Summary
Dipeptidyl peptidase (DPP) 4 (CD26, DPP4) is a multi‐functional protein involved in T cell activation by co‐stimulation via its association with adenosine deaminase (ADA), caveolin‐1, CARMA‐1, CD45, mannose‐6‐phosphate/insulin growth factor‐II receptor (M6P/IGFII‐R) and C‐X‐C motif receptor 4 (CXC‐R4). The proline‐specific dipeptidyl peptidase also modulates the bioactivity of several chemokines. However, a number of enzymes displaying either DPP4‐like activities or representing structural homologues have been discovered in the past two decades and are referred to as DPP4 activity and/or structure homologue (DASH) proteins. Apart from DPP4, DASH proteins include fibroblast activation protein alpha (FAP), DPP8, DPP9, DPP4‐like protein 1 (DPL1, DPP6, DPPX L, DPPX S), DPP4‐like protein 2 (DPL2, DPP10) from the DPP4‐gene family S9b and structurally unrelated enzyme DPP2, displaying DPP4‐like activity. In contrast, DPP6 and DPP10 lack enzymatic DPP4‐like activity. These DASH proteins play important roles in the immune system involving quiescence (DPP2), proliferation (DPP8/DPP9), antigen‐presenting (DPP9), co‐stimulation (DPP4), T cell activation (DPP4), signal transduction (DPP4, DPP8 and DPP9), differentiation (DPP4, DPP8) and tissue remodelling (DPP4, FAP). Thus, they are involved in many pathophysiological processes and have therefore been proposed for potential biomarkers or even drug targets in various cancers (DPP4 and FAP) and inflammatory diseases (DPP4, DPP8/DPP9). However, they also pose the challenge of drug selectivity concerning other DASH members for better efficacy and/or avoidance of unwanted side effects. Therefore, this review unravels the complex roles of DASH proteins in immunology.
Keywords: antigen presentation/processing, CD26, co‐stimulation, DPP4 activity and/or structure homologue proteins (DASH), signal transduction
The dipeptidyl peptidase (DPP)4 family of DPP4 activity and/or structure homologue (DASH) proteins
Within the last two decades a number of enzymes have been discovered to also display DPP4‐like activity or are structural homologues to DPP4. These enzymes/proteins are referred to as DPP4 activity and/or structure homologue (DASH) proteins, and can be grouped into the DPP4 gene family and non‐related DPP4‐like enzymes.
DPP4 gene family
DPP4 belongs to the serine peptidase clan SC, subfamily 9b. Peptidases of the SC clan have a unique catalytic triad in the order of Ser, Asp and His located in an α/β‐hydrolase fold compared to the chymotrypsin catalytic triad of His, Asp and Ser. Currently, six members have been identified as belonging to the dipeptidyl peptidase subfamily 9b, including DPP4, fibroblast activation protein alpha (FAP) 1, DPP8 2, DPP9 3, DPP4‐like protein 1 (DPL1, DPP6, DPPX L, DPPX S) 4 and DPP4‐like protein 2 5. Except for DPL1 and DPL2, all members display DPP4‐like activity with neutral to basic pH optima and similar inhibition profiles 6, 7.
DPP4 (CD26). DPP4 (CD26) is the best‐known DASH protein and has been described in detail elsewhere 7, 8, 9. DPP4 is a multi‐functional protein involved in T cell activation by co‐stimulation via its association with adenosine deaminase (ADA), caveolin‐1, CARMA‐1, CD45, mannose−6‐phosphate/insulin growth factor‐II receptor (M6P/IGFII‐R) and C‐X‐C motif receptor 4 (CXC‐R4). The proline‐specific DPP4 also modulates the bioactivity of several chemokines, as well as neuropeptides and peptide hormones such as neuropeptide Y (NPY), substance P (SP), glucagon‐like peptide (GLP)−1, GIP and glucagon. Indeed, several DPP4 inhibitors (gliptins) are currently on the market as anti‐diabetic drugs 8, 9, 10. Thus it is involved in glucose homeostasis, food uptake, anxiety, stress, cardiovascular, nociception and chemotaxis. The enzyme comprises 766 amino acids and is a type II transmembrane glycoprotein that has also a soluble‐shedded form in serum. It has a molecular weight of 110 kDa and is active as a homodimer. It is distributed ubiquitously, with the highest expression in kidney, lung, liver and small intestine, whereas low expression is found in brain, heart and skeletal muscle. According to kinetic analysis, DPP4 has the highest selectivity for NPY and PYY. The human gene location of DPP4 is 2q24.2 8, 11, 12.
Fibroblast activation protein alpha (FAP). FAP, also referred to as seprase, has the highest sequence identity to DPP4 and is believed to arise from gene duplication due to its gene proximity being at 2q23 6. FAP is a transmembrane protein type II. It consists of 760 amino acids and forms a 170 kDa homodimer. Like DPP4, the monomeric, N‐glycosylated 97 kDa subunits are proteolytically inactive, thus their proteolytic activities are dependent upon subunit association. Furthermore, FAP can form a heterodimeric membrane‐bound proteinase complex with DPP4 6, 7, 13. FAP has been shown to readily hydrolyse NPY, BNP, substance P and PYY as well as to a lower‐rate GLP‐1 and GIP, whereas chemokines were not readily truncated or at a much slower rates by FAP 14. In addition to DPP4 activity, FAP also exhibits gelatinase and collagenase activity, which is collagen type I specific. Furthermore, α‐2‐anti‐plasmin is a natural substrate of serum‐FAP, cleaved at …Gly11‐Pro12‐⇓‐Asn13…, confirming that its endopeptidase activity requires the sequence Xaa‐Gly‐Pro‐Yaa… 15, 16, 17, 18. The crystal structure of FAP has been elucidated, and comparison with the crystal structure of DPP4 points to a lower anchoring of substrates by Glu203–Glu204 due to shielding effects of surrounding hydrophobic residues and lack of Asp663. This, in turn, results in a lower exopeptidase activity and enables its endopeptidase activity as confirmed by site‐directed mutagenesis with subsequent kinetic studies 19. Although FAP expression is restricted to reactive stromal fibroblasts of epithelial cancers, subsets of bone and soft tissue sarcomas, activated stellate cells, arthritic chondrocytes, granulation tissue of healing wounds as well as de‐differentiated adipocytes, co‐expression of DPP4 and FAP in these cells results in the formation of a heteromeric complex 6, 7, 20, 21. The DPP4 activity is maintained by both enzymes in the complex as well as gelatine degradation by FAP 22. In tumorigenic cells and wounds, this heteromeric complex is localised on the advancing portion of invadopodia that is believed to play an important role in tumour invasion, spreading of metastasis, angiogenesis and wound‐healing, respectively 6, 7, 13, 22. Thus, to identify it as a pharmaceutical target, the expression of FAP has been investigated as potential biomarker in several types of cancers 6, 7, 13, 23, 24, 25. Interestingly, it was shown that stromal FAP is more prominent in early‐stage colorectal cancer and smaller tumour xenografts, with increased expression of FAP being an adverse prognostic indicator in patients with advanced metastatic disease 26. In contrast, prolonged survival of patients with breast cancer was associated with high FAP expression, whereas in cervical cancer FAP was correlated with increased dysplasia and carcinoma development, suggesting FAP being an invasion marker 7, 13, 26. Co‐localization of FAP and urokinase‐type plasminogen activator receptor was detected in malignant melanoma by fluorescence resonance energy transfer (FRET), and the complex appeared to be dependent upon both cytoskeleton and integrins 27. Knock‐out mice of FAP confirmed its role in wound‐healing; however, no change of phenotype was observed with regard to cancer 28. Expression of FAP was found to be up‐regulated by interleukin (IL)‐1 and oncostatin M in arthritic chondrocytes from patients with osteoarthritis, while it was down‐regulated in patients with systemic lupus erythematosus 21. Recently, the role FAP in cartilage degradation could be elucidated in FAP‐knock‐out of tumour necrosis factor (TNF)‐α transgenic mice [FAP(–/–) human TNF transgenic (hTNFtg) mice], as these animals revealed less cartilage degradation, but similar inflammation and bone erosion compared to wild‐type hTNFtg mice 29. Cleavage of α2‐anti‐plasmin by soluble serum‐FAP yields a more active form, thereby promoting fibrosis and scar formation 17, 18. Thus, FAP has an opposite physiological role compared to DPP4 that enhances fibrinolysis and scar resolution by activation of plasmin from plasminogen via a quintary complex of ADA, plasminogen 2, DPP4, urinary plasminogen activator (uPA/tPA) and plasminogen‐receptor (Plg‐R) 7. In addition, FAP has been proposed to play a role in neutropenia and anaemia 30.
DPP8. DPP8 consists of 882 amino acids, and its homodimer has a molecular weight of 200 kDa 2, 31. So far, it has been suggested to be located in the cytoplasm as a soluble protein, and until now there has been no evidence for any secretion 2, 6, 7, 13. Recent proteomic screening has revealed phosphorylation of p‐ephrin‐B1 antibody (Tyr331) and mitogen‐activated protein kinase‐activated protein kinase‐2 (MAPK‐APK‐2) (Thr334) 32. Using several chromogenic substrates, DPP8 was shown to display DPP4‐like activity similar to DPP4 2, 31. Hydrolysis of NPY, GLP‐1, GLP‐2, peptide YY (PYY), interferon (IFN)‐induced T cell alpha chemoattractant (ITAC), IFN‐induced protein 10 (IP‐10), stromal cell‐derived factor (SDF)‐1α and SDF‐1β, but not of IFN‐γ‐induced monokine (MIG), growth‐regulated protein b (Groβ) and eotaxin could be demonstrated in vitro, although the rate of cleavage was slower compared to DPP4, in particular for PYY 31, 33, 34. Recent systematic degradomic analysis identified several in‐vivo substrates involved in antigen presentation, signal transduction, cellular energy and nucleotide metabolism 34. DPP8 mRNA is distributed ubiquitously, with its highest expression in testis, prostates, ovaries, placenta and brain 2, 5, 12, 35. Furthermore, it is up‐regulated in activated lymphocytes 2. However, its physiological function is currently unknown and still awaits further studies. The human gene localization is 15q22 6.
DPP9. DPP9 has two variants comprised of 863 and 892 amino acids, respectively 6, 36. The longer DPP9 was found to be enzymatically active as a homodimer with an estimated molecular weight above 200 kDa. DPP9 lacks a transmembrane domain and is found intracellularly near the Golgi apparatus, although secretion from transfected cells has not yet been observed 3, 6, 12, 36. Recently, DPP9 was shown to be associated with mitochondria and to co‐localize strongly with microtubules. Furthermore, DPP9 redistributed towards the ruffling plasma membrane upon stimulation with either phorbol 12‐myristate 13‐acetate or epidermal growth factor. DPP9 was also seen at the leading edge of migrating cells and co‐localised with the focal adhesion proteins, integrin‐1 and talin, resulting subsequently in phosphorylation of focal adhesion kinase and paxillin. This implicates DPP9 to be involved in tissue and tumour growth as well as metastasis 37. A nuclear localization signal was identified at the extended N‐terminal in an alternative spliced variant of long DPP9, targeting it to the nucleus 38. Using several chromogenic substrates, DPP9 exhibited DPP4‐like activity similar to DPP4, and was shown to truncate NPY, GLP‐1, GLP2 and, to a far lesser extent, for PYY in vitro 6, 12, 31. However, the cytoplasmic proteasome‐derived antigenic peptide RU134–42, CXCL10, IL‐1RA, S100‐A10, SET nuclear proto‐oncogene (SET) and human nucleobindin 1 (NUCB1) could be identified as natural substrates of DPP9, suggesting DPP9 to play an important role in peptide turnover and antigen presentation and inflammation 39, 40. Intriguingly, DPP9 was only able to hydrolyse the deglycosylated IL‐1RA isoform. Furthermore, DPP9 was also shown to cleave enzymatically an as‐yet unknown substrate involved in the phosphorylation of protein kinase B (Akt), thereby interfering with epidermal growth factor (EGF) signalling 41. Its binding to Harvey rat sarcoma viral oncogene homologue (H‐RAS) and small ubiquitin‐like modifier (SUMO)1 also confirmed the involvement of DPP9 in signal transduction 42. Recent systematic degradomic analysis and two‐dimensional difference gel electrophoresis (2D DIGE) identified several substrates involved in antigen presentation, signal transduction, cellular energy and nucleotide metabolism 34, 40. Together with DPP4, and contrary to DPP8, DPP9 has a high specificity for the Val–Ala motif, as shown with substrate CSN8 40. DPP9 contains an Arg–Gly–Asp cell attachment motif and two potential glycosylation sites, although deglycosylation revealed no mass differences 3, 5, 36. Like DPP4 and DPP8, DPP9 mRNA is distributed ubiquitously, with its highest expression in liver, heart and skeletal muscle and testis 3, 6, 35, 36. Its physiological function has not yet been elucidated, although an up‐regulation of DPP9 mRNA was detected in human testicular tumour 35. Gene knock‐out in mice with inactive DPP9 turned out to be neonatal‐lethal 43. The gene is located on chromosome 19p13.3 6, 7, 12, 36.
