Abstract
Purpose
Survivors of childhood Hodgkin's lymphoma (HL) are at risk for second malignant neoplasms (SMNs). It is theorized that this risk may be attenuated in patients treated with lower doses of radiation. We report the first long-term outcomes of a cohort of pediatric survivors of HL treated with chemotherapy and low-dose radiation.
Patients and Methods
Pediatric patients with HL (n = 112) treated at Stanford from 1970 to 1990 on two combined modality treatment protocols were identified. Treatment included six cycles of chemotherapy with 15 to 25.5 Gy involved-field radiation with optional 10 Gy boosts to bulky sites. Follow-up through September 1, 2007, was obtained from retrospective chart review and patient questionnaires.
Results
One hundred ten children completed HL therapy; median follow-up was 20.6 years. Eighteen patients developed one or more SMNs, including four leukemias, five thyroid carcinomas, six breast carcinomas, and four sarcomas. Cumulative incidence of first SMN was 17% (95% CI, 10.5 to 26.7) at 20 years after HL diagnosis. The standard incidence ratio for any SMN was 22.9 (95% CI, 14.2 to 35) with an absolute excess risk of 93.7 cases per 10,000 person-years. All four secondary leukemias were fatal. For those with second solid tumors, the mean (± SE) 5-year disease-free and overall survival were 76% ± 12% and 85% ± 10% with median follow-up 5 years from SMN diagnosis.
Conclusion
Despite treatment with low-dose radiation, children treated for HL remain at significant risk for SMN. Sarcomas, breast and thyroid carcinomas occurred with similar frequency and latency as found in studies of children with HL who received high-dose radiation.
INTRODUCTION
With current therapy, most children and adolescents with Hodgkin's lymphoma (HL) are long-term survivors.1 Survivors are at risk for developing second malignant neoplasms (SMNs) including leukemia, sarcomas, breast, thyroid, gastrointestinal, and lung carcinoma.2–10 While secondary leukemia is associated with alkylating agents and epipodophyllotoxin chemotherapy, solid SMN risk is more closely linked to radiation, particularly at higher doses.11,12 Over the past 40 years, treatment for children with HL has evolved from high-dose extended-field radiation to combined-modality therapy with chemotherapy and low-dose involved-field radiation (IFRT). Such treatment protocols have the theoretical benefit of diminished risk of solid SMN due to decreased radiation exposure. Early reports of low SMN incidence in children and young adults after low-dose radiation are promising but suffer from short follow-up (median, 8 to 13 years).13–15
In 1970, in an effort to diminish the deleterious effects of high-dose radiation on growth and musculoskeletal development of children with HL, Stanford investigators pioneered a combined modality treatment protocol with low-dose IFRT and mechlorethamine, vincristine, prednisone, procarbazine (MOPP) chemotherapy.16,17 Children treated on this protocol had normal growth, but secondary leukemias and male infertility were significant concerns. In response, a second protocol was initiated in 1982 combining alternating cycles of MOPP and doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) chemotherapy with low-dose IFRT.18 Median follow-up time for patients treated on these protocols is now longer than 20 years, allowing the first long-term follow-up of pediatric HL survivors treated with chemotherapy and low-dose radiation.
PATIENTS AND METHODS
Patients and Treatment
Between 1970 and 1990, 112 children with newly diagnosed, untreated, biopsy-proven HL were treated at Stanford on two consecutive protocols. Details of eligibility, staging, treatment, and early outcomes have been published previously.17,18 Protocols were approved by the Stanford institutional review board and the parents/guardians of participants provided informed consent.
Ped HD1 protocol enrolled patients from 1970 to 1982. Treatment included six cycles of MOPP chemotherapy and low-dose radiation with dose determined by bone age (range, 15 to 25.5 Gy) followed by 10 Gy boosts for select patients with bulky disease or partial response to treatment. Radiation volumes were tailored to the involved nodal station with appropriate margins—mantle, minimantle, or hemimantle for disease above the diaphragm and modified spade, para-aortic, or inverted Y fields for infradiaphragmatic disease.19 Two patients died before receiving radiation and are excluded from SMN analysis.
Ped HD2 protocol enrolled patients from 1982 to 1990. Treatment included six cycles of chemotherapy (three ABVD, three MOPP) administered in alternating fashion. All patients received 15 Gy IFRT with 10 Gy boosts for select patients with bulky disease or partial response following two cycles of chemotherapy. All enrolled children completed primary therapy and are included in this analysis.
Data Collection
Stanford University institutional review board approval was obtained for this retrospective study. The following data were abstracted from medical records: date of birth, sex, date of HL diagnosis, stage, presence of B symptoms, histology, treatment dates, chemotherapy doses, radiation fields and doses, complications of therapy, date and sites of relapse, relapse therapy, date and cause of death, date and clinical status at last contact. Patients without documented death and with last known address in the United States (n = 95) were sent a follow-up questionnaire regarding interval development of SMNs through September 1, 2007; 46 patients (48%) returned the questionnaire. The Social Security Death Index was queried to ascertain unreported deaths. For those with SMN, date of diagnosis, histology, location, proximity to radiation field, treatment, and clinical outcome were determined. All malignancies counted in the population incidence rates of the registry of the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (including breast ductal carcinoma in situ [DCIS]) were considered SMNs. Nonmelanoma skin cancers, meningiomas, and schwannomas were excluded.
Statistical Analysis
Cumulative incidence of SMN from time of HL diagnosis was estimated using the Kaplan-Meier method with adjustment for competing risks to account for those patients who died of recurrent HL or treatment-related complications.20–22 For patients with multiple SMNs, only the time to the first SMN was included. For patients with solid SMNs, disease-free survival (DFS) was defined as the time from first SMN diagnosis to SMN relapse or death from any cause. Overall survival (OS) was defined as the time from first SMN diagnosis to death. Actuarial curves showing the probability of DFS and OS were constructed according to the Kaplan-Meier method.20
Standardized incidence ratios (SIRs) were calculated as the ratio of the observed SMN cases to expected cases. The absolute excess risk (AER) per 10,000 person-years was calculated as the number of observed cases minus the expected cases divided by person-years of follow-up multiplied by 10,000.23 Age-, sex-, and site-specific SEER cancer incidence rates were applied to person-years of follow-up for the cohort to yield the expected number of cases.24 For patients with multiple SMNs, each SMN was counted in the numerator of the SIR following the methodology of the SEER program incidence calculations.25 Patients were considered to be at risk for SMN from the time of HL diagnosis until death or date of last contact. SIR and AER 95% CIs were determined by the Poisson distribution.26
Due to a concern of ascertainment bias in which those patients who developed SMN were more likely to seek medical care and therefore have more complete follow-up, SIR and AER calculations were repeated with the assumption that all patients without a documented SMN or death had complete follow-up through September 1, 2007, and were healthy without SMN. This additional analysis allows a conservative estimate of the lower bound of SIRs.
