Highlights
-
•
The dynamic load was generated at basitemporal skull if prosthesis was not anchored to this loci.
-
•
Via the parietal tuber-temporozygomatic suture line, this dynamic load was passed to the proximal pterion point region, which eventually generated fatigue effects on the prosthesis and eventually fracture.
-
•
The cranioplasty prosthesis shall be well anchored onto the basitemporal skull to prevent fatigue effects of the prosthesis.
Keywords: Cranioplasty, Prosthesis, Fracture, Anchoring location
Abstract
Introduction
The cranioplasty is a classical surgical procedure to repair large skull defects. The prosthesis fracture was one rare complication following cranioplasty, which was only known to happen in traumatic head injury or child growing skull.
Presentation of case
In the current report, we documented the first reported case of cranioplasty prosthesis fracture in an adult neurological trauma patient at the proximal pterion point region without head trauma. During the first cranioplasty, due to the cerebromalacia at temporal lobe, patient’s temporalis muscle was not stripped from the dura mater and the prosthesis was anchored outside the temporalis muscle. Thus, no screw was used for anchoring the prosthesis at the basitemporal skull. The prosthesis fracture was observed on 12th-month post-surgically at the proximal pterion point region. During the second cranioplasty, the temporalis muscle was semi-partitioned from the back due to cerebromalacia recovery and five screws were used to anchor the prosthesis onto the basitemporal skull. The follow-up result was unremarkable on 21st-month post-second-cranioplasty.
Discussion
A dynamic load was generated on the prosthesis due to head-pillow contact during sleeping. Via the parietal tuber-temporozygomatic suture line, this inward load generates an outward force at the proximal pterion point region, where became a shearing force locating just right below the lowest screw anchoring in this region. This shearing force eventually led to prosthesis fracture at the proximal pterion point due to the fatigue effect.
Conclusion
This case presented the importance of prosthesis anchoring location on the skull, especially when temporalis muscle was required to be preserved due to clinical necessity.
1. Introduction
To meet both cosmetic and functional requirement after large skull defects, cranioplasty is carried out to alleviate the “syndrome of the trephined” due to direct pressure from the atmosphere onto the scalp and dura. Although the surgical procedure is quite straightforward in most cases, various complications following cranioplasty have been reported [1]. Among all complications, prosthesis fracture was extremely rare, which was only subject to traumatic head injury [2], [3], [4] or child growing skull [5]. In the current study, we documented the very first case of cranioplasty titanium mesh prosthesis fracture in an adult, which occurred 13 months post-surgically without any trauma on the head. We used this case to propose the importance of cranioplasty prosthesis anchoring at the basitemporal skull, as otherwise non-traumatic dynamic load from head-pillow contact during sleeping will cause fatigue failure of the prosthesis.
2. Presentation of case
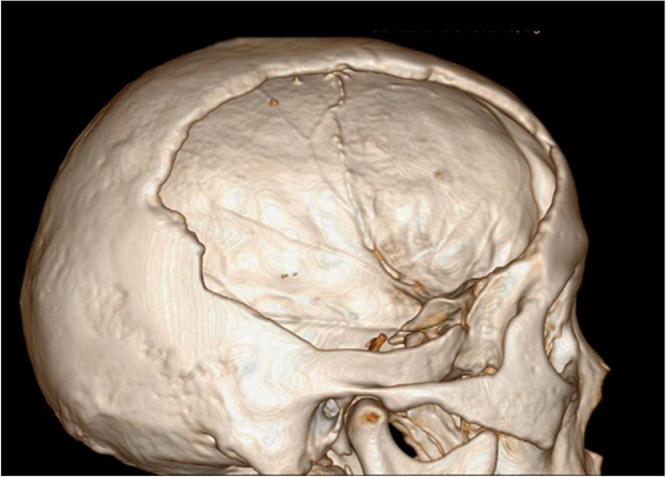
A 50-year-old previously healthy man fell from 6 meter high and hit his head on the ground. He was emergently transferred to a local hospital and diagnosed with a right parietal-temporal skull fracture and right frontal-parietal-temporal epidural hematoma with severe cerebral contusion and laceration. An emergent craniotomy was conducted to clear the epidural hematoma, following the large decompressive craniectomy of the frontal-parietal-temporal skull flap. The patient got apparent clinical improvement 13 months post-surgically and was admitted to our hospital. His neurological examinations were unremarkable. Head CT scans confirmed a 6 × 8 cm bone defect at the right frontal-parietal-temporal skull (Fig. 1). Additionally, multiple loci of decreased density at right posterior temporal lobe were found, which was diagnosed as cerebromalacia.
