Abstract
Omega-3 polyunsaturated fatty acid (PUFA) supplementation has been proposed as (adjuvant) treatment for major depressive disorder (MDD). In the present meta-analysis, we pooled randomized placebo-controlled trials assessing the effects of omega-3 PUFA supplementation on depressive symptoms in MDD. Moreover, we performed meta-regression to test whether supplementation effects depended on eicosapentaenoic acid (EPA) or docosahexaenoic acid dose, their ratio, study duration, participants' age, percentage antidepressant users, baseline MDD symptom severity, publication year and study quality. To limit heterogeneity, we only included studies in adult patients with MDD assessed using standardized clinical interviews, and excluded studies that specifically studied perinatal/perimenopausal or comorbid MDD. Our PubMED/EMBASE search resulted in 1955 articles, from which we included 13 studies providing 1233 participants. After taking potential publication bias into account, meta-analysis showed an overall beneficial effect of omega-3 PUFAs on depressive symptoms in MDD (standardized mean difference=0.398 (0.114–0.682), P=0.006, random-effects model). As an explanation for significant heterogeneity (I2=73.36, P<0.001), meta-regression showed that higher EPA dose (β=0.00037 (0.00009–0.00065), P=0.009), higher percentage antidepressant users (β=0.0058 (0.00017–0.01144), P=0.044) and earlier publication year (β=−0.0735 (−0.143 to 0.004), P=0.04) were significantly associated with better outcome for PUFA supplementation. Additional sensitivity analyses were performed. In conclusion, present meta-analysis suggested a beneficial overall effect of omega-3 PUFA supplementation in MDD patients, especially for higher doses of EPA and in participants taking antidepressants. Future precision medicine trials should establish whether possible interactions between EPA and antidepressants could provide targets to improve antidepressant response and its prediction. Furthermore, potential long-term biochemical side effects of high-dosed add-on EPA supplementation should be carefully monitored.
Introduction
Omega-3 polyunsaturated fatty acids (PUFAs) have been proposed as a treatment for major depressive disorder (MDD). Over the last decade, several meta-analyses have been performed, which suggested variable degrees of beneficial effects of omega-3 PUFAs for MDD, but made critical remarks regarding the quality of the evidence and possible publication bias.1, 2, 3, 4, 5, 6 These meta-analyses evoked academic correspondence, discussing the used inclusion criteria and selection of outcome measures.7, 8 In brief, this correspondence suggested beneficial effects (1) if a higher ratio of omega-3 PUFA eicosapentaenoic acid (EPA) to docosahexaenoic acid (DHA) was being supplemented7 and (2) only in patients with actual MDD as opposed to subjects with merely depressive symptoms.8
Of these meta-analyses, only the three most recent meta-analyses covered the research performed over the last 5 years: (1) Grosso et al.9 included randomized controlled trials (RCTs) published up to August 2013, and concluded that omega-3 PUFAs were effective in depressive patients with or without an MDD diagnosis; (2) Yang et al.10 pooled RCTs published up to March 2015 on the effect of the combination of EPA and DHA on depression in women and observed a beneficial effect; and (3) Appleton et al.4 performed a Cochrane meta-analysis of RCTs published up to May 2015, concluding that there is a small-to-modest, non-clinically beneficial effect of omega-3 PUFAs.
