Summary
The primary objective of the current study was to investigate the potential of the pneumococcal toxin, pneumolysin (Ply), to activate neutrophil extracellular trap (NET) formation in vitro. Isolated human blood neutrophils were exposed to recombinant Ply (5‐20 ng ml−1) for 30–90 min at 37°C and NET formation measured using the following procedures to detect extracellular DNA: (i) flow cytometry using Vybrant® DyeCycle™ Ruby; (ii) spectrofluorimetry using the fluorophore, Sytox® Orange (5 μM); and (iii) NanoDrop® technology. These procedures were complemented by fluorescence microscopy using 4′, 6‐diamino‐2‐phenylindole (DAPI) (nuclear stain) in combination with anti‐citrullinated histone monoclonal antibodies to visualize nets. Exposure of neutrophils to Ply resulted in relatively rapid (detected within 30–60 min), statistically significant (P < 0·05) dose‐ and time‐related increases in the release of cellular DNA impregnated with both citrullinated histone and myeloperoxidase. Microscopy revealed that NETosis appeared to be restricted to a subpopulation of neutrophils, the numbers of NET‐forming cells in the control and Ply‐treated systems (10 and 20 ng ml−1) were 4·3 (4·2), 14.3 (9·9) and 16·5 (7·5), respectively (n = 4, P < 0·0001 for comparison of the control with both Ply‐treated systems). Ply‐induced NETosis occurred in the setting of retention of cell viability, and apparent lack of involvement of reactive oxygen species and Toll‐like receptor 4. In conclusion, Ply induces vital NETosis in human neutrophils, a process which may either contribute to host defence or worsen disease severity, depending on the intensity of the inflammatory response during pneumococcal infection.
Keywords: calcium, chronic granulomatous disease, neutrophils, NETosis, pneumolysin, reactive oxygen species, Toll‐like receptor 4
Introduction
Despite advances in antibiotic therapy and access to sophisticated intensive care facilities, severe community‐acquired pneumonia (CAP), one of the most common infectious diseases, carries an unacceptably high mortality rate of 10–15% in hospitalized patients 1. Those with developing or impaired immune systems are most vulnerable, specifically the very young, elderly people and those with primary and acquired immunodeficiency disorders 1, 2. Of concern, the threat of CAP‐related mortality and morbidity is likely to increase in parallel with a growing elderly population, particularly in developing countries, in whom immunization procedures against the major viral (influenza virus) and bacterial (Streptococcus pneumoniae, also known as the pneumococcus) pathogens are less effective 2, 3.
In the case of invasive pneumococcal disease (IPD), respiratory failure, shock and multi‐organ dysfunction syndrome are leading causes of mortality 4. The major pneumococcal protein toxin, pneumolysin (Ply), contributes significantly to the pathogenesis of acute lung injury, due largely to proinflammatory activities and direct cytotoxic effects on pulmonary epithelium and endothelium, which also promote extrapulmonary dissemination of the pathogen 5, 6, 7. These activities result from the cholesterol‐binding, pore‐forming activities of the toxin 5, 6, 7. In addition Ply, by a mechanism independent of pore‐forming activity, has been reported to activate the production of proinflammatory cytokines, tumour necrosis factor α and interleukin 6 via activation of the pattern recognition receptor, Toll‐like receptor 4 (TLR‐4), on murine macrophages 8. Ply has also been implicated in the pathogenesis of acute coronary events in patients with bacterial CAP, which are also recognized as a significant cause of mortality 9, 10, 11. Mechanistically, evidence from experimental animal models of IPD has demonstrated the involvement of Ply in the formation of microlesions in the myocardium, resulting in myocardial toxicity and dysfunction 12, 13.
Dysregulation of neutrophil extracellular trap (NET) formation is another potential mechanism which may exacerbate lung and cardiac injury in IPD 14, 15, 16, 17, 18. The process of NETosis, which is highly conserved throughout evolution 19, involves the extracellular release of a web of hypercitrullinated chromatin impregnated with predominantly granule‐derived anti‐microbial proteins. NETs entrap and, in some cases, neutralize, microbial and viral pathogens 20. However, if excessive, NETosis poses the potential hazard of cell and organ damage, due not only to the cytotoxic actions of histone constituents 17, 21, but also to pro‐thrombotic activity 14, 15, 18.
The pro‐NETotic activity of Ply as a potential contributor to pulmonary and cardiac dysfunction during severe pneumococcal infection is, however, largely unexplored and represents the primary focus of the current study.
Materials and methods
Recombinant pneumolysin and delta6Ply
Recombinant native Ply was expressed in Escherichia coli and purified from cell extracts 22. The pneumolysoid, delta6Ply, with a double amino acid deletion (alanine 146 and arginine 147), resulting in attenuation of pore‐forming activity, was generated by site‐directed mutagenesis 23. The purities of both toxins were confirmed by sodium dodecyl sulphate‐polyacrylamide gel electrophoresis. The stock protein concentrations of native recombinant Ply and delta6Ply were 493 and 768 μg ml−1, respectively, and were essentially free of contaminating endotoxin. The respective haemolytic activities (using human erythrocytes, 1% final) were 22 700 and 0 haemolytic units pmol−1.
Unless indicated, all other chemicals and reagents were purchased from the Sigma Chemical Co. (St Louis, MO, USA).
