Summary
Noroviruses (NoV) are the most common cause of epidemic gastroenteritis world‐wide. NoV infections are often asymptomatic, although individuals still shed large amounts of NoV in their stool. Understanding the differences between asymptomatic and symptomatic individuals would help in elucidating mechanisms of NoV pathogenesis. Our goal was to compare the serum cytokine responses and faecal viral RNA titres of asymptomatic and symptomatic NoV‐infected individuals. We tested serum samples from infected subjects (n = 26; 19 symptomatic, seven asymptomatic) from two human challenge studies of GI.1 NoV for 16 cytokines. Samples from prechallenge and days 1‐4 post‐challenge were tested for these cytokines. Cytokine levels were compared to stool NoV RNA titres quantified previously by reverse transcription–polymerase chain reaction (RT–qPCR). While both symptomatic and asymptomatic groups had similar patterns of cytokine responses, the symptomatic group generally exhibited a greater elevation of T helper type 1 (Th1) and Th2 cytokines and IL‐8 post‐challenge compared to the asymptomatic group (all P < 0·01). Daily viral RNA titre was associated positively with daily IL‐6 concentration and negatively with daily IL‐12p40 concentration (all P < 0·05). Symptoms were not associated significantly with daily viral RNA titre, duration of viral shedding or cumulative shedding. Symptomatic individuals, compared to asymptomatic, have greater immune system activation, as measured by serum cytokines, but they do not have greater viral burden, as measured by titre and shedding, suggesting that symptoms may be immune‐mediated in NoV infection.
Keywords: adaptive immunity, caliciviruses, gastroenteritis, innate immunity, symptoms
Introduction
Norovirus (NoV) is one of the leading infectious causes of diarrhoea world‐wide, and is considered to be responsible for an estimated 18% of all cases 1. Although it often causes a brief and self‐limited illness, among vulnerable populations, such as elderly people or individuals who are immunosuppressed, NoV illness can be more protracted and have severe outcomes 2, 3, 4.
NoV infection can be symptomatic or asymptomatic; in human challenge studies, approximately 30% of NoV (GI.1 Norwalk)‐infected individuals are asymptomatic 5, 6. Although symptomatic infection is of great concern, both for individual and public health reasons 7, 8, the causes of symptomatic NoV infection are poorly understood. In part, the lack of understanding is the result of a paucity of available animal models of NoV infection, none of which fully recapitulates symptoms observed in humans (reviewed in 9).
Recent advances in NoV vaccine development suggest that although the vaccine candidates provided only modest protection from infection, they demonstrated a greater reduction in the incidence of symptoms and in their severity 10, 11. The mechanism of this protection is unknown. In order to develop more effective vaccines to target both symptom prevention and protection from infection, it is important to understand more clearly the potential immunological drivers of symptoms.
NoV infection leads to a rapid human immune response that is characterized by elevation in serum cytokines 24–48 h post‐infection 12. This occurs at approximately the same time as the development of symptoms, leading to the hypothesis that symptomatic infection is caused by immune‐mediated damage or direct viral activity. There is limited evidence of the association between viral load and symptoms, with some data from two challenge studies suggesting that symptomatic individuals have higher viral RNA titres in stool than asymptomatic individuals 5, 6 and conflicting data, from outbreak studies, indicating that symptomatic and asymptomatic individuals shed virus at similar levels 13.
In this study, we present the largest study to date of the serum cytokine response to NoV infection in symptomatic and asymptomatic individuals who became infected following experimental challenge. The goals were twofold. The first was to assess whether symptoms were associated with greater immune responses to NoV infection, which might indicate immune‐mediated damage as a cause of symptoms. The second was to determine whether symptoms were associated with greater viral burden in a pooled analysis of GI.1 NoV challenge studies, potentially indicating virus‐mediated damage leading to illness.
Methods
Population and samples
The study population was comprised of individuals from two separate NoV challenge studies, described in 14, 15. Overall serum cytokine responses for this population are reported in 12. Both studies enrolled healthy secretor‐positive adults and challenged them with Norwalk virus 8FIIb inoculum. The first study was conducted between May and December 2006 at Emory University Hospital's Clinical Interaction Site, part of the Atlanta Clinical and Translational Science Institute Clinical Interaction Network. Volunteers were challenged with 6.5 × 107 genomic equivalent copies (GEC) of the NoV inoculum, which was seeded into water and left in the dark at room temperature to incubate for varying time‐periods. Stool samples were collected prior to challenge during a 4‐day in‐patient stay during the challenge study, and at planned intervals until the end of shedding post‐challenge.
The second study was a randomized trial of NoV inactivation methods and was conducted between February 2008 and September 2009 at Emory University Hospital's Clinical Interaction Site. Volunteers were assigned to different control or intervention groups and consumed an oyster that had been seeded with 1 × 104 GEC of the NoV inoculum, and then treated with high hydrostatic pressure processing or left untreated as a control. Stool and serum samples were collected prior to challenge during a 4‐day in‐patient stay during the challenge study, and at planned intervals until the end of shedding post‐challenge.
All subjects in both studies consented to allow the future use of all biological specimens. All specimens were stored at −80° C and those used in this study had not been thawed previously. Emory University's Institutional Review Board approved both studies, and they were registered on ClinicalTrials.gov (identifiers NCT00313404 and NCT00674336).
Stool and emesis samples were tested by real‐time reverse transcription–quantitative polymerase chain reaction (RT–qPCR) to determine whether or not individuals had become infected. Infection was defined as having at least one sample test positive by RT–qPCR for NoV RNA following NoV challenge with cumulative viral shedding exceeding the inoculum dose. Between the two studies, a total of 26 individuals became infected with NoV following challenge. Of the 26 who were infected, 19 developed symptoms and seven remained asymptomatic (symptom measurement described below).
