Abstract
The identification of new and more effective treatments for alcohol abuse remains a priority. Alcohol intake activates glucocorticoids, which have a key role in alcohol's reinforcing properties. Glucocorticoid effects are modulated in part by the activity of 11β-hydroxysteroid dehydrogenases (11β-HSD) acting as pre-receptors. Here, we tested the effects on alcohol intake of the 11β-HSD inhibitor carbenoxolone (CBX, 18β-glycyrrhetinic acid 3β-O-hemisuccinate), which has been extensively used in the clinic for the treatment of gastritis and peptic ulcer and is active on both 11β-HSD1 and 11β-HSD2 isoforms. We observed that CBX reduces both baseline and excessive drinking in rats and mice. The CBX diastereomer 18α-glycyrrhetinic acid 3β-O-hemisuccinate (αCBX), which we found to be selective for 11β-HSD2, was also effective in reducing alcohol drinking in mice. Thus, 11β-HSD inhibitors may be a promising new class of candidate alcohol abuse medications, and existing 11β-HSD inhibitor drugs may be potentially re-purposed for alcohol abuse treatment.
Introduction
Alcohol remains the most prevalent abused substance in the United States, with an estimated 6.8 percent of the population aged 12 or older classified as having alcohol dependence or abuse.1 Few pharmacotherapies for alcohol abuse are currently available, and these have shown only limited efficacy and compliance.2, 3, 4, 5 Thus, the development of more effective medications for alcohol abuse is a significant unmet medical need.6
Alcohol disrupts glucocorticoid regulation in both rodents7, 8 and humans.9, 10, 11, 12, 13 Glucocorticoids have been implicated in alcohol's reinforcing effects,14 and activation of glucocorticoids by alcohol is involved in the escalation of alcohol intake in dependent rats and alcohol-seeking and drinking during protracted abstinence.15, 16 Both systemic and intracerebral glucocorticoid receptor antagonism with mifepristone blocked compulsive alcohol drinking in rats.13, 15, 16, 17 In humans, high adrenal sensitivity (cortisol to corticotropin ratio) in response to stress was found to correlate with greater susceptibility to relapse to heavy drinking,12 whereas glucocorticoid receptor antagonism with mifepristone significantly reduced alcohol craving and drinking.13 The effects of glucocorticoids are modulated in target cells by the activity of 11β-hydroxysteroid dehydrogenase (11β-HSD) isozymes acting as pre-receptors that contribute to shape the tissue-specific responsiveness to glucocorticoids.18, 19 In particular, 11β-HSD1, which is usually colocalized with the glucocorticoid receptor, converts 11-keto (inert) glucocorticoids such as cortisone in humans and 11β-dehydrocorticosterone in rodents, into 11-hydroxi (active) glucocorticoids such as cortisol in humans and corticosterone in rodents, respectively, to enhance the effects of glucocorticoids.18, 19 The reverse reaction by 11β-HSD2 attenuates local glucocorticoid responses in some mineralocorticoid receptor (MR)-expressing cells, such as classic aldosterone-selective target tissues (distal nephron, colon, sweat gland), although not in others, such as several MR-expressing brain regions.20 Given the role for glucocorticoids in mediating the reinforcing effects of alcohol,14, 15 the relevance of 11β-HSD to the modulating effects of glucocorticoids on alcohol drinking is unknown.
Carbenoxolone (CBX, 3β-hydroxy-11-oxoolean-12-en-30-oic acid 3-hemisuccinate) is a derivative of glycyrrhetinic acid, a molecule present in licorice.18, 19 CBX is a nonselective 11β-HSD inhibitor21 that has long been used for the treatment of gastritis and peptic ulcer.22 In addition to its modulatory role on glucocorticoid metabolism in target tissues, CBX also inhibits gap junctional communication, at potencies several orders of magnitude higher.23
Here we tested the hypothesis that CBX and its 18α diastereomer, 18α-glycyrrhetinic acid 3β-O-hemisuccinate (αCBX), would reduce alcohol intake in rodents because of their ability to modulate the actions of glucocorticoids. We show that these molecules are capable of reducing alcohol drinking in rodents in both baseline and excessive drinking models, and thus are promising new targets for the treatment of alcohol use disorder. We also show that αCBX is a selective inhibitor of 11β-HSD2 in the mouse.
Materials and methods
Drugs
CBX, 18α-glycyrrhetinic acid and 18β-glycyrrhetinic were purchased from Tocris (Bristol, UK); αCBX was custom synthesized from 18α-glycyrrhetinic acid (Tocris).
Subjects
Adult male Wistar rats (Charles River, Wilmington, MA, USA), weighing 225–275 g at the beginning of the experiments, were housed in groups of two to three per cage. Adult male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were housed four per cage except during drinking sessions. All the rodents were housed in a temperature-controlled (22 °C) vivarium on a 12 h/12 h light/dark cycle with ad libitum access to food and water except during behavioral testing. Operant and limited-access drinking tests were conducted during the dark phase of the light/dark cycle. All the procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Rat operant self-administration
Self-administration sessions were conducted in standard operant conditioning chambers (Med Associates, St. Albans, VT, USA). The rats were trained to self-administer alcohol as previously reported.15 First, the rats were given free-choice access to alcohol (10% w/v) and water for 1 day in their home cages to habituate them to the taste of alcohol. Second, the rats were subjected to an overnight session in the operant chambers with access to one lever (right lever) that delivered water in a fixed-ratio 1 schedule where every lever press is reinforced with delivery of 0.1 ml of solution. Food was available ad libitum during this training. Third, after 1 day off, the rats were subjected to a 2 h session (fixed-ratio 1) for 1 day and a 1 h session (fixed-ratio 1) the next day, with one lever delivering alcohol (right lever). All the subsequent sessions lasted 30 min, and two levers were available (left lever: water; right lever: alcohol) until stable levels of intake were reached. Upon completion of this procedure, the animals were allowed to self-administer a 10% (w/v) alcohol solution and water on a fixed-ratio 1 schedule of reinforcement.