Importantly, these meta-analyses seem to have several issues of interest. First, they did not correct for potential publication bias, for example, by using the trim-and-fill method.11 Second, Grosso et al. included two comparisons of the study of Jazayeri et al.12 This study had three arms using a double-dummy technique (EPA+placebo vs EPA+fluoxetine vs fluoxetine+placebo). Although other meta-analyses only included the fluoxetine+EPA vs fluoxetine+placebo comparison (that is, EPA vs placebo in an add-on design), Grosso et al. additionally included the EPA+placebo vs fluoxetine+placebo comparison (that is, EPA vs fluoxetine during additional placebo treatment). Thereby, Grosso et al. not only compared EPA vs placebo, but additionally included a comparison of EPA vs active antidepressant medication, potentially reducing the overall effect size. Third, Grosso et al. and Yang et al. included three articles that all seem to report on the same RCT,13, 14, 15 that is, (partial) duplicate publication which distorts pooled-effect estimates. Fourth, Grosso et al. and Appleton et al. missed potentially important evidence by not including all RCTs on MDD patients in their analysis (for example, Lesperance et al.16 in Grosso et al.; and Mozaffari-Khosravi et al.17 in Appleton et al.). Although the study of Mozaffari-Khosravi et al. included mild-to-moderate MDD patients, all patients fulfilled MDD criteria, baseline severity was comparable to other included studies and other included studies (for example, Lucas et al.18) also used an upper limit for MDD severity. Fifth, all three meta-analyses only used post-intervention effect size data, while repeated measures are available, which may improve effect size estimation precision. Sixth, by including RCTs that not only included MDD patients but also patients with dysthymia,13, 14 all three meta-analyses did not specifically focus on MDD only. In addition, Appleton et al. included the trial by Peet and Horrobin19 that did not use MDD diagnosis as an inclusion criterion, but rather a cutoff score on a depression symptom questionnaire. Moreover, Appleton et al. and Grosso et al. also included trials on subjects with MDD secondary to neurological or other somatic disorders (for example, Da Silva et al.),20 which may increase heterogeneity due to different underlying pathophysiology.
Therefore, our study aims at continuing the academic debate by performing a revised updated meta-analysis on the effectiveness of omega-3 PUFAs for the treatment of MDD taking into account the above issues of concern. In addition, we performed meta-regression analyses to explain expected heterogeneity, by testing the effects of EPA and DHA dose and their ratio, study duration, participants' age, percentage antidepressant users, baseline MDD symptom severity, publication year and study quality.
Materials and methods
Search strategy
We performed a search in the Medline and Embase databases (from inception up to 8 December 2015). We used a search strategy combining terms regarding MDD and fatty acid supplementation using Boolean operators (See Supplementary Methods). We additionally searched references of selected studies and earlier meta-analyses for potential studies.1, 4, 9
Study selection
Two independent reviewers (RJTM and IH/JA) screened the identified articles for their relevance by title and abstract, and—if necessary—the full text. In case of disagreement between reviewers, inclusion could be conclusively determined by discussion in all the cases. A priori criteria for inclusion of studies were: (1) design was a randomized placebo-controlled trial; (2) inclusion of adult patients with MDD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) as assessed by a standardized clinical interview. We excluded studies that (1) specifically studied perinatal or perimenopausal MDD or (2) MDD secondary to other neuropsychiatric disorders.
With these in- and exclusion criteria, we aimed to test the effect of omega-3 fatty acid supplementation on depressive symptoms specifically in MDD, as opposed to depressive symptoms in the general population (including subclinical depression). Because previous meta-analyses included clinically more heterogeneous populations which theoretically may preclude meta-analysis, we aimed to prevent inclusion of trials where supplementation of omega-3 fatty acids could improve an underlying somatic or (neuro)psychiatric disorder, with a secondary effect on the depression scores. In addition, by excluding specific subgroups like perinatal or perimenopausal MDD, with a potentially different pathophysiology, we aimed to further minimize heterogeneity among included studies. Because of the widespread comorbidity between MDD and cardiovascular disease, we did not exclude studies that specifically studied MDD with comorbid cardiovascular disease or diabetes.21 Nevertheless, we performed sensitivity analyses without the studies that specifically studied MDD with comorbid cardiovascular disease or diabetes.22, 23
Critical appraisal
We assessed the quality of the studies that were selected with the in- and exclusion criteria above using the Jadad scale for reporting RCTs.24
Data extraction
Two independent reviewers (RJTM and IH) extracted the data into specifically designed spreadsheets. We collected the data on descriptives, methods and outcomes. Possible disagreements were resolved by discussion with a third independent reviewer where necessary (JA).
