Abstract
Several lines of evidence suggest aberrant immune response in schizophrenia, including elevated levels of cytokines. These cytokines are thought to be produced by activated microglia, the innate immune cells of the central nervous system. However, increase in translocator protein 18 kDa (TSPO), a marker of activated glia, has not been found in patients with chronic schizophrenia using second-generation radiotracers and positron emission tomography (PET)-based neuroimaging. In this study we focused on patients with recent onset of schizophrenia (within 5 years of diagnosis). Quantified levels of TSPO in the cortical and subcortical brain regions using the PET-based radiotracer [11C]DPA-713 were compared between the patients and healthy controls. Markers of inflammation, including interleukin 6 (IL-6), were assessed in the plasma and cerebrospinal fluid (CSF) in these participants. We observed no significant change in the binding of [11C]DPA-713 to TSPO in 12 patients with recent onset of schizophrenia compared with 14 controls. Nevertheless, the patients with recent onset of schizophrenia showed a significant increase in IL-6 in both plasma (P<0.001) and CSF (P=0.02). The CSF levels of IL-6 were significantly correlated with the levels of IL-6 in plasma within the total study population (P<0.001) and in patients with recent onset of schizophrenia alone (P=0.03). Our results suggest that increased levels of IL-6 may occur in the absence of changed TSPO PET signal in the brains of medicated patients with recent onset of schizophrenia. Future development of PET-based radiotracers targeting alternative markers of glial activation and immune response may be needed to capture the inflammatory signature present in the brains of patients with early-stage disease.
Introduction
Epidemiologic, genetic and preclinical studies support a role of early-life infection and/or altered immune response in the etiology of schizophrenia.1, 2 Perhaps related to initial insult and immune response, elevated levels of circulating cytokines, many with pro-inflammatory roles, have been repeatedly found in patients with schizophrenia, even in the early stages of disease.3, 4, 5 From these findings, a model in which interleukin 6 (IL-6) and other cytokines have detrimental effects on brain maturation and neurotransmission has been proposed.6, 7, 8 Furthermore, biological immunotherapies targeting specific cytokines such as IL-6 have been suggested as future treatment strategies.9 Nevertheless, it is unclear whether levels of peripheral cytokines reflect levels within the central nervous system (CNS), and the temporal relationship between microglial activation and exaggerated cytokine signaling in schizophrenia has not yet been studied in vivo.
Activated microglia and astrocytes in the CNS are the primary sources of cytokines during reactive, inflammatory response, although neurons and endothelial cells may serve as other sources in select conditions within certain signaling cascades.10, 11, 12 Quantitative measures of glial cell activation in the different stages of schizophrenia can be probed in vivo using radiotracers targeting the translocator protein 18 kDa (TSPO) and positron emission tomography (PET).13, 14, 15, 16, 17, 18 Importantly, TSPO expression is greatly increased in activated glial cells in states of brain injury and repair.14 Building on the improved pharmacokinetic characteristics of second-generation radiotracers over the index compound [11C]PK11195,18 two recent in vivo PET studies used second-generation radiotracers ([11C]DAA1106, [18F]FEPPA), and reported no change in binding to TSPO in the brains of patients with chronic schizophrenia.19, 20 However, within both study populations, observed variability in regional binding to TSPO suggested that increased expression of TSPO may vary within patients. Binding of [11C]DAA1106 in the brains of patients with schizophrenia correlated significantly with positive symptoms as well as duration of illness.19 However, the much larger study of patients using [18F]FEPPA PET had several methodological advantages and found no difference in regional brain binding of [18F]FEPPA in patients with active psychotic symptoms compared with matched, healthy controls.20 Finally, a third study by Bloomfield et al.21 used the second-generation radiotracer [11C]PBR28 and PET in patients with chronic schizophrenia as well as those at ultra-high risk for psychosis. There was no difference in binding of [11C]PBR28 between groups using standard kinetic analysis based on the two-tissue compartmental model. However, after applying a new kinetic model that accounts for putative binding to vasculature,22 and then normalizing regional binding to that of whole brain, a significant increase in [11C]PBR28 signal in schizophrenia and in the at-risk state was found in some gray matter regions.21 Each of these three studies included at most one or two patients in their first years of schizophrenia and therefore these studies were unable to evaluate whether TSPO in the brain was increased with hypothesized inflammatory processes in the early stages of disease.
Although results from recent PET-based imaging yield conflicting evidence of TSPO PET signal in the brains of patients, robust cytokine release early in the course of schizophrenia was recently supported by meta-analysis of peripheral cytokine abnormalities in patients, including those with first episode of psychosis (FEP).3, 4, 5 The meta-analysis by Miller et al.4 revealed significantly (P⩽0.001) higher levels of several peripheral cytokines and cytokine-response modifiers in patients with FEP over controls, including plasma levels of IL-6 (effect size (ES)=1.4), soluble interleukin 2 receptor (ES=1.03), interferon γ (IFNγ, ES=0.57), transforming growth factor β (ES=0.48), IL-1β (ES=0.6), tumor necrosis factor α (TNFα, ES=0.81) and IL-12 (ES=0.98). Meta-analysis of cytokine function in medication-naive FEP also demonstrated significant elevations in peripheral IL-1β, sIL-2r, IL-6 and TNFα.5 Some of these same cytokines were also included in two studies of cerebrospinal fluid (CSF) in FEP, although CSF levels of IL-1β were decreased (ES=−0.99, P<0.01) and CSF levels of both IL-6 and IL-12 were unchanged in these samples from unmedicated patients with FEP.4 In our own published studies of CSF from medicated patients with recent onset of schizophrenia (defined as within the first 5 years of disease) and unmedicated FEP, we showed a consistent trend of increased CSF levels of IL-6 in both patient groups compared with levels in controls.23, 24 Still, lack of larger studies of inflammatory markers in CSF of patients with FEP and recent onset of schizophrenia limit the ability to generalize findings of peripheral cytokine abnormalities to the most relevant tissues, namely those within CNS.
