Summary
Complement activation is of major importance in numerous pathological conditions. Therefore, targeted complement inhibition is a promising therapeutic strategy. C1‐esterase inhibitor (C1‐INH) controls activation of the classical pathway (CP) and the lectin pathway (LP). However, conflicting data exist on inhibition of the alternative pathway (AP) by C1‐INH. The inhibitory capacity of C1‐INH for the CP is potentiated by heparin and other glycosaminoglycans, but no data exist for the LP and AP. The current study investigates the effects of C1‐INH in the presence or absence of different clinically used heparinoids on the CP, LP and AP. Furthermore, the combined effects of heparinoids and C1‐INH on coagulation were investigated. C1‐INH, heparinoids or combinations were analysed in a dose‐dependent fashion in the presence of pooled serum. Functional complement activities were measured simultaneously using the Wielisa®‐kit. The activated partial thrombin time was determined using an automated coagulation analyser. The results showed that all three complement pathways were inhibited significantly by C1‐INH or heparinoids. Next to their individual effects on complement activation, heparinoids also enhanced the inhibitory capacity of C1‐INH significantly on the CP and LP. For the AP, significant potentiation of C1‐INH by heparinoids was found; however, this was restricted to certain concentration ranges. At low concentrations the effect on blood coagulation by combining heparinoids with C1‐INH was minimal. In conclusion, our study shows significant potentiating effects of heparinoids on the inhibition of all complement pathways by C1‐INH. Therefore, their combined use is a promising and a potentially cost‐effective treatment option for complement‐mediated diseases.
Keywords: complement, complement 1‐inhibitor, glycosaminoglycans, heparinoids, inhibition
Introduction
The complement system is a major component of innate immunity. Excessive activation or insufficient regulation of the complement cascade causes clinical manifestations of many diseases, such as paroxysmal nocturnal haemoglobinuria 1, atypical haemolytic uraemic syndrome 2 or ischaemia–reperfusion injury in transplantation 3, 4, 5, 6, 7. Therefore, inhibition of complement activation has been recognized as a promising therapeutic strategy.
In renal disease, terminal complement inhibitors such as eculizumab are approved for the treatment of atypical haemolytic uraemic syndrome 1, 2. In transplantation, mounting evidence from animal studies suggests that intervention in complement pathway activation is a promising method for improving allograft outcome 8, 9.
Human C1‐inhibitor (C1‐INH) is a naturally occurring serine protease inhibitor that inhibits activation of the classical pathway (CP) and the lectin pathway (LP). However, conflicting data exist concerning inhibition of the alternative pathway (AP) by C1‐INH 10, 11, 12. In the CP, activation of the C1‐complex results in cleavage of C4 and C2, respectively, leading to the formation of the C4bC2b convertase and thereby activating C3. CP activation is regulated tightly by C1‐INH through binding of activated C1r and C1s and subsequent dissociation from C1q. The LP is activated when mannose‐binding lectin (MBL) and/or ficolins, interact with carbohydrate moieties on microbes and altered self‐surfaces. Activation of MBL‐associated serine proteases 1 (MASP‐1) and MBL‐associated serine proteases 2 (MASP‐2) results in cleavage of C4, C2 and formation of the C4bC2b complex, thereby activating C3. MASPs are also inhibited by C1‐INH, thereby interfering in the LP activation 13, 14. Recently, it was demonstrated that C1‐INH could also inhibit complement activation of the AP. Although the exact mechanism is not exactly clear, it is postulated that C1‐INH binds C3b and inhibits the binding of factor B, thereby blocking the AP convertase 12.
As well as regulation of complement activation, C1‐INH is also involved in the contact system, coagulation and fibrinolysis. C1‐INH has been shown to inhibit activated factor XII 15, 16, kallikrein 17, thrombin 18, platelets 19 and plasmin 20. Treatment using C1‐INH has been approved for hereditary angioedema 21, 22. It is well known that glycosaminoglycans potentiate the inhibitory capacity of C1‐INH. Glycosaminoglycans such as heparin are naturally occurring compounds in the human body. Heparin has been proposed to be one of the most potent enhancers of C1‐INH function 23. In addition, heparin and related compounds possess an anti‐inflammatory effect 24, 25. Low molecular weight heparins, also called heparinoids, are synthetic glycosaminoglycans with similar properties compared to heparin. Currently, the potentiating effect of heparinoids via C1‐INH has been demonstrated only for the CP. Moreover, the effects of clinical heparinoids on complement activation have not yet been investigated. Combined treatment with heparinoids and C1‐INH are considered for clinical use; for example, in the reduction of ischaemia–reperfusion injury during transplantation or other diseases with excessive activation or inappropriate complement regulation. Therefore, the parallel and or combined effects of heparinoids on C1‐INH via the LP and AP should be investigated.
The aim of this study is to investigate the effects of purified plasma C1‐INH in the presence or absence of clinically employed heparinoids on the inhibition of the classical, lectin and alternative pathways in vitro.