Detection of viral shedding
We used RT–qPCR to quantify NoV RNA in all stool and emesis samples from the 2008–09 study by Kirby et al. 6 and from the 2006 study by Newman et al. 12. All samples available from all time‐points were tested as described 6. On average, 10·8 stool samples were tested per subject (range = 4–18). In brief, stool was suspended at a 20% concentration in sterile water and then combined with an equal volume of Vertrel XF (DuPont, Wilmington, DE, USA) to facilitate extraction. The suspension was incubated at 4°C for 2–18 h then centrifuged at 13 000 g for 10 min. The QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA, USA) was used according to the manufacturer's protocol to extract viral RNA from 140 μl of the aqueous phase. All extractions were also conducted with a known NoV‐positive extraction control and a NoV‐negative extraction control of sterile water.
Extracted RNA was stored at −20°C then quantified using RT–qPCR with the Qiagen one‐step RT–PCR kit (Qiagen) on a Bio‐Rad CFX96 system (Bio‐Rad, Hercules, CA, USA) with Norwalk virus‐specific primers and probes (NVKS1, NVKS2 and NVKS3) and in vitro‐transcribed Norwalk virus standards 6, 16 and a limit of detection of 3570 GEC/g stool. Amplification data were analysed and concentrations were calculated with the Bio‐Rad CFX Manager (Bio‐Rad). All samples were tested in duplicate. The average copy number is reported. Samples that had inconsistent results between duplicates (e.g. difference in cycle threshold values > 3) or failed to amplify in either duplicate were retested. All samples were run with successful positive and negative controls. The positive controls were a stool sample known to be positive for NoV RNA and a PCR control of known NoV RNA. The negative controls were a stool sample known to be negative for NoV RNA and sterile water controls for both extraction and PCR.
Daily titres were calculated based on the average viral RNA titre per gram of stool of all samples collected that day. For days when a stool sample was not available, daily titre was calculated as the average of the daily titres from the previous and subsequent days, as long as the previous and subsequent days had NoV‐positive stool samples. If either day did not have a positive stool sample, shedding on the day without an available sample was assumed to be below the limit of detection. Peak viral RNA titre was defined as the highest titre measured for an individual during the entire course of infection. Duration of shedding was calculated as the days from the first positive stool or emesis sample until the mid‐point date between the date of the last positive sample and the date of the first negative sample followed by only negative samples. If the last available sample was positive, the date of that sample was regarded as the end of shedding. Cumulative shedding during the first 5 days post‐challenge was calculated by multiplying the GEC/g stool or emesis for each sample, as measured by RT–qPCR, by the weight of each sample and summing the estimated GEC in the sample over the total number of samples collected during the first 5 post‐challenge days, during which all samples were collected and none were lost.
Gastrointestinal symptoms and scoring
Clinical symptoms of infection were assessed during the challenge studies and scored as reported previously 6. Symptomatic infection was defined as the presence of detectable NoV RNA in stool with (a) diarrhoea alone, (b) emesis plus one additional symptom (i.e. abdominal cramps, nausea, oral temperature ≥ 37·6°C, myalgia, chills, fatigue or headache) or (c) at least one of the following symptoms: nausea, abdominal cramps, headache, chills, myalgia, fatigue or emesis. All symptoms except for fever (oral temperature ≥ 37·6°C) were self‐reported. Asymptomatic infection was defined as having a NoV‐positive stool sample but failing to meet the previous definition for symptomatic infection. Severity of infection was assessed using the 17‐point modified Vesikari score according to previously described methods 6, 11 and was calculated for all individuals included into the study.
Detection of cytokines
For all but one subject, five serum samples from prechallenge and days 1–4 post‐challenge were analysed for cytokines. A sample from day 2 post‐challenge was missing for one subject, and so only four samples were analysed for that individual. Samples were sent to an outside contractor (EMD Millipore Corporation Discovery and Development Solutions, St Charles, MO, USA) and tested using a Milliplex human cytokine 16‐plex assay for interferon (IFN)‐α2, IFN‐γ, interleukin (IL)−1a, IL‐1b, IL‐1ra, IL‐2, IL‐4, IL‐5, IL‐6, IL‐8, IL‐10, IL‐12p40, IL‐12p70, monocyte chemotactic protein 1 (MCP‐1), tumour necrosis factor (TNF)‐α and TNF‐β, as described previously 12. The assay was run with duplicates of all samples and controls. Concentrations were calculated using a five‐parameter logistic model for each cytokine and standard curves from recombinant cytokines, as per EMD Millipore standard protocols. The assay's lower limits of detection (LLOD) were 3·2 pg/ml for IFN‐γ, IL‐10, IL‐12p70, IL‐1b, IL‐2, IL‐5, IL‐6, IL‐8, MCP‐1 and TNF‐α; 16·0 pg/ml for TNF‐β; and 80·0 pg/ml for IFN‐α2, IL‐12p40, IL‐1ra, IL‐1a and IL‐4. All values below LLOD were assigned the value of the LLOD.
Missing data
Cytokine values for missing serum samples (n = 1, i.e. 16 cytokine test results) and invalid cytokine test results (n = 20) were imputed as the average of the concentrations of the same cytokine from the days before and after the missing value for the same subject. A total of two subjects had invalid test results for one or more cytokine tested (20 total invalid results). Using imputation, these invalid results were estimated to be below the LLOD because the observations for the same subject and cytokine before and after the test were below the LLOD.