Univariate associations were evaluated by χ2 test or Fisher's exact test for categoric variables and pooled t-test for continuous variables. Multivariate analysis was undertaken to evaluate the association of SMN with a priori defined potential predictors with P ≤ .1 in univariate analyses. Cox proportional hazards regression was used to evaluate these predictors using chronological age as the time scale to control for the strong association between cancer risk and chronological age.27–29 All pairs of predictors were evaluated for potential interactions. The proportional hazards assumption was evaluated for all variables by generating log-log survival plots for each predictor from the Cox regression model and evaluating at the means of the covariates. Statistical analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Patient Demographics
Fifty-five children were treated on Ped HD1 and 57 on Ped HD2 (Appendix Table A1, online only). Median age at diagnosis was younger for Ped HD1, likely because eligibility was limited to prepubertal children who were felt to be at highest risk for adverse musculoskeletal outcomes with adult high-dose radiation protocols. Based on the promising early results of Ped HD1, Ped HD2 included older adolescents. In addition, children with stage I lymphocyte predominant HL, who tend to be younger, were excluded from Ped HD2.
Ped HD1 Treatment and Outcomes
Fifty three (96.3%) of 55 patients completed primary therapy including radiation to at least one field (eg, right neck, mantle; Appendix Table A2, online only) with median mantle dose of 24 Gy. Median follow-up of these 53 patients is 25.4 years (range, 2.1 to 32.8 years). For those alive at last contact, 27 (56%) of 48 have documented follow-up in the past 5 years and 34 of 48 (73%) have documented follow-up in the past 10 years. Median age at last contact was 33.9 years (range, 7.4 to 44.9 years). Five patients developed relapse at a median of 3.2 years off-therapy (range, 0.6 to 10.6 years); one died of secondary acute myeloid leukemia (AML) after salvage therapy, and another died of complications of bone marrow transplant (BMT) for treatment of relapse. Three are long-term survivors with additional therapy and reported no SMN with 6, 27, and 30 years of follow-up.
Four patients developed secondary leukemia (one acute lymphoblastic leukemia, three AML) at a median of 6.9 years (range, 1.8 to 12.4 years) from HL diagnosis; one occurred after additional therapy for relapse. All died of refractory leukemia. Four patients developed six secondary solid tumors at a median of 17.3 years (range, 8.2 to 29.9 years; Table 1). One patient developed papillary thyroid carcinoma (right lobe after 15 Gy to left neck) and B-precursor acute lymphoblastic leukemia. Two patients with prior stage IV HL with lung involvement developed breast cancer: one with infiltrating ductal carcinoma after 23.5/15 Gy to the mantle/whole lung and one with ductal carcinoma in situ after 25.5/10 Gy mantle/whole lung irradiation. One patient developed three SMNs (bladder paraganglioma, metastatic papillary thyroid carcinoma, and left buttock melanoma) after 25.5 Gy to mantle, para-aortic, and pelvic fields. The melanoma was outside the radiation field.
Table 1.
Characteristics of Pediatric Patients With HL With Solid SMNs
| Age at HL Diagnosis (years) | Sex | Stage | Chemotherapy Regimen (No. of cycles) | Radiation |
Solid SMN | Time to SMN (years) | Age at SMN (years) | Current Status | |
|---|---|---|---|---|---|---|---|---|---|
| Field | Dose (Gy) | ||||||||
| 11.5 | Female | IVA | MOPP (6) | Mantle | 23.5 | Breast invasive ductal carcinoma | 29.9 | 41.4 | Alive, NED |
| Lung | 16 | ||||||||
| 13.6 | Male | IIIA | MOPP (6) | Inverted Y | 24.5 | Bladder paraganglioma | 12.5 | 26.4 | Alive, NED |
| Mantle | 25 | Metastatic papillary thyroid carcinoma | 13.5 | 27.4 | |||||
| Inguinal | 10 | Buttock melanoma (out of radiation field) | 22 | 35.9 | |||||
| 13.4 | Female | IVA | MOPP (4.5) | Mantle | 25 | Breast DCIS | 22.1 | 35.5 | Alive, NED |
| Lung | 10 | ||||||||
| 5 | Male | IA | MOPP (6) | Left hemimantle/spade | 15 | Papillary thyroid carcinoma, right lobe | 8.2 | 13.3 | Dead, refractory leukemia |
| 9.8 | Male | IIA | ABVD/MOPP (6) | Mantle | 15 | Papillary thyroid carcinoma | 18.1 | 27.9 | Alive, NED |
| 14.7 | Female | IIIA | ABVD/MOPP (6) | Minimantle/spade/Waldeyer | 15 | Malignant fibrous histiocytoma, right neck | 9.4 | 24.1 | Alive, NED |
| 15.8 | Female | IVB | ABVD/MOPP (6) | Mantle/inverted Y | 25.2 | Breast invasive ductal carcinoma | 15.4 | 31.3 | Alive, metastatic breast cancer |
| 13.4 | Female | IIA | ABVD/MOPP (6) | Mantle | 25 | Breast DCIS | 12.1 | 25.5 | Alive, NED |
| 12.8 | Female | IVB | ABVD/MOPP (6) | Mantle/inverted Y | 15 | Endometrial stromal sarcoma | 14.3 | 27.1 | Alive, NED |
| 13.9 | Male | IIA | ABVD/MOPP (6) | Mantle | 25 | Chondrosarcoma, scapula | 4 | 17.9 | Alive, disease status unknown |
| 9.3 | Female | IIB | ABVD/MOPP (6) | Mantle | 22.5 | Papillary thyroid carcinoma | 24.4 | 33.7 | Alive, NED |
| 14.7 | Male | IIIA | ABVD/MOPP (6) | Mantle/spade | 15 + 10 Gy boost right neck | Metastatic neuroendocrine tumor (thyroid) | 13.3 | 27.9 | Death |
| 15.1 | Male | IIIB | ABVD/MOPP (6) | Mantle/spade | 15 + 15 Gy boost left neck | MPNST, L3 nerve root | 17.7 | 32.8 | Alive, NED |
| 14.6 | Female | IIA | ABVD/MOPP (6) | Mantle | 15 + 10 Gy boost mediastinum | Breast DCIS | 17.6 | 32.2 | Alive, NED |
| 13.9 | Female | IIA | ABVD/MOPP (6) | Mantle | 15 | Breast invasive ductal carcinoma | 15.6 | 29.6 | Alive, metastatic breast cancer |
NOTE. Radiation fields—mantle: bilateral axillary, mediastinal, hilar, cervical, supra- and infraclavicular lymph nodes; hemimantle: unilateral mantle field; minimantle: bilateral cervical, supraclavicular, and axillary lymph nodes; spade: para-aortic lymph nodes and spleen/splenic pedicle; inverted Y: spleen, para-aortic, iliac, hypogastric, and inguinal lymph nodes.
