Abstract
Epigenetic signatures such as methylation of the monoamine oxidase A (MAOA) gene have been found to be altered in panic disorder (PD). Hypothesizing temporal plasticity of epigenetic processes as a mechanism of successful fear extinction, the present psychotherapy-epigenetic study for we believe the first time investigated MAOA methylation changes during the course of exposure-based cognitive behavioral therapy (CBT) in PD. MAOA methylation was compared between N=28 female Caucasian PD patients (discovery sample) and N=28 age- and sex-matched healthy controls via direct sequencing of sodium bisulfite-treated DNA extracted from blood cells. MAOA methylation was furthermore analyzed at baseline (T0) and after a 6-week CBT (T1) in the discovery sample parallelized by a waiting time in healthy controls, as well as in an independent sample of female PD patients (N=20). Patients exhibited lower MAOA methylation than healthy controls (P<0.001), and baseline PD severity correlated negatively with MAOA methylation (P=0.01). In the discovery sample, MAOA methylation increased up to the level of healthy controls along with CBT response (number of panic attacks; T0–T1: +3.37±2.17%), while non-responders further decreased in methylation (−2.00±1.28% P=0.001). In the replication sample, increases in MAOA methylation correlated with agoraphobic symptom reduction after CBT (P=0.02–0.03). The present results support previous evidence for MAOA hypomethylation as a PD risk marker and suggest reversibility of MAOA hypomethylation as a potential epigenetic correlate of response to CBT. The emerging notion of epigenetic signatures as a mechanism of action of psychotherapeutic interventions may promote epigenetic patterns as biomarkers of lasting extinction effects.
Introduction
Panic disorder (PD) is an anxiety disorder characterized by sudden, unexpected attacks of intense fear and anticipatory anxiety—often comorbid with agoraphobia—and a life-time prevalence of 1-3%.1 The pathomechanism of PD is genetically complex with an estimated heritability of 48%.2 Both cognitive behavioral therapy (CBT) and pharmacotherapy are highly effective for a large proportion of patients with anxiety disorders. However, 20–40% of all patients fail to respond sufficiently to the initial treatment along with a particularly poor quality of life, higher rates of suicidal attempts and considerable socioeconomic implications.3 Therefore, there is an urgent need for a better understanding of predictors and mechanisms of action of therapeutic interventions to inform expert treatment decisions towards more personalized medicine in anxiety disorders.4
Several biological and environmental factors have been suggested to confer PD risk and to mediate treatment success and resistance, respectively. Among those, the monoamine oxidase A (MAOA), a key enzyme in the degradation of biogenic amines such as serotonin and dopamine, is to be considered one of the prime candidates on several levels: on a pharmacological level, MAO inhibitors such as phenelzine or moclobemide are effective in the treatment of PD.5 On a genetic level, the more active longer alleles of a functionally relevant 30 bp variable number tandem repeat (VNTR) in the MAOA gene (Xp11.4–p11.3) have repeatedly been found to be associated with PD, specifically in the female subgroup of patients.6, 7 In a therapy-genetic study, the more active longer MAOA alleles predicted impaired response to CBT in patients with PD.8
Epigenetic processes such as methylation of the cytosine pyrimidine ring in cytosine/guanine (CpG) dinucleotides critically influence gene expression, with methylation mainly ‘silencing' DNA transcription.9 In the first epigenetic study in PD, we discerned hypomethylation of particularly three CpG sites in exon 1/intron 1 of the MAOA gene to be associated with the disorder in female patients.10 DNA methylation—and MAOA methylation in particular—has furthermore been shown to be temporally dynamic, possibly in response to environmental influences.11 Mirroring this dynamic nature of epigenetic processes, in female patients with PD and healthy female subjects the occurrence of negative life events was associated with relatively decreased methylation.10 Given the notion of temporal epigenetic plasticity to possibly constitute a key mechanism of successful fear extinction,12, 13 studies assessing the efficacy of therapeutic interventions to reverse epigenetic risk patterns in PD are clearly warranted.
The present study attempted to replicate our previous finding of hypomethylation in PD10 in an independent case–control sample. Furthermore, applying a proof-of-concept psychotherapy-epigenetic approach we for the first time investigated MAOA methylation changes as a potential epigenetic correlate of treatment response to a standardized 6-week CBT in adult patients with PD, as well as in an independent sample of patients with PD undergoing high-dose exposure-based CBT. Given the female-specific associations of MAOA variation (VNTR)7 and MAOA methylation patterns with PD,10 as well as the X-chromosomal location of the MAOA gene entailing hemizygosity in men, we restricted this analysis to all-female samples of patients with PD. Based on our previous finding of MAOA hypomethylation as a potential risk factor of PD,10 we predicted MAOA hypomethylation to be associated with the categorical diagnosis of PD and PD severity, respectively, as well as MAOA methylation to increase and thus ‘normalize' to level of controls along with response to CBT, while non-responders would show either no alteration or even a decrease of MAOA methylation patterns.
Materials and methods
Discovery sample
Patients
Female patients with PD (N=28; age (mean±s.d.): 34.57±8.51 years) with (N=14; 50%) or without agoraphobia were recruited at the Department of Psychiatry, the University of Würzburg, Germany, within the Collaborative Research Centre SFB-TRR-58 ‘Fear, Anxiety, Anxiety Disorders' explicitly for this study. All patients were of Caucasian background for at least two preceding generations. PD diagnosis was ascertained by experienced psychiatrists and/or clinical psychologists on the basis of a structured clinical interview (SCID-I); comorbid axis I diagnoses (except bipolar disorder, psychotic disorders, current alcohol dependence, current abuse or dependence on benzodiazepines and other psychoactive substances) were allowed if PD was the primary diagnosis (depression: N=12; social anxiety disorder: N=3; specific phobias: N=1). Exclusion criteria were current or previous internal or neurological somatic illnesses, any somatic medication, illegal drugs including cannabis (assessed by urine toxicology), pregnancy and excessive alcohol (>15 glasses of alcohol per week) or nicotine (>20 cigarettes per day) use. As smoking behavior has been shown to influence MAOA methylation,14 smoking status was ascertained in detail with the total number of smoked cigarettes per day during the last 4 weeks. Nine patients were classified as smokers (32%) with a total number of 4.64±7.26 (mean±s.d.) smoked cigarettes per day. Consumption of alcohol (data available for all 28 patients; N=14 patients reported regular alcohol consumption with 0.95±1.77 (mean±s.d.) glasses per week) and caffeine (data available for 26 patients; N=20 patients reported regular caffeine consumption with 10.06±8.77 (mean±s.d.) cups per week) was documented. Nineteen patients (68%) received stable psychiatric medication at baseline (selective serotonin re-uptake inhibitors, SSRIs: N=12; selective serotonin and norepinephrine re-uptake inhibitors, SNRIs: N=2; noradrenaline and selective serotonin agonist, NaSSA: N=4; tricyclic antidepressants, TCA: N=3; pregabaline: N=2; quetiapine: N=2; zopiclone: N=1), no patient received any other medication, and medication remained unmodified during the course of CBT (see the section ‘Treatment' below). This study was approved by the ethics committee of the University of Würzburg, Germany, and was conducted according to the ethical principles of the Helsinki Declaration. Written informed consent was obtained from all participants.
