Summary
Chronic non‐healing wounds are significantly bothersome to patients and can result in severe complications. In addition, they are increasing in numbers, and a challenging problem to the health‐care system. Handling of chronic, non‐healing wounds can be discouraging due to lack of improvement, and a recent explanation can be the involvement of biofilm infections in the pathogenesis of non‐healing wounds. Therefore, new treatment alternatives to improve outcome are continuously sought‐after. Autologous leucopatches are such a new, adjunctive treatment option, showing promising clinical effects. However, the beneficial effect of the patches are not understood fully, although a major contribution is believed to be from the release of stimulating growth factors from activated thrombocytes within the leucopatch. Because the leucopatches also contain substantial numbers of leucocytes, the aim of the present study was to investigate the activity of the polymorphonuclear neutrophils (PMNs) within the leucopatch. By means of burst assay, phagocytosis assay, migration assay, biofilm killing assay and fluorescence in‐situ hybridization (FISH) assay we showed significant respiratory burst in PMNs, active phagocytosis and killing of Pseudomonas aeruginosa by the leucopatch. In addition, bacterial‐induced migration of PMNs from the leucopatch was shown, as well as uptake of P. aeruginosa by PMNs within the leucopatch. The present study substantiated that at least part of the beneficial clinical effect in chronic wounds by leucopatches is attributed to the activity of the PMNs in the leucopatch.
Keywords: autologous platelet‐rich fibrin patch, phagocytosis, Pseudomonas aeruginosa, wound‐healing
Introduction
Chronic wounds can be classified as failing to heal spontaneously within 3 months. As 1% of the western population will suffer from non‐healing wounds, they are considered a significant health problem. Socioeconomically, the management of chronic wounds reaches a total and rising cost of 2–4% of the health budget in western countries 1, 2. The major chronic types of wounds are venous stasis wounds (70% of all chronic wounds) 3, 4, followed by wounds associated with ischaemia, diabetes or pressure. Complications to non‐healing wounds are vast, and patients are at risk of severe pain, sepsis, hospitalization and, in some cases, amputations. The pathophysiology of chronic wounds is not understood fully, and several theories have been provided 5, 6, 7, 8. An explanation for the various and partly conflicting hypotheses for establishment of chronic wounds could be that different categories of non‐healing wounds are described as a whole 9.
However, one important event in the establishment of a non‐healing wound is the emergence of a defective skin barrier caused by structural damage, which increases the susceptibility of a wound to infection 10. Substantial evidence now supports that the establishment of chronic infections residing deep in the wounds in the form of biofilms is a pivotal contributor to the impaired healing of chronic wounds 11, 12. The mechanism is probably a continuous induction of suboptimal host responses unable to eliminate the biofilms and the continuous oxidative milieu caused by activated polymorphonuclear neutrophils (PMNs), resulting in collateral damage of the host tissue, and thereby contributing to the pathophysiology 13.
Handling and design of therapy depends partly on the distinct pathophysiology of the chronic wounds. In addition, the choice of treatment is multi‐modal and is characterized by a relative lack of randomized controlled trials 9. The implementation of novel alternative treatment modalities is compromised by the equivocal interpretation of the evidence due to heterogenic study designs and populations 14, 15. One explanation could be missing acknowledgement of the significance of biofilms within the wounds. Another reason could be the inclusion of chronic wounds of different pathophysiology in the studies, and thereby possibly important different responses to the tested treatments. In addition, the group of patients with chronic wounds are often further heterogenic due to factors such as obesity, diabetes, smoking, malnutrition and age, to mention a few. However, by careful grouping of patients with different backgrounds for their chronic wounds and taking biofilms into account there is an improved potential for testing new treatment options of chronic wounds.
One relatively recent treatment option for non‐healing wounds is the so‐called platelet‐rich plasma or platelet‐rich fibrin, which has been introduced for treatment of various recalcitrant wounds 16, 17. A major effect of the those products has been attributed to the cytokines and growth factors from activated platelets inducing tissue repair, angiogenesis and inflammation, especially platelet‐derived growth factor (PDGF) 18.
The recent development of a multi‐layered platelet‐rich fibrin patch (LeucoPatch®) including leucocytes on the wound‐contacting surface and its positive effect in treating hitherto recalcitrant wounds has led us to investigate further the actual mechanisms of these leucopatches on chronic wounds 19.
The present study was designed to analyse the activity of phagocytes within LeucoPatch®, generated using the LeucoPatch® system by a 3CP™ three‐step process to prepare autologous patches at the point of care (Reapplix Aps, Birkeroed, Denmark) 18, 19. As Pseudomonas aeruginosa has been shown to be one of the most important pathogens of biofilms deep in chronic wounds 20, 21, the leucopatches were tested for their respiratory burst, bacterial killing of P. aeruginosa, phagocytic effect as well as their effect on alginate‐embedded P. aeruginosa‐resembling biofilm mode of growth.
Materials and methods
Study design
Whole blood from normal human volunteers was used for all the experiments. Seven donors participated (four males), aged between 30 and 50 years. All donors were physically well and not taking any medication. Informed consent was obtained from each donor and guidance for the approval process for the study was received from the local ethics committee for research in human subjects, in accordance with the Declaration of Helsinki.