We recently demonstrated that PET-based neuroimaging using the second-generation radiotracer [11C]DPA-713 provided improved delivery of radiotracer to the brain and properties consistent with improved specific binding to TSPO compared with the first-generation radiotracer, [11C]PK11195.25 Importantly, we also showed that [11C]DPA-713 PET was sufficiently sensitive to detect increases in TSPO in the brains of patients with human immunodeficiency virus-associated dementia26 and those with a history of sports-related, repetitive mild traumatic brain injury.27
Here we aimed to use [11C]DPA-713 and high-resolution PET to compare binding of the radiotracer in the brains of patients with recent onset of schizophrenia to that of healthy controls who were matched in age, gender, highest educational level and body mass index. In parallel, we tested these same individuals for changes in IL-6 levels in CSF and peripheral tissue, along with other markers of peripheral immune response. Through the use of these complementary methods in vivo, we sought to characterize better the neuropathological signature of inflammation in patients with recent onset of schizophrenia.
Materials and methods
Human subjects
This study was approved by the Johns Hopkins Institutional Review Board. All the participants provided informed consent. Patients with recent onset of schizophrenia (defined as within 5 years of diagnosis) were recruited from the Johns Hopkins Medical Institutions and from hospitals in the surrounding greater Baltimore–Washington, DC area. Inclusion criteria included diagnosis of schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition after completion of diagnostic and clinical assessment administered by a board-certified psychiatrist (JMC). This assessment included the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition Axis I Disorders-Clinician Version,28 Scales for the Assessment of Positive and Negative Symptoms (SAPS and SANS)29 and the Calgary Depression Scale.30 Patients were excluded if they had (1) history of neurological disorder, structural brain abnormality or history of traumatic brain injury with loss of consciousness; (2) history of special education or known learning disability; (3) history of an inflammatory medical condition including but not limited to diabetes, human immunodeficiency virus and/or hepatitis; (4) benzodiazepine use in the past 6 months; (5) substance abuse (for example, cannabis, alcohol) in the previous 6 months or any history of substance dependence except for nicotine; (6) contraindication to participation in magnetic resonance imaging (MRI); or (7) contraindication to participation in PET including pregnancy. Summary severity scores for three dimensions of symptoms (positive, negative and disorganized) were calculated using the sums of global scores collected from the SAPS and SANS.31 Chlorpromazine equivalents were calculated based on the report by Andreasen et al.32
Healthy adults were recruited from flyers posted in the greater Baltimore–Washington, DC area and by word of mouth. All the healthy controls underwent a careful clinical interview and were without history of medical disease or surgery during the previous year. Healthy controls were excluded if they had any of the above-mentioned exclusion criteria or a Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition Axis I disorder. All subjects in both the patient and control groups were also assessed for TSPO (rs6971) genotype as previously described.26
Neuropsychological assessment
All the participants completed a 2-h battery of neuropsychological tests to assess the cognitive function in five domains, namely processing speed, verbal memory, visual memory, ideational fluency and executive function.33 Neuropsychological tests (Supplementary Table S1) were administered and scored according to standard instructions by the same study team neuropsychologist who was blind to the clinical and imaging data of all the participants. The factor scores were calculated for each domain after controlling for age, sex, race and premorbid intelligence based on a normative sample.34, 35 Premorbid intelligence was estimated using the Hopkins Adult Reading Test.36
Radiotracer synthesis
[11C]DPA-713 was synthesized by O-alkylation of its corresponding desmethyl phenolic precursor with [11C]methyl triflate in acetone.37 [11C]Methyl triflate was obtained from dry phase, high specific activity [11C]methyl iodide prepared in a General Electric MeI MicroLab (Milwaukee, WI, USA) from [11C]carbon dioxide produced in a General Electric PETtrace cyclotron by proton irradiation of a target consisting of oxygen 5% in nitrogen ultra high purity. The radiotracer was purified by reverse-phase high-performance liquid chromatography and formulated by solid phase extraction as a sterile, apyrogenic solution of 14:1 0.9% saline:ethanol. Radiochemical purity at the end of synthesis was >99% with an average specific activity of 303±118 GBq per micromole (8176±3179 mCi per micromole). The radiotracer product met all USP Chapter <823> acceptance criteria.
Brain imaging acquisition
Each participant was fitted with a thermoplastic facemask to minimize the head motion and underwent radial artery catheter insertion for repeated blood sampling. [11C]DPA-713 was delivered via an intravenous bolus injection at the onset of a 90-min dynamic list mode PET acquisition. The average injected dose was 682.3 (±24.1) MBq. Measurement of the arterial plasma input function was conducted as previously described25 through the collection of 25–35 blood samples (1 ml) over the course of each 90-min PET scan. An additional eight serial 4 ml samples were collected for radiolabeled metabolite measurements.38
The PET scans were acquired using a second-generation High Resolution Research Tomograph scanner (Siemens Healthcare, Knoxville, TN, USA), an LSO-based, dedicated brain PET system with 2.5 mm resolution. The 90 min list mode data were binned into 30 frames (four 15-s, four 30-s, three 1-min, two 2-min, five 4-min and twelve 5-min frames). The data were then reconstructed using the iterative three-dimensional ordered subset expectation maximization algorithm (with six iterations and 16 subsets), with correction for radioactive decay, dead time, attenuation, scatter and randoms.39 The attenuation maps were generated from a 6-min transmission scan performed with a 137Cs point source before the emission scan. The reconstructed image space consisted of cubic voxels, each 1.22 mm3 in size, and spanning dimensions of 31 cm × 31 cm (transaxially) and 25 cm (axially).