Rat alcohol vapor exposure
Rats were made dependent by chronic, intermittent exposure to alcohol vapors as previously described.15 They underwent cycles of 14 h on (blood alcohol levels during vapor exposure ranged between 150 and 250 mg%) and 10 h off, during which behavioral testing for acute withdrawal occurred (that is, 6–8 h after vapor was turned off when brain and blood alcohol levels are negligible).24 In this model, rats exhibit motivational and somatic withdrawal signs.15, 17, 25, 26 Dependent rats were exposed to vapor for at least two months before testing. Non-dependent rats were placed in vapor chambers but were exposed to air for the purpose of control.
A separate cohort of rats was tested for self-administration of saccharin-sweetened water. We used a low saccharin concentration (0.004% w/v) based on previous studies15 to maintain approximately similar response rates as alcohol. Training for this experiment was identical to, and as described above, for alcohol, except that saccharin solution was used.
To investigate the effect of CBX on alcohol self-administration, we tested rats trained to self-administer alcohol with and without a history of alcohol vapor exposure sufficient to induce dependence and increased alcohol intake. CBX was administered acutely intraperitoneally in saline 90 min before testing at doses of 0, 20 and 40 mg kg−1, which are in line with the scientific literature,27, 28, 29, 30 in a within-subject Latin Square design.
Mouse two-bottle choice and chronic intermittent ethanol exposure
To evaluate the effects of CBX on drinking in nondependent and dependent mice, we used the chronic intermittent ethanol exposure paradigm.31, 32 C57BL/6J mice had access to two bottles, one containing water and the other containing 15% (v/v) ethanol, for 2 h starting 0.5 h before onset of the dark phase. Following acquisition of stable alcohol intake, half of the mice were subjected to repeated bouts of ethanol vapor exposure consisting of 16 h per day for 4 days. Before each exposure to ethanol vapor, mice were intraperitoneally injected with a solution of 1.5 g kg−1 ethanol and 68.1 mg kg−1 pyrazole and immediately placed into ethanol vapor chambers (La Jolla Alcohol Research, La Jolla, CA, USA). Tail blood sampling for blood ethanol level determination was carried out every other day. Target blood ethanol levels were 175–250 mg%. Seventy-two hours following removal from the chambers, mice received access to water vs 15% (v/v) ethanol for 2 h, and again over the next 4 days. The following week, the mice were re-exposed to the ethanol vapor/control conditions and again tested for two-bottle choice drinking for 5 days. Three vapor bouts followed by two-bottle choice were carried out. Mice were weighed every 4–6 days throughout the two-bottle choice sessions and daily during the vapor exposure bouts. Food and water were available ad libitum and the mice were group housed except during the ethanol drinking sessions.
Mouse drinking in the dark
To evaluate the effects of CBX on binge-like drinking, mice were tested in the drinking-in-the-dark (DID) paradigm.33, 34 The DID paradigm capitalizes on the circadian rhythm in drinking of mice utilizing a discrete time of exposure to ethanol to obtain pharmacologically significant ethanol drinking in a 4-day procedure.33, 34 Blood alcohol levels of C57BL/6J mice in DID are reliably over 100 mg dl−1 (1 mg ml−1) following the final drinking bout and produce behavioral intoxication.34 In the DID procedure, the water bottle is replaced with a bottle containing 20% (v/v) ethanol for 2 h in the home cage 3 h after lights go off. The design involves three daily drinking sessions of 2 h and a fourth of 4 h.33, 34 The effects of CBX and αCBX were tested in the fourth, 4 h session. Compounds were administered acutely intraperitoneally 90 min before testing at doses of 0, 20 and 40 mg kg−1.
11β-HSD activity
11β-HSD1 and 11β-HSD2 activities were measured by homogeneous time-resolved fluorescence assays,35 conducted by SB Drug Discovery (Glasgow, UK) using recombinant human and mouse 11β-HSD1 and 11β-HSD2.
Statistics
Statistical tests were run and graphs created in GraphPad Prism Version 6 (San Diego, CA, USA). Data are presented as mean±s.e.m. Appropriate statistical analyses were chosen based on experimental design. The specific statistical analysis used is indicated in the text and in each figure caption for all the studies. Bonferroni post hoc tests were used for one-way analysis of variance, two-way analysis of variance and repeated-measures analysis of variance. Significance threshold was set to P<0.05 for all the analyses. Sample sizes, although appropriate for relative studies, were generally too small to test variance; however, in instances where an unpaired two-tailed t-test was used, there were no differences in variances. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications. Data collection and quantification were performed blinded whenever possible; final analyses were not performed blind to the conditions of the experiments. However, when possible, behavioral analyses and experiments were performed blind to the experimenter.