Bacterial preparation
P. aeruginosa PAO1 strain was cultured overnight in Luria–Bertani (LB) broth at 37°C on a platform shaker to attain stationary phase organisms. Exponential phase bacteria were obtained from diluting overnight culture in fresh LB broth and shaking at 37°C to reach optical density (OD)600 = 0·5 [approximately 108 colony‐forming units (CFU)/ml]. Bacteria were washed twice in phosphate‐buffered saline (PBS) before application.
Leucopatch preparation
Peripheral whole blood (18 ml) from healthy volunteers was drawn directly into LeucoPatch® devices (Reapplix Aps) using a winged blood sampling set (Terumo Quick Fit; Terumo, Leuven, Belgium) fitted with the LeucoPatch® needle‐holder 18. The device was transferred directly to the centrifuge holder provided and was centrifuged in a two‐step process separated by 10 min. The initial centrifugation for 8 min at 3000 g was followed by a secondary centrifugation at 3000 g for 2 min. After removing the device lid, the patches formed were used immediately or stored at 5ºC or room temperature in Krebs–Ringer buffer supplemented with 10 mM glucose (KRB) until later use.
Neutrophil isolation
PMNs were isolated from whole blood. Erythrocytes were allowed to sediment in 5% dextran solution and the subsequent leucocyte‐enriched plasma was layered on Lymphoprep (Axis‐Shield, Oslo, Norway) and centrifuged. To remove any remaining erythrocytes, the formed pellets enclosing PMNs were lysed with hypertonic saline and the purified PMNs were resuspended in KRB and adjusted to 107 cells/ml.
Respiratory burst assay
The leucopatches were cut into 6‐mm circular fragments; 25 µl of freshly isolated PMNs (107/ml) or leucopatch fragments were transferred onto a 96‐well microtitre plate and phagocytosis was initiated by adding 50 µl of P. aeruginosa PAO1 (5 × 107 CFU/ml), zymosan‐activated serum (ZAS) (10 mg/ml of zymosan opsonized in 30% AB+ serum) or the protein kinase C agonist phorbol myristate acetate (10 mM PMA), and finally 175 µl luminol (30 nM) to the wells. Ten µl of diphenylene iodonium (DPI) (50 µM DPI), a specific inhibitor of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, was employed as control in one experiment. The luminol‐enhanced chemiluminescence analysing phagocyte‐derived reactive oxygen species (ROS) was measured using a luminometer (Wallac 1420 Victor2; Perkin Elmer, Waltham, MA, USA) at 37°C for 1 h.
Bactericidal assay
Leucopatch‐mediated bacterial killing was evaluated by mixing 500 µl of serum‐opsonized P. aeruginosa PAO1 with leucopatches and determining the loss of bacterial viability over time by plating diluted samples overnight followed by colony counting. Washed P. aeruginosa PAO1 were diluted in KRB to a final concentration, in 10% human AB‐positive serum, of 5 × 107 CFU/ml or 5 × 108 CFU/ml. The mixture was rotated on an orbital microplate shaker with a speed of 120 rpm for 30 min at 37°C and transferred to six‐well plates containing freshly prepared leucopatches. Samples were removed at 0, 20, 60 and 90 min for bacterial killing examination, where 50 µl of the reaction mixture was removed and added to 2·45 ml H2O pH 11, thus initiating cellular lyses for 5 min before vortexing vigorously and plating onto modified Conradi–Drigalski plates (Statens Serum Institute, Copenhagen, Denmark) and incubated overnight at 37°C.
Alginate biofilm bactericidal assay
Protanal LF 10/60 (FMC BioPolymer N‐3002; Drammen, Norway) extracted from brown seaweed was dissolved in 0·9% NaCl to an alginate concentration of 1% and sterile‐filtered. The bacterial culture was diluted 1 : 20 in seaweed alginate solution and transferred to a 10‐ml syringe and placed into a syringe pump (Graseby 3100; Ardus Medical Inc., Watford, UK). A vertically orientated tube was connected to the syringe and the syringe pump fed the alginate through the exit orifice of the tube into a gelling bath (0·1 M, pH 7·0 Tris HCL buffer containing 0·1 M CaCl2) creating alginate beads of approximately 50 μl. A magnetic stirrer (IKA® RCT Basic; IKA‐Werke GmbH & Co. KG, Staufen, Germany) was placed underneath the gelling bath to prevent the beads from sticking together during gelling. The distance between the tube exit and gelling bath was 8 cm. The magnetic stirrer was kept constant (280 rpm) and the beads were left for stabilization in the gelling bath for 1 h and finally washed twice in 0·9% NaCl containing 0·1 M CaCl2. After washing, 20 ml 0·9% NaCl containing CaCl2 was added.
The alginate discs (volume = 250 μl) were created by pipetting the alginate/bacterial solution into a 24‐well microtitre plate. The formed discs were transferred immediately to a 50‐ml tube containing gelling medium (0·1 M, pH 7·0 Tris HCL buffer containing 0·1 M CaCl2) for stabilization overnight.
