Abstract
Introduction:
Whole slide imaging (WSI) permits intraoperative consultations (frozen sections) to be performed remotely. However, WSI files are large and can be problematic if there are tissue artifacts (e.g., tissue folds) or when slides are scanned without multiplanes (Z-stacks) to permit focusing. The Panoptiq dynamic imaging system allows users to create their own digital files that combine low power panoramic digital images with regions of interest that can be imaged using high power Z-stacks. The aim of this study was to determine the utility of the Panoptiq dynamic imaging system for frozen section telepathology.
Materials and Methods:
Twenty archival randomly selected genitourinary surgical pathology frozen sectional cases were evaluated using conventional light microscopy (glass slides), panoramic images, and whole slide images. To create panoramic images glass slides were digitized using a Prosilica GT camera (model GT1920C, Allied Vision Technologies) attached to an Olympus B × 45 microscope and Dell Precision Tower 810 computer (Dell). Panoptiq 3 version 3.1.2 software was used for image acquisition and Panoptiq View version 3.1.2 to view images (ViewsIQ, Richmond, BC, Canada). Image acquisition using Panoptiq software involved a pathology resident, who manually created digital maps (×4 objective) and then selected representative regions of interest to generate Z-stacks at higher magnification (×40 objective). Whole slide images were generated using an Aperio XT Scanscope (Leica) and viewed using ImageScope Software (Aperio ePathology, Leica). Three pathologists were asked to render diagnoses and rate image quality (1–10) and their diagnostic confidence (1–10) for each modality.
Results:
The diagnostic concordance with glass slides was 98.3% for panoramic images and 100% for WSI. Panoptiq images were comparable to the glass slide viewing experience in terms of image quality and diagnostic confidence. Complaints regarding WSI included poor focus near tissue folds and air bubbles. Panoptiq permitted fine focusing of tissue folds and air bubbles. Issues with panoramic images included difficulty interpreting low-resolution ×4 image maps and the presence of tiling artifacts. In some cases, Z-stacked areas of Panoptiq images were limited or not representative of diagnostic regions. The image file size of Panoptiq was more than 14 times smaller than that of WSI files.
Conclusions:
The Panoptiq imaging system is a novel tool that can be used for frozen section telepathology. Panoramic digital images were easy to generate and navigate, of relatively small file size, and offered a mechanism to overcome focusing problems commonly encountered with WSI of frozen sections. However, the acquisition of representative Panoptiq images was operator dependent with the individual creating files that may impact the final diagnosis.
Keywords: Digital pathology, frozen section, panoramic, telepathology, whole slide imaging, Z-stack
INTRODUCTION
Telepathology was first described in 1986 as a tool to deliver pathology services over a distance.[1] In the age of increasing subspecialization and associated centralization of pathology services, telepathology for primary frozen section diagnosis may be warranted to have experts render intraoperative diagnoses.[2,3] Telepathology was used for primary frozen section diagnosis as early as 1989 in Norway, where pathologists provided intraoperative consultation to several distant hospitals with 100% accuracy using videotelemicroscopy.[4] Telepathology has since been used by many other Pathology Departments, including consultation for frozen sections.[5,6,7,8,9] Major limitations to the widespread use of telepathology for frozen sections are the cost of equipment, time and staff required to set up and maintain these programs, and perhaps pathologist technophobia.[10] Telepathology may be slow due to poor connectivity or handling huge file sizes. Moreover, there is always the possibility of an inaccurate diagnosis either due to suboptimal image quality, sampling issues, or focusing problems that might lead to inappropriate intraoperative management with adverse patient outcomes and medicolegal consequences. The latter apprehension persists, despite numerous studies validating an average diagnostic accuracy rate of 96% (ranging from 89% to 100%) when telepathology was used for primary frozen section diagnosis.[2]
For routinely reading frozen sections, most pathologists scan glass slides at × 20 magnification. Scanning slides at higher magnification would likely entail prolonged scanning time and working with larger file sizes, which may hamper turnaround time. Digital slides can be difficult to interpret if there are tissue artifacts (e.g., thick sections and tissue folds) or slide imperfections (e.g., air bubbles, tissue extending beyond the coverslip, excess mounting medium, obscuring dirt), especially when glass slides are scanned without multiplanes (Z-stacks) to permit focusing.