Treatment
PD psychotherapy in a regular outpatient clinical setting consisted of six semi-standardized sessions over ~6 weeks according to a shortened version of the exposure-based CBT manual as applied in the ‘Mechanisms of Action for CBT' (MAC) study within the BMBF network ‘Improving the Treatment of Panic Disorder'.15 All therapists were experienced graduate or clinical psychologists having participated in a training workshop on this manual. During the study, therapists were involved in weekly supervision to maintain therapy integrity. The first three sessions were conducted within 2 to 3 weeks each lasting ~90 min and covering psychoeducational information (for example, physiological, mental and behavioral components of anxiety, vicious circle of anxiety, vulnerability–stress model). A second three-session block within the subsequent 3–4 weeks comprised interoceptive exercises (for example, hyperventilation, straw breathing running) for all patients. Those exposure exercise sessions were conducted approximately once a week and lasted 100–240 min per session. Furthermore, these sessions were followed by intensive homework adapted to the individual's particular fears of situations. Within the last session, therapeutic gains and individual plans for continued exposure exercises, as well as relapse prevention were discussed. Medicated patients (N=19; see section ‘Patients' above) were only included in the study, when medication was stable for at least 2 weeks. Pharmacological treatment remained unmodified during the course of CBT. Also, patients were instructed to keep smoking behavior constant during the time course of therapy.
Given the focus on interoceptive exposure and thus the reduction of panic attacks per se rather than the reduction of avoidance behavior as the primary target of the current short-term, proof-of-principle treatment design (six sessions in 6 weeks), the number of panic attacks per week was assessed before (T0) and after (T1) therapy as the primary indicator of disease severity and treatment response, respectively. Patients showing a decrease in the number of experienced attacks at T1 compared with T0 (T1–T0<0) were defined as responders (N=11), patients not differing in the number of panic attacks or even experiencing more attacks after CBT (T1–T0⩾0) were defined as non-responders (N=17). Responders and non-responders did not differ regarding age, MAOA VNTR genotype, smoked cigarettes per day, alcohol and caffeine consumption, medication status or comorbidity with agoraphobia, depression, social anxiety disorder and specific phobias (data not shown; all P>0.05). The Mobility Inventory (MI)—a self-report questionnaire measuring agoraphobic avoidance in specific situations with (MI-Accompanied subscale) or without (MI-Alone subscale) company of a trusted person—was ascertained as a complementary psychometric index.16
Healthy controls
The control group consisted of 28 healthy female subjects of Caucasian descent explicitly recruited for the present study and matched to the discovery patient sample by age (age (mean±s.d.): 34.96±9.02 years; T=0.17, P=0.867) and smoking status according to the number of smoked cigarettes (eight smokers with a total number of smoked cigarettes per day of 5.59±8.82 (mean±s.d.); T=0.44, P=0.663). Absence of mental axis 1 disorders was established by experienced psychologists on the basis of a SCID (Mini International Neuropsychiatric Interview (MINI)) according to the criteria of DSM-IV. The same exclusion criteria as listed for the patient sample applied to the control sample. Healthy volunteers were evaluated at T0 and—in parallel to the course of CBT in the discovery sample—after a 6-week waiting period (T1).
Replication sample
Patients
From a total sample of 154 patients included in the second multicenter (Greifswald, Münster, Würzburg, Bremen, Marburg) clinical trial of the MAC study within the BMBF network ‘Improving the Treatment of Panic Disorder',15 for 20 female patients (age (mean±s.d.): 33.55±11.15 years) with a primary diagnosis of PD with (N=14; 70%) or without agoraphobia DNA samples were available pre- and post-therapy DNA (for details see ‘DNA sampling' below). These 20 patients were thus utilized as an independent replication sample. All patients were of Caucasian descent. Diagnoses were established using a standardized computer-administered face-to-face interview (CAPI-WHO-CIDI). CIDI was administered by expert interviewers who took part in a 3-day training and a subsequent certification supervised by certified CIDI assessors of the clinical coordination center (Bremen). Inclusion criteria were: (a) a current primary DSM-IV-TR diagnosis of PD with or without agoraphobia, (b) age 18–65 years, (c) ability and availability to regularly attend treatment sessions, (d) a score ⩾4 on the Clinical Global Impression scale (CGI); comorbid axis I diagnosis (except bipolar disorder, psychotic disorders, current alcohol dependence, current abuse or dependence on benzodiazepines and other psychoactive substances) was allowed if PD with or without agoraphobia was the primary diagnosis (specific phobia: N=12; depression: N=8; social phobia: N=5; generalized anxiety disorder: N=3; obsessive-compulsive disorder: N=1). Exclusion criteria were current suicidal intent, borderline personality disorder, ongoing psychotherapeutic or psychopharmacological treatment for any mental disorder and physician-verified contraindications of exposure-based CBT (that is, severe cardiovascular, renal and neurological diseases). Again, smoking status was ascertained in detail with the total number of smoked cigarettes per day during the last 4 weeks. Ten patients were classified as smokers (50%), the mean number of smoked cigarettes per day in the overall sample was 6.10±7.82 (mean±s.d.). Given the exclusion criteria, none of the patients received any kind of drugs including psychiatric medication. Patients gave written informed consent after receiving a detailed description of the study program. The study was approved by the Ethics Committee of the German Psychological Society and was conducted according to the ethical principles of the Helsinki Declaration.
Treatment
Patients with a primary diagnosis of PD with agoraphobia received a 12-session written manualized treatment protocol focusing on in situ exposure to target avoidance behavior that was implemented over 6 weeks and was highly comparable to the protocol used in the first clinical trial of the MAC study15 with some modifications. Content, structure and doses of therapy were identical to the MAC study. Briefly, the protocol of the two different CBT variants, to which patients could be randomized, consisted of psychoeducation and an individualized behavioral analysis of the patient's symptoms and coping behavior, providing the treatment rationale for exposure and implementing interoceptive and in situ exposure exercises. The used protocol differed from the one of the first clinical trial only in the implementation of in situ exposure exercises: in both of the two possible CBT variants, the in situ exposure exercises were accompanied by the therapist. However, in one of the two therapy conditions, patients were additionally instructed to provoke bodily symptoms during the tasks (for example, by doing interoceptive exposure exercises). PD patients without comorbid agoraphobia received the same protocol except for in situ exposure exercises resulting in six therapy sessions over 3 weeks. Due to the limited sample size, we pooled all patients and analyzed the effect of therapy on MAOA methylation irrespective of treatment conditions.