P. aeruginosa PAO1 embedded in alginate beads were positioned on a modified Conradi–Drigalski agar plate (Statens Serum Institute) and covered with prepared leucopatches. After 2 h the leucopatches were removed and the alginate beads transferred to tubes containing PBS and homogenized (Heidolph Silent Crusher M; Heidolph Instruments, Schwabach, Germany), serially diluted and plated onto Conradi–Drigalski plates (Statens Serum Institute) for quantification by colony‐counting the next day.
The alginate discs containing P. aeruginosa PAO1 were added to the bottom of a 24‐well microtitre plate and covered with prepared leucopatches (alginate disc to leucopatch ratio 1 : 1). After 2 h the leucopatches were removed and the quantification of bacterial content were determined as described previously.
Leucocyte chemotaxis assay
Transwell chambers were used to mimic chemotactic leucocyte migration. Leucopatches in 200 μl KRB were added to the upper wells of 5‐μm pore size polycarbonate filters in 6·5‐mm diameter 24‐well Transwell chambers (Corning/Sigma, St Louis, MO, USA), with 600 μl chemoattractant or medium (KRB) in the lower wells. Formyl–methionyl–leucyl–phenylalanine (fMLP) or P. aeruginosa PAO1 were used as leucocyte activators/chemoattractants. At specified time‐points, filters were removed from the wells and the number of cells migrated into the bottom chambers were counted with a flow cytometer (FACSCanto; BD Biosciences, San Jose, CA, USA). The identification of leucocytes was performed using fluorescein isothiocyanate (FITC)‐conjugated mouse anti‐human CD15, allophycocyanin (APC)‐conjugated mouse anti‐human CD14 and peridinin chlorophyll (PerCP)‐conjugated mouse anti‐human CD45 monoclonal antibodies (all purchased from BD Biosciences).
PNA fluorescence in‐situ hybridization (FISH) imaging
Prepared leucopatches were mixed with P. aeruginosa PAO1 in a microtitre plate to commence phagocytosis. The microtitre plate was placed on a platform rocker for 60 min at 37°C; subsequently, the leucopatches were transferred to 10% formalin (formaldehyde 4% aqueous‐buffered solution). The fixated patches were paraffin‐embedded and cut into ultrathin (3–5 μm) sections using a microtome and placed on microscope slides, dewaxed and processed through xylene and ethanol to water before probe hybridization. The deparaffinized leucopatch sections were examined by FISH using peptide nucleic acid (PNA) probes. A Texas Red‐labelled P. aeruginosa‐specific PNA probe in hybridization solution (AdvanDx, Inc., Woburn, MA, USA) was added to each section and hybridized in a PNA FISH workstation covered by a lid at 55°C for 90 min. The slides were washed for 30 min at 55°C in wash solution (AdvanDx) and the slides were covered with a coverslip. Slides were read using a fluorescence microscope equipped with Texas Red and 4',6‐diamidino‐2‐phenylindole (DAPI) filters.
Statistics
GraphPad Prism (version 6·0d) software was used for statistical work. Chemiluminescence data were logged automatically into Microsoft Excel. The non‐parametric Mann–Whitney U‐test was used to determine differences between groups if the D'Agostino–Pearson test demonstrated that not all data sets were distributed normally. In the case of normally distributed data sets the parametric t‐test was applied. A level of P ≤ 0·05 was used for assigning statistical significance.
Results
The production of reactive oxygen species (ROS) is sustained in PMNs residing in leucopatches
Reactive oxygen species (ROS) are anti‐microbial metabolites produced during phagocytosis or equivalent stimulation of PMNs. The respiratory burst describes the generation of ROS by the electron‐transfer system NADPH oxidase, where electrons are delivered to oxygen present in phagosomes or the extracellular milieu generating bactericidal oxygen metabolites. The oxidation of luminol generates excited state metabolites and emits light on dropping to the ground state. Thus, the luminol‐enhanced chemiluminescence assay quantifies ROS production during microbial phagocytosis, activation of PMNs by zymosan or the simulation of phagocytosis by stimulating PMNs with PMA.
Figure 1 depicts the level of chemiluminescence detected during stimulation of PMNs residing in leucopatches. When leucopatches are challenged with increasing concentrations of P. aeruginosa there is a prominent chemiluminescence response [6094 counts per second (cps)max to 29886 cpsmax] in a concentration‐dependent manner. Moreover, the chemiluminescence is reduced significantly by addition of DPI, which inhibits NADPH oxidase and impedes the respiratory burst (P < 0·0005). Thus, mixing P. aeruginosa and leucopatches prompts a noticeable ROS production, indicating strongly that the inherent PMNs in leucopatches have preserved their phagocytic competence. ZAS and PMA are employed as activators of PMNs and are indeed capable of generating a chemiluminescence response in leucopatches (Fig. 1); consequently, various stimulants of PMN function seem to provoke a conspicuous response in leucopatches.
Figure 1.
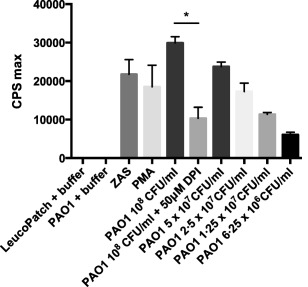
Chemiluminescence assay represents the production of reactive oxygen species (ROS) in leucopathes during 60 min of stimulation with Pseudomonas aeruginosa PAO1, zymosan‐activated serum (ZAS) or the protein kinase C agonist phorbol myristate acetate (PMA). Diphenylene iodonium (DPI) a specific inhibitor of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, was employed as control in one experiment. The luminol‐enhanced NADPH oxidase is employed as control. Results are expressed as counts per second (cps) max ± standard deviation (s.d.). Experiments were performed in triplicate. *P < 0·0005.