Abbreviations: HL, Hodgkin's lymphoma; SMN, second malignant neoplasm; MOPP, mechlorethamine, vincristine, procarbazine, prednisone; NED, no evidence of disease; DCIS, ductal carcinoma in situ; ABVD, doxorubicin, bleomycin; vinblastine; dacarbazine; MPNST, malignant peripheral nerve sheath tumor; L3, third lumbar vertebrae.
Ped HD2 Treatment and Outcomes
All 57 patients completed primary therapy including radiation to at least one field (eg, right neck, mantle; Appendix Table A3, online only) with median mantle dose 22 Gy. Median follow-up is 19 years (range, 2 to 25.4 years). For those alive at last contact, 38 (72%) of 53 have documented follow-up in the past 5 years and 43 (81%) of 53 have documented follow-up in the past 10 years. Median age at last follow-up is 30.6 years (range, 8.9 to 41.6 years). Four patients developed relapse at a median of 1.6 years (range, 0.9 to 3.9 years); one is a long-term survivor without SMN 16 years after autologous BMT. Three patients died following relapse—one from refractory HL, one from disseminated cytomegalovirus infection after BMT, and one from pulmonary fibrosis 13 years after BMT.
There were no secondary leukemias. Eleven patients (19.3%) developed a second solid tumor at a median of 15.4 years (range, 4 to 24.4 years). Each SMN occurred in or adjacent to a prior radiation field (Table 1). Three patients developed thyroid carcinoma after receiving 15 to 25.5 Gy radiation to the neck; one died of metastatic undifferentiated neuroendocrine tumor thought to have originated from the thyroid. Four (19%) of 21 women developed breast cancer after receiving 15 to 25.5 Gy to the mantle field including the axillae. Three presented with localized disease, one of whom relapsed with metastatic disease after initial therapy while the fourth presented with widely metastatic disease. Four patients developed localized sarcomas in fields irradiated to 15 to 25.5 Gy.
Survival and Cumulative Incidence of SMN
For the combined cohort of 110 patients, the estimated cumulative incidence of first SMN was 17% at 20 years (95% CI, 10.5 to 26.7) and 29.4% at 30 years (95% CI, 16 to 50) after HL diagnosis (Fig 1). The majority of the risk is due to solid tumors with a cumulative incidence of 14.3% at 20 years (95% CI, 8.4 to 24) and 27.2% at 30 years (95% CI, 13.9 to 49) while the cumulative incidence of secondary leukemia plateaued at 4% at 15 years (95% CI, 1.5 to 10.3). Only one SMN (AML) occurred in a patient who received additional therapy for HL relapse; all other patients who developed SMN had received only primary HL therapy. The actuarial death rate was 4.1% for secondary leukemia, 4.1% for refractory HL or complications of therapy (ie, infection), and 1.3% for secondary solid tumor. Of the 15 patients who developed a solid SMN, two have died and two are alive with metastatic breast cancer, with mean (± SE) 5-year OS 85 ± 10% and 5-year DFS 76 ± 12%.
Fig 1.
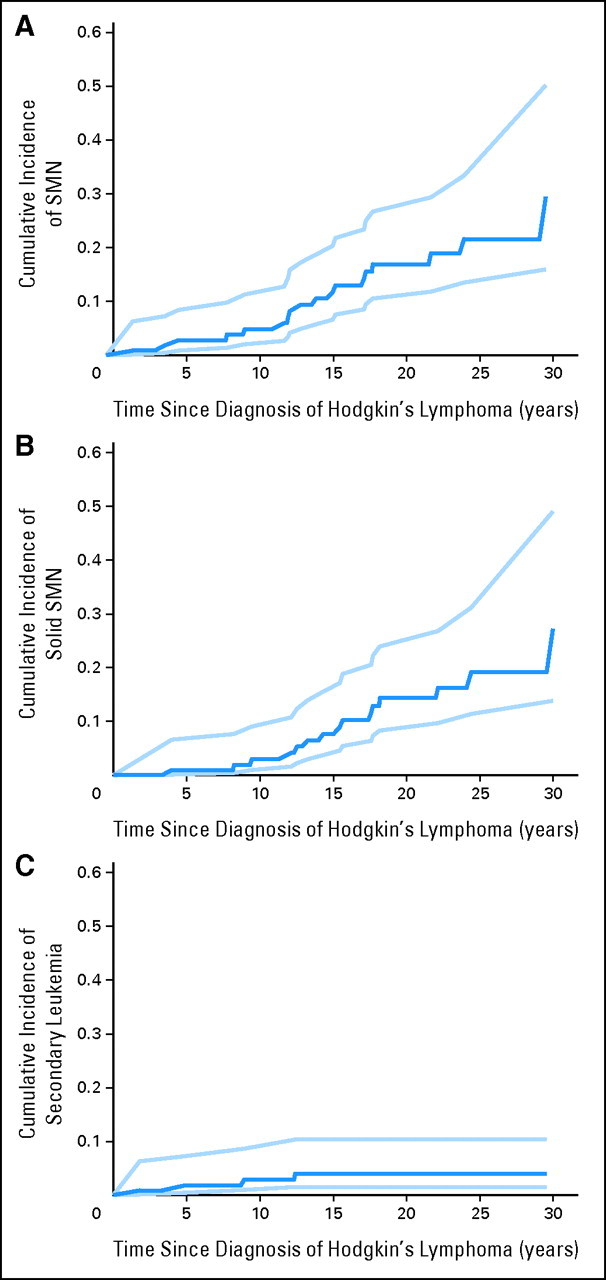
Cumulative incidence (dark blue line) of (A) any second malignant neoplasm (SMN), (B) solid SMN, and (C) secondary leukemia from the date of Hodgkin's lymphoma (HL) diagnosis with 95% CIs (light blue lines). For subjects with multiple SMNs, only the time to the first SMN was included.