Results
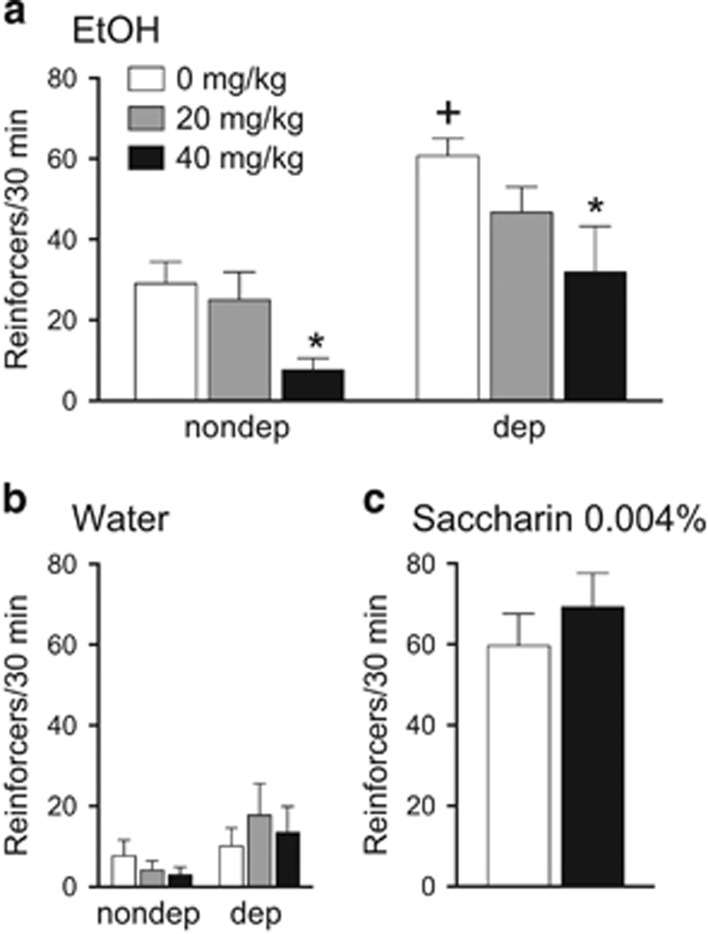
We first tested the effect of CBX on nondependent and dependent alcohol drinking in rats. An established strategy was used to induce alcohol dependence through chronic exposure to intermittent alcohol vapors.15, 24, 26, 36, 37, 38 Alcohol-dependent rats, as in previous studies, showed increased lever press responding for alcohol compared with nondependent rats (Figure 1a; group effect: F1,17=32.9; P<0.0001). Acute intraperitoneal administration of CBX 90 min before testing dose-dependently reduced responding for alcohol in both dependent and nondependent rats (Figure 1a; dose effect: F2,34=5.0; P<0.05). No significant effects of CBX were found for water responding (Figure 1b; dose effect: F2,34=0.23; P=0.80) or in self-administration of saccharin-sweetened water (Figures 1c; t(18)=0.83; P=0.42). Note that responding levels for saccharine are equivalent to those for alcohol in the dependent group.
Figure 1.
CBX reduces ethanol intake in rats in an operant self-administration paradigm. (a) Acute, systemic administration of CBX decreases operant alcohol self-administration both in dependent (dep) and nondependent (nondep) rats. (b) CBX did not influence water intake in any group. (c) Acute, systemic administration of CBX does not affect operant self-administration of saccharin-sweetened water. Rats were given CBX (0, 20 and 40 mg kg−1 or 0 and 40 mg kg−1; intraperitoneally) 90 min before alcohol (10%, w/v), water or saccharin (0.004%) self-administration (30 min session; fixed-ratio 1). The data represent means and s.e.m. *P<0.05, significant difference from respective vehicle; +P<0.05, significant difference from vehicle (saline)-treated nondependent rats. N=9–10 per group.
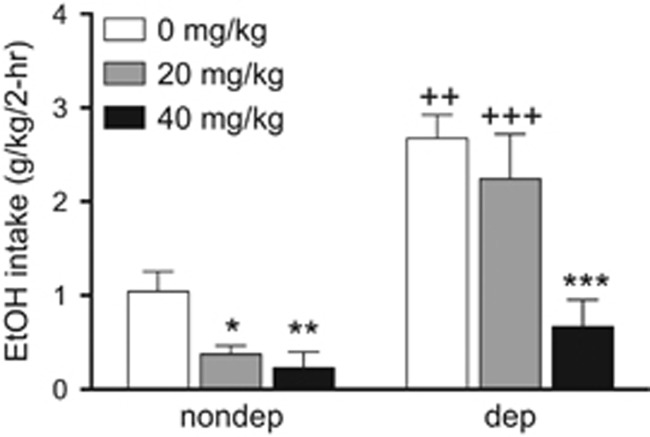
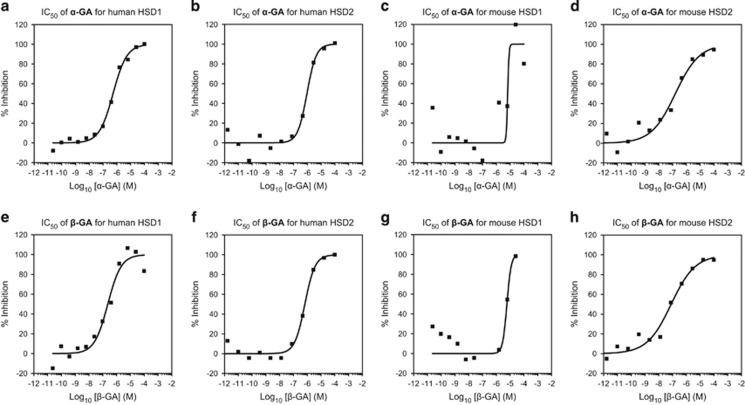
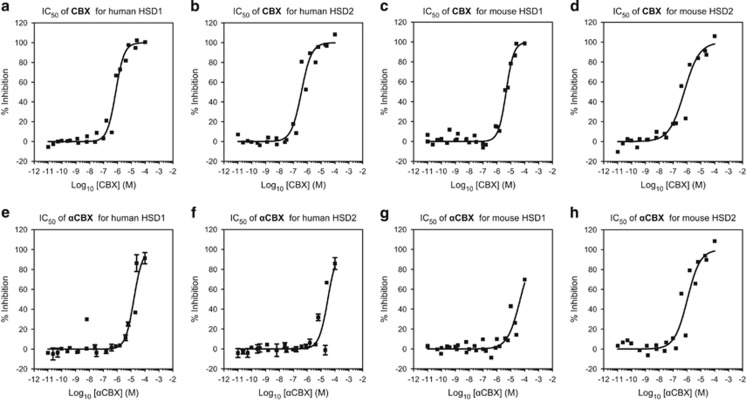
We then tested CBX in nondependent mice in a limited-access two-bottle choice paradigm as well as in mice subjected to repeated bouts of ethanol vapors to induce dependence.31, 32 CBX decreased alcohol intake more effectively in nondependent than in dependent mice at a dose of 40 mg kg−1 (Figure 2). Because 18α-glycyrrhetinic acid has been reported to be a selective inhibitor of human 11β-HSD1,39 we also synthesized αCBX, the 18α diastereomer of CBX (18α-glycyrrhetinic acid 3β-O-hemisuccinate), to explore its potential isozyme selectivity. We observed that 18α-glycyrrhetinic acid and 18β-glycyrrhetinic acid are active both on mouse and human 11β-HSD1 and 11β-HSD2 isoforms (Figure 3), whereas αCBX proved to be a selective inhibitor of mouse 11β-HSD2 with comparable, although slightly lower, potency on mouse HSD2 than CBX (Figure 4).