We used the primary outcome measure reported by the study. We used the unadjusted intention-to-treat repeated-measures statistics where available. If multiple primary outcomes were reported, we used the Hamilton Depression Rating Scale (HDRS).25 If unavailable, we used another clinician-rated outcome. If unavailable, we used a self-rated depression scale. We performed sensitivity analyses using unpublished additional data derived from the authors where available.4, 12, 26, 27
Statistical analysis
We performed the statistical analyses with Comprehensive Meta-Analysis version 2. We used standardized mean differences as the summary statistic for meta-analysis. We assessed heterogeneity by calculating the I2 statistic.11 Given the expected heterogeneity, we a priori used a random-effects model.11 We performed the sensitivity analyses using fixed-effects models because they are considered to be less vulnerable for publication bias. We assessed publication bias by plotting a funnel plot, and reporting the classic and Orwin's fail-safe N, Begg and Mazumdar rank correlation and Egger's regression intercept.11 Finally, we adjusted the values using the Duval and Tweedie's trim-and-fill method.
We performed meta-regression using the method of moments computational option.11 We a priori planned to test the univariate effects of EPA and DHA dose, study duration, participants' age, percentage antidepressant users, baseline MDD severity, publication year and study quality, on the effect size of trials. To compare EPA- and DHA-dose effects for studies where multiple subgroups were available, we regarded these subgroups as independent, assuming independence where necessary.11 For baseline severity, we converted scores to the 17-item Hamilton Depression Rating Scales using available conversion tables where needed.28, 29
Results
Selection of studies
The search strategy resulted in 1955 articles for consideration in the present meta-analysis. The studies were excluded for several reasons, including not applying a randomized placebo-controlled trial design, no inclusion of subjects with MDD as ascertained by a structured clinical interview,30, 31, 32, 33 or specifically including patients with perinatal or perimenopausal MDD, or MDD secondary to other neuropsychiatric disorders20, 34 (Supplementary Figure S1). After applying the in- and exclusion criteria, we included 15 studies,12, 16, 17, 18, 22, 23, 26, 35, 36, 37, 38, 39, 40, 41, 42 including a total of 1253 MDD subjects. Study-characteristics are provided in Supplementary Table 1. Of these 15 studies, 1 small preliminary study reports unpublished data (Coryell et al., related to Fiedorowicz et al.42), and 1 small study has a considerable drop-out rate (>50%).41 Given the relatively preliminary nature of these two studies, we included them only in separate sensitivity analyses.
Meta-analysis
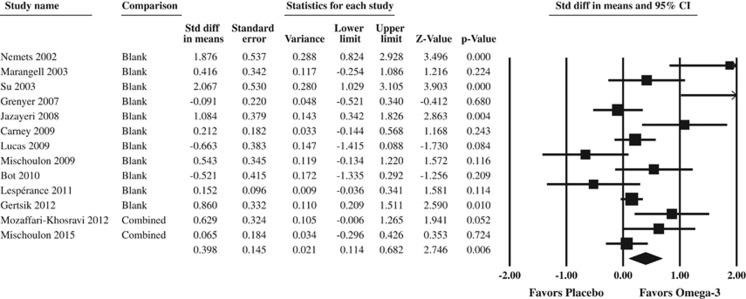
The meta-analysis on 13 RCTs in 1233 MDD subjects showed an overall beneficial effect of omega-3 PUFAs on depressive symptoms in MDD (standardized mean difference=0.398 (0.114–0.682), P=0.006, random-effects model, Figure 1). For the sensitivity analyses, see Supplementary Results.
Figure 1.
Forest plot of the meta-analysis on the effect of omega-3 fatty acid supplementation on depressive symptoms in major depressive disorder. For the comparison column, ‘blank' means that there was only one comparison in the study, ‘combined' means that different comparisons were combined for an overall study estimate. Studies are sorted by publication year.