Materials and methods
Drugs
Heparin (5000 IU/ml) was purchased from Leo Pharma, Amsterdam, the Netherlands, dalteparin (Fragmin©, 10000 IU/ml) from Pfizer, New York, USA, enoxaparin (Clexane©, 10000 IU/ml) from Sanofi‐Aventis, Paris, France and nadroparin (Fraxiparin©, 9500 IU/ml) from GlaxoSmithKline, Brentford, UK. Plasma‐derived C1‐inhibitor (Cinryze®) was a gift from Viropharma, Exton, PA, USA.
Blood samples
Blood samples were taken from 10 healthy volunteers and stored immediately on ice to prevent in‐vitro complement activation. Serum samples were centrifuged, pooled and stored in aliquots at −80°C until further analysis. For plasma samples, blood was drawn into 3·2% sodium citrate, after which the plasma samples were pooled and stored in aliquots at −80°C until use.
Complement pathway activity in human serum
The Wieslab Complement System Screen COMPL300 kit (Euro‐Diagnostica AB, Malmö, Sweden) was used for the assessment of serum complement functional activity in classical, alternative and lectin pathways 26. In this kit, wells are precoated with immunoglobulin (Ig)M (CP), lipopolysaccharide (LPS) (AP) and mannan (LP) to assess the specific activity of each complement pathway. Pooled human sera from healthy volunteers were diluted in specific buffers according to each complement pathway. For CP activity, serum was diluted 1 : 100 in Veronal buffer containing both free calcium and magnesium. For LP activity, serum was diluted 1 : 100 in Veronal buffer containing free calcium, magnesium and monoclonal anti‐C1q antibodies. For AP activity, serum was diluted in 1 : 18 in magnesium ethyleneglycol tetraacetic acid (Mg‐EGTA) buffer containing free magnesium without calcium. Sera in pathway‐specific buffers were incubated in the presence or absence of C1‐INH (range 0–6 U/ml), heparin, dalteparin, enoxaparin and/or nadroparin (range 0–1 IU/ml). These mixtures were incubated for 1 h at 37ºC. After three washes, plates were incubated with phosphatase‐conjugated anti‐human C5b‐9 (aE11) for 30 min at room temperature. After another three washes, plates were then incubated with substrate solution for 30 min. After the reaction was stopped with 0·5 M H2SO4, the amount of reacted substrate was measured at optical density (OD) 450 nm. Data are expressed as the percentage inhibition compared to the positive control (normal human serum) for each pathway.
Blood coagulation time
The activated partial thrombin time (APTT) was determined in plasma samples using an automated coagulation analyser (Behring coagulation system, BCS) with reagents and protocols from the manufacturer (Siemens Healthcare Diagnostics, Marburg, Germany).
Statistics
Data are displayed as mean and ± standard error of the mean. Statistical analyses were performed using BM spss statistics version 22, and P ‐values less than 0·05 were assigned as statistically significant. Dose–response data of C1‐INH and heparinoids alone were analysed by linear regression. For linear regression, normal distribution of the residuals was tested and confirmed using normal probability plots. If needed, data were ln‐transformed for normality. The area under the curve (AUC) was used to compare the combined effect of C1‐INH and heparinoids with the individual effect. AUC was calculated using the trapezoidal rule and compared by Student's t‐test.
Ethics
The study was approved by the Regional Ethical Committee.
Results
Complement pathway activity in the presence of C1‐INH (Fig. 1)
Figure 1.
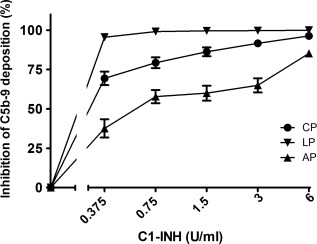
The dose‐dependent inhibitory effect of C1‐inhibitor (C1‐INH) on the three complement pathways. Increasing concentrations of C1‐INH were added to pooled human serum (x‐axis, log2 scale). The inhibition of C5b‐9 deposition was calculated by dividing the optical density (OD) of the sample with added C1‐INH by the control, which had no extra C1‐INH (y‐axis). Data are mean and standard error of the mean of three experiments.
To investigate the direct effect of C1‐INH on the complement system, the activity of each pathway was studied. Analysis of the data by linear regression showed significant dose‐dependent inhibition of the CP (P < 0·001), LP (P = 0·009) and AP (P < 0·001) by C1‐INH. Complement activities of the CP, LP and AP were reduced by 96, 100 and 85%, respectively, using 6 U/ml of C1‐INH.
Complement pathway activity in the presence of heparinoids (Fig. 2)
Figure 2.