The Panoptiq dynamic imaging system (Panoptiq™ 3, ViewsIQ, Richmond, BC, Canada) allows users to create their own digital files with the option to combine low power panoramic digital images with regions of interest that can be imaged using high power Z-stacks. The Panoptiq system works with any microscope that can accommodate a mounted digital camera. While an individual examines a glass slide with the microscope, Panoptiq software digitally stitches together multiple fields of view into a single panoramic view in real-time. The system is also capable of digitizing slides at multiple magnifications and focal planes (Z-stacks). An acquired panoramic image can be transmitted as a dynamic live image or saved to a distant location. To date, the Panoptiq system has been evaluated as a dynamic telepathology tool to triage peripheral blood smears for external diagnostic consultation.[11] Preliminary investigations by our group have also shown that this system is applicable for imaging microbiology and cytology slides.[12,13]
The aim of this study was to determine the utility of the Panoptiq dynamic imaging system, compared to whole slide imaging (WSI), for frozen section telepathology.
MATERIALS AND METHODS
Image Acquisition
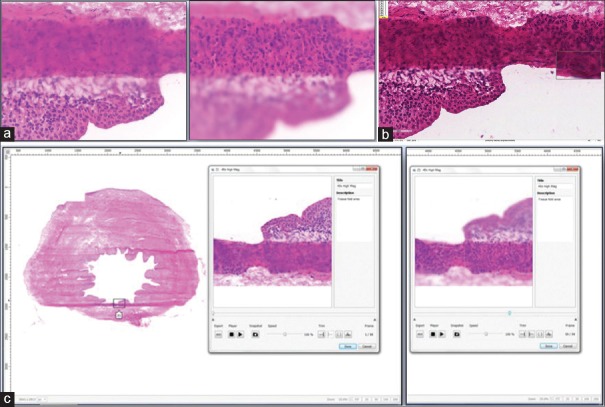
The Panoptiq dynamic imaging system consisted of a Prosilica GT digital video camera (model GT1920C, Allied Vision Technologies) mounted to an Olympus B × 45 microscope [Figure 1]. The microscope had × 4, ×10, ×20, and × 40 objectives (Olympus UPlanApo × 4, UPlanApo × 10, UPlanApo × 20, UPlanFI × 40). The camera was attached to a Dell Precision Tower 810 computer (Dell). Panoptiq 3 version 3.1.2 software was installed on this computer and used for image acquisition. Panoptiq View version 3.1.2 software was required to view images (ViewsIQ, Richmond, BC, Canada). When a user at the microscope moved a glass slide on the microscope stage, the software digitally captured the field of view and stitched it to the previous fields of view in real-time, creating a single panoramic (mosaic) image (.svs file format). We accomplished this using a variety of objective lenses (×2, ×10 and × 20). The Panoptiq Z feature was used to capture a particular area of interest on a slide, at higher magnification (×40) while the user focused up and down. This Z-stack area with multiple focal planes (frames, or collection of images at different depths of field or focus) was pinned over the corresponding area of the low power digital image [Figure 2]. A Z-stack recording up to 90-s is permitted. When a Z-stack gets created an Extensible Markup Language (XML) annotation file gets saved along with the image, defining the position of the Z-stack relative to the scan and other annotations. The Z-stack video file is saved in. zvs format, which is a proprietary format. The size of this file depends on the number of frames in the Z-stack video. The Panoptiq camera captures Z-stack videos at 15 frames per second. Therefore, a 4-s video Z-stack containing sixty frames should be under 30 MB (i.e., 0.4–0.5 MB per frame). When viewing a panoramic image, if one clicks on a saved Z-stack file a video plays showing all captured frames, replicating the focusing action that was performed with the microscope. There is no limit to the number of Z-stacked areas that can be obtained. While playing a Z-stack file the user can stop at any desired Z-plane (frame) to examine the image.
Figure 1.

Prosilica GT camera attached via a C-mount to a microscope was used to manually acquire panoramic digital images
Figure 2.