In analogy to the discovery sample, responders (N=8) and non-responders (N=8) to CBT were defined according to the number of panic attacks at T1 compared with T0. However, given the focus on intensified exposure-based CBT mainly targeting avoidance behavior, the MI score—particularly suitable to indicate changes of pathological avoidance behavior in patients with PD and comorbid agoraphobia16—was chosen as the primary indicator of disease severity and treatment response, respectively.
DNA sampling
EDTA blood was collected from all patients of the discovery sample (N=28) before (baseline, T0) and directly after completing the therapeutic intervention (T1). For the healthy control sample (N=28), EDTA blood was collected at two time points parallel to the time course of the discovery sample, that is, at T0 and after a 6-week waiting period at T1. In the replication sample (N=20), DNA was available for all 20 patients at T0, for 16 of them also at T1 (post therapy) and for 10 of them at T2 (follow-up at 6 months), with DNA being available at all three time points for 6 patients. DNA was isolated using the FlexiGene DNA Kit (QIAGEN, Hilden, Germany) (discovery sample, healthy control sample) or a salting out procedure (replication sample).
MAOA methylation analysis
Aliquots of isolated DNA were treated with sodium bisulfite using the EpiTect 96 Bisulfite Kit (QIAGEN) according to the manufacturer's protocol for all samples in one batch and in randomized order to eliminate possible batch effects; see refs 10, 17 and 18.
An amplicon comprising MAOA exon 1 and parts of intron 1 (chromosome X, GRCh38.p2 Primary Assembly, NCBI Reference Sequence: NC_000023.11, 43656260–43656613) was chosen for DNA methylation analyses in analogy to previous studies on MAOA methylation10, 19 covering the CpGs most significantly associated with PD.10 The amplicon was PCR-amplified following a published protocol10 and sequenced by LGC Genomics, Berlin, Germany, on an ABI 3730XL sequencer (Life Technologies, Darmstadt, Germany).
The obtained sequences were quantitatively analyzed using the freely available Epigenetic Sequencing Methylation Software (ESME)20 as successfully applied previously to study DNA methylation profiles in mental disorders.10, 17, 18, 19, 21, 22 To account for run variability, all samples were tested in duplicate, yielding a mean individual methylation score for each CpG, as well as an individual s.d. for each duplicate. The s.d. of each duplicate was used as a first step of quality control with methylation values of duplicates with s.d.>0.1 set as missing values. In a second step, outliers (⩾3 s.d. from mean methylation of the respective CpG site) were defined as missing data. A cutoff of >20% of missing data was defined as an exclusion criterion. No participant had to be excluded from the reported analyses when applying this quality control criterion; cf, ref. 23.
Electropherograms were robustly readable for 13 CpG sites in patients of the discovery sample, as well as in the healthy control sample matched to the discovery sample (CpGs 1–13), and 12 CpG sites in the replication sample (CpGs 2–13), respectively. CpGs were numbered in analogy to a previous study on MAOA methylation in PD:10 CpG1=43,656,316; CpG2=43,656,327; CpG3=43,656,362; CpG4=43,656,368; CpG5=43,656,370; CpG6=43,656,383; CpG7=43,656,386; CpG8=43,656,392; CpG9=43,656,398; CpG10=43,656,427; CpG11=43,656,432; CpG12=43,656,514; CpG13=43,656,553. All non-template controls were negative. Fully methylated and non-methylated DNAs (Human Methylated & Non-methylated DNA Set, Zymo Research, Freiburg im Breisgau, Germany) were used as controls for complete bisulfite conversion.
According to published protocols with minor modifications,10 all patients and controls of the discovery sample were genotyped for the MAOA VNTR (patients: 3/3: N=3; 3/4: N=14; 3a(3.5)/4: N=1; 4/4: N=8, data missing for two patients; controls: 3/3: N=3; 3/4: N=13; 3a(3.5)/4: N=1; 3/5: N=2; 4/4: N=7, data missing for two controls). When grouping the sample into to low (33/34/3a4/35: N=18 patients; N=19 controls) and high expression (44/45: N=8 patients, N=7 controls) genotype groups according to previous studies,10, 19 patients and controls did not differ in genotype group distribution (P=1.000; Fisher's exact test).
Statistical analysis
Differences in baseline methylation between PD patients of the discovery sample and matched controls were tested using mixed linear models for repeated measures; cf, ref. 24, with MAOA methylation as within factor and group (PD patients vs healthy controls) as between factor with the number of smoked cigarettes as covariate; cf, ref. 14, followed by univariate analysis of variance (ANOVA), again controlled for the number of smoked cigarettes (see legend of Table 1). Associations between methylation and number of panic attacks and MI score at baseline, were evaluated by Pearson's correlations.
Table 1. MAOA methylation in the discovery sample of patients with panic disorder and healthy controls at baseline (T0).
| Patients (N=28) M (s.e.) | Controls (N=28) M (s.e.) | P-value | 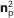 |
|
|---|---|---|---|---|
| Average methylation | 0.404 (0.005) | 0.435 (0.007) | <0.001*** | 0.282 |
| CpG1 | 0.341 (0.008) | 0.369 (0.011) | 0.033* | 0.121 |
| CpG2 | 0.326 (0.006) | 0.358 (0.010) | 0.019* | 0.138 |
| CpG3 | 0.343 (0.007) | 0.387 (0.011) | <0.001*** | 0.275 |
| CpG4 | 0.395 (0.008) | 0.420 (0.007) | 0.049* | 0.108 |
| CpG5 | 0.268 (0.008) | 0.281 (0.011) | 0.169 | 0.065 |
| CpG6 | 0.321 (0.008) | 0.364 (0.008) | <0.001*** | 0.250 |
| CpG7 | 0.414 (0.005) | 0.450 (0.006) | <0.001*** | 0.314 |
| CpG8 | 0.288 (0.007) | 0.330 (0.010) | 0.004** | 0.190 |
| CpG9 | 0.442 (0.006) | 0.478 (0.006) | 0.001*** | 0.238 |
| CpG10 | 0.461 (0.005) | 0.485 (0.007) | 0.013* | 0.152 |
| CpG11 | 0.317 (0.013) | 0.293 (0.010) | 0.233 | 0.053 |
| CpG12 | 0.870 (0.009) | 0.910 (0.008) | <0.001*** | 0.374 |
| CpG13 | 0.473 (0.010) | 0.553 (0.015) | <0.001*** | 0.369 |
Abbreviations: ANOVA, analysis of variance; VNTR, variable number tandem repeat.