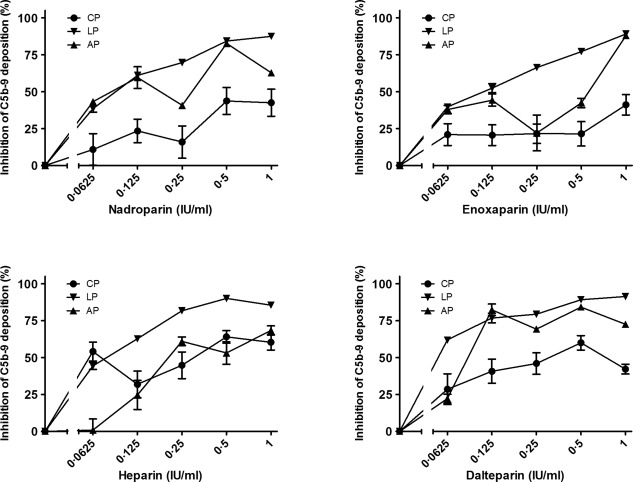
The inhibitory effect of nadroparin, enoxaparin, heparin and dalteparin on the three complement pathways. Increasing concentrations were added to pooled human serum samples (x‐axis, log2 scale). The inhibition of C5b‐9 deposition was calculated by dividing the optical density (OD) of the sample with heparinoid by the control, the sample without heparinoid (y‐axis). Data are expressed as the mean and standard error of the mean of three experiments.
The direct effects of heparinoids on complement were analysed prior to the potentiating effect of heparinoids on C1‐INH. Once again, analysis by linear regression showed that all three pathways were inhibited significantly by increasing concentrations of heparin (P < 0·01), dalteparin (P < 0·05), nadroparin (P < 0·05) and enoxaparin (P < 0·001). Interestingly, the different heparinoids vary in their ability to inhibit the different complement pathways. Heparin was the strongest inhibitor of the CP, with 54% inhibition at the lowest concentrations (0·0625 IU/ml). Dalteparin was the most powerful inhibitor for the LP, with 62% inhibition at the lowest concentrations (0·0625 IU/ml). However, the most potent inhibitor was strictly concentration‐dependent for the AP.
Complement pathway activity in the presence of C1‐INH combined with heparinoids (Figs 3, 4, 5, 6)
Figure 3.
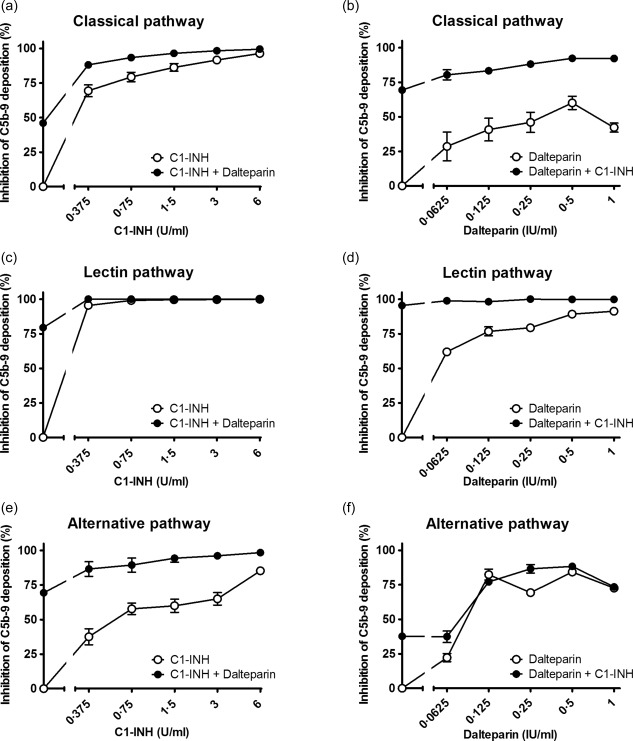
Inhibition of C1‐inhibitor (C1‐INH) in combination with dalteparin on the classical pathway (a,b), lectin pathway (c,d) and the alternative pathway (e,f). Increasing concentrations of C1‐INH were co‐incubated with 0·25 IU/ml dalteparin (a,c,e) or increasing concentrations of dalteparin were co‐incubated with a 0·375 U/ml C1‐INH (b,d,f) in pooled human serum (x‐axis, log2 scale). The inhibition of C5b‐9 deposition was calculated by dividing the optical density (OD) of the sample with heparinoid by the control, the sample without heparinoid (y‐axis). Data are expressed as the mean and standard error of the mean of three experiments.
Figure 4.