Ethics approval
The study was approved by the Research Ethics Committee of the Faculty of Health Sciences, University of Pretoria (Approval no. 31/2014).
Neutrophils
Neutrophils were obtained from the blood of a total of 19 healthy, non‐smoking volunteers (mean age = 41, range = 23–66). Exclusion criteria included the use of any medication on the day of venepuncture. Neutrophils were prepared as described previously 24, 25. The cells, which were routinely of high purity (> 90%) and viability (> 95%), were suspended in indicator‐free Hanks's balanced salt solution (HBSS, pH 7·4; Highveld Biological Co., Johannesburg, South Africa) and used immediately in assays of NET formation.
Exposure of neutrophils to Ply
Following preincubation for 10 min at 37°C, Ply (5–20 ng ml−1) or an equal volume of HBSS (40 μl, control system) was added to the neutrophils (4 × 106 cells in 4 ml HBSS) and the tubes incubated for 30, 60 and 90 min. After incubation, 1 ml of cell suspension was removed from each tube and allocated for flow cytometric detection of citrullinated histone‐ and myeloperoxidase (MPO)‐associated extracellular DNA, as described below. The tubes containing the residual volume of 3 ml were centrifuged to pellet the cells, after which the supernatant fluids were removed and the cell pellets resuspended in 1 ml of HBSS and cell viability measured as described below. The supernatants from each tube were then aliquoted into 2·8 ml and 100 µl volumes for detection of extracellular DNA using spectrofluorimetry and NanoDrop® technology, as described below.
Flow cytometric detection of NETs
Neutrophil suspensions were aliquoted into three flow cytometry analysis tubes (Beckman Coulter, Miami, FL, USA) and stained with 5 μl of mouse anti‐human CD16‐allophycocyanin (APC) monoclonal antibody (Beckman Coulter), 1 μl of Vybrant® DyeCycle™ (VDC) Ruby (final concentration 0·5 μM; Life Technologies, Johannesburg, South Africa) and one of the following in each tube: 5 μl mouse anti‐human MPO‐fluorescein isothiocyanate (FITC) monoclonal antibody (Beckman Coulter), 4 μl of anti‐histone H4 (citrulline 3) rabbit polyclonal antibody (Merck Millipore, Billerica, MA, USA) or 4 μl goat anti‐rabbit immunoglobulin (Ig)G (H + L)‐Alexa Fluor® 488 secondary antibody (Life Technologies). The tubes were mixed gently and incubated in the dark for 10 min at room temperature followed by the addition of 4 μl goat anti‐rabbit IgG (H + L)‐Alexa Fluor® 488 secondary antibody to the tube treated with the purified anti‐histone H4 (citrulline 3) rabbit polyclonal antibody. After an additional 10‐min period of incubation, flow cytometry analyses were performed using a Gallios flow cytometer (Beckman Coulter). Extracellular DNA was identified as CD16–/VDC Ruby+/MPO+ or citrullinated histone H4+ events.
Spectrofluorimetric detection of NETs
In the case of the spectrofluorimetric procedure, the cell‐free supernatants (2·8 ml) were mixed with 3 μl of the DNA‐binding fluorophore, Sytox® Orange (5 µM final; Life Technologies) 26, transferred to cuvettes and placed in the cuvette holder of a Hitachi 650 10S fluorescence spectrophotometer (Hitachi Ltd, Tokyo, Japan) with the excitation and emission wavelengths set at 530 nm and 590 nm, respectively, followed by measurement of fluorescence intensity. The results are expressed as metered fluorescence units (MFUs) following subtraction of the values of time‐matched background control systems, which were held at room temperature (25°C).
NanoDrop® procedure
Extracellular DNA concentrations were determined in the cell‐free supernatants using a NanoDrop ND‐1000 spectrophotometer (Thermo Scientific, Johannesburg, South Africa), according to a previously published protocol 27, and results recorded as nanograms (ng) µl−1.
For this and the spectrofluorimetric procedure a maximum incubation time of 60 min was used, as this gave optimum discrimination between the control and Ply‐treated systems.
A series of additional experiments was performed using the spectrofluorimetric and NanoDrop® procedures. These were: (i) comparison of the pro‐NETotic effects of native Ply with those of delta6Ply (10 and 20 ng ml−1) 23, 28; (ii) measurement of the requirement for extracellular Ca2+ in the pro‐NETotic actions of Ply by suspending the cells in Ca2+‐free medium; (iii) measurement of the requirement for cellular generation of reactive oxygen species (ROS) in Ply‐activated NETosis, first by pretreating the cells with the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor, diphenyleneiodonium chloride (DPI, 10 μM final) 5 min prior to the addition of the toxin, and secondly, by using cells from a 44‐year‐old male with the autosomal recessive form of chronic granulomatous disease (CGD, deficiency of p47phox, GT deletion in exon 2) 29; (iv) a comparison of the pro‐NETotic actions of Ply (20 ng ml−1) with those of the potent activator of NADPH oxidase, phorbol 12‐myristate 13‐acetate (6·25 ng ml−1, final); and (vi) a comparison of the effects of native Ply and the pneumolysoid, delta6Ply, on NETosis.