Publication bias
The funnel plot for all the available data is shown in Supplementary Figure 2. The classic fail-safe N was 95, Orwin's fail-safe N was 19 with criterion for a ‘trivial' standardized difference in means as 0.1 and mean standardized difference in means in missing studies as 0. This implied that at least 19 studies without any effect must have been left unpublished to reduce the overall effect to a trivial effect. Regarding the Begg and Mazumbar rank-correlation test, Kendall's taus with and without continuity correction were 0.21 (P2-sided=0.28), and 0.22 (P2-sided=0.26), respectively, indicative of no publication bias. Egger's regression intercept was 1.13 (95% confidence interval: −0.63 to 2.91; P2-sided=0.19), also suggesting no significant publication bias. Duval and Tweedie's trim-and-fill method used the random-effects model looking for missing studies to the left of the mean, that is, less positive effect of supplementation, and showed that no studies needed to be trimmed. See Supplementary Results for sensitivity analyses.
Meta-regression
Method-of-moments meta-regressions were performed to explain significant heterogeneity (Q=45.04, P<0.001, I2=73.36). Higher EPA dose (β=0.00037 (0.00009–0.00065), P=0.009), higher percentage antidepressant users (β=0.0058 (0.00017–0.01144), P=0.044) and earlier publication year (β=−0.0735 (−0.143 to −0.004), P=0.04) were significantly associated with a better outcome for PUFA administration, whereas DHA dose (β=0.00015 (−0.00029 to 0.00059), P=0.498), EPA/(EPA+DHA) ratio (β=0.0044 (−0.0034 to 0.012), P=0.272), study duration (β=−0.012 (−0.026 to 0.002), P=0.086), baseline severity (β=−0.00023 (−0.14 to 0.14), P=0.997), age (β=−0.036 (−0.075 to 0.002), P=0.06) and Jadad score (β=−0.0791 (−0.327 to 0.169), P=0.531) were not significantly associated. See Supplementary Results for the sensitivity analyses.
Discussion
Our revised updated meta-analysis adds nuance to the continuing debate on the effect of omega-3 PUFA supplementation on depressive symptoms in MDD. By addressing several issues as noted in the introduction, the present meta-analysis studied effects of omega-3 PUFA supplementation in all available evidence in a specific relatively homogeneous group of MDD subjects. Overall, with an standardized mean difference of 0.398 present meta-analysis shows a beneficial effect of omega-3 PUFAs that is comparable to effects reported in meta-analyses of antidepressants.43 Of note, this effect seemed larger in studies (1) supplementing higher doses of EPA and (2) performed in patients using antidepressants (augmentation/add-on), while it was independent of baseline depression severity or EPA/(EPA+DHA) ratio. However, more recent trials had smaller effect sizes, independent of trial quality.
By including a relatively homogeneous group of patients with MDD according to DSM criteria as assessed using a standardized clinical interview, we aimed to enhance internal validity and generalizability of the present meta-analysis. Nevertheless, the included MDD patients still form a heterogeneous group including both subjects that will benefit from omega-3 PUFA supplementation and those that experience no or even negative effects.21 Several other unreported factors may influence the response to omega-3 PUFA, which could not be tested in the present meta-analysis and meta-regression, for example, measurements of inflammation or nutrigenetics.44, 45, 46, 47
Nevertheless, we were able to test sample and trial-related factors that may influence omega-3 PUFA response. In the present meta-analysis, response was independent of baseline MDD symptom severity. This may be because we included a relatively homogeneous sample of MDD patients in comparison with earlier meta-analyses that also included subjects with depressive symptoms.1 In contrast to the lack of effect of baseline severity, we noticed that the effects of supplementation seemed larger in patients being treated with antidepressants compared with patients not being concurrently treated with antidepressants. Of note, MDD baseline severity was not associated with percentage antidepressant use across trials (P=0.778). Therefore, this larger effect in trials where patients were being treated with antidepressants suggests an interaction between antidepressant use and omega-3 PUFAs at the biological level, for example, due to PUFAs' modulating effect on neuronal membrane–antidepressant interactions or on inflammatory pathways.21, 45, 48, 49 In addition, omega-3 PUFAs may interfere with serotonergic neurotransmission,50 or antidepressant transport across the blood–brain barrier by influencing p-glycoprotein.51 It could be highly clinically relevant to follow up on this finding that omega-3 PUFAs seem to have more effect in studies where participants use antidepressants, by further investigating the interaction between omega-3 PUFAs and antidepressants from (integrated) biological and clinical perspectives.