Statistical analysis
Cytokines and symptoms
To investigate the association between serum cytokine response and symptoms at individual time‐points, the significance of unadjusted differences between pre‐ and post‐challenge serum cytokine levels in symptomatic and asymptomatic individuals were tested using the Wilcoxon's signed‐rank test because the data were not distributed normally. To investigate the association between serum cytokine response and symptoms during the first 4 days post‐challenge, we used a mixed linear model to test the association between log10 cytokine concentration (outcome) and day post‐challenge (exposure), controlling for challenge study group (i.e. water study or shellfish study) to account for inoculum and storage time, stratifying by symptom status (yes/no), with a subject‐specific random effect, and an autoregressive correlation structure to account for correlation between time‐points for each individual. The parameter estimates for the effect of day on cytokine concentration were then averaged to create a single measure of cytokine change over time. Student's t‐test was used to compare these measures of cytokine change between symptomatic and asymptomatic individuals.
Cytokines and viral RNA shedding
To assess the relationships between serum cytokine response (outcome, i.e. fold change in serum cytokine level from pre‐challenge) and daily viral RNA titre (exposure, i.e. log10 GEC/g stool) during the first 4 days post‐challenge, a mixed‐effects model was used. It included fixed effects for the effect of day post‐challenge, viral RNA titre each day and challenge study group, plus a subject‐specific random effect. To examine whether prechallenge serum cytokine levels were predictive of duration of viral shedding, we used unadjusted Kaplan–Meier survival curves to test the association between cytokines divided into three categories (below LLOD, above the LLOD and below the median prechallenge serum cytokine level, and above the LLOD and above the median prechallenge serum cytokine level) and time to the end of viral shedding.
Symptoms and viral RNA shedding
To investigate the association between symptoms and viral shedding, Wilcoxon's signed‐rank tests with a normal approximation and Kaplan–Meier survival analysis were used. The associations between symptoms and three viral shedding parameters (as described above) were assessed: peak viral RNA titre, cumulative shedding during the first 5 days post‐challenge and duration of viral shedding. Associations between symptoms and peak viral RNA titre and cumulative shedding were assessed using Wilcoxon's test because the data were not distributed normally. The association between symptoms and the duration of viral shedding was assessed using a Kaplan–Meier survival analysis of time from the onset of shedding until the end of detectable shedding.
All analyses were conducted using sas version 9·4 (SAS Institute, Cary, NC, USA). P‐values < 0·05 were considered significant.
Results
Among the 26 individuals who had stool or emesis samples that tested positive for NoV by RT–qPCR following experimental challenge, 19 developed symptoms and seven remained asymptomatic (Table 1). Subjects were relatively young (mean age = 26·9 years). All individuals who were symptomatic reported feeling nausea, even if they did not vomit or have diarrhoea.
Table 1.
Characteristics of subjects included in the study.
| All (n = 26) | Symptomatic (n = 19) | Asymptomatic (n = 7) | |
|---|---|---|---|
| Characteristic | Mean (s.d.) | Mean (s.d.) | Mean (s.d.) |
| Age (years) | 26·9 (7·5) | 27·1 (5·8) | 25·6 (11·6) |
| Female (n, %) | 16 (61·5%) | 11 (57·9%) | 5 (71·4%) |
| Modified Vesikari score | 4·1 (2·4) | 5·3 (1·8) | 1 (0) |
| Duration of shedding (days) | 23·7 (9·8) | 25·3 (9·2) | 19·3 (10·0) |
| Cumulative shedding in first 5 days (log10 GEC) | 11·1 (11·3) | 11·1 (11·2) | 11·2 (11·5) |
| Mean peak viral RNA titre (log10 GEC) | 9·3 (9·5) | 9·2 (9·5) | 9·3 (9·7) |
GEC = genomic equivalence copies; s.d. = standard deviation.
Studies of human cytokine responses can often have many values measured below the LLOD 17 Therefore, we examined the distribution of each cytokine measured (Table 2, Supporting information, Figs S1 and S2). Three cytokines measured (IFN‐γ, IL‐8 and MCP‐1) had fewer than 5% of observations below the LLOD, and two additional cytokines (IL‐10 and TNF‐α) had fewer than 30% of observations below the LLOD. For many cytokines, asymptomatic individuals had a greater percentage of observations below the LLOD compared to symptomatic individuals (Table 2). Generally, the cytokines had a wide range of observed concentrations.
Table 2.
Serum cytokine responses of subjects included in the study, stratified by symptom status.
| All | Symptomatic | Asymptomatic | ||||
|---|---|---|---|---|---|---|
| Cytokines | Median (IQR) | % below LLOD | Median (IQR) | % below LLOD | Median (IQR) | % below LLOD |
| IFN‐α2 | 80·0 (80·0–138·3) | 71·5% | 80·0 (80·0–136·8) | 70·5% | 80·0 (80·0–166·8) | 74·3% |
| IFN‐γ | 183·7 (67·8–634·6) | 1·5% | 240·0 (79·0–592·7) | 0·0% | 118·5 (56·8–936·5) | 5·7% |
| IL‐1a | 3·2 (3·1–16·5) | 40·8% | 185·2 (80·0–503·1) | 40·0% | 3·2 (3·2–7·5) | 42·9% |
| IL‐1b | 177·2 (80·0–412·8) | 52·3% | 3·2 (3·2–20·8) | 50·5% | 163·4 (80·0–316·7) | 57·1% |
| IL‐1ra | 80·0 (80·0–393·0) | 52·3% | 88·1 (80·0–407·5) | 47·3% | 80·0 (80·0–390·2) | 65·7% |
| IL‐2 | 6·7 (3·2–36·6) | 45·4% | 7·0 (3·2–39·6) | 43·2% | 3·2 (3·2–17·5) | 51·4% |
| IL‐4 | 80·0 (80·0–170·3) | 71·5% | 80·0 (80·0–188·2) | 68·4% | 80·0 (80·0–80·0) | 80% |
| IL‐5 | 3·2 (3·2–6·3) | 66·2% | 3·2 (3·2–7·5) | 64·2% | 3·2 (3·2–4·0) | 71·4% |
| IL‐6 | 27·8 (3·2–94·7) | 30·8% | 32·4 (3·2–91·3) | 30·5% | 21·1 (3·2–96·9) | 31·4% |
| IL‐8 | 81·7 (33·2–200·0) | 3·8% | 85·2 (35·2–196·5) | 4·2% | 64·5 (26·5–222·3) | 2·9% |
| IL‐10 | 17·0 (3·2–50·3) | 26·2% | 11·6 (3·2–50·3) | 32·6% | 19·2 (6·9–50·4) | 8·6% |
| IL‐12p40 | 80·0 (80·0–85·8) | 72·3% | 80·0 (80·0–80·0) | 77·9% | 80·0 (80·0–94·5) | 57·1% |
| IL‐12p70 | 19·5 (3·2–85·3) | 32·3% | 15·4 (3·2–111·6) | 33·7% | 21·8 (3·2–46·0) | 28·6% |
| MCP‐1 | 695·6 (510·6–949·5) | 0·0% | 711·2 (517·5–1,062·5) | 0·0% | 603·5 (415·5–804·1) | 0·0% |
| TNF‐α | 15·2 (8·2–25·2) | 12·3% | 16·8 (9·0–38·9) | 11·6% | 12·0 (5·9–20·2) | 14·3% |
| TNF‐β | 16·0 (16·0–100·7) | 64·6% | 16·0 (16·0–207·0) | 62·1% | 16·0 (16·0–19·2) | 71·4% |
IQR = interquartile range; LLOD = lower limit of detection; IFN = interferon; IL = interleukin; MCP = monocyte chemoattractant protein; TNF = tumour necrosis factor.