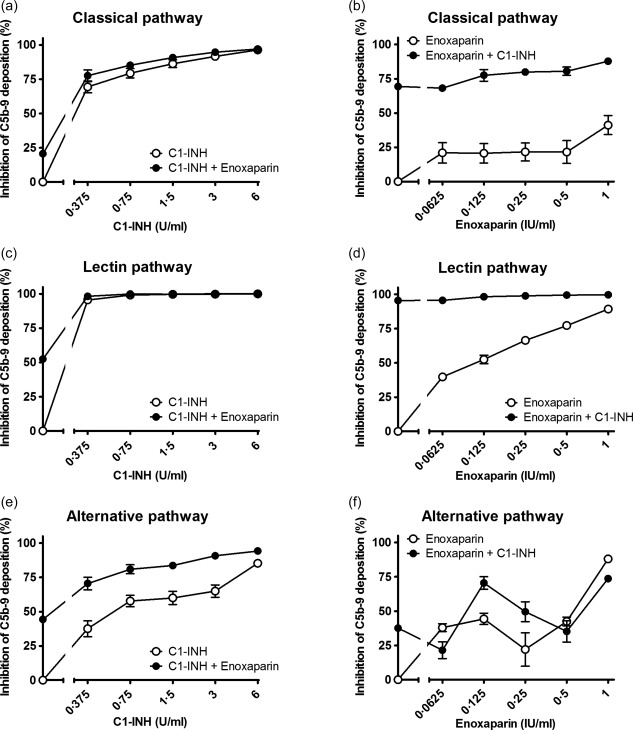
Inhibition of C1‐inhibitor (C1‐INH) in combination with enoxaparin on the classical pathway (a,b), lectin pathway (c,d) and the alternative pathway (e,f). Increasing concentrations of C1‐INH were co‐incubated with 0·125 IU/ml enoxaparin (a,c,e) or increasing concentrations of enoxaparin were co‐incubated with 0·375 U/ml C1‐INH (b,d,f) in pooled human serum (x‐axis, log2 scale). The inhibition of C5b‐9 deposition was calculated by dividing the optical density (OD) of the sample with heparinoid by the control, the sample without heparinoid (y‐axis). Data are expressed as the mean and standard error of the mean of three experiments.
Figure 5.
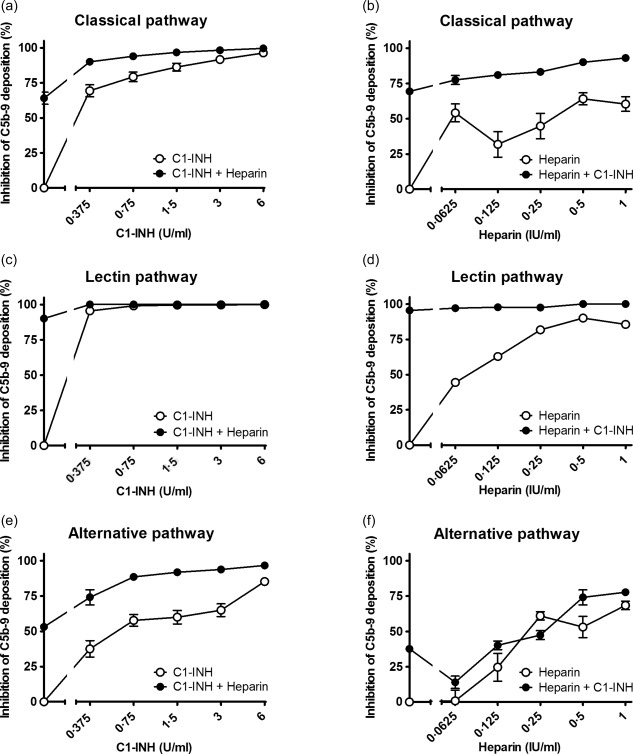
Inhibition of C1‐inhibitor (C1‐INH) in combination with heparin on the classical pathway (a,b), the lectin pathway (c,d) and the alternative pathway (e,f). Increasing concentrations of C1‐INH were co‐incubated with 0·5 IU/ml heparin (a,c,e) or increasing concentrations of heparin were co‐incubated with 0·375 U/ml C1‐INH (b,d,f) in pooled human serum (x‐axis, log2 scale). The inhibition of C5b‐9 deposition was calculated by dividing the optical density (OD) of the sample with heparinoid by the control, the sample without heparinoid (y‐axis). Data are expressed as the mean and standard error of the mean of three experiments.
Figure 6.
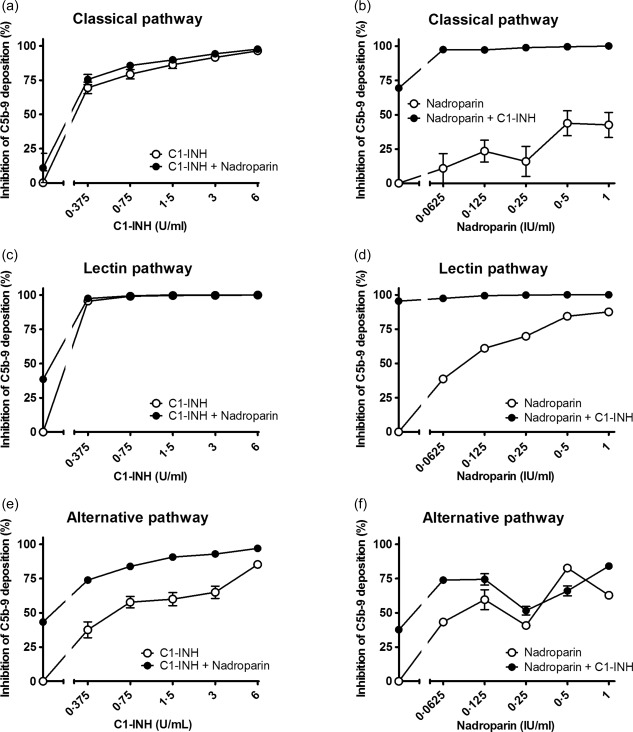
Inhibition of C1‐inhibitor (C1‐INH) in combination with nadroparin on the classical pathway (a,b), the lectin pathway (c,d) and the alternative pathway (e,f). Increasing concentrations of C1‐INH were co‐incubated with 0·0625 nadroparin (a,c,e) or increasing concentrations of nadroparin were co‐incubated with 0·375 U/ml C1‐INH (b,d,f) in pooled human serum (x‐axis, log2 scale). The inhibition of C5b‐9 deposition was calculated by dividing the optical density (OD) of the sample with heparinoid by the control, the sample without heparinoid (y‐axis). Data are expressed as the mean and standard error of the mean of three experiments.