Figure 2.
CBX reduces ethanol intake in nondependent (nondep) and dependent (dep) mice in a limited-access two-bottle choice paradigm. The mice trained to drink alcohol in a limited-access (2 h) two-bottle choice paradigm were either exposed to alcohol vapor to induce dependence or air for the purpose of control, and tested for the effect of CBX on drinking. CBX reduced ethanol intake in nondependent mice at doses of 20 and 40 mg kg−1 intraperitoneally (left), and in dependent mice at 40 mg kg−1, intraperitoneally (right). The two-way analysis of variance revealed a significant effect of vapor exposure (F2,35=33.38, P<0.0001), dose (F2,35=13.04, P<0.0001) and interaction of vapor exposure and dose (F2,35=3.902, P=0.0295). *P<0.05, **P<0.01 and ***P<0.001 represent significant difference from the respective vehicle (saline)-treated group; ++P<0.01 and +++P<0.001 represent significant difference from the respective nondependent group.
Figure 3.
Activity of 18α-glycyrrhetinic acid and 18β-glycyrrhetinic on mouse and human 11β-HSD1 and 11β-HSD2. We tested the IC50 of α- and β-glycyrrhetinic acid (GA) against human and mouse 11β-HSD1 and 11β-HSD2 by means of homogeneous time-resolved fluorescence (HTRF) assays. (a and c) α−GA yielded IC50 values of 532.1 nm for human 11β-HSD1 and 6.63 μm for mouse 11β-HSD1. (b and d) α−GA yielded IC50 values of 942.6 nm for human 11β-HSD2 and 159.7 nm for mouse 11β-HSD2. (e and g) β−GA yielded IC50 values of 232.3 nm for human 11β-HSD1 and 5.85 μm for mouse 11β-HSD1. (f and h) β−GA yielded IC50 values of 674.5 nm for human 11β-HSD2 and 79.7 nm for mouse 11β-HSD2.
Figure 4.
Activity of carbenoxolone (CBX, 18β-glycyrrhetinic acid 3β-O-hemisuccinate) and αCBX (18α-glycyrrhetinic acid 3β-O-hemisuccinate) on mouse and human 11β-HSD1 and 11β-HSD2. We tested the IC50 of 18α−18β-glycyrrhetinic acid 3β-O-hemisuccinate against human and mouse 11β-HSD1 and 11β-HSD2 by means of homogeneous time-resolved fluorescence (HTRF) assays. (a and c) CBX yielded IC50 values of 753.1 nm for human 11β-HSD1 and 4.62 μm for mouse 11β-HSD1. (b and d) CBX yielded IC50 values of 379.6 nm for human 11β-HSD2 and 628.7 nm for mouse 11β-HSD2. (e and g) αCBX yielded IC50 values of 15.92 μm for human 11β-HSD1 and 48.25 μm for mouse 11β-HSD1. (f and h) αCBX yielded IC50 values of 30.67 μm for human 11β-HSD2 and 1.06 μm for mouse 11β-HSD2. Results for CBX are the average of two to three independent replicates; results for αCBX are the average of three to four independent replicates.