All the subjects also underwent brain MRI to facilitate anatomical delineation of regions of interest (ROIs) on brain PET images after PET-MRI co-registration (detailed below). T1-weighted MRIs were obtained on either a 1.5 T Signa Advantage system (GE Medical Systems, Waukesha, WI, USA) or on a Phillips Achieva 3 T scanner (Andover, MA, USA) with a 32-channel head coil to obtain a 1 × 1 × 1 mm three-dimensional MP-RAGE sequence as previously described.26
Measurement of fP
Plasma free fraction (fP) was measured using rapid equilibrium dialysis (RED). Plasma samples were isolated by centrifugation from whole blood withdrawn from each participant before radiotracer injection. A 100 μl sample of plasma was spiked with 1 μl of [3H]DPA-713 (83 Ci mmol−1; Quotient Bioresearch) and added into the sample chamber of Single-Use RED Plate with Inserts (Thermo Scientific, Rockford, IL, USA) with an 8 K molecular-weight cutoff. Then, 300 μl of PBS was added to the buffer chamber and the plate was incubated on an Incubating Microplate Shaker (Fisher Scientific, Waltham, MA, USA) at 37 °C for 4 h. Ten microliters of plasma and 30 μl of PBS were transferred to scintillation vials and mixed with 10 ml of Bio-Safe II (Research Products International, Mount Prospect, IL, USA) counting fluid. All the samples were measured in triplicate. The radioactivity was measured using an LS 6500 Multi-Purpose Scintillation Counter (Beckman Coulter, Pasadena, CA, USA) to obtain the radioactivity in the plasma (CP) and buffer (CU). The free, unbound fraction (fP) was calculated as: fP=CU/CP × 100 (%).
PET image analysis
The software package PMOD (v3.3, PMOD Technologies, Zurich, Switzerland) was used in the initial PET image processing and kinetic analysis. Inter-frame motion correction and PET-MRI co-registration were completed as previously described.27 Based on the T1-weighted MR images, automated cortical reconstruction and volumetric segmentation were performed with the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/).
Eight ROIs were selected and generated by combining the corresponding subregions provided by FreeSurfer (Supplementary Figure S1). These ROIs included six cortical regions (insula, cingulate, parietal, frontal, temporal and occipital) and two subcortical regions (hippocampus, amygdala). These ROIs were selected to represent the widespread cortical and subcortical involvement found in meta-analyses of structural reduction in brain volume even in patients with first episode of psychosis.40, 41, 42 An ROI containing the entire cortical gray matter (GM) was also defined to serve as a normalization factor in the secondary analysis of regional binding relative to GM, as discussed in the previous PET studies using [11C]R-PK-11195 (ref. 43) and [11C]DPA-713.44 Finally, total intracranial volume (ICV) was also defined using Freesurfer, for use in secondary analyses exploring the effect of ROI volume normalized to ICV on regional total distribution volume (VT).
PET time–activity curves (TACs) were generated for all the subjects using the above-mentioned ROI definitions. Based on the time–activity curves obtained, the VT within each ROI was estimated using Logan graphical analysis45 (t*=30 min) with the metabolite-corrected arterial plasma input function. Following the proposed nomenclature for reversibly binding radioligands,46 VT represents the ratio of the radioligand concentration in the brain tissue to that in the plasma at equilibrium, which is proportional to the receptor density in the defined ROI. Regional values of GM-normalized VT (GMVT) and VT corrected for plasma free fraction (VT/fP) were also calculated.
CSF and plasma acquisition
All the participants underwent lumbar puncture after informed consent. All the lumbar puncture procedures were performed at a standardized time (between 1000 h and noon) within the Johns Hopkins Hospital Outpatient Lumbar Puncture Clinic. Upon collection, CSF samples were immediately placed on ice, aliquoted into small volumes and then quickly transferred into a storage freezer at −80 °C. The plasma samples were isolated from whole-blood specimens using centrifugation as described above and were also quickly stored at −80 °C. No samples were thawed more than twice before analysis.
Measurement of cytokine levels
The plasma and CSF samples were tested for concentrations of IL1β, IFNγ, IL-10 and IL-6 using a V-Plex Custom Human Biomarkers kit (Human Proinflammatory Panel 1), and for TNFα using a Human TNFα kit (MSD (Meso Scale Discovery), Rockville, MD, USA). Each kit provided a 96-well plate pre-coated with anti-cytokine antibodies (four-spot or monospot) at the base of each well. All the reagents were provided in each kit. The control samples were reconstituted in diluent according to the manufacturer's instructions. The human plasma samples were diluted twofold and CSF samples were run undiluted. Sixty microliters of each MSD SULFO-TAG anti-human antibody (anti-IL-1β, anti-IFNγ, anti-IL-6 and anti-IL-10) were combined with the diluent. Sixty microliters of Sulfo-Tag Anti-human TNFα antibody was added to the diluent. Fifty microliters of each prepared control or sample preparation was added to each well and incubated on an orbital shaker for 2 h at room temperature. Each plate was washed three times and 25 μl of detection antibody solution was added to each well. The plates were then incubated on an orbital shaker for 2 h at room temperature, then washed three times and detection buffer was added to each well. Electrochemiluminescence of each MSD SULFO-TAG was captured and quantified using a MESO QuickPlex SQ 120 instrument. The raw data were then analyzed using the Discovery Workbench 4.0 software (MSD) by fitting signal from the control calibration curves to a four-parameter logistic model and then back-fitting the electrochemiluminescence signal from each sample to calculate the unknown concentration. The samples were run in duplicate. Calculated mean concentrations with a percentage coefficient of variance >25% were excluded from the analysis.
The plasma levels of IL-1β were below the levels of detection of this assay for almost all the samples and are not presented. In CSF, the levels of IL-1β, IFNγ, IL-10 and TNFα were often below the levels of detection of this assay. Thus, CSF levels of IL-6 are presented here.