Microscopic detection of NETs
Microscopy was performed to confirm that the extracellular DNA demonstrated by other techniques was the product of NETosis. Neutrophil suspension (1·25 × 105 cells in 250 µl) was allowed to adhere to glass coverslips for 30 min. Ply (10 and 20 ng ml−1 final) or an equal volume of HBSS was added to the adherent cells and the coverslips incubated for 90 min at 37°C 5% CO2. The cells were then fixed with 4% paraformaldehyde for 10 min, washed three times in phosphate‐buffered saline (PBS) and blocked with HBSS containing 5% goat serum and 5% bovine serum albumin for 30 min at 37°C. Neutrophils were incubated overnight with polyclonal rabbit anti‐histone H4 (citrulline 3; Merck Millipore) and washed three times in PBS. Following the addition of Alexa Fluor 488‐labelled goat anti‐rabbit secondary antibody (Life Technologies), DNA present in the specimen was stained with the DNA‐binding dye, 4′,6‐diamidino‐2‐phenylindole (DAPI; Life Technologies) for 2 min and analysed for NET formation by fluorescence microscopy using a Zeiss Axio Vert. A1 fluorescence microscope and Axio Vision Software (Zeiss, Johannesburg, South Africa). Representative photomicrographs were taken, and used for the generation of counts by a single non‐blinded observer. Results are expressed as percentage of NET‐forming cells.
Assessment of pore‐forming activity
The pore‐forming activities of Ply (10 and 20 ng ml−1) were determined spectrofluorimetrically according to the magnitude of influx of Ca2+ into neutrophils using fura‐2 acetoxymethylester (fura‐2 AM) as the Ca2+‐sensitive, fluorescent indicator. Briefly, neutrophils (1 × 107 ml−1 in PBS) were incubated with fura‐2 AM (2 μM, final) for 25 min at 37°C, washed and resuspended in HBSS. The fura‐2‐loaded cells (1 × 106) were then preincubated for 10 min at 37°C and transferred to cuvettes which were maintained at 37°C in the Hitachi 650 10S fluorescence spectrophotometer with the excitation and emission wavelengths set at 340 nm and 500 nm, respectively. After a stable baseline was obtained (± 1 min), Ply or delta6Ply was added to the cells and alterations in fluorescence intensity monitored over a 5–10‐min time–course.
Measurement of reactive oxygen species (ROS)
A luminol (5‐amino‐2,3‐dihydro‐1,4‐phthalazinedione)‐enhanced chemiluminescence (LECL) procedure, which is a composite measure of phagocyte‐derived ROS, was used to investigate the generation of ROS by Ply (20 ng ml−1) in the absence and presence of DPI (10 μM). Briefly, the neutrophils (1 × 106 ml−1) in HBSS containing 0·1 mM luminol were preincubated for 10 min at 37°C, without and with DPI, followed by addition of Ply and measurement of LECL during a 20‐min time–course using a thermoregulated chemiluminometer (LKB Wallac 1251 Chemiluminometer; LKB Wallac, Turku, Finland) and results expressed as the peak LECL responses in mV s−1. In addition, a lucigenin (bis‐N‐methylacridium nitrate, 0·2 mM, final)‐enhanced chemiluminescence method was used to demonstrate the generation of superoxide by phorbol myristate acetate (PMA) (6·25 ng ml−1)‐activated neutrophils from the CGD subject.
Cell viability
This was measured at each time interval following addition of Ply or PMA to neutrophils using a flow cytometric propidium iodide‐based dye exclusion assay. The cells (4 × 106 ml−1) were incubated for 5 min with propidium iodide (50 μg ml−1, DNA Prep‐Stain; Beckman Coulter) and cell viability assessed flow cytometrically. Results are expressed as percentage viable cells.
Expression and statistical analyses of results
The results of each series of experiments are expressed as the mean values and standard deviations (s.d.), with the exception of the spectrofluorimetric measurements of Ca2+ influx, for which the traces are shown. In all cases, the ‘n’ values represent the number of different donors used in each series of experiments. The exceptions are studies using CGD cells, in which several replicates were performed on a single donor. For flow cytometric data, statistical analyses were performed using GraphPad Prism 6 version 6·07 (GraphPad Software, San Diego, CA, USA). A two‐way analysis of variance (anova) with Sidak's multiple comparisons test was used to determine statistically significant differences between the untreated control groups and the Ply‐treated groups (various concentrations) over the different time‐points. For bivariate hypothesis testing, t‐tests were used. A P‐value of < 0·05 was taken as statistically significant.
Results
Induction of NETosis by Ply
A representative gating strategy used to identify citrullinated histone+/MPO+ extracellular DNA fragments following treatment of neutrophils with Ply (20 ng ml−1) for 90 min at 37ºC is shown in Fig. 1a–d. The various panels show exclusion of intact CD16+ neutrophils (a); the non‐specific staining control using secondary antibody only (b); indication of extracellular DNA fragment size of CD16–/VDC Ruby+/citrullinated histone+ events (c); and identification of CD16–/VDC Ruby+/citrullinated histone+ or MPO+ extracellular DNA fragments. Due probably to shear pressure applied to the fragments during flow cytometric analysis, the majority of these extracellular DNA fragments, equivalent to 79·59% (7·43), displayed low forward‐scatter properties (Fig. 1d). Only 20·41% (7·43) of the fragments were comparable in size to intact cells (intermediate/high forward‐scatter).
Figure 1.