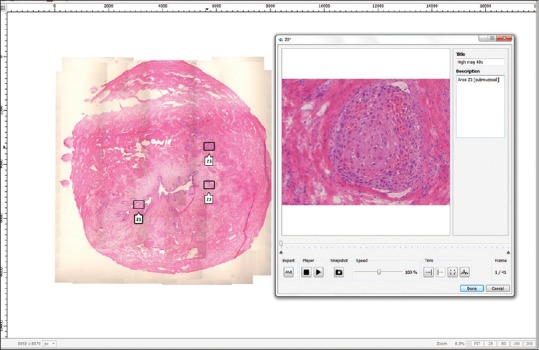
Panoramic digital image shown of a ureter margin frozen section with invasive squamous cell carcinoma (H & E, stain). The left image shows a thumbnail overview of the digital map created at × 4. Note the focal linear stitching artifact. Three regions of interest digitally captured at higher magnification (×40) while focusing up and down with the microscope's fine focus can be seen superimposed as Z-stack boxes (Z1, Z2, and Z3). Box Z1 when opened (right image), shows an invasive focus of squamous cell carcinoma at × 40. Note that this Z1 file is composed of 41 stacked frames of sequential focal planes
Whole slide images were generated by automated scanning of glass slides at ×20 using an Aperio XT Scanscope (Leica). The scanner used a ×20 lens (Olympus UPlanSApo) with a numerical aperture of 0.75. Glass slides were scanned without Z-stacking, producing uniplanar digital slides. Digital slides (.svs format) were viewed using ImageScope software (Aperio ePathology, Leica Biosystems; http://www.leicabiosystems.com/digital-pathology/digital-pathology-management/imagescope/).
Comparative Study
A total of twenty genitourinary pathology frozen section cases were randomly selected and de-identified. These cases represented surgical margins (distal ureter margins, bladder, urethra, and penis), organs (kidney) and other tissues (perirectal soft tissue and paracaval mass) that had been previously submitted for frozen section. A variety of benign (e.g., reactive urothelium and fibromuscular stroma), atypical (e.g., urothelial dysplasia), and neoplastic (e.g., high-grade urothelial carcinoma) diagnoses were included. A single glass slide (H and E stain) from each case was used for this study. Study cases with histology imperfections were not intentionally chosen. A pathology resident (DP) manually acquired all Panoptiq images, all of which included a low magnification digital map (×4 magnification) and Z-stacks (×40 magnification) based on regions of interest that he determined to be diagnostically important. Three board certified anatomic pathologists (SM, AVP, and LP) rendered diagnoses on each case using the three different modalities: Glass slides with a conventional light microscope, Panoptiq images saved on the Dell workstation, and Aperio whole slide images saved on a remote server. All digital images were viewed on HP ZR24w (1920 × 1200) monitors. Pathologists were also asked to rate the image quality and their diagnostic confidence for each modality on a scale of 1–10 (ten being the best quality of slide and the highest diagnostic confidence, respectively), as well as describe any difficulties faced during their evaluation of slides or images. Reviewers were blinded to the original diagnosis. Cases were reviewed with a washout period of 2 weeks between each modality.
RESULTS
Table 1 summarizes the results of this study. All pathologists were able to correctly diagnose almost all of the cases, with overall high diagnostic concordance among the different modalities. The diagnostic concordance with glass slides was 98.3% for Panoptiq images and 100% for Aperio WSI. The single case where the diagnosis was missed with the Panoptiq modality was due to the inclusion of nonrepresentative Z-stacks, which was not diagnostic of the lesion. Panoramic images were comparable to viewing glass slides under a conventional microscope in terms of image quality and diagnostic confidence and were reported by participants to be superior to WSI. Complaints regarding WSI included out of focus areas near tissue folds [Figure 3] and under air bubbles [Figure 4]. Panoptiq permitted fine focusing of tissue folds and air bubbles in Z-stacked files [Figure 5], allowing pathologists to reliably interpret tissue in these challenging areas. Issues with Panoptiq images included difficulty interpreting low-resolution (×4 magnification) image maps and the presence of tiling artifacts. In some cases, the Z-stacked areas of Panoptiq images were considered by pathologists to be limited or not representative of diagnostic regions. The image file size of Panoptiq images was more than 14 times smaller than that of Aperio WSI files. The XML files of Z-stacks that accompany each of our saved panoramic SVS files were only 1–2 KB in size.
Table 1.