Statistical analysis
The statistical analyses of the primary PET outcome measure, regional VT, were performed with multivariate general linear modeling using SPSS Statistics (Version 22.0, IBM, Armonk, NY, USA) so that the effects of several factors could be examined. Specifically, we modeled the VT values obtained from the eight selected ROIs based on their relationship to between-subject factors, including cohort (patient or control) and TSPO genotype (C/C: high affinity binder, C/T: mixed affinity binder) as independent fixed factors. Patients with genotype T/T were excluded from this analysis of PET data due to the low affinity of [11C]DPA-713 for TSPO in individuals with the T/T genotype. Similar analyses were repeated for regional values VT/fP.
All other statistical analyses were performed using R.47 Demographic and clinical characteristics of patients vs controls were compared using two sample t-tests for continuous variables and Fisher's exact test for categorical variables, with the threshold for significance set to P<0.05. Group differences in CSF and plasma inflammatory markers, and in GMVT, were compared using the nonparametric Mann–Whitney U-test due to the small sample size. Correlation between CSF and plasma levels of IL-6 was evaluated using Pearson's r test. Secondary multivariate analysis using linear regression was used to (1) evaluate effects of clinical characteristics on VT in GM and (2) assess the effect of ROI volume normalized to total ICV on VT in each ROI, while controlling for genotype in these analyses. Patients with genotype T/T were excluded from the multivariate analysis of PET data. The data were expressed as mean±s.d., unless otherwise noted.
The threshold for significance in all the statistical tests involving regional VT was set as P<0.006, taking into account multiple comparisons for the eight ROIs using the Bonferroni correction (0.05/8≈0.006). The threshold for significance in all the statistical tests involving the four peripheral markers tested was set as P<0.013 (≈0.05/4). Statistical significance was otherwise defined as P<0.05, except when otherwise noted.
Results
Study population
Demographic characteristics of the study population and clinical characteristics of the patients are presented in Table 1. A total of 14 patients with recent onset of schizophrenia (ages 19–30 years) and 16 healthy control subjects (ages 18–36 years) participated in this study. None of the healthy subjects were using prescribed or over-the-counter medications, with the exception of inclusion of two female participants taking an oral contraceptive. Two of the healthy controls and three of the patients were cigarette smokers. The patients and controls were well matched in age, gender and highest level of education. Two patients were non-adherent with prescribed medications and therefore were not taking antipsychotic medication in the month before the PET scan. One patient was on two second-generation antipsychotic medications and all the other patients were taking antipsychotic monotherapy. The range of chlorpromazine equivalents within the study population was 0–1119.
Table 1. Clinical and demographic characteristics.
| HC (n=16)a | SZ (n=14)a | Pb | |
|---|---|---|---|
| Age (years) | 24.9 (4.7) | 24.1 (3.1) | 0.58 |
| Gender (male) | 9 (56%) | 11 (79%) | 0.26 |
| Years of education | 13.0 (2.0) | 11.5 (2.3) | 0.08 |
| Body mass index | 24.9 (4.0) | 27.6 (4.0) | 0.08 |
| Years of disease | 2.2 (1.4) | ||
| CPZ equivalents | 474.5 (355.2) | ||
| Atypical antipsychotic (% using) | 85.7 | ||
| Typical antipsychotic (% using) | 14.3 | ||
| SAPS/SANS | |||
| Negative symptom dimension | 8.9 (4.1) | ||
| Positive symptom dimension | 3.8 (2.5) | ||
| Disorganized symptom dimension | 2.9 (2.0) | ||
| Calgary Depression Scale | 0.13 (0.34) | 3.36 (5.08) | 0.03 |
| Neurocognitive domains | |||
| Processing speed (HC/SZ: 13/12)c | 95.5 (12.1) | 79.4 (13.6) | 0.005d |
| Attention (HC/SZ: 9/7) | 104.3 (13.9) | 92.1 (15.6) | 0.12 |
| Verbal learning and memory (HC/SZ: 13/12) | 108.3 (14.7) | 81.5 (19.1) | <0.001d |
| Visuospatial memory (HC/SZ: 13/12) | 99.6 (12.3) | 81.3 (16.8) | 0.006d |
| Ideational fluency (HC/SZ: 13/12) | 105.3 (11.7) | 89.5 (15.7) | 0.01 |
| Executive function (HC/SZ: 13/12) | 102.4 (10.9) | 92.7 (17.8) | 0.12 |
Abbreviations: CPZ, chlorpromazine; HC, healthy controls; SAPS, Scale for the Assessment of Positive Symptom; SANS, Scale for the Assessment of Negative Symptom; SZ, patients with recent onset of schizophrenia.
The data are presented as N (%) or mean (s.d.).
P-values for t-test or Fisher's exact test as appropriate.
Numbers of subjects where data are available.
Indicates significance (P<0.008).
Two patients and three healthy controls did not participate in the neuropsychological testing. Testing for in the domain of attention was added after the initiation of this study and therefore five additional patients and two additional controls lack neuropsychological performance scores in the domain of attention. The patients showed significant deficits in performance in tests of processing speed, verbal learning and memory, and visuospatial memory compared with healthy controls (P<0.05/6=0.008).
ROI volumes
The quantitative analysis of the MR-based segmentation results for the eight ROIs showed no significant evidence of regional brain atrophy in the patients with recent onset of schizophrenia compared with controls after Bonferroni correction for multiple comparisons (P>0.05/8=0.006; Table 2). There were also no significant differences between patients and controls in the volume of the total gray matter and ICV.
Table 2. Comparison of measured volumes of each ROI between patients with recent onset schizophrenia and healthy controls who underwent [11C]DPA-713 PET.
| ROI | HC (n=14)a | SZ (n=12)a | Pb |
|---|---|---|---|
| Hippocampus | 8.87 (0.90) | 8.41 (0.64) | 0.143 |
| Amygdala | 3.84 (0.51) | 3.36 (0.43) | 0.015 |
| Frontal cortex | 170.70 (16.43) | 157.55 (24.83) | 0.134 |
| Temporal cortex | 101.61 (11.14) | 93.36 (16.46) | 0.158 |
| Parietal cortex | 111.96 (13.67) | 103.90 (19.30) | 0.241 |
| Occipital cortex | 45.36 (5.22) | 44.08 (7.38) | 0.620 |
| Cingulate cortex | 20.86 (2.58) | 18.25 (2.09) | 0.009 |
| Insular cortex | 29.31 (3.92) | 28.89 (4.23) | 0.794 |
| Total gray matter | 633.20 (63.45) | 602.17 (86.13) | 0.315 |
| Intracranial volume | 1454.10 (157.73) | 1434.68 (139.87) | 0.742 |
Abbreviations: HC, healthy controls; PET, positron emission tomography; ROI, region of interest; SZ, patients with recent onset of schizophrenia.