SIRs
Observed and expected numbers of SMN by age, sex, and site are presented in Table 2. The SIR for any SMN was 22.9 (95% CI, 14.2 to 35) with an AER of 93.7 cases per 10,000 person-years (95% CI, 56.4 to 145.5). SIRs and AERs were elevated for leukemia, thyroid carcinoma, breast carcinoma, and sarcomas; there were no reported cases of gastrointestinal or lung carcinoma. SIRs remained elevated even when recalculated with the “best-case scenario” assumption that those with incomplete follow-up were alive and free from SMN through September 1, 2007 (Table 3).
Table 2.
SIRs and AER of SMN
| Parameter | Patients | Person-Years | Observed SMN | Expected SMN | SIR | 95% CI | AER* | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Any SMN | 110 | 2,143.4 | 21 | 0.916 | 22.9 | 14.2 to 35 | 93.7 | 56.4 to 145.5 |
| Sex | ||||||||
| Male | 75 | 1,435.3 | 11 | 0.505 | 21.8 | 10.9 to 39 | 73.1 | 34.7 to 133.6 |
| Female | 35 | 708.1 | 10 | 0.392 | 25.5 | 12.2 to 46.9 | 135.7 | 62.2 to 254.2 |
| Age at HL diagnosis, years | ||||||||
| < 11 | 53 | 1,057.8 | 4 | 0.357 | 11.2 | 3.1 to 28.7 | 34.4 | 6.9 to 93.4 |
| ≥ 11 | 57 | 1,085.6 | 17 | 0.557 | 30.5 | 17.8 to 48.9 | 151.5 | 86.1 to 245.6 |
| SMN site | ||||||||
| Leukemia | 110 | 2,143.4 | 4 | 0.044 | 90.9 | 24.8 to 232.8 | 18.5 | 4.9 to 47.6 |
| Solid tumor | 110 | 2,143.4 | 17 | 0.86 | 19.8 | 11.5 to 31.7 | 75.3 | 42.2 to 123.0 |
| Thyroid | 110 | 2,143.4 | 5 | 0.094 | 53.2 | 17.3 to 124.1 | 22.9 | 7.1 to 54.0 |
| Sarcoma | 110 | 2,143.4 | 4 | 0.045 | 88.9 | 24.2 to 227.6 | 18.5 | 4.9 to 47.6 |
| Breast (females) | 35 | 708.1 | 6 | 0.082 | 72.3 | 26.5 to 157.3 | 83.6 | 29.9 to 183.3 |
Abbreviations: SIR, standardized incidence ratio; AER, absolute excess risk; SMN, second malignant neoplasm; HL, Hodgkin's lymphoma.
Calculated per 10,000 person-years.
Table 3.
Comparison of SIRs Based on Actual Versus Theoretical Complete Follow-Up
| Parameter | Actual Follow-Up |
Complete Follow-Up* |
||
|---|---|---|---|---|
| SIR | 95% CI | SIR | 95% CI (lower bound) | |
| Any SMN | 22.9 | 14.2 to 35 | 17 | 10.5 |
| Sex | ||||
| Male | 21.8 | 10.9 to 39 | 14.3 | 7.1 |
| Female | 25.5 | 12.2 to 46.9 | 21.1 | 10.1 |
| Age at HL diagnosis, years | ||||
| < 11 | 11.2 | 3.1 to 28.7 | 7.4 | 2 |
| ≥ 11 | 30.5 | 17.8 to 48.9 | 22 | 12.8 |
| SMN site | ||||
| Leukemia | 90.9 | 24.8 to 232.8 | 74.1 | 20.2 |
| Solid tumor | 19.8 | 11.5 to 31.7 | 13.7 | 8 |
| Thyroid | 53.2 | 17.3 to 124.1 | 37 | 12 |
| Sarcoma | 88.9 | 24.2 to 227.6 | 71.4 | 19.5 |
| Breast (female) | 72.3 | 26.5 to 157.3 | 56.1 | 20.6 |
Abbreviations: SIR, standardized incidence ratio; HL, Hodgkin lymphoma; SMN, second malignant neoplasm.
Theoretical complete follow-up assumes that those lost to follow-up are alive without SMN through September 1, 2007.
Analysis of Factors Associated With SMN
In univariate analysis, only female sex and older age at HL diagnosis (≥ 11 years) were associated with SMN (Table 4) and therefore included in the Cox proportional hazards model. Mean follow-up time and age at the time of last contact did not differ between those with and without SMN. Multivariate analysis revealed nonstatistically significant associations of SMN with older age at HL diagnosis (HR, 2.7; 95% CI, 0.7 to 10.4) and female sex (HR, 2.0; 95% CI, 0.8 to 5.2). Stage, chemotherapy regimen, and maximum radiation doses did not significantly alter parameter estimates. Six (17%) of 35 women developed breast cancer at a median of 16.6 years (range, 12.1 to 29.9 years); univariate analysis revealed no statistically significant risk factors (Appendix Table A4, online only), although only one received pelvic radiation and none reported a history of ovarian failure or early menopause.
Table 4.