The present meta-regression finding that higher EPA dose was associated with better response, nuances an earlier meta-analysis that observed significant effects after applying a dichotomous cutoff at 60% EPA content.7 First, although it remained relatively unclear how this previous cutoff was derived, our regression model suggested a linear relationship in all the available data without using an artificial cutoff. In addition, the present meta-analysis also showed that EPA/(EPA+DHA) ratio had no significant effect, nor had DHA dose. Altogether, this suggests that it is not the ratio of EPA vs DHA that is important, but rather the higher EPA dose. Although hypothesized, it does not seem that DHA counteracts the effects of EPA (for example, by competition for target proteins or membrane incorporation);6, 52, 53 DHA simply has no detectable pooled effect on the MDD symptoms. Nevertheless, it remains surprising that EPA seems to be responsible for the beneficial effects of omega-3 PUFA supplementation while DHA concentrations appear to differ more between patients and controls.54 In addition, the regression effect of EPA dose on RCT outcome depended to some extent on one trial that supplemented the highest EPA concentration.26 On the basis of these findings, it could be argued that the beneficial effects of omega-3 PUFA supplementation are not because supplementation corrects a membrane DHA ‘deficit', but rather due to the anti-inflammatory characteristics of EPA's oxidation products.21 Future mechanistic studies in a more continuous dose range should follow up on these findings.
However, these oxidation products may not have positive effects only. Relatively little is known about the precise role and effect of the great diversity of PUFA oxidation products. Although adverse events reported during the studies were usually mild and gastrointestinal in nature (for example, belching, constipation, fishy aftertaste), exposure to PUFA oxidation products with unknown effects may pose unknown risks in the long term.21, 47, 55 It may therefore be advisable to measure these oxidation products during future studies with a longer follow-up to obtain more insight in their potential toxicity. In addition, fishy aftertaste may result in unblinding, potentially distorting effect sizes.4, 56
The finding that more recent studies showed smaller effects remains puzzling. It seems independent from study quality, as we observed no association between study effect size and study quality operationalized as Jadad score. It may be that differences in background diet over time contributed to smaller effects of additional omega-3 PUFA supplementation. For example, even though studies usually excluded participants that used omega-3 PUFA supplements on their own initiative at baseline, it may be that participants started using omega-3 PUFA supplements during the study owing to their increasing popularity, thereby diminishing outcome differences in more recent trials. Unfortunately, not enough trials provided uniform data regarding baseline omega-3 PUFA status or intake to formally test this,57 which would be interesting in future research.
Limitations and strengths
Despite several strengths of this meta-analysis, some limitations should be noted. First, the present meta-analysis has not been performed on patient level data. It would be interesting to confirm these findings on a patient level. Moreover, this may also lead to additional predictors of supplementation outcome, giving rise to extra targets for future precision medicine studies. We hope researchers will be motivated to share and pool available data in the future. Furthermore, while the present meta-analysis had enough power to detect small-to-medium effect sizes, smaller regression effects may have been lost as a result of the smaller number of studies due to the specific in- and exclusion criteria. In addition, several concerns regarding the quality of the available evidence could be made,4 like in most meta-analyses. Further high-quality evidence to support a beneficial effect of EPA in (antidepressant using) MDD patients could lead to more precise estimates of overall effect size. Finally, to maintain power for the meta-regression regarding the effect of percentage antidepressant users on supplementation outcome, we pooled all concurrent antidepressant classes together, while the effects may differ per class. It would be interesting to further test this in future studies including users of different antidepressants.
Nonetheless, the present study/analyses focused on studies that specifically included patients with MDD according to diagnostic criteria as ascertained with a structured clinical interview. We thus limited clinical heterogeneity while still maintaining a substantial overall sample size. As a second strength, by performing extensive nuanced a priori planned publication bias analyses and meta-regression analyses, we revealed new evidence for (1) an effect of EPA dose as opposed to EPA to DHA ratio and (2) an interaction between omega-3 PUFAs and antidepressants, both of which may be highly relevant for clinical practice.