Regional volumes reported in mm3 for the patients and control subjects who underwent PET imaging.
P-values for t-tests across the eight selected ROIs, as well as the total gray matter and the total intracranial volume.
Accounting for the eight selected ROIs, the threshold for significance was P<0.05/8=0.006.
[11C]DPA-713 PET imaging
Among the 16 control subjects and 14 patients with recent onset schizophrenia, two healthy controls and two patients were found to have the T/T genotype and were excluded from the PET image analysis. Eight patients had C/C genotype and four patients had C/T genotype. Nine controls had the C/C genotype and five had the C/T genotype.
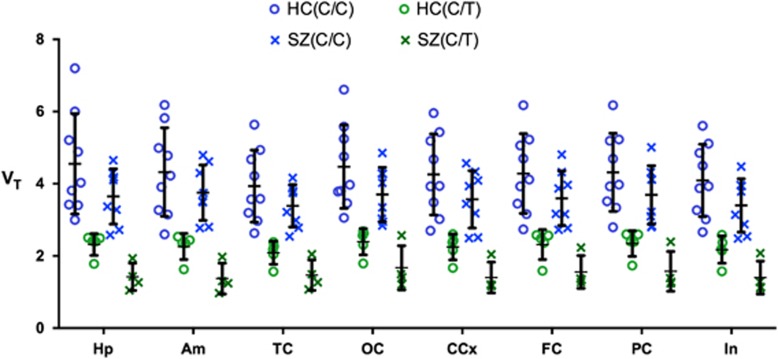
Using two-way analysis of variance with genetic group (C/C vs C/T) and cohort (patients with schizophrenia vs controls) as independent, fixed factors, VT values between patients with schizophrenia and controls were not significantly different in all ROIs tested (Figure 1, Supplementary Table S2). Use of both GMVT (Supplementary Table S3) and VT corrected for plasma free fraction (VT/fP) did not change these results. Multivariate regression analysis using data from patients and controlling for genotype showed no significant effect of ROI volume normalized to total ICV on VT in each of the eight ROIs (P>0.05/8=0.006).
Figure 1.
Scatter plot of total distribution volume values (VT) in the cortical and subcortical regions in patients with recent onset of schizophrenia (SZ) and healthy controls (HC) injected with [11C]DPA-713. Individual patient and healthy control VT data are shown along with mean values and standard deviation. Using two-way analysis of variance with genetic group (C/C vs C/T) and cohort (SZ vs HC) as independent, fixed factors, regional VT values in each of the six cortical regions (insula (In), cingulate (CCx), parietal (PC), frontal (FC), temporal (TC) and occipital (OC)) and two subcortical regions (hippocampus (Hp), amygdala (Am)) did not significantly differ. The threshold for significance after accounting for multiple comparisons was P<0.06/8=0.006.
CSF IL-6 levels
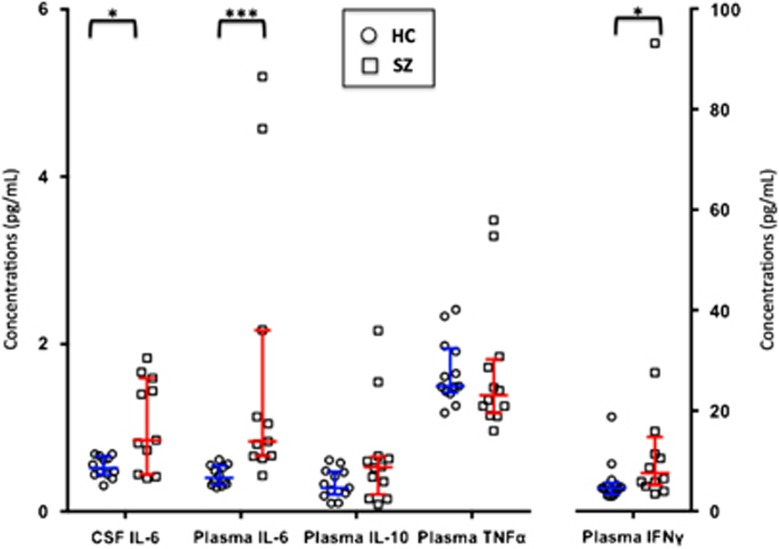
Three patients and four controls declined the lumbar puncture for provision of CSF. The concentration of IL-6 in CSF from 11 patients (interquartile range=0.59–1.52 pg ml−1, median=0.85 pg ml−1) was significantly higher than that in 12 controls (interquartile range=0.44–0.64 pg ml−1, median=0.52 pg ml−1; P=0.02).
Plasma inflammatory marker levels
The results from CSF IL-6 and plasma markers in patients with recent-onset schizophrenia and controls are shown in Figure 2. Twelve patients and 13 controls provided a plasma sample to test for peripheral markers of inflammation and immune response (IL-6, IFNγ, TNFα, IL-10). After Bonferroni correction for the four peripheral markers tested, the threshold for significance was set to P<0.013. The plasma levels of IL-6 were found to be significantly higher in the patients than in the controls (P<0.001). Plasma IFNγ was not significantly higher in patients with schizophrenia compared with controls (P=0.03). As there were two values of plasma IL-6 levels and one value of plasma IFNγ level in patients where the concentration was measured as five times that of other patients, we ran secondary analysis excluding these potential outliers. The plasma IL-6 and IFNγ levels were significantly higher in patients with recent onset of schizophrenia compared with controls after excluding these values (P=0.001 and 0.009, respectively). Plasma levels of the pro-inflammatory marker TNFα and the anti-inflammatory marker IL-10 did not differ between patients and controls.