The intensity of chemiluminescence during phagocytosis differs between freshly isolated PMNs and leucopatches
Comparison of the detected chemiluminescence during phagocytosis of P. aeruginosa by freshly isolated PMNs and leucopatches originating from equivalent donors is represented in Fig. 2. When freshly isolated PMNs are phagocytizing P. aeruginosa the subsequent mean chemiluminescence signal (cpsmax 78095) is twice as high compared to the mean chemiluminescence (cpsmax 34205) detected from leucopatches (P < 0·005). However, the difference in chemiluminescence between free PMNs and leucopatches may be attributable to the relative reduction in phagocytosis‐primed cells in the leucopatch, as probably only a fraction of the absolute amount is conditioned to initiate phagocytosis.
Figure 2.
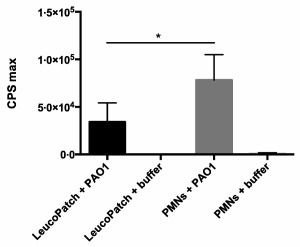
Chemiluminescence assay compares the production of reactive oxygen species (ROS) during phagocytosis of Pseudomonas aeruginosa PAO1 [5 × 107 colony‐forming units (CFU)/ml] in leucopathes and isolated polymorphonuclear neutrophils (PMNs) (107 cells/ml) from identical donor blood. Results are expressed as counts per second (cps) max ± standard deviation (s.d.). Experiments were performed in triplicate. *P < 0·005.
The durability of ROS production in leucopatches is maintained
With the intention of evaluating the durability of PMN‐mediated ROS production, the prepared leucopatches were left in their devices at room temperature for 24 h. The chemiluminescence detected by stimulating leucopatches with either PMA or P. aeruginosa after 8 and 24 h were compared to the initial chemiluminescence of freshly prepared leucopatches (Fig. 3). After 8 h the chemiluminescence is reduced, although not significantly, by 9% (PMA stimulation) and 8% (bacterial stimulation) compared to that of recently prepared leucopatches. Twenty‐four h post‐producing the leucopatches the chemiluminescence is reduced by 68% (PMA stimulation) and 27% (bacterial stimulation) compared to the preliminary quantity. Although the respiratory burst elicited by stimulated leucopatches appears to diminish over time, the chemiluminescence level is preserved within 8 h after generation of the leucopatches, indicating that the phagocytic capacity of PMNs in the leucopatches remains sufficient beyond several hours after preparation.
Figure 3.
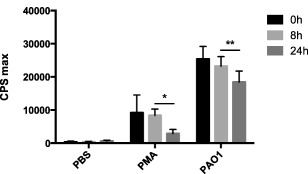
Chemiluminescence assay compares the production of reactive oxygen species (ROS) in leucopathes at various time‐points after preparation. The leucopatches were stored at room temperature in the device for 8 or 24 h before stimulation with Pseudomonas aeruginosa (PAO1) or the protein kinase C agonist phorbol myristate acetate (PMA) for 60 min. Results are expressed as counts per second (cps) max ± standard deviation (s.d.). Experiments were performed in triplicate. *Not significant (n.s.); **n.s.
The conserved chemiluminescence in PMNs during a longer time‐period of 7 days was assessed on leucopatches maintained in a cellular buffer (KRB) and stored at 5ºC (Fig. 4). In these conditions the respiratory burst by PMNs exposed to P. aeruginosa was reduced by 54% after 2 days (P < 0·04) and 91% after 4 days (P < 0·002). The chemiluminescence after 7 days of storage was undetectable. The phagocytic activity of PMNs is obviously influenced by several factors, and optimal cellular milieu to ensure appropriate metabolism is imperative. Nevertheless, the respiratory burst activity of leucopatches remained detectable several days after their production.
Figure 4.
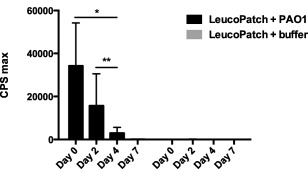
Chemiluminescence assay compares the production of reactive oxygen species (ROS) in leucopatches at different time‐points after preparation. The leucopatches were used immediately after preparation or stored in Krebs–Ringer buffer supplemented with 10 mM glucose (KRB) at 5°C prior to stimulation with Pseudomonas aeruginosa PAO1 [5 × 107 colony‐forming units (CFU)/ml] for 60 min. Results are expressed as counts per second (cps) max ± standard deviation (s.d.). Experiments were performed in triplicate and leucopatches were prepared from seven donors. *P < 0·04; **P < 0·002.