Fig. 1.
The 3-D reconstruction CT imaging revealing a 6 × 8 cm skull defect at the right frontal-parietal-temporal skull prior to the cranioplasty.
4 days after admission, the patient underwent the parietal-temporal cranioplasty under general anesthesia. Skin incision following the original craniotomy was made and the pericranial tissue corresponding to the area of the estimated edge of the skull was stripped. A computer-based patient-matched titanium mesh implant was anchored by the matched titanium screws directly onto the skull and confirmed by surgeons that the implant completely covered the skull defect area. Anatomically speaking, the temporal branches of middle meningeal artery anastomoses with the temporal arteries in the temporalis muscle [6]. Thus, partition the temporalis muscle could break blood flow between these two arteries and therefore decrease brain blood supply, which is a negative factor for brain trauma patient to recover (cerebromalacia after trauma in our case). In order to maintain proper temporal lobe blood flow, the integrity of the temporalis muscle was kept and not stripped from the dura mater during the surgery. The temporalis muscle was simply covered by the titanium mesh and thus no titanium screw could be used for anchoring prosthesis at the basitemporal skull. The patient recovered to a great degree post-surgically without negative complaints. Physical and laboratory examination were unremarkable and 3-D reconstruction CT imaging was performed right before discharge, showing the implant was intact and in an ideal position.
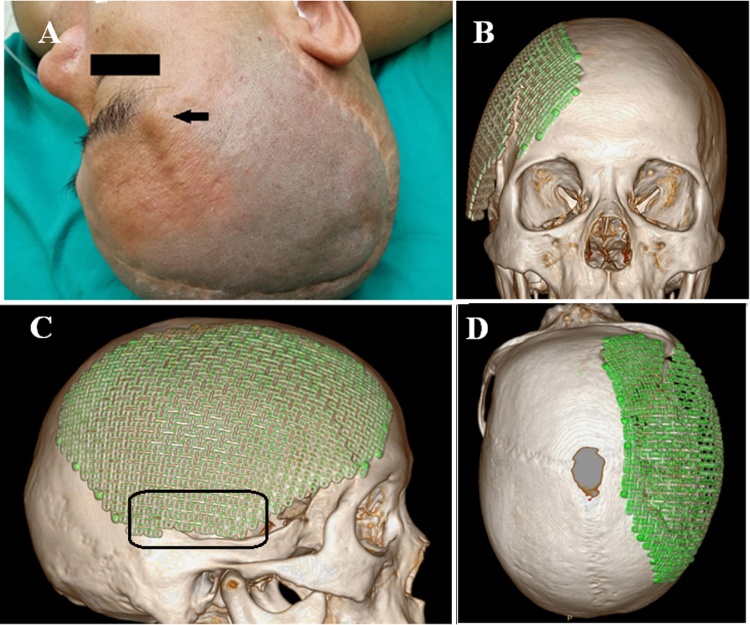
Thirteen months later, the patient re-visited our outpatient clinic and complained a prominence at the right upper orbital region appeared approximately 12 months post the first cranioplasty (Fig. 2A). No trauma or intensive force on the head could be recalled by the patient. However, the patient did mention that he started to use the right lateral position during sleep since the 6th-month post the first cranioplasty, which allowed extra load on the titanium mesh implants. Neurological examinations were unremarkable while the physical examinations identified a linear deformity, facing back and up at 45° and starting from proximal pterion point to an unclear ending point. 3-D reconstruction CT imaging revealed the clear edge of titanium mesh implant fracture, which was a 6 cm linear crack and extended from the proximal pterion point to the parietal bone (Fig. 2B–D). However, no screw fracture or prolapsed out of the skull. Based on these findings, the patient was diagnosed with a cranioplasty prosthesis fracture and underwent the same frontal-parietal-temporal cranioplasty with titanium mesh implant from the same medical instrument supplier 3 days after admission. Because the patient had been greatly recovered from the previous trauma and showed no increased cerebromalacia size in CT scan when compared to the previous one, we semi-partitioned the temporalis muscle from the back during this cranioplasty and anchored five titanium screws at about 1 cm above the basitemporal skull. Meanwhile, about the same amount of screws were used for anchoring prosthesis to other skull regions. The patient received a full recovery post-surgically and was discharged on the fourteenth day from admission before another 3-D reconstruction CT imaging (Fig. 3A and B). The CT imaging showed the implant was intact and in an ideal position. The cracked titanium implant was sent back to manufacture for quality testing and no issue of any kind was found. During the last phone follow-up at 21st-month post second cranioplasty, the patient had no negative complaint and no deformity was found.