Figure 2.
Scatter plot of inflammatory marker levels in the cerebrospinal fluid (CSF) and plasma of patients with recent onset of schizophrenia (SZ) vs healthy controls (HC). Using Mann–Whitney U-testing, the concentration of interleukin 6 (IL-6) in CSF from patients with recent onset of schizophrenia was significantly higher than that in controls (P=0.02). The plasma concentrations of IL-6 were significantly increased in patients with recent onset of schizophrenia compared with controls (P<0.05/4=0.013). Individual patient and healthy control data are shown along with median values and interquartile range. IFNγ, interferon gamma; IL-10, interleukin 10; TNFα, tumor necrosis factor alpha. *P<0.05; ***P<0.001.
Finally, the concentration of IL-6 in CSF was found to correlate with IL-6 in plasma within our total study population (r=0.70, P<0.001) and within the patient cohort alone (r=0.63, P=0.03).
Effect of IL-6 on VT
The secondary multivariate analysis using linear regression revealed no significant effect of both CSF IL-6 and plasma IL-6 on [11C]DPA-713 VT in GM in the total population after controlling for TSPO genotype (P=0.83 and P=0.21, respectively). Exploratory analyses of the effects of clinical characteristics, neurocognitive performance and levels of other plasma immune markers on [11C]DPA-713 VT in GM are presented in Supplementary Table S4. Among these results, there was only one nonsignificant and small, negative effect of chlorpromazine equivalents on VT in GM (−0.001, P=0.05).
Discussion
Converging evidence from the epidemiologic, genetic, preclinical and clinical studies of schizophrenia suggest a key role of inflammation and/or altered immune response in schizophrenia, particularly in the early stages of disease.2, 4, 5, 24, 48 Activation of microglia, the resident immune cells of the CNS, may not be necessarily deleterious and could be a normal response to independent pathologic processes. Accordingly, the characterization of glial cell activation at the onset and over the early course of disease may inform the temporal relationship to other early pathologic markers and symptomatology. PET-based imaging of TSPO offers a unique opportunity to probe for this marker of activated glial cells (microglia, astrocytes) in vivo, based on the increased expression of TSPO by activated glia in states of brain injury or repair.14, 15, 16, 17, 18, 49 We recently showed [11C]DPA-713 PET allows us to measure increases in TSPO in other neurologic diseases associated with inflammation.26, 27
Interestingly, the results from this study are inconsistent with the hypothesized increase of TSPO by activated glia in early schizophrenia. Because of the variability of the regional VT values within these patients, we also examined the results using GMVT. Briefly, the use of GMVT has been shown to be an effective empirical approach26 for eliminating genotypic differences while improving the consistency of the data (including intrasubject reproducibility and intersubject agreement among healthy controls). Still, regional GMVT values did not differ in patients relative to controls. There was also no significant difference in fP and VT/fP between patients and controls, which is important as [11C]DPA-713 free plasma fractions (~10%) are higher than those reported for other second-generation radiotracers and therefore are more accurately estimated. Finally, as atrophy of the brain has been shown even in patients with FEP,40, 41, 42, 50 we also examined the effect of ROI volume normalized to ICV on the VT in each respective ROI, but found no effect.
Our results are similar to those from PET-based neuroimaging of patients with chronic schizophrenia, in which use of [18F]FEPPA, [11C]DAA1106 and [11C]PBR28 revealed no change in regional binding (VT or BPND) of patients compared with controls.19, 20, 21 Bloomfield et al.21 found higher regional [11C]PBR28 PET signal normalized to that of whole brain in patients at ultra-high risk for schizophrenia and in chronic patients using compartmental modeling that also accounted for putative irreversible binding in vasculature (2TCM-1K).22 Investigation of this irreversible binding component in [11C]DPA-713 PET imaging is ongoing. However, as kinetic modeling of [11C]DPA-713 PET data from several healthy controls poorly fits the kinetic model proposed by Rizzo et al.22 (our unpublished data), and biological evidence for this trapping to vasculature is still lacking, we present outcome variables generated using Logan analysis consistent with previously published literature.26, 27 The consistency of our results using VT and GMVT supports the absence of higher [11C]DPA-713 PET signal in patients with early-stage disease.
It is important to note that [11C]DPA-713 PET signal is only an indirect measure of microglial activation in the living brain. Future translational directions should focus on improved methods for in vitro experiments looking for markers of activated microglia directly, in carefully selected postmortem tissue51, 52 from early-stage disease. As our patient population showed an increase in both CSF and plasma concentrations of IL-6, a cytokine released by microglia during inflammation, we cannot discount a key role of glial cell activation and immune response in recent-onset schizophrenia. Indeed, an increase in TSPO may be most robust in very early transformation of resting microglia to the activated state and may not necessarily be as marked in chronically active microglia or astrocytes.53 On the other hand, we have shown that increased binding of [11C]DPA-713 was detectable in the brains of patients with chronic human immunodeficiency virus and those with a history of remote, repeated, sports-related mild traumatic brain injury.26, 27 An alternative explanation is that lack of significantly changed TSPO, and perhaps even the downward trend in TSPO PET signal, is linked to dysfunction of mitochondria54 on which this protein is expressed, or linked to the more general, aberrant glial cell activity implicated in the pathophysiology of schizophrenia.55 These alternatives require further exploration.