Conclusion
In conclusion, the present meta-analysis observed a beneficial overall effect of omega-3 PUFA supplementation in patients with MDD according to diagnostic criteria, which seemed larger in studies that supplemented higher doses of EPA and included patients taking antidepressants. Future precision/personalized medicine trials should establish whether possible interactions between EPA and antidepressants could provide targets to improve antidepressant response and its prediction.48 Nevertheless, potential long-term biochemical side effects of high-dosed add-on EPA supplementation should be carefully monitored.21
Acknowledgments
RJTM is supported by a PhD scholarship from the Academic Medical Center of the University of Amsterdam. HGR is supported by an NWO/ZonMW VENI-Grant #016.126.059. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Bloch MH, Hannestad J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatry 2012; 17: 1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton KM, Hayward RC, Gunnell D, Peters TJ, Rogers PJ, Kessler D et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. Am J Clin Nutr 2006; 84: 1308–1316. [DOI] [PubMed] [Google Scholar]
- Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr 2010; 91: 757–770. [DOI] [PubMed] [Google Scholar]
- Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R. Omega-3 fatty acids for depression in adults. Cochrane Database Syst Rev 2015; 11: CD004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr 2009; 28: 525–542. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of Eicosapentaenoic Acid (EPA) in clinical trials in depression. J Clin Psychiatry 2011; 72: 1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry 2012; 17: 1144–1149. [DOI] [PubMed] [Google Scholar]
- Lin PY, Mischoulon D, Freeman MP, Matsuoka Y, Hibbeln J, Belmaker RH et al. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry 2012; 17: 1161–1163; author reply 1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C et al. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One 2014; 9: e96905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JR, Han D, Qiao ZX, Tian X, Qi D, Qiu XH. Combined application of eicosapentaenoic acid and docosahexaenoic acid on depression in women: a meta-analysis of double-blind randomized controlled trials. Neuropsychiatr Dis Treat 2015; 11: 2055–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M. Introduction to Meta-analysis. John Wiley & Sons: Chichester, UK, 2009, xxviii, pp 421. [Google Scholar]
- Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry 2008; 42: 192–198. [DOI] [PubMed] [Google Scholar]
- Rizzo AM, Corsetto PA, Montorfano G, Opizzi A, Faliva M, Giacosa A et al. Comparison between the AA/EPA ratio in depressed and non depressed elderly females: omega-3 fatty acid supplementation correlates with improved symptoms but does not change immunological parameters. Nutr J 2012; 11: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondanelli M, Giacosa A, Opizzi A, Pelucchi C, La Vecchia C, Montorfano G et al. Effect of omega-3 fatty acids supplementation on depressive symptoms and on health-related quality of life in the treatment of elderly women with depression: a double-blind, placebo-controlled, randomized clinical trial. J Am Coll Nutr 2010; 29: 55–64. [DOI] [PubMed] [Google Scholar]
- Rondanelli M, Giacosa A, Opizzi A, Pelucchi C, La Vecchia C, Montorfano G et al. Long chain omega 3 polyunsaturated fatty acids supplementation in the treatment of elderly depression: effects on depressive symptoms, on phospholipids fatty acids profile and on health-related quality of life. J Nutr Health Aging 2011; 15: 37–44. [DOI] [PubMed] [Google Scholar]
- Lesperance F, Frasure-Smith N, St-Andre E, Turecki G, Lesperance P, Wisniewski SR. The efficacy of omega-3 supplementation for major depression: a randomized controlled trial. J Clin Psychiatry 2011; 72: 1054–1062. [DOI] [PubMed] [Google Scholar]