Comparison of different modalities for reading frozen sections
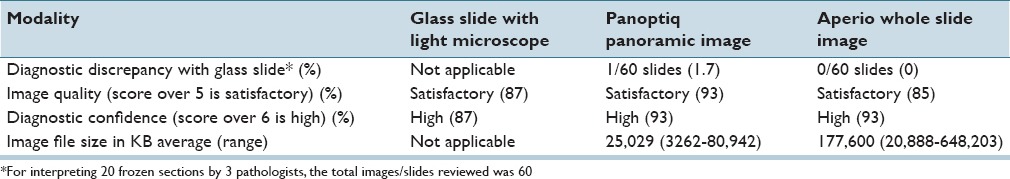
Figure 3.
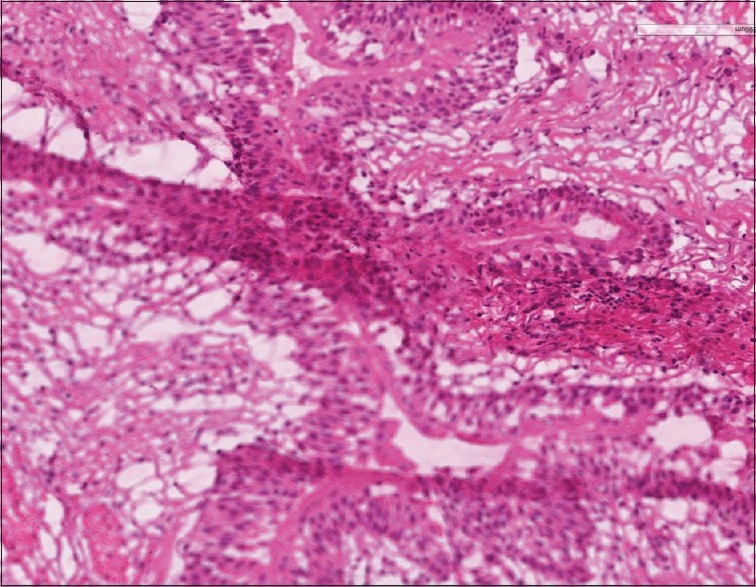
Whole slide image demonstrating that due to the tissue fold in this frozen section most of the scanned tissue (lower half of the image) is out of focus (H & E, stain)
Figure 4.
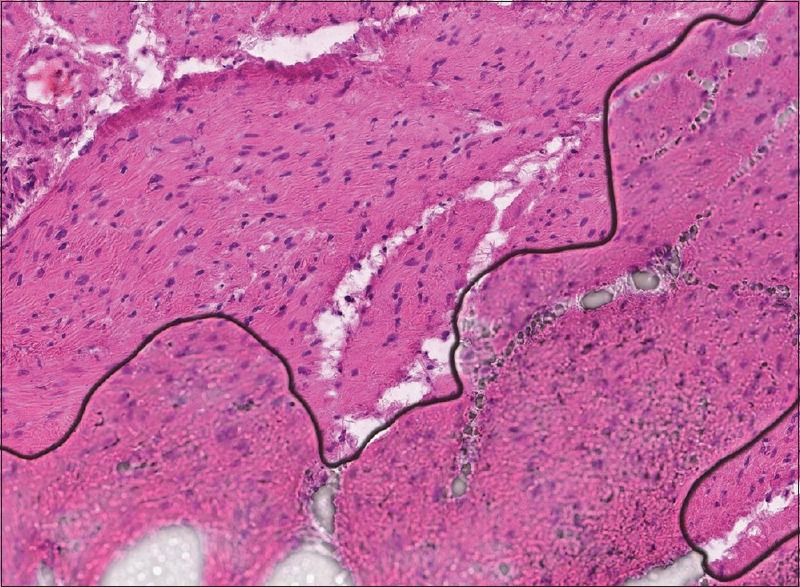
Whole slide image demonstrating that the section of tissue beneath the air bubble (lower half of the image) is out of focus (H & E, stain)
Figure 5.