Fig. 2.
(A) There existed a prominence at the right upper orbital region of the patient during the second visit (as indicated by the black arrow). (B–D) 3-D reconstruction CT imaging at sagittal, coronal and axial plane revealed titanium mesh prosthesis fracture, which was a 6 cm linear crack and extended from the proximal pterion point to the parietal bone. Clearly, the area in the black rectangular area in C was the skull basitemporal locus, which was absent of titanium screw in order to keep the integrity of the temporalis muscle during the first surgery.
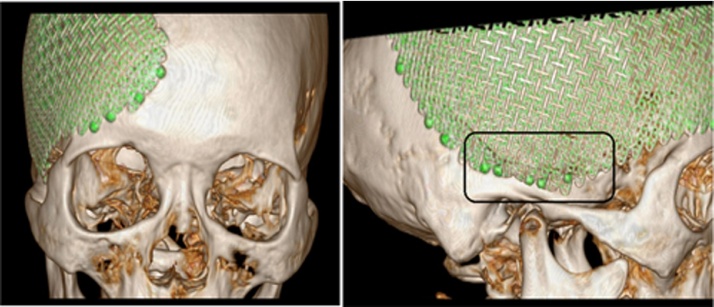
Fig. 3.
(A and B) After the second cranioplasty, 3-D reconstruction CT imaging showed that the new titanium mesh prosthesis was intact and in an ideal position. During this cranioplasty, five screws were used to anchor the prosthesis onto the skull basitemporal locus (indicated as the rectangular area).
3. Discussion
Fracture failure is not rare in orthopedic (long bone [7], [8] and spine [9], [10]) and dental implants [11], [12]. Unlike its counterpart, the cranioplasty prosthesis is not under weight-loading conditions (as orthopedic implants) or bearing obvious extra force (as dental implants). As the patients in our study did not suffer head injury or have a growing skull, the implications of such event can be significant.
Previous studies indicated several contributors for implant fracture: (1) inappropriate implant material [8], (2) bad implant designs [13], (3) inappropriate fixator selection [10], [14], (4) inappropriate surgical technique [9], (5) and (6) biomechanical overload [15], [16]. These factors are deleterious for implants fatigue resistance, which eventually lead to fracture. In the current case, the fractured cranioplasty prosthesis has a computer-based patient-matched design, which has been widely accepted to achieve the best morpho-functional results [17], [18]. The material of the prosthesis is titanium, which remains the most reliable allplastics for cranioplasty because of its advantages, including high malleability and strong resistance to mechanical stress [19], [20]. In our study, the first prosthesis showed no quality issue according to the lab report, fracture due to this possibility was also ruled out. The cranioplasty was well performed by experienced neurosurgeons and matched fixator system was used during surgery. As there was no sign of abutment bone resorption or screw loosening/fracture [12], passive fit for the prosthesis was achieved. Thus, the only possibility we could think of was due to biomechanical overload on the prosthesis.
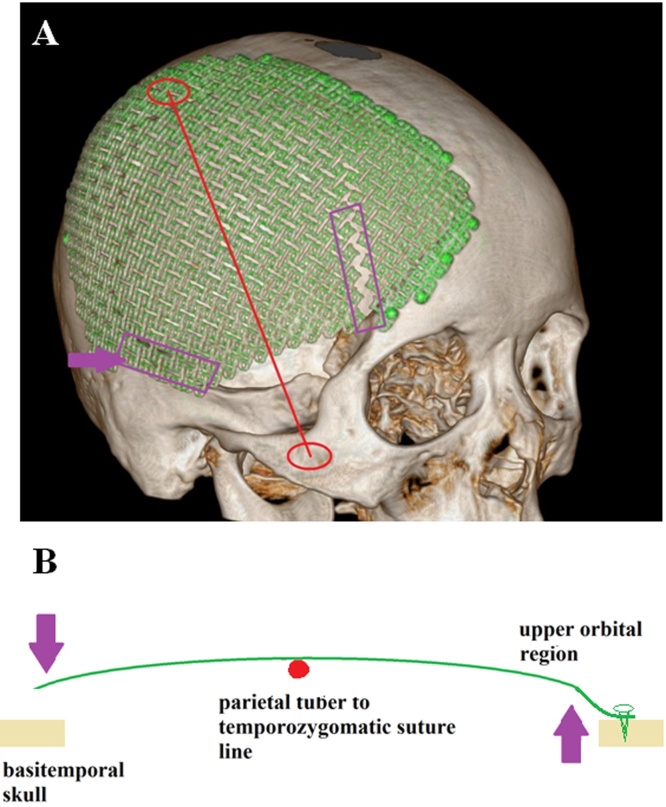
Prosthesis is subject to two types of biomechanical load, the static and the dynamic load. The static load on cranioplasty prosthesis is generated by the direct pressure of the atmosphere, which has been well considered during design and shall be well tolerated. Thus, the dynamic load should play a more important role in the prosthesis fracture. Since the patient started to use the right lateral morpheusthe at the 6th month post-operatively, we conducted a simple biomechanical study to understand the dynamic load (due to head-pillow contact) onto the prosthesis during sleep: (1) first, a normal skull was painted with ink on its lateral side, (2) next, the skull was laid on a clean