So far, one cannot differentiate between DPP8 and DPP9 enzymatic activity due to the lack of selective inhibitors; however, DPP8/DPP9 activity could be detected in human leucocytes, rat brain, lung and testis, bovine testis, murine brain, organs of the immune system such as thymus, spleen, lymph nodes and peripheral blood mononuclear cells (PBMC), testis, skeletal and uterine muscles as well as colon 12, 35, 36, 44, 45, 46, 47. Nevertheless, brain and testis have been the only organs in which DPP8/DPP9 activities precede over DPP4 activity 12, 35, 45, 46. Association of DPP8 and DPP9 with H‐Ras suggests a functional role in signal transduction 41. Furthermore, DPP8/DPP9 appears to be involved in T cell proliferation, thereby releasing IL‐2 as well as macrophage activation causing activation of caspase 1 and induction of IL‐1β 45, 47, 48, 49, 50, 51. However, the suggested cytotoxicity of DPP8/DPP9 inhibition is currently discussed controversially 52, 53, 54, 55, 56. An increase of DPP8/DPP9 activity has been associated with asthma 44. Extra‐enzymatic functions of DPP8/DPP9 include cell adhesion, migration and apoptosis 57. Interestingly, DPP8 and DPP9 are inactivated reversibly by H2O2 oxidation involving two cysteines in each monomer 58. To date, there are no crystal structures of DPP8 and DPP9 available. Nevertheless, molecular modelling based on DPP4 and FAP crystal structures indicate similar overall structures comprised of β‐propeller and α/β‐hydrolase domains, with the active site being located at the interphase of the two domains. However, two loops and one helix of the propeller domain extending to the interphase cavity appear to play a role at the active site, thereby influencing substrate specificity and inhibitor binding 59, 60. Due to the shortest gene sizes, the lowest numbers of exons, the active site being located on one exon and their closest phylogenetic relationship with respect to prokaryotic members of the family, DPP8 and DPP9 are believed to be the ancestral genes of the DPP4 gene family 6.
DPP‐like protein 1 (DPL1) and DPP‐like protein 2 (DPL2). DPP‐like protein 1 (DPL1) and DPP‐like protein 2 (DPL2) lack DPP4‐like activity because of mutations at their active sites. Both of them are type II membrane‐bound glycoproteins, suggested to interact with the voltage‐gated potassium channel Kv4 4, 5, 6, 61, 62, 63. While DPL1 is expressed exclusively in the brain as two variants, i.e. a short and a long form, DPL2 is found in brain, pancreas and adrenal gland 5, 62. The long form DPL1‐L is an 859 amino acid protein with a molecular weight of 97 kDa, whereas the short form, DPL1‐S, consists of 803 amino acids with a reported molecular weight of 91 kDa 4, 6. The human gene localization is 7q36.1–q36.2. DPL1 is associated with amyotrophic lateral sclerosis, familial idiopathic ventricular fibrillation, spinal muscular atrophy and neuroleptic‐induced tardive dyskinesia, whereas DPL2 is linked with asthma 44, 61, 64, 65, 66, 67, 68. The crystal structures of DPL1 and DPL2, respectively, resemble that of DPP4 63, 69. DPL2, better known as DPP10, is a type II membrane protein with a dimeric structure, comprised of alternative splice variants. The long form is expressed as a 796 amino acid protein with a molecular weight of 97 kDa. The human gene localization is 2q14.1 5. Table 1 summarises the properties of the DPP4 gene family members.
Table 1.
| Clan SC | |||||||
|---|---|---|---|---|---|---|---|
| Family S9b (DPP4‐gene family) | S28 | ||||||
| DPP4 | FAP | DPL1 | DPL2 | DPP8 | DPP9 | DPP2 | |
| Synonyms |
DPIV, DPPIV, DAP IV, CD26, ADAbp, Gp108, Gp110, Hep105 |
Seprase, anti‐plasmin cleaving enzyme, APCE |
DP 6, DPP 6, DPPX‐S, DPPX‐L, DPPX |
DP 10, DPP 10, DPP Y |
DPP 8 | DPP 9 | DP II, DPP7, QPP |
| EC number | 3.4.14.5 | 3.4.21.B28 | – | – | – | – | 3.4.14.2 |
| Merops | S09.003 | S09.007 | S09.973 | S09.974 | S09.018 | S09.019 | S28.002 |
| Accession | M80536 | U09278 |
M96859 M96860 |
AY172659 | AF221634 | AF452102 | AF154502 |
| Gene location | 2q24.3 | 2q23 | 7q36.2–3 | 2q12.3–2q14.2 | 15q22 | 19p13.3 | 9q34.3 |
| Gene size (kb) | 81.8 | 72.8 | 659 | 535 | 71 | 48.7 | 2.85 |
| # Exons | 26 | 26 | 26 | 26 | 20 | 22 | 13 |
| Promoter | TATA‐less, GC rich | ? | ? | ? |
TATA‐less, GC rich |
? | ? |
| Regulation of expression |
Tissue‐specific HNFα/β Interferons α/β/γ RA, cytokines |
? | ? | ? | ? | ? | KLF2, TOB1 |
| # Amino acids | 766 | 760 | 803/859 | 796 | 882 | 863 | 492 |
| Mr (kDa) | 110 | 97 | 115/120 | 91 | 100 | 98 | 54.3 |
| Proform | – | – | – | – | – | – | yes (SP) |
| Transmembrane | ✔ | ✔ | ✔ | ✔ | – | – | – |
| Cysteines | 12 | 13 | 11 | 9 | 12 | 17 | 8 |
| # Glycosylation | 9 (2–9)* | 5 | 7 | 10 | ‐ | 2 | 6 |
| Glycosylation | N/O | N | N | N | ‐ | N | N |
| Catalytic triad | ✔ | ✔ | – | – | ✔ | ✔ | ✔ |
| Intron at catalytic S | ✔ | ✔ | – | – | – | – | ✔ |
| Activity | DPP4 | DPP4 + gelatinase | Unknown | Unknown | DPP4 | DPP4 | DPP4 (acidic pH) |
| Quaternary structure | dimer/tetramer | Dimer | Dimer | Dimer | Dimer | Dimer | Dimer |
| Activity needs dimer | ✔ | ✔ | – | – | – | – | ✔ |
| Crystal structure | ✔ | ✔ | ✔ | ✔ | – | – | ✔ |
| Subcellular location | Membrane type II/soluble | Membrane type II/soluble | Membrane type II | Membrane type II | Cytosolic |
Cytosolic/ Nuclear |
Secretory vesicles Lysosomes |
| Binding partners |
ADA, CD45, Cav‐1 Collagen, Glypican‐3, PgR, CARMA‐1, HIV‐TAT, CXCR‐4, M6P/IGFII, FAP |
α3β1 Integrins α5β1 Integrins Annexin 2 uPA‐R, DPP4 |
A‐type K(+) channel (Kv4.2) | A‐type K(+) channel (Kv4.2) | H‐Ras | H‐Ras | ADA |
| Distribution |
Ubiquitous Kidney > small intestine > lung |
Activated‐ Fibroblasts Activated‐ Stellate cells |
Brain | Brain, pancreas, adrenal gland |
Ubiquitous Reproductive Organs, brain |
Ubiquitous Heart, liver Skeletal, brain, muscle, testis |
Ubiquitous kidney, testis,brain |
| Knock‐out | ✔ | ✔ | ✔ | – | – | ✔ | ✔ |
| Transgenic | ✔ | – | – | – | – | – | – |
| Leucocytes |
⇑ act.# T cells, ⇑ act.# B cells ⇑ act.# NK cells ⇑ act.# Macrophages |
– | – | – |
⇑ Peripheral Leucocytes Lymphocytes ≈ Monocytes |
⇑ Peripheral leucocytes Lymphocytes ≈ Monocytes |
⇑ Macrophages Resting Lymphocytes |
| Known physiological functions |
Cell adhesion, Immune response, tumour progression, Psychoneuro‐endocrine function, metabolism |
Cell growth regulation, Tumour progression, wound‐healing Fibrogenesis |
Embryonic development, Signal transduction, regulation of synaptic plasticity, K+ channel |
Signal transduction, Regulation of synapticplasticity, K+ channel |
T‐cellproliferation? Signal Transduction Cell‐survival Cell‐proliferation |
Antigen presenting Signal transduction Cell‐survival Cell‐proliferation |
Neuroprotection? Maintaining lymphocyte quiescence |
| Pathological role |
Diabetes Cancer MS, AIDS Anxiety |
Cancer, Liver Cirrhosis Arthritis Pulmonary fibrosis |
Neurodegeneration Progressive spinal muscular atrophy |
Asthma Amyotrophic lateral sclerosis (ALS) |
Breast ‐, ovary cancer | Prostate cancer |
Apoptosis of leucocytes Neuroprotection diabetes |
| References | 6, 7, 8, 13, 59, 140, 141 | 6, 7, 13, 21, 22, 23, 28, 94 | 4, 6, 7, 44, 63, 64, 65, 66, 133 | 5, 6, 7, 61, 62, 69 | 2, 6, 7, 13, 31, 33, 34, 35, 94 | 6, 7, 13, 35, 36, 38, 39, 40, 41 | 6, 7, 75, 76, 77, 78, 80, 81, 82, 83, 84, 85, 86, 117 |
(2–9)* = Number of glycosylation sites being occupied in the crystal structures; # = activated. ADA = adenosine deaminase.
Non‐related DPP4‐like enzymes
In addition, enzymes structurally unrelated to the DPP4 gene family have been reported to display DPP4 activity 70. These include DPP2, EC3.4.14.2 of SC clan 28, attractin and N‐acetyl alpha‐linked acidic dipeptidases (NAALADases I, NAALADases II and NAALADases L) from the metalloprotease clan MH, family M28B 70. However, detailed kinetic analysis of expressed and purified NAALADase I did not reveal any DPP4‐like activity 71. Similarly, the DPP4‐like activity of attractin in the serum had been discussed controversially for several years, but was later disproved 72, 73, 74.
DPP2, was found to be identical with quiescent proline cell dipeptidase (QPP) and dipeptidyl peptidase 7 (DPP7), based on genetic homology and kinetic parameters 75, 76. The soluble serine protease contains a proform and has a length of 492 amino acids with a molecular weight of 58 kDa 77, 78. Glycosylation and dimerization are required for the catalytic activity and the latter occurs via a leucine zipper motif, which is novel for proteases 79. The homodimer is located in cellular vesicles that are distinct from lysosomes and secretion is regulated by an increased Ca2+ flux 77. Using chromogenic substrates, DPP2 displays post‐proline dipeptidyl aminopeptidase activity similar to DPP4, however, over a broad pH range with an acidic to neutral pH optimum 76, 78, 80. While DPP2 hydrolyses tripeptides readily, its activity decreases rapidly with increasing chain length of peptide. Thus, it was shown to cleave only fragments of substance P1–4, bradykinin1–3 or bradykinin1–5; however, we and others found none of the DPP4 substrates to be cleaved by DPP2 12, 80, 81. DPP2 has been reported to be involved in apoptosis, as a decrease of DPP2 activity caused cells to exit their G0‐phase in quiescent lymphocytes and fibroblasts, resulting in an induction of apoptosis by up‐regulation of p53 and c‐Myc as well as a down‐regulation of Blc‐2 77, 82. Furthermore, DPP2 was found to be essential for maintaining the cell quiescence of lymphocytes, in which the transcription factors Kruppel‐like factor (KLF2) and transducer of ERBB2, 1 (TOB1) regulate the expression of DPP2 83. Nevertheless, another study reports participation in necrosis rather than apoptosis 84. DPP2 is distributed ubiquitously, with high expression in kidney, brain, testis, heart, resting lymphocytes and differentiated macrophages 75, 78, 84, 85. As it was thought previously to be a lysosomal enzyme, its physiological function to date is unknown. However, altered serum activities of DPP2 have been associated with various pathogenic conditions, such as Sjögren syndrome, rheumatoid arthritis (RA), lupus erythematosus, various cancers and Parkinson disease 78. DPP2–/– and constitutive DPP2 knock‐down (kd) are embryonic‐lethal; however, conditional neurogenin 3‐specific DPP2 knockdown mice revealed a phenotype with impaired glucose tolerance, insulin resistance and visceral obesity 86. NGN3 is expressed in all precursors of the enteroendocrine cells and in the pancreas as well as discrete regions of the hypothalamus and brain stem 86. Interestingly, ADA was discovered to also bind to DPP2, although with an order of magnitude lower compared to DPP4 77. The human gene localization is 9q34.3. As DPP2 also belongs to the SC clan, its order of catalytic residues is Ser, Asp and His, located in an α/β hydrolase fold, as summarized in Table 1. Recently, the crystal structure of DPP2 was deposited in the Protein Data Bank as pdb 3JYH, revealing an α/β‐hydrolase domain as well as a novel helical structural domain (SKS) domain, comprised of 5 α‐helices arranged in a helix bundle fold, capping the active site 87. An insertion from the SKS domain to the active site results in steric hindrance of larger substrates and contains Asp334 for anchoring the N‐terminus of the peptide substrate. Prolycarboxy peptidase, also belonging to the S28 family, displays a similar overall structure, whereas the members of the DPP4 gene family are made up of a propeller and an α/β‐hydrolase domain. The propeller has an open architecture and contains eight blades, each made up of four anti‐parallel β‐sheets 87.