Age, MAOA VNTR genotype, comorbidity with agoraphobia or depression and medication did not influence average MAOA methylation at baseline (T0; all P>0.05) in PD patients. However, the number of smoked cigarettes per day correlated inversely with average MAOA methylation (r=−0.379, P=0.047). In the control sample, age and MAOA VNTR genotype did not influence average MAOA methylation (all P>0.05). Again, the number of smoked cigarettes per day correlated inversely with baseline MAOA methylation at CpGs 3, 12 and 13 (r=−0.540 to −0.400, all P<0.05). Thus, all analyses at T0 were corrected for the number of smoked cigarettes per day. P-value (average methylation) from mixed linear model with number of smoked cigarettes as covariate; P-values (single CpG sites) from univariate ANOVA corrected for number of smoked cigarettes. *Significant at P<0.05; **significant at P<0.01; ***significant at P<0.001; bold=significant after Bonferroni correction for multiple testing.  , partial eta squared is reported as an estimate of effect size.
, partial eta squared is reported as an estimate of effect size.
To analyze potential dynamics in absolute methylation during therapy irrespective of treatment response, repeated measures ANOVAs with assessment time points (discovery sample: T0 vs T1; replication sample: T0 vs T1 vs T2) as within-subject variables were conducted. To evaluate possible dynamics in methylation within the healthy controls sample, repeated measures ANOVAs with two time points (T0 vs T1) as within-subject variables were calculated.
Percentage methylation change (T1–T0 in percent of T0: [T1−T0]/T0 × 100) dependent on responder status (see section ‘Treatment' above) was evaluated and controlled for baseline methylation where applicable using univariate ANOVA for average methylation, as well as for individual CpG sites. Baseline methylation differed significantly between therapy responders and non-responders at CpGs 6 (P=0.044) and 12 (P=0.026) only and were thus not included as a covariate in further analyses regarding these two CpG sites. To calculate differences in MAOA methylation between healthy controls and responders/non-responders at baseline (T0) and T1, respectively, multivariate ANOVAs were used. Associations between percentage methylation change (T1–T0 in percent of T0, see above) and MI score change (T1–T0) during therapy were evaluated by Pearson's correlations conducted for average methylation, as well as for individual CpG sites. For the discovery sample, post hoc Bonferroni correction for multiple comparisons regarding percentage methylation change at individual CpG sites (N=13) set the significance level to P⩽0.004. Given a confirmatory approach, no Bonferroni correction was applied to analyses in the replication sample. All data were normally distributed and assumption of equality of variances was met. All tests were carried out two-sided and an alpha-level of <0.05 was considered significant.
Results
Discovery sample
Average MAOA baseline methylation and methylation at the individual 13 CpGs in the discovery sample, as well as in the control sample are given in Table 1.
Case–control association study
Mixed linear models for repeated measures revealed that MAOA methylation differed significantly between PD patients and controls (P<0.001), with decreased average methylation in PD patients compared with healthy controls (P<0.001). Follow-up univariate tests revealed lower methylation in PD patients than in healthy controls at CpG sites 1–4, 6–10, 12 and 13 with P-values ranging from 0.049 to <0.001 (for details see Table 1). After Bonferroni correction for multiple testing, association of average MAOA hypomethylation, as well as hypomethylation at CpGs 3, 6–9, 12 and 13 with PD remained significant (see Table 1).
Baseline MAOA methylation and PD severity
In the patient group, at T0 a negative correlation between MAOA methylation and the number of panic attacks was discerned at CpG4 (r=−0.486, P=0.010). The MI-Alone subscale score at baseline correlated negatively with average baseline methylation (r=−0.519, P=0.005) and with baseline methylation at CpG3 (r=−0.408, P=0.035), CpG4 (r=−0.501, P=0.008), CpG6 (r=−0.491, P=0.009), CpG7 (r=−0.432, P=0.024) and CpG8 (r=−0.585, P=0.001). Also, MI-Accompanied scores correlated inversely with average baseline methylation (r=−0.470, P=0.013) and baseline methylation at CpG4 (r=−0.522, P=0.005), CpG6 (r=−0.387, P=0.046), CpG8 (r=−0.416, P=0.031), CpG12 (r=−0.405, P=0.036) and CpG13 (r=−0.450, P=0.018).
MAOA methylation change during treatment
In the overall patient group irrespective of responder/non-responder status, as well as in the control group, MAOA methylation did not change significantly from T0 to T1 for average methylation or at any individual CpG site (all P>0.05).
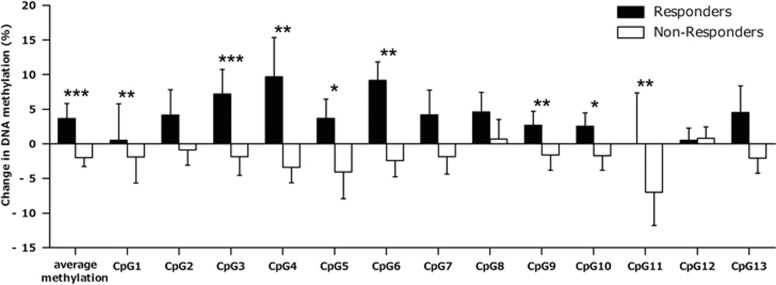
In the main analyses regarding percentage methylation change in the patient sample dependent on responder status according to the number of panic attacks, responders displayed an increase in average methylation after therapy (mean change±s.e., 3.37±2.17%), while non-responders decreased in average methylation (mean change±s.e., −2.00±1.28% P=0.001). This pattern held true for 8 out of 13 single CpG sites, with the results for average methylation and methylation at CpGs 3, 4, 6 and 11 withstanding correction for multiple testing (see Table 2 and Figure 1).
Table 2. MAOA methylation change (% of T0; T1–T0) for responders and non-responders to CBT in the discovery sample of patients with panic disorder.
| Responders (N=11)a M (s.e.) (%) | Non-responders (N=17)a M (s.e.) (%) | P-value | 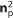 |
|
|---|---|---|---|---|
| Average methylation | 3.37 (2.17) | −2.00 (1.28) | 0.001*** | 0.415 |
| CpG1 | 0.51 (5.28) | −1.90 (3.75) | 0.005** | 0.341 |
| CpG2 | 4.15 (3.69) | −0.87 (2.23) | 0.087 | 0.178 |
| CpG3 | 7.22 (3.53) | −1.85 (2.70) | 0.001*** | 0.446 |
| CpG4 | 9.68 (5.67) | −3.40 (2.23) | 0.003** | 0.365 |
| CpG5 | 3.70 (2.77) | −4.08 (3.82) | 0.040* | 0.228 |
| CpG6 | 9.18 (2.65) | −2.42 (2.33) | 0.003** | 0.286 |
| CpG7 | 4.19 (3.58) | −1.85 (2.54) | 0.098 | 0.170 |
| CpG8 | 4.60 (2.84) | 0.69 (2.84) | 0.085 | 0.179 |
| CpG9 | 2.68 (2.04) | −1.62 (2.19) | 0.009** | 0.313 |
| CpG10 | 2.57 (1.91) | −1.70 (2.12) | 0.027* | 0.251 |
| CpG11 | 0.01 (7.34) | −6.99 (4.79) | 0.002** | 0.390 |
| CpG12 | 0.50 (1.77) | 0.82 (1.62) | 0.898 | 0.001 |
| CpG13 | 4.54 (3.86) | −2.06 (2.21) | 0.276 | 0.098 |
Abbreviations: ANOVA, analysis of variance; T0, baseline; T1, post cognitive behavioral therapy; VNTR, variable number tandem repeat.