paper on a horizontal plate, and (3) finally we monitored the ink density and position on the paper. We found that: (1) the parietal tuber (PT) and temporozygomatic suture (TS) were the two most prominent points on the skull’s sagittal plane; (2) while both were under tension, that of basitemporal skull is significantly stronger than that of the proximal pterion point region; (3) the PT-TS line stands in the middle of the basitemporal skull and the proximal pterion point region and has a similar perpendicular distance towards these two regions. Thus, we created a simple biomechanical model of the lateral lying skull: the PT-TS served as a fulcrum line (Fig. 4A), while the basitemporal skull and upper-orbital region locate at opposite sides of it (Fig. 4B). Since not anchored during the first cranioplasty, the prosthesis has a micro-gap towards basitemporal skull, which generates an inward and dynamic load equaling to skull gravity at the basitemporal locus. Via PT-TP line, this inward load generates an outward force at the proximal pterion point region, where became a shearing force locating just right below the lowest titanium screw anchoring in this region. This shearing force eventually led to a fatigue effect on prosthesis at the proximal pterion point and caused a fracture. Thus, during the second cranioplasty, we anchored the prosthesis to the basitemporal loci and the follow-up showed excellent results.
Fig. 4.
(A and B) A force analysis model of the skull when it was lying on its lateral side on a horizontal plate. On the skull lateral side, the parietal tuber (PT) and temporozygomatic suture (TS) were two most prominent spots, which made every single dot on the PT-TS line serve as a fulcrum. Because the prosthesis was not anchored to the basitemporal skull, the micro gap between prosthesis and skull due to head-pillow contact generated a dynamic load to the prosthesis. Thus, via the PT-TS line, this inward dynamic load at basitemporal locus generated an outward force at proximal pterion point region on the titanium mesh, which led to a fatigue effect and eventually sheared the titanium mesh.
4. Conclusions
The lesson from this case was that the cranioplasty titanium mesh prosthesis should be well anchored onto the basitemporal skull to avoid fatigue effect induced prosthesis fracture failure. Moreover, patient neurological wellness should be fully evaluated prior to the cranioplasty because partial temporalis muscle partition was required for implants anchoring.
Competing interests
The author complains no competing interests in the current study.
Role of funding
There is no funding source supporting the study in the current manuscript.
Ethical approval
This study has been approved by the ethics committee of Shanghai Chang Zheng Hospital.
Consent
The patient in the current study has been fully informed with written consent form. We received the written and signed consent from on second follow-up after the second cranioplasty.
Author contribution
Both cranioplasties were performed by Dr. Ming-kun Yu with the help of Dr. Ying Jiang and Dr. Yun-Kun Wang. Dr. Ming-kun Yu designed the current study and revised the manuscript. Dr. Ying Jiang collected data, wrote the manuscript and revised the manuscript. Dr. Yun-Kun Wang collected data.
Guarantor
Dr. Ming-kun Yu and Dr. Ying Jiang.