- Mozaffari-Khosravi H, Yassini-Ardakani M, Karamati M, Shariati-Bafghi SE. Eicosapentaenoic acid versus docosahexaenoic acid in mild-to-moderate depression: a randomized, double-blind, placebo-controlled trial. Eur Neuropsychopharmacol 2013; 23: 636–644. [DOI] [PubMed] [Google Scholar]
- Lucas M, Asselin G, Merette C, Poulin MJ, Dodin S. Ethyl-eicosapentaenoic acid for the treatment of psychological distress and depressive symptoms in middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. Am J Clin Nutr 2009; 89: 641–651. [DOI] [PubMed] [Google Scholar]
- Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry 2002; 59: 913–919. [DOI] [PubMed] [Google Scholar]
- da Silva TM, Munhoz RP, Alvarez C, Naliwaiko K, Kiss A, Andreatini R et al. Depression in Parkinson's disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord 2008; 111: 351–359. [DOI] [PubMed] [Google Scholar]
- Assies J, Mocking RJ, Lok A, Ruhe HG, Pouwer F, Schene AH. Effects of oxidative stress on fatty acid- and one-carbon-metabolism in psychiatric and cardiovascular disease comorbidity. Acta Psychiatr Scand 2014; 130: 163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: a randomized controlled trial. JAMA 2009; 302: 1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot M, Pouwer F, Assies J, Jansen EH, Diamant M, Snoek FJ et al. Eicosapentaenoic acid as an add-on to antidepressant medication for co-morbid major depression in patients with diabetes mellitus: a randomized, double-blind placebo-controlled study. J Affect Disord 2010; 126: 282–286. [DOI] [PubMed] [Google Scholar]
- Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther 2008; 88: 156–175. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol 2003; 13: 267–271. [DOI] [PubMed] [Google Scholar]
- Mischoulon D, Papakostas GI, Dording CM, Farabaugh AH, Sonawalla SB, Agoston AM et al. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J Clin Psychiatry 2009; 70: 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody TJ, Rush AJ, Bernstein I, Warden D, Brannan S, Burnham D et al. The Montgomery Asberg and the Hamilton ratings of depression: a comparison of measures. Eur Neuropsychopharmacol 2006; 16: 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003; 54: 573–583. [DOI] [PubMed] [Google Scholar]
- Kooshki A, Tofighiyan T. The effects of omega-3 fatty acids on depression in female students. [Persian]. Iran J Obstet Gynecol Infertil 2014; 17: 1–6. [Google Scholar]
- Ginty AT, Conklin SM. Short-term supplementation of acute long-chain omega-3 polyunsaturated fatty acids may alter depression status and decrease symptomology among young adults with depression: a preliminary randomized and placebo controlled trial. Psychiatry Res 2015; 229: 485–489. [DOI] [PubMed] [Google Scholar]
- Park Y, Park YS, Kim SH, Oh DH, Park YC. Supplementation of n-3 polyunsaturated fatty acids for major depressive disorder: a randomized, double-blind, 12-week, placebo-controlled trial in Korea. Ann Nutr Metab 2015; 66: 141–148. [DOI] [PubMed] [Google Scholar]
- Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids 2005; 72: 211–218. [DOI] [PubMed] [Google Scholar]
- Gharekhani A, Khatami MR, Dashti-Khavidaki S, Razeghi E, Noorbala AA, Hashemi-Nazari SS et al. The effect of omega-3 fatty acids on depressive symptoms and inflammatory markers in maintenance hemodialysis patients: a randomized, placebo-controlled clinical trial. Eur J Clin Pharmacol 2014; 70: 655–665. [DOI] [PubMed] [Google Scholar]
- Mischoulon D, Nierenberg AA, Schettler PJ, Kinkead BL, Fehling K, Martinson MA et al. A double-blind, randomized controlled clinical trial comparing eicosapentaenoic acid versus docosahexaenoic acid for depression. J Clin Psychiatry 2015; 76: 54–61. [DOI] [PubMed] [Google Scholar]
- Grenyer BF, Crowe T, Meyer B, Owen AJ, Grigonis-Deane EM, Caputi P et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31: 1393–1396. [DOI] [PubMed] [Google Scholar]
- Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002; 159: 477–479. [DOI] [PubMed] [Google Scholar]