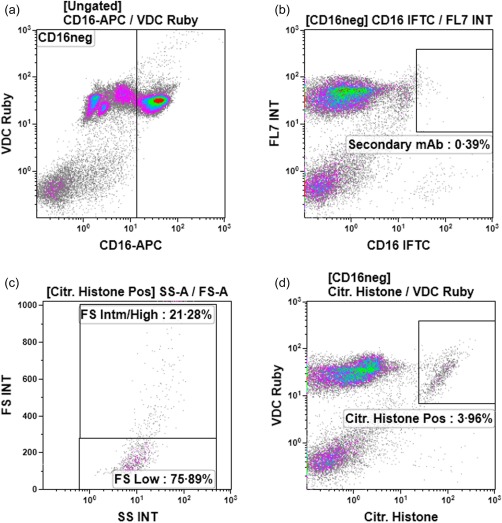
The flow cytometric gating strategy and staining approach to detect extracellular DNA fragments are shown in (a–d). Exclusion of intact CD16+ neutrophils is shown in (a); staining with goat anti‐rabbit immunoglobulin (Ig)G (H + L)‐Alexa Fluor® 488 secondary antibody only (non‐specific staining control) in (b); indication of extracellular DNA fragment size of CD16–/Vybrant® DyeCycle™ (VDC) Ruby+/citrullinated (citr) histone+ events in (c); and identification of CD16–/VDC Ruby+/citrullinated histone+ events in (d).
The effects of addition of Ply (5–20 ng ml−1) on NETosis during a 90‐min time–course, using the flow cytometric procedure, are shown in Fig. 2. Addition of Ply to the cells resulted in a dose‐ and time‐dependent activation of NETosis measured according to the release of citrullinated histone‐positive, extracellular DNA from the cells, which achieved statistical significance at a concentration of 20 ng ml−1 (P < 0·05–P < 0·01) at 60 min, and at concentrations of 10 and 20 ng ml−1 Ply after 90 min exposure (P < 0·01 and P < 0·001, respectively). Similar trends were observed with respect to detection of MPO‐positive extracellular DNA.
Figure 2.
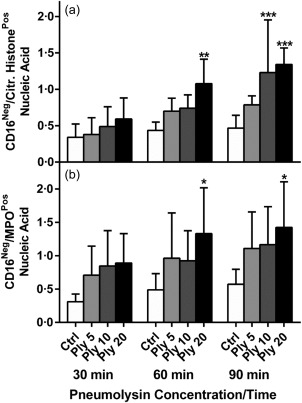
Flow cytometric determination of pneumolysin (Ply) (5, 10 and 20 ng ml−1)‐activated NETosis over a 30–90‐min time–course. The results of five separate experiments (n = 5) are expressed as the mean percentages of total Vybrant® DyeCycle™ (VDC) Ruby+ events [standard deviation (s.d.)], including intact nucleated cells. *P < 0·05; **P < 0·01; ***P < 0·001. A two‐way analysis of variance (anova) with Sidak's multiple comparison test were used to determine significance of the various Ply concentrations at different time‐points.
The effects of Ply (10 and 20 ng ml−1) on NETosis, measured using the spectrofluorimetric and NanoDrop® procedures and a fixed incubation time of 60 min, are shown in Fig. 3.
Figure 3.
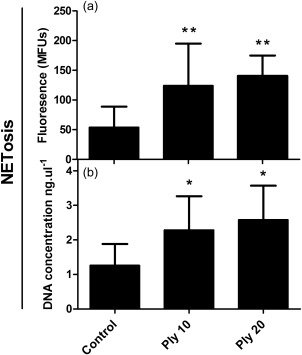
Measurement of pneumolysin (Ply) (10 and 20 ng ml−1)‐activated NETosis according to measurement of extracellular DNA after 60 min incubation using the spectrofluorimetric (a) and NanoDrop® (b) procedures. The results of 10 separate experiments are expressed as the mean values and standard deviations (s.d.) as metered fluorescence units (MFUs) or ng DNA µl−1. *P < 0·01; **P < 0·0001. Data shown in both (a) and (b), data were analysed using the t‐test.
Using both procedures, exposure of neutrophils to Ply at both concentrations resulted in significant increases (P < 0·01–P < 0·0001) in release of extracellular DNA. Importantly, no loss of neutrophil viability was observed following treatment of neutrophils with Ply for up to 60 min, the mean percentage viability (s.d.) being 97·4 (0·9), 98·2 (0·5) and 97·7 (0·7) for the control and Ply (10 and 20 ng ml−1)‐treated systems, respectively (n = 6).
A comparison of Ply (20 ng ml−1)‐ and PMA (6·25 ng ml−1)‐activated NET formation using the spectrofluorimetric procedure is shown in Fig. 4a, with the corresponding cell viability data shown in Fig. 4b. Fixed incubation times of 60 and 90 min were used for Ply and PMA, respectively, as meaningful PMA‐activated NETosis only became detectable at 90 min. PMA, which was a more effective activator of NETosis than Ply, was also associated with loss of viability. A similar time–course of PMA‐activated NETosis has been described 30.
Figure 4.