Although all these enzymes described above display DPP4‐like activity or are structural homologues (Table 1), they exhibit distinct features with respect to cellular compartmentation and glycosylation, as illustrated in Fig. 1. Furthermore, DPP4 also internalizes upon association with binding partners such as CXCR 4 and M6P/IGFII, recycling of terminal carbohydrates and assembled to lipid rafts 8, 9
Figure 1.
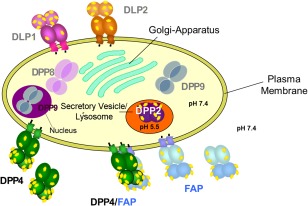
Cellular compartmentation of dipeptidyl peptidase (DPP)4‐like enzymes. DPP4 and fibroblast activation protein alpha (FAP) are located either as homodimers or heteromeric complex on the plasma membrane or shedded into the serum. DPP2, a homodimer, is distributed as a zymogen in secretory vesicles or lysosome. DPP8 and DPP9 are also homodimers and located cytosolically.  , glycosylation.
, glycosylation.
DASH proteins in immune cells
In addition to DPP4, DPP8, DPP9 and DPP2 were also found to be expressed on leucocytes, yet fulfilling different functions, as illustrated in Fig. 2 35, 47, 48, 51, 82, 88, 89. DPP2 plays a vital role in quiescence of resting lymphocytes and its inhibition leads to apoptosis 82, 90. The activity of DPP8 and DPP9 is required for T cell proliferation and is IL‐2‐dependent 40, 48, 49, 52, 90. DPP8 is up‐regulated upon T cell activation, whereas DPP9 plays a role in antigen trimming for antigen presentation 2, 39, 40. DPP4/CD26 is involved in T cell activation, T cell signalling and T cell differentiation due to its interactions with ADA, CD45, caveolin‐1, CARMA‐1 and M6P/IGFII‐R 9. These processes are regulated by the cytokines IL‐2, IL‐6, IL‐10, IL‐12, IL‐17, IL‐29, IFN‐γ and TGF‐β, as well as compartmentation of DPP4/CD26 to either lipid rafts or internalization 8, 9, 48, 88, 89, 91, 92, 93. Furthermore, post‐translation modification of DPP4/CD26 such as sialylation also appears to influence compartmentation and/or the interactions with its binding partners 9, 86, 87, 90. Generally, expression of DPP4/CD26 is up‐regulated in T helper type 1 (Th1) and Th17 cells, but not in Th2 cells. However, comparing CD28 versus CD26 co‐stimulation of CD3 mediated T cell activation, CD26 co‐stimulation was found to induce production of IL‐10 preferentially in human CD4+ T cells mediated via nuclear factor of activated T cells (NFAT) and rapidly accelerated fibrosarcoma–mitogen‐activated protein kinase–extracellular signal‐regulated kinase (Raf–MEK–ERK) pathways, as well as high levels of early growth response 2 (EGR2) mediated possibly via NFAT and activator protein 1 (AP‐1)‐signalling. Furthermore, CD26‐mediated co‐stimulation of CD4+ T cells induced greater lymphocyte‐activation gene 3 (LAG3) expression than CD28‐mediated co‐stimulation 92. Whether or not the other DASH proteins contribute to the overall DPP4‐like activity in Th2 cells such DPP8 still needs to be investigated 2, 7, 13, 51, 88, 94, 95. In addition, DPP4/CD26 is expressed highly on the CD45RO+ CD29+ memory T helper subset CD26bright CD4+, which responds to recall antigens, induces B cell immunoglobulin (Ig)G synthesis and activates cytotoxic T cells 7, 8, 13, 51, 94, 96. CD26/DPP4 also plays a role in chronic pulmonary graft‐versus‐host disease with up‐regulation of IL‐26, involving CD26 and caveolin‐1 interactions 91. Furthermore, DPP4 is up‐regulated in activated natural killer (NK) cells, B cells, eosinophils and macrophages 9, 13, 44, 45, 51, 94. None the less, DPP8, DPP9 and DPP2 are also expressed on macrophages and DPP2 has been detected additionally on mast cells 13, 35, 40, 47, 51, 78, 85. These differentiated leucocytes regulate the expression of the DASH proteins in/on endothelial, fibroblast and epithelial cells via their cytokines, thereby influencing physiological and pathophysiological processes such as vasoconstrictions, vasodilation, angiogenesis, transendothelial migration of lymphocytes, hypothalamic–pituitary–adrenal (HPA) stress axis, wound‐healing, arthritis, cirrhosis, cancer, colitis, inflammatory bowel disease (IBD) and asthma, as illustrated in Fig. 2 6, 7, 9, 13, 21, 29, 35, 40, 44, 51, 94, 97, 98, 99, 100. Substrates of DPP4 and/or DPP8/DPP9 may also be involved in these scenarios, such as the chemokines regulated upon activation normal T cell expressed and secreted (RANTES), SDF‐α and eotaxin, the neuropeptides NPY, SP, vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase‐activating polypeptide (PACAP), as well as the peptide hormones GLP‐2 and GLP‐1 7, 8, 9, 11, 12, 14, 20, 24, 31, 33, 34, 40, 51, 100.
Figure 2.
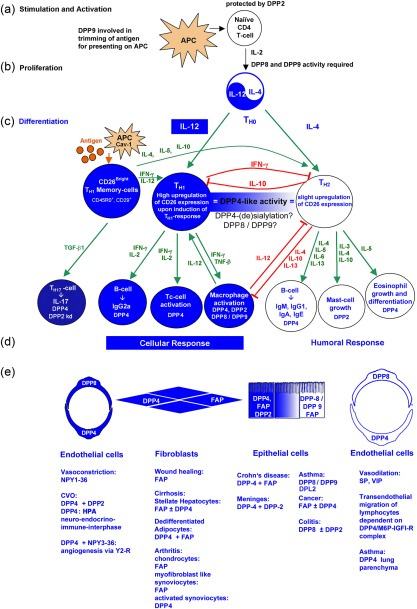
Involvement of dipeptidyl peptidase (DP)4‐like enzymes in T cell effector response. (a) Naive CD4 T cells are protected by DP2. Antigen‐presenting cells (APC) stimulate and activate naive CD4 T cells. (b) Secretion of interleukin (IL)‐2 results in clonal expansion, yielding T helper type 0 (Th0) cells. Enzymatic activities of DP8 and DP9 are required for this process. (c) Secretion of IL‐12 and IL‐4 results in the differentiation of Th1 and Th2 effector cells, respectively. Differentiation into Th1 cells initiates the up‐regulation of DP4 expression. A small subset of CD26bright memory T cells already expresses high amounts of CD26. Upon antigen stimulation, CD26bright memory T cells augment the Th1 and Th2 response by secreting interferon (IFN)‐γ, IL‐12, IL‐4, IL‐5 and IL‐10, respectively. Differentiation into Th2 cells results in only a slight up‐regulation of CD26. The DP4 activity is equal in both T effector cells, due probably to specific DP4 isoforms or DP8 and/or DP9. (d) Differentiated T effector cells secrete specific cytokines that induce differentiation of leucocytes, resulting in cellular response by Th1 and humoral by Th2 effector cells. Leucocytes expressing DP4, DP2 or DP8/9 are indicated. Green arrow = stimulation; red arrow = suppression. 16, 17, 28, 31, 39, 40, 41, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 102, 104.
Knock‐out, deficient and transgenic DPP4‐like animal models
DPP4‐knock‐out, ‐deficient and ‐transgenic animal models have been useful to elucidate the physiological role of DPP4‐like enzymes and in‐vivo substrates. The phenotypes of such animal models are summarised in Table 2. Thus, DPP4(–/–) mice have provided evidence regarding the important role of DPP4 in the incretin metabolism of the insulinotrophic peptides GLP‐1 and GIP. Additional increased energy expenditure and decreased food intake make DPP4 inhibitors a favourite pharmaceutical target compared to other known anti‐diabetic drugs 101, 102. Furthermore, behavioural studies point to a possible negative involvement of DPP4 in stress‐related behaviour, due probably to the modulation and/or inactivation of neuropeptide substrates, therefore identifying DPP4 as a potential pharmaceutical target in stress‐related diseases 102. Intriguingly, DPP4(–/–) seemed to be vital with normal immune responses, although they showed altered cytokine secretion and antibody production upon mitogen stimulation in serum, a down‐regulation of CD4+ T cells as well as up‐regulation of NK cells in the spleen and a marked decrease of peripheral blood CD4+ NK T cells 103. Two substrains of Fischer 344 rats, the F344/Crl(Ger/DPP4–) and F344/CuCrj(Jpn/DPP4–) lack endogenous DPP4 at protein levels, while F344/Crl (USA/DPP4+) represents the wild‐type 104. Studies on these animals confirm the in‐vivo role of DPP4 in diabetes, intestinotrophic peptide GLP‐2 and stress‐related behaviour, whereas isolated PMBC from mutant rats could be activated with mitogens similarly to the wild‐type 105, 106, 107, 108, 109. DPP4‐deficient Fischer rats are used commonly as host animals for the transplantation of hepatocytes or stem cells obtained from wild‐type animals, as DPP4 is a hepatic differentiation marker, and to distinguish between transplanted cells from the host cells 110. Interestingly, mutant and wild‐type F344 displayed the same phenotype with regard to arthritis, and non‐selective DPP4‐like inhibitors were able to suppress induced arthritis in both subspecies, implying the involvement of a DPP4‐like enzyme other than DPP4, such as FAP 7, 21, 28, 29, 111. Similarly, stimulation of neutrophils and erythrocytes from haematopoietic progenitor cells by a non‐selective DPP4‐like inhibitor was observed in both the mutant and wild‐type F344 subspecies, again suggesting an involvement of another DPP4‐like enzyme such as FAP, DPP8 or DPP9 112. A novel congenic DPP4‐deficient DA.F344‐Dpp4m/SvH rat model confirmed the physiological role of DPP4 in glucose metabolism, immunology and stress‐related diseases 113, 114. Like the DPP4‐knock‐out and ‐deficient animal models, transgenic mice with human CD26 displayed a normal immunological phenotype, although thymocyte proliferation as well as CD4+ and CD8+ T cell differentiation and viability was impaired, suggesting an important role of DPP4 in the T lymphocyte homeostasis in peripheral blood 115.
Table 2.