Cytokines and symptoms
To assess whether there was a difference between pre‐ and post‐challenge cytokine concentrations within symptomatic and asymptomatic individuals, we compared pre‐ and post‐challenge concentrations using Wilcoxon's test. Most cytokine concentrations increased post‐challenge among symptomatic and asymptomatic individuals, although the changes were not generally significant (Fig. 1). The only statistically significant increases were for IL‐10, which exhibited significant elevation relative to prechallenge levels among symptomatic individuals on days 2 and 4 post‐challenge; MCP‐1, which exhibited significant elevation relative to prechallenge levels among symptomatic individuals on day 2 post‐challenge; and TNF‐α, which exhibited significant elevation relative to prechallenge levels among symptomatic and asymptomatic individuals on day 2 post‐challenge (Fig. 1).
Figure 1.
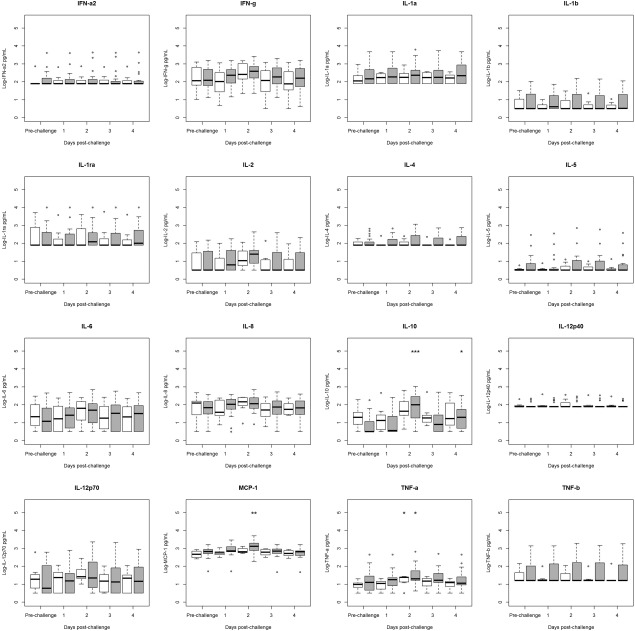
Comparison of pre‐ to post‐challenge serum cytokine concentrations in infected humans from two norovirus challenge studies, stratified by symptom status (symptomatic n = 19, asymptomatic n = 7). Values below the lower limit of detection (LLOD) were assigned to the value of the LLOD. Significance of change from prechallenge value was tested using Wilcoxon's test and is denoted by an asterisk above the relevant category. Interquartile ranges (IQRs) for symptomatic individuals (grey boxes) and for asymptomatic individuals (white boxes) are shown. Dark lines indicate median values. Whiskers indicate the most extreme value that is no more than 1·5 times the IQR away from the bound of the IQR. Circles indicate outliers. *P < 0·05, **P < 0·01, ***P < 0·001.
To compare the overall changes in cytokine concentrations between symptomatic and asymptomatic individuals following NoV challenge, controlling for day post‐challenge and challenge study, we used a mixed linear model stratified by symptom status. We tested this model for the difference between the estimated parameters for symptomatic and asymptomatic individuals using a t‐test. In the adjusted model, symptomatic and asymptomatic individuals remained similar in their pattern of serum cytokine response to NoV, although symptomatic individuals generally had higher concentrations of serum cytokines (Fig. 2). Symptomatic individuals had significantly greater increases in serum IFN‐γ, IL‐1b, IL‐1ra, IL‐2, IL‐4, IL‐6, IL‐8, IL‐10 and TNF‐β during the first 4 days post‐challenge compared to asymptomatic individuals (Fig. 2). Although it was not statistically significant, symptomatic individuals also had greater increases in serum TNF‐α and IL‐12p70 than asymptomatic individuals (both P = 0·054). Asymptomatic individuals had significantly greater changes in IFN‐α2 and IL‐5 concentrations post‐challenge compared to symptomatic individuals, although the magnitude of the difference in change was relatively small compared to the differences observed for other cytokines.
Figure 2.