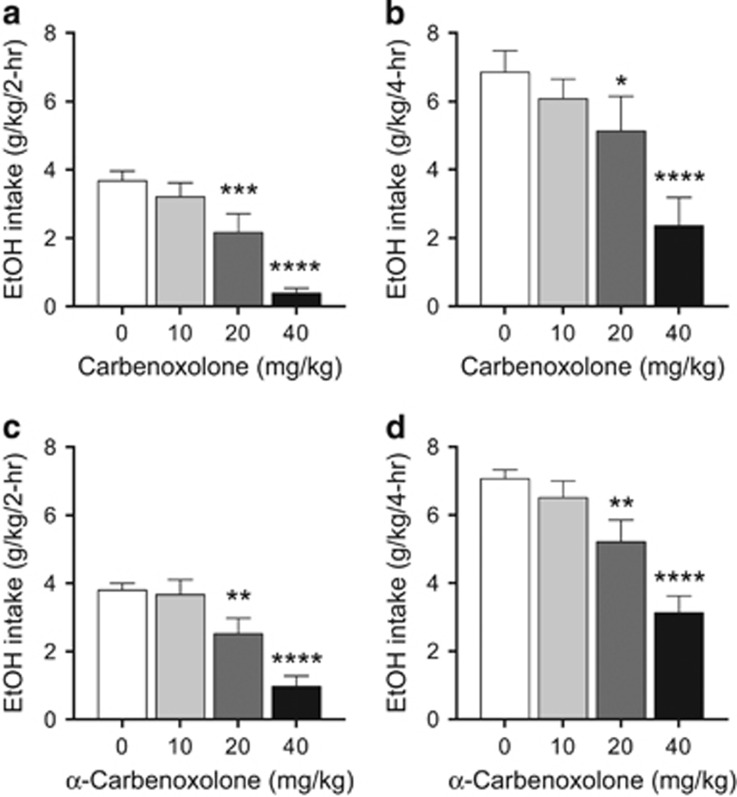
We then tested the effects of CBX and αCBX in the ‘drinking in the dark' (DID) paradigm of binge-like drinking in mice.33 We observed that CBX reduced drinking in the DID paradigm at both 20 and 40 mg kg−1 (Figures 5a and b). αCBX showed similar potency as CBX in reducing drinking in the DID paradigm in mice (Figures 5c and d). As 11β-HSD2 in the kidney contributes to blood pressure regulation, we tested the effects of CBX and αCBX and found that neither compound affected blood pressure in mice (Table 1), consistent with the results of previous studies with CBX in both mice and rats.40, 41
Figure 5.
CBX and αCBX reduce ethanol intake in mice in the drinking-in-the-dark (DID) paradigm. (a and b) CBX reduced ethanol intake in the DID binge-like drinking paradigm. (a) CBX effect on ethanol intake in the first 2 h of the 4-h drinking session (F3,30=26.00, P<0.0001); (b) CBX effect on ethanol intake for the 4-h drinking session (F3,30=10.82, P<0.0001); (c) αCBX effect on ethanol intake in the first 2 h of the 4-h drinking session (F3,86=15.66, P<0.0001); (d) αCBX effect on ethanol intake for the 4-h drinking session (F3,86=18.51, P<0.0001). The effects of CBX and αCBX were more pronounced in the first 2 h of the session, suggesting an effect on initiation of drinking. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, significant difference from respective saline vehicle (N=11–15).
Table 1. Effects of CBX and αCBX on blood pressure (mean±s.e.m.).
| BL | SAL | CBX | αCBX | |
|---|---|---|---|---|
| DIA | 107.73±4.14 | 108.48±4.66 | 100.53±5.66 | 105.61±3.10 |
| SYS | 139.53±4.20 | 140.07±5.57 | 133.29±5.33 | 138.54±4.25 |
| MAP | 118.00±4.09 | 118.68±4.92 | 111.09±5.50 | 116.27±3.31 |
Abbreviations: BL, baseline; CBX, carbenoxolone; DIA, diastolic; SAL, saline; SYS, systolic; MAP, mean arterial pressure.