Univariate Analysis of Risk Factors Associated With SMN Development
| Parameter | SMN (n = 18) | No SMN (n = 92) | P |
|---|---|---|---|
| Sex | |||
| Male | 8 | 67 | .02 |
| Female | 10 | 25 | |
| Age at HL diagnosis, years | |||
| < 11 | 3 | 50 | |
| ≥ 11 | 15 | 42 | |
| Mean | 12.9 | 10.3 | .003 |
| SD | 2.6 | 3.5 | |
| Range | 5-15.8 | 1.7-17.6 | |
| Stage | |||
| I/II | 7 | 43 | .54 |
| III/IV | 11 | 49 | |
| Chemotherapy | |||
| MOPP | 7 | 46 | .39 |
| ABVD/MOPP | 11 | 46 | |
| Mean radiation dose, Gy | |||
| Left neck | 22.1 | 19.1 | .17 |
| Right neck | 19.9 | 19.9 | .99 |
| Mantle/minimantle | 22.2 | 19.8 | .21 |
| Spade/inverted Y | 10.8 | 11.5 | .79 |
| Relapse | |||
| Yes | 1 | 8 | 1.00 |
| No | 17 | 84 | |
| Mean follow-up time, years | 19 | 19.6 | .79 |
| SD | 7.2 | 8.1 | |
| Range | 2.8-30 | 2-32.9 | |
| Mean age at last contact, years | 32 | 29.8 | .34 |
| SD | 7.7 | 8.6 | |
| Range | 15.5-41.8 | 7.4-44.9 |
Abbreviations: SMN, second malignant neoplasm; HL, Hodgkin's lymphoma; SD, standard deviation; MOPP, mechlorethamine, vincristine, procarbazine, and prednisone; ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine.
DISCUSSION
The Stanford combined modality protocols achieved their original goal of providing curative HL therapy while decreasing the musculoskeletal sequelae associated with radiation doses of 40 to 44 Gy in children. Subsequently, as reports of SMN among HL survivors treated with high-dose radiation accumulated, we theorized that low-dose radiation-based protocols might have the additional benefit of decreased SMN incidence or longer latency time to the development of SMN. However, SMN cumulative incidence, SIR, and AER are similar to those from studies in which most patients received higher radiation doses (Table 5), even in the “best-case scenario” with the assumption of complete follow-up without SMN for those lost to follow-up. The median time to solid SMN (15.4 years) is comparable to that reported for the Late Effects Study Group cohort (16.9 years)10 and the cohorts reported by Wolden (15.5 years)2 and Metayer (15 years).4
Table 5.
SIRs, AERs, and Cumulative Incidence of SMN in Pediatric Hodgkin's Lymphoma Cohorts With Median Follow-Up > 15 years
| Reference | No. of Subjects | Median Age at Diagnosis (years) | Median Follow-Up (years) | Any SMN |
Solid SMN |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative Incidence (%) |
SIR | 95% CI | AER* | 95% CI | Cumulative Incidence (%) |
SIR | ||||||
| 20 Year | 30 Year | 20 Year | 30 Year | |||||||||
| Stanford | ||||||||||||
| Actual follow-up | 110 | 11.3 | 20.6 | 17 | 29.4 | 22.9 | 14.2 to 35 | 93.7 | 56.4 to 145.5 | 14.3 | 27.2 | 19.8 |
| Complete follow-up† | 110 | 11.3 | 20.6 | 17 | 10.5 to 25.9 | 74.3 | 44.2 to 116 | 13.7 | ||||
| Bhatia et al10 | 1,380 | 11.7 | 17 | 9.3 | 23.7 | 18.5 | 15.6 to 21.7 | 65 | 5.9 | 20.1 | 18.5 | |
| Green et al6 | 182 | 15.3 (mean) | 17.1 | 12.7 | 26.3 | Male: 9.4 | 4 to 18.5 | |||||
| Female: 10.2 | 5.6 to 17 | |||||||||||
| CCSS7,37,45 | 1,815‡ | ≈14‡ | ≈18‡ | 7.6 | 9.7 | 8.1 to 11.6 | 51.3 | |||||
| Reference | Solid SMN |
Breast Carcinoma |
Thyroid Carcinoma |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SIR 95% CI | AER* | 95% CI | SIR | 95% CI | AER* | 95% CI | SIR | 95% CI | AER* | 95% CI | |
| Stanford | |||||||||||
| Actual follow-up | 11.5 to 31.7 | 75.3 | 42.2 to 123 | 72.3 | 26.5 to 157 | 83.6 | 29.9 to 183.3 | 53.2 | 17.3 to 124 | 22.9 | 7.1 to 54.0 |
| Complete follow-up† | 8 to 21.9 | 59.2 | 32.6 to 97.6 | 56.1 | 20.6 to 122 | 75.2 | 26.7 to 165.2 | 37 | 12 to 86.4 | 18.3 | 5.6 to 43.4 |
| Bhatia et al10 | 15.2 to 22.3 | 51 | 55.5 | 39.5 to 75.9 | 53 | 36.4 | 21.9 to 56.8 | 9 | |||
| Green et al6 | 7.8 | 2.1 to 19.9 | Male: 158.8 | 32.7 to 463.9 | |||||||
| Female: 38 | 7.8 to 111.1 | ||||||||||
| CCSS7,37,45 | 26.3 | 20.2 to 33.7 | 18.3 | 11.4 to 27.6 | |||||||
NOTE. Values and CIs are presented where available from the published studies.
Abbreviations: SIR, standardized incidence ratios; AER, absolute excess risk; SMN, second malignant neoplasm; CCSS, Childhood Cancer Survivor Study.
Calculated per 10,000 person-years.
Assumes that those lost to follow-up are alive without SMN through September 1, 2007.
CCSS publications report entire cohort, not simply patients with Hodgkin's lymphoma, so specific follow-up duration and age data for the Hodgkin's lymphoma subgroup are estimated based on data from different CCSS publications.
Five patients developed thyroid carcinoma, the only solid SMN among those treated for HL before age 10 years. The young age of the patients in our cohort may contribute to the high observed rate of thyroid cancer, consistent with reports that at radiation doses below 20 Gy, thyroid carcinoma risk is highest among patients diagnosed with their primary cancer before age 10 years.30–32 The significant incidence of thyroid carcinoma with low-dose radiation is not surprising given the nonlinear radiation dose-response, in which thyroid SMN risk increases from 0 to 20 Gy and then decreases, with few cases occurring at doses above 40 Gy due to cell killing.33–34 Therefore, with current low-dose radiation regimens, we may expect to see stable or even increasing rates of secondary thyroid carcinoma, particularly in children treated at very young ages.