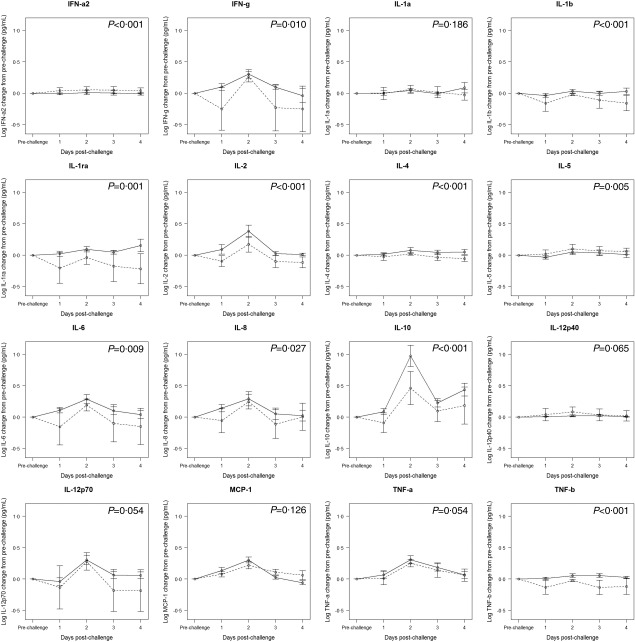
Mixed linear model results for the association between log10 serum cytokine concentration change from pre‐ to post‐challenge by day, with fixed effects for day and a random effect by individual subject. Solid line: symptomatic individuals, dashed line: asymptomatic individuals. Points represent estimates of the effect of day on change in cytokine concentration. Error bars indicate one standard error. P‐value indicates significance of overall elevation in cytokine concentration across days comparing symptomatic to asymptomatic.
Cytokines and viral RNA shedding
To understand more clearly the association between serum cytokine concentrations and NoV shedding, we used a mixed model to test the association during the first 4 days post‐challenge while controlling for post‐challenge day and challenge study group. Based on the adjusted mixed model for the association between log10 cytokine change from prechallenge and log10 daily viral RNA titre (independent variable), increased viral RNA titres were associated significantly with elevated levels of IL‐6 [beta = 0·009, standard error (s.e.) = 0·004, P = 0·023] and significantly decreased levels of IL‐12p40 (beta = 0·002, s.e. = 0·001, P = 0·021) post‐challenge (data not shown). Daily log10 RNA titre was also associated with non‐significantly elevated IFN‐γ (beta = 0·009, P = 0·130), IL‐5 (beta = 0·005, P = 0·072), IL‐8 (beta = 0·020, P = 0·090), and TNF‐α (beta = 0·011, P = 0·104) post‐challenge (data not shown). In addition, we investigated whether prechallenge cytokine levels were predictive of duration of viral shedding, and we found that no prechallenge serum cytokines were significant predictors of duration of viral shedding (data not shown).
Symptoms and viral RNA shedding
We then investigated the associations between symptoms and viral shedding (Supporting information, Fig. S3). We tested the unadjusted association between symptoms and viral shedding using the log‐rank test and Kaplan–Meier curves (Fig. 3). Although symptomatic individuals tended to shed virus for a longer time than asymptomatic individuals (25·3 versus 19·3 days, respectively, Table 1 and Fig. 3), the difference was not significant (P = 0·2855). We also examined the association between peak viral titre and cumulative shedding with symptoms during the first 5 days post‐challenge using Wilcoxon's test. Symptomatic individuals did not appear to have higher peak viral titres than asymptomatic individuals (mean peak titre 1·7 × 109 versus 2·0 × 109 GEC/g stool, respectively, P = 0·119). Similarly, symptomatic individuals did not appear to have meaningfully different levels of cumulative shedding during the first 5 days post‐challenge than asymptomatic individuals (mean cumulative shedding 1·3 × 1011 GEC among symptomatic versus 1·5 × 1011 GEC among asymptomatic individuals, P = 0·215, Table 1).
Figure 3.
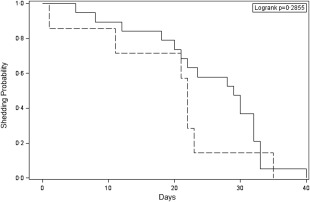
Kaplan–Meier survival curves for association between symptom status and duraion of viral RNA shedding (days) among norovirus (NoV)‐infected individuals. Solid line indicates symptomatic individuals; dashed line indicates asymptomatic individuals.
Discussion
The study goals were to assess the relationships between symptoms and serum cytokine response and between symptoms and shedding. We found that while both symptomatic and asymptomatic individuals showed similar cytokine response patterns overall, symptomatic individuals had significantly higher serum cytokine levels for IFN‐γ, IL‐1b, IL‐1ra, IL‐2, IL‐4, IL‐8, IL‐10 and TNF‐β compared to asymptomatic individuals. Higher daily NoV titres were associated with a significantly higher serum IL‐6 concentration and a significantly lower serum IL‐12p40 concentration, as well as non‐significantly elevated serum IFN‐γ, IL‐8, IL‐5 and TNF‐α concentrations. Symptomatic infection was not associated significantly with greater duration of viral shedding, higher peak viral titres or greater cumulative shedding in the first 5 days post‐challenge compared to asymptomatic infection.
Although previous studies have documented the immune responses to NoV challenge in infected individuals compared to uninfected individuals 12, 18, this is the first study to our knowledge that compares the responses of symptomatic and asymptomatic individuals. We found that individuals who became symptomatic during infection had higher levels of Th1 and some Th‐2 cytokines as well as IL‐8 than asymptomatic individuals (Fig. 2). Studies of rotavirus gastroenteritis have found that serum TNF‐α is associated significantly with diarrhoea incidence when compared to healthy controls and controls with non‐viral diarrhoea 19, 20. Other viral infections, including hepatitis B and dengue virus, induce different proinflammatory cytokine gene expression in symptomatic compared to asymptomatic individuals 21, 22. In the case of dengue, symptomatic individuals had higher expression of many proinflammatory cytokine genes compared to asymptomatic individuals, suggesting that some of dengue's most severe outcomes may be caused partially by an inflammatory response 22. Indeed, cytokines are thought to cause diarrhoea in other infections without primary gastrointestinal tropism (e.g. influenza) 23, 24. The presence of elevated serum cytokines in symptomatic NoV infection thus suggests that symptoms may be immune‐mediated in NoV infection.