- Gertsik L, Poland RE, Bresee C, Rapaport MH. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J Clin Psychopharmacol 2012; 32: 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischoulon D, Papakostas GI, Dording CM, Farabaugh AH, Sonawalla SB, Agoston AM et al. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J Clin Psychiatry 2009; 70: 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry 2003; 160: 996–998. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Mata S, Sanchez P, Gonzalez D, Urbina M, Fazzino F et al. Omega-3 fatty acids as adjunctive of antidepressant therapy and its effects on brain-derived neurotrophic factor in serum, monocytes and lymphocytes. Arch Venezolanos Farmacol Ter 2011; 30: 72–78. [Google Scholar]
- Fiedorowicz JG, Hale N, Spector AA, Coryell WH. Neuroticism but not omega-3 fatty acid levels correlate with early responsiveness to escitalopram. Ann Clin Psychiatry 2010; 22: 157–163. [PMC free article] [PubMed] [Google Scholar]
- Schalkwijk S, Undurraga J, Tondo L, Baldessarini RJ. Declining efficacy in controlled trials of antidepressants: effects of placebo dropout. Int J Neuropsychopharmacol 2014; 17: 1343–1352. [DOI] [PubMed] [Google Scholar]
- Mocking RJ, Lok A, Assies J, Koeter MW, Visser I, Ruhe HG et al. Ala54Thr fatty acid-binding protein 2 (FABP2) polymorphism in recurrent depression: associations with fatty acid concentrations and waist circumference. PLoS One 2013; 8: e82980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry 2016; 21: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocking RJ, Assies J, Koeter MW, Ruhe HG, Lok A, Schene AH. Bimodal distribution of fatty acids in recurrent major depressive disorder. Biol Psychiatry 2012; 71: e3–e5. [DOI] [PubMed] [Google Scholar]
- Mocking RJT, Assies J, Bot M, Jansen EHJM, Schene AH, Pouwer F. Biological effects of add-on eicosapentaenoic acid supplementation in diabetes mellitus and co-morbid depression: a randomized controlled trial. PLoS One 2012; 7: e49431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocking RJ, Verburg HF, Westerink AM, Assies J, Vaz FM, Koeter MW et al. Fatty acid metabolism and its longitudinal relationship with the hypothalamic-pituitary-adrenal axis in major depression: associations with prospective antidepressant response. Psychoneuroendocrinology 2015; 59: 1–13. [DOI] [PubMed] [Google Scholar]
- Fisar Z. Interactions between tricyclic antidepressants and phospholipid bilayer membranes. Gen Physiol Biophys 2005; 24: 161–180. [PubMed] [Google Scholar]
- Kodas E, Galineau L, Bodard S, Vancassel S, Guilloteau D, Besnard JC et al. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J Neurochem 2004; 89: 695–702. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Walker TH, Luo PG, Chen CF. Long-chain polyunsaturated fatty acids promote paclitaxel cytotoxicity via inhibition of the MDR1 gene in the human colon cancer Caco-2 cell line. J Am Coll Nutr 2011; 30: 265–273. [DOI] [PubMed] [Google Scholar]
- Bazinet RP, Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci 2014; 15: 771–785. [DOI] [PubMed] [Google Scholar]
- Russell FD, Burgin-Maunder CS. Distinguishing health benefits of eicosapentaenoic and docosahexaenoic acids. Mar Drugs 2012; 10: 2535–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry 2010; 68: 140–147. [DOI] [PubMed] [Google Scholar]
- Serini S, Fasano E, Piccioni E, Cittadini AR, Calviello G. Dietary n-3 polyunsaturated fatty acids and the paradox of their health benefits and potential harmful effects. Chem Res Toxicol 2011; 24: 2093–2105. [DOI] [PubMed] [Google Scholar]
- Golomb BA, Erickson LC, Koperski S, Sack D, Enkin M, Howick J. What's in placebos: who knows? Analysis of randomized, controlled trials. Ann Intern Med 2010; 153: 532–535. [DOI] [PubMed] [Google Scholar]
- Mocking RJT, Assies J, Lok A, Ruhe HG, Koeter MWJ, Visser I et al. Statistical methodological issues in handling of fatty acid data: percentage or concentration, imputation and indices. Lipids 2012; 47: 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.