No influence of age, MAOA VNTR genotype group, comorbidity with agoraphobia or depression, medication or smoking behavior were detected on percentage average MAOA methylation change (T1–T0). However, methylation at T0 influenced average MAOA methylation change (r=−0.545, P=0.003), necessitating control for methylation at T0 for analyses regarding methylation dynamics. *Significant at P<0.05; **significant at P⩽0.01; ***significant at P⩽0.001; bold=significant after Bonferroni correction for multiple testing. 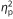 , partial eta squared is reported as an estimate of effect size.
, partial eta squared is reported as an estimate of effect size.
Definition of responders/non-responders, see section ‘Treatment' under ‘Patients and Methods' P-values from univariate ANOVA controlled for baseline methylation of the respective CpG site.
Figure 1.
MAOA methylation change (% of T0; T1–T0) in responders and non-responders to CBT. Change in MAOA methylation (%) from baseline (T0) to post cognitive behavioral therapy (CBT; T1) in the discovery sample stratified for responders (N=11; black bars, mean) and non-responders (N=17; white bars, mean) for average methylation, as well as at single CpG sites (error bars: ±s.e.). For definition of responders/non-responders see section ‘Treatment' under ‘Patients and Methods'. *Significant at P<0.05; **significant at P⩽0.01; ***significant at P⩽0.001.
This ‘normalization' of MAOA methylation patterns in treatment responders was furthermore visible when comparatively assessing absolute MAOA methylation in responders/non-responders and healthy controls at T0 and T1, respectively: While—as expected—baseline average MAOA methylation differed significantly between healthy controls (mean±s.e., 0.435±0.007) and both responders (mean±s.e., 0.405±0.005; P=0.015) and non-responders (mean±s.e., 0.404±0.008; P=0.001), after 6 weeks of waiting time or CBT, respectively, average MAOA methylation did not differ anymore between healthy controls (mean±s.e., 0.432±0.005) and therapy responders (mean±s.e., 0.418±0.007; P=0.148), but remained significantly different for the contrast healthy controls vs non-responders (mean±s.e., 0.395±0.007; P<0.001).
In an explorative approach, an inverse correlation between changes in MI-Alone score and methylation change at CpG12 was discerned in the patient sample (r=−0.431, P=0.022).
Replication sample
Average MAOA baseline methylation across all 12 CpGs readable in the replication sample (see see section ‘MAOA methylation analysis' under ‘Materials and Methods') was 0.39±0.06 (CpG2: mean±s.d., 0.44±0.07; CpG3: mean±s.d., 0.41±0.07; CpG4: mean±s.d., 0.35±0.09; CpG5: mean±s.d., 0.25±0.09; CpG6: mean±s.d., 0.27±0.10; CpG7: mean±s.d., 0.37±0.09; CpG8: mean±s.d., 0.25±0.08; CpG9: mean±s.d., 0.43±0.09; CpG10: mean±s.d., 0.41±0.07; CpG11: mean±s.d., 0.17±0.11; CpG12: mean±s.d., 0.90±0.09; CpG13: mean±s.d., 0.49±0.08).
Baseline MAOA methylation and PD severity
Prior to therapy (T0), no significant correlations between baseline MAOA methylation and the number of panic attacks were discerned (N=20; all P⩾0.05). However, high agoraphobic avoidance as assessed by the MI-Accompanied subscale at T0 was significantly associated with relatively decreased methylation at CpG4 (r=−0.46, P=0.047), CpG7 (r=−0.51, P=0.026), CpG8 (r=−0.55, P=0.014), CpG10 (r=−0.52, P=0.023) and CpG12 (r=−0.51, P=0.027), mirrored by a trend-wise negative correlation between avoidance and average methylation across all CpG sites (r=−0.43, P=0.064). In addition, high MI-Alone subscale scores were trend-wise associated with decreased methylation at CpG8 (r=−0.44, P=0.061).
MAOA methylation change during treatment
In the overall patient group irrespective of responder/non-responder status, MAOA methylation did not change significantly from T0 to T1 for average methylation or at any individual CpG site (N=16; all P>0.05). Accordingly, in those six patients with data available at all three time points no significant change in methylation from T0 to T2 was observed (all P>0.05).
Responders and non-responders defined according to the number of panic attacks did not display differences in methylation dynamics (all P>0.05). However, a reduction of MI-Accompanied subscale scores after therapy mostly went along with an increase in methylation as indexed by percentage increase from T0 to T1 for average methylation (r=−0.42), as well as for methylation at the 12 individual CpGs (r=−0.01–-0.57). This correlation between treatment response and increase in methylation reached statistical significance for methylation at CpG4 (r=−0.56, P=0.025), CpG7 (r=−0.55, P=0.027), CpG8 (r=−0.55, P=0.029), CpG9 (r=−0.55, P=0.028), CpG10 (r=−0.54, P=0.030) and CpG12 (r=−0.57, P=0.020), as well as trend-wise significance for CpG6 (r=−0.48, P=0.062). No significant results were observed for the MI-Alone subscale (all P>0.05).
Discussion
MAOA hypomethylation was discerned to be associated with PD. Accordingly, PD severity was inversely correlated with MAOA methylation at baseline. In a psychotherapy-epigenetic approach, responders and non-responders to a 6-week standardized CBT as defined by the number of panic attacks showed differential dynamics of MAOA methylation during the course of treatment: response was associated with a significant increase in MAOA methylation up to the level of healthy controls, while non-response rather went along with a further decrease in MAOA methylation. Providing approximate replication of this finding, the same direction of methylation patterns and dynamics correlating with PD severity and treatment response, respectively, was identified for agoraphobic avoidance in an independent sample of unmedicated patients with PD.