The PMNs in leucopatches are able to detach and migrate towards leucocyte attractants
Possible chemotactic properties of the PMNs in leucopatches were evaluated by their ability to migrate towards the chemoattractants fMLP and P. aeruginosa in a membrane‐separated two‐phase system (Transwell chambers). Cells able to migrate through the membrane towards a chemoattractant were quantified by flow cytometry (Fig. 5). fMLP is a powerful chemoattractant, inducing a migratory response in more than 105 cells/ml. The efficacy of fMLP to attract PMNs is time‐dependent, thus after 2 h the quantity of attracted cells are 13·3 times higher than after 1 h. Bacteria are also potent chemoattractors; however, the amount of cells migrated towards P. aeruginosa is more than 10 times lower compared to fMLP. As observed previously, time is an important determinant of chemotaxis and the extent of migrated cells after 2 h is 2·8 (106 CFU/ml) and 1·5 times higher compared to the quantity after 1 h. The present chemotaxis assay shows that PMNs are able to detach their fixation in the leucopatches and migrate towards chemotactically active factors, which is a fundamental consequence to ensure sufficient anti‐microbial effects in tissues.
Figure 5.
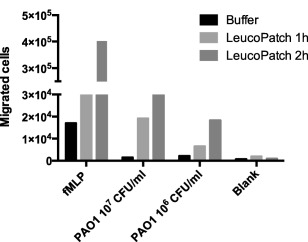
Chemotaxis assay describe the chemotactic migration of leucocytes residing in the leucopatch. Formyl‐methionyl‐leucyl‐phenylalanine (fMLP) and Pseudomonas aeruginosa (PAO1) were applied as leucocyte chemoattractants/activators. The identification and the quantity of leucocytes migrated through 5‐μm pore size polycarbonate filters were determined by flow cytometry using fluorescein isothiocyanate (FITC)‐conjugated mouse anti‐human CD15, allophycocyanin (APC)‐conjugated mouse anti‐human CD14 and peridinin chlorophyll (PerCP)‐conjugated mouse anti‐human CD45 monoclonal antibodies. Experiments were performed twice.
Microscopic imaging visualizes interaction between leucopatch and P. aeruginosa
Adjacent interface between the fraction of PMNs in the leucopatch and bacterial constituents is a premise to concede phagocytic killing. In order to visualize such interaction we utilized fluorescence microscopy. Leucopatches were challenged with planktonic P. aeruginosa and fixed subsequently in formalin prior to paraffin‐embedding. The presence of bacteria in the resulting slides was examined by PNA‐FISH technology by addition of a Texas Red‐labelled P. aeruginosa‐specific PNA probe. Figure 6 presents microscopic images of the leucopatch–bacteria interaction. The red‐fluorescent P. aeruginosa are localized in close contact with the subjacent layer in the leucopatch and are accordingly accessible to PMN mediated phagocytosis.
Figure 6.
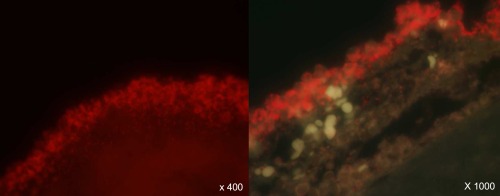
The fluorescent microscopic images visualize the interaction between Pseudomonas aeruginosa and leucopatch constituents. Preformed leucopatches were mixed with P. aeruginosa PAO1 and eventually fixated in formalin and subsequently paraffin‐embedded. Tissue sections were processed by peptide nucleic acid–fluorescence in‐situ hybridization (PNA–FISH) technology allowing the P. aeruginosa to appear fluorescent by hybridization with a Texas Red‐labelled P. aeruginosa‐specific PNA probe.
The bactericidal activity of leucopatches is prominent
The PMN‐mediated killing of planktonic bacteria was determined by exposing leucopatches to serum‐opsonized P. aeruginosa PAO1 followed by plating diluted samples overnight and colony‐counting the next day. The loss of bacterial viability during 90 min of phagocytosis is depicted in Fig. 7. When mixing leucopatch and 5 × 108 CFU/ml of P. aeruginosa the bacterial viability was reduced by 89% after 20 min of leucopatch‐facilitated phagocytosis and decreased further by 98% after 90 min compared to control. The PMN‐mediated phagocytic killing is even more distinct when a 10‐times reduced bacterial concentration (5 × 107 CFU/ml) is subjected to phagocytosis, where the bacterial viability is reduced by more than 99% with respect to the initial inoculum. The lowering of bacterial concentration renders the relation between phagocytic cells and bacteria more favourable and ensures an optimal exploitation of the phagocytic competence of PMNs.
Figure 7.
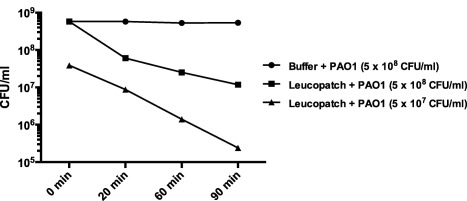
Bactericidal activity of leucopatches during 90 min of stimulation with planktonic Pseudomonas aeruginosa PAO1. Prepared leucopatches are mixed with PAO1, and at specific time‐points post‐mixing the amount of viable bacteria in samples removed from the mixture are determined by serially diluting and subsequent plating onto Conradi–Drigalsky medium followed by colony counting the next day.