This study also found that high daily levels of viral shedding were associated significantly with elevated same‐day levels of IFN‐γ, IL‐6, IL‐8 and TNF‐α. Although it is impossible to establish causality, as other studies of cytokine levels and viral shedding have found 25, this may indicate that individuals with poor viral control (i.e. high viral titres) mount greater proinflammatory responses in an attempt to limit viral replication. Murine NoV research suggests that IFN‐γ and other Th‐1 cytokines are important for limiting viral replication 26, 27. However, murine NoV is able to suppress type 1 IFN production 28. In our study, we observed no significant elevation of IFN‐α2, a type‐1 IFN, post‐challenge, suggesting that human NoV may be able to act similarly. Therefore, this may indicate that individuals with high viral titres because of poor initial viral control may be responding with elevated proinflammatory cytokines in order to limit viral replication, possibly despite NoV inhibition of the immune response.
Our study did not identify any association between serum cytokines and duration of viral shedding. A recent study by Gustavsson et al. found that low CCL5 concentrations were associated with longer duration of viral shedding in a hospitalized cohort infected with GII NoV 29. Although we did not test for CCL5, we measured some CD8+ T cell‐associated cytokines (i.e. IFN‐γ, IL‐2, TNF‐α) 30, and we did not observe any significant association between their levels and duration of viral shedding.
In addition, we found no significant association between symptomatic infection and viral shedding as measured by duration of shedding, peak titre or cumulative shedding. This result is different from the results of Kirby et al.'s earlier analysis, which examined individuals from the 2008–09 challenge study that was included in the present analysis. They found that in the 2008–09 challenge study population there was a significant association between peak titre and symptomatic infection and cumulative shedding in the first 7 days post‐challenge and symptoms 6. The present study analysed a larger population by combining the 2008–09 challenge study with a 2006 challenge study conducted at the same institution, with the same sample and symptom data collection protocols and using the same NoV inoculum. It differs not only from the Kirby et al. study in using an expanded population but also in the use of 5 days' cumulative shedding versus 7 days. However, the population is still relatively small, and may therefore be underpowered to detect a true association, especially in the presence of the high degree of variability observed. Evidence from other studies is mixed, with some suggesting an association between symptoms and higher levels of viral shedding 5 and others showing no significant association 13. However, the Atmar et al. study did not test for the statistical significance of the difference between symptomatic and asymptomatic individuals, whereas Teunis et al.'s study did. Overall, this study's finding that there was no association between viral control and symptoms supports the hypothesis that symptoms may be the result of an individual susceptibility to NoV symptoms or that symptoms may be caused by immune‐mediated mechanisms, such as cytokine storm, rather than the hypothesis that symptoms are the result of direct viral damage. However, it is also possible that this study was underpowered to detect a true association.
As a result of this study's findings and previous NoV and immunological research, we propose a schematic for potential causes of symptomatic NoV infection (Fig. 4). Histological studies of NoV‐infected individuals 31 and select animal models 26, 32, 33 indicate that NoV infection causes damage to the gut mucosa, particularly the epithelium. This damage may lead to immune activation, or the presence of virus alone may stimulate immune activation. Both hypotheses are supported by the finding from this study that daily viral RNA titre is associated with multiple proinflammatory serum cytokines and chemokines. The immune response to NoV has been found to be critical for limiting viral replication and eventually clearing infection 27, 34, 35. However, there may also be immune‐mediated damage, as seen in other enteric infections 36.
Figure 4.
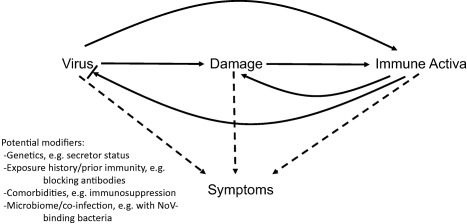
Proposed schematic of potential causes of symptomatic norovirus (NoV) infection. Solid lines indicate stimulation. Bar at the end of an arrow indicates inhibition. Dotted lines indicate hypothesized causes.
The cause of symptoms is ill‐understood, and we suggest three possible hypotheses. First, symptoms may be the direct result of immune activation. Elevated serum cytokines have been shown in rotavirus infection to be associated with symptomatic illness 19 and high levels of serum cytokines and immune activation may cause severe symptoms, including vomiting and diarrhoea, as seen in infections leading to cytokine storm 37. Secondly, symptoms may be the result of physiological damage and may be associated with greater viral load and possibly with direct viral activity. This seems plausible; however, the degree of physiological damage observed in NoV histological specimens is relatively minor 31, and in this study, viral load as assessed by peak titre and cumulative shedding was not associated significantly with symptomatic illness. Thirdly, we suggest that symptoms may be related to all three central variables (i.e. viral load, damage and immune activation) and can be modified potentially by additional factors known to modify immune response, such as genetics 38, immunocompetence 39, previous exposure to similar strains 40, physiological factors (e.g. comorbidities, microbiome) 41 or co‐infection 42. Future research should consider these hypotheses when investigating the causes of symptomatic NoV infection and the role of vaccines in preventing symptoms.