Next, the combined effect of C1‐INH and heparinoids on complement activity was evaluated. Different concentrations of heparinoids were incubated with increasing concentrations of C1‐INH and vice versa (Supporting information). The addition of heparinoids potentiated the inhibitory effect of C1‐INH. To determine the concentration per heparinoid at which the maximal potentiation was reached the AUC was compared. This was performed separately for each heparinoid. The maximal potentiation was achieved at 0.25 IU/ml for dalteparin (Fig. 3), 0.125 IU/ml for enoxaparin (Fig. 4), 0.5 IU/ml for heparin (Fig. 5) and 0.0625 IU/ml for nadroparin (Fig. 6). The AUC was significantly greater (P < 0·05) for the CP, LP and AP when C1‐INH was potentiated maximally with heparin, dalteparin, enoxaparin or nadroparin than C1‐ING alone, as shown in Figs 3, 4, 5, 6 (a,c,e). This demonstrates that inhibition by C1‐INH is potentiated significantly for all three pathways when combined with heparinoids. However, for the AP, potentiation was restricted to certain concentration ranges, and in certain cases addition of higher concentrations of heparinoids to C1‐INH led to less potentiation or even loss of inhibition (Supporting information).
Blood coagulation time in the presence of C1‐INH, heparinoids or both (Figs 7 and 8)
Figure 7.
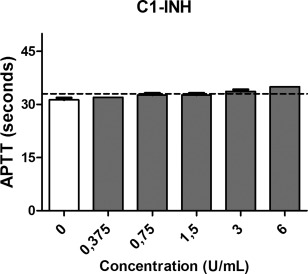
The effect of C1‐inhibitor (C1‐INH) on the activated partial thrombin time (APTT). Increasing concentrations of C1‐INH were added to pooled human plasma samples (x‐axis, log2 scale). The dashed line represents the upper limit of normal (y‐axis). Data are expressed as the mean and standard error of the mean of three experiments.
Figure 8.
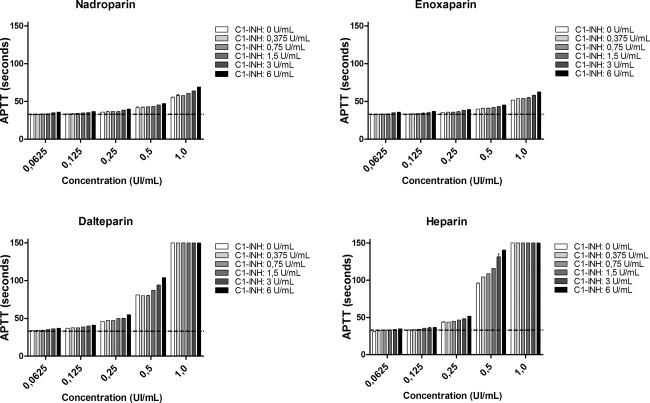
The effect of C1‐inhibitor (C1‐INH) in combination with nadroparin, enoxaparin, dalteparin, and heparin on the activated partial thrombin time (APTT). Increasing concentrations of C1‐INH combined with heparinoids were added to pooled human plasma samples (x‐axis, log2 scale). The dashed line represents the upper limit of normal (y‐axis). Data are expressed as the mean and standard error of the mean of three experiments.
The effects of C1‐inhibitor alone and in combination with heparinoids on the blood coagulation times were analysed. Both C1‐INH and heparinoids prolong the APTT in a dose‐dependent manner (Figs 7 and 8). The APTT was found to be above the normal reference values (23–33 s) at only the highest concentrations of C1‐INH. The APTT was prolonged when C1‐INH was co‐incubated with heparinoids. However, at lower concentrations the combined effect of C1‐INH and heparinoids on the APTT is minimal. Heparin combined with C1‐INH prolonged the APTT most strongly.
Discussion
Therapeutic inhibition of the complement system is considered a promising approach for the treatment of numerous pathological conditions 1, 2, 8, 9. The current study demonstrates that C1‐INH might be a potential therapeutic candidate, because it inhibits activation of all three pathways of the complement system. Furthermore, treatment with C1‐INH in combination with heparinoids could potentially enhance significantly the inhibitory capacity of C1‐INH, considering the in‐vitro data of our study. For the CP and LP, the potentiating effect was minor due to the already profound effect of C1‐INH alone. However, optimal potentiating effects of heparinoids on the CP and LP are found at low concentrations of C1‐INH. There is a strong potentiating effect of heparinoids and C1‐INH on the inhibition of the AP, but this was found to be strictly dose‐dependent. The effect on blood coagulation by the combined use of heparinoids and C1‐INH is minimal as long as both agents are used at low concentrations. This study, therefore, shows a potentiating effect of heparinoids and C1‐INH to inhibit all three complement pathways in vitro. The combined use of these agents is a promising and cost‐effective therapeutic approach for clinical intervention in complement‐mediated diseases.