The present result of MAOA hypomethylation to be associated with the categorical phenotype of PD, as well as with disease severity is in line with our previous observation of MAOA hypomethylation in PD patients,10 providing converging evidence for MAOA hypomethylation as an epigenetic PD risk pattern. Our main finding suggests dynamic changes in MAOA methylation as a potential correlate of response to CBT already visible after 6 weeks of therapy and thus reversibility of an epigenetic risk factor by psychotherapy. This is highly intriguing as it supports and specifies the emerging notion of epigenetically driven neuroplasticity underlying response to extinction-related psychotherapeutic interventions in anxiety disorders,12 already foreshadowed by Kandel's dictum ‘insofar as psychotherapy is successful in bringing about substantive changes in behavior, it does so by producing alterations in gene expression'.25
As in a functional in vitro assay decreased methylation has been shown to activate MAOA expression,26 MAOA hypomethylation might result in a decreased availability of monoamines in the synaptic cleft and thereby confer an increased risk for PD. This concept is supported by evidence from a positron emission tomography (PET) study using [(11)C]clorgyline showing an inverse relation between MAOA methylation and MAOA levels in vivo.27 Against this background and provided confirmation in future functional studies, it could be hypothesized that successful CBT reinstates functional monoamine levels by increasing MAOA methylation with subsequently decreased MAOA expression. This notion extends PET studies reporting changes in serotonin levels in patients with major depressive disorder after psychotherapy partly correlating with clinical improvement,28 by suggesting epigenetic processes as a possible mechanistic link.
The present study adds to the recently burgeoning body of evidence for epigenetic mechanisms possibly constituting dynamic biological correlates of therapeutic interventions. In this respect, our results suggesting reversibility of MAOA hypomethylation by CBT complement a previous study by Roberts et al.29 reporting increases in 5-HTT gene methylation to correlate with remission status after CBT in a sample of children with mixed primary anxiety disorder diagnoses. To the best of our knowledge, so far only four other studies have investigated the dynamics of epigenetic processes as a potential correlate of treatment response in mental disorders or related animal-model phenotypes: In rats with a depression-like phenotype (flinders sensitive line), P11 gene hypermethylation was found to be reversible by antidepressant treatment with escitalopram.30 In patients with major depression, Lopez et al.31 discerned a significant decrease in histone H3 lysine 27 trimethylation (H3K27me3) levels at promoter-IV of the BDNF gene along with response to citalopram after 8 weeks of treatment. In patients with borderline personality disorder, initially increased BDNF gene methylation decreased during a 4-week course of intensive dialectical behavior therapy along with therapy response.32 Finally, Yehuda et al.33 reported FKBP5 methylation to decrease in association with responder status after a 12-week psychotherapy in combat veterans with post-traumatic stress disorder. Given that presently no changes in MAOA methylation were discerned in age- and sex-matched healthy probands when applying a control study design paralleling the time course of psychotherapy in the discovery patient sample, it can be assumed that the changes in MAOA methylation discerned in patients are due to psychotherapy effects. The present observation of non-response to CBT to be accompanied by a further decrease in MAOA methylation underlines some reports of temporary symptom exacerbation during exposure therapy, for example, in a minority of patients with post-traumatic stress disorder particularly when only considering the very early-treatment stages, for example refs. 34 and 35, and suggests MAOA methylation dynamics as a potential epigenetic correlate.
Despite several strengths such as high clinical and demographic homogeneity of the present patient sample, strict inclusion/exclusion criteria, recruitment of a matched healthy control sample, consideration of several potential confounders of epigenetic processes, survival of our main results after conservative Bonferroni correction, as well as the attempt of a replication in an independent sample, the present findings have to be interpreted in the light of some limitations and remarks: owed to the proof-of-concept design, strict inclusion/exclusion criteria and inclusion of female patients only due to the X-chromosomal location of the MAOA gene, sample sizes—particularly of the replication sample, which was not explicitly recruited for this purpose—are relatively small warranting further replication in larger samples including male patients, as well as applying a follow-up design. An a priori calculation of the required total sample size was hindered by the fact that the only published study regarding the relationship between changes in methylation patterns and psychotherapy response in anxiety disorders suggesting medium effect sizes was not fully comparable to the present study given a different study population (children), heterogeneity of anxiety diagnoses (generalized anxiety disorder: N=56, specific phobia: N=20, separation anxiety disorder: N=16, social anxiety disorder: N=14, obsessive-compulsive disorder: N=9, PD/agoraphobia: N=1), a different response criterion and a different gene of interest (5-HTT; SERT).29 However, the presently observed medium to large effect sizes (cf, Tables 1 and 2) suggest sufficient power to detect these effects. Second, focus and intensity of CBT in the discovery and the replication sample were not fully comparable (discovery sample: 6 sessions in 6 weeks, focus on interoceptive exposure; replication sample: 12 sessions in 6 weeks, focus on in situ exposure). Also, the clinical composition of the samples regarding comorbidity with agoraphobia was different (discovery sample: 50% replication sample: 70%). Against this background, it is remarkable that the observed methylation changes as a potential correlate of treatment response applied to different response criteria, that is, panic attacks per se (discovery sample) and avoidance behavior as measured by the MI score (replication sample), potentially reflecting differences in treatment focus and intensity and/or comorbidity rates between the two samples. Thus, replication of the present finding in the current design is to be considered approximate and warrants further evaluation in large independent samples exhibiting a highly parallelized clinical design. Medication with antidepressants might influence methylation status on the one hand, for example ref. 30, and has been shown to potentially aggravate PD symptoms during uptitration of the drug on the other hand.36 However, as in the discovery sample (i) all patients receiving psychotropic medication were stable on a clinically sufficient dose of the respective medication for at least 2 weeks before inclusion into the study, (ii) medication was not changed during CBT, (iii) baseline MAOA methylation differed between medicated and non-medicated patients at one single CpG only and most importantly did not statistically impact methylation change during therapy, and (iv) patients in the replication sample were entirely medication-free, this was probably not a major confounding factor in the present study. Smoking as another possible confounder of the present results—given its known role as a modulator of MAOA methylation14 and as a potential causal factor/facilitator in the development of PD37—has been controlled for on several levels: (i) smoking status was ascertained in detail in both the discovery and the replication sample, (ii) patients were instructed to keep smoking behavior constant during the time course of therapy, (iii) the control group was matched to the discovery patient sample according to smoking status, and (iv) all tests regarding baseline methylation were controlled for smoking behavior. Technically, DNA methylation was measured in blood samples entailing cell-composition effects as a possible confounder. Finally, investigation of epigenetic patterns in peripheral biomaterial such as blood does not allow for direct conclusions regarding methylation correlates in brain tissue.10 However, a recent PET study reporting peripheral MAOA methylation in leukocytes to inversely correlate with brain MAOA levels suggests MAOA methylation in blood as a viable sensor for central processes.27 In general, the role of MAOA methylation in the pathogenesis and/or treatment response mediation in PD needs to be confirmed by epigenome-wide association studies.