Summary of phenotypes obtained from DPP4‐knock‐out (k.o.), DPP4‐deficient and DPP4‐transgenic animal models as well as FAP–/– k.o., DPP9S729A/S729A gki., DPL1–/– k.o. and DPP2–/– k.o.mice 7, 9, 28, 43, 86, 101, 102, 103, 104, 107, 108, 110, 111, 112, 113, 114, 115, 116, 141, 142, 143, 144, 145.
| Model | Investigation | Phenotype |
|---|---|---|
| DPP4 (–/–) mice | Protection from obesity + insulin resistance |
⇑ Energy expenditure, ⇑ serum GLP‐1, leptin, insulin, ⇑ Glucose tolerance ⇓ food intake DPP4(–/–) ≈ DPP4‐inhibition ≠ DPP4(+/+) ⇒ DPP4 = target enzyme |
| Immunology |
Spleen: ⇓ CD4+ T cells, ⇑ NK cells, stimulation (PWM): ⇓ IL‐4,⇑ IL‐10, IFN‐γ Peripheral blood: ⇓ CD4+ NK T lymphocytes After immunization (PWM): ⇓ IgG, IgG1, IgG2a, IgE, ⇓ IL‐4, IL‐2 + delayed IFN‐γ DPP4(–/–) ≈ DPP4(+/+) ⇒ non‐selective + DPP8/DPP9 inhibitors ⇓ T cell proliferation ⇒ DPP8/DPP9 involved |
|
| Nociception | ⇑ Plasma substance P, delayed pain response | |
| Cancer | DPP4(–/–) ≈ DPP4(+/+) ⇒ DPP4‐like inhibitor ⇓ tumour cells | |
| Behaviour |
⇓ Depression‐like behaviour according to tail suspension and forced swim test DPP4Mut ≠ DPP4WT ⇒ DPP4 = target enzyme |
|
| Disease | Experimental colitis: DPP4(–/–) ≈ DPP4(+/+) ⇒ DPP4‐like inhibitor ⇒ intestinal adaptation | |
| Autoimmunity |
MS: ⇓ TGF‐β1, ⇓ Th1 immunity ⇑ clinical experimental autoimmune encephalomyelitis Arthritis: ⇑ serum SDF‐α ⇑ arthritic inflammation |
|
|
F344/Crl (Ger/DPP4–) rats or F344/DuCrji (DPP4–) rats |
Protection from obesity +insulin resistance |
Serum: * ⇑ GLP‐1* ⇑ GIP, * ⇑ glucose tolerance, * ⇑ insulin after high fat‐ or glucose‐diet, ⇓ GLP‐113–36, ⇓ insulin resistance, ⇓ food intake after high fat diet, ⇓ weight gain DPP4Mut ≈ DPP4‐inhibition ≠ DPP4WT ⇒ DPP4 = target enzyme |
| Satiety |
DPP4Mut: ⇑ food intake + weight gain, ≈ postprandial [PYY] after 24 h fast due to PYY1–36
DPP4WT: peripheral administered PYY1‐36 and PYY3‐36: ⇓ food intake but not in DPP4Mut Short term DPP4‐like inhibition: no anorectic effect of peripheral administered PYY1–36 |
|
| Diseases |
Asthma: peritracheal oedema: ⇑ oedema due to ACE inhibitors Glomerulonephritis: DPP4Mut = resistant to experimental induced glomerulonephritis Cholestasis: ⇑ serum DPP4‐activity after induction of cholestasis in DPP4WT rats, no serum DPP4 activity in DPP4Mut Cancer: ⇓ DPP4Mut expression ⇒ ⇓ metastasis + cell adhesion Arthritis: DPP4Mut ≈ DPP4WT ⇒ ⇓ arthritic inflammation with DPP4 ‐like inhibitors ⇒ other DPP4‐like enzymes involved in arthritis |
|
| Immunology |
Isolated PMBC from DPP4WT and DPP4Mut are able to be activated after mitogen activation ⇒ DPP4 may be involved but not necessary for T cell activation in rats Isolated splenic leucocytes from DPP4WT and DPP4Mut have the same proliferative response of in‐vitro stimulation by T cells (Con A), B cells (LPS) and T + B cell (PWM) mitogens ⇒ DPP4 may be involved but not necessary for lymphocyte proliferation in rats. Altered age dependent leucocyte subset + thymic emigration pattern in DPP4Mut Asthma: DPP4WT: ⇑ CD4+/CD26+/CD25+ T cells recruitment in asthma induced lungs of rats ⇑ CD26+: CD26– TCR cells ⇒ ⇑ IgE DPP4Mut: ⇓ CD4+ T cells ⇓ IgE, ⇑ recruitment of eosinophils, ⇓ recruitment of T cells Cancer: ⇓ NK cytotoxicity in DPP4Mut rats ⇒ DPP4 activity sustains NK cytotoxicity Haematopoiesis: DPP4Mut ≈ DPP4WT⇒ DPP4‐like inhibitor ⇑ neutrophils + erythrocytes from progenitor stem cells via G‐CSF⇒DPP4‐like enzyme involved in neutropenia and acute anaemia |
|
| Nociception | ⇑ Sensitivity to non‐habiturated pain stimuly and/or reduced stress‐induced analgaesia | |
| Behaviour |
⇓ Stress response in OF, SI, passive avoidance + EPM ⇓ motor activity DPP4Mut ≠ DPP4WT ⇒ DPP4 = target enzyme |
|
| Small intestine | DPP4Mut ≈ DPP4‐inhibition ⇒⇑ GLP‐2 + ⇑ bowel weight + resistance to gastrointestinal damage | |
|
Assimilation of Pro in kidney + small intestine |
⇑ Excretion proline containing peptides in urine; ⇓ weight in DPP4Mut after fed with gliadin Isolated brush border membranes from small intestines + kidney unable to hydrolyse proline containing peptides |
|
| Transplantation | Used as a model of stem cells/hepatocyte transplantation to distinguish between donor and recipient, DPP4 = HAM.4 = differentiation marker of hepatocytes | |
| Da.F344‐Dpp4m/SvH | Protection from obesity +insulin resistance |
Serum: ⇑ GLP‐1 ⇑ Glucose tolerance ⇑ insulin after OGT ⇓ weight gain after high‐calorie diet ⇓ islet size ⇒ DPP4Mut ≠ DPP4WT ⇒ DPP4 = target enzyme |
| Lipid metabolism | ⇑ Leptin signalling ⇑ bound leptin in plasma ⇓ free leptin in liver ⇓ triglycerides ⇓ alkaline phosphatase ⇓ aminotransferases | |
| Kidney + small intestine | Impaired assimilation of Pro in kidney and small intestine ⇓ weight in DPP4Mut after fed with gliadin | |
| Immunology |
⇓ Lymphocytes ⇓ eosinophils ⇑ NK cells ⇑ B cells ⇓ NK cytoxicity ⇓ T cell proliferation ⇓ IL‐6 Soluble DPP4 derived partially from bone marrow and not kidney according to transplantation studies |
|
| Behaviour |
⇓ ACTH ⇓ corticosterone ⇓ stress‐induced hyperthermia ⇓ stress‐induced analgaesia, ⇑ fear extinction, ⇑ NPY in CNS ⇓ stress response in hole board test, SI + EPM ⇒ DPP4Mut ≠ DPP4WT ⇒ DPP4 = target enzyme |
|
| huCD26tg mice | Immunology |
⇓ Age‐related thymus cellularity with impaired thymocyte proliferation ⇓ peripheral T cell pool with ⇑ apoptosis in CD4+ and CD8+ subpopulations |
| FAP(–/–) mice | General | Delayed wound‐healing |
| FAP(–/–)hTNFtg mice | Arthritis | Ameliorates cartilage destruction in inflammatory destructive arthritis |
| DPP9S729A/S729A | General | GKI‐DPP9S729A/S729A mice with inactive DP9 ⇒ neonatal lethal, DPP9wt/S729A ⇒ indistinguishable from wild‐type |
| DPL1(–/–) mice | General | DPL 1(–/–) ⇒ embryonic lethal, DPL1(+/–) ⇒ pigmentation defect |
| DPP2–/– | General | Lethal |
| NGN3‐specific DPP2(–/–) mice | Metabolism | ⇑ Hyperinsulaemia, ⇑ glucose intolerance, ⇑ insulin resistance, ⇑ liver steatosis, ⇑ adipocytes resulting in visceral obesity |
OP, open field test; SI, social‐interaction test; EPM, elevated plus maze.
Red text: DPP4 inhibition results similar phenotype as DPP4−/− and both are different to wild‐type DPP+/+, confirming DPP4 as pharmaceutical target; green text: no differences between DPP–/– and DPP+/+, but inhibition with non‐selective DPP4‐inhibitor shows pharmaceutical efficacy indicating that DASH‐protein other than DPP4 is involved.
Intriguingly, FAP–/– mice displayed a phenotype with delayed wound‐healing, but no increased susceptibility towards cancer 28. However, FAP(–/–) hTNFtg mice revealed less cartilage degradation, but similar inflammation and bone erosion compared to wild‐type hTNFtg mice 29.
DPL1–/– and DPP2–/– were found to be lethal, whereas DPL1–/+ exhibited pigmentation defects and neurogenin‐3 induced DPP2–/–, a phenotype opposed to DPP4–/– with increased hyperinsulaemia, glucose intolerance, insulin resistance and liver steatosis 86, 116. Furthermore, mutant mice with knock‐down of DPP2 in resting T cells (lck‐DPP2 kd) led to differentiation into IL‐17 releasing Th17 cells after in‐vivo priming and in‐vitro antigen‐specific stimulation 117. Homozygote gene knock‐down DPP9S729A/S729A mice die shortly after birth, while heterozygote DPP9wt/S729A mice were morphologically indistinguishable from the wild‐type. The results imply, on one hand, that enzymatic activity of DPP9 is essential for survival, and on the other hand that no other DASH protein is able to take over the role of DPP9, as none of them were up‐regulated 43. The physiological and pathophysiological role of DPP9 has been elucidated only recently, being involved in antigen presenting and the EGF signalling pathway 38, 39, 40. This suggests that DPP9 plays a role in inflammation as well as cell proliferation and apoptosis 40, 41.
DASH proteins as therapeutic targets
History and development of DPP inhibitors as well as modulators of DASH proteins
The first generation of DPP4 inhibitors were developed prior to the discovery of DPP8 and DPP9, and these include P32/98 (Ile‐Thia), Lys[Z(NO2)]‐pyrrolidine, Lys[Z(NO2)]‐thiazolidide, Lys[Z(NO2)]‐piperidide, LAF‐237 (vildagliptin), NVP‐DP728, L‐Pro‐L‐boroPro, Pro‐Pro‐diphenyl phosphonate esters, aminoacyl‐pyrrolidine‐2‐nitriles, aminoacylpyrrolidides and aminoacyl‐thiazolidides 7, 8, 118. At this stage, data concerning selectivity were available only for DPP4 and DPP2. In retrospect, the importance of DPP4 selectivity over DPP2 has been elucidated by the opposing pathological roles of DPP2 in diabetes compared to DPP4 86. However, toxicological studies of the first‐generation inhibitor P32/98 resulted in high toxicity with bloody diarrhoea, emesis and tenesmus in dogs and alopecia, thrombocytopenia, anaemia, enlarged spleen, multiple histological pathologies and mortality in rats. Subsequently, selective inhibitors were developed, and investigation of the above cytotoxic effects revealed the inhibition of DPP8 and DPP9 to be responsible for the side effects 52. Therefore, the second generation of anti‐diabetic DPP4 inhibitors focused on the selectivity of the various DPP4‐like enzymes 52, 119, 120, 121, 122. Conversely, the requirement of selectivity is currently debated controversially, as some of the selective inhibitors against DPP8/9 were unable to enter the cell, suggesting that the cytotoxic effects observed were not due to inhibition of these cytosolic enzymes 53, 54, 55, 56. Given that DPP4, FAP, DPP8, DPP9 and even DPP2 have their highest sequence and structure similarities at the catalytic domain 6, uncompetitive inhibition at the propeller domain may be more specific for a particular DPP4‐like enzyme. Molecular modelling of DPP8 and DPP9 revealed a P2‐loop at the propeller domain, containing F357 and R358, that seems to be unique to DPP8 and DPP9 and is suggested to influence substrate and inhibitor binding to the P2‐pocket 60.
In addition, administration of DPP4 inhibitors or anti‐DPP4‐monoclonal antibodies (mAb) have been demonstrated to improve additional disease conditions in various animal models, cell cultures or interfering with the interaction of binding partners, as summarised in Table 3 123. For example, a non‐selective DPP4‐like inhibitor, PT‐100, had been in clinical trials II‐III for various types of cancers based on its dual inhibitory action against FAP as well as DPP8 and/or DPP9, although it was discontinued in 2007 30, 49. Dual inhibitor IP10.C8 against DPP4 and APN are currently being investigated for the treatment of autoimmune diseases such as psoriasis, multiple sclerosis (MS) and IBD 48, 124. Intriguingly, DPP4 and DPP2 again appear to have opposing roles regarding Th17. While DPP4 inhibition was shown to suppress the development of Th17 cell differentiation, knock‐down of DPP2 in resting T cells of mutant mice led to differentiation into IL‐17‐releasing Th17 cells 48, 125, 126. In fact, inhibition of DPP4 has been proposed for the treatment of the autoimmune disease diabetes type 1 by suppressing the pathogenic effects of Th1 and Th17 cells and up‐regulating Th2 cells 127. DPP2 selective inhibitor AX8819 was designed for a prognostic marker of B cell chronic leucocytic leukaemia, as well as a potential drug target to induce apoptosis in malignant B cells 126. DASH inhibitors Lys[Z(NO2)]‐Thia, Lys[Z(NO2)]‐Pyr, TMC‐2A, TSL‐225, as well as FAP‐specific inhibitor L‐glutamyl L‐boroproline, have been implemented for the treatment of arthritis 7, 13, 97, 111, 121, 128. FAP inhibitors have been developed to promote fibrinolysis 21. Radioactive labelled anti‐FAP‐monoclonal antibodies have been applied for targeting tumour cells 129. Furthermore, Pentostatin, an ADA inhibitor admitted by the Food and Drug Administration (FDA), was shown to reduce CD26+ T lymphocytes preferentially 130. Chronic administration of haloperidol increased the gene expression of DPL1 in mouse brains. Latter findings indicated an altered response of Kv4/DPP6 to long‐term neuroleptic administration 66.