Acknowledgements
We would like to thank our department and hospital for their support of the current study.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijscr.2016.04.039.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Williams L., Fan K., Bentley R. Custom-made titanium cranioplasty: early and late complications of 151 cranioplasties and review of the literature. Int. J. Oral Maxillofac. Surg. 2015;44:599–608. doi: 10.1016/j.ijom.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Adetchessi A., Pech-Gourg G., Metellus P., Fuentes S. Fracture of macroporous hydroxyapatite prosthesis. Neurochirurgie. 2012;58:382–385. doi: 10.1016/j.neuchi.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Staffa G., Nataloni A., Compagnone C., Servadei F. Custom-made cranioplasty prostheses in porous hydroxyapatite using 3D design techniques: 7 years experience in 25 patiens. Acta Neurochir. 2007;149:161–170. doi: 10.1007/s00701-006-1078-9. [DOI] [PubMed] [Google Scholar]
- 4.Marchac D., Greensmith A. Long-term experience with methylmethacrylate cranioplasty in craniofacial surgery. J. Plast Reconstr. Aesthet. Surg. 2008;6:744–752. doi: 10.1016/j.bjps.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 5.Naim-Ur-Rahman, Jamjoom A., Jamjoom Z. Growing skull fractures: surgical management of difficult and atypical cases. Acta Neurochir. (Wien) 1996;138:1088–1093. doi: 10.1007/BF01412312. [DOI] [PubMed] [Google Scholar]
- 6.Elazab E., Abdel-Hameed F. The arterial supply of the temporalis muscle. Surg. Radiol. Anat. 2006;28:241–247. doi: 10.1007/s00276-006-0096-x. [DOI] [PubMed] [Google Scholar]
- 7.Clement A., Vanderby R.J., Halanski M., Noonan K. Guided growth implant failure is a result of cyclic fatigue: explant analysis with scanning electron microscopy. J. Pediatr. Orthop. 2015 doi: 10.1097/BPO.0000000000000661. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 8.Yeganeh A., Otoukesh B., Kaghazian P., Yeganeh N., Boddohi B., Moghtadaei M. Evaluation of the etiologies of implant fracture in patients with fractures of the implants of lower limbs' long bones. Med. Arch. 2015;69:405–408. doi: 10.5455/medarh.2015.69.405-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J., Sponseller P., Thompson G., Akbarnia B., Emans J., Yazici M. Growing rod fractures: risk factors and opportunities for prevention. Spine. 2011;36:1639–1644. doi: 10.1097/BRS.0b013e31822a982f. [DOI] [PubMed] [Google Scholar]
- 10.Shinohara K., Takigawa T., Tanaka M., Sugimoto Y., Arataki S., Yamane K. Implant failure of titanium versus cobalt-chromium growing rods in early-onset scoliosis. Spine. 2016;41:502–507. doi: 10.1097/BRS.0000000000001267. [DOI] [PubMed] [Google Scholar]
- 11.Shemtov-Yona K., Rittel D., Machtei E., Levin L. Effect of dental implant diameter on fatigue performance. Part II: failure analysis. Clin. Implant Dent. Relat. Res. 2014;16:178–184. doi: 10.1111/j.1708-8208.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 12.Gealh W., Mazzo V., Barbi F., Camarini E. Osseointegrated implant fracture: causes and treatment. J. Oral Implantol. 2011;34:499–503. doi: 10.1563/AAID-JOI-D-09-00135.1. [DOI] [PubMed] [Google Scholar]
- 13.Osman R., Ma S., Duncan W., De Silva R., Siddiqi A., Swain M. Fractured zirconia implants and related implant designs: scanning electron microscopy analysis. Clin. Oral Implants. 2013;24:592–597. doi: 10.1111/j.1600-0501.2011.02411.x. [DOI] [PubMed] [Google Scholar]
- 14.Eckert E., Salinas T. Implant FracturesEtiology, prevention, and treatment. In: Stuart J., editor. Forum Dental Implant Complications Etiology, Prevention, and Treatment. 1st ed. Blackwell Publishing; Singapore: 2010. pp. 100–109. [Google Scholar]
- 15.Vander Sloten J., Labey L., Van Audekercke R., Van der Perre G. Materials selection and design for orthopaedic implants with improved long-term performance. Biomaterials. 1998;19:1455–1459. doi: 10.1016/s0142-9612(98)00058-1. [DOI] [PubMed] [Google Scholar]
- 16.Bartel D., Davy D., Keaveny T. Orthopaedic biomechanics mechanics and design in musculoskeletal systems. Pearson. 2006 [Google Scholar]
- 17.Joffe J., Harris M., Kahugu F., Nicoll S., Linney A., Richards R. A prospective study of computer-aided design and manufacture of titanium plate for cranioplasty and its clinical outcome. Br. J. Neurosurg. 1999;13:576–580. doi: 10.1080/02688699943088. [DOI] [PubMed] [Google Scholar]
- 18.Frodel J.J. Computer-designed implants for fronto-orbital defect reconstruction. Facial Plast. Surg. 2008;24:22–34. doi: 10.1055/s-2007-1021459. [DOI] [PubMed] [Google Scholar]
- 19.Cabraja M., Klein M., Lehmann T. Long-term results following titanium cranioplasty of large skull defects. Neurosurg. Focus. 2009;26:E10. doi: 10.3171/2009.3.FOCUS091. [DOI] [PubMed] [Google Scholar]
- 20.Shah A., Jung H., Skirboll S. Materials used in cranioplasty: a history and analysis. Neurosurg. Focus. 2014;36:E19. doi: 10.3171/2014.2.FOCUS13561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.