In summary, the present psychotherapy-epigenetic study adds to previous evidence for MAOA hypomethylation as a risk marker of PD and for the first time suggests MAOA methylation changes as a potential epigenetic correlate of treatment response to CBT in PD patients. Given robust replication of the present results, MAOA methylation pattern as an accessible biomarker of PD risk may aid in developing resilience-increasing preventive measures for epigenetically defined high-risk groups. In addition, the emerging notion of epigenetic signatures as a core mechanism of action of response to psychotherapeutic interventions is hoped to contribute to the development of a more personalized and thus more effective treatment of PD. For instance, along the lines of an individualized treatment augmentation approach, MAO inhibitors could be probed as an adjunct to psychotherapy in PD patients displaying MAOA hypomethylation. On a more general note, the present findings might contribute to the promotion of psychotherapeutic or pharmacological options inducing epigenetics changes for lasting extinction effects.
Acknowledgments
The present project was supported by the German Research Foundation (DFG), Collaborative Research Centre ‘Fear, Anxiety, Anxiety Disorders' SFB-TRR-58, projects C02 (to KD, K-PL and JD), Z02 (to JD, AR and PP) and A05 (to K-PL), and the German Ministry of Research and Education (BMBF, 01EE1402A, PROTECT-AD, P5 to KD and JD). Patients of the replication sample were obtained from the German multicenter trial: mechanisms of CBT-treatment effects in patients with panic disorder and panic disorder with agoraphobia: the role of interoceptive exposure and fear augmentation (MCBT-PDAS). The study was funded by the German Federal Ministry of Education and Research (BMBF, 01GV0614) as part of the larger BMBF Psychotherapy Research Funding Initiative Improving the Treatment of Panic Disorder. Centers of the multicenter trial: Principal investigators (PI) with respective areas of concentration of the MCBT-PDAS are Alfons Hamm (Greifswald: PI Psychophysiology); Thomas Lang (Bremen: Study Director for the Randomized Clinical Trial (RCT) and Manual Development); Alexander L. Gerlach (Münster: PI Panic subtypes); Christiane Pané-Farré (Greifswald: PI Psychophysiology and Panic Disorder); Tilo Kircher (Marburg: PI for functional neuroimaging) and Jürgen Deckert (Würzburg: PI for Genetics). Additional site directors in the RCT component of the program are Winfried Rief (Marburg), and Paul Pauli (Würzburg). Centers of the Research Network: Volker Arolt (Münster: Overall Network Coordination), Hans-Ulrich Wittchen (Dresden) and Andreas Ströhle (Berlin). Data access and responsibility: all principle investigators took responsibility for the integrity of the respective study data and their components. All authors and co-authors had full access to all study data. Data analysis and manuscript preparation were completed by the authors and co-authors of this article, who took responsibility for its accuracy and content. Acknowledgments and staff members by site: Bremen (coordinating center for the multicenter trial): Veronika Bamann, Sandra Cammin, Sarah Czilwik, Kira Geisler, Sylvia Helbig-Lang, Kirsten Helmes, Anne Kordt, Tanja Leonhard, Mila Plett-Perelshteyn, Christian Soltau, Juliane Sülz, Maxie von Auer; Greifswald (coordinating site for psychophysiology): Anett Hoffmann, Jan Richter; Mannheim (coordinating center for ambulatory assessment): Christoph Biwer, Elisabeth Borgmann, Antje Gerdes, Otto Martin, Kristina Steinbach, Bettina Stemmler, Andrew White; Marburg (coordinating center for functional neuroimaging): Tobias Fehlinger, Andreas Jansen, Nikita Jegan, Carsten Konrad, Marion Mickeler, Silke Rusch, Katrin Schlötterer, Benjamin Straube, Mareike Stumpenhorst, Katrin Wambach, Yunbo Yang; Münster (coordinating site for panic subtypes): Susanne Kettler, Anna Vossbeck-Elsebusch; Würzburg Psychiatry Department (coordinating center for genetics): Carola Gagel, Andreas Reif, Heike Weber; Würzburg Psychology Department: Almut Friedl-Huber, Harald Krebs, Caroline Ott, Nina Steinhäuser; additional support was provided by the coordinating center for clinical studies in Dresden (KKS Dresden): Marko Käppler. The RCT was approved by the Ethics Committee of the German Psychological Society (DGPs, AH11.2009). The study was registered with the NCT01323556. The funding organizations had no role in the design and conduct of the study, in the collection, analysis and interpretation of the data, or in the preparation, review or approval of the manuscript. We also acknowledge the skillful technical support by Carola Gagel.
The authors declare no conflict of interest.