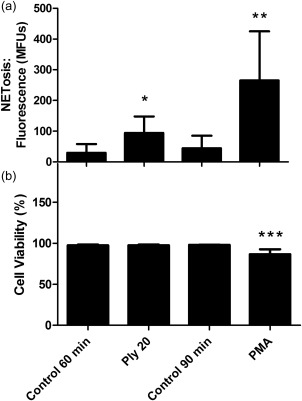
Comparison of the effects of pneumolysin (Ply) (20 ng ml−1) and phorbol myristate acetate (PMA) (6·25 ng ml−1) on: (i) NETosis using the spectrofluorimetric procedure and exposure times of 60 min and 90 min for systems treated with Ply and PMA, respectively (a); and (ii) cell viability using the same exposure times (b). The results of five experiments (n = 5) are presented as the mean values as metered fluorescence units (MFUs) or percentage viability and s.d. for measurement of NETosis and viability, respectively. *P < 0·03; **P < 0·02; ***P < 0·007. Data were analysed using the t‐test.
Microscopic detection of NETosis
According to microscopic analysis, the mean percentages (s.d.) of NETotic cells in the control system and systems treated with 10 and 20 ng ml−1 Ply following 90 min incubation were 4·3 (4·2), 14·3 (9·9) and 16·5 (7·5), respectively (n = 4, P < 0·0001 for comparison of the control with both Ply‐treated systems). A photomicrograph demonstrating Ply (20 ng ml−1)‐induced NETs is shown in Fig. 5.
Figure 5.
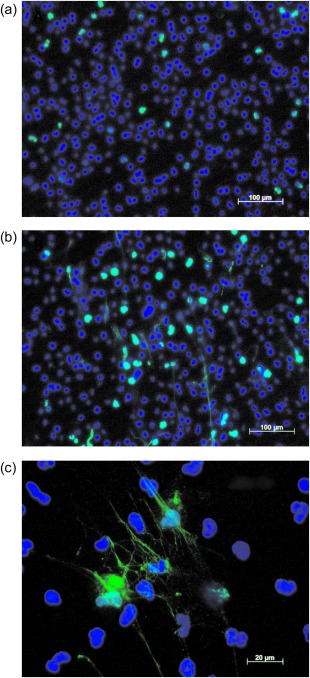
Fluorescence microscopic images of neutrophil extracellular trap formation by control neutrophils (a) and cells treated for 90 min with pneumolysin at a concentration of 20 ng ml−1 (b) stained with 4′, 6‐diamino‐2‐phenylindole (DAPI) (nuclear stain) and citrullinated histone antibody viewed at ×10 magnification. (c) NETotic cells at a higher magnification (×40).
Assessment of the involvement of TLR‐4, ROS and extracellular Ca2+ in the pro‐NETotic actions of Ply
Exposure of neutrophils to delta6Ply which, like Ply, activates TLR‐4 8, failed to induce NETosis, the MFU values (s.d.) being 15·3 (4·1), 96 (52·3) and 10·1 (10·48) for the control system and the systems treated with 20 ng ml−1 Ply or delta6Ply, respectively (n = 3). Similar results were obtained with the NanoDrop® procedure (not shown). These results apparently exclude involvement of TLR‐4 in the pro‐NETotic actions of Ply.
The effects of the NADPH oxidase inhibitor, DPI (10 μM), on Ply‐activated NETosis, as well as the effects of the pore‐forming toxin on CGD cells, are shown in Fig. 6. Treatment of neutrophils with DPI had modest, statistically insignificant effects on Ply‐activated NETosis, while exposure of CGD neutrophils to the toxin, but not to PMA, resulted in significant NETosis. These results appear to exclude meaningful involvement of NADPH oxidase‐derived ROS in the pro‐NETotic actions of Ply, while confirming their role in PMA‐activated NETosis.
Figure 6.
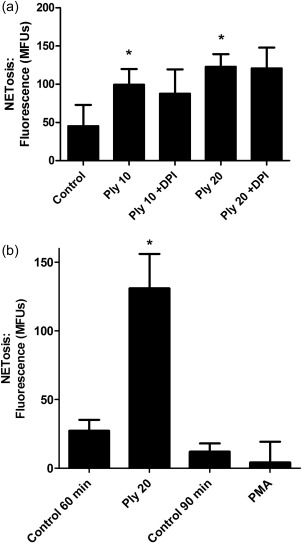
Measurement of the involvement of reactive oxygen species in pneumolysin (Ply) (20 ng ml−1)‐mediated NETosis using: (i) neutrophils from healthy individuals (n = 4) treated with the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor diphenyleneiodonium chloride (DPI, 10 μM) shown in (a) and (ii) neutrophils from a single patient with chronic granulomatous disease (CGD) exposed to Ply (20 ng ml−1) or phorbol myristate acetate (PMA) (6·25 ng ml−1) for 60 and 90 min, respectively (four replicates in each experimental system) as shown in (b). *P < 0·03 for comparison of the Ply‐treated systems at both 10 and 20 ng ml−1 with the untreated control system. Inclusion of DPI had no statistically significant effects on NETosis‐activated by Ply at either 10 or 20 ng ml−1 (a). *P < 0·03 for comparison of the responses of Ply‐exposed chronic granulomatous disease (CGD) cells with the corresponding control system, while no significant differences were evident between the control and PMA‐activated systems (b). Data from both (a) and (b) were analysed using the t‐test.
Exposure of neutrophils suspended in Ca2+‐free medium to Ply (10 ng ml−1) for 60 min resulted in significant cytotoxicity accompanied by a massive increase in extracellular DNA (s.d.), with values of 0·41 (0·25) and 10·35 (1·74) ng DNA µl−1 for the control and Ply‐treated systems, respectively, using the NanoDrop® procedure, with corresponding mean percentages (s.d.) viability of 97·5 (0·5) and 11·7 (11·6), n = 3.