Strengths and limitations
Key strengths of this research include the analysis of a wide range of serum cytokines and use of a large, longitudinal cohort of NoV‐challenged individuals, including many asymptomatic individuals. As such, this is the first study to analyse the serum cytokine response in a large cohort of asymptomatic individuals. An additional strength is the use of robust statistical methods that account for correlated longitudinal data. However, there are limitations to this work. As with many challenge studies, relatively few individuals were included, which limited its statistical power, and prechallenge exposure history could not be assessed. Due to the observational nature of the study, it is impossible to assess causality. There is the possibility that some of the cytokines may have degraded during the time that samples were stored, although we attempted to minimize this by selecting samples that had not been thawed previously. An additional limitation is the potential for measurement error when assessing viral shedding. Viral titres may depend on stool volume, and individuals with similar absolute levels of intestinal viral shedding may have different calculated titres if the virus is diluted in a greater amount of stool (e.g. individuals with high‐fibre diets may have more copious stool output than individuals with low‐fibre diets). We have attempted to avoid some of this error by also considering cumulative shedding; however, this may also be biased in cases of faecal retention or constipation.
Conclusions
NoV illness is a major cause of morbidity and mortality world‐wide, and the causes of symptomatic infection continue to be poorly understood. To help develop better prophylaxis, including vaccines, against both symptomatic and asymptomatic NoV infection, it is important to understand the immunological basis of NoV‐related illness. We found that symptomatic individuals had significantly higher levels of serum cytokines post‐challenge than asymptomatic individuals, although they did not have significantly higher viral titres, longer durations of viral shedding or greater amounts of viral shedding in the 5 days post‐challenge. These findings suggest that symptoms may be driven partially by an immune‐mediated mechanism rather than depend upon viral burden. However, there are probably additional factors involved in symptom development. Future vaccine candidates should capitalize upon the growing understanding of symptomatic infection and leverage existing gains in enhancing protection against severe gastroenteritis.
Disclosure
The authors have no disclosures.
Author contributions
K. L. N., C. A. P., J. S. L., C. L. M. and W. D. F. designed the study. K. L. N. and A. E. K. performed the experiments. K. L. N., W. D. F. and J. S. L. completed the analysis. K. L. N. wrote the paper. All authors edited the paper and provided substantial feedback.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Serum cytokine concentrations of symptomatic norovirus‐infected challenge study volunteers. Prechallenge (day 0) to day 4 post‐challenge, one line per subject.
Fig. S2. Serum cytokine concentrations of asymptomatic norovirus‐infected challenge study volunteers. Prechallenge (day 0) to day 4 post‐challenge, one line per subject.
Fig. S3. Viral shedding in symptomatic (top panel) and asymptomatic (bottom panel) norovirus‐infected challenge study volunteers. Prechallenge (day 0) to end of follow‐up, one line per subject. GEC = genomic equivalence copies.
Acknowledgements
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K. L. N., grant 1F30DK100097; C. A. P., grants R01DK072564, R01DK061379 and R01DK079392), the ARCS Foundation (K. L. N), the National Institute of Allergy and Infectious Diseases (J. S. L., grant 1K01AI087724), the Emory University Global Health Institute (J. S. L.), and NoroCORE (K. L. N., J. S. L., A. E. K., C. L. M, USDA grant 2011‐68003‐30395). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or United States Department of Agriculture.
References
- 1. Ahmed SM, Hall AJ, Robinson AE et al Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta‐analysis. Lancet Infect Dis 2014; 14:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Green KY. Norovirus infection in immunocompromised hosts. Clin Microbiol Infect 2014; 20:717–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gustavsson L, Andersson LM, Lindh M, Westin J. Excess mortality following community‐onset norovirus enteritis in the elderly. J Hosp Infect 2011; 79:27–31. [DOI] [PubMed] [Google Scholar]
- 4. Desai R, Hembree CD, Handel A et al Severe outcomes are associated with genogroup 2 genotype 4 norovirus outbreaks: a systematic literature review. Clin Infect Dis 2012; 55:189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atmar RL, Opekun AR, Gilger MA et al Norwalk virus shedding after experimental human infection. Emerg Infect Dis 2008; 14:1553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirby AE, Shi J, Montes J, Lichtenstein M, Moe CL. Disease course and viral shedding in experimental Norwalk virus and Snow Mountain virus infection. J Med Virol 2014; 86:2055–64. [DOI] [PubMed] [Google Scholar]
- 7. Sukhrie FH, Teunis P, Vennema H et al Nosocomial transmission of norovirus is mainly caused by symptomatic cases. Clin Infect Dis 2012; 54:931. [DOI] [PubMed] [Google Scholar]
- 8. Zelner JL, Lopman BA, Hall AJ, Ballesteros S, Grenfell BT. Linking time‐varying symptomatology and intensity of infectiousness to patterns of norovirus transmission. PLoS One 2013; 8:e68413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Newman KL, Leon JS. Norovirus immunology: of mice and mechanisms. Eur J Immunol 2015; 45:2742–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernstein DI, Atmar RL, Lyon GM et al Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis 2015; 211:870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atmar RL, Bernstein DI, Harro CD et al Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med 2011; 365:2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Newman KL, Moe CL, Kirby AE, Flanders WD, Parkos CA, Leon JS. Human norovirus infection and the acute serum cytokine response. Clin Exp Immunol 2015; 182:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teunis PF, Sukhrie FH, Vennema H, Bogerman J, Beersma MF, Koopmans MP. Shedding of norovirus in symptomatic and asymptomatic infections. Epidemiol Infect 2015; 143:1710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leon JS, Kingsley DH, Montes JS et al Randomized, double‐blinded clinical trial for human norovirus inactivation in oysters by high hydrostatic pressure processing. Appl Environ Microbiol 2011; 77:5476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seitz SR, Leon JS, Schwab KJ et al Norovirus infectivity in humans and persistence in water. Appl Environ Microbiol 2011; 77:6884–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu P, Hsiao HM, Jaykus LA, Moe C. Quantification of Norwalk virus inocula: comparison of endpoint titration and real‐time reverse transcription–PCR methods. J Med Virol 2010; 82:1612–16. [DOI] [PubMed] [Google Scholar]