A challenging frozen section of a distal ureter margin is shown, which was difficult to interpret due to a tissue fold (H & E, stain). (a) With the glass slide examined under a traditional light microscope and using a fine focus control, a pathologist can focus up and down along the vertical plane (Z-axis) to examine the tissue adjacent to the fold (left) and within (right) the fold (×40 magnification). (b) With a uniplanar whole slide image, maximally zoomed to × 40, the tissue fold remains out of focus. (c) With the Panoptiq panoramic image the Z-stacked box (containing 99 frames at different Z-planes) captured at × 40 magnification, allows the tissue to be examined similarly to a light microscope; note that with frame 1 (left) tissue adjacent to the fold is in focus whereas with frame 59 (right) only tissue in the fold is in focus
DISCUSSION
The major benefit of employing telepathology for remotely handling intraoperative consultations is the ability to obtain a distant expert pathologist's opinion in a timely manner, which improves patient care. Imaging technology used for this purpose has advanced and includes several options including live video streaming, robotic microscopy, WSI, and more recently hybrid devices that permit both WSI and real-time robotic telemicroscopy. Improvements in image resolution and slide navigation have resulted in fewer deferrals, increased diagnostic accuracy, and more timely interpretations.[2,3] However, glass slides prepared during frozen sections are often challenging to digitize for several reasons: Their coverslips may move if they have recently applied wet mounting medium, there may be excess mounting medium spilled onto the coverslip if they were prepared manually by someone who is inexperienced, air bubbles are frequently trapped under the coverslip, and they often have tissue folds. All of these artifacts make it difficult to examine frozen sections in digital format. Moreover, these cases typically need to be rapidly interpreted (e.g., 20 min or less).
The application of WSI for frozen sections has several advantages over real-time robotic microscopy such as quicker navigation of digital images and archiving of scanned slides.[2] Unfortunately, WSI acquired at ×20 with a single Z-plane may be insufficient to overcome focusing issues due to some of the aforementioned tissue and/or slide artifacts. Scanning frozen section slides with Z-stacking may be impractical if it takes significantly longer to digitize. Moreover, such multiplanar images involve large file sizes which are harder to manage and more expensive to store. For this reason, many centers performing real-time telepathology of frozen sections have either retained their old robotic microscopes (e.g., Trestle system from Zeiss) or invested in newer hybrid WSI/robotic instruments that still allow live remote viewing.
In this study, we have shown that the Panoptiq imaging system can be used for frozen section telepathology. Panoramic images were comparable to viewing glass slides on a conventional light microscope. This system allows any microscope objective to be used from ×2 to ×100 oil immersion to capture images. Panoptiq digital images were easy to generate, albeit that they had to be created manually with a microscope that has an attached digital camera. Although we did not record the time spent generating images for each case, on average we noted that it took 5-10 min to generate a panoramic image with fewer than five Z-stacks. Moreover, after gaining experience with the system subsequent image acquisition was quicker. Hence, the time required to prepare a suitable digital slide for a confident diagnosis, even if Z-stacking is used, is unlikely to significantly impact turnaround time requirements for single block frozen sections. Panoramic images were easy to navigate, allowing a user to quickly review captured slides at low power to determine the overall architecture, and then review superimposed Z-stacked boxes to examine areas of interest at much higher magnification with focus capabilities. The multiplanar Z-stacks overcame focusing problems commonly encountered with WSI of frozen sections, such as tissue folds. Because Panoptiq images can be saved, this digital imaging solution allows these files to be transmitted and/or later retrieved for review in distant locations. The relatively small file size of Panoptiq images is beneficial because this simplifies image management (e.g., connectivity demands) and the need for expensive storage. The overall file size will increase if many Z-stacks are captured with each panoramic image. On average, most users generate a 200–300 MB file per Z-stack. With the ease of rapidly capturing an image of relatively small file size, this hastens the entire process of acquiring and viewing a case, which is a major consideration for intraoperative consultations. However, we noticed that if image stitching is done too rapidly, the final digital map had tiling artifacts.
The acquisition of representative Panoptiq images, particularly Z-stacks of key regions, was entirely dependent upon the individual creating these digital files, which in one case negatively impacted the final diagnosis. Therefore, the individual creating panoramic images needs to be well trained not only to use this system but also requires a certain level of diagnostic competency to make sure that appropriate areas are reliably captured for subsequent review. For settings with an experienced user (e.g., pathologist, pathology resident), the Panoptiq system could function in the real world for frozen section telepathology. However, a histotechnologist likely does not have the diagnostic expertise to capture areas of subtle diagnostic significance. Therefore, we would not recommend using the Panoptiq system in a situation where there is no on-site pathologist or equivalent suitable user to create a digital panoramic slide. If the process is performed in real-time, the person acquiring the image and the consulting pathologist may engage in a bi-directional dialog and could reselect areas of interest for recording Z-stacks.