One strength of this study is the careful characterization of these patients with early-stage schizophrenia, all of whom have had less than 5 years of disease and treatment. Indeed, even in this early stage of disease, we see predicted, significant deficits in neurocognitive performance. Nevertheless, as mentioned above, we cannot rule out that even the first years of diagnosis are too late along the course of disease to capture the response of activated glia. We must also consider the potential effects of prescribed antipsychotic medication on binding of [11C]DPA-713 to TSPO, which may vary by medication. For example, binding of the first-generation radiotracer, [3H]PK11195, to TSPO was significantly decreased in an ex vivo study of hippocampus from rats treated with sulpiride, thioridazine or risperidone, although binding was significantly increased in this region in rats treated with clozapine.56 Although our study population is too small to examine the effects of particular medications on binding to TSPO, chlorpromazine equivalents showed only a very small, insignificant effect on VT in GM.
Importantly, even in this population of patients, we still showed significant increases in CSF and plasma levels of IL-6, consistent with results from recent meta-analysis of peripheral cytokine changes in schizophrenia.3, 4, 5 To our knowledge, this is the first study to show that an increase in peripheral IL-6 may reflect increased levels of IL-6 in the CNS of patients with schizophrenia. It is important to note that IL-6 has pleotropic activities, and can be secreted by neurons in various states of oxidative stress,10 independent of production by microglia in the brain. This point is underscored by the lack of significant increase in several other pro-inflammatory cytokines in the plasma of patients in our study, though IFNγ levels were significantly higher in patients after the removal of an outlier in post hoc analyses. It is also possible that the origin of IL-6 in CSF is not from cells of the CNS, but from peripheral inflammatory cells. Peripheral IL-6 may enter the CNS through the choroid plexus, or through a subtle disturbance in the integrity of the blood–brain barrier.57 Irrespective of the origin of production, pathologic increase in CNS IL-6 may have detrimental, neuromodulatory effects on the hypothalamic–pituitary–adrenal axis58 and a mechanistic role in cognitive deficits seen in schizophrenia.59, 60 CNS IL-6 has been proposed as an important mediator of altered synaptic connectivity, brain structure and function in schizophrenia, implicating this cytokine as a potential target for immunotherapy.9
By demonstrating lack of significant change in binding of [11C]DPA-713 in the brains of patients compared with controls, we support previously noted absent detection of change in TSPO using other second-generation PET-based radiotracers in patients with schizophrenia. Here we extend from those studies with our focus on patients with recent onset of illness who nevertheless have both predicted cognitive deficits and elevated levels of IL-6 in plasma and CSF. Given the interest in further development of interventions targeting cytokine pathways and immune-modulation in early-stage schizophrenia, our results support the development of other PET-based radiotracers targeting alternative markers of glial activation and immune response. On combining PET results with analysis of CSF (and peripheral) tissue from the same individuals in vivo, we may be able to elucidate the molecular inflammatory signature in early-stage disease and more meaningfully inform translational application of relevant therapeutic interventions.
Acknowledgments
This work was supported by NARSAD (JMC, AS), the Alexander Wilson Schweizer Fellowship (JMC), Mitsubishi Tanabe Parma Corporation, and Silvio O. Conte Center grant MH094268. We thank the Johns Hopkins PET Center for expert provision of [11C]DPA-713.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Brown AS. Further evidence of infectious insults in the pathogenesis and pathophysiology of schizophrenia. Am J Psychiatry 2011; 168: 764–766. [DOI] [PubMed] [Google Scholar]
- Horvath S, Mirnics K. Immune system disturbances in schizophrenia. Biol Psychiatry 2014; 75: 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry 2008; 63: 801–808. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 2011; 70: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res 2014; 155: 101–108. [DOI] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol Psychiatry 2013; 73: 951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landek-Salgado MA, Faust TE, Sawa A. Molecular substrates of schizophrenia: homeostatic signaling to connectivity. Mol Psychiatry 2016; 21: 10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ, Mortensen PB, Sawa A. Schizophrenia. Lancet 2016. (in press). [DOI] [PMC free article] [PubMed]
- Girgis RR, Kumar SS, Brown AS. The cytokine model of schizophrenia: emerging therapeutic strategies. Biol Psychiatry 2014; 75: 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidin M, Bennett MV, Kessler JA. Cultured sympathetic neurons synthesize and release the cytokine interleukin 1 beta. Proc Natl Acad Sci USA 1992; 89: 10440–10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curfs JH, Meis JF, Hoogkamp-Korstanje JA. A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev 1997; 10: 742–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry 2000; 157: 683–694. [DOI] [PubMed] [Google Scholar]
- Dolle F, Luus C, Reynolds A, Kassiou M. Radiolabelled molecules for imaging the translocator protein (18 kDa) using positron emission tomography. Curr Med Chem 2009; 16: 2899–2923. [DOI] [PubMed] [Google Scholar]
- Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther 2008; 118: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching AS, Kuhnast B, Damont A, Roeda D, Tavitian B, Dolle F. Current paradigm of the 18-kDa translocator protein (TSPO) as a molecular target for PET imaging in neuroinflammation and neurodegenerative diseases. Insights Imaging 2012; 3: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wiley CA. Molecular imaging of microglia/macrophages in the brain. Glia 2013; 61: 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Matthews PM. Imaging brain microglial activation using positron emission tomography and translocator protein-specific radioligands. Int Rev Neurobiol 2011; 101: 19–39. [DOI] [PubMed] [Google Scholar]
- Chauveau F, Boutin H, Van Camp N, Dolle F, Tavitian B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging 2008; 35: 2304–2319. [DOI] [PubMed] [Google Scholar]
- Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R et al. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol 2010; 13: 943–950. [DOI] [PubMed] [Google Scholar]
- Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G et al. Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr Bull 2015; 41: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry 2016; 173: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo G, Veronese M, Tonietto M, Zanotti-Fregonara P, Turkheimer FE, Bertoldo A. Kinetic modeling without accounting for the vascular component impairs the quantification of [(11)C]PBR28 brain PET data. J Cereb Blood Flow Metab 2014; 34: 1060–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Ishizuka K, Kano SI, Edwards JA, Seifuddin FT, Shimano MA et al. Marked reduction of soluble superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with recent-onset schizophrenia. Mol Psychiatry 2013; 18: 10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes LN, Severance EG, Leek JT, Gressitt KL, Rohleder C, Coughlin JM et al. Inflammatory molecular signature associated with infectious agents in psychosis. Schizophr Bull 2014; 40: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres CJ, Pomper MG, James M, Uzuner O, Hammoud DA, Watkins CC et al. Initial evaluation of 11C-DPA-713, a novel TSPO PET ligand, in humans. J Nucl Med 2009; 50: 1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ma S, Yue C, Kim PK, Adams AV et al. Regional brain distribution of translocator protein using [(11)C]DPA-713 PET in individuals infected with HIV. J Neurovirol 2014; 20: 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Munro CA, Ma S, Yue C, Chen S et al. Neuroinflammation and brain atrophy in former NFL players: an in vivo multimodal imaging pilot study. Neurobiol Dis 2014; 74: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. Biometrics Research Department, New York State Psychiatric Institute: New York, NY, USA, 2002. [Google Scholar]
- Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry 1982; 39: 789–794. [DOI] [PubMed] [Google Scholar]
- Addington J, Cadenhead KS, Cornblatt BA, Mathalon DH, McGlashan TH, Perkins DO et al. North American Prodrome Longitudinal Study (NAPLS 2): overview and recruitment. Schizophr Res 2012; 142: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DD, Arndt S, Andreasen NC. Alogia, attentional impairment, and inappropriate affect: their status in the dimensions of schizophrenia. Compr Psychiatry 1993; 34: 221–226. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry 2010; 67: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda N, Pena J, Schretlen DJ, Sanchez P, Aretouli E, Elizagarate E et al. Hierarchical structure of the cognitive processes in schizophrenia: the fundamental role of processing speed. Schizophr Res 2012; 135: 72–78. [DOI] [PubMed] [Google Scholar]
- Parmenter BA, Testa SM, Schretlen DJ, Weinstock-Guttman B, Benedict RH. The utility of regression-based norms in interpreting the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 2010; 16: 6–16. [DOI] [PubMed] [Google Scholar]
- Testa SM, Winicki JM, Pearlson GD, Gordon B, Schretlen DJ. Accounting for estimated IQ in neuropsychological test performance with regression-based techniques. J Int Neuropsychol Soc 2009; 15: 1012–1022. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Winicki JM, Meyer SM, Testa SM, Pearlson GD, Gordon B. Development, psychometric properties, and validity of the hopkins adult reading test (HART). Clin Neuropsychol 2009; 23: 926–943. [DOI] [PubMed] [Google Scholar]
- Thominiaux C, Dolle F, James ML, Bramoulle Y, Boutin H, Besret L et al. Improved synthesis of the peripheral benzodiazepine receptor ligand [11C]DPA-713 using [11C]methyl triflate. Appl Radiat Isot 2006; 64: 570–573. [DOI] [PubMed] [Google Scholar]
- Hilton J, Yokoi F, Dannals RF, Ravert HT, Szabo Z, Wong DF. Column-switching HPLC for the analysis of plasma in PET imaging studies. Nucl Med Biol 2000; 27: 627–630. [DOI] [PubMed] [Google Scholar]
- Rahmim A, Lenox M, Reader AJ, Michel C, Burbar Z, Ruth TJ et al. Statistical list-mode image reconstruction for the high resolution research tomograph. Phys Med Biol 2004; 49: 4239–4258. [DOI] [PubMed] [Google Scholar]
- Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D et al. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry 2001; 49: 487–499. [DOI] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry 2006; 188: 510–518. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry 2006; 63: 139–149. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 2009; 50: 1801–1807. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ma S, Yue C, Kim PK, Adams AV et al. Regional brain distribution of translocator protein using [C]DPA-713 PET in individuals infected with HIV. J Neurovirol 2014; 20: 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 1990; 10: 740–747. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007; 27: 1533–1539. [DOI] [PubMed] [Google Scholar]
- R Development Core TeamR: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 2013; 18: 206–214. [DOI] [PubMed] [Google Scholar]
- Scarf AM, Kassiou M. The translocator protein. J Nucl Med 2011; 52: 677–680. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Lambe EK, Kapur S, Mikulis DJ. Cerebral gray matter volume deficits in first episode psychosis. Arch Gen Psychiatry 1998; 55: 540–546. [DOI] [PubMed] [Google Scholar]
- Schnieder TP, Dwork AJ. Searching for neuropathology: gliosis in schizophrenia. Biol Psychiatry 2011; 69: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Picker L, Morrens M, Sabbe BGC, Gentleman S, Nicoll JAR, Boche D. EPA-0759 - Multi-immunostaining for microglial activation in schizophrenia. Eur Psychiatry 2014; 29(Supplement 1): 1.24119631 [Google Scholar]
- Banati RB, Myers R, Kreutzberg GW. PK ('peripheral benzodiazepine')—binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H]PK11195 binding to activated microglia. J Neurocytol 1997; 26: 77–82. [DOI] [PubMed] [Google Scholar]
- Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M et al. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci 2012; 13: 293–307. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Steiner J, Guest PC, Dobrowolny H, Bogerts B. Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophr Res 2015; 161: 4–18. [DOI] [PubMed] [Google Scholar]
- Danovich L, Veenman L, Leschiner S, Lahav M, Shuster V, Weizman A et al. The influence of clozapine treatment and other antipsychotics on the 18 kDa translocator protein, formerly named the peripheral-type benzodiazepine receptor, and steroid production. Eur Neuropsychopharmacol 2008; 18: 24–33. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci 1995; 57: 1011–1026. [DOI] [PubMed] [Google Scholar]
- Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol 1993; 54: 1–78. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci 2008; 28: 13957–13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka T, Tamura M, Kondo MA, Sakaue M, Okada K, Takemoto K et al. Cuprizone short-term exposure: astrocytic IL-6 activation and behavioral changes relevant to psychosis. Neurobiol Dis 2013; 59: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.