C1‐INH is already approved for treatment of patients with hereditary angioedema 21, 22. Our study demonstrates inhibition of all complement pathways by C1‐INH, which is supported by previous studies 10, 11, 12. Although most reports have focused on only one pathway, Nielsen et al. investigated the effects of C1‐INH on all three pathways using the same functional assay. In contrast to Nielsen et al., our study demonstrated stronger inhibitory effects of C1‐INH on all complement pathways. The discrepancy could be explained by the use of different C1‐INHs, suggesting that the purified C1‐INH used in this study is more potent at inhibiting complement in vitro.
The heparinoids used in this study are used clinically and known for a wide variety of biological effects. It has been known for years that heparin inhibits all pathways of complement by blocking MASPs, C1q and by the potentiation of factor H 13, 27, 28. However, to our knowledge this is the first report that shows, in addition to heparin, that low molecular weight heparins, that are widely used clinically, inhibit all three complement pathways significantly. Remarkably, although the different heparinoids are vastly similar in structure and molecular weight, clear differences are found in their ability to inhibit different pathways. Lastly, the pooled human serum used in this study to test the effect of heparinoids on the complement system already contains naturally occurring C1‐INH. One can therefore not exclude the possibility that part of the complement inhibition by heparinoids alone found in our experiment is through potentiation of C1‐INH, naturally present in serum.
The present study also investigated the effect of heparinoids in the presence of C1‐INH on complement activation. In 1920, Gross and Erch reported that heparin could potentiate C1‐INH activity for the CP. The mechanisms by which heparin can potentiate C1‐INH activity includes enhanced binding of C1‐INH with C1s and interference with the binding of C1q to an activator. Therefore, it has been suggested that glycosaminoglycans might be used clinically as complement‐inhibiting agents combined with C1‐INH. Wuillemin et al. studied extensively the potentiating effects of several glycosaminoglycans on C1‐INH to inhibit complement activation. Heparin was concluded to be one of the most potent enhancers of C1‐INH function 23. In addition to previous studies, our study also investigated the potentiating effects of clinical heparinoids on C1‐INH to inhibit complement activation for all three pathways.
For the CP and LP, significant potentiation of C1‐INH by heparinoids was found. In contrast, AP potentiation was found to be strictly heparinoid and dose‐dependent. Certain combinations of heparinoids and C1‐INH were less effective in inhibiting the AP than C1‐INH or the heparinoid alone. This might be explained by the known biphasic response of complement to heparin; at low concentration, heparin can activate the alternative pathway of complement through potentiation of the amplification loop; at high heparin concentration, the alternative pathway is inhibited through potentiation of factor H, thereby preventing C3bBb‐convertase formation 23, 29, 30, 31. Interestingly, we found no biphasic effect for heparin or low molecular weight heparin alone, yet in certain combinations with C1‐INH this biphasic effect occurred for the AP (Supporting information). It is suggested that, as inhibition of the AP by heparin is known to occur mainly through factor H potentiation, C1‐INH either interferes in this binding, binds directly to heparin or competes with the C3b–factor H complex.
The effect on coagulation of heparinoids combined with C1‐INH was also studied. In low concentrations the combined effect on the APTT was minimal. None the less, at higher concentrations (0·5 and 1 IU/ml) the combined effect with C1‐INH is more profound. The prothrombin time (PT) was not influenced significantly by C1‐INH, heparinoids or their combination (data not shown).
In conclusion, this study shows that plasma derived C1‐INH inhibits complement activation of all three pathways in a dose‐dependent fashion. Furthermore, clinically used heparinoids potentiate the inhibitory capacity of C1‐INH on all three pathways. For the AP, we demonstrated that within a range of different concentrations a biphasic effect occurs of both amplifying and reducing complement inhibition by C1‐INH. These results shed new light on the potential use of with C1‐INH in order to target complement activation in the clinical setting. Potentially, heparinoids and C1‐INH could be used combined as a cost‐effective therapeutic option for the treatment of complement‐mediated diseases. However, further in‐vivo studies are needed to confirm the potentiating effect of combining heparinoids with C1‐INH.
Disclosure
This was a company‐sponsored study by ViroPharma Incorporated (now part of the Shire Group of Companies), manufacturer of C1‐esterase inhibitor (CINRYZE®), in collaboration with the University Medical Centre Groningen researchers. ViroPharma employees (J. S. and M. E. U.) were involved in the design of the study and interpretation of the data and contributed as authors to the preparation of the manuscript.