Discussion
Only a limited number of drugs exist with clinical efficacy for the treatment of alcohol abuse.6, 42 Expansion of therapeutic options is needed to improve treatment success at different stages of disease progression and to bring about individualized therapies based on patient genetic makeup.6, 42
Glucocorticoids facilitate drug seeking, brain stimulation reward, dopamine release and are themselves self-administered.43, 44 The present effects of CBX in reducing alcohol intake could be related to the facilitation of reward mechanisms associated with activation of glucocorticoids.14, 43, 45 The present results show that the unselective 11β-HSD inhibitor CBX effectively reduces alcohol intake in both dependent and nondependent rats and mice. This suggests that 11β-HSDs may have a fundamental role in modulating the reinforcing effects of alcohol via their actions in modulating glucocorticoids, and that existing 11β-HSD inhibitor drugs, such as CBX as well as others, for example, see refs 46, 47, can potentially be re-purposed for alcohol abuse. Re-purposing of drugs with known safety profiles in humans for diseases other than the ones for which they were originally developed is a potentially fast and effective way to address an unmet medical need.48
Glucocorticoid receptors in multiple brain regions have been implicated in the effects of alcohol.16 11β-HSD1 is broadly expressed in the adult rat brain, including in brain regions relevant to alcohol reinforcing properties such as the amygdala.49, 50, 51 Thus, the previously shown ability of CBX to inhibit central 11β-HSD1,52, 53 resulting in reduced glucocorticoid signaling, likely contributes to reduced drinking, paralleling the effects of the glucocorticoid receptor inhibitor mifepristone on drinking.15, 16 The comparable effects in the mouse of CBX, which inhibits both mouse 11β-HSD isozymes, and αCBX, a selective 11β-HSD2 inhibitor in the mouse, point to a potential role also for the latter isozyme in alcohol drinking. In the brain, 11β-HSD2 is expressed primarily in a subpopulation of neurons in the nucleus tractus solitarii.54, 55, 56 This neuronal population, denominated HSD2 neurons, receive inputs from the central nucleus of the amygdala and paraventricular nucleus of the hypothalamus, and project to the ventral BNST, polysynaptically to the nucleus accumbens, and both directly and indirectly to the central nucleus of the amygdala.54, 57, 58 Locally, HSD2 neurons are targeted by a group of neurons in the dorsomedial nucleus tractus solitarii that express neurotensin,59 which has been implicated in the regulation of alcohol intake.60 Thus, the connectivity of HSD2 neurons in the nucleus tractus solitarii suggests that inhibition of 11β-HSD2 in this neuronal population may result in central effects on alcohol intake. Future studies are needed to explore the relative contributions of 11β-HSD isozymes to alcohol drinking, as well as the contribution of 11β-HSD to the phenotype of genetic models of excessive alcohol drinking, such as alcohol-preferring rodent lines differing in their glucocorticoid regulation.61, 62
11β-HSD1 inhibitors are being considered as potential cognitive enhancers, as well as for the therapy of type 2 diabetes, metabolic syndrome and obesity.53, 63 11β-HSD1 knockout mice show resistance to diet-induced obesity, increased glucose tolerance and insulin sensitivity.63 In addition, studies with 11β-HSD1 null mice and 11β-HSD1-selective inhibitors indicate a role for 11β-HSD1 in the intake of palatable food,64 but the mechanism behind this effect has been questioned.65 In the mouse, inhibitors of the CRF1 receptor, which is also responsible for CRF signaling at the pituitary, regulate food and fluid intake as well as alcohol intake in the drinking-in-the-dark model of binge alcohol consumption.66 Conversely, in the rat, CRF1 antagonists selectively reduce dependent but not nondependent alcohol drinking.38, 67, 68 In addition to species differences in terms of neurochemistry underlying motivation for alcohol and sweet solutions in rats and mice, in the present study, the rat studies used operant procedures that involve increased motivation/workload (that is, lever press) for access to the solution, whereas the mouse studies involved voluntary drinking that involves low workload and is more affected by consummatory processes. Therefore, the effect of CBX on alcohol drinking in different models of alcohol consumption, species and animal history of alcohol exposure emphasizes the potential of CBX in decreasing alcohol reinforcement across species and levels of motivation for alcohol.
CBX, which inhibits both 11β-HSD isoforms,21 has long been used in the clinic for the therapy of gastritis and peptic and duodenal ulcers.69 However, its use has greatly diminished in favor of other classes of drugs, such as proton pump inhibitors and histamine H2 antagonists. This is in part because of the potential of chronic CBX use to induce pseudohyperaldosteronism,70, 71 which is due to inhibition by CBX of 11β-HSD type 2 isoform (11β-HSD2) in the kidney.46 By inactivating glucocorticoids in the kidney and other mineralocorticoid target tissues, 11β-HSD2 shields the mineralocorticoid receptor from activation by glucocorticoids.46 Thus, inhibition of 11β-HSD2 permits glucocorticoids to act on mineralocorticoid receptors.46 However, this side effect can be managed by combination with an anti-kaliuretic diuretic, such as amiloride, or thiazide diuretics and potassium supplementation.53, 69, 72
The development of new and more effective medications for alcoholism remains a priority.6 Here we showed that CBX and its diastereomer αCBX, which is a selective inhibitor of mouse 11β-HSD2, reduce baseline and excessive drinking in rodents. Collectively, the present results suggest that 11β-HSD inhibitors may represent a promising new class of candidate therapeutic targets to treat alcohol use disorders.
Acknowledgments
This work was supported by NIH grants AA020960, AA021667, AA017371 and AA020893 and by the National Institute on Drug Abuse Intramural Research Program (LFV).
PPS is an inventor on a related patent application. The remaining authors declare no conflict of interest.