Table 3.
Summary of disease conditions, being potential targets for DASH inhibition or modulated by anti‐DPP4‐mononuclear antibody (mAb) and/or anti‐FAP‐mAb 7, 9, 10, 13, 30, 52, 121, 123, 124, 128, 129, 146, 147, 148, 149, 150, 151.
| Target disease | Effect of inhibitor | Development stage | Comments | Inhibitor/mAb |
|---|---|---|---|---|
| Type 2 diabetes | Inhibition of incretin deactivation, improvement of postprandially stimulated insulin secretion and glucose tolerance |
FDA approved: Sitagliptin, Saxagliptin, Linagliptin EMEA: Vildagliptin Japan: Alogliptin Korea: Gemigliptin |
Many pharmaceutical companies are targeting DPP4 inhibition |
Sitagliptin (Januvia) Saxagliptin (Onglyza) Linagliptin (Ondero) Vildagliptin (Galvus) Alogliptin (Nesina) |
| Tumours |
Prevents spreading of metastases, inhibits tumour migration and angiogenesis by inhibiting FAP Inhibition of DPP8 + DPP9: ⇑ IL‐β ⇒ ⇑ cytokines + ⇑ chemokines ⇒ ⇑ neutrophils, ⇑ T‐lymphocytes, ⇑ macrophages |
Phase III: FDA put on hold Phase II studies: Melanomas |
Disapproved treatment on lung and pancreas cancer Clinical trials for melanomas |
Talabostat (PT‐100) (Val‐boroPro) Discontinued |
| Cell‐specific suppression of stromal tumours expression high levels of FAP |
Human Clinical Phase I Animal model |
F19‐antibody radio‐labelled chemotherapy |
FAP‐mAbF19 Oral DNA vaccine against FAP |
|
| Prevents spreading of metastases, inhibition of tumour migration and angiogenesis | Cell culture, animal models | Hetero‐dimerization with FAP involved |
DPP4‐mAb 1F7 Pro‐boroPro |
|
| Anti‐cancer effects and tumour cytotoxicity | Cell culture, animal models | Z‐GP‐Dox, an FAP‐based doxorubicin prodrug | Z‐GP‐Dox with, systemically delivered via mixed nanomicelles | |
| Potent anti‐tumour effects by increased levels of natural killer cells and DCs as well as making murine and human tumour cells more sensitive to antigen‐specific CTL killing | Cell culture, animal models | Pan inhibitor of DASH | ARI‐4175 in combination with a recombinant viral or dendritic cell (DC)‐based tumour‐cell vaccine | |
| Multiple sclerosis | Suppression of EAE, involves Th17 cells | Animal experiments,human primary cell culture | Autoimmune disease |
Lys[Z(NO2)]‐Pyr PETIR‐001 |
| Psoriasis | Reduction of keratinocyte hyperproliferation, involves Th17 cells | Cell culture, animal models, preclinical studies | Autoimmune disease |
Lys[Z(NO2)]‐Thia Lys[Z(NO2)]‐Pyr P32/98 PETIR‐001 |
| Colitis | Suppression of GLP‐2 inactivation | Cell culture, animal models | DPP4, DPP8 and DPP2 may be involved |
P32/98 PETIR‐001 |
| IBD | Suppression of GLP‐2 inactivation, involves Th17 cells | Cell culture, animal models | DPP4 involved |
P32/98 PETIR‐001 |
| Rheumatoid arthritis | Suppression of symptoms via inhibition of DPP4 + FAP (L‐glutamyl L‐boroproline) | Animal models |
DPP4 + DASH inhibition ⇒ PT of CD45 inhibition FAP involved |
Lys[Z(NO2)]‐Thia Lys[Z(NO2)]‐Pyr TMC‐2A TSL‐225 L‐glutamyl L‐boroproline |
| Anxiety/stress | Suppression of NPY cleavage decreases anxiety | Effective in animal models (rats) | Suggested to mainly involve NPY + DPP4 | P32/98 |
| Psychosis/schizophrenia |
DPP4 inhibitor blocks mescaline‐induced scratching + amphetamine‐induced hyperactivity Haloperidol ⇑ Kv4/DPP6 ⇒ ⇓ tardive dyskinesia |
Effective in animal models (mice) |
May also involve endomorphin‐1/2 Haloperidol ⇑ Kv4/DPP‐6 |
AMAC (DPP4) Haloperidol (DPP‐6) |
| Graft rejection | Suppression of graft rejection | Animal experiments | Pro‐Pro diphenyl‐phosphonate ester (prodipine) | |
| Autoimmune disease | General immunosuppressive effects, involves Th17 cells | Cell culture, animal models |
High doses necessary Dual inhibition of DPP4 and AP N (PETIR‐001) |
Lys[Z(NO2)]‐Thia Lys[Z(NO2)]‐Pyr Ala‐boroPro Pro‐boroPro PETIR‐001 |
| Acne | Inhibitors suppressed proliferation and IL‐2 production of Propionibacterium acnes‐stimulated T cells ex vivo + ⇓ TGF‐β | Cell culture |
Dual inhibition of DPP4 and AP N (PETIR‐001) |
Lys[Z(NO2)]‐Thia Lys[Z(NO2)]‐Pyr PETIR‐001 |
| T cell lymphoid malignancies | Enhanced cell cycle arrest at the G1‐S checkpoint, resulting in increased p21 expression | Cell culture, animal models | Influences survival and de‐novo tumour growth |
DPP4‐mAb 1F7 Diprotin A Val‐Pyr |
| B‐CCL | Prognostic marker + drug target | Ex‐vivo apoptosis assay | DPP2 inhibition | AX8819 |
| AIDS | Suppression of SDF‐α cleavage, suppression of gp 120 and DPP4 interaction | Cell culture | Mechanism not fully understood | Phe‐Pyrr‐2‐CN Arg(PMC)‐ Pyrr‐2‐CN |
| Fibrinolysis | Suppression of α‐anti‐plasmin of cleavage | In‐vivo clotting | Involves FAP | Acetyl‐Arg‐(8‐amino‐3,6‐dioxaoctanoic acid)‐D‐Ala‐L‐boroPro |
| Wound‐healing | Promotion of wound‐healing | Cell culture | Heteromeric complex of DPP4 with FAP involved |
DPP4‐mAbE26 DPP4‐mAbE19 DPP4‐mAbE3 |
Red text: inhibitor admitted or disapproved by a regulatory authority. PT, phosphatase activity.
Role of DASH proteins in diseases
Cancer
DPP4 has been proposed as a biomarker for a variety of cancers, such as thyroid, colon, breast, prostate and malignant pleural mesothelioma as well as lymphoma, β cell chronic leucocytic leukaemia and T cell lymphoid malignancies 7, 8, 9, 22, 23, 130, 131. In addition, expression of FAP has been investigated in several types of cancers as potential biomarkers for epithelial colon, gastric, intestinal, oesophageal, undifferentiated thyroid, lung, breast, ovarian and cervical cancers, meningioma, glioma and cutaneous melanoma, as well as aggressive fibromatosus 7, 12, 15, 17. In cancers and capillaries where DPP4 and FAP co‐localize, they form a heteromeric complex with both enzymes still maintaining their activities. This complex protrudes at invadopodia, where gelatin is binding to DPP4 and being degraded by FAP, respectively. Truncation of NPY by DPP4 results in angiogenesis, whereas chemokines such as SDF‐α are involved for migration and invasion. Thus, by means of their associations and substrate specificities, the DPP4/FAP heteromeric complex is responsible for tumour invasion, migration, metastasis and angiogenesis 6, 7, 13, 22, 23, 24.
Furthermore, potential roles of DPP8 and DPP9 have been suggested in breast and ovarian cancer and an up‐regulation of DPP9 has been detected in testicular tumours 35, 94, 132. In addition, DPP9 was found to regulate cell survival and proliferation by inhibiting Akt activation involving the EGF signalling pathway. Moreover, DPP9 and DPP8 associate with H‐Ras, a key signal molecule of the EGF receptor signalling pathway 41. DPP8 and DPP9 have also been proposed to play a role in cell adhesion, migration and apoptosis 57.
Finally, DPP2 appears to be a prognostic biomarker and drug target for B cell chronic leucocytic leukaemia 126.
Asthma
Investigating the effects of airway inflammation in wild‐type and DPP4‐deficient rats, a significant increase of DPP4 enzymatic activity was found in the lung parenchyma as well as DPP8/DPP9 enzymatic activity in the bronchial epithelium. Furthermore, these enzymes also displayed elevated activities in bronchoalveolar lavage fluid. In addition, strong immunohistochemical staining was detected in bronchial epithelium and trachea for DPP8, DPP9 and DPL2, respectively. These results were also confirmed by elevated mRNA levels of DPP8, DPP9 and DPL2 in bronchial epithelium and trachea of asthmatic lungs. In contrast, increased staining of DPP4 and T cells was found in asthmatic lung parenchyma. Thus, the results revealed differential and site‐specific expression of DASH proteins in lung as well as their up‐regulation and functions in asthma 44. In fact, DPL2 has been proposed for asthma susceptibility 133.
Arthritis
Expression and activity of DPP4 in arthritis is up‐regulated in peripheral blood T lymphocytes and reduced in serum and synoviocytes, respectively 134. SDF‐α, one of the best substrates of DPP4, plays an important role in the pathogenesis of arthritis. However, the regulatory mechanism of DPP4 and SDF‐α in arthritis appears to be somewhat complex and has not yet been elucidated 8, 134. Different results were obtained from clinical and epidemiological studies with anti‐diabetic DPP4 inhibitors regarding increased or lower risks of polyarthropathy and autoimmune diseases such as RA in patients with type 2 diabetes mellitus (T2DM) 135, 136. Implementing differential diagnostics of polyarthropathy other than RA, osteoarthritis and crystal‐associated arthritis, as well as analysing various cytokines and chemokines, higher incidences of polyarthropathy, were found in patients treated with DPP4 inhibitors. Intriguingly, the polyarthropathy was associated with reduced levels of SDF‐α in plasma from T2DM patients receiving DPP4 inhibitors. Following cessation of DPP4 inhibitors, the clinical symptoms of polyarthropathy resolved within 3 months and the plasma levels of SDF‐α were restored 135. In contrast, the risks of developing an autoimmune disease such as RA seems to be lower upon treatment with DPP4 inhibitors, according to epidemiological studies 136. A single nucleotide polymorphism (SNP) within an intron of DPP4 has been identified recently as a novel risk locus of RA 137. However, in‐vivo studies with three different DASH inhibitors ameliorated disease symptoms in both wild‐type as well as DPP4‐deficient F344 rats, implying the involvement of additional DPP4‐like enzymes 111. Although FAP was found to be up‐regulated in chondrocytes and myofibroblasts of synoviocyte‐like cells from patients with osteoarthritis and RA, specific inhibition of FAP and DPP4 resulted in increased invasion of activated synoviocytes due to elevated SDF‐α levels, ruling out at least DPP4 and FAP as pharmacological targets against arthritis 21, 98, 128. In contrast, synovial fibroblasts (SF) of FAP(–/–) hTNFtg mice had a reduced cartilage adhesion capacity compared to hTNFtg SF in vitro. This indicates an unknown role of the FAP protein, but not its enzymatic activity in the attachment of SF to cartilage, promoting proteoglycan loss and subsequently cartilage degradation in chronic inflammatory arthritis 29. Investigations of FAP expression in SF from patients with RA and osteoarthritis (OA) revealed elevated FAP levels in RA, thereby confirming FAP being involved in chronic inflammatory arthritis 29.
In contrast to the activity of DPP4 in serum and synovial fluids, DPP2 activity was found to be increased, although its function has not yet been elucidated 78, 138.
IBD
IBD includes Crohn's disease and ulcerative colitis 9. The involvement of DASH proteins has been elucidated based on DPP–/– mice and DPP4/DPP4‐like inhibitors. In Crohn's disease, pharmacological inhibition of DPP4 by two different inhibitors reduced disease activity significantly due to elevated GLP‐2, indicating DPP4 to be a pharmaceutical target in Crohn's disease. However, in colitis DPP4 inhibitors and DPP4–/– mice were less effective, suggesting the involvement of other DASH proteins such as DPP8 and DPP2 97.