The contribution of bacterial biofilms to the pathogenesis of chronic wounds necessitates proper simulation in vitro of the anti‐microbial activity of leucopatches. Thus, we employed alginate‐embedded P. aeruginosa, mimicking the biofilm mode of growth, in our bactericidal assays. Figure 8a shows the efficacy of leucopatches to control bacterial growth in alginate beads. During 2 h of growth the inoculum of 8 × 107 CFU/ml is increased by 111%; however, leucopatches inhibited bacterial growth completely compared to non‐leucopatch control (P < 0·002).
Figure 8.
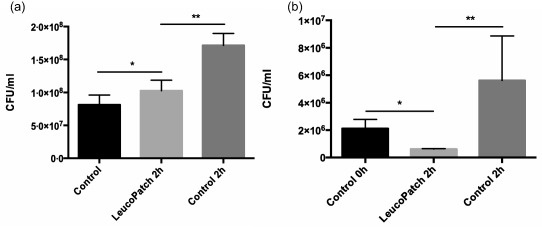
Bacteriostatic activity of leucopatches challenged with alginate‐embedded Pseudomonas aeruginosa (PAO1) in beads mimicking biofilm mode of bacterial growth (a). The alginate beads enclosing PAO1 were positioned in direct contact with leucopatches and transferred subsequently to tubes containing phosphate‐buffered saline (PBS) and homogenized, serially diluted and plated onto Conradi–Drigalski plates followed by colony counting the next day. *Not significant (n.s.); **P < 0·002. Bactericidal activity of leucopatches challenged with a biofilm model of Pseudomonas aeruginosa (PAO1) embedded in alginate discs (b). The freshly prepared leucopatches were layered carefully on the surface, in direct contact with the alginate disc containing PAO1. After 2 h the alginate discs were transferred to tubes containing phosphate‐buffered saline and homogenized, serially diluted and plated onto Conradi–Drigalski plates followed by colony‐counting the next day. *P < 0·005; **P < 0·02.
To ensure optimal physical contact between the PMN fraction of leucopatches and bacterial biofilms, we employed alginate discs encompassing P. aeruginosa; see Fig. 8b. The surface of the discs was sheeted with a size‐approximated leucopatch to allow proper interaction for phagocytosis. When simulating bacterial biofilm in the form of discs, the leucopatches were not only repressing bacterial growth but were indeed able to eradicate the biofilm by reducing the bacterial content by 70% after 2 h (P < 0·005).
Discussion
Chronic wound patients often have systemic and local ischaemia in the affected area, and therefore display a decreased early inflammatory cell infiltration 22, 23, 24. Polymorphonuclear neutrophil granulocytes (PMNs) are the first line of defence upon recognition of invading bacteria. Damage to the protective skin surface and underlying tissue facilitates bacterial colonization in the wound and the subsequent establishment of bacterial biofilms and induction of a suboptimal chronic inflammation unable to clear the infection 12, 25. The biofilm infections cannot be eliminated due to the resilience of the biofilms to host immune response and antibiotic treatment 26. Platelet‐rich fibrin has already been included in wound care of various kinds, and by the inclusion of leucocytes into patches, e.g. as LeucoPatch® promising effects in the treatment of chronic wounds have been observed (19 and Löndahl 2015, submitted for publication). The present study shows an additional mechanism of leucocyte‐containing platelet‐rich patches, apart from the already suggested immune modulating and healing support effect 18.
The observation of biofilms deep in non‐healing wounds has added significant knowledge to the recalcitrant nature of these wounds 12. As the biofilms are not eliminated by the host responses collateral tissue damage occurs instead, and thereby host responses contribute eventually to the chronic nature of the non‐healing wounds 27. Additionally, the necrotic tissue in the wounds may provide a wound environment which limits the migratory skills of the recruited PMNs 28. Furthermore, the release of anti‐microbial host factors in the wound milieu is probably degraded in the proteolytic environment 6. However, with the supplementing of host immune cells from the surface, biofilms are challenged from more sites and on a larger surface. Therefore, part of the effect of leucocyte‐containing patches may be due to oxidative as well as non‐oxidative defence mechanisms, in addition to the growth factors and cytokines from the platelets. Although lower in leucopatches compared to free PMNs, our results showed a substantial oxidative burst as well as phagocytic activity and bacteria‐killing. Accordingly, the respiratory burst and subsequent phagocytic activity of freshly isolated PMNs is superior compared to that of PMNs derived from leucopatches. Such a comparison is only legitimate if the concentration of activated PMNs is equivalent in the two set‐ups. The content of PMNs in leucopatches is estimated theoretically to be approximately 107 cells/ml; however, it is unlikely that the absolute amount is conditioned to accomplish phagocytosis. Because approximately a fraction of 1/17 of one patch is used for respiratory burst assay, presumably around 600 000 PMNs are used. For comparison, approximately 250 000 free PMNs are used as control respiratory burst assay. This means that, even though far from all PMNs in the leucopatch are being exposed to stimulation, the measured respiratory burst is still approximately half of what is observed from free PMNs.
The emigration of circulating leucocytes from the blood into inflamed tissues is a crucial event in the anti‐microbial activity of PMNs. Chemotaxis is a complex process; integrating several components of the host response, however, is finalized by the migratory response to a chemoattractant gradient. Thus, we observed migration of PMNs from the leucopatch both by stimulation with fMLP and P. aeruginosa, suggesting that in the patients PMNs can invade into the wound from the leucopatch and attack the biofilms from two sides. These observations indicate that at least part of the beneficial effect of the leucopatches is due to anti‐bacterial effects.