References
- SAMHSAResults from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2013. [Google Scholar]
- Berrettini W. Opioid pharmacogenetics of alcohol addiction. Cold Spring Harb Perspect Med 2013; 3: a012203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 2014; 174: 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Lehert P. Acamprosate for alcohol dependence: a sex-specific meta-analysis based on individual patient data. Alcohol Clin Exp Res 2012; 36: 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, O'Malley S, Anton R. COMBINE genetics study: the pharmacogenetics of alcoholism treatment response: genes and mechanisms. J Stud Alcohol Suppl 2005; 15: 56–64. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML et al. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol 2012; 17: 513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci 2008; 28: 1641–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res 2000; 24: 1836–1849. [PubMed] [Google Scholar]
- Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ. Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcohol Clin Exp Res 2003; 27: 1420–1427. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res 2000; 24: 651–658. [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addict Biol 2009; 14: 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry 2011; 68: 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE et al. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest 2015; 125: 3193–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Eriksson CJ, Engel JA, Hansen S. Consequence of long-term exposure to corticosterone or dexamethasone on ethanol consumption in the adrenalectomized rat, and the effect of type I and type II corticosteroid receptor antagonists. Psychopharmacology 1995; 117: 216–224. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW Jr., Logrip ML et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci 2012; 32: 7563–7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Shin W, Vendruscolo LF, Lefebvre C, Koob GF, Califano A et al. Identifying candidate drivers of alcohol dependence-induced excessive drinking by assembly and interrogation of brain-specific regulatory networks. Genome Biol 2015; 16: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE et al. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF(1) receptor antagonism. Neuropharmacology 2012; 62: 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K, Holmes M, Seckl J. 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev 2013; 93: 1139–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper N, Stewart PM. 11beta-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J Endocrinol 2005; 186: 251–271. [DOI] [PubMed] [Google Scholar]
- Albiston AL, Obeyesekere VR, Smith RE, Krozowski ZS. Cloning and tissue distribution of the human 11 beta-hydroxysteroid dehydrogenase type 2 enzyme. Mol Cell Endocrinol 1994; 105: R11–R17. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Wallace AM, Atherden SM, Shearing CH, Edwards CR. Mineralocorticoid activity of carbenoxolone: contrasting effects of carbenoxolone and liquorice on 11 beta-hydroxysteroid dehydrogenase activity in man. Clin Sci (Lond) 1990; 78: 49–54. [DOI] [PubMed] [Google Scholar]
- Bertaccini G, Coruzzi G. Pharmacology of the treatment of peptic ulcer disease. Dig Dis Sci 1985; 30(11 Suppl): 43S–51S. [DOI] [PubMed] [Google Scholar]
- Andrews RC, Rooyackers O, Walker BR. Effects of the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone on insulin sensitivity in men with type 2 diabetes. J Clin Endocrinol Metab 2003; 88: 285–291. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN. Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res 2009; 33: 2113–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J Stud Alcohol 2003; 64: 445–449. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology 2000; 22: 581–594. [DOI] [PubMed] [Google Scholar]
- Prasad Sakamuri SS, Sukapaka M, Prathipati VK, Nemani H, Putcha UK, Pothana S et al. Carbenoxolone treatment ameliorated metabolic syndrome in WNIN/Ob obese rats, but induced severe fat loss and glucose intolerance in lean rats. PLoS One 2012; 7: e50216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraki S, Litrus L, Soriano L, Monbureau M, To LK, Braithwaite SP et al. A pharmacological screening approach for discovery of neuroprotective compounds in ischemic stroke. PLoS One 2013; 8: e69233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareri P, Condorelli D, Belluardo N, Russo E, Loiacono A, Barresi V et al. Anticonvulsant effects of carbenoxolone in genetically epilepsy prone rats (GEPRs). Neuropharmacology 2004; 47: 1205–1216. [DOI] [PubMed] [Google Scholar]
- Gareri P, Condorelli D, Belluardo N, Gratteri S, Ferreri G, Donato Di Paola E et al. Influence of carbenoxolone on the anticonvulsant efficacy of conventional antiepileptic drugs against audiogenic seizures in DBA/2 mice. Eur J Pharmacol 2004; 484: 49–56. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res 2004; 28: 1829–1838. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology 2005; 181: 688–696. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 2005; 84: 53–63. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T Jr. et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav 2007; 6: 1–18. [DOI] [PubMed] [Google Scholar]
- Degorce F, Card A, Soh S, Trinquet E, Knapik GP, Xie B. HTRF: A technology tailored for drug discovery—a review of theoretical aspects and recent applications. Curr Chem Genomics 2009; 3: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res 2004; 28: 1676–1682. [DOI] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Repunte-Canonigo V, Hagihara K, Thurbon D, Lekic D et al. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci 2009; 29: 5389–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res 2002; 26: 1494–1501. [DOI] [PubMed] [Google Scholar]
- Classen-Houben D, Schuster D, Da Cunha T, Odermatt A, Wolber G, Jordis U et al. Selective inhibition of 11beta-hydroxysteroid dehydrogenase 1 by 18alpha-glycyrrhetinic acid but not 18beta-glycyrrhetinic acid. J Steroid Biochem Mol Biol 2009; 113: 248–252. [DOI] [PubMed] [Google Scholar]