Conclusion
Taken together, DASH proteins play important roles in the immune system involving quiescence, proliferation, antigen‐presenting, co‐stimulation, T cell activation, signal transduction, differentiation and tissue modelling. Thus, they are involved in many pathophysiological processes and have therefore been proposed for potential biomarkers or even drug targets in various cancers and inflammatory diseases. However, they also pose the challenge of drug selectivity concerning other DASH members for better efficacy and/or avoidance of unwanted side effects. Hence, more knowledge is needed to disentangle the complex roles of DASH proteins in immunology.
Disclosure
The authors have no disclosures to declare.
References
- 1. Scanlan MJ, Raj BK, Calvo B et al Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci USA 1994; 91:5657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abbott CA, Yu DM, Woollatt E, Sutherland GR, McCaughan GW, Gorrell MD. Cloning, expression and chromosomal localization of a novel human dipeptidyl peptidase (DPP) IV homolog, DPP8. Eur J Biochem 2000; 267:6140–50. [DOI] [PubMed] [Google Scholar]
- 3. Olsen C, Wagtmann N. Identification and characterization of human DPP9, a novel homologue of dipeptidyl peptidase IV. Gene 2002; 299:185–93. [DOI] [PubMed] [Google Scholar]
- 4. Wada K, Yokotani N, Hunter C, Doi K, Wenthold RJ, Shimasaki S. Differential expression of two distinct forms of mRNA encoding members of a dipeptidyl aminopeptidase family. Proc Natl Acad Sci USA 1992; 89:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qi SY, Riviere PJ, Trojnar J, Junien JL, Akinsanya KO. Cloning and characterization of dipeptidyl peptidase 10, a new member of an emerging subgroup of serine proteases. Biochem J 2003; 373:179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abbott CA, Gorrell MD. The family of CD26/DP IV and related Ectopeptidases In: Langner J, Ansorge S, eds. Ectopeptidases. New York: Kluwer Academic/Plenum Publishers, 2002:171–95. [Google Scholar]
- 7. Gorrell MD. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin Sci (Lond) 2005; 108:277–92. [DOI] [PubMed] [Google Scholar]
- 8. Lambeir AM, Durinx C, Scharpe S, De Meester I. Dipeptidyl‐peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci 2003; 40:209–94. [DOI] [PubMed] [Google Scholar]
- 9. Klemann C, Wagner L, Von Horsten S, Stephan M. Cut to the chase: CD26/dipeptidyl peptidase‐4 (DPP‐4)'s entanglement in the immune system, a review. Clin Exp Immunol 2015; accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baetta R, Corsini A. Pharmacology of dipeptidyl peptidase‐4 inhibitors: similarities and differences. Drugs 2011; 71:1441–67. [DOI] [PubMed] [Google Scholar]
- 11. Wagner L, Wolf R, Zeitschel U et al Proteolytic degradation of neuropeptide Y (NPY) from head to toe novel NPY‐cleaving enzymes and revealed potential drug interactions. J Neurochem 2015; 135:1019–37. [DOI] [PubMed] [Google Scholar]
- 12. Frerker N, Wagner L, Wolf R et al Neuropeptide Y (NPY) cleaving enzymes: structural and functional homologues of dipeptidyl peptidase 4. Peptides 2007; 28:257–68. [DOI] [PubMed] [Google Scholar]
- 13. Yu DM, Yao TW, Chowdhury S et al The dipeptidyl peptidase IV family in cancer and cell biology. FEBS J 2010; 277:1126–44. [DOI] [PubMed] [Google Scholar]
- 14. Keane FM, Nadvi NA, Yao TW, Gorrell MD. Neuropeptide Y, B‐type natriuretic peptide, substance P and peptide YY are novel substrates of fibroblast activation protein‐alpha. FEBS J 2011; 278:1316–32. [DOI] [PubMed] [Google Scholar]
- 15. Edosada CY, Quan C, Tran T et al Peptide substrate profiling defines fibroblast activation protein as an endopeptidase of strict Gly(2)‐Pro(1)‐cleaving specificity. FEBS Lett 2006; 580:1581–6. [DOI] [PubMed] [Google Scholar]
- 16. Lee KN, Jackson KW, Christiansen VJ, Lee CS, Chun JG, McKee PA. Antiplasmin‐cleaving enzyme is a soluble form of fibroblast activation protein. Blood 2006; 107:1397–404. [DOI] [PubMed] [Google Scholar]
- 17. Lee KN, Jackson KW, Christiansen VJ, Chung KH, McKee PA. Alpha2‐antiplasmin: potential therapeutic roles in fibrin survival and removal. Curr Med Chem Cardiovasc Hematol Agents 2004; 2:303–10. [DOI] [PubMed] [Google Scholar]
- 18. Lee KN, Jackson KW, Christiansen VJ, Chung KH, McKee PA. A novel plasma proteinase potentiates {alpha}2‐antiplasmin inhibition of fibrin digestion. Blood 2004; 103:3783–8. [DOI] [PubMed] [Google Scholar]
- 19. Aertgeerts K, Ye S, Tennant MG et al Crystal structure of human dipeptidyl peptidase IV in complex with a decapeptide reveals details on substrate specificity and tetrahedral intermediate formation. Protein Sci 2004; 13:412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghersi G, Dong H, Goldstein LA et al Seprase‐dPPIV association and prolyl peptidase and gelatinase activities of the protease complex. Adv Exp Med Biol 2003; 524:87–94. [DOI] [PubMed] [Google Scholar]
- 21. Milner JM, Kevorkian L, Young DA et al Fibroblast activation protein alpha is expressed by chondrocytes following a pro‐inflammatory stimulus and is elevated in osteoarthritis. Arthritis Res Ther 2006; 8:R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghersi G, Zhao Q, Salamone M, Yeh Y, Zucker S, Chen WT. The protease complex consisting of dipeptidyl peptidase IV and seprase plays a role in the migration and invasion of human endothelial cells in collagenous matrices. Cancer Res 2006; 66:4652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kotackova L, Balaziova E, Sedo A. Expression pattern of dipeptidyl peptidase IV activity and/or structure homologues in cancer. Folia Biol (Praha) 2009; 55:77–84. [DOI] [PubMed] [Google Scholar]
- 24. Busek P, Mali kR, Sedo A. Dipeptidyl peptidase IV activity and/or structure homologues (DASH) and their substrates in cancer. Int J Biochem Cell Biol 2004; 36:408–21. [DOI] [PubMed] [Google Scholar]
- 25. Lessard J, Pelletier M, Biertho L et al Characterization of dedifferentiating human mature adipocytes from the visceral and subcutaneous fat compartments: fibroblast‐activation protein alpha and dipeptidyl peptidase 4 as major components of matrix remodeling. PLoS One 2015; 10:e0122065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Henry LR, Lee HO, Lee JS et al Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res 2007; 13:1736–41. [DOI] [PubMed] [Google Scholar]
- 27. Artym VV, Kindzelskii AL, Chen WT, Petty HR. Molecular proximity of seprase and the urokinase‐type plasminogen activator receptor on malignant melanoma cell membranes: dependence on beta1 integrins and the cytoskeleton. Carcinogenesis 2002; 23:1593–601. [DOI] [PubMed] [Google Scholar]
- 28. Niedermeyer J, Kriz M, Hilberg F et al Targeted disruption of mouse fibroblast activation protein. Mol Cell Biol 2000; 20:1089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Waldele S, Koers‐Wunrau C, Beckmann D et al Deficiency of fibroblast activation protein alpha ameliorates cartilage destruction in inflammatory destructive arthritis. Arthritis Res Ther 2015; 17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nemunaitis J, Vukelja SJ, Richards D et al Phase I trial of PT‐100 (PT‐100), a cytokine‐inducing small molecule, following chemotherapy for solid tumor malignancy. Cancer Invest 2006; 24:553–61. [DOI] [PubMed] [Google Scholar]
- 31. Bjelke JR, Christensen J, Nielsen PF et al Dipeptidyl peptidase 8 and 9 specificity and molecular characterization compared to dipeptidyl peptidase IV. Biochem J 2006; 396:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu LR, Zhu Z, Chan KC, Issaq HJ, Dimitrov DS, Veenstra TD. Improved titanium dioxide enrichment of phosphopeptides from HeLa cells and high confident phosphopeptide identification by cross‐validation of MS/MS and MS/MS/MS spectra. J Proteome Res 2007; 6:4150–62. [DOI] [PubMed] [Google Scholar]
- 33. Ajami K, Pitman MR, Wilson CH et al Stromal cell‐derived factors 1alpha and 1beta, inflammatory protein‐10 and interferon‐inducible T cell chemo‐attractant are novel substrates of dipeptidyl peptidase 8. FEBS Lett 2008; 582:819–25. [DOI] [PubMed] [Google Scholar]
- 34. Wilson CH, Indarto D, Doucet A et al Identifying natural substrates for dipeptidyl peptidases 8 and 9 using terminal amine isotopic labeling of substrates (TAILS) reveals in vivo roles in cellular homeostasis and energy metabolism. J Biol Chem 2013; 288:13936–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu DM, Ajami K, Gall MG et al The in vivo expression of dipeptidyl peptidases 8 and 9. J Histochem Cytochem 2009; 57:1025–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ajami K, Abbott CA, McCaughan GW, Gorrell MD. Dipeptidyl peptidase 9 has two forms, a broad tissue distribution, cytoplasmic localization and DPIV‐like peptidase activity. Biochim Biophys Acta 2004; 1679:18–28. [DOI] [PubMed] [Google Scholar]
- 37. Zhang H, Chen Y, Wadham C, McCaughan GW, Keane FM, Gorrell MD. Dipeptidyl peptidase 9 subcellular localization and a role in cell adhesion involving focal adhesion kinase and paxillin. Biochim Biophys Acta 2015; 1853:470–80. [DOI] [PubMed] [Google Scholar]
- 38. Justa‐Schuch D, Moller U, Geiss‐Friedlander R. The amino terminus extension in the long dipeptidyl peptidase 9 isoform contains a nuclear localization signal targeting the active peptidase to the nucleus. Cell Mol Life Sci 2014; 71:3611–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geiss‐Friedlander R, Parmentier N, Moller U, Urlaub H, Van den Eynde BJ, Melchior F. The cytoplasmic peptidase DPP9 is rate‐limiting for degradation of proline‐containing peptides. J Biol Chem 2009; 284:27211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang H, Maqsudi S, Rainczuk A et al Identification of novel dipeptidyl peptidase 9 substrates by two‐dimensional differential in‐gel electrophoresis. FEBS J 2015; 282:3737–57. [DOI] [PubMed] [Google Scholar]
- 41. Yao TW, Kim WS, Yu DM et al A novel role of dipeptidyl peptidase 9 in epidermal growth factor signalling. Mol Cancer Res 2011; 9:948–59. [DOI] [PubMed] [Google Scholar]
- 42. Zhang H, Chen Y, Keane FM, Gorrell MD. Advances in understanding the expression and function of dipeptidyl peptidase 8 and 9. Mol Cancer Res 2013; 11:1487–96. [DOI] [PubMed] [Google Scholar]
- 43. Gall MG, Chen Y, Vieira de Ribeiro AJ et al Targeted inactivation of dipeptidyl peptidase 9 enzymatic activity causes mouse neonate lethality. PLOS ONE 2013; 8:e78378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schade J, Stephan M, Schmiedl A et al Regulation of expression and function of dipeptidyl peptidase 4 (DP4), DP8/9, and DP10 in allergic responses of the lung in rats. J Histochem Cytochem 2008; 56:147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rohnert P, Schmidt W, Emmerlich P et al Dipeptidyl peptidase IV, aminopeptidase N and DPIV/APN‐like proteases in cerebral ischemia. J Neuroinflammation 2012; 9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dubois V, Lambeir AM, Vandamme S et al Dipeptidyl peptidase 9 (DPP9) from bovine testes: identification and characterization as the short form by mass spectrometry. Biochim Biophys Acta 2010; 1804:781–8. [DOI] [PubMed] [Google Scholar]
- 47. Maes MB, Dubois V, Brandt I et al Dipeptidyl peptidase 8/9‐like activity in human leukocytes. J Leukoc Biol 2007; 81:1252–7. [DOI] [PubMed] [Google Scholar]