Breast cancer is the most common SMN among female HL survivors and is strongly linked to supradiaphragmatic radiation and younger age (< 20 years) at the time of HL treatment.35–40 In our cohort, breast cancer incidence was similar to other pediatric HL studies despite lower radiation doses (Table 5). In contrast, Inskip and colleagues41 reported a linear relationship between breast cancer risk and radiation dose based on data from the Childhood Cancer Survivor Study, with decreased risk among those women receiving 11.4 to 29.9 Gy compared to those receiving 30 Gy or more. The high breast cancer SIR in our cohort, despite mantle radiation doses between 15 and 25.5 Gy, may be due to small cohort size with wide confidence intervals. In addition, three patients were diagnosed with DCIS through screening; they may represent an ascertainment bias which inflates the SIR. When the DCIS cases are excluded, the SIR remains elevated at 36.1 (95% CI, 7.5 to 105.6) with an AER of 41 cases per 100,000 person-years.
Alternatively, Inskip and colleagues41 noted that breast cancer risk is substantially mitigated by ovarian radiation ≥ 5 Gy at the time of initial treatment. It is possible that the proportion of women in our cohort who received ovarian radiation is lower than the comparison cohorts in Table 5, diminishing our ability to detect a protective effect of lower mantle radiation doses. Also, in the Inskip et al Childhood Cancer Survivor Study cohort, only 50% of patients received any alkylating agent and 30% received any anthracycline, versus 100% and 52% in our cohort, respectively. It is possible that some of the benefit of low-dose radiation is offset by an increased SMN risk conferred by specific chemotherapy agents.
Sarcoma risk increases significantly at radiation doses higher than 30 Gy; most sarcomas are reported in fields treated to at least 35 Gy.2,42 Therefore, a notable decrease in secondary sarcoma incidence might be expected with lower radiation doses. However, our SIR for secondary sarcoma remained highly elevated, and of the four sarcomas, three occurred in 15 Gy radiation fields. The four reported sarcomas occurred in patients treated with MOPP/ABVD chemotherapy. Henderson and colleagues43 found that both alkylating agent and anthracycline chemotherapy are associated with increased risk of secondary sarcomas. In our cohort, the occurrence of more sarcoma cases with fewer cycles of MOPP suggests that the anthracycline exposure from the ABVD cycles may be an important contributor. Alternatively, this finding may reflect the more complete follow-up of the MOPP/ABVD cohort compared to the MOPP only group.
Notably, there were no reported cases of lung or gastrointestinal carcinoma which may reflect the lower radiation doses or the young median age at last follow-up (31 years). It is possible that these SMN subtypes may emerge as cohort members continue to age. However, the lack of these SMN subtypes to date is encouraging because the Late Effects Study Group reported multiple lung and gastrointestinal SMN with younger median age at last follow-up (27.8 years) and shorter median follow-up time (17 v 20.6 years).10
Due to the long latency to the development of solid SMN, current follow-up data necessarily reflect past therapies. HL treatment has continued to evolve since these early Stanford protocols, with refinements in chemotherapy regimens and radiation techniques to limit exposure of normal tissues. Models suggest that lower radiation doses and smaller volumes will decrease the risk of certain SMNs such as breast cancer,41 particularly with shielding of the axillae,44 while risk of thyroid carcinoma is likely to remain elevated. However, the outcomes for this cohort suggest that children who receive combined-modality therapy with low-dose radiation remain at significant risk for morbidity and mortality from sarcomas, thyroid, and breast carcinomas and will continue to require aggressive surveillance. Future therapeutic protocols for pediatric patients with HL should pursue radiation dose reduction or elimination of radiation when feasible. Improved understanding of genetic predisposition and modifying factors such as hormone status and the impact of specific chemotherapy agents such as anthracyclines will help to identify those patients at greatest risk of SMN.
Note Added in Proof
Consistent with the ongoing significant SMN risk observed in this study, two additional patients with SMN have self-identified since the submission of this article. One male from Ped HD2 reported a grade 3 leiomyosarcoma of the groin at 22 years after 15 Gy to pelvic field and MOPP/ABVD chemotherapy, and one male from Ped HD1 reported papillary thyroid carcinoma at 31 years after 25.5 Gy to the neck and MOPP chemotherapy.
Appendix
,,,
Table A1.
Patient Characteristics for Pediatric Hodgkin Lymphoma Protocols Ped HD1 (1970-1982) and Ped HD2 (1982-1990)
| Characteristic | Ped HD1 (n = 55)* |
Ped HD2 (n = 57) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Median age at diagnosis, years | 10.5 | 12.4 | ||
| Range | 1.7-14.9 | 4.4-17.6 | ||
| Sex | ||||
| Male | 40 | 73 | 36 | 63 |
| Female | 15 | 27 | 21 | 37 |
| Stage | ||||
| I/II | 27 | 49 | 23 | 40 |
| III/IV | 28 | 51 | 34 | 60 |
| B symptoms† | 10 | 18 | 18 | 31.6 |
| Histology | ||||
| Nodular sclerosing | 35 | 64 | 42 | 74 |
| Mixed cellularity | 14 | 25 | 9 | 16 |
| Lymphocyte predominant | 5 | 9 | 2 | 3 |
| Other | 1 | 2 | 4 | 7 |
Includes two patients who died due to progressive disease prior to receiving radiation.
B symptoms include persistent unexplained fever, drenching night sweats, and unintentional weight loss > 10% of baseline weight.
Table A2.
Radiation Doses for Patients Treated on the Ped HD1 Protocol
| Dose (Gy) | No. of Patients Treated |
|||
|---|---|---|---|---|
| Left Neck | Right Neck | Mantle/Minimantle | Spade/Inverted Y | |
| 0 | 8 | 8 | 6 | 19 |
| < 15 | 0 | 1 | 0 | 0 |
| 15-20 | 18 | 20 | 22 | 20 |
| 20.01-25.00 | 16 | 14 | 16 | 11 |
| 25.01-30.00 | 6 | 7 | 6 | 3 |
| > 30 | 5 | 3 | 3 | 0 |
| Median dose (excluding those with no radiation to field) | 25 | 24 | 24 | 20 |
Table A3.
Radiation Doses for Patients Treated on the on Ped HD2 Protocol
| Dose (Gy) | No. of Patients Treated |
|||
|---|---|---|---|---|
| Left Neck | Right Neck | Mantle/Minimantle | Spade/Inverted Y | |
| 0 | 3 | 2 | 1 | 23 |
| < 15 | 1 | 1 | 1 | 1 |
| 15-20 | 28 | 27 | 27 | 28 |
| 20.01-25.00 | 16 | 20 | 20 | 4 |
| 25.01-30.00 | 8 | 6 | 7 | 1 |
| > 30 | 1 | 1 | 1 | 0 |
| Median dose (excluding those with no radiation to field) | 15.8 | 18 | 22 | 15 |
Table A4.