Limitations of our study are the small number of cases analyzed. However, this was a proof-of-concept exercise and not intended to represent a complete validation study of this new technology. Additional clinical and education applications are being conducted. Other limitations of this study were that only frozen sections from one subspecialty were evaluated, and only one person acquired all of the images. This may have introduced operator and reviewer bias. Nevertheless, this study demonstrates that using the Panoptiq imaging system for frozen section telepathology exhibits comparable diagnostic performance to conventional microscopy. Further studies are encouraged to confirm if this innovative digital solution can be safely introduced into routine practice for an intraoperative consultation.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
Dr. Pantanowitz consults for Omnyx. Dr. Parwani has intellectual property rights with Omnyx.
Acknowledgment
We would like to thank Herman Lo from ViewsIQ for technical assistance.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2016/7/1/26/181770
REFERENCES
- 1.Weinstein RS, Bloom KJ, Rozek LS. Telepathology and the networking of pathology diagnostic services. Arch Pathol Lab Med. 1987;111:646–52. [PubMed] [Google Scholar]
- 2.Evans AJ, Chetty R, Clarke BA, Croul S, Ghazarian DM, Kiehl TR, et al. Primary frozen section diagnosis by robotic microscopy and virtual slide telepathology: The University Health Network experience. Hum Pathol. 2009;40:1070–81. doi: 10.1016/j.humpath.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Pantanowitz L, Wiley CA, Demetris A, Lesniak A, Ahmed I, Cable W, et al. Experience with multimodality telepathology at the University of Pittsburgh Medical Center. J Pathol Inform. 2012;3:45. doi: 10.4103/2153-3539.104907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordrum I, Engum B, Rinde E, Finseth A, Ericsson H, Kearney M, et al. Remote frozen section service: A telepathology project in Northern Norway. Hum Pathol. 1991;22:514–8. doi: 10.1016/0046-8177(91)90226-f. [DOI] [PubMed] [Google Scholar]
- 5.Horbinski C, Fine JL, Medina-Flores R, Yagi Y, Wiley CA. Telepathology for intraoperative neuropathologic consultations at an academic medical center: A 5-year report. J Neuropathol Exp Neurol. 2007;66:750–9. doi: 10.1097/nen.0b013e318126c179. [DOI] [PubMed] [Google Scholar]
- 6.Liang WY, Hsu CY, Lai CR, Ho DM, Chiang IJ. Low-cost telepathology system for intraoperative frozen-section consultation: Our experience and review of the literature. Hum Pathol. 2008;39:56–62. doi: 10.1016/j.humpath.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Fallon MA, Wilbur DC, Prasad M. Ovarian frozen section diagnosis: Use of whole-slide imaging shows excellent correlation between virtual slide and original interpretations in a large series of cases. Arch Pathol Lab Med. 2010;134:1020–3. doi: 10.5858/2009-0320-OA.1. [DOI] [PubMed] [Google Scholar]
- 8.Ramey J, Fung KM, Hassell LA. Use of mobile high-resolution device for remote frozen section evaluation of whole slide images. J Pathol Inform. 2011;2:41. doi: 10.4103/2153-3539.84276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer TW, Slaw RJ, McKenney JK, Patil DT. Validation of whole slide imaging for frozen section diagnosis in surgical pathology. J Pathol Inform. 2015s;6:49. doi: 10.4103/2153-3539.163988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans AJ, Kiehl TR, Croul S. Frequently asked questions concerning the use of whole-slide imaging telepathology for neuropathology frozen sections. Semin Diagn Pathol. 2010;27:160–6. doi: 10.1053/j.semdp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Goswami R, Pi D, Pal J, Cheng K, Hudoba De Badyn M. Performance evaluation of a dynamic telepathology system (Panoptiq™) in the morphologic assessment of peripheral blood film abnormalities. Int J Lab Hematol. 2015;37:365–71. doi: 10.1111/ijlh.12294. [DOI] [PubMed] [Google Scholar]
- 12.Rhoads DD, Ahmed I, Pantanowitz L. Feasibility of using the Panoptiq imaging system for telemicrobiology. J Pathol Inform. 2015;6:S51–2. [Google Scholar]
- 13.Pantanowitz L, Cuda J, Xing J, Ahmed I, Parwani AV, Monaco SE. Panoramic digital images (Panoptiq) for cytopathology screening and interpretation. J Am Soc Cytopathol. 2015;4:S66–7. [Google Scholar]