- 48. Reinhold D, Goihl A, Wrenger S et al Role of dipeptidyl peptidase IV (DP IV)‐like enzymes in T lymphocyte activation: investigations in DP IV/CD26‐knockout mice. Clin Chem Lab Med 2009; 47:268–74. [DOI] [PubMed] [Google Scholar]
- 49. Eager RM, Cunningham CC, Senzer NN et al Phase II assessment of talabostat and cisplatin in second‐line stage IV melanoma. BMC Cancer 2009; 9:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Waumans Y, Baerts L, Kehoe K, Lambeir AM, De Meester I. The dipeptidyl peptidase family, prolyl oligopeptidase, and prolyl carboxypeptidase in the immune system and inflammatory disease, including atherosclerosis. Front Immunol 2015; 6:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Waumans Y, Baerts L, Kehoe K et al The dipeptidyl peptidase family, prolyl oligopeptidase, and prolyl carboxypeptidase in the immune system and inflammatory disease, including atherosclerosis. Front Immunol 2015; 6:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lankas GR, Leiting B, Roy RS et al Dipeptidyl peptidase IV inhibition for the treatment of type 2 diabetes: potential importance of selectivity over dipeptidyl peptidases 8 and 9. Diabetes 2005; 54:2988–94. [DOI] [PubMed] [Google Scholar]
- 53. Wu JJ, Tang HK, Yeh TK et al Biochemistry, pharmacokinetics, and toxicology of a potent and selective DPP8/9 inhibitor. Biochem Pharmacol 2009; 78:203–10. [DOI] [PubMed] [Google Scholar]
- 54. Kirby M, Yu DM, O'Connor S, Gorrell MD. Inhibitor selectivity in the clinical application of dipeptidyl peptidase‐4 inhibition. Clin Sci (Lond) 2010; 118:31–41. [DOI] [PubMed] [Google Scholar]
- 55. Bank U, Heimburg A, Wohlfarth A et al Outside or inside: role of the subcellular localization of DP4‐like enzymes for substrate conversion and inhibitor effects. Biol Chem 2011; 392:169–87. [DOI] [PubMed] [Google Scholar]
- 56. Burkey BF, Hoffmann PK, Hassiepen U, Trappe J, Juedes M, Foley JE. Adverse effects of dipeptidyl peptidases 8 and 9 inhibition in rodents revisited. Diabetes Obes Metab 2008; 10:1057–61. [DOI] [PubMed] [Google Scholar]
- 57. Yu DM, Wang XM, McCaughan GW, Gorrell MD. Extraenzymatic functions of the dipeptidyl peptidase IV‐related proteins DP8 and DP9 in cell adhesion, migration and apoptosis. FEBS J 2006; 273:2447–60. [DOI] [PubMed] [Google Scholar]
- 58. Park J, Knott HT, Navdi NA et al Reversible inactivation of human dipeptidyl peptidases 8 and 9 by oxidation. Open Enzym Inhib J 2008; 1:52–60. [Google Scholar]
- 59. Rasmussen HB, Branner S, Wiberg FC, Wagtmann N. Crystal structure of human dipeptidyl peptidase IV/CD26 in complex with a substrate analog. Nat Struct Biol 2003; 10:19–25. [DOI] [PubMed] [Google Scholar]
- 60. Rummey C, Metz G. Homology models of dipeptidyl peptidases 8 and 9 with a focus on loop predictions near the active site. Proteins 2007; 66:160–71. [DOI] [PubMed] [Google Scholar]
- 61. Zagha E, Ozaita A, Chang SY et al Dipeptidyl peptidase 10 modulates Kv4‐mediated A‐type potassium channels. J Biol Chem 2005; 280:18853–61. [DOI] [PubMed] [Google Scholar]
- 62. Chen T, Ajami K, McCaughan GW, Gai WP, Gorrell MD, Abbott CA. Molecular characterization of a novel dipeptidyl peptidase like 2‐short form (DPL2‐s) that is highly expressed in the brain and lacks dipeptidyl peptidase activity. Biochim Biophys Acta 2006; 1764:33–43. [DOI] [PubMed] [Google Scholar]
- 63. Strop P, Bankovich AJ, Hansen KC, Garcia KC, Brunger AT. Structure of a human A‐type potassium channel interacting protein DPPX, a member of the dipeptidyl aminopeptidase family. J Mol Biol 2004; 343:1055–65. [DOI] [PubMed] [Google Scholar]
- 64. van Es MA, van Vught PW, van Kempen G, Blauw HM, Veldink JH, van den Berg LH. Dpp6 is associated with susceptibility to progressive spinal muscular atrophy. Neurology 2009; 72:1184–5. [DOI] [PubMed] [Google Scholar]
- 65. Daoud H, Valdmanis PN, Dion PA, Rouleau GA. Analysis of DPP6 and FGGY as candidate genes for amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2010; 11:389–91. [DOI] [PubMed] [Google Scholar]
- 66. Tanaka S, Syu A, Ishiguro H et al DPP6 as a candidate gene for neuroleptic‐induced tardive dyskinesia. Pharmacogenomics J 2013; 13:27–34. [DOI] [PubMed] [Google Scholar]
- 67. Postema PG, Christiaans I, Hofman N et al Founder mutations in the Netherlands: familial idiopathic ventricular fibrillation and DPP6. Neth Heart J 2011; 19:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weiss ST, Raby BA, Rogers A. Asthma genetics and genomics 2009. Curr Opin Genet Dev 2009; 19:279–82. [DOI] [PubMed] [Google Scholar]
- 69. Bezerra GA, Dobrovetsky E, Seitova A, Dhe‐Paganon S, Gruber K. Crystallization and preliminary X‐ray diffraction analysis of human dipeptidyl peptidase 10 (DPPY), a component of voltage‐gated potassium channels. Acta Crystallogr Sect F Struct Biol Cryst Commun 2012; 68:214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sedo A, Malik R. Dipeptidyl peptidase IV‐like molecules: homologous proteins or homologous activities? Biochim Biophys Acta 2001; 1550:107–16. [DOI] [PubMed] [Google Scholar]
- 71. Barinka C, Rinnova M, Sacha P et al Substrate specificity, inhibition and enzymological analysis of recombinant human glutamate carboxypeptidase II. J Neurochem 2002; 80:477–87. [DOI] [PubMed] [Google Scholar]
- 72. Durinx C, Lambeir AM, Bosmans E et al Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is responsible for the release of X‐Pro dipeptides. Eur J Biochem 2000; 267:5608–13. [DOI] [PubMed] [Google Scholar]
- 73. Friedrich D, Hoffmann T, Bar J et al Does human attractin have DP4 activity? Biol Chem 2007; 388:155–62. [DOI] [PubMed] [Google Scholar]
- 74. Duke‐Cohan JS, Morimoto C, Rocker JA, Schlossman SF. Serum high molecular weight dipeptidyl peptidase IV (CD26) is similar to a novel antigen DPPT‐L released from activated T cells. J Immunol 1996; 156:1714–21. [PubMed] [Google Scholar]
- 75. Araki H, Li Y, Yamamoto Y et al Purification, molecular cloning, and immunohistochemical localization of dipeptidyl peptidase II from the rat kidney and its identity with quiescent cell proline dipeptidase. J Biochem (Tokyo) 2001; 129:279–88. [DOI] [PubMed] [Google Scholar]
- 76. Leiting B, Pryor KD, Wu JK et al Catalytic properties and inhibition of proline‐specific dipeptidyl peptidases II, IV and VII. Biochem J 2003; 371:525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chiravuri M, Huber BT. Aminodipeptidase inhibitor‐induced cell death in quiescent lymphocytes: a review. Apoptosis 2000; 5:319–22. [DOI] [PubMed] [Google Scholar]
- 78. Maes MB, Scharpe S, De Meester I. Dipeptidyl peptidase II (DPPII), a review. Clin Chim Acta 2007; 380:31–49. [DOI] [PubMed] [Google Scholar]
- 79. Chiravuri M, Lee H, Mathieu SL, Huber BT. Homodimerization via a leucine zipper motif is required for enzymatic activity of quiescent cell proline dipeptidase. J Biol Chem 2000; 275:26994–9. [DOI] [PubMed] [Google Scholar]
- 80. Mentlein R, Struckhoff G. Purification of two dipeptidyl aminopeptidases II from rat brain and their action on proline‐containing neuropeptides. J Neurochem 1989; 52:1284–93. [DOI] [PubMed] [Google Scholar]
- 81. Brandt I, Lambeir AM, Maes MB, Scharpe S, De Meester I. Peptide substrates of dipeptidyl peptidases. Adv Exp Med Biol 2006; 575:3–18. [DOI] [PubMed] [Google Scholar]
- 82. Mele DA, Bista P, Baez DV, Huber BT. Dipeptidyl peptidase 2 is an essential survival factor in the regulation of cell quiescence. Cell Cycle 2009; 8:2425–34. [DOI] [PubMed] [Google Scholar]
- 83. Bista P, Mele DA, Baez DV, Huber BT. Lymphocyte quiescence factor Dpp2 is transcriptionally activated by KLF2 and TOB1. Mol Immunol 2008; 45:3618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Maes MB, Martinet W, Schrijvers DM et al Dipeptidyl peptidase II and leukocyte cell death. Biochem Pharmacol 2006; 72:70–9. [DOI] [PubMed] [Google Scholar]
- 85. Gossrau R, Lojda Z. Study on dipeptidylpeptidase II (DPP II). Histochemistry 1980; 70:53–76. [DOI] [PubMed] [Google Scholar]
- 86. Danilova OV, Tai AK, Mele DA et al Neurogenin 3‐specific dipeptidyl peptidase‐2 deficiency causes impaired glucose tolerance, insulin resistance, and visceral obesity. Endocrinology 2009; 150:5240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bezerra GA, Dobrovetsky E, Dong A et al Structures of human DPP7 reveal the molecular basis of specific inhibition and the architectural diversity of proline‐specific peptidases. PLOS ONE 2012; 7:e43019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Boonacker E, Van Noorden CJ. The multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell Biol 2003; 82:53–73. [DOI] [PubMed] [Google Scholar]
- 89. Ohnuma K, Takahashi N, Yamochi T, Hosono O, Dang NH, Morimoto C. Role of CD26/dipeptidyl peptidase IV in human T cell activation and function. Front Biosci 2008; 13:2299–310. [DOI] [PubMed] [Google Scholar]
- 90. Vora KA, Porter G, Peng R et al Genetic ablation or pharmacological blockade of dipeptidyl peptidase IV does not impact T cell‐dependent immune responses. BMC Immunol 2009; 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ohnuma K, Hatano R, Aune TM et al Regulation of pulmonary graft‐versus‐host disease by IL‐26+CD26+CD4 T lymphocytes. J Immunol 2015; 194:3697–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hatano R, Ohnuma K, Otsuka H et al CD26‐mediated induction of EGR2 and IL‐10 as potential regulatory mechanism for CD26 costimulatory pathway. J Immunol 2015; 194:960–72. [DOI] [PubMed] [Google Scholar]
- 93. Salgado FJ, Lojo J, Alonso‐Lebrero JL et al A role for IL‐12 in the regulation of T cell plasma membrane compartmentation. J Biol Chem 2003; 278:24849–57. [DOI] [PubMed] [Google Scholar]
- 94. Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci 2009; 30:600–7. [DOI] [PubMed] [Google Scholar]
- 95. Yazbeck R, Howarth GS, Butler RN, Geier MS, Abbott CA. Biochemical and histological changes in the small intestine of mice with dextran sulfate sodium colitis. J Cell Physiol 2011; 226:3219–24. [DOI] [PubMed] [Google Scholar]
- 96. De Meester I, Korom S, Van Damme J, Scharpe S. CD26, let it cut or cut it down. Immunol Today 1999; 20:367–75. [DOI] [PubMed] [Google Scholar]
- 97. Yazbeck R, Howarth GS, Geier MS, Demuth HU, Abbott CA. Inhibiting dipeptidyl peptidase activity partially ameliorates colitis in mice. Front Biosci 2008; 13:6850–8. [DOI] [PubMed] [Google Scholar]
- 98. Bauer S, Jendro MC, Wadle A et al Fibroblast activation protein is expressed by rheumatoid myofibroblast‐like synoviocytes. Arthritis Res Ther 2006; 8:R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Silva AP, Cavadas C, Baisse‐Agushi B, Spertini O, Brunner HR, Grouzmann E. NPY, NPY receptors, and DPP IV activity are modulated by LPS, TNF‐alpha and IFN‐gamma in HUVEC. Regul Pept 2003; 116:71–9. [DOI] [PubMed] [Google Scholar]
- 100. Straub RH, Grum F, Strauch U et al Anti‐inflammatory role of sympathetic nerves in chronic intestinal inflammation. Gut 2008; 57:911–21. [DOI] [PubMed] [Google Scholar]
- 101. Marguet D, Baggio L, Kobayashi T et al Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci USA 2000; 97:6874–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. El YM, Vaugeois JM, Marguet D et al Behavioral characterization of CD26 deficient mice in animal tests of anxiety and antidepressant‐like activity. Behav Brain Res 2006; 171:279–85. [DOI] [PubMed] [Google Scholar]