References
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 2011; 21: 655–679. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry 2001; 158: 1568–1578. [DOI] [PubMed] [Google Scholar]
- Bystritsky A. Treatment-resistant anxiety disorders. Mol Psychiatry 2006; 11: 805–814. [DOI] [PubMed] [Google Scholar]
- Turecki G, Ota VK, Belangero SI, Jackowski A, Kaufman J. Early life adversity, genomic plasticity, and psychopathology. Lancet Psychiatry 2014; 1: 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller JW, Bouwer C, Behnke K. Moclobemide for anxiety disorders: a focus on moclobemide for panic disorder. Int Clin Psychopharmacol 1997; 12: 27–30. [PubMed] [Google Scholar]
- Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D et al. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet 1999; 8: 621–624. [DOI] [PubMed] [Google Scholar]
- Reif A, Weber H, Domschke K, Klauke B, Baumann C, Jacob CP et al. Meta-analysis argues for a female-specific role of MAOA-uVNTR in panic disorder in four European populations. Am J Med Genet B Neuropsychiatr Genet 2012; 159B: 786–793. [DOI] [PubMed] [Google Scholar]
- Reif A, Richter J, Straube B, Höfler M, Lueken U, Gloster AT et al. MAOA and mechanisms of panic disorder revisited: from bench to molecular psychotherapy. Mol Psychiatry 2014; 19: 122–128. [DOI] [PubMed] [Google Scholar]
- Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 2012; 13: 484–492. [DOI] [PubMed] [Google Scholar]
- Domschke K, Tidow N, Kuithan H, Schwarte K, Klauke B, Ambrée O et al. Monoamine oxidase A gene DNA hypomethylation—a risk factor for panic disorder? Int J Neuropsychopharmacol 2012; 15: 1217–1228. [DOI] [PubMed] [Google Scholar]
- Wong CC, Caspi A, Williams B, Craig IW, Houts R, Ambler A et al. A longitudinal study of epigenetic variation in twins. Epigenetics 2010; 5: 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford JM, Lattal KM. Is an epigenetic switch the key to persistent extinction? Neurobiol Learn Mem 2011; 96: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle N, Singewald N. HDAC inhibitors as cognitive enhancers in fear, anxiety and trauma therapy: where do we stand? Biochem Soc Trans 2014; 42: 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert RA, Beach SR, Gunter TD, Brody GH, Madan A, Gerrard M. The effect of smoking on MAOA promoter methylation in DNA prepared from lymphoblasts and whole blood. Am J Med Genet B Neuropsychiatr Genet 2010; 153B: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloster AT, Wittchen HU, Einsle F, Lang T, Helbig-Lang S, Fydrich T et al. Psychological treatment for panic disorder with agoraphobia: a randomized controlled trial to examine the role of therapist-guided exposure in situ in CBT. J Consult Clin Psychol 2011; 79: 406–420. [DOI] [PubMed] [Google Scholar]
- Chambless DL, Caputo GC, Jasin SE, Gracely EJ, Williams C. The mobility inventory for agoraphobia. Behav Res Ther 1985; 23: 35–44. [DOI] [PubMed] [Google Scholar]
- Domschke K, Tidow N, Schrempf M, Schwarte K, Klauke B, Reif A et al. Epigenetic signature of panic disorder: a role of glutamate decarboxylase 1 (GAD1) DNA hypomethylation? Prog Neuropsychopharmacol Biol Psychiatry 2013; 46: 189–196. [DOI] [PubMed] [Google Scholar]
- Ziegler C, Dannlowski U, Bräuer D, Stevens S, Laeger I, Wittmann H et al. Oxytocin receptor gene methylation: converging multilevel evidence for a role in social anxiety. Neuropsychopharmacology 2015; 40: 1528–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Tidow N, Schwarte K, Ziegler C, Lesch KP, Deckert J et al. Pharmacoepigenetics of depression: no major influence of MAO-A DNA methylation on treatment response. J Neural Transm 2015; 122: 99–108. [DOI] [PubMed] [Google Scholar]
- Lewin J, Schmitt AO, Adorjan P, Hildmann T, Piepenbrock C. Quantitative DNA methylation analysis based on four-dye trace data from direct sequencing of PCR amplificates. Bioinformatics 2004; 20: 3005–3012. [DOI] [PubMed] [Google Scholar]
- Domschke K, Tidow N, Schwarte K, Deckert J, Lesch KP, Arolt V et al. Serotonin transporter gene hypomethylation predicts impaired antidepressant treatment response. Int J Neuropsychopharmacol 2014; 17: 1167–1176. [DOI] [PubMed] [Google Scholar]
- Tadic A, Muller-Engling L, Schlicht KF, Kotsiari A, Dreimüller N, Kleinmann A et al. Methylation of the promoter of brain-derived neurotrophic factor exon IV and antidepressant response in major depression. Mol Psychiatry 2014; 19: 281–283. [DOI] [PubMed] [Google Scholar]
- Unternaehrer E, Meyer AH, Burkhardt SC, Dempster E, Staehli S, Theill N et al. Childhood maternal care is associated with DNA methylation of the genes for brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) in peripheral blood cells in adult men and women. Stress 2015; 18: 451–461. [DOI] [PubMed] [Google Scholar]
- Schuster R, Kleimann A, Rehme MK, Taschner L, Glahn A, Groh A et al. Elevated methylation and decreased serum concentrations of BDNF in patients in levomethadone compared with diamorphine maintenance treatment. Eur Arch Psychiatry Clin Neurosci; e-pub ahead of print 22 January 2016; doi: 10.1007/s00406-016-0668-7. [DOI] [PubMed]
- Kandel ER. A new intellectual framework for psychiatry. Am J Psychiatry 1998; 155: 457–469. [DOI] [PubMed] [Google Scholar]
- Checknita D, Maussion G, Labonté B, Comai S, Tremblay RE, Vitaro F et al. Monoamine oxidase A gene promoter methylation and transcriptional downregulation in an offender population with antisocial personality disorder. Br J Psychiatry 2015; 206: 216–222. [DOI] [PubMed] [Google Scholar]
- Shumay E, Logan J, Volkow ND, Fowler JS. Evidence that the methylation state of the monoamine oxidase A (MAOA) gene predicts brain activity of MAO A enzyme in healthy men. Epigenetics 2012; 7: 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson H, Hirvonen J, Salminen J, Hietala J. Increased serotonin receptor 1A binding in major depressive disorder after psychotherapy, but not after SSRI pharmacotherapy, is related to improved social functioning capacity. Psychother Psychosom 2013; 82: 260–261. [DOI] [PubMed] [Google Scholar]
- Roberts S, Lester KJ, Hudson JL, Rapee RM, Creswell C, Cooper PJ et al. Serotonin tranporter methylation and response to cognitive behaviour therapy in children with anxiety disorders. Transl Psychiatry 2014; 4: e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melas PA, Rogdaki M, Lennartsson A, Björk K, Qi H, Witasp A et al. Antidepressant treatment is associated with epigenetic alterations in the promoter of P11 in a genetic model of depression. Int J Neuropsychopharmacol 2012; 15: 669–679. [DOI] [PubMed] [Google Scholar]
- Lopez JP, Mamdani F, Labonte B, Beaulieu MM, Yang JP, Berlim MT et al. Epigenetic regulation of BDNF expression according to antidepressant response. Mol Psychiatry 2013; 18: 398–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud N, Salzmann A, Prada P, Nicastro R, Hoeppli ME, Furrer S et al. Response to psychotherapy in borderline personality disorder and methylation status of the BDNF gene. Transl Psychiatry 2013; 3: e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Daskalakis NP, Desarnaud F, Makotkine I, Lehrner AL, Koch E et al. Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Front Psychiatry 2013; 4: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Zoellner LA, Feeny NC, Hembree EA, Alvarez-Conrad J. Does imaginal exposure exacerbate PTSD symptoms? J Consult Clin Psychol 2002; 70: 1022–1028. [DOI] [PubMed] [Google Scholar]
- Nishith P, Resick PA, Griffin MG. Pattern of change in prolonged exposure and cognitive-processing therapy for female rape victims with posttraumatic stress disorder. J Consult Clin Psychol 2002; 70: 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair LI, Christmas DM, Hood SD, Potokar JP, Robertson A, Isaac A et al. Antidepressant-induced jitteriness/anxiety syndrome: systematic review. Br J Psychiatry 2009; 194: 483–490. [DOI] [PubMed] [Google Scholar]
- Cosci F, Knuts IJ, Abrams K, Griez EJ, Schruers KR. Cigarette smoking and panic: a critical review of the literature. J Clin Psychiatry 2010; 71: 606–615. [DOI] [PubMed] [Google Scholar]