Univariate Analysis of Risk Factors Associated With Development of Secondary Breast Cancer in Female Patients With HL (n = 35)
| Factor | Breast Cancer (n = 6) | No Breast Cancer (n = 29) | P* |
|---|---|---|---|
| Age at HL diagnosis, years | |||
| < 11 | 0 | 9 | |
| ≥ 11 | 6 | 20 | .31 |
| Mean | 13.8 | 11.8 | |
| SD | 1.4 | 3.1 | |
| Range | 11.5-15.8 | 4.3-15 | .13 |
| Stage | |||
| I/II | 3 | 14 | 1.00 |
| III/IV | 3 | 15 | |
| Chemotherapy | |||
| MOPP | 2 | 12 | 1.00 |
| ABVD/MOPP | 4 | 17 | |
| Mean mantle radiation dose, Gy | 23.3 | 18.5 | .29 |
| Range | 15-25.6 | 0-43 | |
| Pelvic radiotherapy | |||
| Yes | 1 | 8 | 1.00 |
| No | 5 | 21 | |
| Mean follow-up time, years | 22.9 | 19.7 | .39 |
| SD | 3.3 | 6.8 | |
| Range | 17.2-30 | 2-32.9 | |
| Mean age at last contact | 36.7 | 31.4 | .14 |
| SD | 2.7 | 6.6 | |
| Range | 30.6-41.8 | 14.5-44.1 |
Abbreviations: HL, Hodgkin's lymphoma; SD, standard deviation; MOPP mechlorethamine, vincristine, procarbazine, prednisone; ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine.
Footnotes
Supported in part by the Diana R. & Daniel J. Riccio Fund, Stanford University.
Presented in part at the 45th Annual Meeting of the American Society of Clinical Oncology, Orlando, FL, May 29-June 2, 2009.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Maureen M. O'Brien, Alice S. Whittemore, Michael P. Link
Administrative support: Michael P. Link
Provision of study materials or patients: Maureen M. O'Brien, Michael P. Link
Collection and assembly of data: Maureen M. O'Brien
Data analysis and interpretation: Maureen M. O'Brien, Raymond R. Balise, Alice S. Whittemore
Manuscript writing: Maureen M. O'Brien, Sarah S. Donaldson, Alice S. Whittemore, Michael P. Link
Final approval of manuscript: Maureen M. O'Brien, Sarah S. Donaldson, Raymond R. Balise, Alice S. Whittemore, Michael P. Link
REFERENCES
- 1.Hudson MM, Donaldson SS. Treatment of pediatric Hodgkin's lymphoma. Semin Hematol. 1999;36:313–323. [PubMed] [Google Scholar]
- 2.Wolden SL, Lamborn KR, Cleary SF, et al. Second cancers following pediatric Hodgkin's disease. J Clin Oncol. 1998;16:536–544. doi: 10.1200/JCO.1998.16.2.536. [DOI] [PubMed] [Google Scholar]
- 3.Van Leeuwen FE, Klokmann WJ, vant Veer MB, et al. Long-term risk of second malignancies in survivors of Hodgkin's disease treated during adolescence or young adulthood. J Clin Oncol. 2000;18:487–497. doi: 10.1200/JCO.2000.18.3.487. [DOI] [PubMed] [Google Scholar]
- 4.Metayer C, Lynch CF, Clarke A, et al. Second cancers among long-term survivors of Hodgkin's disease diagnosed in childhood and adolescence. J Clin Oncol. 2000;18:2435–2443. doi: 10.1200/JCO.2000.18.12.2435. [DOI] [PubMed] [Google Scholar]
- 5.Beaty O, Hudson MM, Greenwald C, et al. Subsequent malignancies in children and adolescents after treatment for Hodgkin's disease. J Clin Oncol. 1995;13:603–609. doi: 10.1200/JCO.1995.13.3.603. [DOI] [PubMed] [Google Scholar]
- 6.Green DM, Hyland A, Barcos MP, et al. Second malignant neoplasms after treatment for Hodgkin's disease in childhood or adolescence. J Clin Oncol. 2000;18:1492–1499. doi: 10.1200/JCO.2000.18.7.1492. [DOI] [PubMed] [Google Scholar]
- 7.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 8.Sankila R, Garwicz S, Olsen JH, et al. Risk of subsequent malignant neoplasms among 1641 Hodgkin's disease patients diagnosed in childhood and adolescence: A population-based cohort study in the five Nordic countries. J Clin Oncol. 1996;14:1442–1446. doi: 10.1200/JCO.1996.14.5.1442. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin's disease. N Engl J Med. 1996;334:745–751. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia S, Yasui Y, Robison LL, et al. High-risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 11.Schellong G, Riepenhausen M, Creutzig U, et al. Low risk of secondary leukemias after chemotherapy without mechlorethamine in childhood Hodgkin's disease: German-Austrian Pediatric Hodgkin's Disease Group. J Clin Oncol. 1997;15:2247–2253. doi: 10.1200/JCO.1997.15.6.2247. [DOI] [PubMed] [Google Scholar]
- 12.Van Leeuwen FE, Klokman WJ, Hagenbeek A, et al. Second cancer risk following Hodgkin's disease: A 20-year follow-up study. J Clin Oncol. 1994;12:312–325. doi: 10.1200/JCO.1994.12.2.312. [DOI] [PubMed] [Google Scholar]
- 13.Salloum E, Doria R, Schubert W, et al. Second solid tumors in patients with Hodgkin's disease cured after radiation or chemotherapy plus adjuvant low-dose radiation. J Clin Oncol. 1996;14:2435–2443. doi: 10.1200/JCO.1996.14.9.2435. [DOI] [PubMed] [Google Scholar]
- 14.Koontz BF, Kirkpatrick JP, Clough RW, et al. Combined modality therapy versus radiotherapy alone for treatment of early-stage Hodgkin's disease: Cure balanced against complications. J Clin Oncol. 2006;24:605–611. doi: 10.1200/JCO.2005.02.9850. [DOI] [PubMed] [Google Scholar]
- 15.Chow LM, Nathan PC, Hodgson DC, et al. Survival and late effects in children with Hodgkin's lymphoma treated with MOPP/ABV and low-dose, extended-field irradiation. J Clin Oncol. 2006;24:5735–5741. doi: 10.1200/JCO.2006.05.6879. [DOI] [PubMed] [Google Scholar]
- 16.Willman KY, Cox RS, Donaldson SS. Radiation induced height impairment in pediatric Hodgkin's disease. Int J Radiat Oncol Biol Phys. 1994;28:85–92. doi: 10.1016/0360-3016(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson SS, Link MP. Combined modality treatment with low-dose radiation and MOPP chemotherapy for children with Hodgkin's disease. J Clin Oncol. 1987;5:742–749. doi: 10.1200/JCO.1987.5.5.742. [DOI] [PubMed] [Google Scholar]
- 18.Hunger SP, Link MP, Donaldson SS. ABVD/MOPP and low-dose involved-field radiotherapy in pediatric Hodgkin's disease: The Stanford experience. J Clin Oncol. 1994;12:2160–2166. doi: 10.1200/JCO.1994.12.10.2160. [DOI] [PubMed] [Google Scholar]
- 19.Hudson MM, Donaldson SS. Hodgkin's disease. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. ed 4. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. pp. 645–674. [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:475–481. [Google Scholar]
- 21.Haesook TK. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13:559–565. doi: 10.1158/1078-0432.CCR-06-1210. [DOI] [PubMed] [Google Scholar]
- 22.Allison PD. Cary, NC: SAS Institute Inc; 1995. Survival Analysis Using SAS®: A practical guide; pp. 185–208. [Google Scholar]
- 23.Travis LB. Evaluation of the risk of therapy-associated complications in survivors of pediatric Hodgkin lymphoma. Am Soc Hematol Ed Book. 2007:192–196. doi: 10.1182/asheducation-2007.1.192. [DOI] [PubMed] [Google Scholar]
- 24. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use, Nov 2006 Sub (1973-2004) - Linked To County Attributes - Total U.S., 1969-2004 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007, based on the November 2006 submission.