Assessment of the pore‐forming activities of Ply and delta6Ply
Pore‐forming activity was assessed spectrofluorimetrically using fura‐2/AM as the Ca2+‐sensitive fluorescent probe and these results, which are typical traces from a single representative experiment, are shown in Fig. 7. Following a short lag, treatment of neutrophils with native Ply, but not delta6Ply, caused significant influx of Ca2+.
Figure 7.
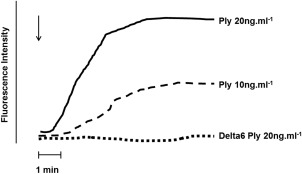
Fura‐2/acetoxymethylester (AM) fluorescence traces from a single representative experiment showing the effect of addition (denoted by the arrow, ↓) of either pneumolysin (Ply) (10 and 20 ng ml−1) or delta6Ply (20 ng ml−1) on the influx of Ca2+ into neutrophils recorded as increased fluorescence intensity as a measure of pore‐forming activity.
Measurement of ROS
Exposure of neutrophils to Ply resulted in a modest increase in the generation of ROS, which was abolished completely by treatment of the cells with DPI (P = 0·03). The mean peak values (s.d.) for the control Ply‐free system and those treated with 20 ng ml−1 Ply without and with DPI were 1140 (286), 1427 (376) and 36 (36) mV s−1, respectively (n = 3). In the case of superoxide generation by the CGD cells, the peak values for unstimulated and PMA (6·25 ng ml−1) ‐activated neutrophils from a healthy control subject were 877 (212) and 5100 (246) mV s−1, respectively, while the corresponding values from the CGD subject were 87 (43) and 72 (35) mV s−1.
Discussion
This study, using a range of mutually confirmatory procedures, has demonstrated that exposure of human neutrophils to Ply at pathologically relevant concentrations 31 results in activation of NETosis. If such interactions occur in the clinical setting this may explain, at least in part, the mechanisms underlying the cardiopulmonary complications of severe pneumococcal disease which are associated with a high mortality despite seemingly appropriate anti‐microbial therapy 9, 32.
The pro‐NETotic actions of Ply resulted in mobilization of citrullinated histone‐ and MPO‐associated nuclear DNA, but in contrast to those of the prototype pro‐oxidative activator of NETosis, phorbol ester (PMA), which induces late suicidal NETosis 30, 33, these were detected earlier (within 30–60 min), were independent of activation of NADPH oxidase and occurred in the setting of retention of cell viability. These findings are consistent with the process of vital NETosis reported previously to be initiated by activators such as the Staphylococcus aureus pore‐forming toxin, Panton–Valentine leucocidin (PVL) 33, and the calcium ionophore, ionomycin 34. In both cases, there are some similarities with those of Ply‐activated NETosis 33. These include: (i) relative rapidity of onset; (ii) lack of involvement of ROS; (iii) mobilization of nuclear DNA; (iv) retention of cell viability; and (v) restriction of vital NETosis to < 20% of the cell population, consistent with the involvement of a neutrophil subpopulation in this process 35. Previous reports using non‐opsonized 6‐h bacterial cultures have suggested that pneumococci are less effective inducers of NETosis than S. aureus 33. However, as release of Ply occurs late in the growth of pneumococci due to autolysis, 6 h of culture is too early for optimal release of Ply. In addition, induction of NETosis occurs most effectively in the presence of opsonized bacteria 36.
With respect to alternative potential mechanisms of Ply‐mediated NETosis the toxin, in both its native and toxoid forms, has been reported to interact with and activate TLR‐4 on macrophages 8, also present on neutrophils, representing a potential mechanism of induction of NETosis. This can be excluded, however, on the basis of the observed inactivity of delta6Ply observed in the current study, underscoring the requirement for the pore‐forming activity of the intact native toxin in activation of NETosis. In this context, we have reported previously that Ply augments several early‐occurring inflammatory activities of neutrophils by a mechanism dependent on pore‐formation and influx of extracellular Ca2+ 24, 25. These activities include increased generation of ROS, prostanoids and eicosanoids, as well as release of the primary granule enzyme, elastase 24, 25. In the current study, however, a link between Ply‐mediated NETosis and Ca2+ influx was difficult to establish. This was due to augmentation of the cytotoxic actions of Ply in Ca2+‐free medium, a phenomenon we have observed previously 24, which is seemingly related to the requirement for Ca2+ to repair Ply‐mediated membrane pore formation 37. The lack of activity of delta6Ply therefore represents the major line of evidence, albeit indirect, supporting a pore‐forming/Ca2+‐dependent mechanism in the pro‐NETotic actions of Ply. It is, however, also supported by the similarities to the pro‐NETotic actions of ionomycin, which also occur within 30–60 min, in the setting of lack of involvement of ROS 34. In this context, it is noteworthy that neutrophils from a CGD individual were responsive to Ply‐induced NETosis in keeping with previous reports of the existence of NADPH‐oxidase‐independent mechanisms of NETosis operative in CGD neutrophils 16, 38, 39.