We chose to test the anti‐bacterial effect of the leucopatches on P. aeruginosa as this pathogen has been shown to be an important contributor to the pathophysiology of chronic wounds 21. Whether the effect is similar against other wound pathogens is unknown. However, Moojen et al. showed a bacterial killing effect against Staphylococcus aureus by a comparable platelet‐leucocyte gel 29. In addition, the platelet‐leucocyte gels used in that study had substantial levels and activity of myeloperoxidase (MPO) compared to controls. The number of leucocytes in the platelet‐leucocyte gels (17·9 ± 2·62 × 106/ml) and in the leucopatch (55 × 106/patch) is difficult to compare due to the differences in three‐dimensional structures; however, both contain viable leucocytes 18, 29.
The clinical standard use of LeucoPatch® is application for 1 week in the chronic wound and replacement of the patch with a new patch produced just before replacement 19. Therefore, we also investigated the activity of patches over several days, and although the activity was reduced over time the oxidative burst could still be observed after 7 days. This observation is interesting, as it is generally accepted that the lifespan of PMNs is short (< 1 day), although a recent study using in‐vivo labelling in humans have estimated the average circulatory neutrophil lifespan to be 5·4 days 30. In addition, a relatively large interindividual variation could be observed between patches from the different donors. Whether this can also be correlated with interindividual treatment effects or whether it would actually be possible to stimulate the patches further to improve their effect still remains to be studied.
As mentioned above, a significant characteristic of chronic wounds are the presence of biofilms. Because bacteria are significantly more resistant to host response when growing in biofilms compared to planktonic growth, we used our biofilm model of embedding bacteria in seaweed alginate to resemble the extracellular matrix in biofilms 31. Using this model system, we observed a substantial reduction in bacterial reduction compared to the experiments using planktonic growing bacteria. However, and more importantly, we still showed a reduction in the bacterial load in the model biofilms where P. aeruginosa is embedded in seaweed alginate. The 40 times lower baseline bacterial burden in the alginate discs, compared to the equivalent concentration in the alginate beads, probably balances the ratio between PMNs and bacteria in favour of phagocytic killing. The kinetics of phagocytosis is a saturable process. Consequently, the effect is dependent upon initial bacterial burden to allow the PMNs to contribute optimally to bacterial clearance. This indicates that the anti‐bacterial effects of the LeucoPatch® can also be expected against the biofilm‐growing bacteria in the chronic wounds. Surgical debridement is the traditional remedy to remove or break up formed biofilm; however, these are often reformed within 48–96 h 32.This study confirms the potential of leucopatches to kill bacteria and inhibit the reformation of biofilm.
Previously, it was shown that production and release of rhamnolipids from a quorum sensing competent P. aeruginosa could lyse PMNs effectively in vitro and thereby adds significantly to the virulence of biofilm infections 33. Although to a lesser extent, this was confirmed in vivo in a lung infection mouse model as well as in sputum from patients with cystic fibrosis 33. Rhamnolipid release from P. aeruginosa biofilms in non‐healing wounds could probably contribute to the pathogenesis. As rhamnolipids effect is mediated through binding to the cell membranes of the host cells, one could speculate that part of the beneficial effects of the leucopatch may be due to neutralization of rhamnolipids by the high presence of thrombocytes and their membranes.
Finally, modifications can be performed, including activation of the patches for augmented effect. In addition, potential anti‐inflammatory effects have not been studied until now. Nevertheless, and in conclusion, the LeucoPatch® system is an easily obtained treatment modality, using the patient's own material, and is therefore well tolerated.
Disclosures
C. M. has received funding for research carried out for this work.
Author contributions
K. T., H. T., L. C. and N. H. state no disclosures. R. L. is a co‐inventor of the LeucoPatchTM technology.
Acknowledgements
The studies described are supported by Reapplix Aps.
References
- 1. Gottrup F. Optimizing wound treatment through health care structuring and professional education. Wound Repair Regen 2004; 12:129–33. [DOI] [PubMed] [Google Scholar]
- 2. Hjort A, Gottrup F. Cost of wound treatment to increase significantly in Denmark over the next decade. J Wound Care 2010; 19:173–4, 176, 178, 180, 182, 184. [DOI] [PubMed] [Google Scholar]
- 3. Nelzen O, Bergqvist D, Lindhagen A. Venous and non‐venous leg ulcers: clinical history and appearance in a population study. Br J Surg 1994; 81:182–87. [DOI] [PubMed] [Google Scholar]
- 4. Baker SR, Stacey MC, Jopp‐McKay AG, Hoskin SE, Thompson PJ. Epidemiology of chronic venous ulcers. Br J Surg 1991; 78:864–7. [DOI] [PubMed] [Google Scholar]
- 5. Trengove NJ, Bielefeldt‐Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non‐healing and healing chronic leg ulcers. Wound Repair Regen 2000; 8:13–25. [DOI] [PubMed] [Google Scholar]
- 6. Trengove NJ, Stacey MC, MacAuley S et al Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999; 7:442–52. [DOI] [PubMed] [Google Scholar]
- 7. Drinkwater SL, Burnand KG, Ding R, Smith A. Increased but ineffectual angiogenic drive in nonhealing venous leg ulcers. J Vasc Surg 2003; 38:1106–12. [DOI] [PubMed] [Google Scholar]
- 8. Wysocki AB, Staiano‐Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP‐2 and MMP‐9. J Invest Dermatol 1993; 101:64–8. [DOI] [PubMed] [Google Scholar]
- 9. Trøstrup H. (2013) What is new in the understanding of non healing wounds. Ulcers 2013; Article ID 625934, 8 pages.