- 25.Horner MJ, Ries LAG, Krapcho M, et al., editors. Bethesda, MD: National Cancer Institute, based on November 2008 SEER data submission, posted to the SEER web site; 2009. SEER Cancer Statistics Review, 1975-2006. http://seer.cancer.gov/csr/1975_2006/ [Google Scholar]
- 26.Bailar JC, Ederer F. Significance factors for the ratio of a Poisson variable to its expectation. Biometrics. 1964;20:639–643. [Google Scholar]
- 27.Yasui Y, Liu Y, Neglia JP, et al. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158:1108–1113. doi: 10.1093/aje/kwg278. [DOI] [PubMed] [Google Scholar]
- 28.Korn EL, Graubard BI, Midthune G. Time-to-event analysis of longitudinal follow-up of a survey: Choice of the time scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 29.Cheung YB, Gao F, Khoo KS. Age at diagnosis and the choice of survival analysis methods in cancer epidemiology. J Clin Epidemiol. 2003;56:38–43. doi: 10.1016/s0895-4356(02)00536-x. [DOI] [PubMed] [Google Scholar]
- 30.Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: A pooled analysis of seven studies. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 31.Tucker MA, Morris-Jones PH, Boice JD, et al. Therapeutic radiation at a young age is linked to secondary thyroid cancer: The Late Effects Study Group. Cancer Res. 1991;51:2885–2888. [PubMed] [Google Scholar]
- 32.Sigurdson AJ, Ronckers CM, Mertens AC, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet. 2005;365:2014–2023. doi: 10.1016/S0140-6736(05)66695-0. [DOI] [PubMed] [Google Scholar]
- 33.Ronckers CM, Sigurdson AJ, Stovall M, et al. Thyroid cancer in childhood cancer survivors: A detailed evaluation of radiation dose response and its modifiers. Radiat Res. 2006;166:618–628. doi: 10.1667/RR3605.1. [DOI] [PubMed] [Google Scholar]
- 34.Gray LH. Radiation biology and cancer. Cellular Radiation Biology: A Collection of Papers Presented at the Eighteenth Annual Symposium on Fundamental Cancer Research, 1964.; Baltimore, MD: Williams and Wilkins; 1965. pp. 7–25. [Google Scholar]
- 35.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among women with Hodgkin disease. JAMA. 2003;290:465–474. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 36.Guibout C, Adjadj E, Rubino C, et al. Malignant breast tumors after radiotherapy for a first cancer during childhood. J Clin Oncol. 2005;23:197–204. doi: 10.1200/JCO.2005.06.225. [DOI] [PubMed] [Google Scholar]
- 37.Kenney LB, Yasui Y, Inskip OD, et al. Breast cancer after childhood cancers: A report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141:590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 38.Van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin's disease. J Natl Cancer Inst. 2003;95:972–980. doi: 10.1093/jnci/95.13.971. [DOI] [PubMed] [Google Scholar]
- 39.Boice JD., Jr Radiation and breast carcinogenesis. Med Pediatr Oncol. 2001;36:508–513. doi: 10.1002/mpo.1122. [DOI] [PubMed] [Google Scholar]
- 40.Hancock SL, Tucker MA, Hoppe RT. Breast cancer after treatment of Hodgkin's disease. J Natl Cancer Inst. 1993;85:25–31. doi: 10.1093/jnci/85.1.25. [DOI] [PubMed] [Google Scholar]
- 41.Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:3901–3907. doi: 10.1200/JCO.2008.20.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menu-Branthomme A, Rubino C, Shamsaldin A, et al. Radiation dose, chemotherapy, and risk of soft tissue sarcoma after solid tumors during childhood. Int J Cancer. 2004;110:87–93. doi: 10.1002/ijc.20002. [DOI] [PubMed] [Google Scholar]
- 43.Henderson TO, Whitton J, Stovall M, et al. Secondary sarcomas in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2007;99:300–308. doi: 10.1093/jnci/djk052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodgson DC, Koh ES, Tran TH, et al. Individualized estimates of second cancer risks after contemporary radiation therapy for Hodgkin lymphoma. Cancer. 2007;110:2576–2586. doi: 10.1002/cncr.23081. [DOI] [PubMed] [Google Scholar]
- 45.Sklar C, Whitton J, Mertens A, et al. Abnormalities of the thyroid in survivors of Hodgkin's disease: Data from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2000;85:3227–3232. doi: 10.1210/jcem.85.9.6808. [DOI] [PubMed] [Google Scholar]