Pneumococcal pneumonia is associated with marked neutrophilic infiltration 37 and the presence of NETs in the lungs of mice infected experimentally with the pneumococcus 40. Although unproven, the events involved in induction of NETosis in this setting are likely to be related to the pro‐NETotic actions of Ply described in the current study, augmented possibly by pneumococcal alpha‐enolase, which has also been reported to induce NET formation, albeit via an indirect mechanism 41. Although NETs are intended to trap and possibly kill microbial pathogens, the pneumococcus via expression of the polysaccharide capsule 42 and the surface endonuclease, EndA 43, is particularly adept at evading and escaping from NETs. Induction and subversion of NETosis therefore represents a potential strategy favouring persistence and possibly invasiveness of the pathogen resulting from alveolar epithelial and endothelial damage mediated by the cytotoxic components of NETs, especially histones 17, 21.
The findings of the current study not only extend the repertoire of potentially harmful proinflammatory, proinfective activities of Ply, but may also provide novel insights into the immunopathogenesis of acute lung injury and acute cardiac events during severe pneumococcal infection. Notwithstanding direct cytotoxic effects 12, 13, 14, 15, 16, 17, 18 and proinflammatory actions of the toxin 24, 25, indirect activities related to activation of NETosis, including cytotoxic and pro‐thrombotic activities of NETs 14, 15, 18, are also likely to contribute to toxin‐mediated pulmonary and cardiac injury. These findings underscore the potential of Ply‐targeted therapies such as macrolide and macrolide‐like antibiotics which are potent inhibitors of the production of Ply 44, as well as cholesterol‐rich liposomes which neutralize the toxin 45. In addition, pharmacological inhibitors of peptidylarginine deiminases, key enzymes in the induction of NETosis, are currently in preclinical development 46.
Disclosure
None of the authors have any disclosures to declare.
References
- 1. Steel HC, Cockeran R, Anderson R, Feldman C. Overview of community‐acquired pneumonia and the role of inflammatory mechanisms in the immunopathogenesis of severe pneumococcal disease. Mediators Inflamm 2013; 2013:490346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Welte T, Torres A, Nathwani D. Clinical and economic burden of community‐acquired pneumonia among adults in Europe. Thorax 2012; 67:71–9. [DOI] [PubMed] [Google Scholar]
- 3. Feldman C, Anderson R. Review: current and new generation pneumococcal vaccines. J Infect 2014; 69:309–25. [DOI] [PubMed] [Google Scholar]
- 4. Aliberti S, Ramirez JA. Cardiac diseases complicating community‐acquired pneumonia. Curr Opin Infect Dis 2014; 27:295–301. [DOI] [PubMed] [Google Scholar]
- 5. Feldman C, Munro NC, Jeffery PK et al Pneumolysin induces the salient histologic features of pneumococcal infection in the rat lung in vivo . Am J Respir Cell Mol Biol 1991; 5:416–23. [DOI] [PubMed] [Google Scholar]
- 6. Witzenrath M, Gutbier B, Hocke AC et al Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit Care Med 2006; 34:1947–54. [DOI] [PubMed] [Google Scholar]
- 7. Garcia‐Suarez Mdel M, Florez N, Astudillo A et al The role of pneumolysin in mediating lung damage in a lethal pneumococcal pneumonia murine model. Respir Res 2007; 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malley R, Henneke P, Morse SC et al Recognition of pneumolysin by Toll‐like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci USA 2003; 100:1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Musher DM, Rueda AM, Kaka AS, Mapara SM. The association between pneumococcal pneumonia and acute cardiac events. Clin Infect Dis 2007; 45:158–65. [DOI] [PubMed] [Google Scholar]
- 10. Corrales‐Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis 2010; 10:83–92. [DOI] [PubMed] [Google Scholar]
- 11. Corrales‐Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ. Cardiac complications in patients with community‐acquired pneumonia: incidence, timing, risk factors, and association with short‐term mortality. Circulation 2012; 125:773–81. [DOI] [PubMed] [Google Scholar]
- 12. Alhamdi Y, Neill DR, Abrams ST et al Circulating pneumolysin is a potent inducer of cardiac injury during pneumococcal infection. PLOS Pathog 2015; 11:e1004836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown AO, Mann B, Gao G et al Streptococcus pneumoniae translocates into the myocardium and forms unique microlesions that disrupt cardiac function. PLOS Pathog 2014; 10:e1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mangold A, Alias S, Scherz T et al Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST‐elevation acute coronary syndrome are predictors of ST‐segment resolution and infarct size. Circ Res 2015; 116:1182–92. [DOI] [PubMed] [Google Scholar]
- 15. Borissoff JI, ten Cate H. From neutrophil extracellular traps release to thrombosis: an overshooting host‐defense mechanism? J Thromb Haemost 2011; 9:1791–4. [DOI] [PubMed] [Google Scholar]
- 16. Narayana Moorthy A, Narasaraju T, Rai P et al In vivo and in vitro studies on the roles of neutrophil extracellular traps during secondary pneumococcal pneumonia after primary pulmonary influenza infection. Front Immunol 2013; 4:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ward PA, Grailer JJ. Acute lung injury and the role of histones. Transl Respir Med 2014; 2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stakos DA, Kambas K, Konstantinidis T et al Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J 2015; 36:1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robb CT, Dyrynda EA, Gray RD, Rossi AG, Smith VJ. Invertebrate extracellular phagocyte traps show that chromatin is an ancient defence weapon. Nat Commun 2014; 5:4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brinkmann V, Reichard U, Goosmann C et al Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532–5. [DOI] [PubMed] [Google Scholar]