- Nuotio-Antar AM, Hachey DL, Hasty AH. Carbenoxolone treatment attenuates symptoms of metabolic syndrome and atherogenesis in obese, hyperlipidemic mice. Am J Physiol Endocrinol Metab 2007; 293: E1517–E1528. [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Lindsay RM, Edwards CR, Seckl JR. Inhibition of 11-beta-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension 1996; 27: 1200–1204. [DOI] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci 2011; 12: 670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci 1998; 19: 67–74. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Deminiere JM, Le Moal M, Simon H. Rats orally self-administer corticosterone. Brain Res 1993; 622: 315–320. [DOI] [PubMed] [Google Scholar]
- Lu YL, Richardson HN. Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction. Neuroscience 2014; 277: 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning TM. Human hydroxysteroid dehydrogenases and pre-receptor regulation: insights into inhibitor design and evaluation. J Steroid Biochem Mol Biol 2011; 125: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JS, Goldberg FW, Turnbull AV. Medicinal chemistry of inhibitors of 11beta-hydroxysteroid dehydrogenase type 1 (11beta-HSD1). J Med Chem 2014; 57: 4466–4486. [DOI] [PubMed] [Google Scholar]
- Strittmatter SM. Overcoming drug development bottlenecks with repurposing: old drugs learn new tricks. Nat Med 2014; 20: 590–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier G, Luu-The V, Li S, Bujold G, Labrie F. Localization and glucocorticoid regulation of 11beta-hydroxysteroid dehydrogenase type 1 mRNA in the male mouse forebrain. Neuroscience 2007; 145: 110–115. [DOI] [PubMed] [Google Scholar]
- Kanitz E, Puppe B, Tuchscherer M, Heberer M, Viergutz T, Tuchscherer A. A single exposure to social isolation in domestic piglets activates behavioural arousal, neuroendocrine stress hormones, and stress-related gene expression in the brain. Physiol Behav 2009; 98: 176–185. [DOI] [PubMed] [Google Scholar]
- Ong SX, Chng K, Meaney MJ, Buschdorf JP. Decreased hippocampal mineralocorticoid:glucocorticoid receptor ratio is associated with low birth weight in female cynomolgus macaque neonates. J Mol Endocrinol 2013; 51: 59–67. [DOI] [PubMed] [Google Scholar]
- Jellinck PH, Monder C, McEwen BS, Sakai RR. Differential inhibition of 11 beta-hydroxysteroid dehydrogenase by carbenoxolone in rat brain regions and peripheral tissues. J Steroid Biochem Mol Biol 1993; 46: 209–213. [DOI] [PubMed] [Google Scholar]
- Sandeep TC, Yau JL, MacLullich AM, Noble J, Deary IJ, Walker BR et al. 11Beta-hydroxysteroid dehydrogenase inhibition improves cognitive function in healthy elderly men and type 2 diabetics. Proc Natl Acad Sci USA 2004; 101: 6734–6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Aldosterone in the brain. Am J Physiol Renal Physiol 2009; 297: F559–F576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland BL, Li KX, Funder JW. Hybridization histochemical localization of 11 beta-hydroxysteroid dehydrogenase type 2 in rat brain. Endocrinology 1995; 136: 4697–4700. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Kawata M, Loewy AD. Aldosterone-sensitive neurons in the rat central nervous system. J Comp Neurol 2006; 494: 515–527. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary tract: bidirectional connections with the central nucleus of the amygdala. J Comp Neurol 2006; 497: 646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhtman E, Geerling JC, Loewy AD. Aldosterone-sensitive neurons of the nucleus of the solitary tract: multisynaptic pathway to the nucleus accumbens. J Comp Neurol 2007; 501: 274–289. [DOI] [PubMed] [Google Scholar]
- Sequeira SM, Geerling JC, Loewy AD. Local inputs to aldosterone-sensitive neurons of the nucleus tractus solitarius. Neuroscience 2006; 141: 1995–2005. [DOI] [PubMed] [Google Scholar]
- Lee MR, Hinton DJ, Song JY, Lee KW, Choo C, Johng H et al. Neurotensin receptor type 1 regulates ethanol intoxication and consumption in mice. Pharmacol Biochem Behav 2010; 95: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Chu HP, Crabbe JC, Keith LD. Differential modulation by the stress axis of ethanol withdrawal seizure expression in WSP and WSR mice. Alcohol Clin Exp Res 1991; 15: 412–417. [DOI] [PubMed] [Google Scholar]
- Apter SJ, Eriksson CJ. The role of social isolation in the effects of alcohol on corticosterone and testosterone levels of alcohol-preferring and non-preferring rats. Alcohol Alcohol 2006; 41: 33–38. [DOI] [PubMed] [Google Scholar]
- Gathercole LL, Stewart PM. Targeting the pre-receptor metabolism of cortisol as a novel therapy in obesity and diabetes. J Steroid Biochem Mol Biol 2010; 122: 21–27. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Birtles S, de Schoolmeester J, Swales J, Moody G, Hislop D et al. Inhibition of 11beta-hydroxysteroid dehydrogenase type 1 reduces food intake and weight gain but maintains energy expenditure in diet-induced obese mice. Diabetologia 2006; 49: 1333–1337. [DOI] [PubMed] [Google Scholar]
- Harno E, Cottrell EC, Yu A, DeSchoolmeester J, Gutierrez PM, Denn M et al. 11beta-Hydroxysteroid dehydrogenase type 1 (11beta-HSD1) inhibitors still improve metabolic phenotype in male 11beta-HSD1 knockout mice suggesting off-target mechanisms. Endocrinology 2013; 154: 4580–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Ryabinin AE. CRF1 receptor signaling regulates food and fluid intake in the drinking-in-the-dark model of binge alcohol consumption. Alcohol Clin Exp Res 2013; 37: 1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD et al. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol Biochem Behav 2008; 88: 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry 2007; 61: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder RM, Brogden RN, Sawyer PR, Speight TM, Spencer R, Avery GS. Carbenoxolone: a review of its pharmacological properties and therapeutic efficacy in peptic ulcer disease. Drugs 1976; 11: 245–307. [DOI] [PubMed] [Google Scholar]
- Turpie AG, Runcie J, Thomson TJ. Clinical trial of deglydyrrhizinized liquorice in gastric ulcer. Gut 1969; 10: 299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpie AG, Thomson TJ. Carbenoxolone sodium in the treatment of gastric ulcer with special reference to side-effects. Gut 1965; 6: 591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk J, Schmack B, Rosch W, Domschke W. Controlled trial of carbenoxolone sodium vs cimetidine in duodenal ulcer. Scand J Gastroenterol Suppl 1980; 65: 103–107. [PubMed] [Google Scholar]