Author contributions
Research idea and study design by J. D., J. S., M. R. D., H. G. L., M. U. and M. A. J. S.; data acquisition by F. P., J. D. and E. L. d. V.; data analysis/interpretation by F. P., J. D., J. S., M. R. D., H. G. L., M. U. and M. A. J. S.; statistical analysis by F. P. and J. G. M. B; F. P. and J. D. wrote the manuscript.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1a. Percentage inhibition of classical pathway activity by C1‐INH in combination with heparin, dalteparin, nadroparin or enoxaparin. The inhibition of C5b‐9 deposition was calculated by dividing the optical density (OD) of the sample with heparinoid by the control, the sample without heparinoid or C1‐INH. Data are expressed as the mean and standard error of the mean of three experiments.
Table S1b. Percentage inhibition of lectin pathway activity by C1‐INH in combination with heparin, dalteparin, nadroparin or enoxaparin. The inhibition of C5b‐9 deposition was calculated by dividing the optical density (OD) of the sample with heparinoid by the control, the sample without heparinoid or C1‐INH. Data are expressed as the mean and standard error of the mean of three experiments.
Table S1c. Percentage inhibition of alternative pathway activity by C1‐INH in combination with heparin, dalteparin, nadroparin or enoxaparin. The inhibition of C5b‐9 deposition was calculated by dividing the optical density (OD) of the sample with heparinoid by the control, the sample without heparinoid or C1‐INH. Data are expressed as the mean and standard error of the mean of three experiments.
Acknowledgement
We thank Ryanne S. Hijmans for her editorial support with preparation of this manuscript.
References
- 1. Heitlinger E. Learnings from over 25 years of PNH experience: the era of targeted complement inhibition. Blood Rev 2013; 27:S1–6. [DOI] [PubMed] [Google Scholar]
- 2. Legendre CM, Licht C, Muus P et al Terminal complement inhibitor eculizumab in atypical hemolytic–uremic syndrome. N Engl J Med 2013; 368:2169–81. [DOI] [PubMed] [Google Scholar]
- 3. Damman J, Seelen MA, Moers C et al Systemic complement activation in deceased donors is associated with acute rejection after renal transplantation in the recipient. Transplantation 2011; 92:163–9. [DOI] [PubMed] [Google Scholar]
- 4. Damman J, Nijboer WN, Schuurs TA et al Local renal complement C3 induction by donor brain death is associated with reduced renal allograft function after transplantation. Nephrol Dial Transplant 2011; 26:2345–54. [DOI] [PubMed] [Google Scholar]
- 5. Berger SP, Roos A, Mallat MJK, Fujita T, De Fijter JW, Daha MR. Association between mannose‐binding lectin levels and graft survival in kidney transplantation. Am J Transplant 2005; 5:1361–6. [DOI] [PubMed] [Google Scholar]
- 6. Zhou W, Farrar CA, Abe K et al Predominant role for C5b‐9 in renal ischemia/reperfusion injury. J Clin Invest 2000; 105:1363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown KM, Kondeatis E, Vaughan RW et al Influence of donor C3 allotype on late renal‐transplantation outcome. N Engl J Med 2006; 354:2014–23. [DOI] [PubMed] [Google Scholar]
- 8. Sacks SH, Karegli J, Farrar CA et al Targeting complement at the time of transplantation. Adv Exp Med Biol 2013; 735:247–55. [DOI] [PubMed] [Google Scholar]
- 9. Damman J, Hoeger S, Boneschansker L et al Targeting complement activation in brain‐dead donors improves renal function after transplantation. Transpl Immunol 2011; 24:233–7. [DOI] [PubMed] [Google Scholar]
- 10. Nielsen EW, Waage C, Fure H et al Effect of supraphysiologic levels of C1‐inhibitor on the classical, lectin and alternative pathways of complement. Mol Immunol 2007; 44:1819–26. [DOI] [PubMed] [Google Scholar]
- 11. Castellano G, Melchiorre R, Loverre A et al Therapeutic targeting of classical and lectin pathways of complement protects from ischemia‐reperfusion‐induced renal damage. Am J Pathol 2010; 176:1648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang H, Wagner E, Zhang H, Frank MM. Complement 1 inhibitor is a regulator of the alternative complement pathway. J Exp Med 2001; 194:1609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Presanis JS, Hajela K, Ambrus G, Gál P, Sim RB. Differential substrate and inhibitor profiles for human MASP‐1 and MASP‐2. Mol Immunol 2004; 40:921–9. [DOI] [PubMed] [Google Scholar]
- 14. Paréj K, Dobó J, Závodszky P, Gál P. The control of the complement lectin pathway activation revisited: both C1‐inhibitor and antithrombin are likely physiological inhibitors, while α2‐macroglobulin is not. Mol Immunol 2013; 54:415–22. [DOI] [PubMed] [Google Scholar]