- 21. Saffarzadeh M, Juenemann C, Queisser MA et al Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLOS ONE 2012; 7:e32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saunders FK, Mitchell TJ, Walker JA, Andrew PW, Boulnois GJ. Pneumolysin, the thiol‐activated toxin of Streptococcus pneumoniae, does not require a thiol group for in vitro activity. Infect Immun 1989; 57:2547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirkham LA, Kerr AR, Douce GR et al Construction and immunological characterization of a novel nontoxic protective pneumolysin mutant for use in future pneumococcal vaccines. Infect Immun 2006; 74:586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cockeran R, Theron AJ, Steel HC et al Proinflammatory interactions of pneumolysin with human neutrophils. J Infect Dis 2001; 183:604–11. [DOI] [PubMed] [Google Scholar]
- 25. Cockeran R, Steel HC, Mitchell TJ, Feldman C, Anderson R. Pneumolysin potentiates production of prostaglandin E(2) and leukotriene B(4) by human neutrophils. Infect Immun 2001; 69:3494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berkes E, Oehmke F, Tinneberg HR, Preissner KT, Saffarzadeh M. Association of neutrophil extracellular traps with endometriosis‐related chronic inflammation. Eur J Obstet Gynecol Reprod Biol 2014; 183:193–200. [DOI] [PubMed] [Google Scholar]
- 27. Desjardins P, Conklin D. NanoDrop microvolume quantitation of nucleic acids. J Vis Exp 2010, doi:10.3791/2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cockeran R, Steel HC, Theron AJ, Mitchell TJ, Feldman C, Anderson R. Characterization of the interactions of the pneumolysoid, Delta6 PLY, with human neutrophils in vitro . Vaccine 2011; 29:8780–2. [DOI] [PubMed] [Google Scholar]
- 29. Tintinger GR, Theron AJ, Steel HC, Anderson R. Accelerated calcium influx and hyperactivation of neutrophils in chronic granulomatous disease. Clin Exp Immunol 2001; 123:254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barrientos L, Marin‐Esteban V, de Chaisemartin L et al An improved strategy to recover large fragments of functional human neutrophil extracellular traps. Front Immunol 2013; 4:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spreer A, Kerstan H, Bottcher T et al Reduced release of pneumolysin by Streptococcus pneumoniae in vitro and in vivo after treatment with nonbacteriolytic antibiotics in comparison to ceftriaxone. Antimicrob Agents Chemother 2003; 47:2649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mongardon N, Max A, Bougle A et al Epidemiology and outcome of severe pneumococcal pneumonia admitted to intensive care unit: a multicenter study. Crit Care 2012; 16:R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pilsczek FH, Salina D, Poon KK et al A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus . J Immunol 2010; 185:7413–25. [DOI] [PubMed] [Google Scholar]
- 34. Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol 2012; 92:841–9. [DOI] [PubMed] [Google Scholar]
- 35. Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood 2014; 124:710–9. [DOI] [PubMed] [Google Scholar]
- 36. Mitchell TJ, Dalziel CE. The biology of pneumolysin. Subcell Biochem 2014; 80:145–60. [DOI] [PubMed] [Google Scholar]
- 37. Wolfmeier H, Schoenauer R, Atanassoff AP et al Ca(2)(+)‐dependent repair of pneumolysin pores: a new paradigm for host cellular defense against bacterial pore‐forming toxins. Biochim Biophys Acta 2015; 1853:2045–54. [DOI] [PubMed] [Google Scholar]
- 38. Rada B, Jendrysik MA, Pang L et al Pyocyanin‐enhanced neutrophil extracellular trap formation requires the NADPH oxidase. PLOS ONE 2013; 8:e54205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arai Y, Nishinaka Y, Arai T et al Uric acid induces NADPH oxidase‐independent neutrophil extracellular trap formation. Biochem Biophys Res Commun 2014; 443:556–61. [DOI] [PubMed] [Google Scholar]
- 40. Kadioglu A, Andrew PW. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol 2004; 25:143–9. [DOI] [PubMed] [Google Scholar]
- 41. Mori Y, Yamaguchi M, Terao Y, Hamada S, Ooshima T, Kawabata S. Alpha‐Enolase of Streptococcus pneumoniae induces formation of neutrophil extracellular traps. J Biol Chem 2012; 287:10472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wartha F, Beiter K, Albiger B et al Capsule and D‐alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol 2007; 9:1162–71. [DOI] [PubMed] [Google Scholar]
- 43. Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques‐Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol 2006; 16:401–7. [DOI] [PubMed] [Google Scholar]
- 44. Anderson R, Steel HC, Cockeran R et al Comparison of the effects of macrolides, amoxicillin, ceftriaxone, doxycycline, tobramycin and fluoroquinolones, on the production of pneumolysin by Streptococcus pneumoniae in vitro . J Antimicrob Chemother 2007; 60:1155–8. [DOI] [PubMed] [Google Scholar]
- 45. Henry BD, Neill DR, Becker KA et al Engineered liposomes sequester bacterial exotoxins and protect from severe invasive infections in mice. Nat Biotechnol 2015; 33:81–8. [DOI] [PubMed] [Google Scholar]
- 46. Bozdag M, Dreker T, Henry C et al Novel small molecule protein arginine deiminase 4 (PAD4) inhibitors. Bioorg Med Chem Lett 2013; 23:715–9. [DOI] [PubMed] [Google Scholar]