- 103. Yan S, Marguet D, Dobers J, Reutter W, Fan H. Deficiency of CD26 results in a change of cytokine and immunoglobulin secretion after stimulation by pokeweed mitogen. Eur J Immunol 2003; 33:1519–27. [DOI] [PubMed] [Google Scholar]
- 104. Karl T, Chwalisz WT, Wedekind D et al Localization, transmission, spontaneous mutations, and variation of function of the Dpp4 (dipeptidyl‐peptidase IV; CD26) gene in rats. Regul Pept 2003; 115:81–90. [DOI] [PubMed] [Google Scholar]
- 105. Drucker DJ. Biological actions and therapeutic potential of the glucagon‐like peptides. Gastroenterology 2002; 122:531–44. [DOI] [PubMed] [Google Scholar]
- 106. Nagakura T, Yasuda N, Yamazaki K et al Improved glucose tolerance via enhanced glucose‐dependent insulin secretion in dipeptidyl peptidase IV‐deficient Fischer rats. Biochem Biophys Res Commun 2001; 284:501–6. [DOI] [PubMed] [Google Scholar]
- 107. Pederson RA, Kieffer TJ, Pauly R, Kofod H, Kwong J, McIntosh CH. The enteroinsular axis in dipeptidyl peptidase IV‐negative rats. Metabolism 1996; 45:1335–41. [DOI] [PubMed] [Google Scholar]
- 108. Karl T, Hoffmann T, Pabst R, Von Horsten S. Extreme reduction of dipeptidyl peptidase IV activity in F344 rat substrains is associated with various behavioral differences. Physiol Behav 2003; 80:123–34. [DOI] [PubMed] [Google Scholar]
- 109. Yasuda N, Inoue T, Nagakura T et al Enhanced secretion of glucagon‐like peptide 1 by biguanide compounds. Biochem Biophys Res Commun 2002; 298:779–84. [DOI] [PubMed] [Google Scholar]
- 110. Laconi S, Pillai S, Porcu PP, Shafritz DA, Pani P, Laconi E. Massive liver replacement by transplanted hepatocytes in the absence of exogenous growth stimuli in rats treated with retrorsine. Am J Pathol 2001; 158:771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tanaka S, Murakami T, Horikawa H, Sugiura M, Kawashima K, Sugita T. Suppression of arthritis by the inhibitors of dipeptidyl peptidase IV. Int J Immunopharmacol 1997; 19:15–24. [DOI] [PubMed] [Google Scholar]
- 112. Jones B, Adams S, Miller GT, Jesson MI, Watanabe T, Wallner BP. Hematopoietic stimulation by a dipeptidyl peptidase inhibitor reveals a novel regulatory mechanism and therapeutic treatment for blood cell deficiencies. Blood 2003; 102:641–8. [DOI] [PubMed] [Google Scholar]
- 113. Frerker N, Raber K, Bode F et al Phenotyping of congenic dipeptidyl peptidase 4 (DP4) deficient Dark Agouti (DA) rats suggests involvement of DP4 in neuro‐, endocrine, and immune functions. Clin Chem Lab Med 2009; 47:275–87. [DOI] [PubMed] [Google Scholar]
- 114. Wang Z, Grigo C, Steinbeck J, Von Horsten S, Amann K, Daniel C. Soluble DPP4 originates in part from bone marrow cells and not from the kidney. Peptides 2014; 57:109–17. [DOI] [PubMed] [Google Scholar]
- 115. Simeoni L, Rufini A, Moretti T, Forte P, Aiuti A, Fantoni A. Human CD26 expression in transgenic mice affects murine T‐cell populations and modifies their subset distribution. Hum Immunol 2002; 63:719–30. [DOI] [PubMed] [Google Scholar]
- 116. Hough RB, Lengeling A, Bedian V, Lo C, Bucan M. Rump white inversion in the mouse disrupts dipeptidyl aminopeptidase‐like protein 6 and causes dysregulation of Kit expression. Proc Natl Acad Sci USA 1998; 95:13800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mele DA, Sampson JF, Huber BT. Th17 differentiation is the default program for DPP2‐deficient T‐cell differentiation. Eur J Immunol 2011; 41:1583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Augustyns K, Van der Veken P, Senten K, Haemers A. The therapeutic potential of inhibitors of dipeptidyl peptidase IV (DPP IV) and related proline‐specific dipeptidyl aminopeptidases. Curr Med Chem 2005; 12:971–98. [DOI] [PubMed] [Google Scholar]
- 119. Jiaang WT, Chen YS, Hsu T et al Novel isoindoline compounds for potent and selective inhibition of prolyl dipeptidase DPP8. Bioorg Med Chem Lett 2005; 15:687–91. [DOI] [PubMed] [Google Scholar]
- 120. Chen SJ, Jiaang WT. Current advances and therapeutic potential of agents targeting dipeptidyl peptidases‐IV, ‐II, 8/9 and fibroblast activation protein. Curr Top Med Chem 2011; 11:1447–63. [DOI] [PubMed] [Google Scholar]
- 121. Van der Veken P, Haemers A, Augustyns K. Prolyl peptidases related to dipeptidyl peptidase IV: potential of specific inhibitors in drug discovery. Curr Top Med Chem 2007; 7:621–35. [DOI] [PubMed] [Google Scholar]
- 122. Van Goethem S, Van der Veken P, Dubois V et al Inhibitors of dipeptidyl peptidase 8 and dipeptidyl peptidase 9. Part 2: isoindoline containing inhibitors. Bioorg Med Chem Lett 2008; 18:4159–62. [DOI] [PubMed] [Google Scholar]
- 123. Hoffmann T, Demuth H‐U. Therapeutic strategies exploiting DP IV inhibition ‐ target disease: type 2 diabetes In: Langner J, Ansorge S, eds. Ectopeptidases. New York, USA: Kluwer Academic/Plenum Publishers, 2002:259–78. [Google Scholar]
- 124. Bank U, Tadje J, Helmuth M et al Dipeptidylpeptidase IV (DPIV) and alanyl‐aminopeptidases (AAPs) as a new target complex for treatment of autoimmune and inflammatory diseases‐proof of concept in a mouse model of colitis. Adv Exp Med Biol 2006; 575:143–53. [DOI] [PubMed] [Google Scholar]
- 125. Bengsch B, Seigel B, Flecken T, Wolanski J, Blum HE, Thimme R. Human Th17 cells express high levels of enzymatically active dipeptidylpeptidase IV (CD26). J Immunol 2012; 188:5438–47. [DOI] [PubMed] [Google Scholar]
- 126. Danilov AV, Danilova OV, Brown JR, Rabinowitz A, Klein AK, Huber BT. Dipeptidyl peptidase 2 apoptosis assay determines the B‐cell activation stage and predicts prognosis in chronic lymphocytic leukemia. Exp Hematol 2010; 38:1167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zhao Y, Yang L, Wang X, Zhou Z. The new insights from DPP‐4 inhibitors: their potential immune modulatory function in autoimmune diabetes. Diabetes Metab Res Rev 2014; 30:646–53. [DOI] [PubMed] [Google Scholar]
- 128. Ospelt C, Mertens JC, Jungel A et al Inhibition of fibroblast activation protein and dipeptidylpeptidase 4 increases cartilage invasion by rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 2010; 62:1224–35. [DOI] [PubMed] [Google Scholar]
- 129. Tanswell P, Garin‐Chesa P, Rettig WJ et al Population pharmacokinetics of antifibroblast activation protein monoclonal antibody F19 in cancer patients. Br J Clin Pharmacol 2001; 51:177–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sato K, Dang NH. CD26: a novel treatment target for T‐cell lymphoid malignancies? [Review]. Int J Oncol 2003; 22:481–97. [PubMed] [Google Scholar]
- 131. Fujimoto N, Ohnuma K, Aoe K et al Clinical significance of soluble CD26 in malignant pleural mesothelioma. PLOS ONE 2014; 9:e115647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wilson CH, Abbott CA. Expression profiling of dipeptidyl peptidase 8 and 9 in breast and ovarian carcinoma cell lines. Int J Oncol 2012; 41:919–32. [DOI] [PubMed] [Google Scholar]
- 133. Weiss ST, Raby BA. Asthma genetics 2003. Hum Mol Genet 2004; 13:R83–9. [DOI] [PubMed] [Google Scholar]
- 134. Busso N, Wagtmann N, Herling C et al Circulating CD26 is negatively associated with inflammation in human and experimental arthritis. Am J Pathol 2005; 166:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Saito T, Ohnuma K, Suzuki H et al Polyarthropathy in type 2 diabetes patients treated with DPP4 inhibitors. Diabetes Res Clin Pract 2013; 102:e8–12. [DOI] [PubMed] [Google Scholar]
- 136. Kim SC, Schneeweiss S, Glynn RJ, Doherty M, Goldfine AB, Solomon DH. Dipeptidyl peptidase‐4 inhibitors in type 2 diabetes may reduce the risk of autoimmune diseases: a population‐based cohort study. Ann Rheum Dis 2015; 74:1968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Jiang L, Yin J, Ye L et al Novel risk loci for rheumatoid arthritis in Han Chinese and congruence with risk variants in Europeans. Arthritis Rheumatol 2014; 66:1121–32. [DOI] [PubMed] [Google Scholar]
- 138. Gotoh H, Hagihara M, Nagatsu T, Iwata H, Miura T. Activities of dipeptidyl peptidase II and dipeptidyl peptidase IV in synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Clin Chem 1989; 35:1016–8. [PubMed] [Google Scholar]
- 139. Bjelke JR, Kanstrup AB, Rasmussen HB. Selectivity among dipeptidyl peptidases of the s9b family. Cell Mol Biol (Noisy‐le‐Grand) 2006; 52:3–7. [PubMed] [Google Scholar]
- 140. Davoodi J, Kelly J, Gendron NH, MacKenzie AE. The Simpson–Golabi–Behmel syndrome causative glypican‐3, binds to and inhibits the dipeptidyl peptidase activity of CD26. Proteomics 2007; 7:2300–10. [DOI] [PubMed] [Google Scholar]
- 141. Canneva F, Golub Y, Distler J et al. DPP4‐deficient congenic rats display blunted stress, improved fear extinction and increased central NPY. Psychoneuroendocrinology 2015; 53:195–206. [DOI] [PubMed] [Google Scholar]
- 142. Guieu R, Fenouillet E, Devaux C et al CD26 modulates nociception in mice via its dipeptidyl‐peptidase IV activity. Behav Brain Res 2006; 166:279–85. [DOI] [PubMed] [Google Scholar]
- 143. Nagakura T, Yasuda N, Yamazaki K, Ikuta H, Tanaka I. Enteroinsular axis of db/db mice and efficacy of dipeptidyl peptidase IV inhibition. Metabolism 2003; 52:81–6. [DOI] [PubMed] [Google Scholar]
- 144. Drucker DJ, Boushey RP, Wang F, Hill ME, Brubaker PL, Yusta B. Biologic properties and therapeutic potential of glucagon‐like peptide‐2. JPEN J Parenter Enteral Nutr 1999; 23:S98–100. [DOI] [PubMed] [Google Scholar]
- 145. Conarello SL, Li Z, Ronan J et al Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci USA 2003; 100:6825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lautar SL, Rojas C, Slusher BS et al DPP IV inhibitor blocks mescaline‐induced scratching and amphetamine‐induced hyperactivity in mice. Brain Res 2005; 1048:177–84. [DOI] [PubMed] [Google Scholar]
- 147. Donahue RN, Duncan BB, Fry TJ et al A pan inhibitor of DASH family enzymes induces immunogenic modulation and sensitizes murine and human carcinoma cells to antigen‐specific cytotoxic T lymphocyte killing: implications for combination therapy with cancer vaccines. Vaccine 2014; 32:3223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Thielitz A, Reinhold D, Vetter R et al Inhibitors of dipeptidyl peptidase IV and aminopeptidase N target major pathogenetic steps in acne initiation. J Invest Dermatol 2007; 127:1042–51. [DOI] [PubMed] [Google Scholar]
- 149. Zhang Y, Zhang X, Liu H, Cai S, Wu B. Mixed nanomicelles as potential carriers for systemic delivery of Z‐GP‐Dox, an FAPalpha‐based doxorubicin prodrug: formulation and pharmacokinetic evaluation. Int J Nanomedicine 2015; 10:1625–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Acs G, Esposito NN, Rakosy Z, Laronga C, Zhang PJ. Invasive ductal carcinomas of the breast showing partial reversed cell polarity are associated with lymphatic tumor spread and may represent part of a spectrum of invasive micropapillary carcinoma. Am J Surg Pathol 2010; 34:1637–46. [DOI] [PubMed] [Google Scholar]
- 151. Argyrakopoulou G, Doupis J. DPP4 inhibitors: from sitagliptin monotherapy to the new alogliptin‐pioglitazone combination therapy. Adv Ther 2009; 26:272–80. [DOI] [PubMed] [Google Scholar]