- 10. Tuttle MS. Association between microbial bioburden and healing outcomes in venous leg ulcers: a review of the evidence. Adv Wound Care 2015; 4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bjarnsholt T, Kirketerp‐Møller K, Jensen PO et al Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen 2008; 16:2–10. [DOI] [PubMed] [Google Scholar]
- 12. Kirketerp‐Møller K, Jensen PO, Fazli M et al Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol 2008; 46:2717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schultz GS, Sibbald RG, Falanga V et al Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003; 11 (Suppl 1):S1–28. [DOI] [PubMed] [Google Scholar]
- 14. Martí‐Carvajal AJ, Gluud C, Nicola S et al. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev 2015; 10:CD008548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma C, Hernandez MA, Kirkpatrick VE, Liang LJ, Nouvong AL, Gordon II. Topical platelet‐derived growth factor vs placebo therapy of diabetic foot ulcers offloaded with windowed casts: a randomized, controlled trial. Wounds 2015; 27:83–91. [PubMed] [Google Scholar]
- 16. Burnouf T, Goubran HA, Chen TM, Ou KL, El‐Ekiaby M, Radosevic M. Blood‐derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev 2013; 27:77–89. [DOI] [PubMed] [Google Scholar]
- 17. Villela DL, Santos VL. Evidence on the use of platelet‐rich plasma for diabetic ulcer: a systematic review. Growth Factors 2010; 28:111–6. [DOI] [PubMed] [Google Scholar]
- 18. Lundquist R, Holmstrom K, Clausen C, Jorgensen B, Karlsmark T. Characteristics of an autologous leukocyte and platelet‐rich fibrin patch intended for the treatment of recalcitrant wounds. Wound Repair Regen 2013; 21:66–76. [DOI] [PubMed] [Google Scholar]
- 19. Jørgensen B, Karlsmark T, Vogensen H, Haase L, Lundquist R. A pilot study to evaluate the safety and clinical performance of Leucopatch, an autologous, additive‐free, platelet‐rich fibrin for the treatment of recalcitrant chronic wounds. Int J Low Extrem Wounds 2011; 10:218–23. [DOI] [PubMed] [Google Scholar]
- 20. Fazli M, Bjarnsholt T, Kirketerp‐Moller K et al Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J Clin Microbiol 2009; 47:4084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gjødsbøl K, Christensen JJ, Karlsmark T, Jørgensen B, Klein BM, Krogfelt KA. Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 2006; 3:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galkowska H, Wojewodzka U, Olszewski WL. Low recruitment of immune cells with increased expression of endothelial adhesion molecules in margins of the chronic diabetic foot ulcers. Wound Repair Regen 2005; 13:248–54. [DOI] [PubMed] [Google Scholar]
- 23. Jorneskog G. Why critical limb ischemia criteria are not applicable to diabetic foot and what the consequences are. Scand J Surg 2012; 101:114–8. [DOI] [PubMed] [Google Scholar]
- 24. Ochoa O, Torres FM, Shireman PK. Chemokines and diabetic wound healing. Vascular 2007; 15:350–5. [DOI] [PubMed] [Google Scholar]
- 25. Pradhan L, Nabzdyk C, Andersen ND, LoGerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Rev Mol Med 2009; 11:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999; 284:1318–22. [DOI] [PubMed] [Google Scholar]
- 27. Bjarnsholt T, Alhede M, Alhede M et al The in vivo biofilm. Trends Microbiol 2013; 21:466–74. [DOI] [PubMed] [Google Scholar]
- 28. Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J Immunol 2013; 190:1746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moojen DJ, Spijkers SN, Schot CS et al Identification of orthopaedic infections using broad‐range polymerase chain reaction and reverse line blot hybridization. J Bone Joint Surg Am 2007; 89:1298–305. [DOI] [PubMed] [Google Scholar]
- 30. Pillay J, den Braber I, Vrisekoop N et al In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 2010; 116:625–7. [DOI] [PubMed] [Google Scholar]
- 31. Christophersen LJ, Trøstrup H, Malling Damlund DS et al Bead‐size directed distribution of Pseudomonas aeruginosa results in distinct inflammatory response in a mouse model of chronic lung infection. Clin Exp Immunol 2012; 170:222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Attinger C, Wolcott R. Clinically addressing biofilm in chronic wounds. Adv Wound Care 2012; 1:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jensen PO, Bjarnsholt T, Phipps R et al Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum‐sensing‐controlled production of rhamnolipid by Pseudomonas aeruginosa . Microbiology 2007; 153:1329–38. [DOI] [PubMed] [Google Scholar]