- 17. Wong HL, Pfeiffer RM, Fears TR, Vermeulen R, Ji S, Rabkin CS. Reproducibility and correlations of multiplex cytokine levels in asymptomatic persons. Cancer Epidemiol Biomarkers Prev 2008; 17:3450–6. [DOI] [PubMed] [Google Scholar]
- 18. Lindesmith L, Moe C, Lependu J, Frelinger JA, Treanor J, Baric RS. Cellular and humoral immunity following Snow Mountain virus challenge. J Virol 2005; 79:2900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang B, Snipes‐Magaldi L, Dennehy P et al Cytokines as mediators for or effectors against rotavirus disease in children. Clin Diagn Lab Immunol 2003; 10:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Azim T, Zaki MH, Podder G et al Rotavirus‐specific subclass antibody and cytokine responses in Bangladeshi children with rotavirus diarrhoea. J Med Virol 2003; 69:286–95. [DOI] [PubMed] [Google Scholar]
- 21. Lian JQ, Yang XF, Zhao RR et al Expression profiles of circulating cytokines, chemokines and immune cells in patients with hepatitis B virus infection. Hepat Mon 2014; 14:e18892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yeo AS, Azhar NA, Yeow W et al Lack of clinical manifestations in asymptomatic dengue infection is attributed to broad down‐regulation and selective up‐regulation of host defence response genes. PLoS One 2014; 9:e92240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reisinger EC, Fritzsche C, Krause R, Krejs GJ. Diarrhea caused by primarily non‐gastrointestinal infections. Nat Clin Pract Gastroenterol Hepatol 2005; 2:216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J, Li FQ, Wei HM, Lian ZX, Sun R, Tian ZG. Respiratory influenza virus infection induces intestinal immune injury via microbiota‐mediated Th17 cell‐dependent inflammation. J Exp Med 2014; 211:2397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehta SK, Crucian BE, Stowe RP et al Reactivation of latent viruses is associated with increased plasma cytokines in astronauts. Cytokine 2013; 61:205–9. [DOI] [PubMed] [Google Scholar]
- 26. Mumphrey SM, Changotra H, Moore TN et al Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1‐dependent interferon responses. J Virol 2007; 81:3251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW IV. STAT1‐dependent innate immunity to a Norwalk‐like virus. Science 2003; 299:1575–8. [DOI] [PubMed] [Google Scholar]
- 28. McFadden N, Bailey D, Carrara G et al Norovirus regulation of the innate immune response and apoptosis occurs via the product of the alternative open reading frame 4. PLOS Pathog 2011; 7:e1002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gustavsson L, Skovbjerg S, Lindh M, Westin J, Andersson LM. Low serum levels of CCL5 are associated with longer duration of viral shedding in norovirus infection. J Clin Virol 2015; 69:133–7. [DOI] [PubMed] [Google Scholar]
- 30. Marques RE, Guabiraba R, Russo RC, Teixeira MM. Targeting CCL5 in inflammation. Expert Opin Ther Targets 2013; 17:1439–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Troeger H, Loddenkemper C, Schneider T et al Structural and functional changes of the duodenum in human norovirus infection. Gut 2009; 58:1070–7. [DOI] [PubMed] [Google Scholar]
- 32. Souza M, Azevedo MS, Jung K, Cheetham S, Saif LJ. Pathogenesis and immune responses in gnotobiotic calves after infection with the genogroup II.4‐HS66 strain of human norovirus. J Virol 2008; 82:1777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheetham S, Souza M, Meulia T, Grimes S, Han MG, Saif LJ. Pathogenesis of a genogroup II human norovirus in gnotobiotic pigs. J Virol 2006; 80:10372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chachu KA, LoBue AD, Strong DW, Baric RS, Virgin HW. Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog 2008; 4:e1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chachu KA, Strong DW, LoBue AD, Wobus CE, Baric RS, Virgin HW IV. Antibody is critical for the clearance of murine norovirus infection. J Virol 2008; 82:6610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Navaneethan U, Giannella RA. Mechanisms of infectious diarrhea. Nat Clin Pract Gastroenterol Hepatol 2008; 5:637–47. [DOI] [PubMed] [Google Scholar]
- 37. Martina BE. Dengue pathogenesis: a disease driven by the host response. Sci Prog 2014; 97:197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lindesmith L, Moe C, Marionneau S et al Human susceptibility and resistance to Norwalk virus infection. Nat Med 2003; 9:548–53. [DOI] [PubMed] [Google Scholar]
- 39. Saif MA, Bonney DK, Bigger B et al Chronic norovirus infection in pediatric hematopoietic stem cell transplant recipients: a cause of prolonged intestinal failure requiring intensive nutritional support. Pediatr Transplant 2011; 15:505–9. [DOI] [PubMed] [Google Scholar]
- 40. Lindesmith LC, Donaldson E, Leon J et al Heterotypic humoral and cellular immune responses following Norwalk virus infection. J Virol 2010; 84:1800–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baldridge MT, Nice TJ, McCune BT et al Commensal microbes and interferon‐lambda determine persistence of enteric murine norovirus infection. Science 2015; 347:266–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Osborne LC, Monticelli LA, Nice TJ et al Coinfection. Virus–helminth coinfection reveals a microbiota‐independent mechanism of immunomodulation. Science 2014; 345:578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Serum cytokine concentrations of symptomatic norovirus‐infected challenge study volunteers. Prechallenge (day 0) to day 4 post‐challenge, one line per subject.
Fig. S2. Serum cytokine concentrations of asymptomatic norovirus‐infected challenge study volunteers. Prechallenge (day 0) to day 4 post‐challenge, one line per subject.
Fig. S3. Viral shedding in symptomatic (top panel) and asymptomatic (bottom panel) norovirus‐infected challenge study volunteers. Prechallenge (day 0) to end of follow‐up, one line per subject. GEC = genomic equivalence copies.


