ, Poller DN, Waisman JR, et al. Prognostic classification of breast ductal carcinoma-in-situ. Lancet. 1995;345:1154–1157. doi: 10.1016/s0140-6736(95)90982-6. [DOI] [PubMed] [Google Scholar]
- 17.Silverstein MJ. The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am J Surg. 2003;186:337–343. doi: 10.1016/s0002-9610(03)00265-4. [DOI] [PubMed] [Google Scholar]
- 18.Silverstein MJ, Lagios MD. Choosing treatment for patients with ductal carcinoma in situ: Fine tuning the University of Southern California/Van Nuys Prognostic Index. J Natl Cancer Inst Monogr. 2010;2010:193–196. doi: 10.1093/jncimonographs/lgq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverstein MJ, Lagios MD, Craig PH, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer. 1996;77:2267–2274. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2267::AID-CNCR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 20.Smith GL, Smith BD, Haffty BG. Rationalization and regionalization of treatment for ductal carcinoma in situ of the breast. Int J Radiat Oncol Biol Phys. 2006;65:1397–1403. doi: 10.1016/j.ijrobp.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 21. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. http://seer.cancer.gov/
- 22.Lund JL, Harlan LC, Yabroff KR, et al. Should cause of death from the death certificate be used to examine cancer-specific survival? A study of patients with distant stage disease. Cancer Invest. 2010;28:758–764. doi: 10.3109/07357901003630959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sagara Y, Mallory MA, Wong S, et al. Survival benefit of breast surgery for low-grade ductal carcinoma in situ: A population-based cohort study. JAMA Surg. 2015;150:739–745. doi: 10.1001/jamasurg.2015.0876. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horton NJ, Kleinman KP. Much ado about nothing: A comparison of missing data methods and software to fit incomplete data regression models. Am Stat. 2007;61:79–90. doi: 10.1198/000313007X172556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stürmer T, Rothman KJ, Avorn J, et al. Treatment effects in the presence of unmeasured confounding: Dealing with observations in the tails of the propensity score distribution—A simulation study. Am J Epidemiol. 2010;172:843–854. doi: 10.1093/aje/kwq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 29.Walker GV, Giordano SH, Williams M, et al. Muddy water? Variation in reporting receipt of breast cancer radiation therapy by population-based tumor registries. Int J Radiat Oncol Biol Phys. 2013;86:686–693. doi: 10.1016/j.ijrobp.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patorno E, Grotta A, Bellocco R, et al. Propensity score methodology for confounding control in health care utilization databases. Epidemiol Biostat Public Health. 2013;10:e8940-1–e8940-16. [Google Scholar]
- 31.Wehner P, Lagios MD, Silverstein MJ. DCIS treated with excision alone using the National Comprehensive Cancer Network (NCCN) guidelines. Ann Surg Oncol. 2013;20:3175–3179. doi: 10.1245/s10434-013-3176-2. [DOI] [PubMed] [Google Scholar]
- 32.Esserman LJ, Thompson IM, Reid B, et al. Addressing overdiagnosis and overtreatment in cancer: A prescription for change. Lancet Oncol. 2014;15:e234–e242. doi: 10.1016/S1470-2045(13)70598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302:1685–1692. doi: 10.1001/jama.2009.1498. [DOI] [PubMed] [Google Scholar]
- 34.Harding C, Pompei F, Burmistrov D, et al. Breast cancer screening, incidence, and mortality across US counties. JAMA Intern Med. 2015;175:1483–1489. doi: 10.1001/jamainternmed.2015.3043. [DOI] [PubMed] [Google Scholar]
- 35.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 36.National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology: Breast cancer version 2, 2015. http://www.nccn.com. [DOI] [PubMed]
- 37.Punglia RS, Schnitt SJ, Weeks JC. Treatment of ductal carcinoma in situ after excision: Would a prophylactic paradigm be more appropriate? J Natl Cancer Inst. 2013;105:1527–1533. doi: 10.1093/jnci/djt256. [DOI] [PubMed] [Google Scholar]
- 38.Baxter NN, Virnig BA, Durham SB, et al. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96:443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 39.Buchholz TA, Theriault RL, Niland JC, et al. The use of radiation as a component of breast conservation therapy in National Comprehensive Cancer Network Centers. J Clin Oncol. 2006;24:361–369. doi: 10.1200/JCO.2005.02.3127. [DOI] [PubMed] [Google Scholar]
- 40.Rakovitch E, Nofech-Mozes S, Hanna W, et al. A population-based validation study of the DCIS Score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res Treat. 2015;152:389–398. doi: 10.1007/s10549-015-3464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105:701–710. doi: 10.1093/jnci/djt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noone A-M, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with Medicare claims. Med Care. doi: 10.1097/MLR.0000000000000073. [epub ahead of print on March 15, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virnig BA, Warren JL, Cooper GS, et al. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40:IV-49–IV-54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]