- 15. Schreiber AD, Kaplan AP, Austen KF. Inhibition by C1INH of Hagemann factor fragment activation of coagulation, fibrinolysis, and kinin generation. J Clin Invest 1973; 52:1402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Agostini A, Lijnen HR, Pixley RA, Colman RW, Schapira M. Inactivation of factor XII active fragment in normal plasma. Predominant role of C1‐inhibitor. J Clin Invest 1984; 73:1542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pappalardo E, Zingale LC, Terlizzi A, Zanichelli A, Folcioni A, Cicardi M. Mechanisms of C1‐inhibitor deficiency. Immunobiology 2002; 205:542–51. [DOI] [PubMed] [Google Scholar]
- 18. Cugno M, Bos I, Lubbers Y, Hack CE, Agostoni A. In vitro interaction of C1‐inhibitor with thrombin. Blood Coagul Fibrinolysis 2001; 12:253–60. [DOI] [PubMed] [Google Scholar]
- 19. Coppola L, Guastafierro S, Verrazzo G, Coppola A, De Lucia D, Tirelli A. C1 inhibitor infusion modifies platelet activity in hereditary angioedema patients. Arch Pathol Lab Med 2002; 126:842–5. [DOI] [PubMed] [Google Scholar]
- 20. Harpel PC. Studies on human plasma alpha2‐macroglobulin‐enzyme interactions. J Exp Med 1973; 138:508–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cicardi M, Zingale LC. The deficiency of C1 inhibitor and its treatment. Immunobiology 2007; 212:325–31. [DOI] [PubMed] [Google Scholar]
- 22. Zuraw BL, Busse PJ, White M et al Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med 2010; 363:513–22. [DOI] [PubMed] [Google Scholar]
- 23. Wuillemin WA, te Velthuis H, Lubbers YT, de Ruig CP, Eldering E, Hack CE. Potentiation of C1 inhibitor by glycosaminoglycans: dextran sulfate species are effective inhibitors of in vitro complement activation in plasma. J Immunol 1997; 159:1953–60. [PubMed] [Google Scholar]
- 24. Diaz JA, Wrobleski SK, Alvarado CM et al P‐selectin inhibition therapeutically promotes thrombus resolution and prevents vein wall fibrosis better than enoxaparin and an inhibitor to von Willebrand factor. Arterioscler Thromb Vasc Biol 2015; 35:829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maugeri N, de Gaetano G, Barbanti M, Donati MB, Cerletti C. Prevention of platelet‐polymorphonuclear leukocyte interactions: new clues to the antithrombotic properties of parnaparin, a low molecular weight heparin. Haematologica 2005; 90:833–9. [PubMed] [Google Scholar]
- 26. Seelen MA, Roos A, Wieslander J et al Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. J Immunol Methods 2005; 296:187–98. [DOI] [PubMed] [Google Scholar]
- 27. Boackle RJ, Caughman GB, Vesely J, Medgyesi G, Fudenberg HH. Potentiation of factor H by heparin: a rate‐limiting mechanism for inhibition of the alternative complement pathway. Mol Immunol 1983; 20:1157–64. [DOI] [PubMed] [Google Scholar]
- 28. McKay EJ, Laurell AB, Mårtensson U, Sjöholm AG. Activation of Cl, the first component of complement, the generation of Clr‐Cls and Cl‐ inactivator complexes in normal serum by heparin‐affinity chromatography. Mol Immunol 1981; 18:349–57. [DOI] [PubMed] [Google Scholar]
- 29. Logue GL. Effect of heparin on complement activation and lysis of paroxysmal nocturnal hemoglobinuria (PNH) red cells. Blood 1977; 50:239–47. [PubMed] [Google Scholar]
- 30. Keil LB, Jimenez E, Guma M, De Bari Va. Biphasic response of complement to heparin: fluid‐phase generation of neoantigens in human serum and in a reconstituted alternative pathway amplification cycle. Am J Hematol 1995; 50:254–62. [DOI] [PubMed] [Google Scholar]
- 31. LV Galebskaia, IL Solovtsova, EV Riumina. Modification of proteolytic complement cascade after treatment with exogenous heparin. Vopr Med Khim 2001; 47:91–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1a. Percentage inhibition of classical pathway activity by C1‐INH in combination with heparin, dalteparin, nadroparin or enoxaparin. The inhibition of C5b‐9 deposition was calculated by dividing the optical density (OD) of the sample with heparinoid by the control, the sample without heparinoid or C1‐INH. Data are expressed as the mean and standard error of the mean of three experiments.
Table S1b. Percentage inhibition of lectin pathway activity by C1‐INH in combination with heparin, dalteparin, nadroparin or enoxaparin. The inhibition of C5b‐9 deposition was calculated by dividing the optical density (OD) of the sample with heparinoid by the control, the sample without heparinoid or C1‐INH. Data are expressed as the mean and standard error of the mean of three experiments.
Table S1c. Percentage inhibition of alternative pathway activity by C1‐INH in combination with heparin, dalteparin, nadroparin or enoxaparin. The inhibition of C5b‐9 deposition was calculated by dividing the optical density (OD) of the sample with heparinoid by the control, the sample without heparinoid or C1‐INH. Data are expressed as the mean and standard error of the mean of three experiments.


